Introduction
Atherosclerosis, a chronic inflammatory disease, is
a major factor leading to cardiovascular complications (1). Following the upregulation of
inflammatory chemokines, monocytes adhere to endothelial cells in
the arterial wall and differentiate into macrophages, which ingest
lipoproteins and form foam cells. Vascular smooth muscle cells
proliferate and synthesize extracellular matrix proteins. The above
processes lead to plaque formation (2).
Wnt5a is a highly conserved secreted glycoprotein of
the Wnt protein family, which activates the β-catenin-independent
signaling pathway, including the planar cell polarity (PCP) pathway
and the Ca2+ pathway, which then further activates
downstream signals, including Jun N-terminal kinase (JNK), protein
kinase C (PKC) and Ca2+/calmodulin-dependent kinase II
(CaMKII) (3). Wnt5a has been
implicated in inflammatory diseases, including rheumatoid
arthritis, atherosclerosis, psoriasis and sepsis, suggesting that
it plays a critical biological role in the regulation of
inflammation (4–7). In vitro studies have
demonstrated that Wnt5a promotes the expression of inflammatory
cytokines, chemokines and matrix metalloproteinases (MMPs)
(8). Wnt5a can activate nuclear
factor-κB (NF-κB) which is involved in the expression of
inflammatory genes (9). Wnt5a
also stimulates chemotactic migration and chemokine production by
activating the p38 and extracellular signal-regulated kinase (ERK)
pathways (10).
Christman et al (5) reported that Wnt5a expression was
expressed in human and murine atherosclerotic lesions. It has
recently been demonstrated that Wnt5a levels are elevated in the
serum of atherosclerotic patients relative to healthy controls and
that the expression of Wnt5a is increased in advanced human
atherosclerotic lesions (11).
These data suggest an active role of Wnt5a in the pathogenesis of
atherosclerosis. However, the direct effects of Wnt5a on
atherosclerotic lesions and the underlying mechanisms have not been
well delineated.
In the present study, we used adenovirus
(Ad)-mediated small interfering RNA (siRNA) to target Wnt5a in a
mouse model of atherosclerosis. Subsequently, the effects of Wnt5a
knockdown on atherosclerotic lesions and the potential mechanisms
involved were investigated.
Materials and methods
Animal model
Eight-week-old male apolipoprotein E-deficient
(ApoE−/−) mice with a C57BL/6J genetic background were
obtained from Beijing University of Medicine Laboratory (Beijing,
China). All experimental protocols were approved by the
Institutional Animal Care Committee of Henan Provincial People’s
Hospital, Zhengzhou, China. All the mice were kept at 25°C on a
12-h light/dark cycle and fed a high-fat diet (15% fat, 0.25%
cholesterol). All efforts were made to minimize suffering. Mice
were euthanized by an injection with pentobarbital sodium (100
mg/kg) intraperitoneally.
Construction of recombinant adenovirus
carrying siRNA
According to the Wnt5a gene sequence (GenBank
accession no. NM_009524), an effective siRNA for Wnt5a (sense,
5′-GAAGCCCAUUGGAAUAUUATT-3′ and antisense,
5′-UAAUAUUCCAAUGGGCUUCTT-3′) was designed and synthesized by
Genepharma Co., Ltd. (Shanghai, China). Non-specific siRNA
sequences (sense, 3′-UUCUCCGAACG UGUCACGUUU-5′ and antisense,
3′-ACGUGACACGUUC GGAGAAUU-5′) were used as the control. The
recombinant adenovirus was constructed as previously described
(12). The siRNA sequences were
amplified and subcloned into the pAdTrack-cytomegalovirus (CMV)
plasmid, an adenoviral shuttle plasmid. Subsequently, the
recombinant shuttle plasmids, pAdTrack-CMV and pAdEasy-1, were
homologously recombined in Escherichia coli strain BJ5183.
The obtained recombinant plasmids were then transfected into human
embryonic kidey (HEK)-293 cells (Type Culture Collection of the
Chinese Academy of Sciences, Shanghai, China) to generate
recombinant adenovirus. The virus was amplified and purified, and
titers were determined using the p24 ELISA kit (Cell Biolabs, Inc.,
San Diego, CA, USA).
Treatment of mice with recombinant
adenovirus
After 10 weeks on a high-fat diet, the mice were
randomly divided into 3 groups (n=15 in each group): the mock group
which received a 200-μl phosphate-buffered saline (PBS) injection;
the Ad-NC group which received a 200-μl (1×1010
plaque-forming units of virus) injection of recombinant adenovirus
expressing non-specific siRNA; the Ad-Wnt5a siRNA group which
received 200-μl (1×1010 plaque-forming units) injection
of recombinant adenovirus expressing Wnt5a siRNA. Two weeks later,
a second injection was administered in a similar fashion as
described above, and the mice were euthanized for analysis 2 weeks
after the second injection. Blood samples were collected by cardiac
puncture from mice fasted overnight and concentrations of plasma
total cholesterol (TC), triglyceride (TG), high-density lipoprotein
(HDL) and low-density lipoprotein (LDL) were detected using the
ELISA kit (Shanghai Bangyi Biotechnology Co., Ltd., Shanghai,
China) on an automatic analyzer (Roche P800; Roche Diagnostics,
Indianapolis, IN, USA).
Analysis of atherosclerosis
Prior to carotid artery isolation, the mouse hearts
were perfused with PBS, followed by 4% paraformaldehyde for 30 min
under physiological pressure. Afterwards, the isolated carotid
artery was fixed with 4% paraformaldehyde for 12 h, then embedded
in paraffin and cut into 5-μm serial sections. The paraffin
sections were prepared and stained with hematoxylin and eosin
(H&E) (Sigma, St. Louis, MO, USA). For the detection of
collagen, the sections were stained with picrosirius red (Sigma).
Lipid-rich lesions were identified by Oil Red O (Sigma) staining.
The plaque area, collagen area and lipid area were observed and
calculated using Image-Pro Plus 6.0 software (Media Cybernetics
Inc., Rockville, MD, USA).
Quantitative reverse
transcription-polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the carotid arteries
using the RNeasy Fibrous Tissue mini kit (Qiagen, Valencia, CA,
USA) and reverse transcribed into cDNA using a PrimeScript™ 1st
Strand cDNA Synthesis kit (Takara, Dalian, China). Quantitative PCR
was performed using SYBR® Premix DimerEraser™ (Takara)
to detect the mRNA expression of Wnt5a, cyclooxygenase-2 (COX-2),
MMP-2, MMP-9 and monocyte chemotactic protein-1 (MCP-1). The primer
sequences used are listed in Table
I. Quantitative measurements were determined using the ΔΔCt
method and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was
used as the internal standard for all samples. The RT-qPCR
reactions were performed in triplicate.
 | Table IPrimers used for RT-qPCR. |
Table I
Primers used for RT-qPCR.
| Gene | Forward primer
(5′→3′) | Reverse primer
(5′→3′) |
|---|
| Wnt5a |
AATCCACGCTAAGGGTTCCTATGAG |
AGCCAGCACGTCTTGAGGCTA |
| COX-2 |
CCCAGAGCTCCTTTTCAACC |
ATTTGGCACATTTCTTCCCC |
| MMP-2 |
ACCCAGATGTGGCCAACTAC |
TACTTTTAAGGCCCGAGCAA |
| MMP-9 |
ATGATGGAGGAGAAGCAGTC |
AGGTGAAGGGAAAGTGACAT |
| MCP-1 |
TTAAAAACCTGGATCGGAACCAA |
GCATTAGCTTCAGATTTACGGG |
| GAPDH |
AAGGGTCATCATCTCTGCCC |
GTGATGGCATGGACTGTGGT |
Western blot analysis
Proteins from the carotid arteries were isolated
using RIPA Lysis buffer (Beyotime, Haimen, China) containing 100
μg/ml phenylmethylsulfonyl fluoride (PMSF; Beyotime). Protein
concentrations were determined using a BCA assay. Equal amounts of
protein were separated by SDS-PAGE and then transferred onto
nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The
membranes were blocked in 5% skimmed milk for 1 h at room
temperature and incubated with primary antibodies overnight at 4°C.
Subsequently, they were incubated with horseradish peroxidase
(HRP)-conjugated secondary antibodies for 1 h at room temperature.
The membranes were visualized using an Odyssey imaging system
(LI-COR Biosciences, Lincoln, NE, USA). The following antibodies
were used: rabbit anti-Wnt5a (0.5 μg/ml), rabbit anti-MMP-2
(1:500), rabbit anti-MMP-9 (1:600), rabbit anti-COX-2 (1:600),
rabbit anti-GAPDH (0.5 μg/ml), goat anti-rat IgG H&L (HRP)
(1:2,000), goat anti-rabbit IgG H&L (HRP) (1:2,000) (all
purchased from Abcam, Cambridge, MA), rabbit anti-MCP-1 (1:600),
rabbit anti-phospho-specific Thr202/Tyr204-ERK1/2 (1:1,000), rabbit
anti-phospho-SAPK/JNK Thr183/Tyr185 (1:1,000), rabbit
antiphospho-p38 mitogen-activated protein kinase (MAPK)
Thr180/Tyr182 (1:1,000) and rabbit anti-phospho-NF-κB p65 Ser536
(1:1,000) (all purchased from Cell Signaling Technology (Danvers,
MA, USA).
Statistical analysis
Values are expressed as the means ± standard
deviation (SD). Statistical significance of differences between two
groups was determined using the Student’s t-test, and that among
multiple groups was determined by one-way ANOVA. A P-value of
<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using SPSS
software version 11.5 (SPSS Inc., Chicago, IL, USA).
Results
Effective knockdown of Wnt5a by
Ad-mediated siRNA in ApoE−/− mice
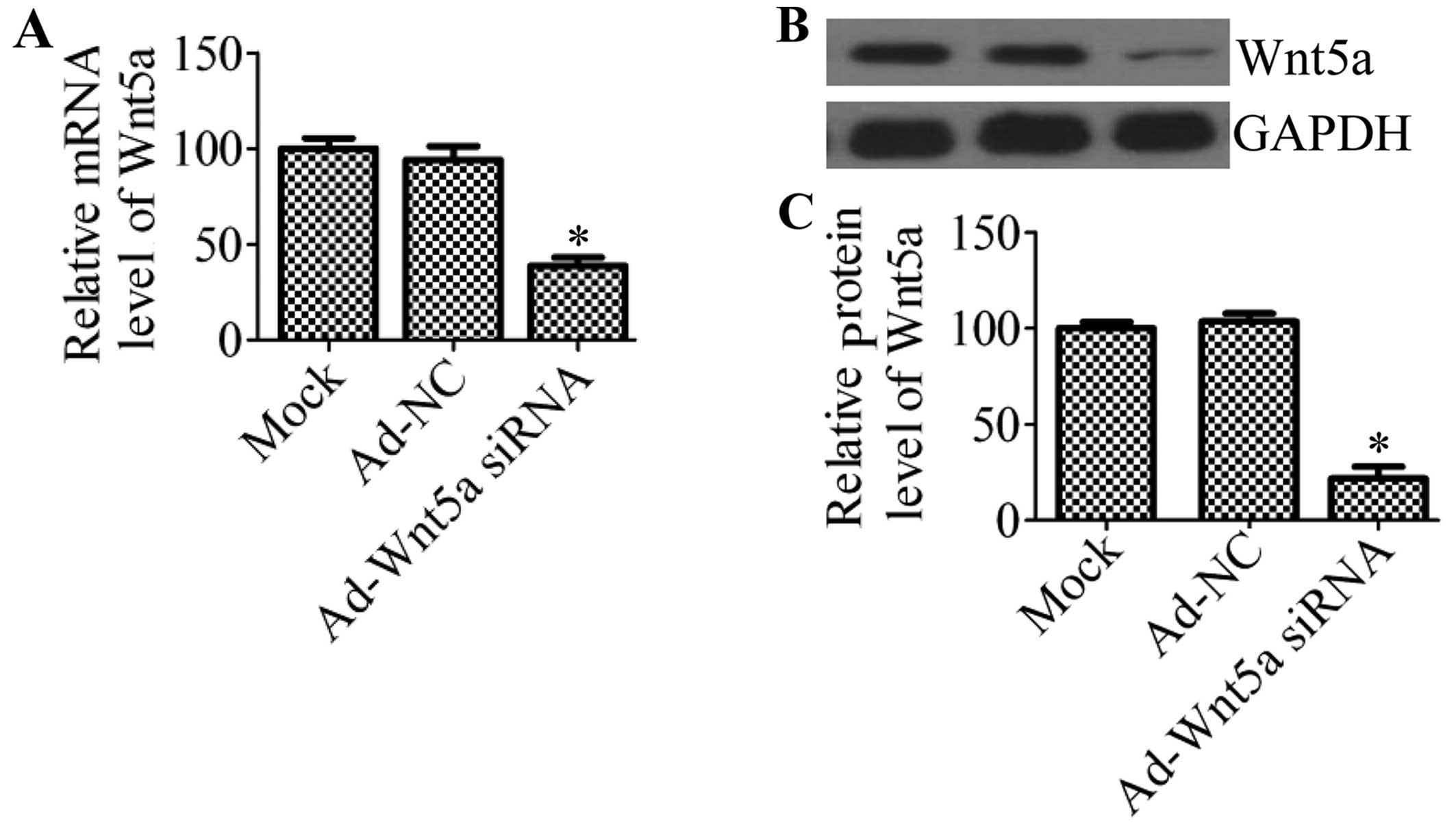
After adenovirus carrying Wnt5a siRNA was
administered to the mice in vivo, Wnt5a expression in the
carotid artery tissues was determined by RT-qPCR and western blot
analysis. The results revealed that the mRNA expression of Wnt5a
was significantly decreased by 61.3% compared with that of the
Ad-NC group and mock group (P<0.05) (Fig. 1A). These results were further
confirmed by western blot analysis (Fig. 1B), which indicated that the
protein expression of Wnt5a was also markedly decreased by 76.5%
(Fig. 1C) in the mice treated
with Ad-Wnt5a siRNA compared with the controls.
Ad-Wnt5a siRNA has no effect on plasma
lipid levels
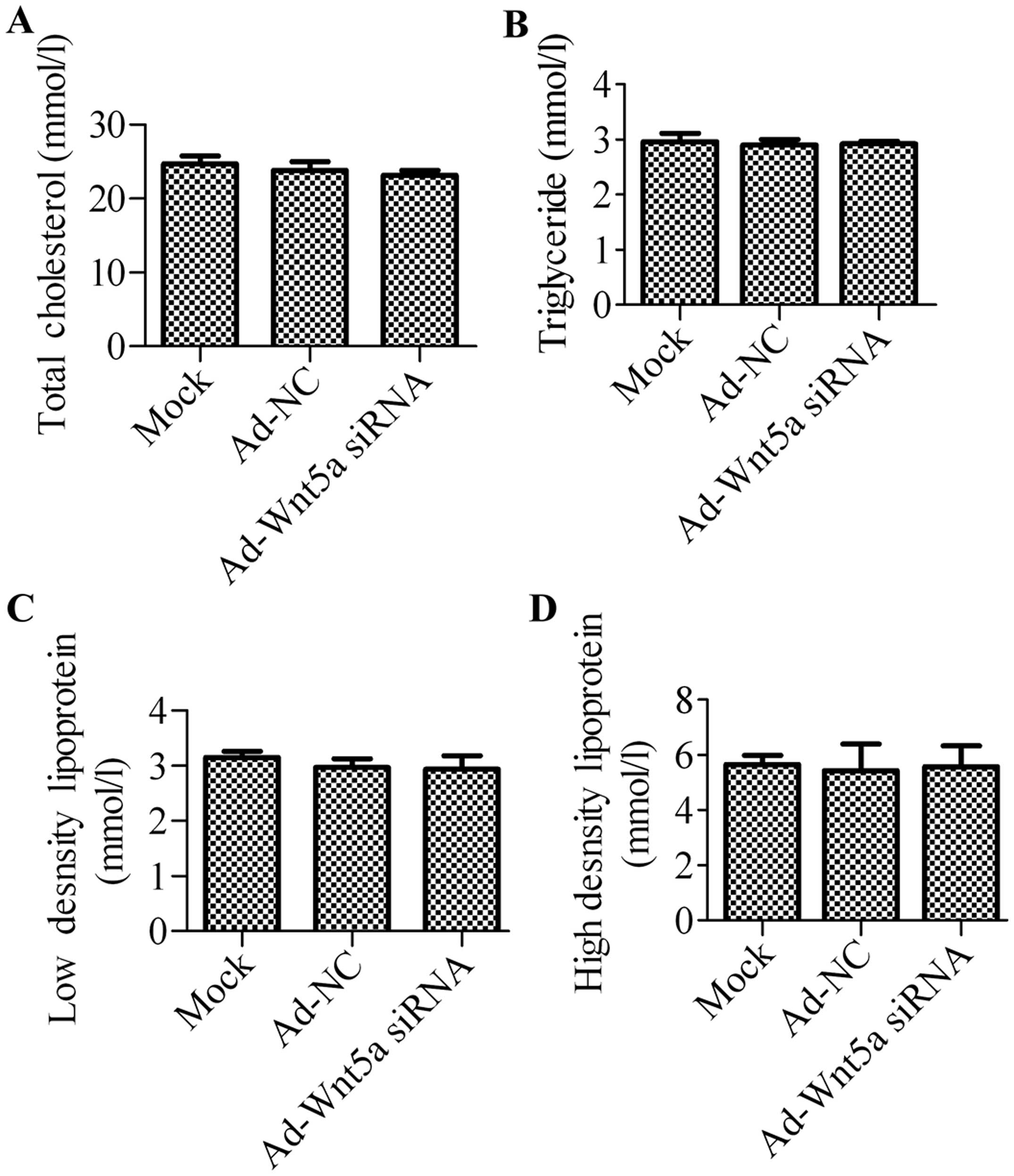
To determine the effects of Wnt5a knockdown on the
mouse model of atherosclerosis, the serum lipid levels of the mice
in the 3 groups were detected. There was no significant difference
in the serum levels of TC, TG, HDL and LDL between the mock group
and NC group, suggesting that treatment with Ad-NC had no effects
on the serum lipid profiles of the ApoE−/− mice
(Fig. 2). Compared with the mock
group or NC group, the administration of Ad-Wnt5a siRNA also had no
significant effect on blood lipid levels.
Treatment with Ad-Wnt5a siRNA improves
the stability of atherosclerotic plaque
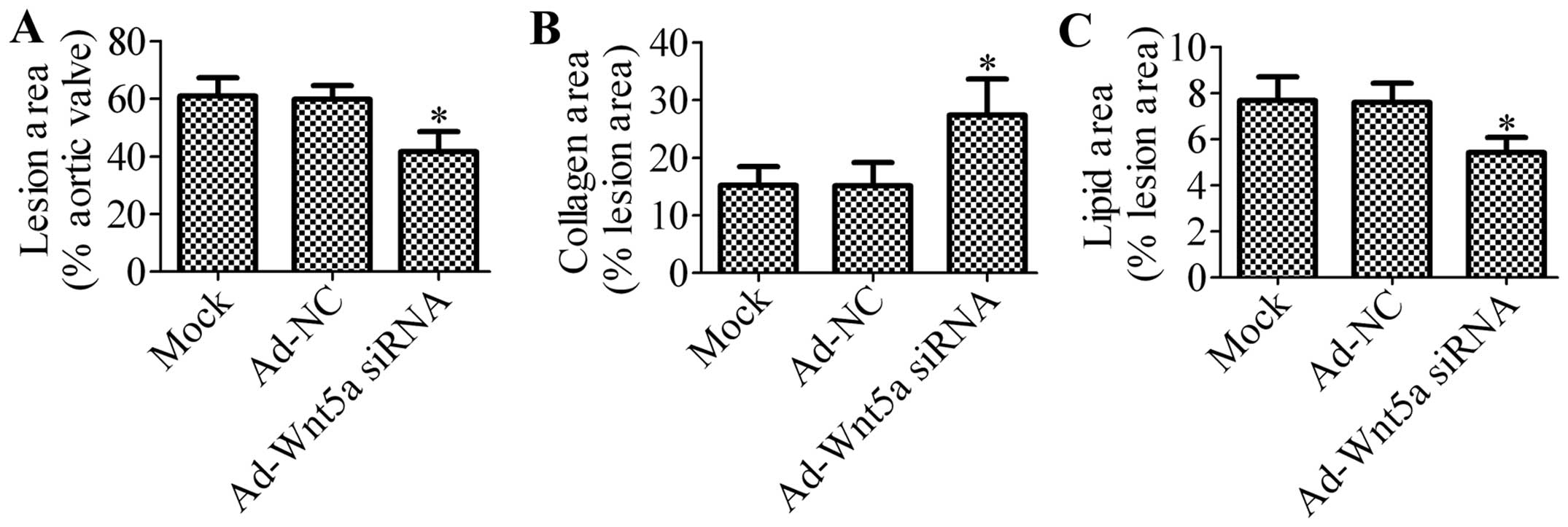
In order to further investigate the effects of
treatment with Ad-Wnt5a siRNA on atherosclerosis, we determined the
atherosclerotic lesion areas by H&E staining. Compared with the
mock group or Ad-NC group, the mice which were transfected with
Ad-Wnt5a siRNA exhibited a decreased lesion area (Fig. 3A), suggesting that the knockdown
of Wnt5a suppressed atherosclerotic development in the
ApoE−/− mice. In order to assess whether the silencing
of Wnt5a expression affects plaque stability, we analyzed the
collagen content and lipid area in atherosclerotic plaque. Using
picrosirius red staining, we found that siRNA targeting Wnt5a
significantly increased the collagen area (Fig. 3B). Moreover, we found that
treatment with Ad-Wnt5a siRNA decreased the lipid content in the
plaque compared with the mock or NC group (Fig. 3C).
Silencing of Wnt5a alters the expression
of inflammatory mediators
Inflammatory factors have been reported to play an
important role in atherosclerotic plaque formation and stability
(13). Additionally, Wnt5a is
involved in the regulation of inflammation (7). Thus, we then investigated whether
the knockdown of Wnt5a has an effect on the inflammatory factors
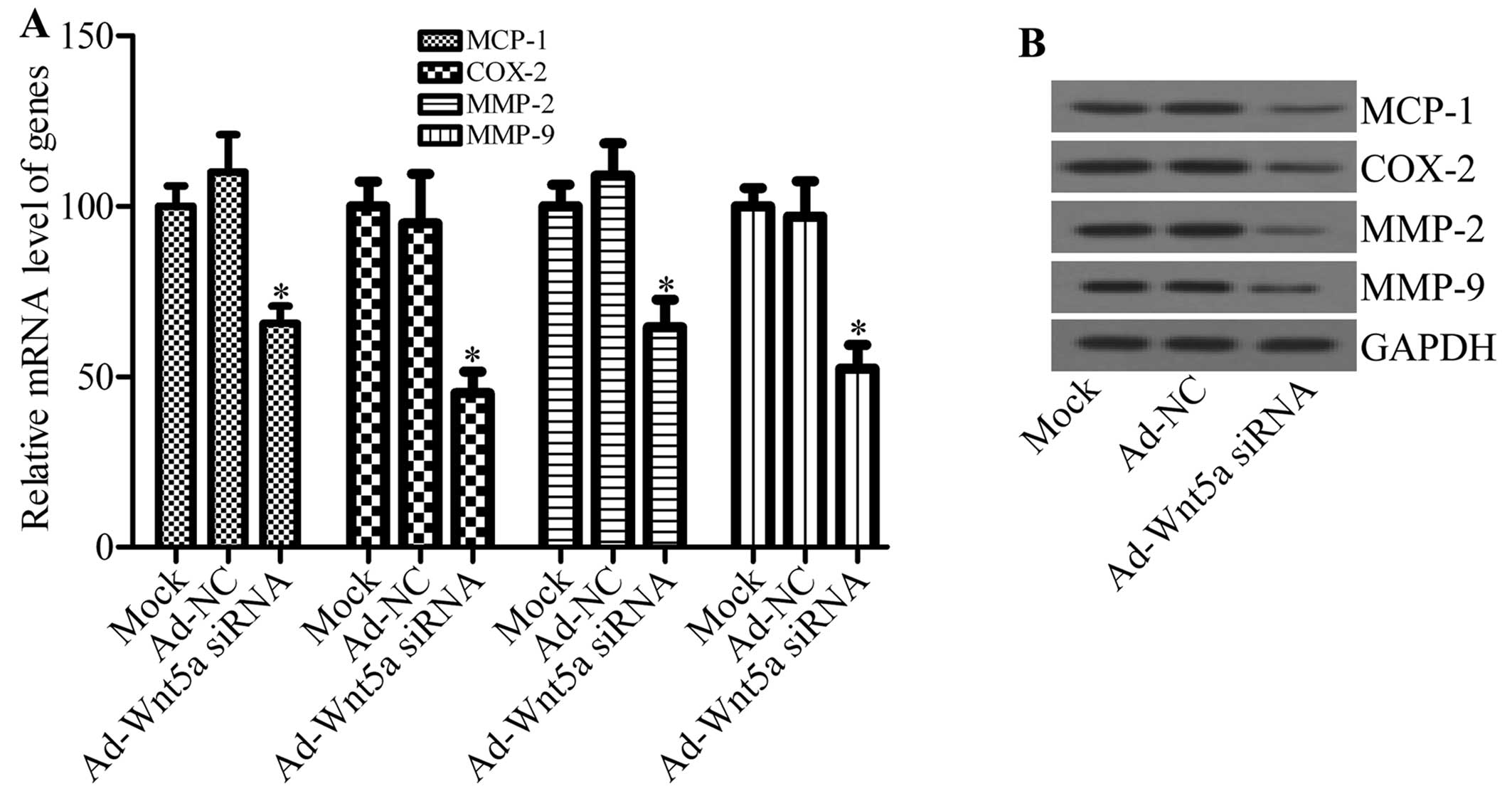
involved in atherosclerotic plaque formation and stability. The
levels of inflammatory factors, such as MCP-1, COX-2, MMP-2 and
MMP-9 were analyzed by RT-qPCR and western blot analysis. Compared
with the mock and NC groups, Ad-Wnt5a siRNA significantly
downregulated the mRNA expression of MCP-1, COX-2, MMP-2 and MMP-9
in the atherosclerotic lesions (Fig.
4A). Moreover, the protein expression of MCP-1, COX-2, MMP-2
and MMP-9 was also decreased by Ad-Wnt5a siRNA (Fig. 4B). These results indicate that the
knockdown of Wnt5a inhibits the inflammatory response in
atherosclerosis.
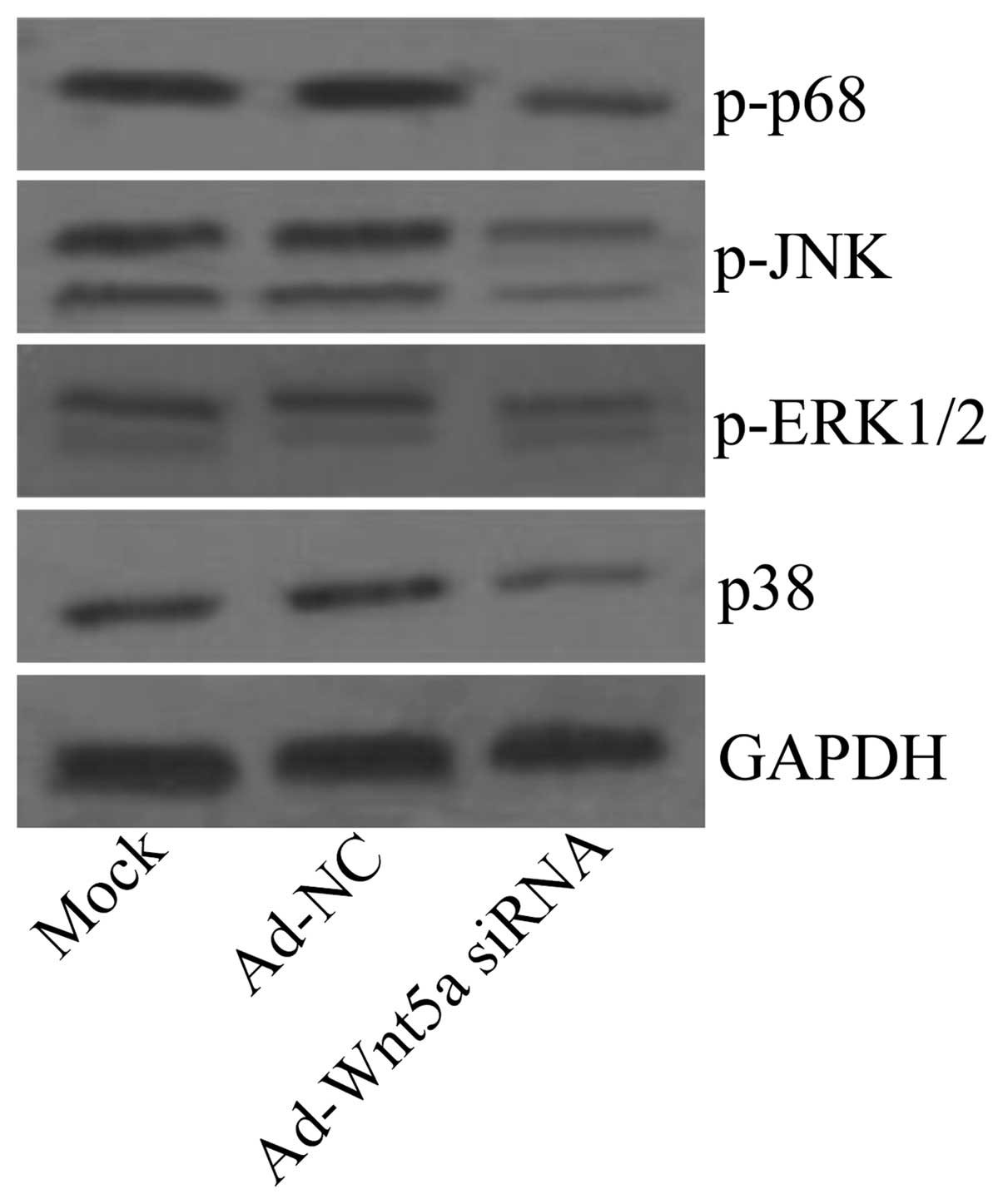
Ad-Wnt5a siRNA inhibits the activation of
the NF-κB and MAPK signaling pathways
The activation of the NF-κB and MAPK pathways is
known to result in the production of inflammatory cytokines and
chemokines (14,15), which is constitutively activated
in atherosclerosis. To investigate whether Ad-Wnt5a siRNA is
capable of activating NF-κB and MAPK pathways, we examined the
effects of treatment with Ad-Wnt5a siRNA on p65, JNK, ERK1/2 and
p38 phosphorylation in by western blot analysis. The results
revealed that Ad-Wnt5a siRNA attenuated p65, JNK, ERK1/2 and p38
phosphorylation compared with the mock and Ad-NC groups (Fig. 5), suggesting that the knockdown of
Wnt5a inhibited the NF-κB and MAPK signaling pathways.
Discussion
In the present study, we found that the silencing of
Wnt5a in vivo reduced the plaque area and increased plaque
stability in an animal model of atherosclerosis. The potential
mechanisms may involve the NF-κB and MAPK pathways, through which
Wnt5a downregulated inflammatory mediators. To the best of our
knowledge, our study is the first to delineate the direct effects
of Ad-Wnt5a siRNA on atherosclerotic lesions and the underlying
mechanisms in ApoE−/− mice in vivo.
Atherosclerosis is a serious inflammatory disorder
that is associated with the upregulation of inflammatory makers.
Therefore, atherosclerotic development may be inhibited by reducing
the levels of inflammatory mediators. COX-2 is a key regulator of
inflammatory processes and is expressed in atherosclerotic lesions
from humans and mice (16,17).
COX-2 has been shown to promote early atherosclerotic lesion
formation in ApoE−/− mice (18). It has been demonstrated that Wnt5a
induces the expression of COX-2 in endothelial cells (9). MCP-1 is a member of the chemokine
family and plays an important role in the initiation of
atherosclerosis. It has previously been demonstrated that the
absence of MCP-1 exerts protective effects against macrophage
recruitment and atherosclerotic lesion formation in apolipoprotein
B (ApoB) transgenic mice (19).
In the present study, we found that the silencing of Wnt5a
downregulated the expression of COX-2 and MCP-1, and decreased the
plaque area of the aortic root in ApoE−/− mice,
suggesting that the silencing of Wnt5a attenuates atherosclerotic
lesion formation by reducing the levels of COX-2 and MCP-1.
The majority of atherosclerotic plaques are stable,
but a few become vulnerable and lead to myocardial infarction (MI)
and stroke (20–22). Plaque rupture is a major type of
vulnerable plaque (23). A
previous study indicated that an increased collagen-to-lipid ratio
is an important indicator of plaque rupture (24). MMP-2 and MMP-9, as mediators of
extracellular cell matrix degradation, play important roles in
plaque rupture by degrading collagen (25). In the current study, we found a
reduced collagen-to-lipid ratio and a decreased expression of MMP-2
and MMP-9 in the Ad-Wnt5a siRNA group, suggesting that the
silencing of Wnt5a enhances plaque stability.
In the progression of an atherosclerotic lesion,
NF-κB activation promotes the expression of COX, cytokines,
chemokines and adhesion molecules (14). NF-κB has also been reported to
regulate metalloproteinase expression, particularly that of MMP-2
and MMP-9 (26). The inhibition
of NF-κB signaling attenuates the pathogenesis of atherosclerosis
(27). It has previously been
demonstrated that MAPK activation facilitates foam cell formation
and MAPK inactivation is an interesting target for drug therapy to
reduce atherosclerotic lesion formation (28). In this study, we measured NF-κB
activity and found that the silencing of Wnt5a decreased p65, JNK,
ERK1/2 and p38 phosphorylation. This indicates that the silencing
of Wnt5a may suppress NF-κB and MAPK activity, reducing the levels
of COX-2, MCP-1, MMP-2 and MMP-9.
Taken together, our results demonstrate that the
silencing of Wnt5a inhibits NF-κB and MAPK signaling, thereby
modulating the level of inflammation in atherosclerotic conditions
and protecting against vascular injury in atherosclerosis. The
present study provides evidence that Wnt5a may be used as an
effective molecular target for the gene therapy of atherosclerosis.
However, further investigations are warranted to delineate the
precise molecular mechanisms of Wnt5a in regulating the
inflammatory process in atherosclerosis.
Acknowledgements
This study was supported by the State Key Clinical
Specialty Construction Project and the Key Project of Science and
Technology of Henan (grant no. 132102310080).
Abbreviations:
|
ApoE−/−
|
apolipoprotein E-deficient
|
|
COX-2
|
cyclooxygenase-2
|
|
JNK
|
Jun N-terminal kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MCP-1
|
monocyte chemotactic protein-1
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
NF-κB
|
nuclear factor-κB
|
|
PBS
|
phosphate-buffered saline
|
|
siRNA
|
small interfering RNA
|
|
TC
|
total cholesterol
|
|
TG
|
triglyceride
|
|
HDL
|
high-density lipoprotein
|
|
LDL
|
low-density lipoprotein.
|
References
|
1
|
Glass CK and Witztum JL: Atherosclerosis.
the road ahead. Cell. 104:503–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li AC and Glass CK: The macrophage foam
cell as a target for therapeutic intervention. Nat Med.
8:1235–1242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kikuchi A, Yamamoto H, Sato A and
Matsumoto S: Wnt5a: its signalling, functions and implication in
diseases. Acta Physiol (Oxf). 204:17–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sen M, Lauterbach K, El-Gabalawy H, et al:
Expression and function of wingless and frizzled homologs in
rheumatoid arthritis. Proc Natl Acad Sci USA. 97:2791–2796. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Christman MA II, Goetz DJ, Dickerson E, et
al: Wnt5a is expressed in murine and human atherosclerotic lesions.
Am J Physiol Heart Circ Physiol. 294:H2864–H2870. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reischl J, Schwenke S, Beekman JM, et al:
Increased expression of Wnt5a in psoriatic plaques. J Invest
Dermatol. 127:163–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pereira C, Schaer DJ, Bachli EB, Kurrer MO
and Schoedon G: Wnt5A/CaMKII signaling contributes to the
inflammatory response of macrophages and is a target for the
antiinflammatory action of activated protein C and interleukin-10.
Arterioscler Thromb Vasc Biol. 28:504–510. 2008. View Article : Google Scholar
|
|
8
|
Halleskog C, Dijksterhuis JP, Kilander MB,
et al: Heterotrimeric G protein-dependent WNT-5A signaling to
ERK1/2 mediates distinct aspects of microglia proinflammatory
transformation. J Neuroinflammation. 9:1112012. View Article : Google Scholar
|
|
9
|
Kim J, Kim J, Kim DW, Ha Y, et al: Wnt5a
induces endothelial inflammation via beta-catenin-independent
signaling. J Immunol. 185:1274–1282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jung YS, Lee HY, Kim SD, et al: Wnt5a
stimulates chemotactic migration and chemokine production in human
neutrophils. Exp Mol Med. 45:e272013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malgor R, Bhatt PM, Connolly BA, et al:
Wnt5a, TLR2 and TLR4 are elevated in advanced human atherosclerotic
lesions. Inflamm Res. 63:277–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan X, Baxter RC, Perbal B and Firth SM:
The aminoterminal insulin-like growth factor (IGF) binding domain
of IGF binding protein-3 cannot be functionally substituted by the
structurally homologous domain of CCN3. Endocrinology.
147:5268–5274. 2006. View Article : Google Scholar
|
|
13
|
Libby P, Ridker PM and Hansson GK:
Inflammation in atherosclerosis: from pathophysiology to practice.
J Am Coll Cardiol. 54:2129–2138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kutuk O and Basaga H: Inflammation meets
oxidation: NF-kappaB as a mediator of initial lesion development in
atherosclerosis. Trends Mol Med. 9:549–557. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chan ED and Riches DW: IFN-gamma + LPS
induction of iNOS is modulated by ERK, JNK/SAPK, and p38(mapk) in a
mouse macrophage cell line. Am J Physiol Cell Physiol.
280:C441–C450. 2001.PubMed/NCBI
|
|
16
|
Schönbeck U, Sukhova GK, Graber P, Coulter
S and Libby P: Augmented expression of cyclooxygenase-2 in human
atherosclerotic lesions. Am J Pathol. 155:1281–1291.
1999.PubMed/NCBI
|
|
17
|
Burleigh ME, Babaev VR, Oates JA, et al:
Cyclooxygenase-2 promotes early atherosclerotic lesion formation in
LDL receptor-deficient mice. Circulation. 105:1816–1823. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burleigh ME, Babaev VR, Yancey PG, et al:
Cyclooxygenase-2 promotes early atherosclerotic lesion formation in
ApoE-deficient and C57BL/6 mice. J Mol Cell Cardiol. 39:443–452.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gosling J, Slaymaker S, Gu L, et al: MCP-1
deficiency reduces susceptibility to atherosclerosis in mice that
overexpress human apolipoprotein B. J Clin Invest. 103:773–778.
1999. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Falk E: Pathogenesis of atherosclerosis. J
Am Coll Cardiol. 47(Suppl 8): C7–C12. 2006. View Article : Google Scholar
|
|
21
|
Davies MJ: The pathophysiology of acute
coronary syndromes. Heart. 83:361–366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schaar JA, Muller JE, Falk E, et al:
Terminology for high-risk and vulnerable coronary artery plaques.
Report of a meeting on the vulnerable plaque, June 17 and 18, 2003,
Santorini, Greece. Eur Heart J. 25:1077–1082. 2004.PubMed/NCBI
|
|
23
|
Ylä-Herttuala S, Bentzon JF, Daemen M, et
al: Stabilisation of atherosclerotic plaques. Position paper of the
European Society of Cardiology (ESC) Working Group on
atherosclerosis and vascular biology. Thromb Haemost. 106:1–19.
2011.PubMed/NCBI
|
|
24
|
Naghavi M, Libby P, Falk E, et al: From
vulnerable plaque to vulnerable patient: a call for new definitions
and risk assessment strategies: Part I. Circulation. 108:1664–1672.
2003. View Article : Google Scholar
|
|
25
|
Newby AC: Metalloproteinase expression in
monocytes and macrophages and its relationship to atherosclerotic
plaque instability. Arterioscler Thromb Vasc Biol. 28:2108–2114.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bond M, Fabunmi RP, Baker AH and Newby AC:
Synergistic upregulation of metalloproteinase-9 by growth factors
and inflammatory cytokines: an absolute requirement for
transcription factor NF-kappa B. FEBS Lett. 435:29–34. 1998.
View Article : Google Scholar
|
|
27
|
Wang J, Zhang R, Xu Y, et al: Genistein
inhibits the development of atherosclerosis via inhibiting
NF-kappaB and VCAM-1 expression in LDLR knockout mice. Can J
Physiol Pharmacol. 86:777–784. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muslin AJ: MAPK signalling in
cardiovascular health and disease: molecular mechanisms and
therapeutic targets. Clin Sci (Lond). 115:203–218. 2008. View Article : Google Scholar : PubMed/NCBI
|