Introduction
Ulcerative colitis (UC) is an inflammatory bowel
disease that predisposes for colorectal cancer. The risk of
developing malignancies increases with disease duration and also
with age at disease onset (1).
Malignant development in UC is a multistep progression through
inflammation, regeneration and dysplasia, leading to
adenocarcinoma. The process also includes a number of important
molecular changes. The colonic mucosa of patients with UC may
harbour severe molecular abnormalities due to chromosomal
instability (CIN), leading to DNA aneuploidy. Aneuploidy is
regarded as an early event in the malignant development of UC
(2) and may be present in both
dysplastic, as well as in non-dysplastic colonic mucosa (3,4).
Aneuploidy in UC relates to disease duration
(5–8) and is regarded as an independent risk
factor for the development of adenocarcinoma in UC (9,10).
The consequence of aneuploidy in non-neoplastic cells is growth
arrest or cell death, but is in UC suggested to be a precursor of
future malignancies (6). More
than half of the colorectal adenocarcinomas developing from UC
present DNA aneuploidy (7,9),
and it is thus considered a major contributor to the neoplastic
phenotype (11,12).
The term aneuploidy refers to either structural
errors or copy number errors in chromosomes, and aneuploid cells
usually contain a combination of these two errors. Structural
errors are most likely products of chromosomal breakage, and,
whereas multiple mechanisms may underlie chromosomal breakage
(13–15), copy number errors are achieved
mainly through errors in chromosomal segregation (16,17). The spindle checkpoint (or mitotic
checkpoint) is crucial for the separation of chromosomes to both
daughter cells during mitosis. It is a complex signalling cascade
that will arrest mitosis upon faulty alignment of chromosomes or if
the spindle fails to attach to kinetochores properly (18–20). A dysfunctional spindle checkpoint
is considered to be a major cause of aneuploidy in malignancies
(16,17,21).
p53 is a tumour suppressor protein with multiple
functions in the regulation of the cell cycle and chromosomal
stabilization (22). In cancers,
there are often mutations and/or loss of heterozygosity in the
TP53 gene, resulting in loss of function. In UC-related
carcinogenesis, evidence points to the inactivation of p53 as being
a relatively early event (22–24), whereas it is considered a late
event in the development of sporadic colorectal cancers (25). p53 and Aurora A are reportedly
involved in a mitotic feedback loop: p53 is considered to be a
negative regulator of Aurora A expression, whereas Aurora A can
phosphorylate p53 rendering it incapable of binding to DNA, or
marking it for degradation (22,26–28). If wild-type p53 is assumed to be a
negative regulator of the mitotic spindle kinase Aurora A (22,29), the loss of functional p53 may have
serious implications for regulation of the spindle checkpoint. Loss
of wild-type p53 function may result in centrosome amplification,
faulty chromosomal segregation and aneuploidy. In the absence of
TP53 mutations, the accumulation of p53 in a UC colon can
also be due to a programmed p53 response to various reactive
oxidative species present in inflamed tissue (30).
Overexpression of Aurora A is implicated in abnormal
centrosome amplification and in the abrogation of the spindle
checkpoint (31). The gene coding
for Aurora A is located on 20q13.2, a chromosomal arm frequently
amplified in solid tumours, including colorectal tumours (32). The expression of Aurora A has been
reported to be elevated in several tumour types (33,34), as well as in the colonic mucosa of
patients with UC (35).
In this study, we have assessed both the mutational
frequency of TP53 and the protein levels of p53 in a set of
colectomies from patients suffering from longstanding UC. We also
re-evaluated previously published data on Aurora A expression
assessed by immunohistochemical staining in the same colectomies
(35). The colectomies were
stratified as progressors and non-progressors, as previously
presented (36,37). Within the progressors, we assessed
the results from Aurora A in association with DNA ploidy status and
advancing degrees of dysplasia, as well as with the protein levels
of p53 and the TP53 mutation status.
Materials and methods
UC colectomies and patients
Thirty patients suffering from longstanding UC were
included in this study. All patients had suffered from UC for
>10 years prior to the colectomy, some as long as 30 years.
Patients also varied widely with respect to age at the time of the
first presentation of symptoms (from 10 to 60 years old).
The colectomy specimens have been previously
described (35–37). We divided the colectomies into
progressors and non-progressors, revealing 10 non-progressors that
did not present any dysplastic lesions or DNA aneuploidy, and 20
progressors that all presented at least one area of
dysplasia/cancer, where the majority of cases also presented
lesions with DNA aneuploidy.
At least 8 locations from each colectomy were
examined, and within the progressors, we found 83 non-dysplastic
areas, 31 areas indefinite for dysplasia, 29 areas with dysplasia
and 8 adenocarcinomas. A total of 18 non-dysplastic and 20
dysplastic areas revealed DNA aneuploidy. The aneuploid, dysplastic
areas included 8 areas of indefinite dysplasia and 5
adenocarcinomas. By definition, all non-progressor lesions were
diploid and non-dysplastic. Detailed distributions of dysplasia and
aneuploid lesions within the progressors have been previously
described (35,37).
Ethical considerations
The use of this material for research purposes has
ethical approval from the Regional Ethics Committee (approval no.
REK S-06062).
Tissue microarray evaluation
Tissue microarrays (TMAs) from 8 locations within
each colon specimen were prepared using a Beecher tissue microarray
instrument (Beecher Instruments, Inc., Sun Prairie, WI, USA) as
previously described (35). The
core size was 0.6 mm. All cores had been previously evaluated by an
experienced pathologist (O.P.C.). At least 2 tissue cores from each
mucosal region were sampled.
TMAs do not consistently display full colonic crypts
as whole sections do. p53 staining was performed on whole sections
since the detectable accumulation of p53 is heterogeneous
(positively- and negatively-stained areas in the same section). If
tissue cores for TMAs were sampled from areas negative for p53,
this may have led to an increased number of false negatives. The
expression of Aurora A was homogeneous (staining was evenly
distributed throughout the section); thus, TMAs were regarded as
reliable for the estimation of Aurora A protein expression.
Immunohistochemistry (IHC)
Immunohistochemical staining for p53 and Aurora A
was performed as described in our previous publications (35,38,39).
p53 accumulation was assessed microscopically, by
manually counting positive nuclei in whole sections, as previously
described (38). At least 1,200
nuclei were counted, and a section was scored as positive for p53
if >5% of the cells in a section showed nuclear staining as
previously presented by our research group (40).
Aurora A expression was assessed from TMAs. Aurora A
protein expression was defined for each sample as the percentage of
positive cells out of at least 300 randomly selected mucosal
epithelial cells from each included tissue core. Typical staining
of Aurora A and p53 is presented in Fig. 1. With increasing degree of
dysplasia, an increasing amount of cells with nucleic positivity of
Aurora A also presented cytoplasmic staining.
p53 mutation analysis
Mutation analysis for TP53 exons 5–8 was
performed by cycling temperature capillary electrophoresis (CTCE),
as previously described (41,42). This procedure detects the presence
of mutations. We did not sequence the mutation-positive cases in
order to determine the actual mutation. The primer sequences for
the mutation analyses are presented in the study by Bjørheim et
al (43).
Statistical analysis
p53/TP53 correlations were examined in cross
tabulation and assessed by Pearson’s χ2 test. Assessment
of Aurora A protein levels in association with the DNA ploidy
status, mucosal morphology and p53 accumulation, as well as the
TP53 mutational status, was performed using a multilevel
model compensating for patient differences, as each patient
included in this study contributed with more than one biopsy. A
linear mixed model (LMM) with restricted maximum likelihood (REML)
estimations and a Bonferroni post hoc test were used. Tests were
performed with PASW statistics 18 (Chicago, IL, USA). All tests
were two-sided and a p-value of 0.05 was considered to indicate a
statistically significant difference.
Results
p53 immunohistochemistry
No accumulation of p53 was detected within the 10
non-progressor colectomies. Of the 20 progressor colectomies, 60%
(12/20) harboured areas with accumulation of p53, but no colectomy
specimens showed p53 accumulation through all 8 lesions. The 20
progressors had a total of 130 lesions available for p53
assessment, and 20.8% (27/130) of the lesions were positive for p53
accumulation. Within the positive lesions, 22.2% (6/27) also
contained aneuploid populations. In precolectomy biopsies from
patients with colectomies positive for p53 accumulation, we
detected p53 accumulation up to 14 years prior to colectomy. A
summary of colectomy lesions positive for p53 accumulation,
including DNA ploidy status and mucosal morphology, is presented in
Table I.
 | Table IImmunohistochemistry results from
p53-positive lesions (n=27) in progressors. |
Table I
Immunohistochemistry results from
p53-positive lesions (n=27) in progressors.
| >5% p53
staining |
|---|
|
|
|---|
| Diploid | Aneuploid |
|---|
| Non-dysplasia | 8 | 0 |
| Indefinite
dysplasia | 5 | 1 |
| Dysplasia | 5 | 2 |
| Adenocarcinoma | 3 | 3 |
Mutation analysis of the TP53 gene (exons
5–8)
TP53 mutations were found in both progressors and
non-progressors. A total of 70% (7/10) of the non-progressors and
55% (11/20) of the progressors harboured areas with mutations in
one of the TP53 mutation hotspots (exons 5–8).
Of the 70 non-progressor lesions available for
TP53 mutation analysis 20% (14/70) had a TP53
mutation. The 20 progressor colectomies yielded 129 lesions
available for TP53 mutation analysis. Of these 129 lesions,
11.6% (15/129), harboured a mutation in one of the mutation
hotspots examined (exons 5–8). A total of 20.8% (9/15) of the
mutated lesions also had aneuploid cell populations. Table II shows a summary of lesions with
TP53 mutations, with DNA ploidy and mucosal morphology.
 | Table IIUlcerative colitis colectomy lesions
with mutated TP53. |
Table II
Ulcerative colitis colectomy lesions
with mutated TP53.
| | Mutated
TP53 |
|---|
| |
|
|---|
| Colon | Morphology | Diploid | Aneuploid |
|---|
|
Non-progressors | Non-dysplasia | 14 | 0 |
| Progressors | Non-dysplasia | 2 | 4 |
| Indefinite
dysplasia | 3 | 2 |
| Dysplasia | 0 | 1 |
| Adenocarcinoma | 1 | 2 |
No correlation was detected between mutations in
TP53 and the accumulation of p53 in this material. Three
progressor lesions had both a TP53 mutation and accumulation
of p53. All 3 lesions originated from separate colons and included
two adenocarcinomas and one lesion indefinite for dysplasia. One of
the adenocarcinomas was also aneuploid.
Aurora A expression in UC progressors and
non-progressors
We have previously demonstrated that the expression
of Aurora A in UC mucosa is elevated compared to non-UC control
samples (35). In the present
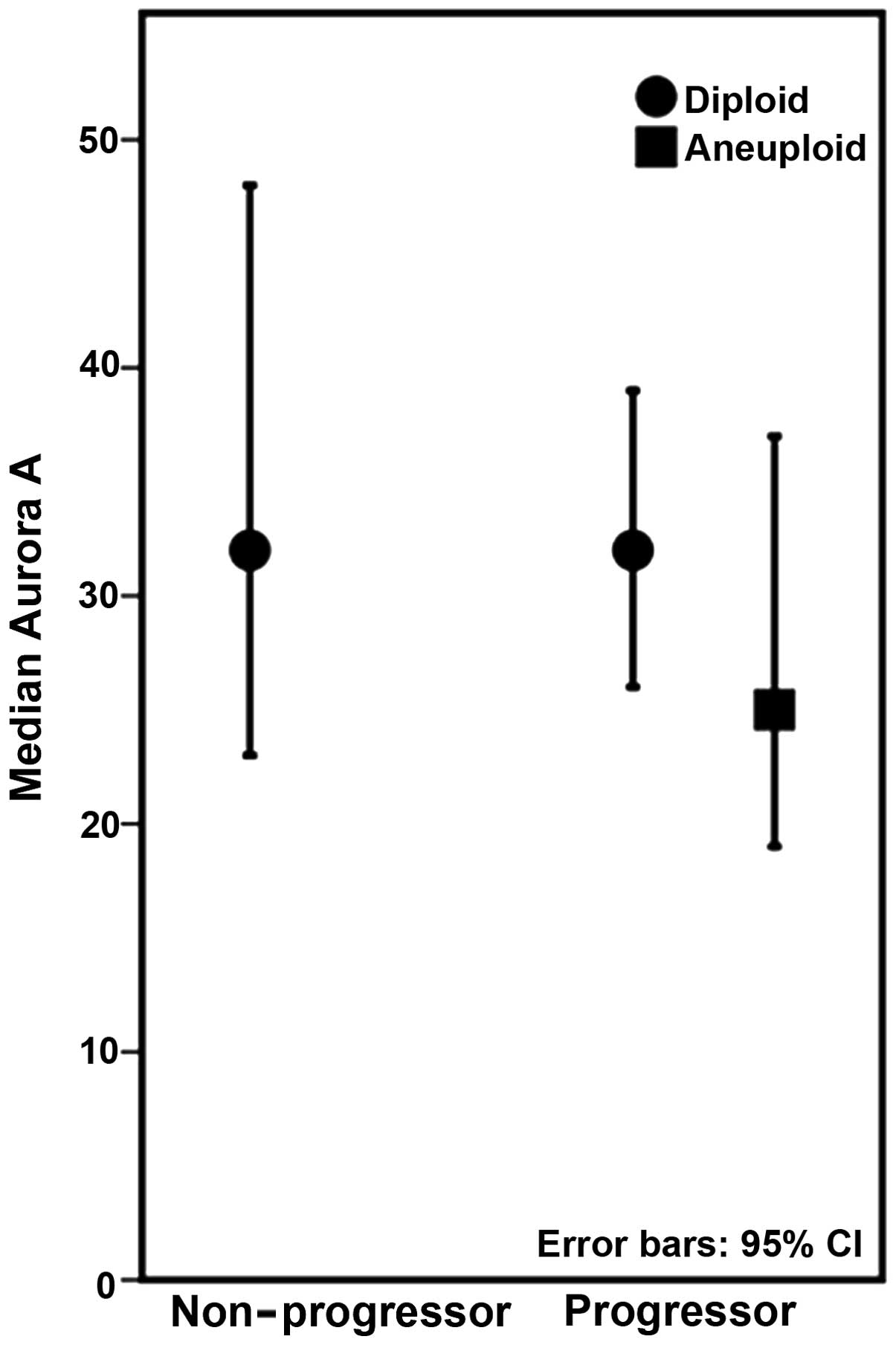
study, Aurora A expression was not found to differ between the
progressors and non-progressors, neither when including all types
of progressor lesions, nor when only diploid, non-dysplastic
progressor lesions were included.
Within the progressors, we found a significant
association between Aurora A and DNA ploidy status (p=0.020), with
lower levels of Aurora A present in lesions harbouring aneuploid
populations (Fig. 2). The
expression of Aurora A within the progressor lesions decreased with
increasing severity of dysplasia, but when accounting for patient
variation this was not statistically significant. The lowest values
of Aurora A expression were observed within high-grade dysplasia.
Adenocarcinomas harboured increased levels of Aurora A expression
(Fig. 3A). As only 6 lesions were
diagnosed as high-grade dysplasia, these were combined with
low-grade dysplasia for statistical purposes. Excluding the 6
colectomies harbouring adenocarcinomas, a significant decrease in
Aurora A expression associated with increasing degrees of dysplasia
was observed in the 14 remaining colectomies (p=0.025) (Fig. 3B).
Expression of Aurora A associated with
p53 accumulation/TP53 mutation
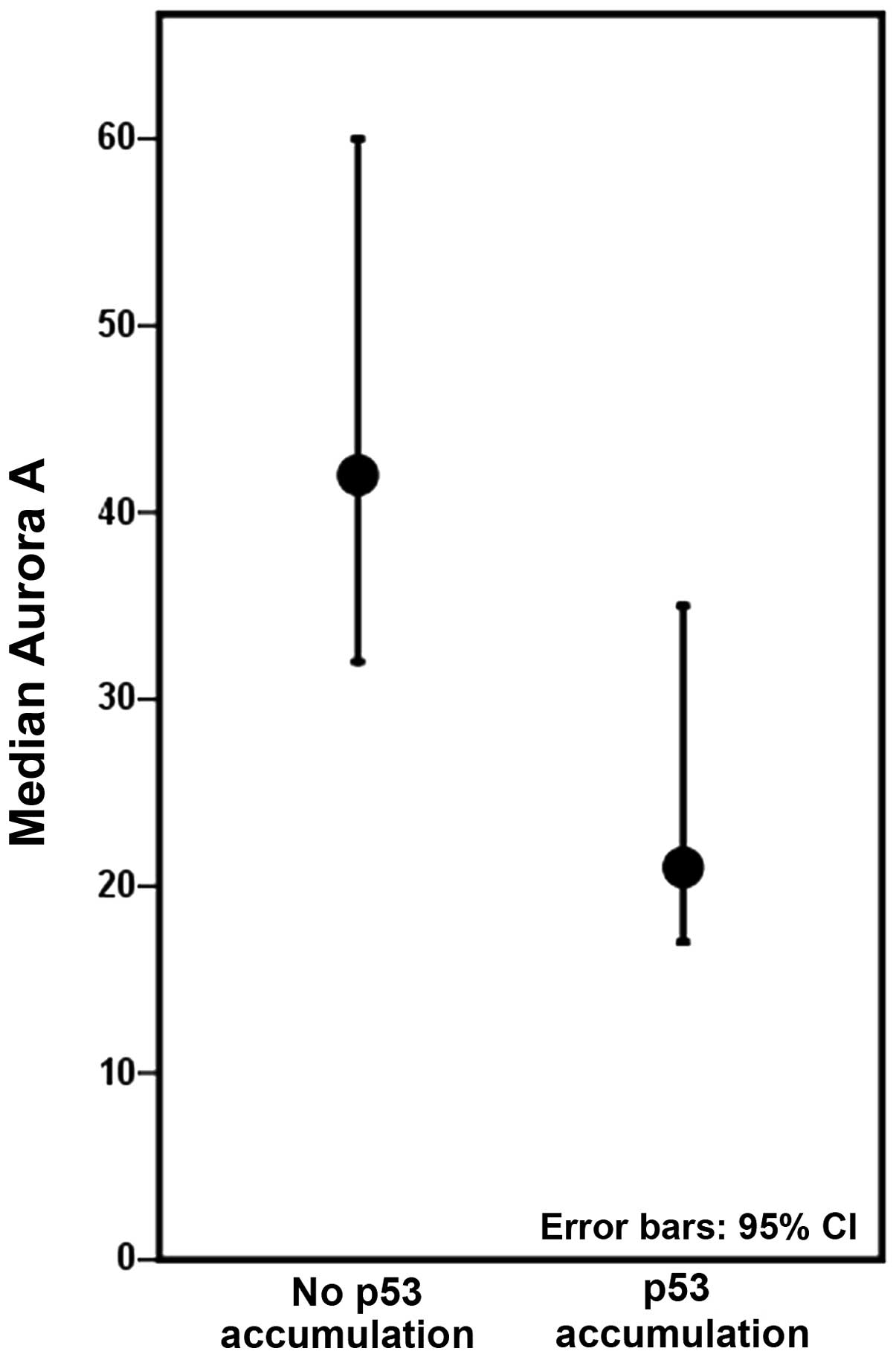
Colectomies harbouring at least one lesion with p53
accumulation displayed decreased levels of Aurora A, compared to
colectomies with no p53 accumulation (Fig. 4), although not to a significant
degree when inter-patient differences were accounted for. The
expression of Aurora A was not significantly associated with the
p53 mutation status in our study material.
Discussion
It has long been known that protein levels of Aurora
A are upregulated in the majority of solid tumours (31,44), linked to CIN and aneuploidy
(33,45) and associated with a poor prognosis
(46). It is also known that
Aurora A is mapped to chromosome 20q13.2, a region highly amplified
in, for example, sporadic colorectal cancers (32). UC colonic mucosa is subjected to
rapid cell division (47) and
high levels of oxidative stress (48), regardless of its status as
progressor or non-progressor. Oxidative stress has been shown to
induce spindle checkpoint override in cell lines, as it can inhibit
the anaphase-promoting complex/cyclosome (APC/C) (49). Aurora A in normal functioning
cells is targeted by APC/C for degradation during late mitosis, a
function essential for mitotic exit. Persistent Aurora A may be
able to prolong the anaphase and induce separation of chromatids
(50). Both progressors and
non-progressors in our material presented elevated levels of Aurora
A compared to non-UC control samples, but only progressors revealed
dysplastic development and DNA ploidy changes. This may suggest
that the general increase in Aurora A levels observed in UC colonic
mucosa is consistent with enhanced spindle checkpoint activity as a
natural response to an accelerated cellular proliferation, as well
as elevated levels of oxidative stress. Other factors however, are
most likely also required to override the checkpoint function,
inducing CIN and DNA aneuploidy.
We have previously presented findings of similar
levels of human telomerase reverse transcriptase (hTERT) protein
expression and equal shortening of mean telomere length in the
colonic mucosa of progressors compared to non-progressors from the
same UC patient material (36,37). These results are in accordance
with UC being a disease that accelerates the ageing of the colonic
mucosa (51); however, these
parameters are unable to differentiate a progressor from a
non-progressor UC colon. Likewise, our results indicate that the
expression of Aurora A is not an ideal biomarker for
differentiating progressor from non-progressor UC colons.
In this study, TP53 mutations were detected
in both progressors and non-progressors, consistent with the
observation that the frequency of TP53 mutation increases
after at least 10 years of UC duration, and without association to
malignancies (52), and the
observation that TP53 mutations are frequent in the inflamed
tissue of UC colons (53). Of
note, the majority of mutations were found within the
non-progressors; however, no p53 accumulation was observed in the
non-progressors. The reason for this is unclear. Lack of such
correlation has been previously shown at the single crypt level in
UC (54). The lack of detectable
p53 may be due to nonsense mutations and premature stop codons,
rather than a missense mutation; since with a missense mutation,
the accumulation of p53 is to be expected. It has been observed
that less than 20% of TP53 mutations of human cancers are
nonsense mutations or stop codon mutations (55,56). It has also been observed that the
mutation of TP53 occurs prior to loss of heterozygosity in
the colonic mucosa of UC progressors (57), and our results may be indicative
of an early mutation of a single TP53 allele, whereas the
remaining allele provides functional p53 in these non-progressors.
Since we did not sequence the cases positive for TP53
mutation, this aspect of our study remains unclear.
Pre-colectomy biopsies from the patients included in
our study made it possible to track p53 positivity retrospectively.
A total of 11 patients displayed p53 accumulation in the
pre-colectomy biopsies. All 11 had developed progressor traits by
colectomy. Six cases had indeed developed adenocarcinomas. The
finding that only progressors showed p53 accumulation indicates
that p53 accumulation may be a potential biomarker of a progressor
colon, which is consistent with previous reports of p53 expression
associating with dysplastic development in UC (58–60).
The expression levels of Aurora A within the
progressors did not differ to a statistically significant degree
when a comparison was made between the advancing degrees of
dysplasia, including adenocarcinomas. However, when the 6 colectomy
specimens with cancer were removed, a statistically significant
decrease in Aurora A expression was observed with increasing levels
of dysplasia. Adenocarcinomas presented elevated levels of Aurora A
compared to dysplastic lesions (Fig.
3A). In addition, Aurora A expression was also significantly
associated with DNA aneuploidy within the progressors (Fig. 2). This association was masked when
progressors and non-progressors were combined (35). In our material, the aneuploid
lesions had decreased levels of Aurora A compared to diploid
lesions, again with the exception of adenocarcinomas, where the
aneuploid cancers had elevated levels of Aurora A compared to the
diploid cancers (data not shown). As colon cancer has been shown to
harbour high levels of 20q amplification (32), a trait not often observed within
the non-cancerous UC mucosa (2),
this may be an explanation for the elevated levels of Aurora A in
adenocarcinomas compared to the dysplastic lesions in our study
material.
Recently, a study of Aurora A expression in
dysplasia and cancer in gastric mucosa demonstrated increased
levels of Aurora A in dysplastic gastric lesions (61). As this is in contrast to our
findings in the UC mucosa, it may indicate that different
mechanisms are involved in dysplastic development in the colonic
and gastric mucosa.
p53 has been shown to be an important negative
regulator of Aurora A. Loss of p53 may lead to the abnormal
regulation of Aurora A and dysregulated mitosis (29,62,63). An increase in Aurora A expression
may induce a protective mechanism, oncogene-induced senescence,
against malignant development, possibly dependent on the loss of
functional p53 (64–66). As our data show decreased Aurora A
expression in areas harbouring aneuploid populations, it may be
possible that the development of CIN and aneuploidy is necessary to
overcome this protection. Our results showing that non-progressor
lesions with wild-type TP53 have higher Aurora A levels than
mutated TP53 non-progressor lesions, [although the
difference was not statistically significant when inter-patient
differences were re-accounted for (data not shown)], are also
consistent with this hypothesis.
Wild-type p53 is difficult to detect in normal
unstressed cells. The detection of p53 protein becomes possible due
to the extended half-life of a mutated, non-functioning protein or
by the stabilisation of p53 as a natural response to, for example,
cellular stress and inflammation (30,67). p53 accumulation is also a known
response to excess shortening of telomeres (68,69). As we have previously shown that
the mucosa of UC progressor cases harbours significantly more
ultra-short telomeres than those found in the non-progressor cases
(36), this could indicates that
telomeric repeat-induced activation of p53 is a possibility in our
progressor cases. The lack of detectable p53 in non-progressors
perhaps also indicates that the elevated levels of Aurora A in the
non-progressors phosphorylate p53, targeting it for degradation.
This is consistent with a previous report, demonstrating that
Aurora A phosphorylates p53 at Ser315, leading to murine double
minute 2 (MDM2)-mediated ubiquitination and degradation of p53
(62).
Our findings of no p53 accumulation detected in the
non-progressors differ from those of previous studies (70). This may be due to our definition
of a non-progressor; we included only non-dysplastic patients
[described as having regenerative or inflamed mucosa by two
experienced pathologists (O.P.C. and S.N.A.)] with no detectable
DNA aneuploidy. We selected this definition as it has been shown
that even UC patients with only one lesion indefinite for
dysplasia, or with DNA aneuploidy alone, may develop adenocarcinoma
(71,72).
In conclusion, our findings indicate that p53
accumulation may be a good biomarker for progressor UC cases, as no
accumulation was detected in the non-progressors, and the
progressors showed p53 accumulation in biopsies collected several
years prior to colectomy. The expression of Aurora A did not differ
between progressor and non-progressor UC colectomies. Within the
progressor cases, the levels of Aurora A were decreased in
association with both aneuploidy and dysplasia, but increased in
adenocarcinomas. p53 and Aurora A appear to regulate each other in
a different manner in progressors and non-progressors.
Acknowledgements
This study was made possible by the generous funding
from the South-Eastern Norway Regional Health Authority and by
Stiftelsen UNI. These organisations had no role in collecting,
analysing, or interpreting the data or in writing of the
report.
References
|
1
|
Salk JJ, Bansal A, Lai LA, et al: Clonal
expansions and short telomeres are associated with neoplasia in
early-onset, but not late-onset, ulcerative colitis. Inflamm Bowel
Dis. 19:2593–2602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Willenbucher RF, Aust DE, Chang CG, et al:
Genomic instability is an early event during the progression
pathway of ulcerative-colitis-related neoplasia. Am J Pathol.
154:1825–1830. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rubin CE, Haggitt RC, Burmer GC, et al:
DNA aneuploidy in colonic biopsies predicts future development of
dysplasia in ulcerative colitis. Gastroenterology. 103:1611–1620.
1992.PubMed/NCBI
|
|
4
|
Rabinovitch PS, Dziadon S, Brentnall TA,
et al: Pancolonic chromosomal instability precedes dysplasia and
cancer in ulcerative colitis. Cancer Res. 59:5148–5153.
1999.PubMed/NCBI
|
|
5
|
Hammarberg C, Slezak P and Tribukait B:
Early detection of malignancy in ulcerative colitis. A
flow-cytometric DNA study. Cancer. 53:291–295. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fozard JB, Quirke P, Dixon MF, Giles GR
and Bird CC: DNA aneuploidy in ulcerative colitis. Gut.
27:1414–1418. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meling GI, Clausen OP, Bergan A,
Schjølberg A and Rognum TO: Flow cytometric DNA ploidy pattern in
dysplastic mucosa, and in primary and metastatic carcinomas in
patients with longstanding ulcerative colitis. Br J Cancer.
64:339–344. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meyer KF, Nause SL, Freitag-Wolf S, et al:
Aneuploidy characterizes adjacent non-malignant mucosa of
ulcerative colitis-associated but not sporadic colorectal
carcinomas: a matched-pair analysis. Scand J Gastroenterol.
48:679–687. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gerling M, Meyer KF, Fuchs K, et al: High
frequency of aneuploidy defines ulcerative colitis-associated
carcinomas: a comparative prognostic study to sporadic colorectal
carcinomas. Ann Surg. 252:74–83. 2010. View Article : Google Scholar
|
|
10
|
Gerling M, Nousiainen K, Hautaniemi S, et
al: Aneuploidy-associated gene expression signatures characterize
malignant transformation in ulcerative colitis. Inflamm Bowel Dis.
19:691–703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lengauer C, Kinzler KW and Vogelstein B:
Genetic instabilities in human cancers. Nature. 396:643–649. 1998.
View Article : Google Scholar
|
|
12
|
Davoli T and de Lange T: The causes and
consequences of polyploidy in normal development and cancer. Annu
Rev Cell Dev Biol. 27:585–610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Londoño-Vallejo JA: Telomere instability
and cancer. Biochimie. 90:73–82. 2008. View Article : Google Scholar
|
|
14
|
Cheung AL and Deng W: Telomere
dysfunction, genome instability and cancer. Front Biosci.
13:2075–2090. 2008. View
Article : Google Scholar
|
|
15
|
Kong CM, Lee XW and Wang X: Telomere
shortening in human diseases. FEBS J. 280:3180–3193. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kops GJ, Weaver BA and Cleveland DW: On
the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev
Cancer. 5:773–785. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bharadwaj R and Yu H: The spindle
checkpoint, aneuploidy, and cancer. Oncogene. 23:2016–2027. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Musacchio A and Salmon ED: The
spindle-assembly checkpoint in space and time. Nat Rev Mol Cell
Biol. 8:379–393. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Musacchio A and Hardwick KG: The spindle
checkpoint: structural insights into dynamic signalling. Nat Rev
Mol Cell Biol. 3:731–741. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kops GJ, Foltz DR and Cleveland DW:
Lethality to human cancer cells through massive chromosome loss by
inhibition of the mitotic checkpoint. Proc Natl Acad Sci USA.
101:8699–8704. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weaver BA and Cleveland DW: Decoding the
links between mitosis, cancer, and chemotherapy: The mitotic
checkpoint, adaptation, and cell death. Cancer Cell. 8:7–12. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aylon Y and Oren M: p53: guardian of
ploidy. Mol Oncol. 5:315–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sato A and MacHinami R: p53
immunohistochemistry of ulcerative colitis-associated with
dysplasia and carcinoma. Pathol Int. 49:858–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klump B, Holzmann K, Kühn A, et al:
Distribution of cell populations with DNA aneuploidy and p53
protein expression in ulcerative colitis. Eur J Gastroenterol
Hepatol. 9:789–794. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baker SJ, Preisinger AC, Jessup JM, et al:
p53 gene mutations occur in combination with 17p allelic deletions
as late events in colorectal tumorigenesis. Cancer Res.
50:7717–7722. 1990.PubMed/NCBI
|
|
26
|
Mao JH, Wu D, Perez-Losada J, et al:
Crosstalk between Aurora-A and p53: Frequent deletion or
downregulation of Aurora-A in tumors from p53 null mice. Cancer
Cell. 11:161–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ha GH and Breuer EK: Mitotic kinases and
p53 signaling. Biochem Res Int. 2012:1959032012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katayama H, Wang J, Treekitkarnmongkol W,
et al: Aurora kinase-A inactivates DNA damage-induced apoptosis and
spindle assembly checkpoint response functions of p73. Cancer Cell.
21:196–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu CC, Yang TY, Yu CT, et al: p53
negatively regulates Aurora A via both transcriptional and
posttranslational regulation. Cell Cycle. 11:3433–3442. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Forrester K, Ambs S, Lupold SE, et al:
Nitric oxide-induced p53 accumulation and regulation of inducible
nitric oxide synthase expression by wild-type p53. Proc Natl Acad
Sci USA. 93:2442–2447. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nikonova AS, Astsaturov I, Serebriiskii
IG, Dunbrack RL Jr and Golemis EA: Aurora A kinase (AURKA) in
normal and pathological cell division. Cell Mol Life Sci.
70:661–687. 2013. View Article : Google Scholar :
|
|
32
|
De Angelis PM, Clausen OP, Schjølberg A
and Stokke T: Chromosomal gains and losses in primary colorectal
carcinomas detected by CGH and their associations with tumour DNA
ploidy, genotypes and phenotypes. Br J Cancer. 80:526–535. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou H, Kuang J, Zhong L, et al: Tumour
amplified kinase STK15/BTAK induces centrosome amplification,
aneuploidy and transformation. Nat Genet. 20:189–193. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marumoto T, Zhang D and Saya H: Aurora-A-a
guardian of poles. Nat Rev Cancer. 5:42–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burum-Auensen E, De Angelis PM, Schjølberg
AR, Røislien J, Andersen SN and Clausen OP: Spindle proteins Aurora
A and BUB1B, but not Mad2, are aberrantly expressed in dysplastic
mucosa of patients with longstanding ulcerative colitis. J Clin
Pathol. 60:1403–1408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Friis-Ottessen M, Bendix L, Kølvraa S,
Norheim-Andersen S, De Angelis PM and Clausen OP: Telomere
shortening correlates to dysplasia but not to DNA aneuploidy in
longstanding ulcerative colitis. BMC Gastroenterol. 14:82014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Friis-Ottessen M, De Angelis PM,
Schjølberg AR, Andersen SN and Clausen OP: Reduced hTERT protein
levels are associated with DNA aneuploidy in the colonic mucosa of
patients suffering from longstanding ulcerative colitis. Int J Mol
Med. 33:1477–1483. 2014.PubMed/NCBI
|
|
38
|
Schjølberg AR, Clausen OPF, Burum-Auensen
E and De Angelis PM: Aneuploidy is associated with TP53 expression
but not with BRCA1 or TERT expression in sporadic colorectal
cancer. Anticancer Res. 29:4381–4387. 2009.PubMed/NCBI
|
|
39
|
Burum-Auensen E, De Angelis PM, Schjølberg
AR, Kravik KL, Aure M and Clausen OP: Subcellular localization of
the spindle proteins Aurora A, Mad2, and BUBR1 assessed by
immunohistochemistry. J Histochem Cytochem. 55:477–486. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Clausen OP, Lothe RA, Børresen-Dale AL, et
al: Association of p53 accumulation with TP53 mutations, loss of
heterozygosity at 17p13, and DNA ploidy status in 273 colorectal
carcinomas. Diagn Mol Pathol. 7:215–223. 1998. View Article : Google Scholar
|
|
41
|
Ekstrøm PO, Warren DJ and Thilly WG:
Separation principles of cycling temperature capillary
electrophoresis. Electrophoresis. 33:1162–1168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ekstrøm PO, Khrapko K, Li-Sucholeiki XC,
Hunter IW and Thilly WG: Analysis of mutational spectra by
denaturing capillary electrophoresis. Nat Protoc. 3:1153–1166.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bjørheim J, Gaudernack G and Ekstrøm PO:
Mutation analysis of TP53 exons 5–8 by automated constant
denaturant capillary electrophoresis. Tumour Biol. 22:323–327.
2001. View Article : Google Scholar
|
|
44
|
Bischoff JR, Anderson L, Zhu Y, et al: A
homologue of Drosophila aurora kinase is oncogenic and amplified in
human colorectal cancers. EMBO J. 17:3052–3065. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baba Y, Nosho K, Shima K, et al: Aurora-A
expression is independently associated with chromosomal instability
in colorectal cancer. Neoplasia. 11:418–425. 2009.PubMed/NCBI
|
|
46
|
Goos JA, Coupe VM, Diosdado B, et al:
Aurora kinase A (AURKA) expression in colorectal cancer liver
metastasis is associated with poor prognosis. Br J Cancer.
109:2445–2452. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Greco V, Lauro G, Fabbrini A and Torsoli
A: Histochemistry of the colonic epithelial mucins in normal
subjects and in patients with ulcerative colitis. A qualitative and
histophotometric investigation. Gut. 8:491–496. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Roessner A, Kuester D, Malfertheiner P and
Schneider-Stock R: Oxidative stress in ulcerative
colitis-associated carcinogenesis. Pathol Res Pract. 204:511–524.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
D’Angiolella V, Santarpia C and Grieco D:
Oxidative stress overrides the spindle checkpoint. Cell Cycle.
6:576–579. 2007. View Article : Google Scholar
|
|
50
|
Floyd S, Pines J and Lindon C: APC/C Cdh1
targets aurora kinase to control reorganization of the mitotic
spindle at anaphase. Curr Biol. 18:1649–1658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Risques RA, Lai LA, Brentnall TA, et al:
Ulcerative colitis is a disease of accelerated colon aging:
evidence from telomere attrition and DNA damage. Gastroenterology.
135:410–418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lang SM, Stratakis DF, Heinzlmann M,
Heldwein W, Wiebecke B and Loeschke K: Molecular screening of
patients with long standing extensive ulcerative colitis: detection
of p53 and Ki-ras mutations by single strand conformation
polymorphism analysis and differential hybridisation in colonic
lavage fluid. Gut. 44:822–825. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hussain SP, Amstad P, Raja K, et al:
Increased p53 mutation load in noncancerous colon tissue from
ulcerative colitis: a cancer-prone chronic inflammatory disease.
Cancer Res. 60:3333–3337. 2000.PubMed/NCBI
|
|
54
|
Yoshida T, Mikami T, Mitomi H and Okayasu
I: Diverse p53 alterations in ulcerative colitis-associated
low-grade dysplasia: full-length gene sequencing in microdissected
single crypts. J Pathol. 199:166–175. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
van Schaik FD, Oldenburg B, Offerhaus GJ,
et al: Role of immunohistochemical markers in predicting
progression of dysplasia to advanced neoplasia in patients with
ulcerative colitis. Inflamm Bowel Dis. 18:480–488. 2012. View Article : Google Scholar
|
|
56
|
Harris CC and Hollstein M: Clinical
implications of the p53 tumor-suppressor gene. N Engl J Med.
329:1318–1327. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Brentnall TA, Crispin DA, Rabinovitch PS,
et al: Mutations in the p53 gene: an early marker of neoplastic
progression in ulcerative colitis. Gastroenterology. 107:369–378.
1994.PubMed/NCBI
|
|
58
|
Wong NA, Mayer NJ, MacKell S, Gilmour HM
and Harrison DJ: Immunohistochemical assessment of Ki67 and p53
expression assists the diagnosis and grading of ulcerative
colitis-related dysplasia. Histopathology. 37:108–114. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shigaki K, Mitomi H, Fujimori T, et al:
Immunohistochemical analysis of chromogranin A and p53 expressions
in ulcerative colitis-associated neoplasia: neuroendocrine
differentiation as an early event in the colitis-neoplasia
sequence. Hum Pathol. 44:2393–2399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Harpaz N, Peck AL, Yin J, et al: p53
protein expression in ulcerative colitis-associated colorectal
dysplasia and carcinoma. Hum Pathol. 25:1069–1074. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Katsha A, Soutto M, Sehdev V, et al:
Aurora kinase A promotes inflammation and tumorigenesis in mice and
human gastric neoplasia. Gastroenterology. 145:1312–1322.e-8. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Katayama H, Sasai K, Kawai H, et al:
Phosphorylation by aurora kinase A induces Mdm2-mediated
destabilization and inhibition of p53. Nat Genet. 36:55–62. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Vader G and Lens SM: The Aurora kinase
family in cell division and cancer. Biochim Biophys Acta.
1786:60–72. 2008.PubMed/NCBI
|
|
64
|
Bartkova J, Rezaei N, Liontos M, et al:
Oncogene-induced senescence is part of the tumorigenesis barrier
imposed by DNA damage checkpoints. Nature. 444:633–637. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Suram A, Kaplunov J, Patel PL, et al:
Oncogene-induced telomere dysfunction enforces cellular senescence
in human cancer precursor lesions. EMBO J. 31:2839–2851. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang D, Shimizu T, Araki N, et al: Aurora
A overexpression induces cellular senescence in mammary gland
hyperplastic tumors developed in p53-deficient mice. Oncogene.
27:4305–4314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ashcroft M and Vousden KH: Regulation of
p53 stability. Oncogene. 18:7637–7643. 1999. View Article : Google Scholar
|
|
68
|
Milyavsky M, Mimran A, Senderovich S, et
al: Activation of p53 protein by telomeric (TTAGGG)n repeats.
Nucleic Acids Res. 29:5207–5215. 2001. View Article : Google Scholar
|
|
69
|
d’Adda di Fagagna F, Reaper PM,
Clay-Farrace L, et al: A DNA damage checkpoint response in
telomere-initiated senescence. Nature. 426:194–198. 2003.
View Article : Google Scholar
|
|
70
|
Risques RA, Lai LA, Himmetoglu C, et al:
Ulcerative colitis-associated colorectal cancer arises in a field
of short telomeres, senescence, and inflammation. Cancer Res.
71:1669–1679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gorfine SR, Bauer JJ, Harris MT and Kreel
I: Dysplasia complicating chronic ulcerative colitis: is immediate
colectomy warranted? Dis Colon Rectum. 43:1575–1581. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ullman TA, Loftus EV Jr, Kakar S, Burgart
LJ, Sandborn WJ and Tremaine WJ: The fate of low grade dysplasia in
ulcerative colitis. Am J Gastroenterol. 97:922–927. 2002.
View Article : Google Scholar : PubMed/NCBI
|