Introduction
It is considered that the dysfunction of podocytes,
which are a type of glomerular epithelial cell, plays an important
role in progressive renal diseases, including focal segmental
glomerulosclerosis (FSGS) and diabetic nephropathy (DN) (1,2).
Podocyte injury is also associated with a higher degree of
albuminuria and leads to the development of glomerulosclerosis. It
has recently been demonstrated that the Notch pathway plays an
important role in the onset and development of glomerular disease
(3). Niranjan et al
(4) reported that the Notch
pathway was activated in podocytes in patients with FSGS and DN and
in mouse models of these diseases. The Notch pathway is an
evolutionarily conserved and widely used intercellular signaling
pathway that influences cellular proliferation and differentiation
(5,6). The binding of a ligand induces a
conformational change in the Notch receptor. This allows an
extracellular metalloprotease to cleave the receptor, which then
allows the γ-secretase-mediated protease to release the Notch
intracellular domain (NICD). NICD travels into the nucleus where it
activates the transcription of downstream genes, such as the hairy
and enhancer of split (Hes) gene.
It is known that alterations in hemodynamics
contribute to the development of glomerular disease. Alterations in
hemodynamics in glomerular disease activate angiotensin II (Ang
II), an important member of the renin-angiotensin system, which
damages podocytes and results in the accumulation of extracellular
matrix (ECM) components and basement membrane thickening in the
glomeruli (7,8). It is well recognized that in
glomerular disease, Ang II increases the synthesis of ECM
components, including collagens and laminin (9,10).
Koshizaka et al (11)
found that Ang II induced podocyte apoptosis through the activation
of the Notch pathway in cultured podocytes. However, it is unclear
as to whether the Notch pathway mediates the Ang II-induced ECM
synthesis in podocytes.
Matrix metalloproteinases (MMPs) constitute a family
of extracellular soluble or membrane-bound proteases that
collectively degrade or proteolytically modify essentially all the
main components of the ECM, including collagens, laminin and
proteoglycans. Gelatinases (e.g., MMP-2 and MMP-9), which degrade
type IV collagen and laminin, are important constitutional elements
of the basement membrane of the glomeruli (12,13). It is well established that
transforming growth factor-β1 (TGF-β1) is a potent stimulator of
ECM production in glomerular injury and may be the most important
growth factor in determining the extent of renal fibrosis following
injury. The exposure of cultured podocytes and mesangial cells to
TGF-β1 has been shown to increase the production of type IV
collagen and laminin (14–16).
Previous studies have demonstrated that Ang II decreases matrix
degradation due to a reduction in MMP activity and promotes the
synthesis of ECM components through TGF-β1 (17,18).
In the present study, we aimed to investigate the
hypothesis that Ang II may induce the activation of the Notch
pathway in podocytes. The activation of the Notch pathway may alter
the production of ECM through MMPs and TGF-β1 in Ang II-stimulated
podocytes. The inhibition of the activation of the Notch pathway
can prevent ECM accumulation and glomerulosclerosis.
Materials and methods
Cell culture
Conditionally immortalized mouse podocytes were
purchased from the Cell Resource Center (Peking Union Medical
College, Beijing, China). In this cell line, a
temperature-sensitive mutant of the SV40 virus large T-cell antigen
(tsA58 Tag) is controlled by a γ-interferon-inducible
H-2Kb promoter. The podocytes were firstly cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS)
(both from Gibco-BRL, Gaithersburg, MD, USA) and 10 U/ml
γ-interferon (PeproTech, Rochy Hill, NJ, USA) in a 33°C 5%
CO2 atmosphere to induce proliferation, and were then
incubated in RPMI-1640 medium supplemented with 10% FBS and
deprived of γ-interferon in a 37°C 5% CO2 atmosphere for
14 days to induce quiescence and the differentiated phenotype, as
previously described (19). The
podocytes were grown to 75–85% confluence under growth restrictive
conditions and growth-arrested in serum-free RPMI-1640 for 24 h to
synchronize cell growth. After this time period, the medium was
changed to fresh serum-free medium containing Ang II
(10−6 mol/l; Sigma, St. Louis, MO, USA) at the indicated
time points, as previously described (11). The transient transfection of the
podocytes with a vector carrying short hairpin RNA (shRNA)
targeting Notch1 (sh-Notch1) or a negative control vector
(sh-Scramble) (Jingsai Corp., Wuhan, China) was carried out using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. For inhibition experiments, the cells
were treated with γ-secretase inhibitor (GSI; Sigma) at 1
μmol/l for 30 min prior to stimulation with Ang II.
Western blot analysis
The cells were lysed in lysis buffer (20 mmol/l
Tris·HCl, 2.5 mmol/l EDTA, 10% glycerol, 0.1% SDS, 1% Triton X-100,
1% sodium deoxycholate, 10 mmol/l sodium pyrophosphate, 50 mmol/l
NaF, 1 mmol/l sodium vanadate and 1 mmol/l PMSF) and the protein
concentrations were measured by Coomassie brilliant blue assay.
Protein (40 μg) was loaded and separated on a
SDS-polyacrylamide gel and then transferred onto a polyvinylidene
fluoride (PVDF) membrane (Millipore Corp., Billerica, MA, USA). The
membrane was blocked with 5% dry milk and incubated overnight at
4°C with rabbit anti-Notch1 (ab65297; 1:200 dilution), anti-NICD1
(ab52301; 1:200 dilution), anti-Hes1 (ab71599; 1:2,000 dilution)
(all from Abcam, Cambridge, MA, USA), anti-MMP-2 (10737-2-AP; 1:500
dilution; Proteintech, Chicago, IL, USA), anti-MMP-9 (sc-10737;
1:400 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), anti-TGF-β1 (18978-1-AP; 1:1,000 dilution), anti-type IV
collagen (19797-1-AP; 1:1,000 dilution), anti-laminin (19698-1-AP;
1:1,000 dilution) (all from Proteintech) and anti-β-actin
(sc-130656; 1:1,000 dilution; Santa Cruz Biotechnology, Inc.)
polyclonal antibodies. After washing, the membrane was incubated
with goat anti-rabbit IgG horseradish peroxidase conjugate
(SA00001-2; 1:5,000 dilution; Proteintech). Proteins in western
blot analysis were quantified following acquisition and analysis of
the image using the software of a UVP Image Station Lab Works
version 4.5. Proteins expression was quantified by comparison with
the internal control, β-actin.
Reverse transcription PCR (RT-PCR)
Total RNA was extracted from the podocytes using
TRIzol reagent (Invitrogen) according to the instructions of the
manufacturer and reverse transcribed using oligo(dT) primers in the
presence of avian myeloblastosis virus reverse transcriptase to
yield cDNA. The cDNA was amplified on a 7900HT Sequence Detection
system (Applied Biosystems, Foster City, CA, USA) at default
thermal cycling conditions: 2 min at 50°C, 10 min at 95°C for
enzyme activation and then 40 cycles of 15 sec at 95°C for
denaturation and 1 min at 60°C for annealing and extension. The
results were analyzed using the relative standard curve method of
analysis/ΔCt method of analysis. The primers used for
PCR were as follows: 18S forward, 5′-CGC CGC TAG AGG TGA AAT TC-3′
and reverse, 5′-CCA GTC GGC ATC GTT TAT GG-3′ (149 bp); Notch1
forward, 5′-GTG GAT GAC CTA GGC AAG TCG-3′ and reverse, 5′-GTC TCC
TCC TTG TTG TTC TGC A-3′ (118 bp); Hes1 forward, 5′-CAC GAC ACC GGA
CAA ACC A-3′ and reverse, 5′-GCC GGG AGC TAT CTT TCT TAA GTG-3′
(148 bp); MMP-2 forward, 5′-CAG GGA ATG AGT ACT GGG TCT ATT-3′ and
reverse, 5′-ACT CCA GTT AAA GGC AGC ATC TAC-3′ (118 bp); MMP-9
forward, 5′-CAA TCC TTG CAA TGT GGA TG-3′ and reverse, 5′-TAA GGA
AGG GGC CCT GTA AT-3′ (128 bp); TGF-β1 forward, 5′-ACC GCA ACA ACG
CAA TCT ATG-3′ and reverse, 5′-ATT CCG TCT CCT TGG TTC AG-3′ (196
bp); type IV collagen forward, 5′-GTC AAA CTA CTG CTA TCC CTC CGT
GTC-3′ and reverse, 5′-CAT TCT ATA AAT GGA CTG GCT CGG AAT-3′ (162
bp); laminin forward, 5′-CCT GCC AAA TTC CTC GGT AAC-3′ and
reverse, 5′-ACA TCG TAG GCA GAC GGC TG-3′ (101 bp).
Enzyme activity assay
The activity of MMP-2 and MMP-9 in the serum-free
conditioned medium was assayed using a cell active MMP-2 and MMP-9
fluorescence assay kit (GenMed Scientifics, Arlington, MA, USA),
according to the manufacturer’s instructions. The relative
fluorescence units were determined with an excitation wavelength of
330 nm and an emission wavelength of 400 nm. The consistency of the
fluorescent polypeptide segments was calculated on the basis of the
relative fluorescence units. MMP-2 and MMP-9 activity was expressed
as nmol/mg/min.
Enzyme-linked immunosorbent assay
(ELISA)
After the cells were cultured in 6-well plates under
the different experimental conditions, the supernatants were
collected. The TGF-β1, type IV collagen and laminin protein levels
were quantified using a commercial quantikine enzyme-linked
immunosorbent assay kit (ELISA; R&D Systems, Minneapolis, MN,
USA) according to the manufacturer’s instructions.
Statistical analysis
Data presented as bar graphs are the means ±
standard deviation (SD) of at least 3 independent experiments.
Statistical analysis was performed using the Student’s t-test with
SPSS version 13.0 software (SPSS Inc., Chicago, IL, USA). The
results were considered statistically significant at P<0.05.
Results
Ang II activates the Notch pathway in
podocytes
To determine the effects of Ang II on the activation
of the Notch pathway, we measured the Notch1, NICD1 and Hes1 levels
in the podocytes stimulated with Ang II for 0, 12, 24, 48 and 72 h
(Fig. 1). Notch1 protein and mRNA
expression increased within 12 h of Ang II stimulation, peaked at
24 h, and then slightly decreased at 48 and 72 h (all P<0.01);
the maximum protein expression levels of NICD1 were detected as
early as 12 h and then the expression levels declined, but did not
return to the basal levels (Ang II stimulation at 0 h) (all
P<0.01; Fig. 1A and B); Hes1
protein and mRNA expression also peaked at 12 h and then decreased
at 24 and 48 h (P<0.01; Fig. 1A
and C). No changes in Hes1 protein and mRNA expression were
detected in the cultured podocytes at 0 and 72 h (P>0.05;
Fig. 1).
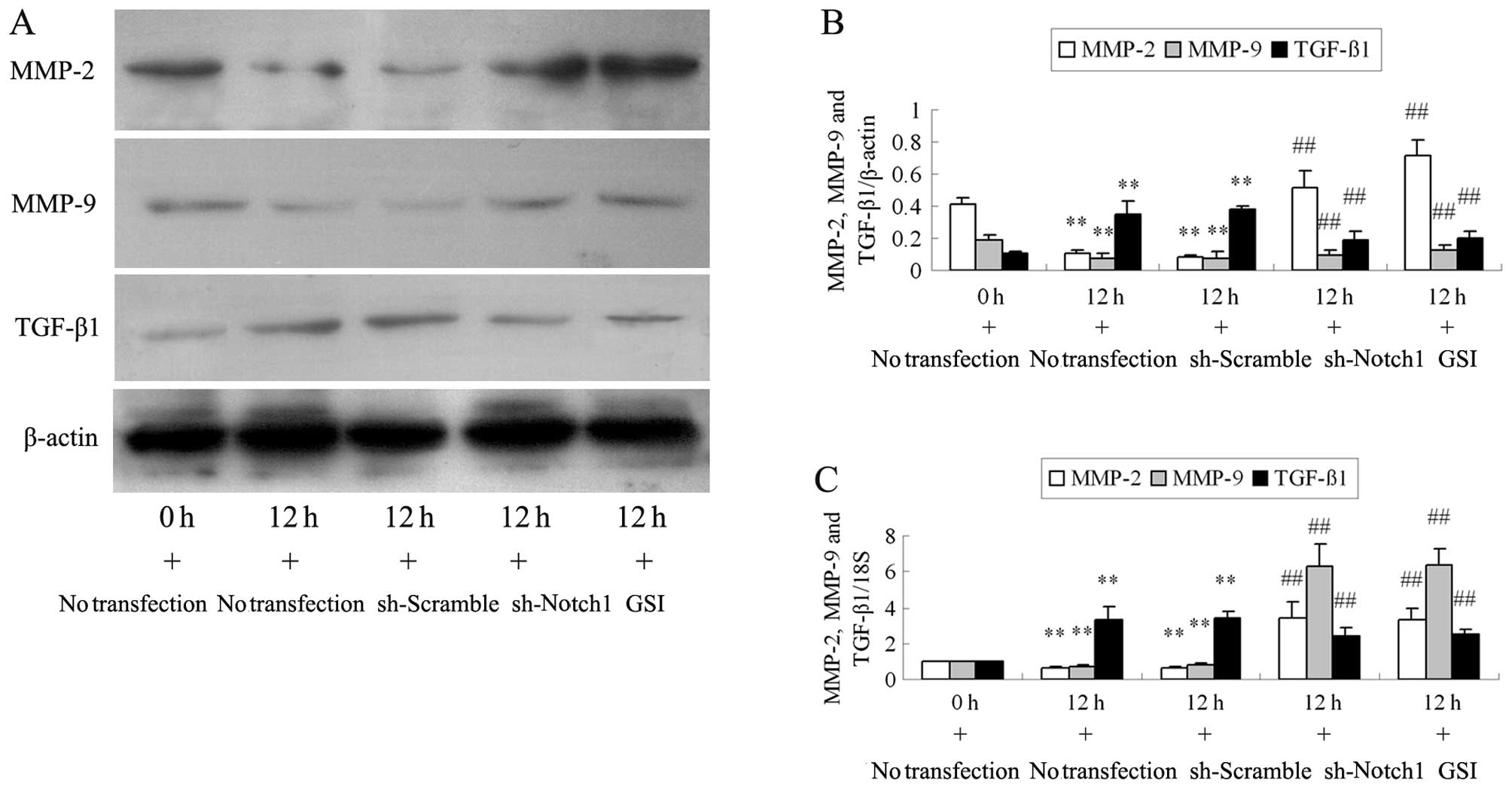
Transfection with sh-Notch1 or
pre-treatment with GSI increases MMP and decreases TGF-β1
expression in the Ang II-stimulated podocytes
Compared with the cells stimulated with Ang II at 0
h, the protein levels of Notch1, NICD1 and Hes1 significantly
increased at 12 h (Fig. 2A and B;
all P<0.01). Transfection with sh-Notch1 decreased the Ang
II-induced the protein overexpression of Notch1, NICD1 and Hes1 in
the podocytes (all P<0.01; Fig. 2A
and B). RT-PCR revealed similar changes in Notch1 and Hes1 mRNA
expression following transfection (Fig. 2C). GSI decreased the NICD1 and
Hes1 protein and the Hes1 mRNA expression in the podocytes
stimulated with Ang II at 12 h (all P<0.01; Fig. 2). Western blot analysis and RT-PCR
revealed that GSI did not inhibit Notch1 overexpression induced by
Ang II at 12 h (all P>0.05). Ang II stimulation decreased MMP-2
and MMP-9 protein and mRNA expression at 12 h (all P<0.01;
Fig. 3). Compared with the cells
stimulated with Ang II, the MMP-2 and MMP-9 protein and mRNA levels
significantly increased in the podocytes transfected with sh-Notch1
or pre-treated with GSI (all P<0.01). Incubation with Ang II for
12 h resulted in a significant upregulation in TGF-β1 protein and
mRNA expression compared with the cells stimulated with Ang II at 0
h (all P<0.01). However, the changes observed in the TGF-β1
expression level in the Ang II-stimulated podocytes were reversed
by transfection with sh-Notch1 or by the addition of GSI to the Ang
II culture medium (all P<0.01).
Transfection with sh-Notch1 or
pre-treatment with GSI increases MMP activity in the Ang
II-stimulated podocytes
When the podocytes were incubated with Ang II for 12
h, Ang II markedly decreased MMP-2 and MMP-9 activity. However,
transfection with sh-Notch1 or pre-treatment with GSI enhanced the
Ang II-induced inhibition of MMP-2 and MMP-9 activity (all
P<0.01; Fig. 4).
Transfection with sh-Notch1 or
pre-treatment with GSI decreases type IV collagen and laminin
expression in the Ang II-stimulated podocytes
As shown by western blot analysis (Fig. 5A and B), type IV collagen and
laminin protein expression was induced by Ang II at 12 h compared
with the podocytes treated with Ang II at 0 h; these expression
levels were efficiently inhibited by transfection with sh-Notch1 or
pre-treatment with GSI (P<0.05 or P<0.01). Compared with the
podocytes stimulated with Ang II at 0 h, the mRNA levels of type IV
collagen and laminin were increased in the Ang II-stimulated
podocytes at 12 h. Their mRNA expression was inhibited following
transfection with sh-Notch1 or pretreatment with GSI (all
P<0.01; Fig. 5C).
Transfection with sh-Notch1 or
pre-treatment with GSI decreases Ang II-induced TGF-β1, type IV
collagen and laminin secretion
We also examined the concentrations of TGF-β1, type
IV collagen and laminin in the culture medium of the podocytes
using ELISA (Fig. 6). We found
that the podocytes stimulated with Ang II for 12 h showed higher
levels of TGF-β1, type IV collagen and laminin in the supernatants
than those cultured with Ang II at 0 h (P<0.05 or P<0.01).
Compared to the podocytes stimulated with Ang II, which induced the
overexpression of TGF-β1, type IV collagen and laminin in the
supernatants, transfection with sh-Notch1 or pre-treatment with GSI
significantly decreased the expression of TGF-β1, type IV collagen
and laminin (P<0.05 or P<0.01; Fig. 6).
Discussion
The Notch pathway is an evolutionarily conserved
local cell-signaling mechanism that participates in a variety of
cellular processes and is important in glomerular development
(20,21). It has been found that the
activation of the Notch pathway in podocytes plays a role in the
development of glomerular disease (4). In our previous study, it was
revealed that the activation of the Notch pathway in high
glucose-stimulated podocytes altered the apoptotic pathway and
induced apoptosis (22).
Alterations in hemodynamics in glomerular disease induce higher
levels of Ang II, which also interacts with the Notch pathway in
podocytes (11). In this study,
we also observed the activation of the Notch pathway in Ang
II-stimulated podocytes. Notch1 seems to be a modulatory target of
Ang II in podocytes. Ang II induces Notch1 cleavage in podocytes
and the release of NICD1, which travels to the nucleus where it
activates the Hes1 gene. The activation of the Notch pathway
induced by Ang II was inhibited by Notch1 shRNA or GSI.
ECM accumulation in glomeruli is considered the most
common destructive pathway associated with chronic glomerular
disease, which is characterized by the remodeling of the
interstitial ECM, resulting in the excessive deposition of ECM
components, including type IV collagen and laminin (23). MMPs are zinc-containing
endopeptidases that are involved in the remodeling of the ECM and
are crucial for tissue development and homeostasis. MMP-2 and MMP-9
cleave the denatured collagens and laminin, as well as some
chemokines. In view of their matrix-degrading capacity, MMP-2 and
MMP-9 were originally considered to be beneficial to chronic renal
fibrogenesis due to their perceived potential to lessen
interstitial matrix accumulation and deposition (12,13,24). Studies on renal fibrosis have
demonstrated that a decrease in MMP levels promotes the deposition
of ECM in the context of renal fibrosis (11,25). Ang II has been shown to inhibit
the angiotensin converting enzyme II (ACE2)-induced MMP-2 activity
through the Ang II type-1 receptor (AT1R) and extracellular
signal-regulated kinase 1 and 2 (ERK1/2) signaling pathway in human
cardiofibroblasts (26). Under
normal glucose conditions, Ang II induces a dose-dependent
downregulation in MMP-2; on the contrary, MMP-9 is upregulated by
Ang II (27). However, we found
that Ang II inhibited the protein and mRNA expression, as well as
the activity of MMP-2 and MMP-9 in podocytes. An enhanced MMP
activity has been shown to be responsible for the development of
disease due to their ability to degrade type IV collagen and
laminin (28,29). Zhou et al (30) found that the downregulation of
Notch1 decreased the migration and invasion capacities of
hepatocellular carcinoma cells by regulating CD44v6, E-cadherin,
MMP-2, MMP-9 and urokinase-type plasminogen activator (uPA). In
this study, after the activation of the Notch pathway was inhibited
by Notch1 shRNA or GSI in Ang II-stimulated podocytes, MMP-2 and
MMP-9 expression and activity increased. These results indicated
that Ang II inhibited MMP-2 and MMP-9 through the Notch pathway in
podocytes.
It is well established that TGF-β1 is a potent
stimulator of ECM production in glomerular injury and may be the
most important growth factor in determining the extent of renal
fibrosis following injury. It has been demonstrated that TGF-β1
mediates the production of type IV collagen and laminin in
mesangial cells under conditions of high glucose in vitro
(31). The exposure of cultured
conditionally immortalized human podocytes to TGF-β1 has also been
shown to increase the production of the basement membrane
components (32). In the present
study, we found that the stimulation of podocytes with Ang II
induced type IV collagen and laminin accumulation by increasing
TGF-β1 mRNA and protein synthesis. Ang II has been shown to induce
the expression and production of TGF-β1 through a mechanism
dependent on reactive oxygen species production in mouse skeletal
muscle cells (33). In another
study, Ang II-induced cardiac hypertrophy and fibrosis were
significantly enhanced by the increase in TGF-β1 expression in
fibroblast growth factor 16 (Fgf16) knockout mice (34). Furthermore, our finding that the
inhibition of the Notch pathway by Notch1 shRNA or GSI markedly
prevented the Ang II-induced upregulation of TGF-β1 suggests that
Ang II induces TGF-β1 expression through the Notch pathway.
Aoyagi-Ikeda et al (35)
found that Notch induced myofibro-blast differentiation through a
TGF-β-Smad3 pathway that activated SMA gene transcription in
alveolar epithelial cells, and increased migratory behavior in
pulmonary fibrosis. In a previous study, blocking Notch signaling
by the γ-secretase inhibitor, DAPT, was shown to significantly
attenuate liver fibrosis, decrease the expression of TGF-β1 and
suppress the endothelial-to-mesenchymal transition (EMT) process in
a rat hepatic stellate cell line (36). In this study, we found that the
activity of MMP-2 and MMP-9 was reduced, and TGF-β1 expression was
increased in Ang II-stimulated podocytes. Imbalances between the
synthesis and degradation of ECM proteins are considered to play
important roles in the progression of glomerular sclerosis in
glomerular disease (37).
In conclusion, our data demonstrate that the Ang
II-induced activation of the Notch pathway, which inhibited MMP
activity, increased TGF-β1 expression and increased the synthesis
of ECM components in podocytes. Thus, the activation of the Notch
pathway plays a role in Ang II-induced glomerular injury. The
blockade of the Notch pathway may thus be an effective method for
the treatment of glomerular disease.
Acknowledgments
This study was supported by grants from the Hebei
Natural Science Foundation of China (H2014206294) and the
Department of Health of Hebei Province of China (ZL20140030).
References
|
1
|
Ikezumi Y, Suzuki T, Karasawa T, Yamada T,
Hasegawa H, Nishimura H and Uchiyama M: Low birthweight and
premature birth are risk factors for podocytopenia and focal
segmental glomerulosclerosis. Am J Nephrol. 38:149–157. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma R, Liu L, Liu X, Wang Y, Jiang W and Xu
L: Triptolide markedly attenuates albuminuria and podocyte injury
in an animal model of diabetic nephropathy. Exp Ther Med.
6:649–656. 2013.PubMed/NCBI
|
|
3
|
Sirin Y and Susztak K: Notch in the
kidney: development and disease. J Pathol. 226:394–403. 2012.
View Article : Google Scholar
|
|
4
|
Niranjan T, Bielesz B, Gruenwald A, Ponda
MP, Kopp JB, Thomas DB and Susztak K: The Notch pathway in
podocytes plays a role in the development of glomerular disease.
Nat Med. 14:290–298. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou W, Wang G and Guo S: Regulation of
angiogenesis via Notch signaling in breast cancer and cancer stem
cells. Biochim Biophys Acta. 1836:304–320. 2013.PubMed/NCBI
|
|
6
|
Ji X, Wang Z, Geamanu A, Sarkar FH and
Gupta SV: Inhibition of cell growth and induction of apoptosis in
non-small cell lung cancer cells by delta-tocotrienol is associated
with notch-1 down-regulation. J Cell Biochem. 112:2773–2783. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang W, Li L, Zhou Z, Gao J and Sun Y:
Effect of spironolactone combined with angiotensin-converting
enzyme inhibitors and/or angiotensin II receptor blockers on
chronic glomerular disease. Exp Ther Med. 6:1527–1531.
2013.PubMed/NCBI
|
|
8
|
Ding Y, Stidham RD, Bumeister R, Trevino
I, Winters A, Sprouse M, Ding M, Ferguson DA, Meyer CJ, Wigley WC
and Ma R: The synthetic triterpenoid, RTA 405, increases the
glomerular filtration rate and reduces angiotensin II-induced
contraction of glomerular mesangial cells. Kidney Int. 83:845–854.
2013. View Article : Google Scholar :
|
|
9
|
Siragy HM and Carey RM: Role of the
intrarenal renin-angiotensin-aldosterone system in chronic kidney
disease. Am J Nephrol. 31:541–550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang S, Yao B, Zhou Y, Yin H, Zhang MZ and
Harris RC: Intrarenal dopamine modulates progressive angiotensin
II-mediated renal injury. Am J Physiol Renal Physiol.
302:F742–F749. 2012. View Article : Google Scholar :
|
|
11
|
Koshizaka M, Takemoto M, Sato S, Tokuyama
H, Fujimoto M, Okabe E, Ishibashi R, Ishikawa T, Tsurutani Y,
Onishi S, Mezawa M, He P, Honjo S, Ueda S, Saito Y and Yokote K: An
angiotensin II type 1 receptor blocker prevents renal injury via
inhibition of the Notch pathway in Ins2 Akita diabetic mice. Exp
Diabetes Res. 2012:1598742012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou TB, Qin YH, Lei FY, Su LN, Zhao YJ
and Huang WF: Less gelatinases is associated with apolipoprotein E
accumulation in glomerulosclerosis rats. Histol Histopathol.
27:249–256. 2012.
|
|
13
|
Ji L, Yin XX, Wu ZM, Wang JY, Lu Q and Gao
YY: Ginkgo biloba extract prevents glucose-induced accumulation of
ECM in rat mesangial cells. Phytother Res. 23:477–485. 2009.
View Article : Google Scholar
|
|
14
|
Wang B, Komers R, Carew R, Winbanks CE, Xu
B, Herman-Edelstein M, Koh P, Thomas M, Jandeleit-Dahm K,
Gregorevic P, Cooper ME and Kantharidis P: Suppression of
microRNA-29 expression by TGF-β1 promotes collagen expression and
renal fibrosis. J Am Soc Nephrol. 23:252–265. 2012. View Article : Google Scholar :
|
|
15
|
Schena FP and Gesualdo L: Pathogenetic
mechanisms of diabetic nephropathy. J Am Soc Nephrol. 16(Suppl 1):
S30–S33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh LP, Green K, Alexander M, Bassly S
and Crook ED: Hexosamines and TGF-beta1 use similar signaling
pathways to mediate matrix protein synthesis in mesangial cells. Am
J Physiol Renal Physiol. 286:F409–F416. 2004. View Article : Google Scholar
|
|
17
|
Han SY, Jee YH, Han KH, Kang YS, Kim HK,
Han JY, Kim YS and Cha DR: An imbalance between matrix
metalloproteinase-2 and tissue inhibitor of matrix
metalloproteinase-2 contributes to the development of early
diabetic nephropathy. Nephrol Dial Transplant. 21:2406–2416. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu GX, Li YQ, Huang XR, Wei L, Chen HY,
Shi YJ, Heuchel RL and Lan HY: Disruption of Smad7 promotes Ang
II-mediated renal inflammation and fibrosis via
Sp1-TGF-β/Smad3-NF-κB-dependent mechanisms in mice. PLoS One.
8:e535732013. View Article : Google Scholar
|
|
19
|
Mundel P, Reiser J, Zúñiga Mejía Borja A,
Pavenstädt H, Davidson GR, Kriz W and Zeller R: Rearrangements of
the cytoskeleton and cell contacts induce process formation during
differentiation of conditionally immortalized mouse podocyte cell
lines. Exp Cell Res. 236:248–258. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng HT, Kim M, Valerius MT, Surendran K,
Schuster-Gossler K, Gossler A, McMahon AP and Kopan R: Notch2, but
not Notch1, is required for proximal fate acquisition in the
mammalian nephron. Development. 134:801–811. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arumugam TV, Chan SL, Jo DG, Yilmaz G,
Tang SC, Cheng A, Gleichmann M, Okun E, Dixit VD, Chigurupati S,
Mughal MR, Ouyang X, Miele L, Magnus T, Poosala S, Granger DN and
Mattson MP: Gamma secretase-mediated Notch signaling worsens
braindamage and functional outcome in ischemic stroke. Nat Med.
12:621–623. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao F, Yao M, Shi Y, Hao J, Ren Y, Liu Q,
Wang X and Duan H: Notch pathway is involved in high
glucose-induced apoptosis in podocytes via Bcl-2 and p53 pathways.
J Cell Biochem. 114:1029–1038. 2013. View Article : Google Scholar
|
|
23
|
Lennon R, Byron A, Humphries JD, Randles
MJ, Carisey A, Murphy S, Knight D, Brenchley PE, Zent R and
Humphries MJ: Global analysis reveals the complexity of the human
glomerular extracellular matrix. J Am Soc Nephrol. 25:939–951.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng S, Pollock AS, Mahimkar R, Olson JL
and Lovett DH: Matrix metalloproteinase 2 and basement membrane
integrity: a unifying mechanism for progressive renal injury. FASEB
J. 20:1898–1900. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsai JP, Liou JH, Kao WT, Wang SC, Lian JD
and Chang HR: Increased expression of intranuclear matrix
metalloproteinase 9 in atrophic renal tubules is associated with
renal fibrosis. PLoS One. 7:e481642012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuan TC, Chen MY, Liao YC, Ko L, Hong YH,
Yen CY, Hsieh WY, Cheng KS, Wu CL and Lin CS: Angiotensin II
down-regulates ACE2-mediated enhancement of MMP-2 activity in human
cardiofibroblasts. Biochem Cell Biol. 91:435–442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Solini A, Rossi C, Santini E, Madec S,
Salvati A and Ferrannini E: Angiotensin-II and rosuvastatin
influence matrix remodeling in human mesangial cells via
metalloproteinase modulation. J Hypertens. 29:1930–1939. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Czech KA, Bennett M and Devarajan P:
Distinct metalloproteinase excretion patterns in focal segmental
glomerulosclerosis. Pediatr Nephrol. 26:2179–2184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun H, Ge N, Shao M, Cheng X, Li Y, Li S
and Shen J: Lumbrokinase attenuates diabetic nephropathy through
regulating extracellular matrix degradation in
Streptozotocin-induced diabetic rats. Diabetes Res Clin Pract.
100:85–95. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou L, Zhang N, Song W, You N, Li Q, Sun
W, Zhang Y, Wang D and Dou K: The significance of Notch1 compared
with Notch3 in high metastasis and poor overall survival in
hepatocellular carcinoma. PLoS One. 8:e573822013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang DQ, Wei YQ, Yin XX, Lu Q, Hao HH,
Zhai YP, Wang JY and Ren J: In vitro suppression of quercetin on
hypertrophy and extracellular matrix accumulation in rat glomerular
mesangial cells cultured by high glucose. Fitoterapia. 82:920–926.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Herman-Edelstein M, Thomas MC,
Thallas-Bonke V, Saleem M, Cooper ME and Kantharidis P:
Dedifferentiation of immortalized human podocytes in response to
transforming growth factor-β: a model for diabetic podocytopathy.
Diabetes. 60:1779–1788. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morales MG, Abrigo J, Meneses C, Simon F,
Cisternas F, Rivera JC, Vazquez Y and Cabello-Verrugio C: The
Ang-(1–7)/Mas-1 axis attenuates the expression and signalling of
TGF-β1 induced by AngII in mouse skeletal muscle. Clin Sci (Lond).
127:251–264. 2014. View Article : Google Scholar
|
|
34
|
Matsumoto E, Sasaki S, Kinoshita H, Kito
T, Ohta H, Konishi M, Kuwahara K, Nakao K and Itoh N: Angiotensin
II-induced cardiac hypertrophy and fibrosis are promoted in mice
lacking Fgf16. Genes Cells. 18:544–553. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aoyagi-Ikeda K, Maeno T, Matsui H, Ueno M,
Hara K, Aoki Y, Aoki F, Shimizu T, Doi H, Kawai-Kowase K, Iso T,
Suga T, Arai M and Kurabayashi M: Notch induces myofibroblast
differentiation of alveolar epithelial cells via transforming
growthfactor-{beta}-Smad3 pathway. Am J Respir Cell Mol Biol.
45:136–144. 2011.PubMed/NCBI
|
|
36
|
Chen Y, Zheng S, Qi D, Zheng S, Guo J,
Zhang S and Weng Z: Inhibition of Notch signaling by a γ-secretase
inhibitor attenuates hepatic fibrosis in rats. PLoS One.
7:e465122012. View Article : Google Scholar
|
|
37
|
Tsioufis C, Bafakis I, Kasiakogias A and
Stefanadis C: The role of matrix metalloproteinases in diabetes
mellitus. Curr Top Med Chem. 12:1159–1165. 2012. View Article : Google Scholar : PubMed/NCBI
|