Introduction
Ovarian cancer is the leading cause of
cancer-related mortality among women worldwide and it predominately
affects postmenopausal women, with approximately 204,000 women
being diagnosed with the disease each year (1). More than 80% of patients with
ovarian cancer eventually have a relapse with chemoresistant
disease (2,3); therefore, causes a negative impact
on the treatment of ovarian cancer.
Cancer stem cells (CSCs) are a small subset of cells
capable of initiating and sustaining tumor growth (4). The CSC hypothesis posits that CSCs
are a minority population of self-renewing cancer cells that fuel
tumor growth and remain in patients after conventional therapy has
been completed (5). Therefore,
therapies focusing on CSCs seem to be more effective for the
treatment of cancer.
Several studies have identified the existence of
CSCs in an increasingly longer list of solid tumors (4), including ovarian cancer (6–10).
Recurrent ovarian tumors are known to be enriched with CSC-like
cells (11). It has been
suggested that ovarian CSCs (OCSCs) have the capacity to survive
treatment and they may recreate the original patient tumor in
animal models (12,13).
A recent study on cancer biology demonstrated that
CSCs and the tumor microenvironment seem to be related (14). Tumors can influence the
microenvironment by releasing extracellular signals, promoting
tumor angiogenesis and inducing peripheral immune tolerance, while
immune cells in the microenvironment can affect the growth and
evolution of cancer cells, such as in immunoediting (15–17). A recent study revealed that OCSC
factors can influence the tumor microenvironment and prognosis
(18). The current study was
designed to investigate the role of OCSCs in the M1/M2 polarization
of macrophages, as well as to elucidate the underlying molecular
mechanisms.
Materials and methods
Cell culture
The mouse monocyte macrophage cell line, Raw264.7,
was obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM; Gibco-BRL, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (Invitrogen Life
Technologies, Carlsbad, CA, USA) at 37°C in a humidified atmosphere
of 5% CO2. The peroxisome proliferator-activated
receptor γ (PPARγ) antagonist, GW9662, was purchased from Cayman
Chemical Co. (Ann Arbor, MI, US) and dissolved in 25% DMSO. The
Raw264.7 cells were treated with GW9662 at the concentration of 0.1
μM.
Isolation of OCSCs
The mouse ovarian cancer cell line, OVHM, was
obtained from the Key Laboratory of Gynecological Oncology, Qilu
Hospital of Shandong University, Jinan, China and cultured in DMEM
(Gibco-BRL) supplemented with 10% fetal bovine serum (Invitrogen
Life Technologies) at 37°C with 5% CO2. A stem-like cell
subpopulation from the ovarian cancer cells was obtained and
separated through the suspension culture of the OVHM cells in
serum-free medium. The OVHM cells were cultured in serum-free DMEM
supplemented with epidermal growth factor (20 ng/ml; Invitrogen
Life Technologies), basic fibroblast growth factor (20 ng/ml;
Invitrogen Life Technologies) and B27 (2%; Gibco-BRL). The cells in
suspension formed spheroid structures. The primary spheres were
dissociated to generate single cells and serially diluted to plate
into 96-well plates at one cell per well. The cells were visualized
under a microscope (TS100; Nikon, Tokyo, Japan), and the wells
containing one single cell were marked. The secondary spheres were
dissociated and plated into serum-free medium at 50
cells/cm2. Sphere-forming cells were passaged up to
passage (P)7 and then used for the experiments.
Co-culture of Raw264.7 cells with
OCSCs
For co-culture experiments, 0.4-μm pore size
Transwell inserts (Corning Inc., Corning, NY, USA) were plated into
a 6-well plate. The Raw264.7 cells were seeded at the bottom well,
while the OCSCs were seeded onto the inserts.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted from the cells
using TRIzol reagent (Invitrogen Life Technologies). The reverse
transcription of 1 μg of RNA was performed using a
RevertAid™ First Strand cDNA Synthesis kit (Fermentas, Vilnius,
Lithuania), following the manufacturer’s instructions. Quantitative
PCR (qPCR) was performed using SYBR-Green PCR master mix (Applied
Biosystems, Foster City, CA, USA) in a 7900HT fast Real-Time PCR
System (Applied Biosystems). The relative levels of gene expression
were calculated by the comparative CT method.
Western blot analysis
The specific primary antibodies were rabbit
polyclonal to PPARγ (1:800; sc-7196; Santa Cruz Biotechnology,
Santa Cruz, CA, USA), rabbit polyclonal to nuclear factor κB
(NF-κB; 1:1000; sc-372; Santa Cruz Biotechnology) and rabbit
polyclonal to GAPDH (1:2000; ab37168; Abcam, Cambridge, MA, USA).
The cells were lysed in ice-cold cell lysis buffer (25 mM Tris-HCl,
pH 7.6, 150 mM NaCl, 0.1% Triton X-100, 0.1% Nonidet P-40, 1%
sodium deoxycholate, 1% SDS and 2 mM phenylmethylsulfonyl
fluoride). Total cellular protein (20 mg) was loaded per lane,
separated by 10% SDS-polyacrylamide gel electrophoresis and then
transferred onto a polyvinylidene fluoride membrane (Millipore,
Billerica, MA, USA) by electroblotting. After blocking with 5%
non-fat milk (Inner Mongolia Yili Industrial Group Co., Ltd., Inner
Mongolia, China) at 4°C overnight, the membrane was incubated with
primary antibodies for 2 h at room temperature followed by
incubation with horseradish peroxidase-conjugated anti-rabbit
secondary antibody (1:2000; sc-2004; Santa Cruz Biotechnology) for
1 h at room temperature. The signals were probed using an ECL
Western Blotting kit (Pierce Biotechnology, Inc., Rockford, IL,
USA).
Immunofluorescence staining
CD117 and CD44 are specific markers of OCSCs
(6,19). Immunofluorescence staining was
used in the present study to detect the expression of CD117 and
CD44 in the OCSCs. After washing with PBS, the cells were fixed in
4% paraformaldehyde (Sigma, St. Louis, MO, USA) at 4°C for 1 h. The
cells were then permeabilized by exposure to 0.2% TritonX-100
(Sigma) at 4°C for 1 h, followed by blocking with normal goat serum
(Maixin, Fuzhou, Fujian, China) at 4°C for a further 1 h. The cells
were then incubated with primary antibodies [rabbit polyclonal to
CD117 (1:800; ab5506) and rabbit monoclonal to CD44 (1:400;
ab51037); Abcam] for 2 h at room temperature. After washing with
PBS, [Alexa Fluor 488 labeled Goat anti-rabbit IgG (1:400;
ab150077); Abcam] was added and the cells were incubated at 37°C
for 1 h. The nucleus was stained with DAPI (Invitrogen Life
Technologies) and the slides were visualized under a fluorescence
microscope (80i; Nikon).
Statistical analysis
The results were analyzed using SPSS statistical
software version 19.0 (SPPS, Inc., Chicago, IL, USA). All the data
are presented as the means ± SD. Statistically significant
differences between 2 groups were compared using the Student’s
t-test, and P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Identification of OCSCs
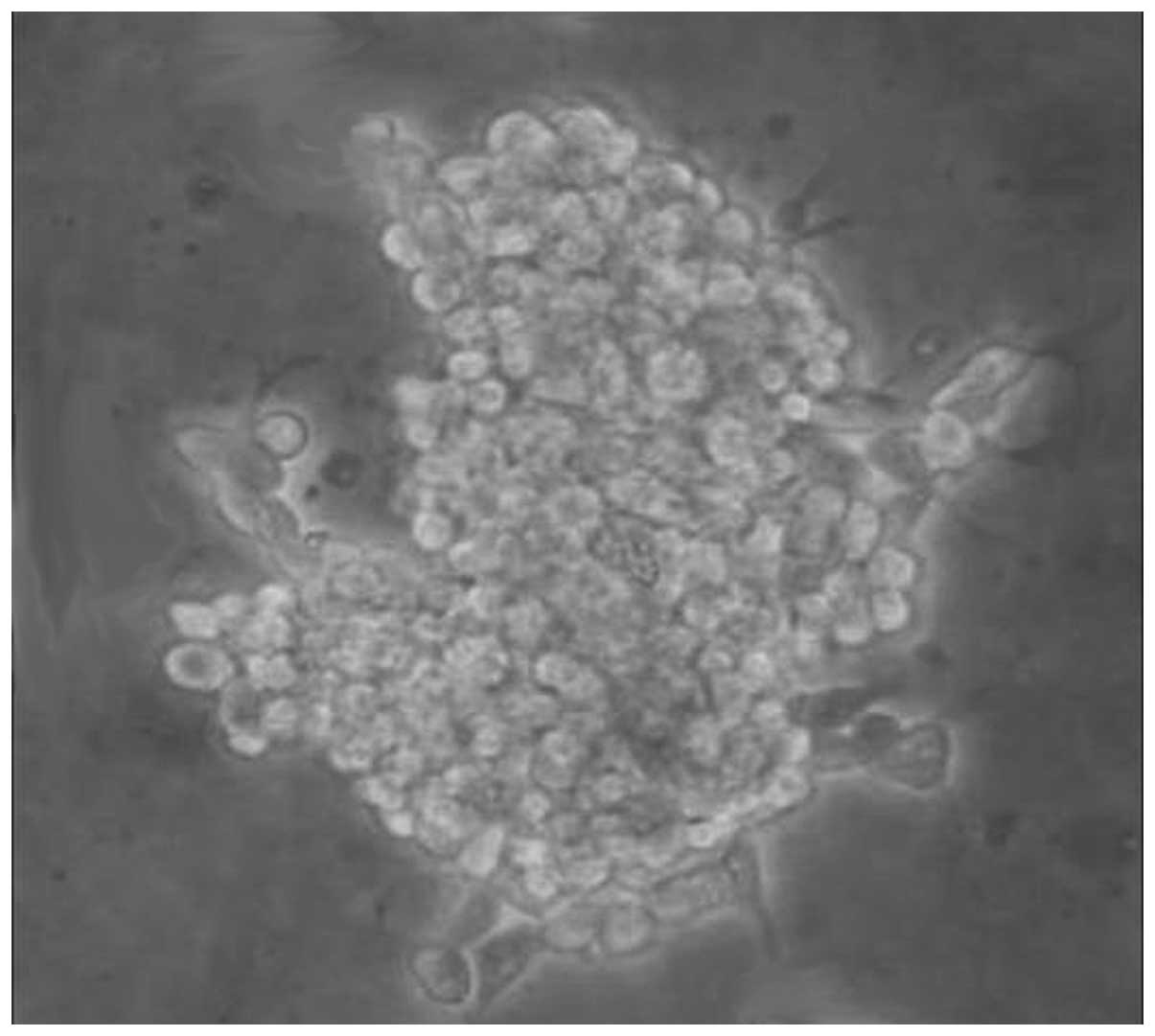
In the present study, a self-renewing, stem-like
cell subpopulation from the ovarian cancer cell line, OVHM, was
isolated using non-adherent suspension culture. Fig. 1 shows the spheroid structure of
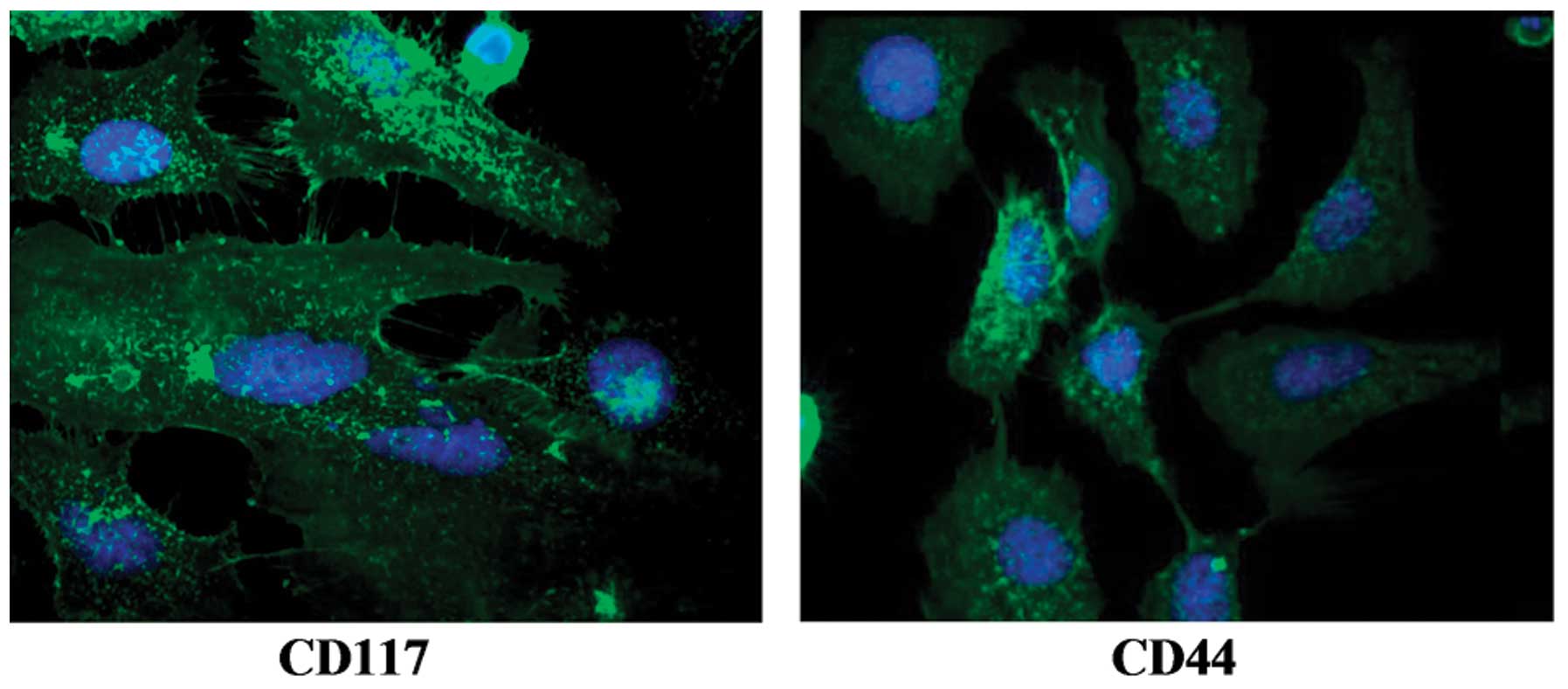
the OCSCs in serum-free medium. Immunofluorescence staining was
used to confirm the specific markers expressed in the isolated
OCSCs. The images from immunofluorescence staining are presented in
Fig. 2. The OCSCs showed
positively staining for CD117 and CD44.
OCSCs promote the M2 polarization of
macrophages
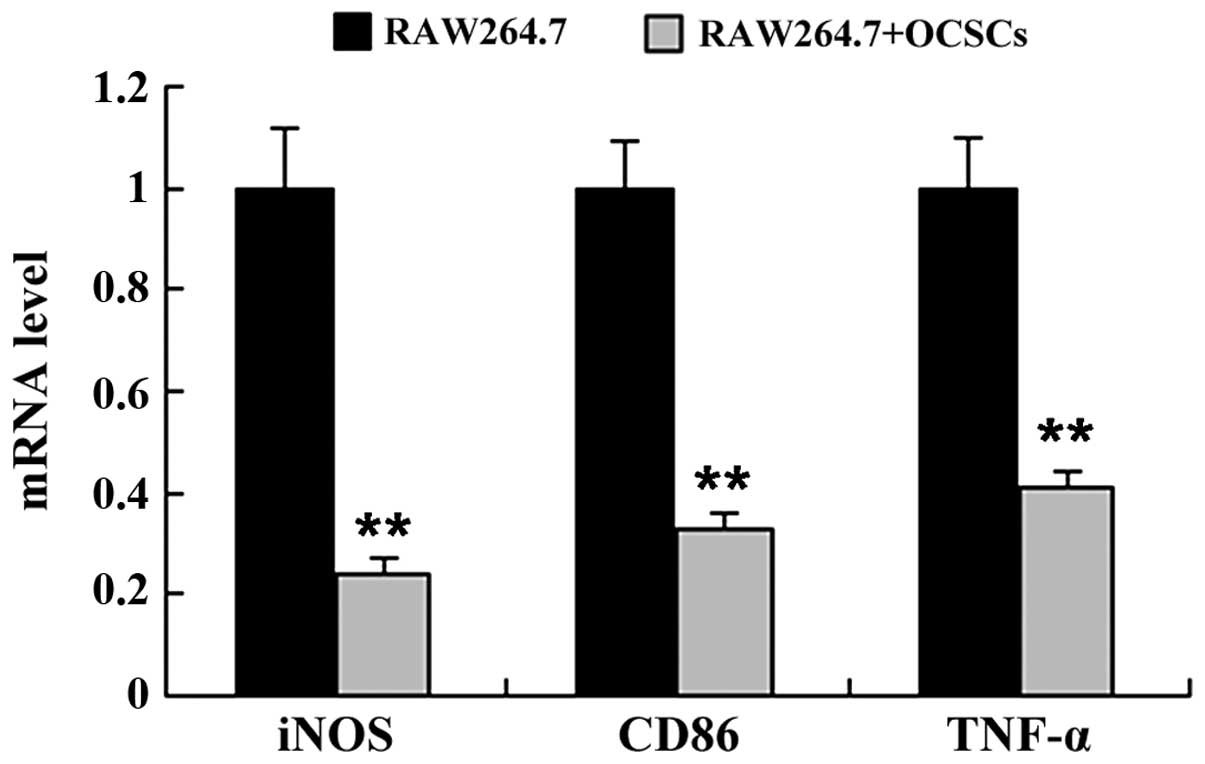
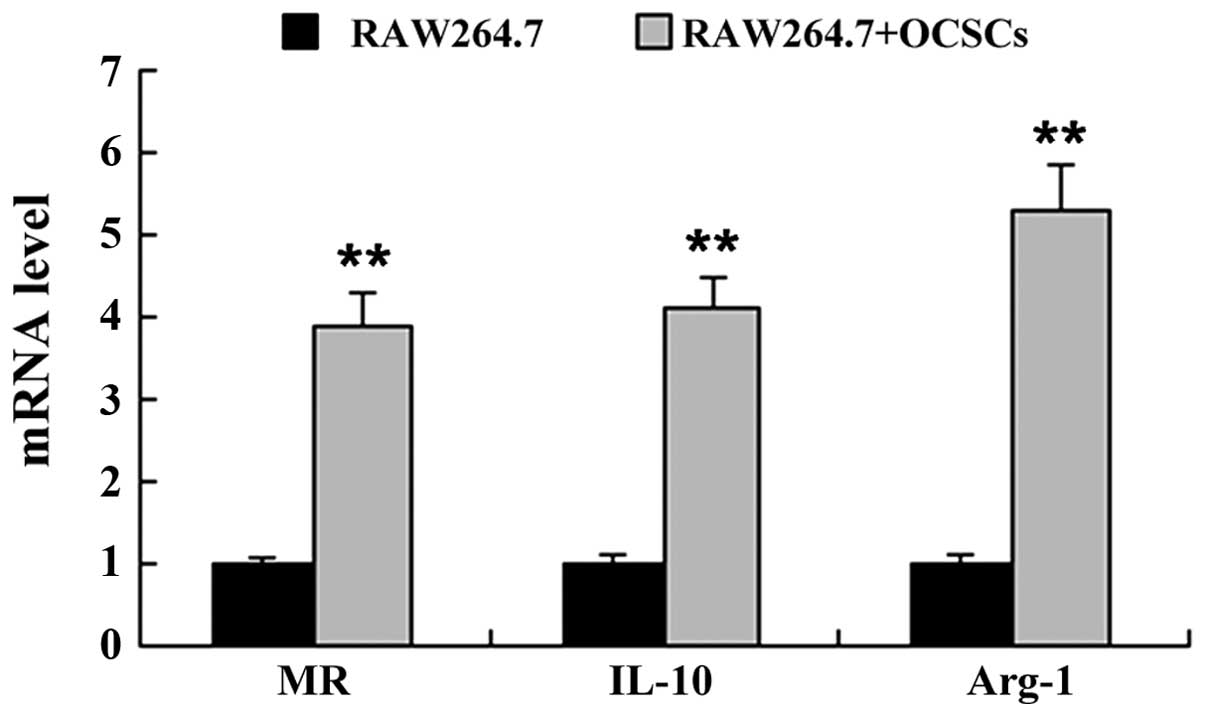
To determine whether OCSCs affect macrophage
polarization, the Raw264.7 cells were co-cultured with the OCSCs,
and the expression levels of markers of the M1 macrophage phenotype
[inducible nitric oxide synthase (iNOS), CD86 and tumor necrosis
factor (TNF)-α] and the M2 macrophage phenotype [mannose receptor
(MR), interleukin (IL)-10 and arginase-1 (Arg-1)] were measured by
RT-qPCR. As shown in Figs. 3 and
4, co-culture of the Raw264.7
cells with OCSCs significantly induced the mRNA expression of
markers of the M2 macrophage phenotype, including MR, IL-10 and
Arg-1, whereas the mRNA expression of markers of the M1 macrophage
phenotype, including iNOS, CD86 and TNF-α was markedly
decreased.
OCSCs affects PPARγ/NF-κB expression in
Raw264.7 cells
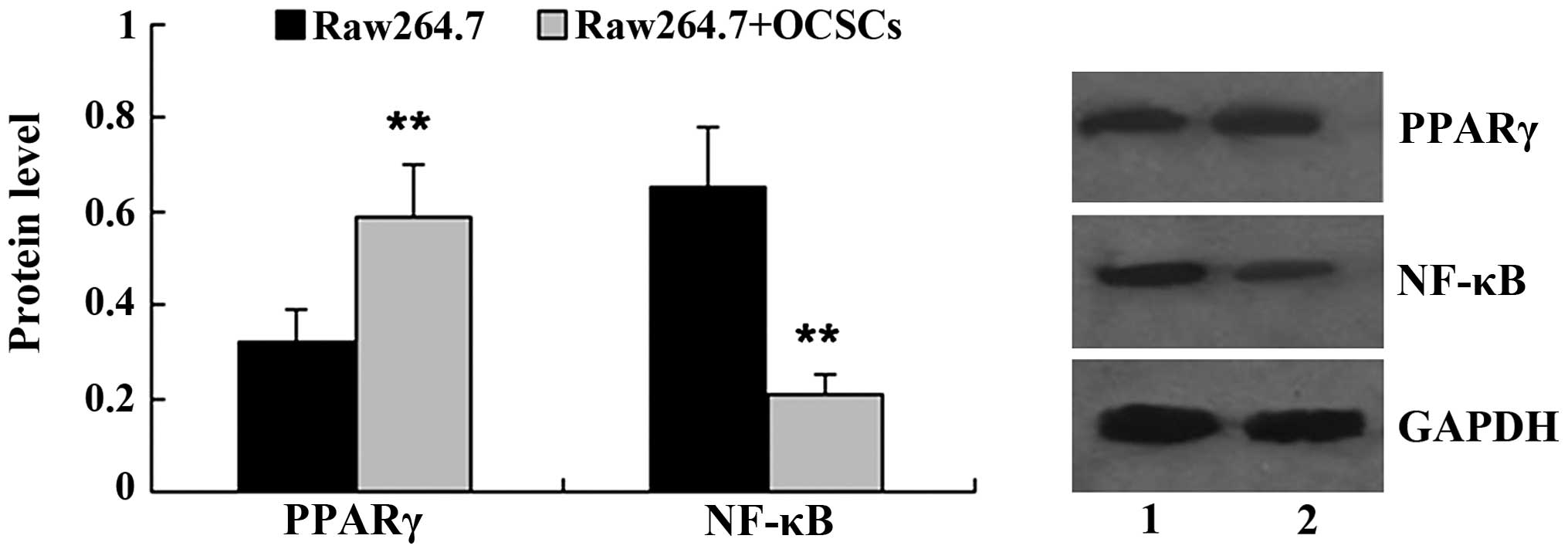
Subsequently, we examined the expression of PPARγ
and NF-κB in the Raw264.7 cells co-cultured with OCSCs. Western
blot analysis revealed that, in comparison with the Raw264.7 cells
cultured alone, co-culture with OCSCs significantly increased the
relative protein level of PPARγ, while it decreased the relative
protein level of NF-κB (Fig.
5).
GW9662 affects PPARγ/NF-κB expression in
Raw264.7 cells
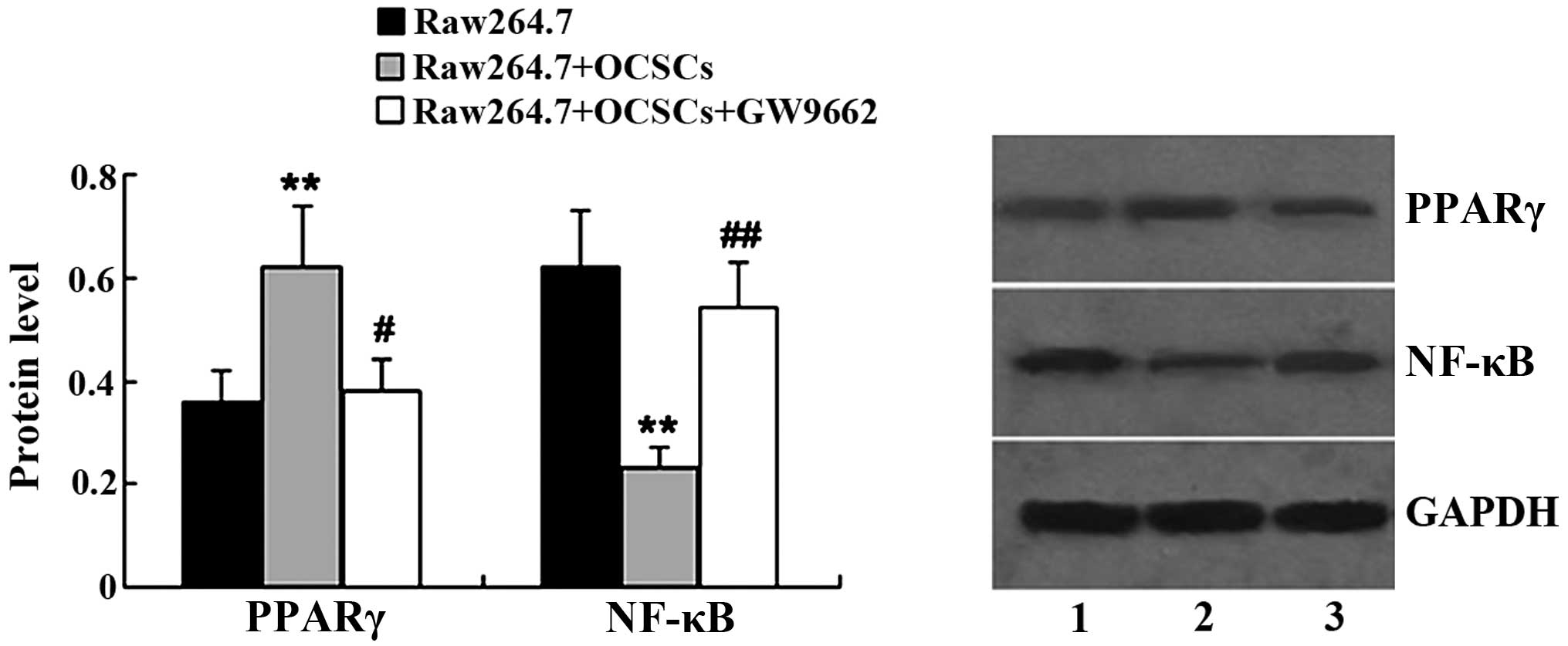
GW9662 was used to treat the Raw264.7 cells
co-cultured with OCSCs, and the protein expression levels of PPARγ
and NF-κB in the Raw264.7 cells were the measured by western blot
analysis. As is shown in Fig. 6,
following treatment with GW9662, the expression of PPARγ was
significantly decreased in the Raw264.7 cells co-cultured with the
OCSCs. In addition, NF-κB expression in the Raw264.7 cells
co-cultured with the OCSCs was altered following treatment with
GW9662. GW9662 led to an increase in NF-κB expression in the
Raw264.7 cells co-cultured with the OCSCs.
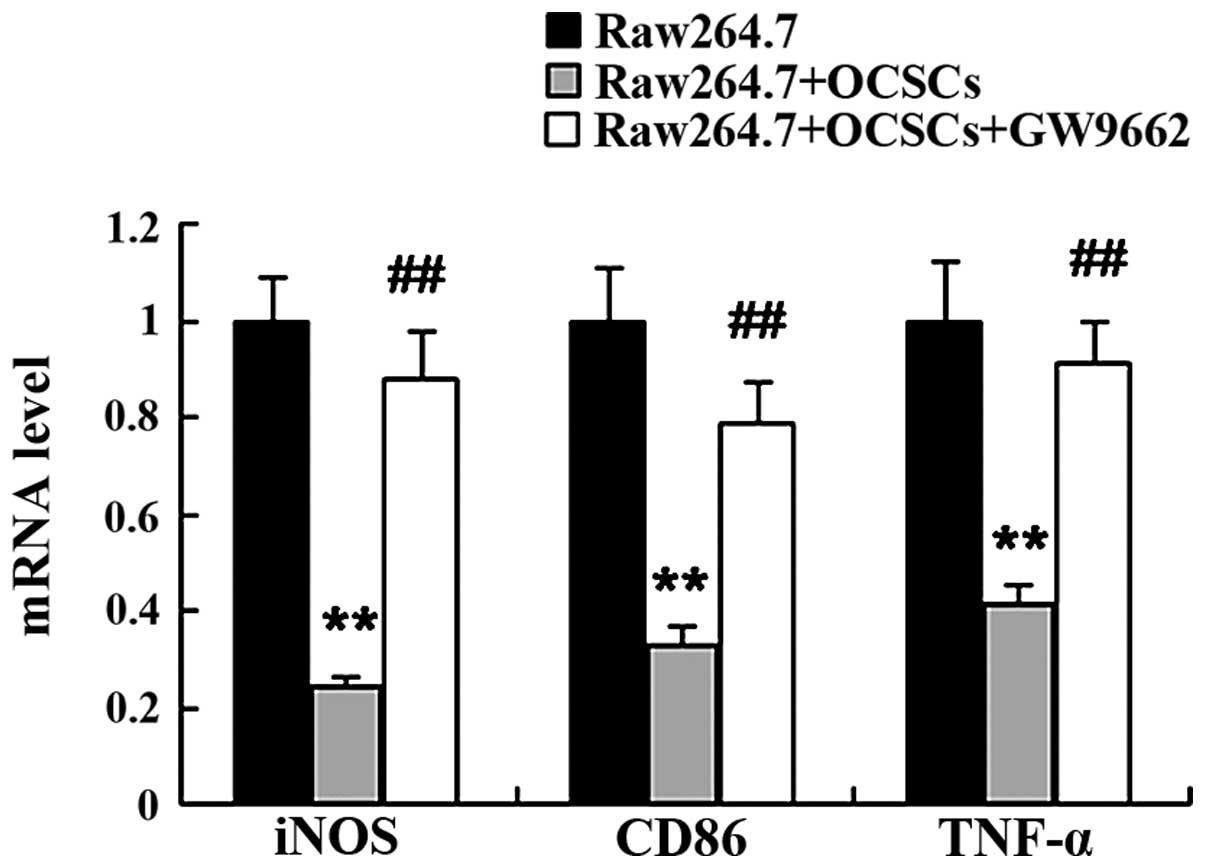
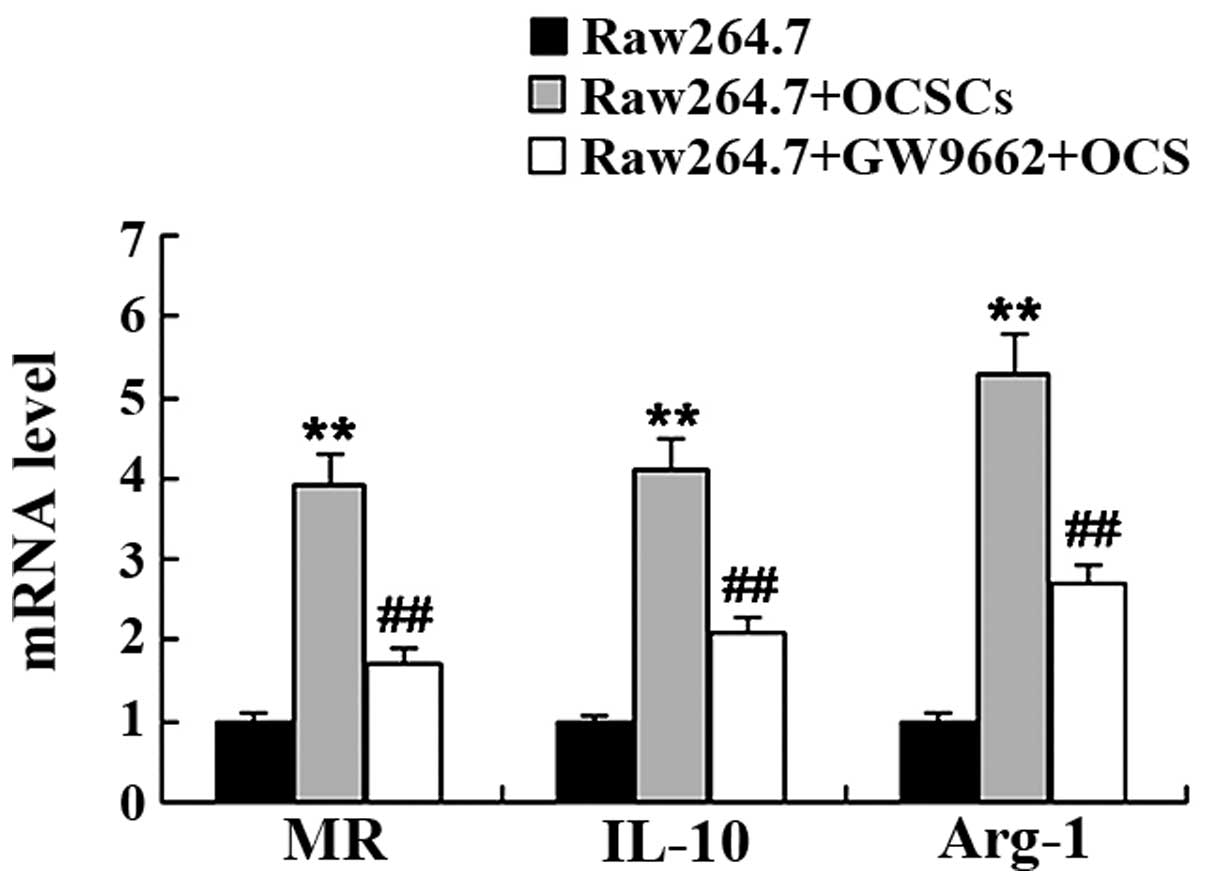
Inhibition of PPARγ attenuates the
promoting effects of OCSCs on the M2 polarization of
macrophages
To examine the role of PPARγ in mediating the
promoting effects of OCSCs on the M2 polarization of macrophages,
PPARγ was inhibited by treatment with GW9662. We found that the
OCSCs promoted the M2 polarization of macrophages with an increased
mRNA expression of MR, IL-10 and Arg-1, and a decreased mRNA
expression of iNOS, CD86 and TNF-α. However, this effect was
reversed by treatment with GW9662. Compared with the Raw264.7 cells
co-cultured with the OCSCs, the expression of markers of the M2
macrophage phenotype was significantly decreased, while that of
markers of the M1 macrophage phenotype was significantly increased
following treatment with GW9662 (Figs. 7 and 8).
Discussion
The tumor microenvironment is a complex cellular
environment in which the tumor exists; it consists of cancer cells,
fibroblasts, immune cells, surrounding blood vessels, cytokines,
chemicals and the extracellular matrix (20–25). The interaction between cancer
cells and the tumor microenvironment plays an important role in
tumor progression.
Macrophages are a heterogeneous cell population
which exists in abundance in the tumor microenvironment, adapting
and responding to a large variety of tumor-secreted cytokines
(26). Tumor-associated
macrophages (TAMs) can influence tumor growth, invasion,
angiogenesis and metastasis. There are mainly two types of
polarized macrophages, termed M1 and M2 macrophages. Th1 cytokines,
such as lipopolysaccharide (LPS), IL-1β and interferon γ (IFNγ
promote monocyte differentiation into a ‘classical’ M1 macrophage
phenotype, while Th2 cytokines, such as IL-4 and IL-13, lead to an
‘alternative’ M2 macrophage phenotype. M1 macrophages are generally
anti-tumoral and they primarily produce pro-inflammatory cytokines,
such as TNF-α, IL-6 and IL-12 (27). M2 macrophages on the other hand
exert protumoral effects and they produce anti-inflammatory
factors, including transforming growth factor (TGF)-β, IL-10 and
IL-1 receptor antagonist, as well as promote angiogenesis and
tissue remodeling (27–29). Moreover, upon specific signals,
macrophages can switch from an activated M1 state back to an M2
state and vice versa (30).
Raw264.7 macrophages have been identified as
‘innate’ macrophages that can differentiate into M1 or M2
macrophages (31). In the present
study, we demonstrated the interplay between OCSCs and macrophages.
Following co-culture with OCSCs, the macrophages acquired an M2
phenotype characterized by an increased expression of MR, IL-10 and
Arg-1, while the expression of M1 macrophages markers, including
TNF-α, iNOS and CD86, was suppressed.
NF-κB is one of the major transcription factors
responsible for the development of immune responses. NF-κB
activation has been associated with the enhanced transcriptional
activity of chemokines, cytokines and adhesion molecules, which can
facilitate the recruitment and activation of inflammatory cells to
the site of NF-κB activation. Gao et al found that mouse
mesenchymal stem cells induced macrophage M2 activation through
NF-κB (32). As expected, in the
present study, the expression of NF-κB in the Raw264.7 cells was
inhibited by OCSCs.
PPARγ, a ligand-activated nuclear receptor, is
abundantly expressed in macrophages. PPARγ can be activated by
natural and synthetic ligands, such as 15d-PGJ2 (33), antidiabetic thiazolidinediones
(TZDs) and the GW1929 compound (34). During the differentiation of
monocytes into macrophages, the expression of PPARγ is rapidly
induced (35). PPARγ modulates
the immune inflammatory response with anti-inflammatory properties
(36). It has been suggested that
PPARγ inhibits the activation of inflammatory response genes (such
as IL-2, IL-6, IL-8, TNF-α and metalloproteases) by negatively
interfering with NF-κB (37). In
the present study, we futher investigated the effects of OCSCs on
PPARγ activation in macrophages. The results revealed that the
expression of PPARγ was significantly increased in the Raw264.7
cells co-cultured with OCSCs.
To determine whether PPARγ and NF-κB play a role in
mediating the effects of OCSCs on the M2 polarization of
macrophages, GW9662, a potent and selective antagonist of
full-length PPARγ was added to block PPARγ activation. Our results
revealed that GW9662 effectively suppressed PPARγ activation in the
Raw264.7 cells co-cultured with OCSCs, while the expression of
NF-κB increased. Furthermore, treatment with GW9662 influenced M1
and M2 marker expression in the Raw264.7 cells co-cultured with
OCSCs. These results support the view that the effects of OCSCs on
the M2 polarization of macrophages may be attenuated by treatment
with PPARγ inhibitor.
Taken together, the results of the present study
suggest a potential functional association between OCSCs and the
M1/M1 polarization of macrophages. OCSCs are capable of promoting
the M2 polarization of macrophages by affecting the PPARγ/NF-κB
pathway.
References
|
1
|
Ozols RF, Bookman MA, Connolly DC, Daly
MB, Godwin AK, Schilder RJ, Xu X and Hamilton TC: Focus on
epithelial ovarian cancer. Cancer Cell. 5:19–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clarke-Pearson DL: Clinical practice.
Screening for ovarian cancer. N Engl J Med. 361:170–177. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwartz PE: Current diagnosis and
treatment modalities for ovarian cancer. Cancer Treat Res.
107:99–118. 2002.PubMed/NCBI
|
|
4
|
Ailles LE and Weissman IL: Cancer stem
cells in solid tumors. Curr Opin Biotechnol. 18:460–466. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells - perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang S, Balch C, Chan MW, Lai HC, Matei
D, Schilder JM, Yan PS, Huang TH and Nephew KP: Identification and
characterization of ovarian cancer-initiating cells from primary
human tumors. Cancer Res. 68:4311–4320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bapat SA, Mali AM, Koppikar CB and Kurrey
NK: Stem and progenitor-like cells contribute to the aggressive
behavior of human epithelial ovarian cancer. Cancer Res.
65:3025–3029. 2005.PubMed/NCBI
|
|
8
|
Burleson KM, Casey RC, Skubitz KM,
Pambuccian SE, Oegema TR Jr and Skubitz AP: Ovarian carcinoma
ascites spheroids adhere to extracellular matrix components and
meso-thelial cell monolayers. Gynecol Oncol. 93:170–181. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Curley MD, Therrien VA, Cummings CL, et
al: CD133 expression defines a tumor initiating cell population in
primary human ovarian cancer. Stem Cells. 27:2875–2883.
2009.PubMed/NCBI
|
|
10
|
Wani AA, Sharma N, Shouche YS and Bapat
SA: Nuclear-mitochondrial genomic profiling reveals a pattern of
evolution in epithelial ovarian tumor stem cells. Oncogene.
25:6336–6344. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steg AD, Bevis KS, Katre AA, Ziebarth A,
Dobbin ZC, Alvarez RD, Zhang K, Conner M and Landen CN: Stem cell
pathways contribute to clinical chemoresistance in ovarian cancer.
Clin Cancer Res. 18:869–881. 2012. View Article : Google Scholar :
|
|
12
|
Alvero AB, O’Malley D, Brown D, Kelly G,
Garg M, Chen W, Rutherford T and Mor G: Molecular mechanism of
phenoxodiol-induced apoptosis in ovarian carcinoma cells. Cancer.
106:599–608. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alvero AB, Fu HH, Holmberg J, Visintin I,
Mor L, Marquina CC, Oidtman J, Silasi DA and Mor G: Stem-like
ovarian cancer cells can serve as tumor vascular progenitors. Stem
Cells. 27:2405–2413. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Albini A, Cesana E and Noonan DM: Cancer
stem cells and the tumor microenvironment: Soloists or choral
singers. Curr Pharm Biotechnol. 12:171–181. 2011. View Article : Google Scholar
|
|
15
|
Stockmann C, Schadendorf D, Klose R and
Helfrich I: The impact of the immune system on tumor: Angiogenesis
and vascular remodeling. Front Oncol. 4:692014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chouaib S, Kieda C, Benlalam H, Noman MZ,
Mami-Chouaib F and Rüegg C: Endothelial cells as key determinants
of the tumor microenvironment: Interaction with tumor cells,
extracellular matrix and immune killer cells. Crit Rev Immunol.
30:529–545. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mantovani A and Sica A: Macrophages,
innate immunity and cancer: Balance, tolerance, and diversity. Curr
Opin Immunol. 22:231–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma W, Ma J, Xu J, Qiao C, Branscum A,
Cardenas A, Baron AT, Schwartz P, Maihle NJ and Huang Y: Lin28
regulates BMP4 and functions with Oct4 to affect ovarian tumor
microenvironment. Cell Cycle. 12:88–97. 2013. View Article : Google Scholar :
|
|
19
|
Alvero AB, Chen R, Fu HH, et al: Molecular
phenotyping of human ovarian cancer stem cells unravels the
mechanisms for repair and chemoresistance. Cell Cycle. 8:158–166.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alvaro T, de la Cruz-Merino L,
Henao-Carrasco F, Villar Rodríguez JL, Vicente Baz D, Codes Manuel
de Villena M and Provencio M: Tumor microenvironment and immune
effects of antineoplastic therapy in lymphoproliferative syndromes.
J Biomed Biotechnol. 2010:1–17. 2010. View Article : Google Scholar
|
|
21
|
Banchereau J, Briere F, Caux C, Davoust J,
Lebecque S, Liu YJ, Pulendran B and Palucka K: Immunobiology of
dendritic cells. Annu Rev Immunol. 18:767–811. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cretu A and Brooks PC: Impact of the
non-cellular tumor micro-environment on metastasis: Potential
therapeutic and imaging opportunities. J Cell Physiol. 213:391–402.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oluwadara O, Giacomelli L, Brant X,
Christensen R, Avezova R, Kossan G and Chiappelli F: The role of
the microenvironment in tumor immune surveillance. Bioinformation.
5:285–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piersma SJ: Immunosuppressive tumor
microenvironment in cervical cancer patients. Cancer Microenviron.
4:361–375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sautès-Fridman C, Cherfils-Vicini J,
Damotte D, Fisson S, Fridman WH, Cremer I and Dieu-Nosjean MC:
Tumor micro-environment is multifaceted. Cancer Metastasis Rev.
30:13–25. 2011. View Article : Google Scholar
|
|
26
|
Mantovani A, Schioppa T, Porta C, Allavena
P and Sica A: Role of tumor-associated macrophages in tumor
progression and invasion. Cancer Metastasis Rev. 25:315–322. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gordon S: Alternative activation of
macrophages. Nat Rev Immunol. 3:23–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mantovani A, Locati M, Vecchi A, Sozzani S
and Allavena P: Decoy receptors: A strategy to regulate
inflammatory cytokines and chemokines. Trends Immunol. 22:328–336.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Porcheray F, Viaud S, Rimaniol AC, Léone
C, Samah B, Dereuddre-Bosquet N, Dormont D and Gras G: Macrophage
activation switching: An asset for the resolution of inflammation.
Clin Exp Immunol. 142:481–489. 2005.PubMed/NCBI
|
|
31
|
Murray PJ and Wynn TA: Protective and
pathogenic functions of macrophage subsets. Nat Rev Immunol.
11:723–737. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao S, Mao F, Zhang B, et al: Mouse bone
marrow-derived mesenchymal stem cells induce macrophage M2
polarization through the nuclear factor-κB and signal transducer
and activator of transcription 3 pathways. Exp Biol Med (Maywood).
239:366–375. 2014. View Article : Google Scholar
|
|
33
|
Kliewer SA, Lenhard JM, Willson TM, Patel
I, Morris DC and Lehmann JM: A prostaglandin J2 metabolite binds
peroxisome proliferator-activated receptor gamma and promotes
adipocyte differentiation. Cell. 83:813–819. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Willson TM, Brown PJ, Sternbach DD and
Henke BR: The PPARs: From orphan receptors to drug discovery. J Med
Chem. 43:527–550. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chinetti G, Griglio S, Antonucci M, Torra
IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J and Staels
B: Activation of proliferator-activated receptors alpha and gamma
induces apoptosis of human monocyte-derived macrophages. J Biol
Chem. 273:25573–25580. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chinetti G, Fruchart JC and Staels B:
Peroxisome proliferator-activated receptors: New targets for the
pharmacological modulation of macrophage gene expression and
function. Curr Opin Lipidol. 14:459–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chinetti G, Fruchart JC and Staels B:
Peroxisome proliferator-activated receptors (PPARs): Nuclear
receptors at the crossroads between lipid metabolism and
inflammation. Inflamm Res. 49:497–505. 2000. View Article : Google Scholar : PubMed/NCBI
|