Introduction
Spinal cord injury (SCI) leads to the consequent
death of neurocytes, thus causing the dysfunction of signal
transmissions between neurons and axons, which could in turn induce
apoptosis of neurons. As reported, axons of the injured neurons in
the adult mammalian central nervous system (CNS) can seldom
spontaneously regenerate (1).
This phenomenon has caused confusion for the SCI treatment. There
are relevant studies that have restored the regenerative ability of
the injured axons (2–11). For example, various neurotrophin
treatments, including nerve growth factor (NGF) and brain-derived
neurotrophic factor, have been effectively proved to promote
neurons branching and sprouting (12,13), and to promote axon regeneration
(14,15). However, the mechanism underlying
neurotrophin treatments has not been fully investigated or
established.
PC12 cells originate from a rat pheochromocytoma
tumor cell line, which is sensitive to NGF treatment by
differentiating into neuron-like cells (16,17). Therefore, PC12 cells have been
employed as a promising, unique and frequently used cell model for
neural development and protection studies (17,18). Akt is a type of neurocyte protein
kinase that is associated with stress response to growth factors
(19), which also has a role in
tumor growth (20). The nuclear
factor E2-related factor 2 (Nrf2) is a type of transcription
factor, which could initiate antioxidant response element (ARE)
transcription. The Nrf2 gene products include a scope of
antioxidative factors participating in antioxidant function, such
as heme oxygenase-1 (HO-1), NAD(P) H:quinone oxidoreductase-1
(NQO-1) and γ-glutamylcysteine synthetase (γ-GCS) (21-24). The present study aimed to further
understand whether Akt could activate Nrf2/ARE antioxidant systems
to decrease apoptosis of neurocytes and contribute to axon
regeneration.
The NGF-differentiated PC12 cells were used to
investigate the effect of Akt on axon growth. Changes in axon
regrowth produced by silence and overexpression of Akt were
examined and the function of antioxidant enzyme activities in the
presence of NGF in PC12 cells was identified. Understanding the
mechanisms between Akt and the Nrf2/ARE pathway in PC12 cells is
important for the development of new methods to prevent or treat
neurodegenerative diseases, such as SCI-caused axon
degeneration.
Materials and methods
Cell culture and differentiation
PC12 cells were purchased from Riken Cell Bank
(Tsukuba, Ibaraki, Japan) and cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% (v/v) horse serum and
5% (v/v) fetal bovine serum (FBS) (all from Hyclone, Logan, UT,
USA). The dishes had been previously coated with poly-L-lysine
(Sigma-Aldrich, St. Louis, MO, USA). The cells were incubated at
37°C in a humidified 5% CO2 atmosphere. PC12 cells were
differentiated with 100 ng/ml NGF (Invitrogen, Carlsbad, CA, USA)
for ≤72 h.
Recombinant adenovirus construction and
transfection
Recombinant adenovirus vectors were purchased from
Genomeditech Biotechnologies (Shanghai, China). Briefly, the genes
encoding Akt were amplified and identified, followed by conjugation
with shuttle vector pAdTrack-CMV. The pAdTrack-CMV and adenoviral
gene expression vector pAdEasy-1 were co-transfected into HEK293
cells in non-serum DMEM medium to produce recombinant adenovirus
using Lipofectamine 2000 (Invitrogen). The recombinant adenoviruses
were harvested, amplified, concentrated and purified, and the
titers were measured prior to use. Cells treated with empty carrier
LacZ instead of recombinant adenovirus were used as negative
control.
Preparation of small interference RNA
(siRNA) and transfection
The siRNA was synthesized by GenePharma Co., Ltd.
(Shanghai, China). Briefly, the medium had been changed to
non-serum medium 30 min before transfection. siRNA (5 µl;
Akt siRNA sc-108059; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) was added into 245 µl of non-serum DMEM (solution A).
Lipofectamine 2000 (10 µl; Invitrogen) was also diluted in
245 µl of non-serum DMEM (solution B) and incubated for 5
min at room temperature. Subsequently, solution B was gently added
into solution A, mixed and incubated for 20 min at room
temperature. The mixtures were equally distributed into the 6-well
cultured cells at drop speed with successive agitation, followed by
incubation at 37°C, prior to conducting further analysis. Cells
treated with siRNA instead of Akt siRNA were used as the negative
control.
Neurite outgrowth measurement of PC12
cells
The length of axons that extended from cell bodies
was measured by Image-Pro Plus 6.0 software (Media Cybernetics,
Silver Spring, MD, USA). Subsequently, the number of neurites
extending from cells was also calculated by counting the number and
percentage to determine differentiation efficiency.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay for cell viability evaluation
Cell viability was evaluated by the MTT assay.
Briefly, PC12 cells were cultured in 96-well plates with a density
of 2×104 cells/well. Subsequently, cells were incubated
with 20 µl MTT solution (5 mg/ml) in fresh medium (10% FBS)
for 4 h in a 37°C incubator. Following this, the mixtures were
centrifuged at 12,890 × g for 15 min and the supernatant was
carefully discarded using a vacuum pump, and formazan crystals were
dissolved in dimethylsulfoxide (0.1% final concentration;
Sigma-Aldrich). The absorbance of samples was measured at 490 nm
using the EnVision® Multilabel Reader (Perkin-Elmer,
Waltham, MA, USA).
Hoechst assay and terminal
deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)
experiment for apoptosis evaluation
For the Hoechst assay, cells were seeded at a
density of 1×104/well in 96-well plates, followed by the
addition of 200 µl fresh medium and incubation at 37°C in 5%
CO2. When cells grew to a confluence of 80%, apoptosis
was detected via the Hoechst Staining kit (Beyotime, Beijing,
China) according to the manufacturer's instructions. For the TUNEL
experiment, cells were firstly fixed with 4% paraformaldehyde for
10 min at room temperature. Subsequently, cells were washed with
phosphate-buffered saline (PBS) twice and permeabilized by 0.1%
Triton X-100 under ice-cold incubation for 10 min. Following
washing with PBS again, cell apoptosis was evaluated by a TUNEL
Apoptosis Detection kit (Merck Millipore, Billerica, MA, USA)
following the manufacturer's instructions. Cells were observed
under a fluorescent microscope (Olympus, Tokyo, Japan). The
positive cells were counted in randomly selected fields.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA of targeted PC12 cells was isolated using
the TRIzol reagent (Life Technologies, Rockville, MD, USA). Reverse
transcription was conducted using 1 µg of total RNA from
each sample via the oligo(dT) primer, using the RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Waltham, MA,
USA). RT-qPCR analysis, including Akt, HO-1, NQO-1 and γ-GCS, was
performed using the SYBR-Green PCR kit (Takara, Shiga, Japan) on a
Bio-Rad CFX96 Real-Time PCR Detection system. β-actin served as the
reference gene and data were further analyzed using the ΔCt
method.
Western blot analysis
Proteins were harvested using
radioimmunoprecipitation assay buffer supplemented with protease
inhibitor phenylmethanesulfonylfluoride (both from Sigma-Aldrich).
A total of 20 µg proteins were fractionated via sodium
dodecyl sulfate-polyacrylamide gel electrophoresis to be
transferred onto a nitrocellulose (NC) membrane (Amersham, Little
Chalfont, UK). Subsequently, the NC membrane was incubated in
blocking buffer consisting of 4% bovine serum albumin
(Sigma-Aldrich) in Tris-buffered saline to block non-specific
binding for 1 h. Subsequently, the membrane was incubated with
primary antibodies (rabbit antibodies including Akt (sc-8312;
1:500), HO-1 (sc-10789; 1:500), NQO-1 (sc-16464; 1:600) and γ-GCS
(sc-22755; 1:500), all from Santa Cruz Biotechnology, Inc.) diluted
in blocking buffer overnight at 4°C. On the following day, the NC
membrane was incubated with horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (sc-2004; diluted at 1:1,000; Santa
Cruz Biotechnology, Inc.) for 1 h. The protein signal was
visualized using the Amersham ECL™ Plus Western Blotting Detection
kit (GE Healthcare, Piscataway, NJ, USA).
Statistical analysis
All the data are presented as mean ± standard
deviation. Comparisons between the two groups and among multiple
groups were performed by Student's t-test and one-way analysis of
variance, respectively. P<0.05 was considered to indicate a
statistically significant difference. All the statistical analyses
were performed using SPSS version 19.0 (IBM Corp., Armonk, NY,
USA).
Results
NGF promotes neural differentiation of
PC12 cells
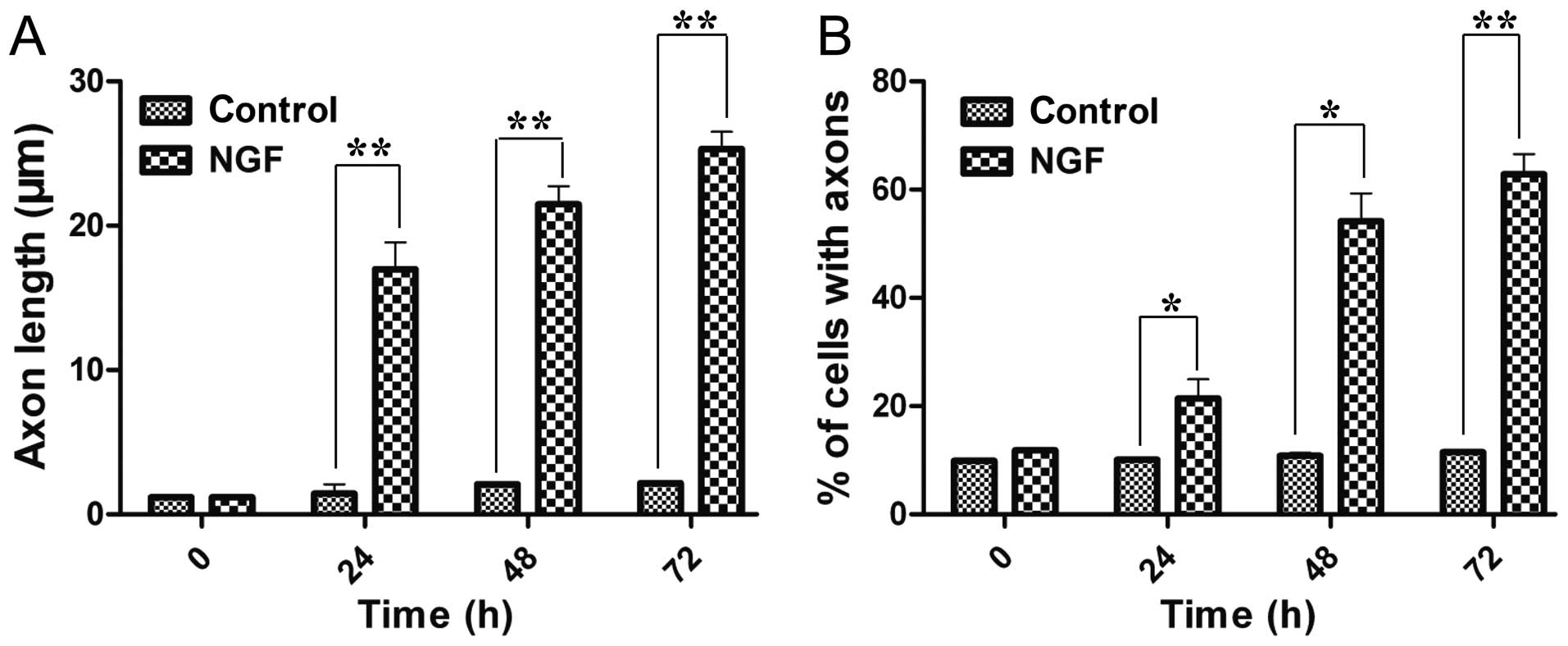
The study first confirmed the function of NGF to
induce neural differentiation of PC12 cells. NGF-treated cells had
a significant increase in the neurites length and also in the
number of neurite-possessing cells in a time-dependent manner
(Fig. 1). After 72 h induction,
the average axon length was 21.4-fold longer than previously and
the percentage of axon-attached neurocytes increased to 62.8±3.8%.
These results demonstrated that NGF could induce neural
differentiation of PC12 cells.
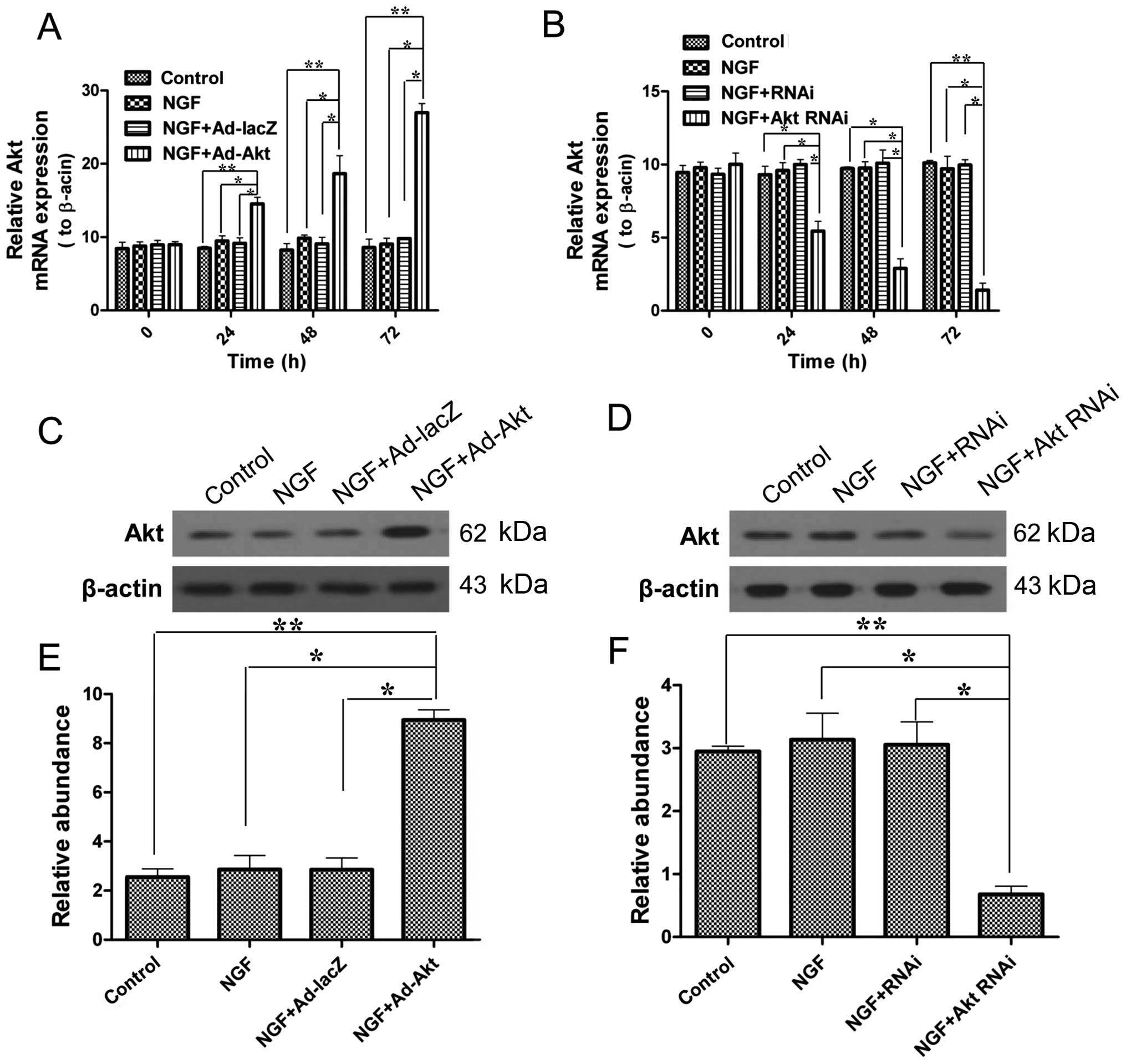
Akt is overexpressed or silenced in PC12
cells
Subsequently, in order to conduct the further
experiments under defined conditions, the effects of adenovirus
transfection and siRNA on PC12 cells were confirmed. Fig. 2 shows that Akt mRNA and protein
expression levels were significantly upregulated by adenovirus
transfection, and by contrast, siRNA knocked down Akt
expression.
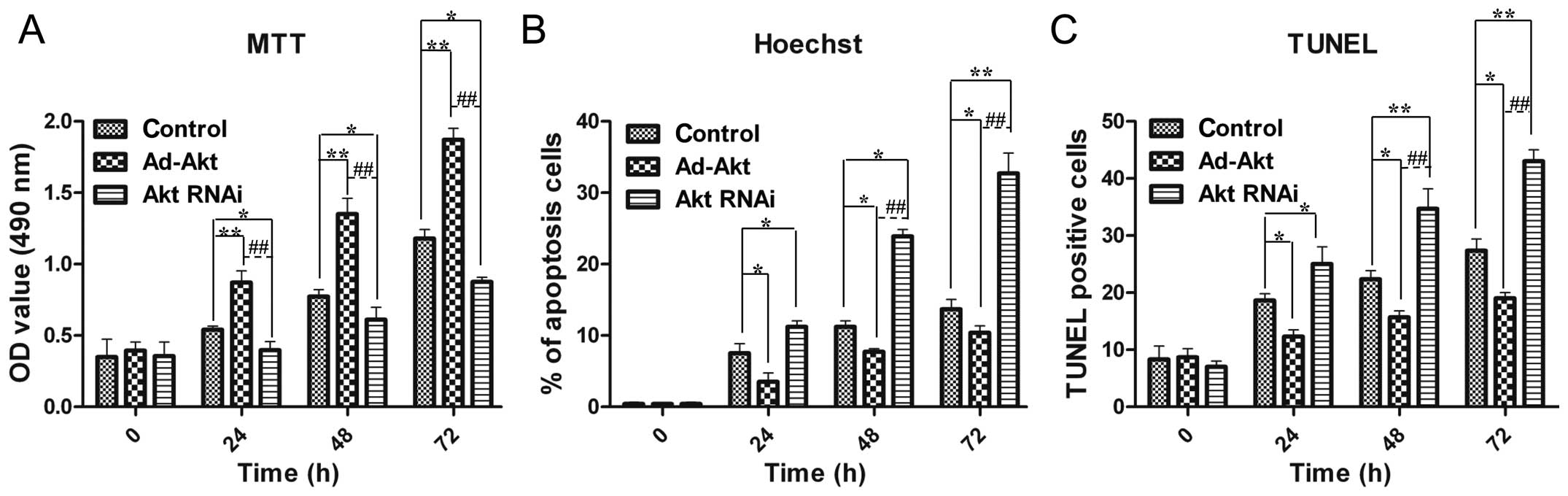
Akt promotes the proliferation of PC12
cells
Cell viability is important for neural repair and
regeneration, and therefore, PC12 cells were subjected to MTT
assays. The results showed that cell proliferation was
significantly improved in the existence of Akt overexpression, and
by contrast, cell growth was inhibited by Akt silencing (Fig. 3A).
Akt inhibits the apoptosis of PC12
cells
As Akt could promote PC12 cells proliferation, Akt
was assumed to be able to decrease apoptosis for cell accumulation.
The Hoechst and TUNEL experiments were performed to verify this
hypothesis. The results proved that Akt overexpression reduced the
apoptosis rate to 10.4±1.0%, while Akt silencing contributed to
cell death (32.7±2.8%) (Fig. 3B).
TUNEL results exhibited similar results as the above descriptions
(Fig. 3C). These results
confirmed the role of Akt in alleviating apoptosis of PC12 cells,
which contributed to cell number increase collaborating with cell
viability promotion.
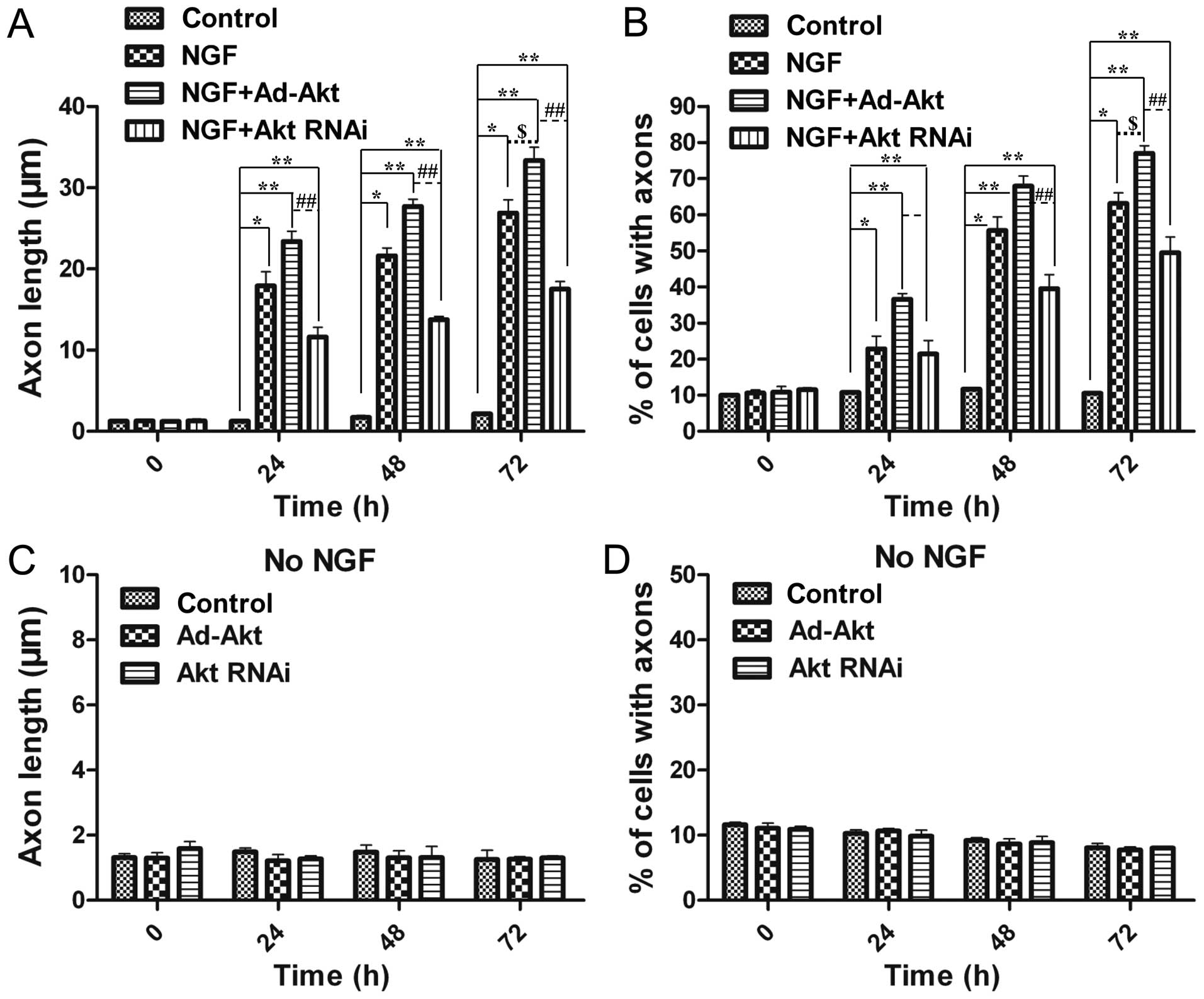
Overexpression of Akt enhances axon
growth induced by NGF
As NGF was proved to promote neural differentiation,
the present study aimed to discover a synergetic effector to
enhance this function. Overexpression of Akt significantly improved
the average axon length (33.4±1.6 µm) compared with the
NGF-treated (26.9±1.7 µm) and control groups (2.14±0.14
µm) (Fig. 4A).
Furthermore, the number of differentiated cells was also increased
by 18.1 and 86.2% compared with the NGF-treated and control groups,
respectively (Fig. 4B).
Silencing of Akt diminishes axon growth
induced by NGF
To further identify the function of Akt in axon
growth, its expression was knocked out via siRNA. Fig. 4A and B showed that 24 h after
siRNA transfection, there was a 1.5-fold decrease of the average
length in the siRNA-treated group, while 48 h later there was a
1.6-fold decline, compared with the NGF-treated group.
Additionally, the differentiated cells were shown to have an
average 21% decrease (from 24 to 48 h after siRNA transfection). As
a result, knockdown of Akt expression attenuated NGF-induced axons
outgrowth.
Akt cannot influence axon growth without
NGF
The above results confirmed the effects of Akt on
the proliferation, apoptosis and axon sprouting of PC12 cells.
However, all the aforementioned results were acquired in the
presence of NGF, and therefore cannot distinguish whether, how and
to what extent Akt alone had participated in these functions.
Therefore, the present experiment was conducted to monitor neural
differentiation without NGF. The results proved that Akt alone
could not promote or attenuate neurite growth, so therefore, Akt
did not influence axon growth in the absence of NGF (Fig. 4C and D). This phenomenon confirmed
the necessary role of NGF to foster the growth of axons, and Akt
could reinforce this effect induced by NGF.
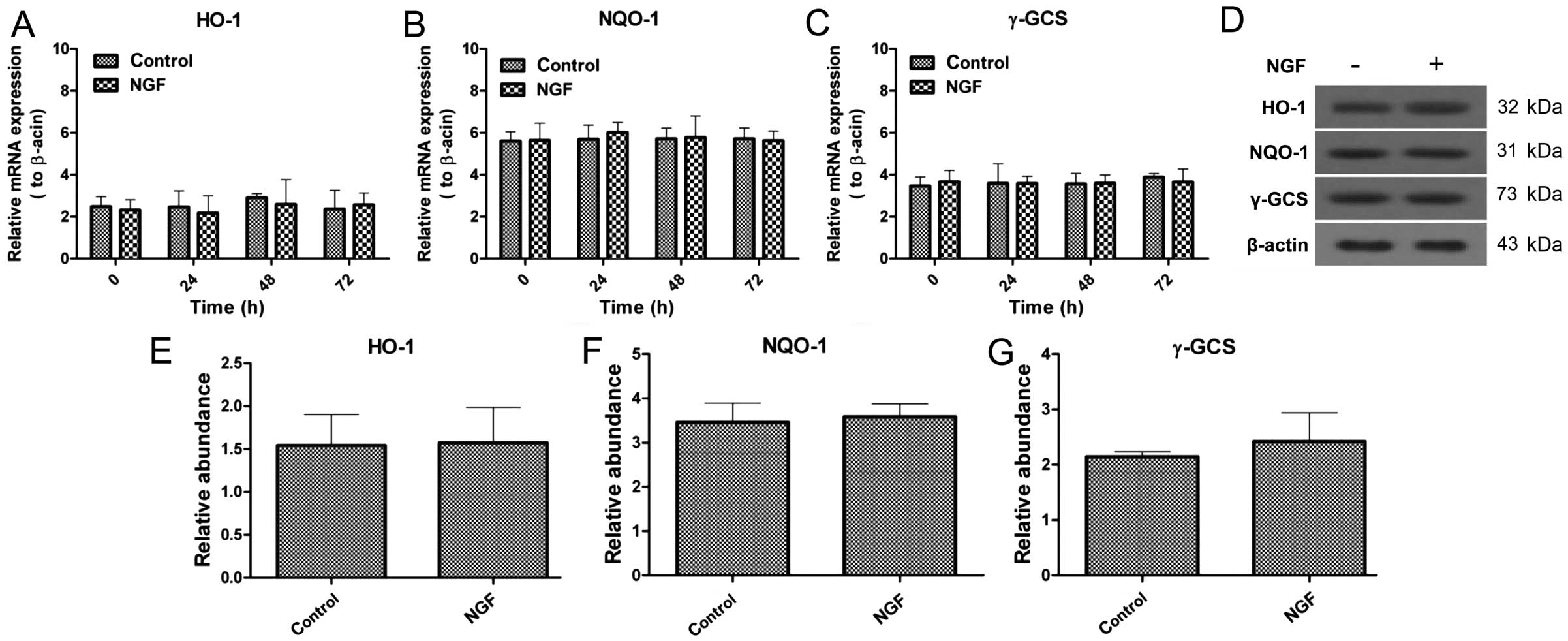
NGF cannot alter the expression of HO-1,
NQO-1 and γ-GCS
As aforementioned, NGF is a necessity for axon
extending. Therefore, whether NGF-induced axon growth is associated
with the Nrf2/ARE signaling pathway was examined. NGF-treated and
non-NGF-treated cells were collected to evaluate the changes of
HO-1, NQO-1 and γ-GCS expression in mRNA and protein levels. The
mRNA expression was not significantly changed in the presence and
absence of NGF, which was also similar with the protein expression,
and NGF was not closely associated with Nrf2/ARE signaling
(Fig. 5).
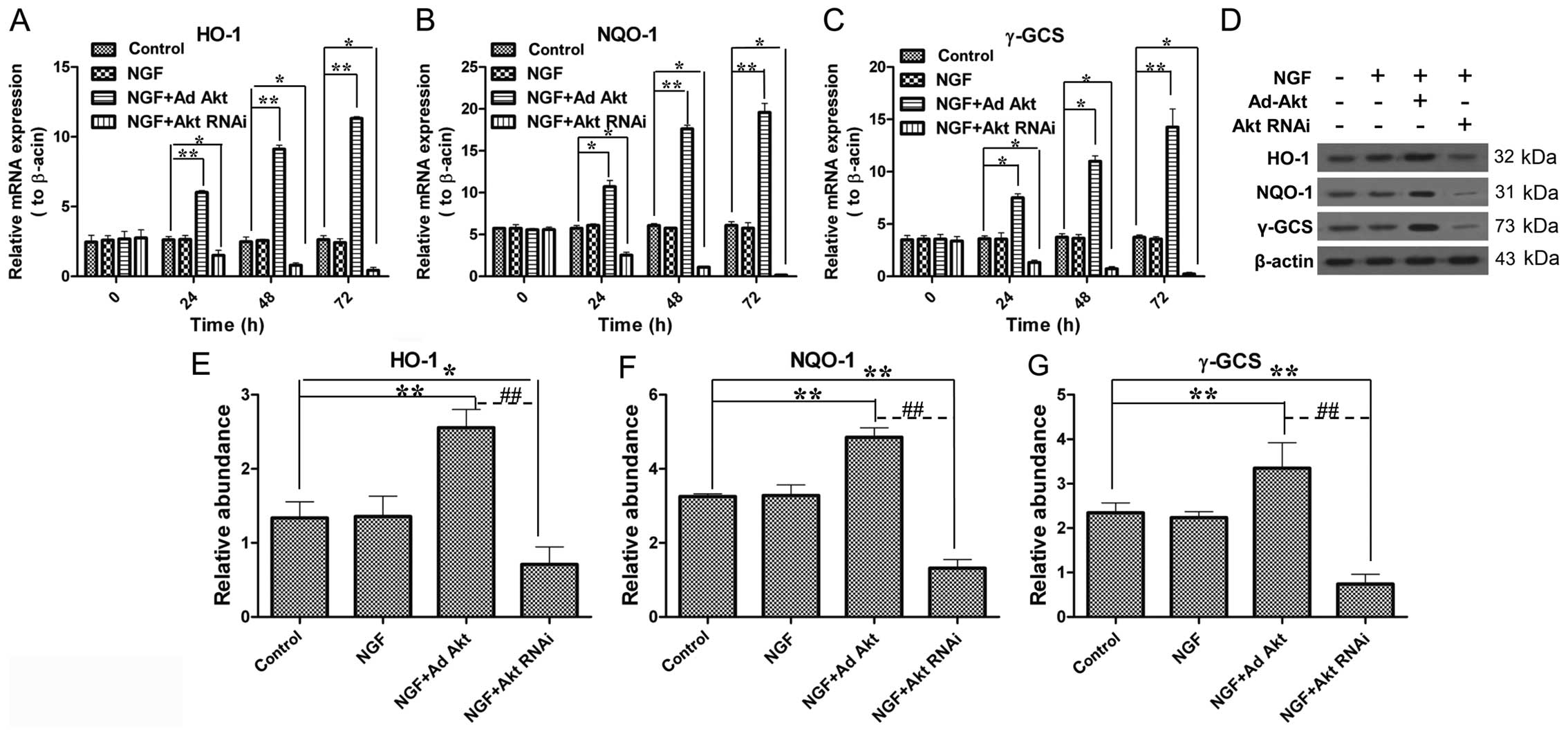
Akt increases the expression of HO-1,
NQO-1 and γ-GCS during axon growth
Subsequently, whether Akt has a role in the Nrf2/ARE
signaling pathway was investigated. The results showed that Akt
overexpression upregulated HO-1, NQO-1 and γ-GCS expression to
4.3-, 3.2- and 3.8-fold in the mRNA level compared with the control
group, respectively (Fig. 6A–C);
the western blot results also showed a significant increase in the
protein level. By contrast, the knockdown of Akt led to a
significant decrease of HO-1, NQO-1 and γ-GCS expression at the
mRNA and protein levels (Fig.
6D–G). These results confirmed that Akt participates in
Nrf2/ARE signaling.
Discussion
The spine consists of 26 hollow vertebras filled
with vast spinal cord containing abundant neurocytes. The axons
extending from the neurocytes are responsible for the signal
transmission from and to the brain (25,26). SCI is the most serious
complication resulting from spinal injury, leading to severe
dysfunction and disorder below the injured segment, such as
paralysis and quadriplegia. The loss of the function and
intercurrent sequelae are mainly due to the death and apoptosis of
neurocytes surrounding the lesion sites (27), cutting the signal transmission and
losing control of body parts, and the axons could rarely
spontaneously regenerate. Therefore, it is important to find
techniques for efficient axon regeneration.
Numerous studies have proposed certain methods to
promote axon regeneration in animal models. The methods include
transplantation of neural stem cell grafts (28); injection of human induced
pluripotent stem cells into the lesion sites (29); inhibition of myelin-associated
glycoprotein (MAG) to promote neurites sprouting from transplanted
neurons (30,31); avoidance of myelinated tracts
(4,32); removal of myelin (33); and the application of antibodies
to inhibit myelin inhibitors (2,34).
Another means to induce axon regeneration has relied on
neurotrophin treatments. For example, different neurotrophin
treatments have not only increased neuron regeneration in adult
CNS, but also stimulated axonal growth and sprouting following
injury (12,13,35,36). All the above methods merely focus
on promoting axon regeneration, however, none of the associated
signaling mechanisms have been further excavated or
investigated.
In the present study, the effects of NGF to promote
neural differentiation in PC12 cells were first confirmed. On the
basis of NGF, the role of Akt in promoting proliferation and
inhibiting apoptosis of PC12 cells was subsequently investigated.
Akt was overexpressed and silenced via adenovirus and siRNA
transfection. The results showed that increased Akt expression
could promote axon growth, contrary to the growth inhibition by Akt
silence. Of note, the promoting or inhibitory effects had a
precondition that PC12 cells must be treated with NGF. Therefore,
NGF is a determinant of axon growth. The Nrf2/ARE signaling pathway
is an antioxidative system for neuroprotection. HO-1, NQO-1 and
γ-GCS are three molecules that have important roles in this
antioxidant system. Whether this known neuroprotection is
associated with the Akt effects requires elucidating. As expected,
Akt overexpression upregulated HO-1, NQO-1 and γ-GCS expression. By
contrast, Akt knockdown had a negative effect on HO-1, NQO-1 and
γ-GCS expression. Therefore, Akt has a positive correlation with
Nrf2/ARE signaling. To distinguish the role of NGF in Nrf2/ARE, an
experiment was conducted to evaluate HO-1, NQO-1 and γ-GCS levels
with or without NGF. The results indicated that NGF could not
affect Nrf2/ARE signaling.
In the present study, Akt not only promoted the
proliferation, but also inhibited the apoptosis of PC12 cells.
These all contributed to neuroprotection, such as in SCI treatment,
as neurocyte death and reduction are the main reason for SCI
complication (37). Additionally,
the promotive effect toward axon growth contributed to branching
and sprouting of neurocytes to carry signals effectively. However,
the present study is limited of animal experiments to verify Akt
effects for SCI recovery in vivo, which requires further
analysis. In the present study, a synergistic effect was discovered
between Akt and NGF. Therefore, only Akt could activate the
Nrf2/ARE signaling pathway and its downstream genes. NGF was
responsible for neural differentiation, Akt alone had no influence;
Akt was able to boost neural differentiation induced by NGF, which
was likely to be involved in the Nrf2/ARE pathway. The Nrf2/ARE
signaling pathway is a main protective mechanism versus oxidative
damage. The upregulation of HO-1, NQO-1 and γ-GCS by Akt was
coordinated with axon growth induced by NGF; they all contribute
synergistically and systematically to neuroprotection and
functional recovery of neurocytes.
In conclusion, an association between Akt and a
potential of the Nrf2/Akt signaling pathway to enhance NGF-induced
axon growth was reported, which contributes to the treatment of
neural degenerative diseases, such as SCI and its subsequent
complications.
Abbreviations:
|
SCI
|
spinal cord injury
|
|
NGF
|
nerve growth factor
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
ARE
|
antioxidant response element
|
|
CNS
|
central nervous system
|
|
HO-1
|
heme oxygenase-1
|
|
NQO-1
|
NAD(P)H:quinone oxidoreductase-1
|
|
γ-GCS
|
γ-glutamylcysteine synthetase
|
Acknowledgments
The present study was supported by the foundation
item of human resource development of the Second Affiliated
Hospital of Zhengzhou University.
References
|
1
|
Schwartz M, Cohen I, Lazarov-Spiegler O,
Moalem G and Yoles E: The remedy may lie in ourselves: Prospects
for immune cell therapy in central nervous system protection and
repair. J Mol Med Berl. 77:713–717. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schnell L and Schwab ME: Axonal
regeneration in the rat spinal cord produced by an antibody against
myelin-associated neurite growth inhibitors. Nature. 343:269–272.
1990. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang DW, McKerracher L, Braun PE and
David S: A therapeutic vaccine approach to stimulate axon
regeneration in the adult mammalian spinal cord. Neuron.
24:639–647. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng H, Cao Y and Olson L: Spinal cord
repair in adult paraplegic rats: Partial restoration of hind limb
function. Science. 273:510–513. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Howland DR, Bregman BS, Tessler A and
Goldberger ME: Transplants enhance locomotion in neonatal kittens
whose spinal cords are transected: A behavioral and anatomical
study. Exp Neurol. 135:123–145. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rapalino O, Lazarov-Spiegler O, Agranov E,
Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A,
Belkin M, et al: Implantation of stimulated homologous macrophages
results in partial recovery of paraplegic rats. Nat Med. 4:814–821.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Kim D, Himes BT, Chow SY, Schallert
T, Murray M, Tessler A and Fischer I: Transplants of fibroblasts
genetically modified to express BDNF promote regeneration of adult
rat rubrospinal axons and recovery of forelimb function. J
Neurosci. 19:4370–4387. 1999.PubMed/NCBI
|
|
8
|
McDonald JW, Liu XZ, Qu Y, Liu S, Mickey
SK, Turetsky D, Gottlieb DI and Choi DW: Transplanted embryonic
stem cells survive, differentiate and promote recovery in injured
rat spinal cord. Nat Med. 5:1410–1412. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramón-Cueto A, Cordero MI, Santos-Benito
FF and Avila J: Functional recovery of paraplegic rats and motor
axon regeneration in their spinal cords by olfactory ensheathing
glia. Neuron. 25:425–435. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Davies SJ, Goucher DR, Doller C and Silver
J: Robust regeneration of adult sensory axons in degenerating white
matter of the adult rat spinal cord. J Neurosci. 19:5810–5822.
1999.PubMed/NCBI
|
|
11
|
Moon LD, Brecknell JE, Franklin RJ,
Dunnett SB and Fawcett JW: Robust regeneration of CNS axons through
a track depleted of CNS glia. Exp Neurol. 161:49–66. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schnell L, Schneider R, Kolbeck R, Barde
YA and Schwab ME: Neurotrophin-3 enhances sprouting of
corticospinal tract during development and after adult spinal cord
lesion. Nature. 367:170–173. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sawai H, Clarke DB, Kittlerova P, Bray GM
and Aguayo AJ: Brain-derived neurotrophic factor and
neurotrophin-4/5 stimulate growth of axonal branches from
regenerating retinal ganglion cells. J Neurosci. 16:3887–3894.
1996.PubMed/NCBI
|
|
14
|
Kobayashi NR, Fan DP, Giehl KM, Bedard AM,
Wiegand SJ and Tetzlaff W: BDNF and NT-4/5 prevent atrophy of rat
rubrospinal neurons after cervical axotomy, stimulate GAP-43 and
Talpha1-tubulin mRNA expression, and promote axonal regeneration. J
Neurosci. 17:9583–9595. 1997.
|
|
15
|
Bregman BS, Broude E, McAtee M and Kelley
MS: Transplants and neurotrophic factors prevent atrophy of mature
CNS neurons after spinal cord injury. Exp Neurol. 149:13–27. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tischler AS and Greene LA: Nerve growth
factor-induced process formation by cultured rat pheochromocytoma
cells. Nature. 258:341–342. 1975. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greene LA and Tischler AS: Establishment
of a noradrenergic clonal line of rat adrenal pheochromocytoma
cells which respond to nerve growth factor. Proc Natl Acad Sci USA.
73:2424–2428. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mesner PW, Winters TR and Green SH: Nerve
growth factor withdrawal-induced cell death in neuronal PC12 cells
resembles that in sympathetic neurons. J Cell Biol. 119:1669–1680.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahn JY: Neuroprotection signaling of
nuclear akt in neuronal cells. Exp Neurobiol. 23:200–206. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar
|
|
21
|
Kaspar JW, Niture SK and Jaiswal AK:
Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol
Med. 47:1304–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sarvestani NN, Khodagholi F, Ansari N and
Farimani MM: Involvement of p-CREB and phase II detoxifying enzyme
system in neuroprotection mediated by the flavonoid calycopterin
isolated from Dracocephalum kotschyi. Phytomedicine. 20:939–946.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
González-Burgos E, Carretero ME and
Gómez-Serranillos MP: Nrf2-dependent neuroprotective activity of
diterpenoids isolated from Sideritis spp. J Ethnopharmacol.
147:645–652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kole MH and Stuart GJ: Signal processing
in the axon initial segment. Neuron. 73:235–247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O'Donnell M, Chance RK and Bashaw GJ: Axon
growth and guidance: Receptor regulation and signal transduction.
Annu Rev Neurosci. 32:383–412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McKerracher L: Spinal cord repair:
Strategies to promote axon regeneration. Neurobiol Dis. 8:11–18.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu P, Kadoya K and Tuszynski MH: Axonal
growth and connectivity from neural stem cell grafts in models of
spinal cord injury. Curr Opin Neurobiol. 27:103–109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu P, Woodruff G, Wang Y, Graham L, Hunt
M, Wu D, Boehle E, Ahmad R, Poplawski G, Brock J, et al:
Long-distance axonal growth from human induced pluripotent stem
cells after spinal cord injury. Neuron. 83:789–796. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McKeon RJ, Schreiber RC, Rudge JS and
Silver J: Reduction of neurite outgrowth in a model of glial
scarring following CNS injury is correlated with the expression of
inhibitory molecules on reactive astrocytes. J Neurosci.
11:3398–3411. 1991.PubMed/NCBI
|
|
31
|
Davies SJ, Fitch MT, Memberg SP, Hall AK,
Raisman G and Silver J: Regeneration of adult axons in white matter
tracts of the central nervous system. Nature. 390:680–683.
1997.PubMed/NCBI
|
|
32
|
David S and Aguayo AJ: Axonal elongation
into peripheral nervous system 'bridges' after central nervous
system injury in adult rats. Science. 214:931–933. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Keirstead HS, Hasan SJ, Muir GD and
Steeves JD: Suppression of the onset of myelination extends the
permissive period for the functional repair of embryonic spinal
cord. Proc Natl Acad Sci USA. 89:11664–11668. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bregman BS, Kunkel-Bagden E, Schnell L,
Dai HN, Gao D and Schwab ME: Recovery from spinal cord injury
mediated by antibodies to neurite growth inhibitors. Nature.
378:498–501. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Weidner N, Ner A, Salimi N and Tuszynski
MH: Spontaneous corticospinal axonal plasticity and functional
recovery after adult central nervous system injury. Proc Natl Acad
Sci USA. 98:3513–3518. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Coumans JV, Lin TT, Dai HN, MacArthur L,
McAtee M, Nash C and Bregman BS: Axonal regeneration and functional
recovery after complete spinal cord transection in rats by delayed
treatment with transplants and neurotrophins. J Neurosci.
21:9334–9344. 2001.PubMed/NCBI
|
|
37
|
Wang J, Zheng Q, Zhao M and Guo X:
Neurocyte apoptosis and expressions of caspase-3 and Fas after
spinal cord injury and their implication in rats. J Huazhong Univ
Sci Technolog Med Sci. 26:709–712. 2006. View Article : Google Scholar
|