Introduction
Glaucoma is a degenerative nerve disorder, which is
characterized by optic atrophy and visual field defects, and
results in irreversible blindness. It has been reported that almost
64.3 million patients suffered from glaucoma worldwide in 2013, and
this number is likely to increase to 76 million in 2020 and 111.8
million in 2040 (1). The hallmark
of glaucoma is the apoptosis of retinal ganglion cells (RGCs),
which are the only efferent neurons that convey visual signals from
the retina to the brain. Several risk factors, including elevated
intraocular pressure (2),
oxidative stress (3), elevated
glutamate (4) and aging (5), have been considered to accelerate
RGC apoptosis in glaucoma, among which, oxidative stress is
considered as the final common pathway in glaucoma (6). Several studies have reported that
oxidative stress can lead to apoptosis of RGCs through activation
of the mitochondrial pathway (7),
and that apoptosis is the main cause of RGC loss (8). Thus, identifying a way of inhibiting
oxidative stress-induced apoptosis in RGCs may provide an effective
therapy for glaucoma.
Oxidative stress is able to destroy mitochondrial
membrane potential (ΔΨm), induce mitochondrial DNA damage and the
release of apoptosis-related factors, and thus, trigger apoptosis
(9). Mitochondria play an
important role in the functioning and survival of RGCs (10,11), and mitochondrial dysfunction has
been observed in glaucoma patients (12). Mitochondrial dysfunction is
regarded as an early event in the mitochondrial apoptotic pathway.
In the mitochondrial apoptotic pathway, mitochondrial dysfunction
and the activation of pro-apoptotic Bcl-2 family members has been
demonstrated to induce the release of cytochrome c, which
forms the apoptosome complexes, and contributes to the activation
of caspase-9 and the cleavage of caspase-3 (8,13,14). The mitochondrial-dependent
apoptosis of RGCs has been previously investigated (15). As an important consequence of
mitochondrial dysfunction caused by oxidative stress, excessive
reactive oxygen species (ROS) are capable of mediating
mitochondrial permeability transition and the release of
pro-apoptotic proteins, and thus, stimulate the mitochondrial
apoptotic pathway (9).
Saffron is a traditional medicine that is frequently
used in clinical therapy. The clinical therapeutic effects of
saffron have been demonstrated in cancer (16), hypertension (17), insomnia and anxiety (18), cerebral ischemia (19) and depression (20). The effect of saffron on retinal
diseases has also been demonstrated by improving focal macular
electrorectinogram parameters (21), inhibiting cell death induced by
intense light (22), and treating
macula lutea and ischemic retinopathy caused by old age (23). Crocetin and crocin are the two
major active ingredients of saffron. Crocetin can is capable of
preventing the retinal damage induced by oxidative and endoplasmic
reticulum stresses through inhibition of the activity of caspase-3
and -9 (24), and of protecting
the retina from ischemic damage via the inhibition of oxidative
stress (25). Crocin has also
been shown to exert a protective effect on retinal
ischemia/reperfusion (IR) injury-induced apoptosis of RGCs
(26). However, the mechanism by
which crocin protects against oxidative stress-induced damage toof
RGCs remains unclear.
In the present study, we investigated the protective
effects of crocin on RGCs under oxidative stress. Hydrogen peroxide
(H2O2) was used to establish a model of
oxidative stress injury in RGCs to mimic RGC injury in glaucoma
in vitro. The anti-apoptotic effect of crocin was
determined, and the mitochondrial-mediated apoptosis pathway was
examined to determine the anti-apoptotic mechanism of crocin. In
addition, the activity of phosphorylated nuclear factor-κB
(p-NF-κB) p65 was also measured using western blot analysis.
Materials and methods
Cell culture
RGC-5 cells, obtained from the American Type Culture
Collection (Cat. no. PT6600; ATCC, Manassas, VA, USA), were
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% fetal bovine serum (FBS) (both from Gibco, Carlsbad, CA,
USA), 100 U/ml penicillin and 100 μg/ml streptomycin (both
from Sigma-Aldrich, St. Louis, MO, USA). Cells were grown in a
humidified incubator with 5% CO2 at 37°C and passaged
every three days. Second generation RGCs were used in our
experiments.
Establishment of a model of oxidative
stress injury in RGCs and crocin treatment
The cells were equally divided into five groups,
which were treated with different concentrations of
H2O2 (0, 200, 400, 800 and 1,000 μM)
for 16 h. Cell viability and lactic dehydrogenase (LDH) release
were tested to investigate the cell injury induced by
H2O2 in RGCs, and an appropriate
concentration was chosen to establish the model of oxidative stress
injury in RGCs.
RGC-5 cells were pre-treated with 0.1 and 1
μM of crocin (Sigma-Aldrich) for 24 h, and no drugs were
added to the control group. The cells were then subjected to
oxidative insult with H2O2 (800 μM)
for 16 h and collected for subsequent experiments.
WST-1 cell proliferation assay
Cell viability was determined using a WST-1 assay
(Roche Diagnostics GmbH, Mannheim, Germany). Briefly, the cells
were cultured as described above. WST-1 reagent (10 μl) was
then added to each well, and incubated for 4 h at 37°C. The optical
density (OD) was read at 440 nm using a microplate reader (FLUOstar
Omega, BMG Labtech, Ortenberg, Germany).
LDH release assay
LDH release was determined using a LDH cytotoxicity
detection kit (Takara Bio, Tokyo, Japan). Briefly, the cells were
cultured in 96-well plates and 100 μl cell suspensions of
RGCs were collected to assess the LDH activity. Fresh reaction
mixture (100 μl) was then added to each well and incubated
at room temperature for 30 min. The absorbance was determined at
490 nm using a microplate reader (Bio-Rad, Hercules, CA, USA).
Annexin V/FITC assay
Apoptotic cells were quantified using a FITC Annexin
V apoptosis detection kit (BD Biosciences, Piscataway, NJ, USA).
Approximately 1×105 cells were collected and resuspended
with 100 μl of binding buffer. Then, 5 μl of FITC
Annexin V and 5 μl of propidium iodide (PI) were added to
stain cells for 15 min at room temperature in the dark.
Subsequently, 400 μl of 1X binding buffer was added prior to
analysis by flow cytometry (BD Biosciences).
ROS assay
The level of intracellular ROS was evaluated using a
ROS assay kit (Beyotime Biotech, Jiangsu, China). Briefly, the
cells were harvested and washed by 1X buffer, and then stained with
20 μM of dichloro-dihydro-fluorescein diacetate (DCFH-DA;
1:1,000) for 20 min at 37°C. The signal was read at
excitation/emission (Ex/Em) wavelengths of 488/525 nm filter after
the cells were further washed three times using PBS. Cells treated
with ROSup (provided with the ROS assay kit) only were used as
negative controls.
Measurement of ΔΨm
JC-1 fluorescent dye 9 (Beyotime Biotech) was used
to measure ΔΨm. The cells were collected and incubated with JC-1
staining solution at 37°C for 15 min in a 5% CO2
incubator, and then resuspended with 500 μl of preheated
incubation buffer. The green fluorescence (JC-1-monomer) was viewed
at Ex/Em 490/530 nm, and the red fluorescence (JC-1-aggregate) was
viewed at Ex/Em wavelengths of 525/590 nm.
Caspase-3 activity assay
The enzymatic activity of caspase-3 was detected by
a caspase-3 assay kit (Abcam, Cambridge, MA, USA). Briefly, the
cells were suspended in lysis buffer and incubated on ice for 10
min. Reaction buffer and DEVD-AFC substrate were then added prior
to being read at Ex/Em wavelengths of 400/505 nm.
Western blot analysis
The total protein was extracted from RGC-5 cells
using RIPA (Beyotime Biotech) and its concentration was determined
using a bicinchoninic acid (BCA) assay. The proteins were
electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and then transferred onto nitrocellulose
membranes (Amersham; GE Healthcare Europe GmbH, Freiburg, Germany).
The membranes were blocked with 5% (v/v) dried milk and probed with
anti-Bax, anti-Bcl-2, anti-cytochrome c (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and anti p-NF-κB p65
(Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C
overnight. Subsequently, HRP-conjugated goat anti-mouse IgG
(Bioworld Technology, Inc., St. Louis Park, MN, USA) was added and
incubated with the membranes for 1 h at room temperature. β-actin
(Cell Signaling Technology, Inc.,) was used as the reference
protein.
Statistical analysis
Data are presented as the means ± SEM. Statistical
comparisons were performed using the Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
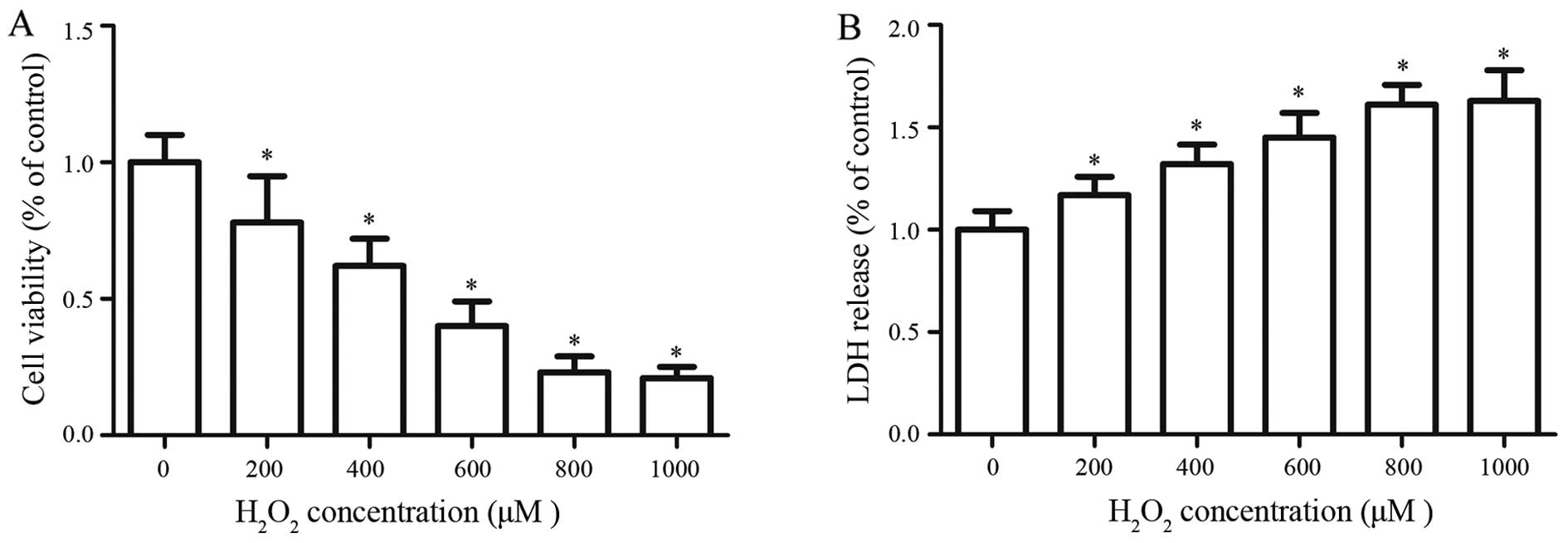
Effects of different concentrations of
H2O2 on cell viability and LDH release in
RGC-5 cells
To establish a model of oxidative stress injury in
RGCs, different concentrations of H2O2 were
used to evaluate the cytotoxicity to select an appropriate
concentration. The results showed that H2O2
is capable of decreasing RGC-5 cell viability in a dose-dependent
manner; however, there was no significant difference between
concentrations of 800 and 1,000 μM (Fig. 1A). As the concentration of
H2O2 increased, there was a corresponding
gradual increase in the release of LDH (Fig. 1B). The LDH release assay and the
WST-1 assay showed a very low gradient from 800 to 1,000 μM.
Thus, an H2O2 concentration of 800 μM
was used to establish the a model of oxidative stress injury in
RGCs.
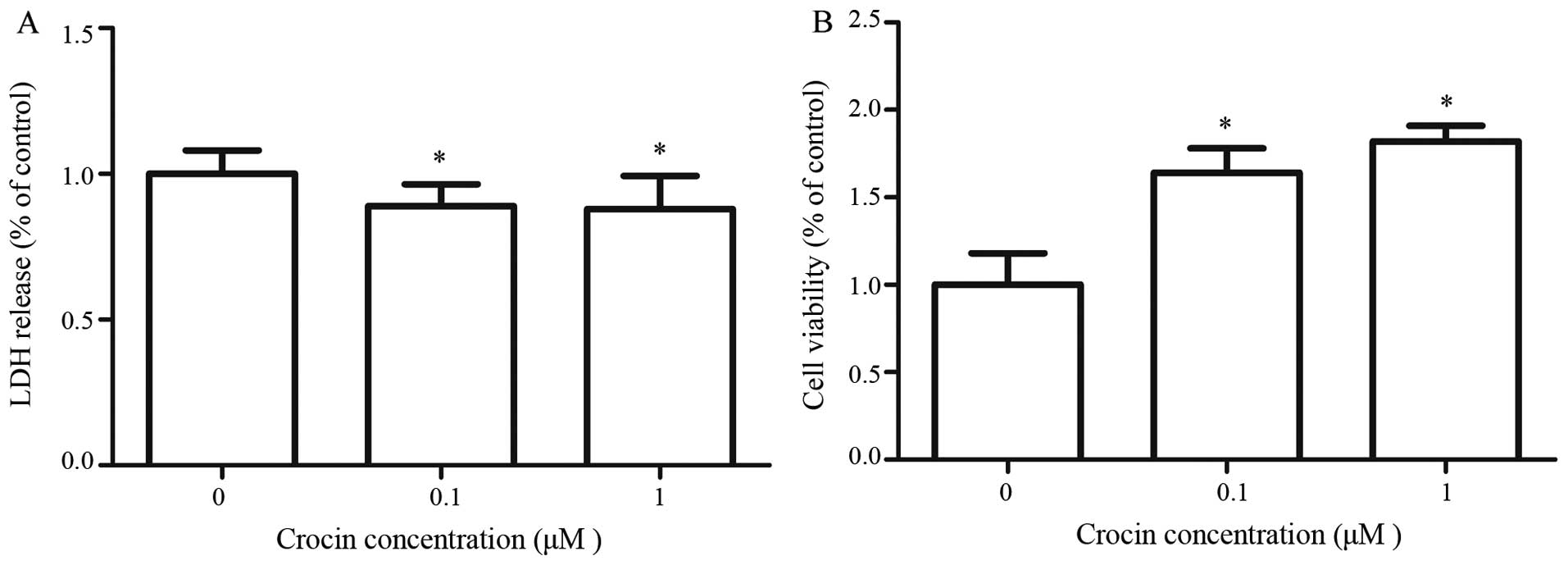
Effects of crocin on cell viability and
LDH release in oxidative stress-injured RGC-5 cells
LDH is a stable cytoplasmic enzyme that is present
in all cells. When the plasma membrane is damaged, LDH is rapidly
released into the culture supernatant, thus, LDH release in the
culture supernatant is a measure of cytotoxicity. To evaluate the
anti-cytotoxic effect of crocin on RGC-5 cells, an LDH assay was
performed using an LDH cytotoxicity assay kit. LDH release in RGC-5
cells was significantly decreased in the presence of crocin. There
was no significant difference in the LDH release between crocin
concentrations of 0.1 and 1 μM (P>0.05) (Fig. 2A). To understand the
cytoprotective effects of crocin in
H2O2−insulted RGC-5 cells, a WST-1 assay was
performed to determine cell viability. Crocin significantly
enhanced RGC-5 cell viability in
H2O2-insulted cells (P<0.05), and this
effect was not concentration-dependent (P>0.05) (Fig. 2B). These results indicated that
crocin could enhanced the cell viability of RGC-5 cells that have
been injured by H2O2.
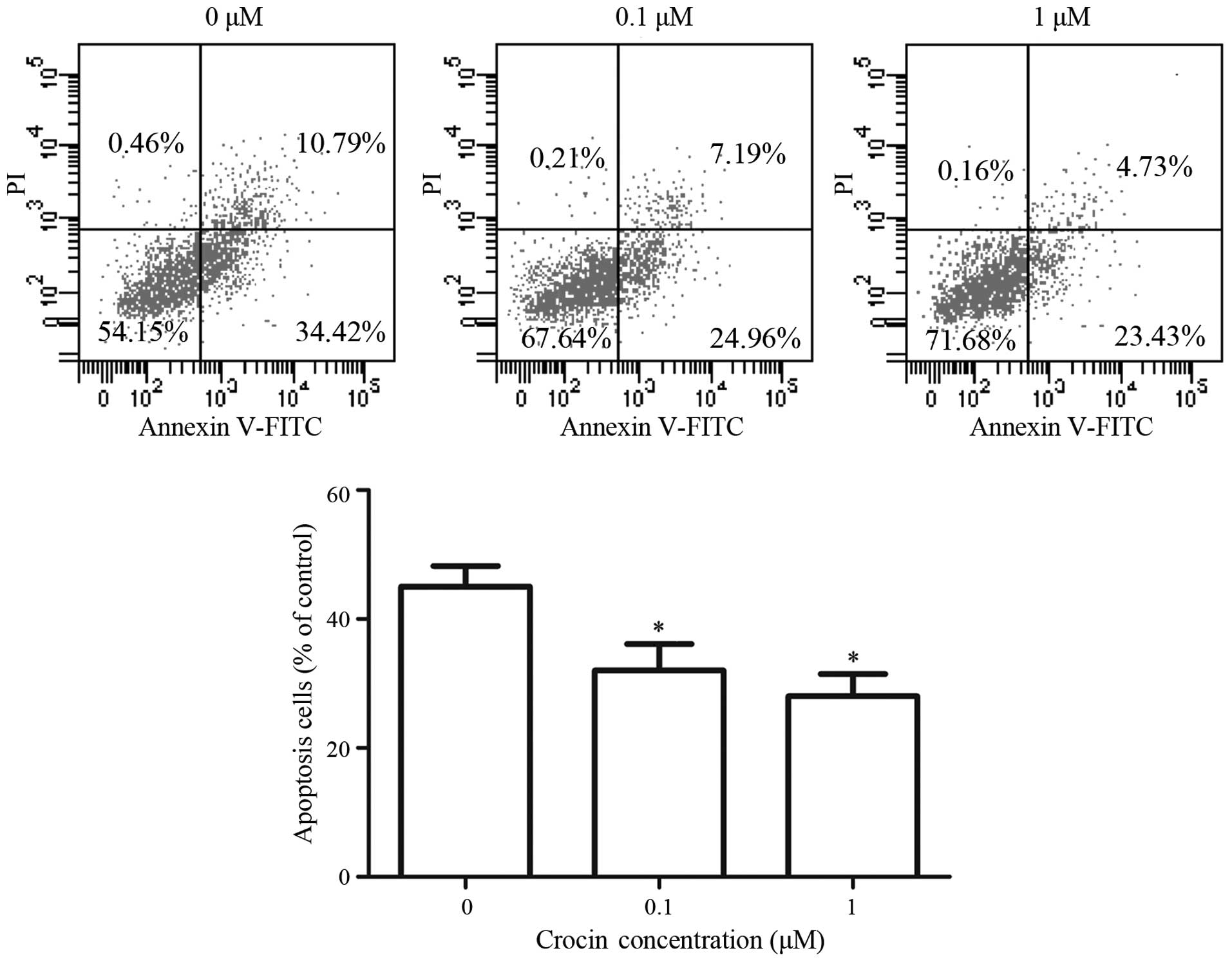
Effect of crocin on the apoptosis of
oxidative stress-injured RGC-5 cells
H2O2-induced apoptosis of
RGC-5 cells was analyzed using flow cytometry through Annexin
V-FITC/PI staining. The percentages of apoptotic cells decreased
from 45.39% without crocin, to 32.15% in the presence of 0.1
μM and 28.16% in the presence of 1 μM crocin
(Fig. 3). Crocin significantly
inhibited H2O2-induced apoptosis in RCG-5
cells and there was no significant difference in the percentages of
apoptotic cells between crocin concentrations of 0.1 and 1
μM.
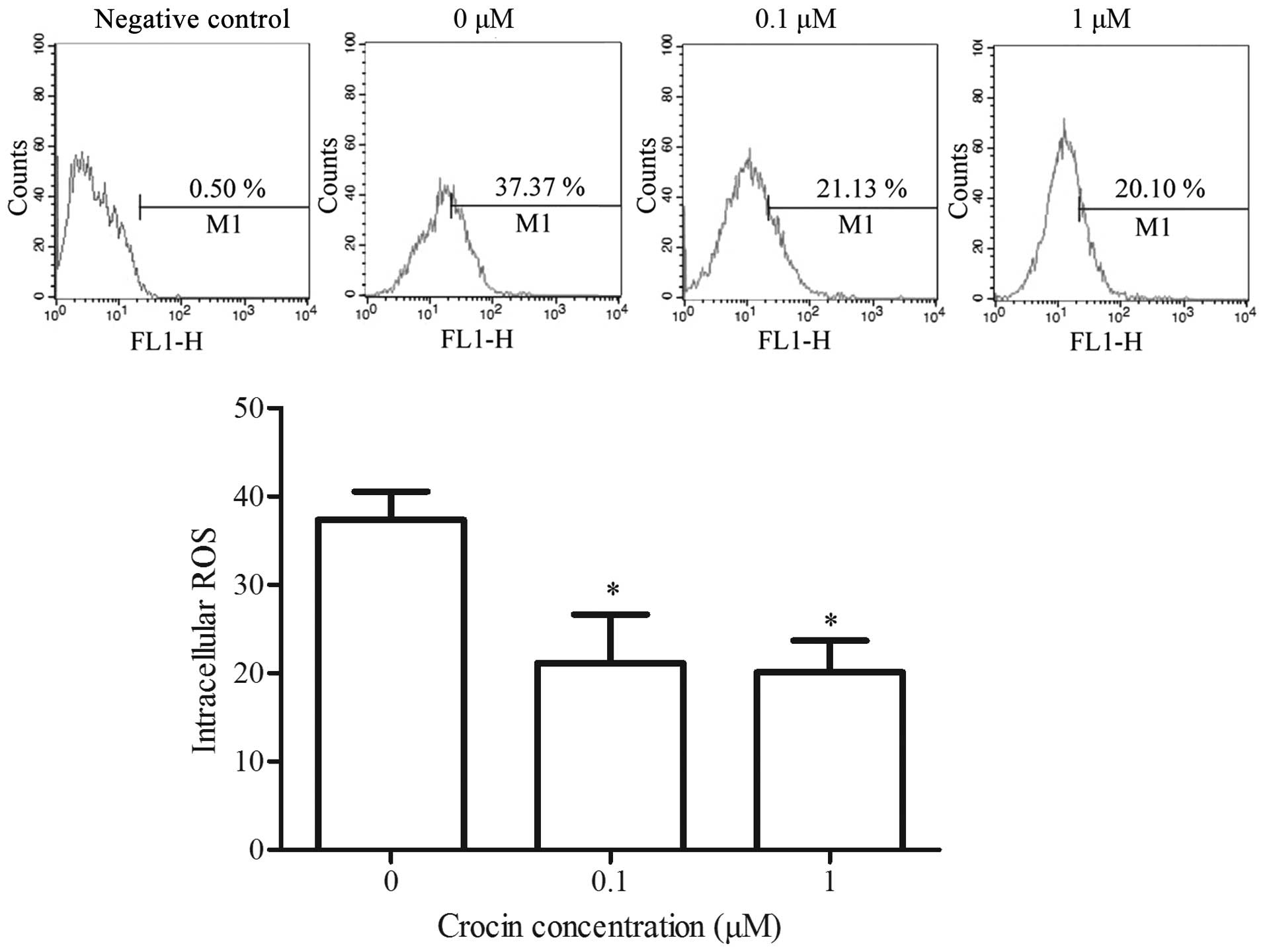
Effect of crocin on the production of ROS
in oxidative stress-injured RGC-5 cells
Intracellular ROS is an oxidative stress indicator
in cells that plays an important role in apoptosis induction under
physiological and pathological conditions (27). ROS are released from the
mitochondria, and excessive ROS are able to disrupt the ΔΨm in
return (27). In the present
study, excessive ROS was generated in
H2O2-injured cells (Fig. 4). With the addition of crocin, the
intracellular ROS content was markedly reduced compared with the
control group, and the difference between crocin concentrations of
0.1 and 1 μM was not significant. These results indicated
that crocin exerted an antioxidant effect on the oxidative
stress-injured RGC-5 cells.
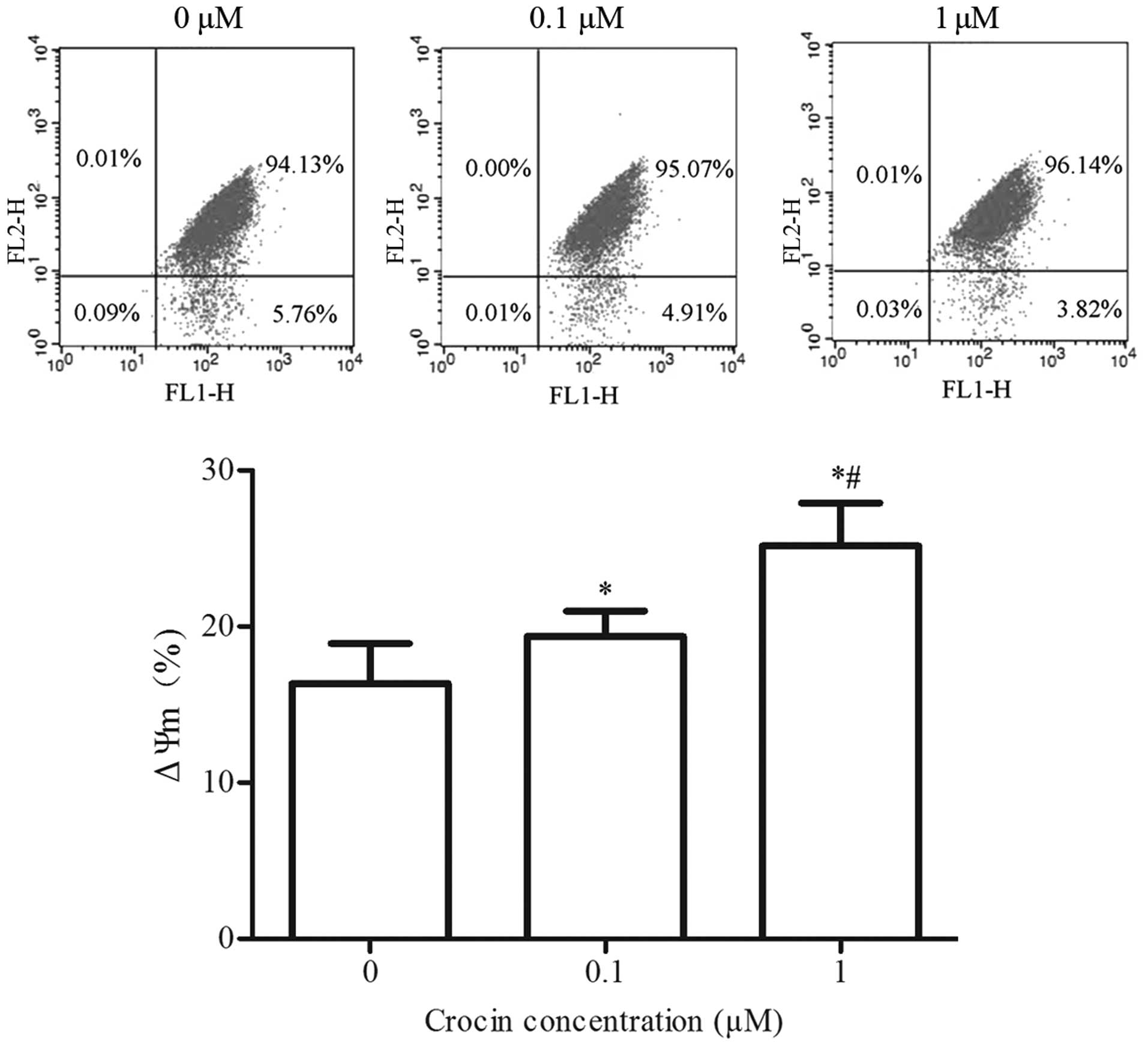
Effect of crocin on ΔΨm in oxidative
stress-injured RGC-5 cells
Mitochondria are closely associated with cell
apoptosis, and a decrease in ΔΨm is considered as one of the
earliest hallmark events in the cascade reaction process of
apoptosis (28). To examien the
effect of crocin on H2O2-induced ΔΨm
disruption, the lipophilic cation JC-1 was used to evaluate ΔΨm.
Crocin significantly increased ΔΨm in oxidative stress-injured
RGC-5 cells (P<0.05). A significant difference between crocin
concentrations of 0.1 and 1 μM was identified, which
suggested that the mitochondria-dependent pathway may be involved
in the protective effect of crocin on
H2O2-injured cells (Fig. 5).
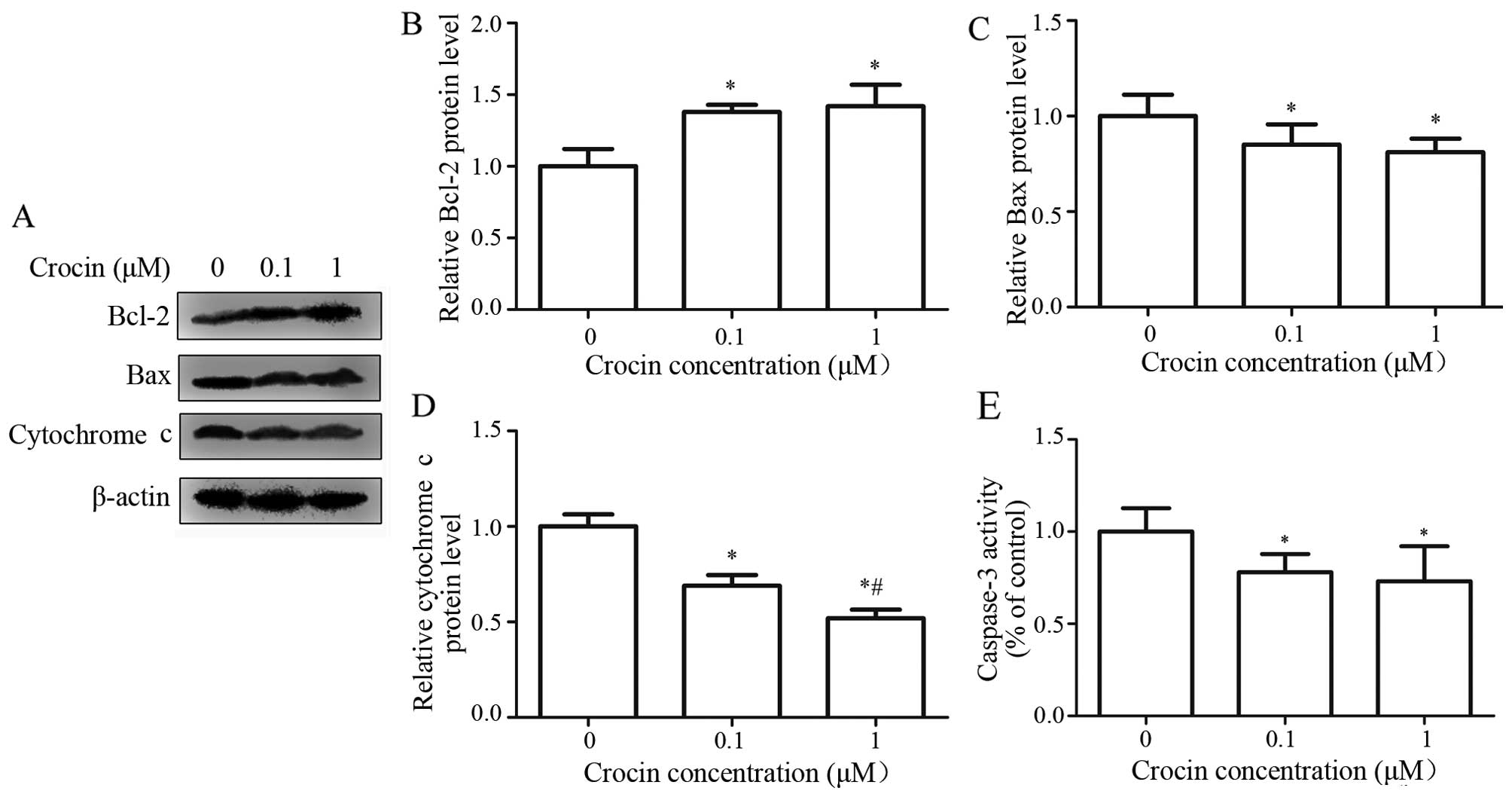
Effects of crocin on the activity of
caspase-3 and the expression of Bcl-2, Bax and cytochrome c in
oxidative stress-injured RGC-5 cells
Caspase-3 is the final effector in the
mitochondria-mediated apoptotic pathway (9). Cytochrome c, the
anti-apoptotic factor Bcl-2 and pro-apoptotic factor Bax are the
key regulating factors in the mitochondrial pathway (14). To investigate the effect of crocin
on the apoptosis of RGC-5 cells, we detected the expression of
Bcl-2, Bax and cytochrome c using western blot analysis. As
shown in Fig. 6A, the expression
level of Bcl-2 was markedly higher in the crocin groups than the
control group (P<0.05), and the difference between 0.1 and 1
μM crocin was not significant (Fig. 6B). There was also a significant
difference in the expression of Bax between the crocin groups and
the control group (P<0.05) (Fig.
6C), while no significant difference was observed between 0.1
and 1 μM crocin. Cytochrome c release in
oxidative-stress-injured RGC-5 cells was significantly suppressed
at crocin concentrations of 0.1 and 1 μM (Fig. 6D), and the inhibitory effect of 1
μM of crocin was significantly stronger than that of 0.1
μM of crocin.
Caspases are aspartic acid proteases containing
cysteine, which selectively cleave the target protein of aspartate
residue, and thus, induce cell apoptosis. In caspase-dependent
signaling, caspase-3 is one of the most important effector
caspases, and its activation is the final step of apoptosis
(29). To investigate the effect
of crocin on H2O2-induced activation of
caspase-3, we used a caspase-3 assay kit to detect caspase-3
activity. The results showed that crocin treatment significantly
inhibited the activation of caspase-3 activity, while there was no
significant difference between 0.1 and 1 μM crocin (Fig. 6E).
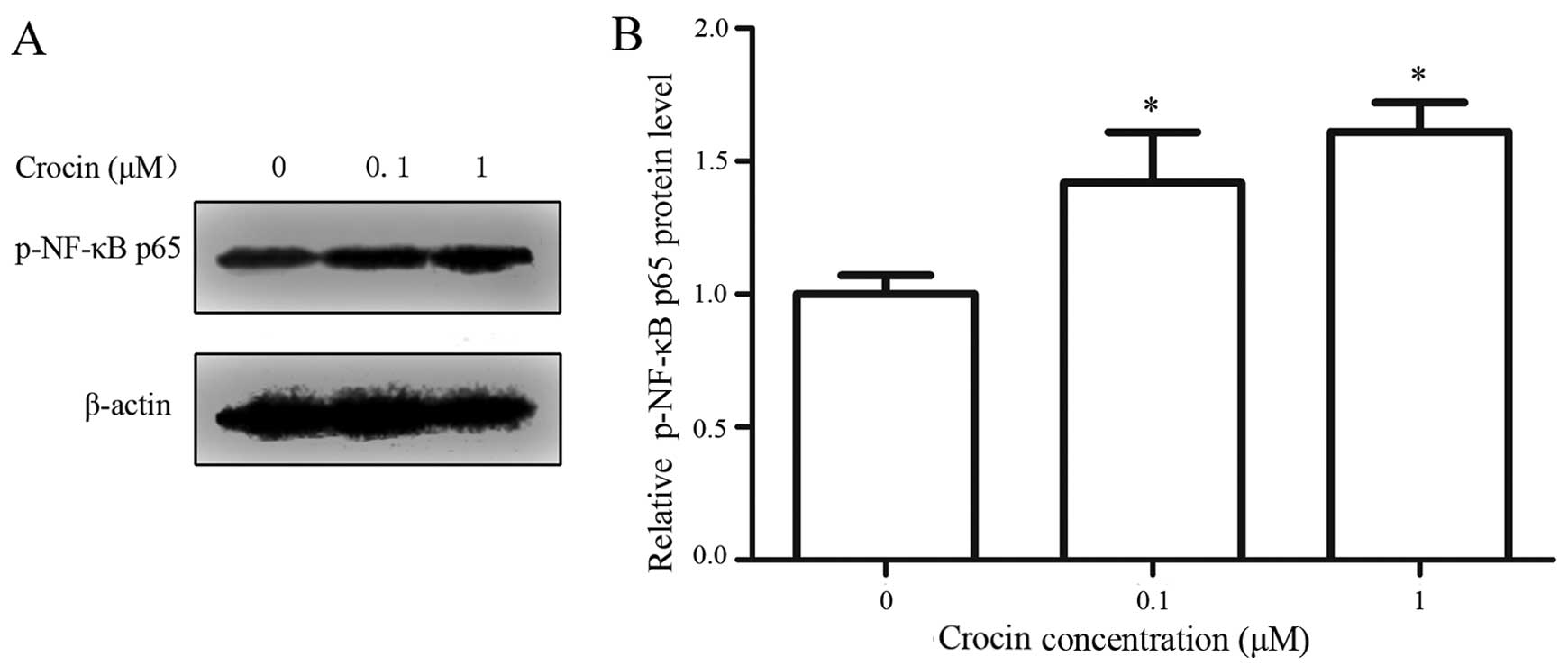
Effect of crocin on p-NF-κB 65 in
oxidative stress-injured RGC-5 cells
NF-κB is a family of nuclear transcription factors
that include the subunits Rel, p65, RelB, p50 and p52, which
influence cell apoptosis by regulating the expression of cell
survival genes (30). The
phosphorylation of the transactivation p65 subunit is essential for
efficient transcriptional activation by NF-κB (31). Thus, the level of p-NF-κB p65 was
measured using western blot analysis in the present experiment. The
results showed that the relative p-NF-κB p65 level was markedly
increased in the presence of crocin (Fig. 7), which indicates that crocin
upregulated the activity of NF-κB in oxidative stress-injured RGC-5
cells.
Discussion
In glaucoma, elevated intraocular pressure is the
most significant risk factor for accelerated RGC death. It is
widely accepted that oxidative damage in response to pressure
elevation is an important underlying mechanism of elevated
intraocular pressure-induced cell damage and neuronal death
(32,33). Thus, H2O2
was used to establish a model of oxidative stress injury in RGCs to
mimic RGC injury in glaucoma in vitro. The LDH and WST-1
assay results showed that H2O2 decreased cell
viability and increased LDH release. Efficiency was highest at a
concentration of 800 μM, therefore a concentration of 800
μM H2O2 was used to establish the
model of oxidative stress injured in RGCs for subsequent
experiments.
Crocin is one of the active ingredients of saffron,
which is frequently used as a traditional medicine for its
antitoxic properties (34). The
anti-apoptotic and antioxidant effects of crocin have been stated
in several studies (35,36). Qi et al (26) have reported that crocin injections
prevented apoptosis of RGCs subsequent to retinal IR injury. In the
present study, we detected changes in the cell viability and
apoptosis of H2O2−insulted RGC-5 cells by
WST-1 and Annexin V/PI staining in vitro, respectively. Our
results were consistent with those of Mehri et al (37), who reported that crocin enhanced
cell viability and reduced apoptosis. In addition, we also detected
LDH release in H2O2-insulted RGCs using an
LDH cytotoxicity assay kit. LDH release was significantly decreased
by crocin concentrations of 0.1 and 1 μM. Taken together,
these results suggest that crocin prevented
H2O2-induced damage to RGCs.
One of the important mechanisms by which crocin
exerts its biological effects is its ability to modulate the redox
status of organisms. Evidence has suggested that overproduction of
ROS plays an important role in the protective effects of crocin in
serum-deprived and hypoxic PC12 cells (38). Mousavi et al (39) have confirmed that crocin decreased
the production of ROS induced by glucose in PC12 cells. To
determine the effects of crocin on the production of ROS in
H2O2-injured RGC-5 cells, we determined the
production of ROS by performing a cellular ROS assay. The results
showed that H2O2-induced production of ROS
was significantly suppressed by crocin, suggesting that crocin is
capable of reducing the ROS level and suppressing
H2O2-induced oxidative stress in RGC-5
cells.
There are two main pathways of oxidative
stress-induced apoptosis: mitochondrial- and death
receptor-mediated pathways (40).
In the mitochondrial pathway, Bcl-2 and Bax are the key regulators.
Bcl-2 inhibits apoptosis by suppressing cytochrome c release
and caspase activation, while Bax promotes apoptosis by inducing
the release of cytochrome c, which then triggers the
downstream apoptosis event (29).
On the other hand, the release of apoptosis-related factor
cytochrome c may also be inhibited by the rise of the ΔΨm,
which decreased intimal permeability (41). Our results show that crocin
effectively prevented H2O2-induced apoptosis
by increasing ΔΨm, downregulating Bax and cytochrome c and
caspase-3, and upregulating Bcl-2. This finding indicates that
crocin stabilized the mitochondria and inhibited apoptosis mediated
by the mitochondrial pathway, thereby protecting RGCs from
apoptosis.
NF-κB activity helps cells to avoid the sustained
phase of JNK activation which has been demonstrated to activate the
mitochondrial apoptotic pathway (42), and thus, promotes cell survival
(43,44). NF-κB plays an important role in
the apoptosis of RGCs mediated by H2O2
(45,46). The present study revealed that the
level of p-NF-κB p65 was significantly higher in the crocin groups
than in the control group. This result suggests that crocin
initiated the activation of NF-κB in the presence of
H2O2, thereby reducing
H2O2-induced apoptosis.
Taken together, our results demonstrate that crocin
is capable of protecting H2O2-injured RGC-5
cells from apoptosis through the mitochondrial pathway, and by
upregulating the activity of NF-κB.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81273902).
Abbreviations:
|
RGCs
|
retinal ganglion cells
|
|
ΔΨm
|
mitochondrial membrane potential
|
|
LDH
|
lactic dehydrogenase
|
|
IR
|
ischemia/reperfusion
|
|
ROS
|
reactive oxygen species
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
OD
|
optical density
|
References
|
1
|
Tham YC, Li X, Wong TY, Quigley HA, Aung T
and Cheng CY: Global prevalence of glaucoma and projections of
glaucoma burden through 2040: a systematic review and
meta-analysis. Ophthalmology. 121:2081–2090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khan AK, Tse DY, van der Heijden ME, Shah
P, Nusbaum DM, Yang Z, Wu SM and Frankfort BJ: Prolonged elevation
of intraocular pressure results in retinal ganglion cell loss and
abnormal retinal function in mice. Exp Eye Res. 130:29–37. 2015.
View Article : Google Scholar
|
|
3
|
Wang Z, Pan X, Wang D, Sun H, Han F, Lv C
and Zhang X: Protective effects of protocatechuic acid on retinal
ganglion cells from oxidative damage induced by
H2O2. Neurol Res. 37:159–166. 2015.
View Article : Google Scholar
|
|
4
|
Harada T, Harada C, Nakamura K, Quah HM,
Okumura A, Namekata K, Saeki T, Aihara M, Yoshida H, Mitani A and
Tanaka K: The potential role of glutamate transporters in the
pathogenesis of normal tension glaucoma. J Clin Invest.
117:1763–1770. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levkovitch-Verbin H, Vander S, Makarovsky
D and Lavinsky F: Increase in retinal ganglion cells'
susceptibility to elevated intraocular pressure and impairment of
their endogenous neuroprotective mechanism by age. Mol Vis.
19:2011–2022. 2013.PubMed/NCBI
|
|
6
|
Chrysostomou V, Rezania F, Trounce IA and
Crowston JG: Oxidative stress and mitochondrial dysfunction in
glaucoma. Curr Opin Pharmacol. 13:12–15. 2013. View Article : Google Scholar
|
|
7
|
Sancho P, Fernández C, Yuste VJ, Amrán D,
Ramos AM, de Blas E, Susin SA and Aller P: Regulation of
apoptosis/necrosis execution in cadmium-treated human promonocytic
cells under different forms of oxidative stress. Apoptosis.
11:673–686. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Almasieh M, Wilson AM, Morquette B, Cueva
Vargas JL and Di Polo A: The molecular basis of retinal ganglion
cell death in glaucoma. Prog Retin Eye Res. 31:152–181. 2012.
View Article : Google Scholar
|
|
9
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang XJ, Ge J and Zhuo YH: Role of
mitochondria in the pathogenesis and treatment of glaucoma. Chin
Med J (Engl). 126:4358–4365. 2013.
|
|
11
|
Lascaratos G, Garway-Heath DF, Willoughby
CE, Chau KY and Schapira AH: Mitochondrial dysfunction in glaucoma:
Understanding genetic influences. Mitochondrion. 12:202–212. 2012.
View Article : Google Scholar
|
|
12
|
Abu-Amero KK, Morales J and Bosley TM:
Mitochondrial abnormalities in patients with primary open-angle
glaucoma. Invest Ophthalmol Vis Sci. 47:2533–2541. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Estaquier J, Vallette F, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng QF, Lv J, Ge H, Zhang L, Xue F, Zhu Y
and Liu P: Overexpressed mutant optineurin (E50K) induces retinal
ganglion cells apoptosis via mitochondrial pathway. Mol Biol Rep.
39:5867–5873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abdullaev FI and Espinosa-Aguirre JJ:
Biomedical properties of saffron and its potential use in cancer
therapy and chemoprevention trials. Cancer Detect Prev. 28:426–432.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Imenshahidi M, Hosseinzadeh H and
Javadpour Y: Hypotensive effect of aqueous saffron extract (Crocus
sativus L.) and its constituents, safranal and crocin, in
normotensive and hypertensive rats. Phytother Res. 24:990–994.
2010.
|
|
18
|
Hosseinzadeh H and Noraei NB: Anxiolytic
and hypnotic effect of Crocus sativus aqueous extract and its
constituents, crocin and safranal, in mice. Phytother Res.
23:768–774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hosseinzadeh H, Sadeghnia HR, Ghaeni FA,
Motamedshariaty VS and Mohajeri SA: Effects of saffron (Crocus
sativus L.) and its active constituent, crocin, on recognition and
spatial memory after chronic cerebral hypoperfusion in rats.
Phytother Res. 26:381–386. 2012.
|
|
20
|
Akhondzadeh Basti A, Moshiri E, Noorbala
AA, Jamshidi AH, Abbasi SH and Akhondzadeh S: Comparison of petal
of Crocus sativus L. and fluoxetine in the treatment of depressed
outpatients: A pilot double-blind randomized trial. Prog
Neuropsychopharmacol Biol Psychiatry. 31:439–442. 2007. View Article : Google Scholar
|
|
21
|
Falsini B, Piccardi M, Minnella A,
Savastano C, Capoluongo E, Fadda A, Balestrazzi E, Maccarone R and
Bisti S: Influence of saffron supplementation on retinal flicker
sensitivity in early age-related macular degeneration. Invest
Ophthalmol Vis Sci. 51:6118–6124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maccarone R, Di Marco S and Bisti S:
Saffron supplement maintains morphology and function after exposure
to damaging light in mammalian retina. Invest Ophthalmol Vis Sci.
49:1254–1261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moghaddasi MS: Saffron chemicals and
medicine usage. J Med Plants Res. 4:427–430. 2010.
|
|
24
|
Yamauchi M, Tsuruma K, Imai S, Nakanishi
T, Umigai N, Shimazawa M and Hara H: Crocetin prevents retinal
degeneration induced by oxidative and endoplasmic reticulum
stresses via inhibition of caspase activity. Eur J Pharmacol.
650:110–119. 2011. View Article : Google Scholar
|
|
25
|
Ishizuka F, Shimazawa M, Umigai N,
Ogishima H, Nakamura S, Tsuruma K and Hara H: Crocetin, a
carotenoid derivative, inhibits retinal ischemic damage in mice.
Eur J Pharmacol. 703:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi Y, Chen L, Zhang L, Liu WB, Chen XY and
Yang XG: Crocin prevents retinal ischaemia/reperfusion
injury-induced apoptosis in retinal ganglion cells through the
PI3K/AKT signalling pathway. Exp Eye Res. 107:44–51. 2013.
View Article : Google Scholar
|
|
27
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar
|
|
28
|
Mignotte B and Vayssiere JL: Mitochondria
and apoptosis. Eur J Biochem. 252:1–15. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Granville DJ and Gottlieb RA:
Mitochondria: Regulators of cell death and survival. Scientific
World Journal. 2:1569–1578. 2002. View Article : Google Scholar
|
|
30
|
Tang G, Minemoto Y, Dibling B, Purcell NH,
Li Z, Karin M and Lin A: Inhibition of JNK activation through
NF-kappaB target genes. Nature. 414:313–317. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhong H, Voll RE and Ghosh S:
Phosphorylation of NF-κB p65 by PKA stimulates transcriptional
activity by promoting a novel bivalent interaction with the
coactivator CBP/p300. Mol Cell. 1:661–671. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ju WK, Liu Q, Kim KY, Crowston JG, Lindsey
JD, Agarwal N, Ellisman MH, Perkins GA and Weinreb RN: Elevated
hydrostatic pressure triggers mitochondrial fission and decreases
cellular ATP in differentiated RGC-5 cells. Invest Ophthalmol Vis
Sci. 48:2145–2151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Q, Ju WK, Crowston JG, Xie F, Perry G,
Smith MA, Lindsey JD and Weinreb RN: Oxidative stress is an early
event in hydrostatic pressure induced retinal ganglion cell damage.
Invest Ophthalmol Vis Sci. 48:4580–4589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huynh TP, Mann SN and Mandal NA: Botanical
compounds: effects on major eye diseases. Evid Based Complement
Alternat Med. 2013:5491742013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Soeda S, Ochiai T, Paopong L, Tanaka H,
Shoyama Y and Shimeno H: Crocin suppresses tumor necrosis
factor-alpha-induced cell death of neuronally differentiated PC-12
cells. Life Sci. 69:2887–2898. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ochiai T, Soeda S, Ohno S, Tanaka H,
Shoyama Y and Shimeno H: Crocin prevents the death of PC-12 cells
through sphingomyelinase-ceramide signaling by increasing
glutathione synthesis. Neurochem Int. 44:321–330. 2004. View Article : Google Scholar
|
|
37
|
Mehri S, Abnous K, Mousavi SH, Shariaty VM
and Hosseinzadeh H: Neuroprotective effect of crocin on
acrylamide-induced cytotoxicity in PC12 cells. Cell Mol Neurobiol.
32:227–235. 2012. View Article : Google Scholar
|
|
38
|
Ochiai T, Shimeno H, Mishima K, Iwasaki K,
Fujiwara M, Tanaka H, Shoyama Y, Toda A, Eyanagi R and Soeda S:
Protective effects of carotenoids from saffron on neuronal injury
in vitro and in vivo. Biochim Biophys Acta. 1770:578–584. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mousavi SH, Tayarani NZ and Parsaee H:
Protective effect of saffron extract and crocin on reactive oxygen
species-mediated high glucose-induced toxicity in PC12 cells. Cell
Mol Neurobiol. 30:185–191. 2010. View Article : Google Scholar
|
|
40
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hao M, Li Y, Lin W, Xu Q, Shao N, Zhang Y
and Kuang H: Estrogen prevents high-glucose-induced damage of
retinal ganglion cells via mitochondrial pathway. Graefes Arch Clin
Exp Ophthalmol. 253:83–90. 2015. View Article : Google Scholar
|
|
42
|
Weston CR and Davis RJ: The JNK signal
transduction pathway. Curr Opin Cell Biol. 19:142–149. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kamata H, Honda S, Maeda S, Chang L,
Hirata H and Karin M: Reactive oxygen species promote
TNFalpha-induced death and sustained JNK activation by inhibiting
MAP kinase phosphatases. Cell. 120:649–661. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakano H, Nakajima A, Sakon-Komazawa S,
Piao JH, Xue X and Okumura K: Reactive oxygen species mediate
crosstalk between NF-kappaB and JNK. Cell Death Differ. 13:730–737.
2006. View Article : Google Scholar
|
|
45
|
Gupta VK, You Y, Li JC, Klistorner A and
Graham SL: Protective effects of 7,8-dihydroxyflavone on retinal
ganglion and RGC-5 cells against excitotoxic and oxidative stress.
J Mol Neurosci. 49:96–104. 2013. View Article : Google Scholar
|
|
46
|
Ozawa Y, Yuki K, Yamagishi R, Tsubota K
and Aihara M: Renin-angiotensin system involvement in the oxidative
stress-induced neurodegeneration of cultured retinal ganglion
cells. Jpn J Ophthalmol. 57:126–132. 2013. View Article : Google Scholar
|