Introduction
Acute liver failure (ALF) is a serious condition
resulting from the development of hepatocellular dysfunction over a
period of several days or a few weeks (1). ALF is very harmful to patient
health, and has a mortality rate of 80% (2). Thus, ALF is associated with a high
rate of mortality, and many patients require liver transplantation.
Therefore, there is a need to improve our understanding of the
underlying mechanisms in order that novel therapies be
developed.
The lipoxins comprise a group of arachidonic acid
metabolites first discovered by Serhan in 1984 (3). Unlike other arachidonic acid
metabolites that are pro-inflammatory, such as prostaglandins and
leukotrienes, lipoxins play a role in the resolution of
inflammation. During the resolution phase of acute inflammation,
the production of prostaglandins and leukotrienes ceases and the
production of lipoxins begins; this is known as the 'lipid mediator
conversion' (4,5). Lipoxins then promote macrophage
clearance of the apoptotic polymorphonuclear leukocytes. The
lipoxins include lipoxin A4 (LXA4) and lipoxin B4, both of which
play an anti-inflammatory role in a number of pathological
processes (3).
LXA4 is an important endogenous mediator that
promotes the resolution of inflammation, serving as a 'braking
signal' (3). Previous studies
have shown that LXA4 can attenuate airway inflammation following
lipopolysaccharide (LPS)-induced lung injury in mice (6), reduce systemic inflammation and
improve survival rates in a rat model of sepsis (7), suppress inflammation-induced
mechanical hypersensitivity in rats (8), and inhibit pulmonary and renal
fibrosis in animal models (9,10).
In addition, the levels of LXA4 are decreased in patients with
asthma (11). The activation of
nuclear factor-κB (NF-κB) is necessary for many inflammatory
reactions, and LXA4 has been proven to reduce the expression of
tumor necrosis factor-α (TNF-α) and the activation of NF-κB in a
rabbit model of paracetamol-induced acute hepatic injury (12). LXA4 has also been demonstrated to
inhibit NF-κB activation in pulmonary capillary epithelial cells
(13), peritoneal macrophages
(7), and dorsal root ganglia in
the spinal cord (14).
To the best of our knowledge, it has not yet been
reported whether or not LXA4 plays a role in ALF. However, LXA4 is
known to play protective, anti-inflammatory roles in cases of liver
injury induced by a high-fat diet/endotoxin (15), paracetamol (12), or acetaminophen (16), and also in cases of liver fibrosis
induced by carbon tetrachloride (17). LXA4 also attenuates the acute
rejection of transplanted livers (18), and suppresses hepatocellular
carcinoma (19). Given the
special role which LXA4 plays in inflammatory processes, in the
present study we decided to explore the effects of LXA4 in a rat
model of ALF, which was induced by intraperitoneal injection of
D-galactosamine (D-GalN) and LPS.
Materials and methods
Materials
LXA4 was purchased from the Cayman Chemical Co. (Ann
Arbor, MI, USA) as well as D-GalN. Type IV collagenase was from
Biosharp (Hefei, China). The Percoll separation solution was from
Wuhan Boster Biological Engineering (Wuhan, China). Unless
otherwise specified, all other reagents were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Animal models
Eight-week old male Wister rats, weighing 180–220 g,
were provided by the Experimental Animal Research Center of Hubei,
China. The rats were randomly divided into six groups: control
group (n=3), model group (n=6), three LXA4-treated groups
(low/medium/high doses; n=6 each) and the pyrrolidine
dithiocarbamate (PDTC)-treated group (n=6). The animals in the
control group received an intra-peritoneal injection of
phosphate-buffered saline (PBS). Rats in the other experimental
groups received an intraperitoneal injection of D-GalN (300 mg/kg)
to the lower left abdomen, which was followed 30 min later by an
intraperitoneal injection of LPS (50 µg/kg) to the lower
right abdomen. The low-, medium- and high-dose LXA4 treatment
groups also received intraperitoneal injections of 0.5, 1 and 2
µg/kg LXA4, respectively, 30 min before D-GalN injection.
For the PDTC group, PDTC (100 mg/kg; an inhibitor of NF-κB) was
administered with D-GalN. The rats were sacrificed 24 h after LPS
injection. All procedures and animal experiments were approved by
the Animal Care and Use Committee of the Animal Experimental
Center, Hubei University of Medicine.
Liver histology
The livers were removed from certain rats, fixed
with 4% paraformaldehyde, embedded in paraffin, and cut into
8-µm sections. The sections were stained with hematoxylin
and eosin (H&E) and examined under an optical microscope
(CKX41; Olympus, Tokyo, Japan). Liver injury was assessed by the
scoring of five randomly selected fields viewed at x400
magnification: 0, no or minimal damage; 1, mild damage, cell
swelling, a limited number of cells showing pyknosis; 2, moderate
damage, extensive nuclear pyknosis, enhanced eosin staining in the
cytoplasm, the appearance of bridging necrosis; 3, severe necrosis,
disappearance of hepatic cords, bleeding, massive inflammatory cell
infiltration. Liver tissues and blood samples were taken from
certain rats, while others were used for the extraction of liver
cells.
Measuring alanine transaminase (ALT) and
aspartate transaminase (AST) levels
Whole blood samples (totaling 33) were taken 24 h
after rats were injected with LPS. These were kept at room
temperature for 2 h, and then centrifuged at 3,000 × g for 5 min.
The supernatant was collected, and ALT and AST levels were
determined without delay using an automatic biochemical analyzer
(Hitachi 7020; Hitachi, Tokyo, Japan).
Detection of TNF-α and interleukin-6
(IL-6)
In the present study, serum TNF-α and IL-6 were both
detected using an enzyme-linked immunosorbent assay (ELISA) kit
(purchased from R&D Systems, Minneapolis, MN, USA) according to
the manufacturer's instructions. Briefly, 50 µl assay
diluent was added to each well. After adding 50 µl standard,
control and samples, these were mixed and incubated for 2 h at room
temperature. After aspiration and washing, 100 µl rat TNF-α
conjugate was added to each well and incubated. Aspiration and
washing was repeated, 100 µl Substrate Solution was added,
and this was followed by incubation for 30 min; 100 µl Stop
Solution was then added to each well. A microplate reader (450 nm)
was used to determine the OD. The OD values were then used to
determine the concentration of each sample.
Detection of TNF-α mRNA and IL-6 mRNA in
liver tissues
Hepatic tissues were placed in liquid nitrogen and
ground to a powder. Total RNA was extracted using TRIzol
(Invitrogen, Carlsbad, CA, USA) and cDNA was produced by reverse
transcription and amplification. The primers used were as follows:
IL-6, 5′-TTG CCT TCT TGG GAC TGA TGT-3′ (sense) and 5′-TAC TGG TCT
GTT GTG GGT GGT-3′ (antisense); TNF-α, 5′-GCC ACC ACG CTC TTC TGT
C-3′ (sense) and 5′-GCT ACG GGC TTG TCA CTC G-3′ (antisense); and
actin, 5′-CGT TGA CAT CCG TAA AGA CCT C-3′ (sense) and 5′-TAG GAG
CCA GGG CAG TAA TCT-3′ (antisense). The PCR conditions were as
follows: initial denaturation for 1 min at 95°C, followed by 40
cycles at 95°C for 15 sec, 58°C for 15 sec, and 72°C for 45 sec.
The threshold cycle (Ct) values were assessed for each sample, and
the relative expression of the target genes were determined using
the 2−ΔΔCt method, where ΔΔCt = (Cttarget −
Ctactin)sample − (Cttarget −
Ctactin)control.
Detection of liver cell apoptosis
Hepatocyte apoptosis was assessed using a commercial
terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) assay with diaminobenzidine (DAB) staining, in accordance
with the manufacturer's instructions (Hoffmann-La Roche, Basel,
Switzerland). Briefly, tissue sections were dewaxed and rehydrated,
and then incubated with Pro-teinase K working solution and rinsed
with PBS. Slides were incubated with permeabilization solution and
rinsed again with PBS. TUNEL reaction mixture or Label Solution
(for the control) were added. DNase I was added for the positive
control. These were incubated for 60 min in a humidified atmosphere
in the dark and then rinsed with PBS. After adding 50 µl
Converter-POD, slides were again incubated and washed, and this was
followed by the addition of 100 µl DAB substrate, incubation
and rinsing with PBS. Samples were counterstained with hematoxylin
and analyzed using a light microscope.
Measurement of caspase-3 activity in
liver tissues
The activity of caspase-3 in the liver tissues was
determined using a commercial kit (Beyotime Institute of
Biotechnology, Shanghai, China) in strict accordance with the
manufacturer's instructions. Briefly, PNA was added (concentrations
0, 10, 20, 50, 100 and 200 µM) and a microplate reader was
used to read standard absorbance (405 nm; A405). PNA absorbance and
the standard curve were calculated, 100 µl lysate buffer/10
mg liver tissue was added. After homogenization on ice,
centrifugation was undertaken (15 min at 4°C; 16,000 rpm). The
supernatant was transferred and put on ice. The reaction system was
prepared, and Ac-DEVD-PNA (2 mM) was added. After incubation for 90
min at 37°C, absorbance was measured using a microplate reader
(A405). PNA absorbances of samples catalyzed by caspase-3 were
subtracted (A405 of blank, free PNA, from A450 of standards). The
amount of PNA catalyzed by samples was then calculated according to
the standard curve.
Isolation of hepatocytes and Kupffer
cells
Cell isolation was carried out using a modified
version of the method described by Knook (20–22). Briefly, rats were anesthetized
with an intraperitoneal injection of sodium pentobarbital.
Pre-warmed (37°C) calcium- and magnesium-free Hanks' perfusion
solution was infused through a catheter inserted into the portal
vein. After 15 min, the solution was changed to one containing
0.05% collagenase IV (Biosharp) and the infusion was continued for
a further 20 min. Rats were sacrificed by spinal dislocation. These
livers were then removed, placed in 0.05% collagenase IV solution,
and digested at 37°C for 30 min. The digestion solution was
filtered through a 200-µm mesh strainer, washed twice with
Hanks' solution, and centrifuged at 50 × g for 5 min at 4°C. Both
the supernatant and cell pellet were collected. The cell pellet was
washed and centrifuged a further three times to obtain purified
hepatocytes. The viability of the hepatocytes was examined by
Trypan blue exclusion. The supernatant was centrifuged at 400 × g
at 4°C for 5 min to remove cell debris. Five milliliters 50%
Percoll solution and 4 ml 25% Percoll solution (Wuhan Boster
Biological Engineering) were added sequentially to a 15-ml
centrifuge tube, followed by 3 ml of the supernatant containing the
cells. The tube was centrifuged at 600 × g for 20 min at 4°C. The
cell layer (which appeared white) was collected, and the cells were
re-plated on 6-well plates at 106 cells/ml. After 2 h,
the non-adherent cells were washed off, leaving Kupffer cells as
the remaining adherent cells.
The purity of the Kupffer cells was examined by
immunostaining using monoclonal anti-ED1 (anti-CD68) antibody
(Advanced Technology and Industrial, Tai Kok Tsui, Kowloon, Hong
Kong). The majority of cells were found to be ED1 positive. The
purity of the culture was calculated as the percentage of the
number of ED1-positive cells (green) over the number of stained
nuclei (red) from five randomly selected fields. The purity was
determined to be 96.3±1.46% (data not shown).
Electrophoretic mobility shift assay
(EMSA) for detection of NF-κb
An EMSA (Pierce Chemical Co., Rockford, IL, USA) for
the detection of NF-κB in nucleoprotein from liver tissue and
Kupffer cells was carried out using a nucleoprotein extraction kit
(Biovision, Milpitas, CA, USA) to extract nucleoprotein from liver
tissues and Kupffer cells, together with a commercial NF-κB probe
(Beyotime Institute of Biotechnology) according to the instructions
provided.
Statistical analysis
Data are expressed as the means ± standard deviation
(SD). Comparisons between groups were studied using analysis of
variance (ANOVA) followed by the SNK (Student-Newman-Keuls) post
hoc test. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
LXA4 improves liver function
In the control group, the enzymatic activity of ALT
and AST was below the level of detection. The enzymatic activity of
ALT and AST in the model group was substantially elevated,
indicating liver damage; this suggests that the model was valid.
Administration of LXA4 significantly lowered the enzymatic activity
of ALT and AST in a dose-dependent manner. In the PDTC group, the
enzymatic activity of ALT and AST was lower than in the model
group, but higher than in the medium- and high-dose LXA4 groups
(Fig. 1).
LXA4 improves the histological appearance
of the liver
In the H&E-stained liver tissues from the model
group, we observed liver cell degeneration, severe necrosis, the
disappearance of hepatic cords, bleeding and also infiltration of
numerous inflammatory cells in the portal area (Fig. 2). Administration of LXA4 was
associated with dose-dependent improvements in tissue appearance
and a decrease in liver injury scores, as compared to the model
group (Fig. 2). The liver injury
score in the PDTC group was significantly lower than that of the
model group, but higher than that of the high-dose LXA4 group
(Fig. 2).
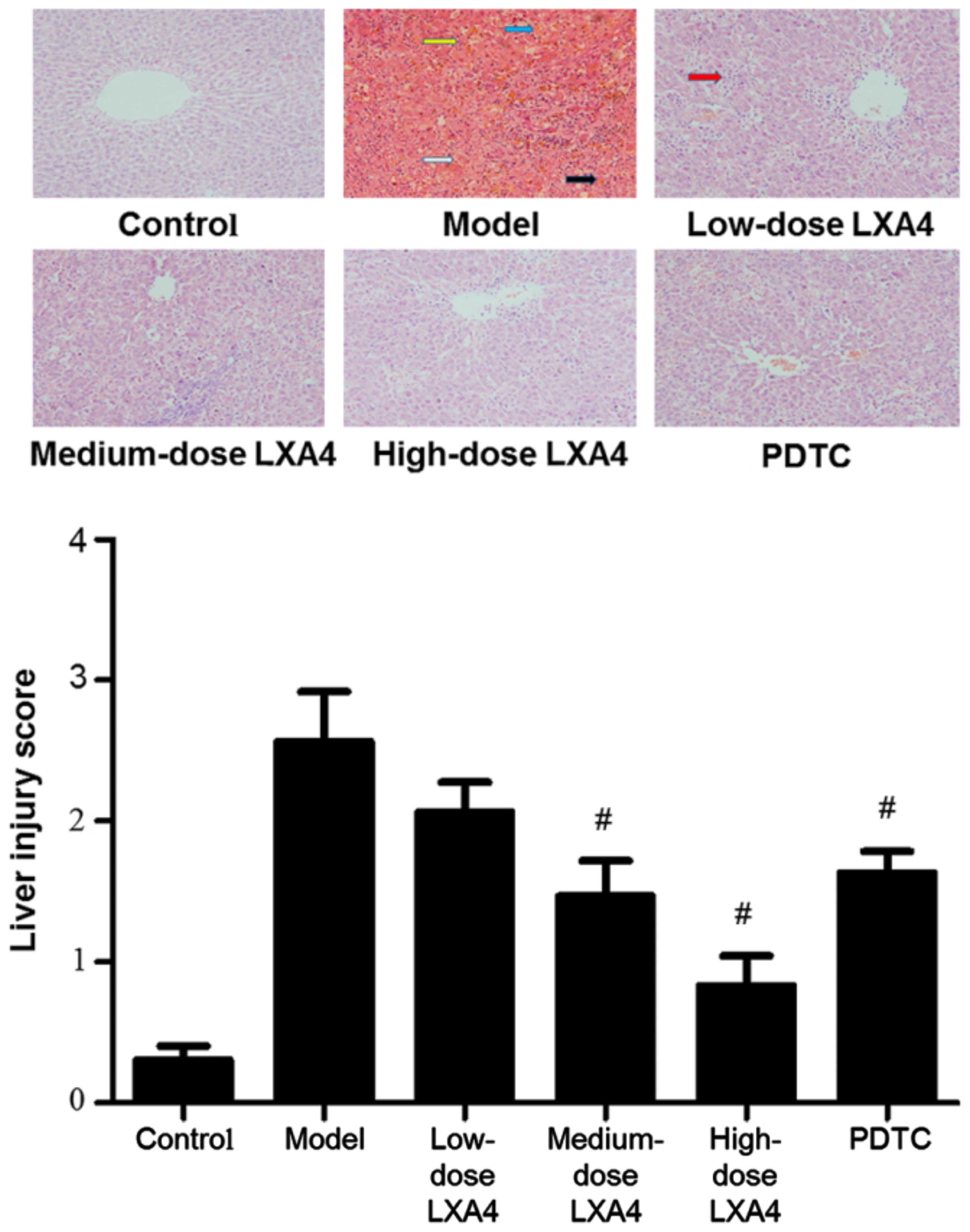 | Figure 2Lipoxin A4 (LXA4) improves the
histological appearance of the liver in our model of acute liver
failure (ALF). Paraffin sections of the liver tissue were stained
with hemotoxylin and eosin (H&E); representative examples from
each experimental group are shown. Yellow arrows indicate the
disappearance of the hepatic cord; blue arows indicate bleeding;
white arrows indicate degeneration; black arrows indicate severe
necrosis. Liver injury was assessed by the scoring of five randomly
selected fields under ×400 magnification, as follows: 0, no or only
minimal damage; 1, mild damage, cell swelling, a limited number of
cells showing pyknosis; 2, moderate damage, extensive nuclear
pyknosis, enhanced eosin staining in the cytoplasm, the appearance
of bridging necrosis; 3, severe necrosis, disappearance of hepatic
cords, bleeding, massive inflammatory cell infiltration.
#P<0.05 compared to the model group. |
LXA4 attenuates the elevation of serum
TNF-α and IL-6 levels
Compared to the control group, the serum levels of
IL-6 and TNF-α were significantly higher in the model group
(Fig. 3). LXA4 caused
dose-dependent reductions in IL-6 and TNF-α levels compared to the
model group (Fig. 4). In the
PDTC-treated group, TNF-α and IL-6 levels were significantly
decreased compared to the model group; furthermore, the level of
IL-6 but not TNF-α was significantly higher than in the high-dose
LXA4 group (Fig. 3).
LXA4 attenuates the increased hepatic
expression of TNF-α and IL-6 mRNA
Compared to the control group, the mRNA levels of
IL-6 and TNF-α were significantly increased in the model group
(Fig. 4). mRNA expression levels
of IL-6 and TNF-α were significantly lower in all three
LXA4-treated groups than in the model group. In the PDTC-treated
group, IL-6 and TNF-α mRNA levels were significantly decreased
compared to the model group, and were comparable to those in the
high-dose LXA4 group (Fig.
4).
LXA4 decreases the number of
TUNEL-positive hepatic cells
The number of TUNEL-positive cells (used as a
measure of apoptosis) in the model group was significantly higher
than in the control group (Fig.
5). All three LXA4 groups had significantly lower numbers of
TUNEL-positive cells than the model group, with a dose-dependent
decrease also evident. In the PDTC-treated group, the number of
TUNEL-positive cells was significantly decreased compared to the
model group, and was comparable to the high-dose LXA4 group
(Fig. 5).
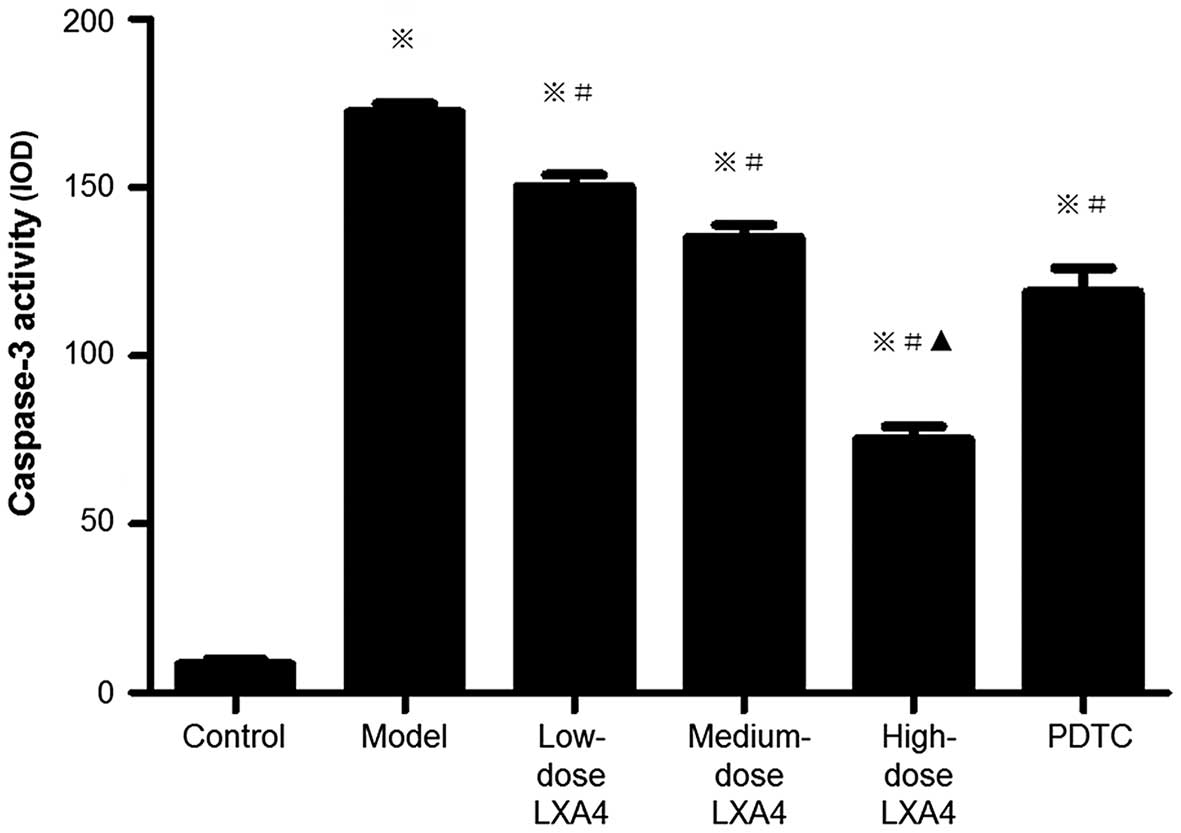
LXA4 attenuates the elevation of hepatic
caspase-3 activity
Caspase-3 activity in liver tissues was used as an
additional measure of apoptosis. Caspase-3 activity was
significantly higher in the model group compared to the control
group, and was significantly lower in all three LXA4 groups
compared to the model group, and we also observed a dose-dependent
reduction (Fig. 6). In the
PDTC-treated group, caspase-3 activity was significantly decreased
compared to the model group, and was similar to the medium-dose
LXA4 group (Fig. 6). These data
were in agreement with those which were obtained using the TUNEL
method.
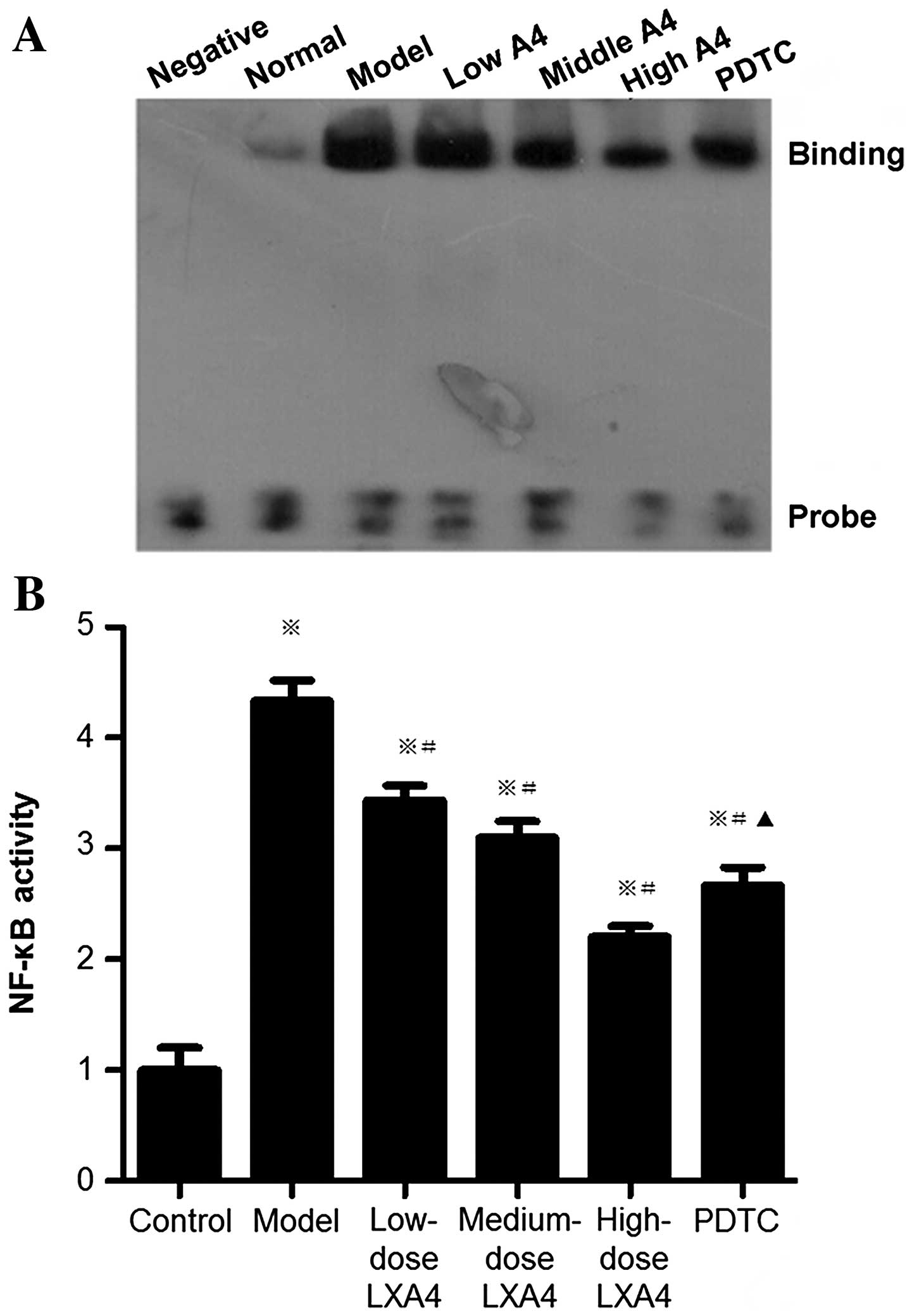
LXA4 attenuates the elevation of hepatic
NF-κB activity
The activity of NF-κB (examined by EMSA) was
significantly higher in the model group than in the control group,
and significantly lower in the LXA4-treated groups than in the
model group (Fig. 7). In the PDTC
group, NF-κB activity was significantly decreased compared to the
model group, and significantly increased compared to the high-dose
LXA4 group (Fig. 7).
LXA4 attenuates the elevation of NF-κB
activity in purified Kupffer cells
NF-κB activation (assessed using EMSA) in the model
group was significantly higher than in the control group (Fig. 8). In the LXA4 groups, NF-κB
activity was significantly reduced compared to the model group
(Fig. 8). In the PDTC-treated
group, NF-κB activity was significantly lower than that in the
model group, and was comparable to that in the high-dose LXA4 group
(Fig. 8).
Discussion
The combined use of D-GalN and LPS has been shown to
result in injury to the liver (23), and this combination has been used
in previous research to generate an animal model of ALF (24). In the present study, the model
group exhibited significantly elevated liver transaminase activity
(with serum AST >8,000 U/l) as well as disorders of liver
structure, including severe inflammatory necrosis and hemorrhage.
In addition, the mortality rate in the model group was 50% (data
not shown). These results indicate that a model of ALF had been
generated successfully.
In this study, LXA4 administration significantly
alleviated the signs of ALF, demonstrated by significantly reduced
levels of serum ALT and AST, reduced liver tissue inflammation and
necrosis, and improved survival (data not shown). These findings
suggest that LXA4 plays a protective role against ALF, a statement
which is supported by a previous study on rabbits (12).
The induction of TNF-α is one of the earliest events
that occur during inflammation of the liver. TNF-α initiates a
cascade of circulating pro-inflammatory cytokines, leading to the
necrosis of liver cells. Depending on the micro-environment, TNF-α
is capable of either inducing the proliferation of liver cells or
promoting apoptosis and necrosis (25). The anti-apoptotic effects of TNF-α
are mediated via activation of NF-κB, whereas the pro-apoptotic
actions are mediated via caspases (26). Elevated TNF-α levels have been
demonstrated in the serum and liver tissues of patients with acute
and chronic hepatitis B and C (27–31). Serum TNF-α levels are also
increased significantly in patients with ALF (32). The finding that mice deficient in
the TNF-p55 receptor for TNF-α were resistant to fatal liver injury
induced by co-administration of D-GlaN/LPS demonstrates the central
role of TNF-α in this model (33). A previous study has shown that
LXA4 suppressed the effect of LPS on NF-κB (34). In the present study, we noted that
LXA4 significantly reduced the serum TNF-α level, and decreased the
mRNA expression of TNF-α, the number of TUNEL-positive cells and
caspase-3 activity in liver tissue. This strongly suggests that the
hepatoprotective effect of LXA4 is effected through inhibition of
the actions of TNF-α.
It is known that, in the liver, IL-6 is secreted by
Kupffer cells and hepatocytes (35,36). IL-6 induces the production of
acute-phase proteins during acute inflammation in the liver, and
serum levels of IL-6 correlate positively with the severity of the
disease (26). Blockade of the
IL-6 signaling pathway significantly increases the sensitivity of
mouse hepatocytes to LPS-induced liver failure (26), indicating that IL-6 causes damage
to liver cells. The results of the present study showed that LXA4
significantly reduced the serum IL-6 level and IL-6 mRNA expression
in liver tissues, implying that the protective effect exerted by
LXA4 on the liver is partially related to a reduction in IL-6
levels. A previous study has shown that LXA4 protected against
obesity-induced systemic diseases, which are mostly inflammatory in
nature (37), ans this supports
the results of the present study.
TNF-α is produced by Kupffer cells in the liver;
inhibition of NF-κB activation in Kupffer cells has been shown to
block LPS-induced TNF-α generation, thereby inhibiting the death of
liver cells sensitized with Propionibacterium acnes
(38). Previous research on rats
has demonstrated that the inhibition of NF-κB activation in Kupffer
cells reduces the production of pro-inflammatory cytokines,
attenuates D-GlaN/LPS-induced liver damage, and improves survival,
without affecting anti-apoptotic proteins in liver tissues
(39). This raises the
possibility that the hepatoprotective effect exerted by LXA4
observed in the present study is due to the inhibition of NF-κB in
Kupffer cells. We found that NF-κB activity in Kupffer cells was
significantly increased in cases of ALF, and that this was
accompanied by elevations in serum TNF-α and IL-6 levels, as well
as enhanced mRNA expression of TNF-α and IL-6 in liver tissues.
Administration of LXA4 significantly attenuated these increases,
suggesting that LXA4 inhibits excessive activation of Kupffer cells
in cases of ALF and reduces the generation of pro-inflammatory
cytokines.
The aim of the present study was to probe the
association between NF-κB activation and Kupffer cells in a rat
model of ALF. PDTC acts as a membrane-permeable inhibitor of NF-κB
activation in a variety of cells, and it has been reported that
PDTC exerts protective effects against LPS-induced acute liver
injury (40). Our results
revealed that the high dose of LXA4 was superior to PDTC at
alleviating ALF, raising the possibility that the protective
effects exerted by LXA4 include mechanisms other than NF-κB
inhibition. In the present study, LXA4 and PDTC inhibited the
expression of mRNA levels of IL-6 and TNF-α in the liver. However,
PDTC did not inhibit the expression of TNF-α in the serum as
significantly as medium and high doses of LXA4 did. This indicates
that LXA4 regulates TNF-α expression through various signaling
pathways besides the NF-κB pathway. However, additional studies are
necessary to address this issue.
This study has shown that LXA4 reduces serum levels
of TNF-α and IL-6 and alleviates liver cell apoptosis and necrosis
in a D-GlaN/LPS-induced rat model of ALF. One possible mechanism is
that LXA4 inhibits NF-κB activation in Kupffer cells. In addition,
we suggest that LXA4 promotes homeostasis of the internal
environment of liver cells during acute liver dysfunction, reducing
the secretion of inflammatory cytokines, and decreasing the
excessive activation of NF-κB in hepatocytes. This prevents
large-scale necrosis of liver tissue, and longer term would likely
reduce the possibility of genetic mutations and thus malignancy
during regeneration of the liver. Our results have also
demonstrated that LXA4 exerts a better protective effect than PDTC
during ALF, suggesting that LXA4 acts via mechanisms which do not
only involve NF-κB inhibition.
Acknowledgments
We thank Professor Deying Tian (Wuhan Tongji
Hospital) for helpful suggestions about the design of the study.
This study was supported by the Hubei Provincial Natural Science
Youth Foundation (no. 2014CFB215) and the Hubei Provincial
Department of Education Instruction Projects (no. B2014050). This
study was also supported by the National Science and Technology
Major Project in the 11th five-year plan (no. 2008ZX10005-007), the
12th five-year plan (no. 2012ZX10005-005) and the Open Project of
Hubei Key Laboratory of Wudang Local Chinese Medicine Research
(Hubei University of Medicine; no. WDCM006).
References
|
1
|
Panackel C, Thomas R, Sebastian B and
Mathai SK: Recent advances in management of acute liver failure.
Indian J Crit Care Med. 19:27–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bernal W, Auzinger G, Dhawan A and Wendon
J: Acute liver failure. Lancet. 376:190–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Serhan CN: Pro-resolving lipid mediators
are leads for resolution physiology. Nature. 510:92–101. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buckley CD, Gilroy DW and Serhan CN:
Proresolving lipid mediators and mechanisms in the resolution of
acute inflammation. Immunity. 40:315–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Serhan CN, Krishnamoorthy S, Recchiuti A
and Chiang N: Novel anti-inflammatory - pro-resolving mediators and
their receptors. Curr Top Med Chem. 11:629–647. 2011. View Article : Google Scholar :
|
|
6
|
Jin SW, Zhang L, Lian QQ, Liu D, Wu P, Yao
SL and Ye DY: Posttreatment with aspirin-triggered lipoxin A4
analog attenuates lipopolysaccharide-induced acute lung injury in
mice: the role of heme oxygenase-1. Anesth Analg. 104:369–377.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walker J, Dichter E, Lacorte G, Kerner D,
Spur B, Rodriguez A and Yin K: Lipoxin A4 increases survival by
decreasing systemic inflammation and bacterial load in sepsis.
Shock. 36:410–416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdelmoaty S, Wigerblad G, Bas DB,
Codeluppi S, Fernandez-Zafra T, El-Awady S, Moustafa Y, Abdelhamid
AD, Brodin E and Svensson CI: Spinal actions of lipoxin A4 and
17(R)-resolvin D1 attenuate inflammation-induced mechanical
hypersensitivity and spinal TNF release. PLoS One. 8:e755432013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Börgeson E, Docherty NG, Murphy M, Rodgers
K, Ryan A, O'Sullivan TP, Guiry PJ, Goldschmeding R, Higgins DF and
Godson C: Lipoxin A4 and benzo-lipoxin A4 attenuate experimental
renal fibrosis. FASEB J. 25:2967–2979. 2011. View Article : Google Scholar
|
|
10
|
Martins V, Valença SS, Farias-Filho FA,
Molinaro R, Simões RL, Ferreira TP, e Silva PM, Hogaboam CM, Kunkel
SL, Fierro IM, et al: ATLa, an aspirin-triggered lipoxin A4
synthetic analog, prevents the inflammatory and fibrotic effects of
bleomycin-induced pulmonary fibrosis. J Immunol. 182:5374–5381.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Planagumà A, Kazani S, Marigowda G,
Haworth O, Mariani TJ, Israel E, Bleecker ER, Curran-Everett D,
Erzurum SC, Calhoun WJ, et al: Airway lipoxin A4 generation and
lipoxin A4 receptor expression are decreased in severe asthma. Am J
Respir Crit Care Med. 178:574–582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xia J, Zhou XL, Zhao Y, Zhu YQ, Jiang S
and Ni SZ: Roles of lipoxin A4 in preventing paracetamol-induced
acute hepatic injury in a rabbit model. Inflammation. 36:1431–1439.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu SH, Liao PY, Dong L and Chen ZQ: Signal
pathway involved in inhibition by lipoxin A(4) of production of
interleukins induced in endothelial cells by lipopolysaccharide.
Inflamm Res. 57:430–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun T, Yu E, Yu L, Luo J, Li H and Fu Z:
LipoxinA(4) induced antinociception and decreased expression of
NF-κB and pro-inflammatory cytokines after chronic dorsal root
ganglia compression in rats. Eur J Pain. 16:18–27. 2012. View Article : Google Scholar
|
|
15
|
Nishiokada A, Miyoshi M, Fujiwara M,
Aoyama-Ishikawa M, Nishiyama Y, Kai M, Maeshige N, Takahashi M,
Hamada Y, Usami Y and Usami M: Changes of hepatic lipid mediators
associated with intake of high-fat diet for 12 weeks in endotoxemic
rats using LC-ESI-MS/MS. Clin Nutr. 33:S22–S23. 2014. View Article : Google Scholar
|
|
16
|
El-Agamy DS, Makled MN and Gamil NM:
Protective effects of BML-111 against acetaminophen-induced acute
liver injury in mice. J Physiol Biochem. 70:141–149. 2014.
View Article : Google Scholar
|
|
17
|
Zhou XY, Yu ZJ, Yan D, Wang HM, Huang YH,
Sha J, Xu FY, Cai ZY and Min WP: BML-11, a lipoxin receptor
agonist, protected carbon tetrachloride-induced hepatic fibrosis in
rats. Inflammation. 36:1101–1106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao W, Zeng F, Kang K, Qi Y, Yao L, Yang
H, Ling L, Wu N and Wu D: Lipoxin A4 attenuates acute rejection via
shifting TH1/TH2 cytokine balance in rat liver transplantation.
Transplant Proc. 45:2451–2454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hao H, Liu M, Wu P, Cai L, Tang K, Yi P,
Li Y, Chen Y and Ye D: Lipoxin A4 and its analog suppress
hepatocellular carcinoma via remodeling tumor microenvironment.
Cancer Lett. 309:85–94. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knook DL, Blansjaar N and Sleyster EC:
Isolation and characterization of Kupffer and endothelial cells
from the rat liver. Exp Cell Res. 109:317–329. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kono H, Wheeler MD, Rusyn I, Lin M, Seabra
V, Rivera CA, Bradford BU, Forman DT and Thurman RG: Gender
differences in early alcohol-induced liver injury: role of CD14,
NF-kappaB, and TNF-alpha. Am J Physiol Gastrointest Liver Physiol.
278:G652–G661. 2000.PubMed/NCBI
|
|
22
|
Su GL, Goyert SM, Fan MH, Aminlari A, Gong
KQ, Klein RD, Myc A, Alarcon WH, Steinstraesser L, Remick DG and
Wang SC: Activation of human and mouse Kupffer cells by
lipopolysaccharide is mediated by CD14. Am J Physiol Gastrointest
Liver Physiol. 283:G640–G645. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Z, Kong X, Zhang T, Ye J, Fang Z and
Yang X: Pseudo-ephedrine/ephedrine shows potent anti-inflammatory
activity against TNF-α-mediated acute liver failure induced by
lipopolysaccharide/D-galactosamine. Eur J Pharmacol. 724:112–121.
2014. View Article : Google Scholar
|
|
24
|
Tuñón MJ, Alvarez M, Culebras JM and
González-Gallego J: An overview of animal models for investigating
the pathogenesis and therapeutic strategies in acute hepatic
failure. World J Gastroenterol. 15:3086–3098. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cosgrove BD, Cheng C, Pritchard JR, Stolz
DB, Lauffenburger DA and Griffith LG: An inducible autocrine
cascade regulates rat hepatocyte proliferation and apoptosis
responses to tumor necrosis factor-alpha. Hepatology. 48:276–288.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tacke F, Luedde T and Trautwein C:
Inflammatory pathways in liver homeostasis and liver injury. Clin
Rev Allergy Immunol. 36:4–12. 2009. View Article : Google Scholar
|
|
27
|
Aroucha DC, do Carmo RF, Moura P, Silva
JL, Vasconcelos LR, Cavalcanti MS, Muniz MT, Aroucha ML, Siqueira
ER, Cahú GG, et al: High tumor necrosis factor-α/interleukin-10
ratio is associated with hepatocellular carcinoma in patients with
chronic hepatitis C. Cytokine. 62:421–425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Głowacki MK, Cieśla A, Cibor D, Owczarek
D, Mach T, Wiliński J and Wiliński B: Selected apoptotic markers in
serum of patients with chronic viral hepatitis C. Przegl Lek.
71:369–373. 2014.
|
|
29
|
Hammam O, Mahmoud O, Zahran M, Sayed A,
Salama R, Hosny K and Farghly A: A Possible role for TNF-α in
coordinating inflammation and angiogenesis in chronic liver disease
and hepatocellular carcinoma. Gastrointest Cancer Res. 6:107–114.
2013.PubMed/NCBI
|
|
30
|
Kiki I, Yilmaz O, Erdem F, Gundogdu M,
Demircan B and Bilici M: Tumour necrosis factor-alpha levels in
hepatitis B virus-related chronic active hepatitis and liver
cirrhosis and its relationship to Knodell and Child-Pugh scores.
Int J Clin Pract. 60:1075–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zou Z, Li B, Xu D, Zhang Z, Zhao JM, Zhou
G, Sun Y, Huang L, Fu J, Yang Y, et al: Imbalanced intrahepatic
cytokine expression of interferon-gamma, tumor necrosis
factor-alpha, and interleukin-10 in patients with acute-on-chronic
liver failure associated with hepatitis B virus infection. J Clin
Gastroenterol. 43:182–190. 2009. View Article : Google Scholar
|
|
32
|
Mao WL, Chen Y, Chen YM and Li LJ: Changes
of serum cytokine levels in patients with acute on chronic liver
failure treated by plasma exchange. J Clin Gastroenterol.
45:551–555. 2011. View Article : Google Scholar
|
|
33
|
Nowak M, Gaines GC, Rosenberg J, Minter R,
Bahjat FR, Rectenwald J, MacKay SL, Edwards CK III and Moldawer LL:
LPS-induced liver injury in D-galactosamine-sensitized mice
requires secreted TNF-alpha and the TNF-p55 receptor. Am J Physiol
Regul Integr Comp Physiol. 278:R1202–R1209. 2000.PubMed/NCBI
|
|
34
|
Huang YH, Wang HM, Cai ZY, Xu FY and Zhou
XY: Lipoxin A4 inhibits NF-κB activation and cell cycle progression
in RAW264.7 cells. Inflammation. 37:1084–1090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nemeth E, Baird AW and O'Farrelly C:
Microanatomy of the liver immune system. Semin Immunopathol.
31:333–343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Norris CA, He M, Kang LI, Ding MQ, Radder
JE, Haynes MM, Yang Y, Paranjpe S, Bowen WC and Orr A: Synthesis of
IL-6 by hepatocytes is a normal response to common hepatic stimuli.
PLoS One. 9:e960532014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Börgeson E, Johnson AM, Lee YS, Till A,
Syed GH, Ali-Shah ST, Guiry PJ, Dalli J, Colas RA, Serhan CN, et
al: Lipoxin A4 attenuates obesity-induced adipose inflammation and
associated liver and kidney disease. Cell Metab. 22:125–137. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ogushi I, Iimuro Y, Seki E, Son G, Hirano
T, Hada T, Tsutsui H, Nakanishi K, Morishita R, Kaneda Y and
Fujimoto J: Nuclear factor kappa B decoy oligodeoxynucleotides
prevent endotoxin-induced fatal liver failure in a murine model.
Hepatology. 38:335–344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hoffmann F, Sass G, Zillies J, Zahler S,
Tiegs G, Hartkorn A, Fuchs S, Wagner J, Winter G, Coester C, et al:
A novel technique for selective NF-kappaB inhibition in Kupffer
cells: contrary effects in fulminant hepatitis and
ischaemia-reperfusion. Gut. 58:1670–1678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hagar HH: An insight into the possible
protective effect of pyrrolidine dithiocarbamate against
lipopolysaccharide-induced oxidative stress and acute hepatic
injury in rats. Saudi Pharm J. 17:259–267. 2009. View Article : Google Scholar : PubMed/NCBI
|