Introduction
Cancer is one of the most life-threatening diseases
worldwide, and the incidence and mortality of cancer in Korea have
continuously increased due to various acquired risk factors,
including a Western diet and environmental factors (1). Oral cancer accounts for
approximately 3–5% of all cancer cases (2,3);
however, unlike cancers in other parts of the body, damage to the
facial region can cause psychological disorders in patients and
negatively affect their daily life, particularly in terms of eating
or speaking. Thus, there is great interest in exploring treatments
for oral cancer (4). Oral cancer
poses a risk of invasion to adjacent organs and a higher
possibility of metastatic relapse. Thus, surgical therapy,
radiation and medication in combination are usually used to treat
oral cancer; however, severe adverse effects and low treatment
efficacy, as well as the ineffective inhibition of cancer cell
growth and micrometastasis, results in patients with oral cancer
tending to have a poor prognosis (5). It is therefore necessary to find
naturally-derived substances to serve as anticancer drugs that are
capable of specifically targeting cancer cells, with limited
side-effects and potent anticancer effects.
Mangosteen (Garcinia mangostana L.) is well
known as the 'queen of tropical fruit' due to its delicious taste,
and has long been used for medicinal purposes, particularly for the
treatment of dermatitis, ulcers and diarrhea, in parts of
SoutheastEastern South Asia such as Malaysia, Indonesia, Taiwan,
Philippines, India and Sri Lanka (6). It has been reported that xanthone, a
component contained within the pericarp (rind or peel) of the
mangosteen fruit, has been shown to exert various biological
effects, including antioxidant (7), anticancer (8), antibacterial (9,10),
anti-inflammatory (11),
anti-allergic and antiviral effects (12). Xanthone has also been widely used
as an inhibitor of enzymes involved in the oxidation of low-density
lipoprotein (LDL) cholesterol (13), as well as those associated with
infections, such as prostaglandin E2 (PGE2)
and cyclo-oxygenase-2 (COX-2) (14). Thus far, various xanthones have
been found in fruit, fruit skin, tree bark, moss and mold, and
approximately 40 different xanthones have been found in the
mangosteen fruit (15).
α-mangostin is a key, physiologically active
substance contained within the fruit skin of mangosteens that has
been demonstrated to inhibit the cell cycle and induce the
apoptosis of various cancer cell lines, including colorectal,
mammary, liver and prostate cancer cells (8,16–19). In particular, the anticancer
effects and the inhibitory effects on lymph node metastasis of
α-mangostin have been reported using tumor xenograft mouse models
of mammary cancer (19).
The mitogen-activated protein kinase (MAPK) cascade,
a pathway used to send external signals to internal cells, is
involved in various processes, including cell proliferation and
fragmentation, apoptosis and survival. There are also subgroups of
MAPKs, which include extracellular signal-regulated kinase (ERK),
p38 kinase, and c-jun N-terminal kinase/stress-activated protein
kinase (JNK/SAPK). Each group is controlled by its own pathway and
performs distinct functions. ERK is mainly involved in cell
survival, whereas SAPK and p38 kinase mainly regulate apoptosis
(20).
However, the anticancer effects of α-mangostin on
oral cancer remain unknown. Thus, in this study, we aimed to
investigate the anticancer effects of α-mangostin on oral (tongue)
cancer, which is a type of cancer with severe adverse effects and
lower treatment efficacy compared with other types of cancer. The
naturally-derived substance, α-mangostin, was evaluated in YD-15
cells, a tongue mucoepidermoid carcinoma cell line, in order to
examine its inhibitory effects on cancer progression in terms of
apoptosis. Accordingly, we focused on the ERK1/2 and p38 MAPK
signaling pathways in an aim to elucidate the underlying molecular
mechanisms.
Materials and methods
Chemicals, drugs and antibodies
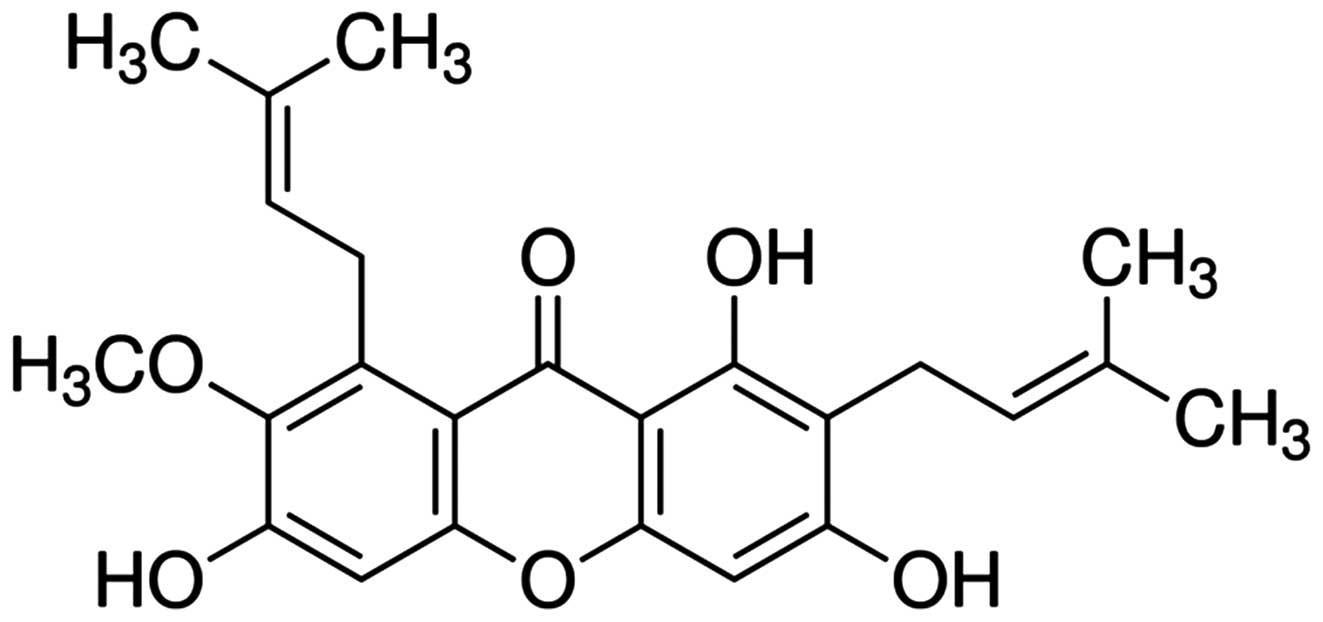
α-mangostin (chemical structure shown in Fig. 1) was purchased from Sigma-Aldrich
(St. Louis, MO, USA), dissolved in dimethyl sulfoxide (DMSO) and
stored at −20°C. RPMI-1640 medium, penicillin-streptomycin,
trypsin-EDTA and fetal bovine serum (FBS) were purchased from
HyClone Laboratories, Inc. (Logan, UT, USA).
3-(4,5-Dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and DMSO were obtained from Sigma-Aldrich. Cell lysis buffer and
4′,6-diamidino-2-phenylindole (DAPI) were purchased from Invitrogen
Life Technologies (Carlsbad, CA, USA). The fluorescein
isothiocyanate (FITC)-conjugated Annexin V Apoptosis Detection kit
was purchased from BD Biosciences (San Diego, CA, USA).
Anti-β-actin (#4967), anti-Bax (#2772), anti-Bcl-2 (#2876),
anti-caspase-3 (#9662), anti-cleaved caspase-3 (#9661),
anti-caspase-9 (#9502), anti-poly(ADP-ribose) polymerase (PARP;
#9542), anti-ERK1/2 (#9102), anti-phosphorylated (p)-ERK1/2
(#4376), anti-p38 (#9212), anti-p-p38 (#4631), anti-c-myc (#9027),
anti-Ki-67 (#9027) and goat anti-rabbit horseradish peroxidase
(HRP)-conjugated (#7074) antibodies were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA). The DeadEnd™
fluorometric terminal deoxynucleotidyl transferase-mediated dUTP
nick-end labeling (TUNEL) assay kit was purchased from Promega
Corp. (Madison, WI, USA).
Cell lines and culture
The human tongue mucoepidermoid carcinoma cell line,
YD-15, was purchased from the Korean Cell Line Bank (Seoul, Korea)
and maintained in RPMI-1640 medium supplemented with 10% FBS and 1%
penicillin-streptomycin at 37°C in a humidified 5% CO2
atmosphere. The culture medium was replaced every 2–3 days. For
α-mangostin treatment, the YD-15 cells were seeded at a density of
approximately 3×104 cells/cm2 in a
175-cm2 flask and were allowed to adhere overnight.
Cell viability assay
The effects of α-mangostin on YD-15 cell survival
were determined by MTT assay. The YD-15 cells were seeded in
96-well plates at a density of 2×104 cells/ml in a
volume of 200 µl/well. Following 24 h of incubation, the
cells were treated with 10, 15, 20, 25 or 30 µM α-mangostin
for 24 h in triplicate. Following treatment, the medium was
discarded and 40 µl 5 mg/ml MTT solution were added followed
by incubation for an additional 2 h. The medium was then aspirated,
and the formazan product generated by the viable cells was
solubilized by the addition of 100 µl DMSO. The absorbance
of the solutions at 595 nm was determined using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The percentage of
viable cells relative to the untreated (control) cells was
estimated.
Nuclear staining
To assess apoptosis, the nuclei of the YD-15 cells
were stained with DAPI. The cells were seeded onto 60-mm dishes at
a density of 1×105 cells/ml and incubated with 10 or 15
µM α-mangostin for 24 h. Following treatment, the cells were
fixed in phosphate-buffered saline (PBS) containing 4%
paraformaldehyde for 15 min in an incubator. Following fixation,
the cells were washed twice with PBS and the cell nuclei were
stained with DAPI in PBS. Fluorescence signals were visualized
using a fluorescence microscope (BX41; Olympus Co., Tokyo, Japan)
at ×200 magnification.
Annexin V staining for the analysis of
apoptosis
The Annexin V/propidium iodide (PI) assay was
performed according to the manufacturer's instructions (BD
Biosciences). Briefly, the YD-15 cells were treated with or without
10 or 15 µM α-mangostin for 24 h, washed twice with cold
PBS, and incubated with fluorescein isothiocyanate
(FITC)-conjugated Annexin V and phycoerythrin (PE)-conjugated PI in
binding buffer at room temperature for 15 min in the dark. The
samples were analyzed using a FACSCalibur™ flow cytometer (BD
Biosciences).
Flow cytometric analysis of the cell
cycle
Cell cycle progression was assayed by measuring DNA
fragmentation with PI staining. The YD-15 cells were treated with
or without 10 or 15 µM α-mangostin for 24 h, washed twice
with PBS and fixed with 70% ethanol for 30 min. Following fixation,
the DNA fragments were stained in PBS containing PI and RNase
(Sigma-Aldrich) for 30 min at room temperature. After sorting out
the viable cells, the fluorescence intensity was measured using a
FACSCalibur™ flow cytometer (BD Biosciences).
Western blot analysis
The cells were grown in culture flasks under the
same conditions as described above and treated with 10 or 15
µM α-mangostin for 24 h. The cells were washed with PBS and
treated with trypsin-EDTA for 1 min. Cell pellets were obtained by
centrifugation, lysed in lysis buffer (Invitrogen Life
Technologies) and centrifuged at 13,000 rpm for 5 min at 4°C to
obtain whole-cell lysates. Protein concentrations were determined
using a Bradford Protein assay kit (Bio-Rad Laboratories Inc.). The
samples were stored at −80°C. The proteins were resolved by sodium
dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred electrophoretically onto nitrocellulose membranes
(Bio-Rad Laboratories Inc.). The membranes were blocked with
Tris-buffered saline (TBS) containing 5% non-fat dry milk and 0.1%
Tween-20 at 4°C for 2 h. After blocking, the membranes were
incubated with anti-β-actin, anti-Bax, anti-Bcl-2, anti-caspase-3,
anti-caspase-9, anti-PARP, anti-ERK1/2, anti-p-ERK1/2, anti-p38,
anti-pp38 and anti-c-myc antibodies overnight at 4°C, with gentle
shaking. Following incubation with the primary antibodies, the
membranes were incubated with HRP-conjugated goat anti-rabbit IgG
secondary antibodies for 2 h at room temperature with gentle
shaking. After washing the membranes 3 times for 10 min in TBS
containing 0.1% Tween-20, bands were detected using enhanced
chemiluminescence (ECL) western blotting detection reagents
(Pierce, Rockford, IL, USA) according to the manufacturer's
instructions. β-actin was used as a loading control. Band density
was measured using the ImageJ software (NIH, Bethesda, MD, USA)
program.
Animal experiments
Five-week-old male BALB/c nude (nu/nu) mice were
purchased from the animal production company of Orient Bio, Inc.
(Gyeonggi-do, Korea) and maintained in a controlled environment at
23±5°C with 40±10% relative humidity with artificial lighting from
8:00 a.m. to 8:00 p.m. in facilities approved by the Companion and
Laboratory Animal Science Department of Kongju National University
(Chungnam, Korea). The animals were housed in cages and allowed
access to sterilized water and commercial rodent chow (Biopia,
Seoul, Korea) ad libitum. All animal experiments were
performed following the approval of the Institutional Animal Care
and Use Committee according to the guidelines of Kongju National
University.
Tumor xenografts
The YD-15 cells were maintained in RPMI-1640
supplemented with 10% FBS and 1% penicillin-streptomycin at 3°C in
a humidified 5% CO2 atmosphere. The YD-15 cells were
harvested by exposure to trypsin-EDTA. The cells were then washed
twice and resuspended in RPMI-1640 medium. The YD-15 cells were
then injected subcutaneously (1×107 cells/0.2 ml
medium/animal) into the left and right flanks of the mice using a
27-gauge needle. When the tumors were palpable, the mice were
assigned randomly into 3 groups with 3 mice in each (the
vehicle-treated controls, and the groups treated with 10 or 20
mg/kg body weight α-mangostin). The doses of α-mangostin (10 and 20
mg/kg) were selected for the in vivo experiments using mice
based on the results of a previous study by Akao et al
(8), in which mice administered
>20 mg/kg α-mangostin exhibited a significant increase in
natural killer (NK) cell activity. Therefore, we selected 20 mg/kg
as the dose for use in the present study, as this is the dose that
others have reported has no harmful effect. For administration,
α-mangostin was dissolved in 0.1% DMSO and further diluted in PBS
before injection. α-mangostin was administered intraperitoneally 5
times per week at a dose of 10 or 20 mg/kg body weight, while the
control group mice were administered the vehicle only (DMSO in
PBS). Tumor weight and size were monitored twice each week. Tumor
size was measured using Vernier calipers (Mitutoyo, Kawasaki,
Japan). The mice were sacrificed by ether inhalation at 22 days
following treatment and the tumors were excised for the measurement
of tumor weight. A portion of the tumor was embedded in paraffin
and used for TUNEL assays and immunohistochemical analysis.
TUNEL assay
Apoptotic cell death was quantified using a Promega
DeadEnd Colorimetric TUNEL system kit according to the
manufacturer's instructions (Promega Corp.). Briefly, the tumor
tissues were fixed in 10% formalin overnight and embedded in
paraffin. The blocks were then cut into 5-µm-thick slices.
The tissue sections attached to microscopic slides were then
deparaffinized by immersion in xylene and the slides were then
washed with 100% ethanol. The samples were rehydrated by sequential
immersion in a graded ethanol series (95, 85, 70 and 50%). The
tumor sections were visualized using 3′-diaminobenzidine
tetrahydrochloride (DAB) solution, treated with mounting reagent,
and observed under a microscope (BX41; Olympus Co.) at x200
magnification.
Immunohistochemical analysis
The tumor sections were deparaffinized with two
changes of xylene for 10 min, rehydrated with two changes each of
100 and 95% ethanol for 1 min, and rinsed with tap water for 10
min. The sections were then incubated at 4°C with anti-cleaved
caspase-3, anti-p-ERK1/2, anti-p-p38 and anti-Ki-67 antibodies
overnight and incubated for 1 h at room temperature with a
HRP-conjugated goat anti-rabbit antibody followed by incubation for
1 h. The tumor sections were visualized using DAB solution, treated
with mounting reagent, and observed under a microscope (×200
magnification).
Statistical analysis
The results are all expressed as the means ±
standard deviations (SD). Differences between mean values for the
individual groups were assessed by one-way analysis of variance
(ANOVA) with Dunnett's t-tests. A P<0.05 was considered to
indicate a statistically significant difference.
Results
Inhibitory effects of α-mangostin on the
cell survival rate and morphological changes in YD-15 cells
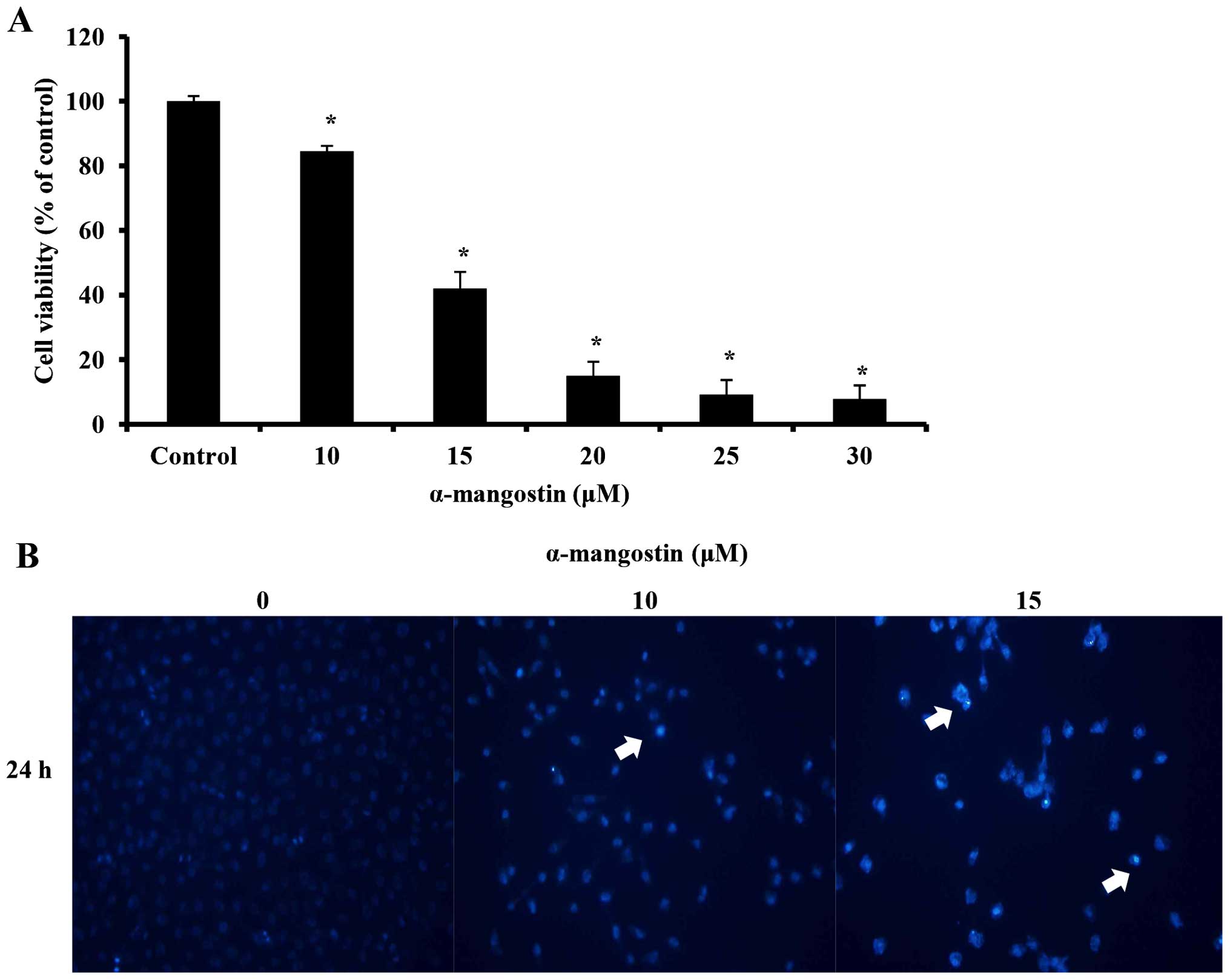
An MTT assay was conducted to examine the effects of
α-mangostin on YD-15 cell viability. The YD-15 cells were treated
with 0, 10, 15, 20, 25 and 30 µM α-mangostin for 24 h. Cell
viability was inhibited at a concentration of 10 µM
(Fig. 2A). Compared with the
untreated control cells, the cells treated with 10 µM
α-mangostin displayed approximately 15% inhibition, whereas those
treated with 15 µM α-mangostin exhibited approximately 58%
inhibition. Moreover, the number of viable cells decreased in a
concentration-dependent manner. DAPI staining was performed to
identify the morphological changes associated with nuclear and
chromosomal condensation. The YD-15 cells were treated with 0, 10
and 15 µM α-mangostin for 24 h. DAPI staining was utilized
to observe the cells by fluorescence microscopy. As a result,
increased apoptosis (indicated by increased chromatin condensation)
was observed in the cells treated with 10 and 15 µM
α-mangostin compared to the control group (Fig. 2B). These results indicate that
α-mangostin decreased the viability of the YD-15 cells, creating
apoptotic bodies, thus leading to cellular apoptosis.
Effects of α-mangostin on YD-15 cell
apoptosis
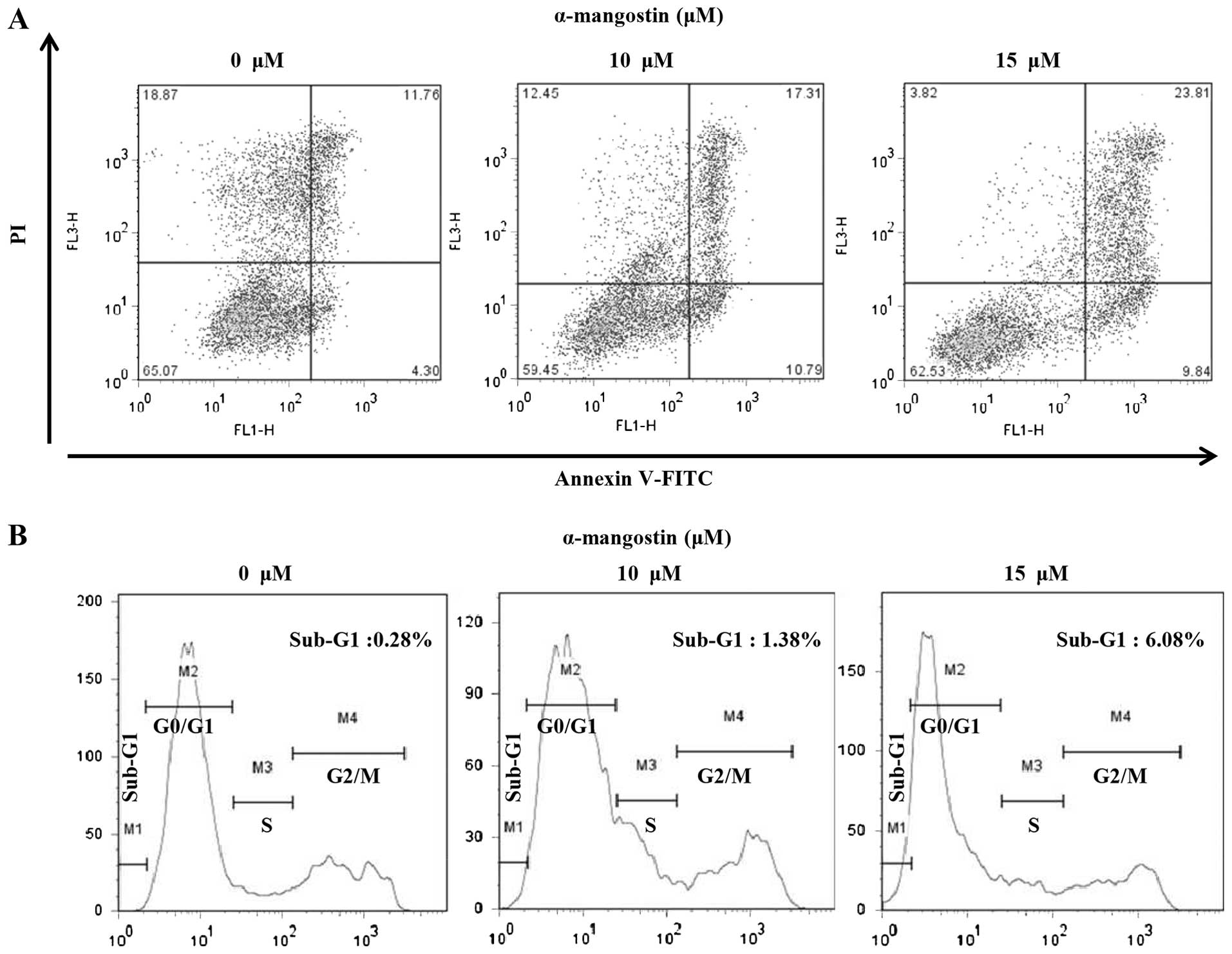
Apoptotic cells were analyzed quantitatively by
Annexin-V FITC/PI staining to examine the effects of α-mangostin on
YD-15 cells. The percentage of apoptotic cells in the untreated
(control) group was 16.06% (early apoptotic cells, 4.30%; late
apoptotic cells, 11.76%). However, the number of apoptotic cells
increased significantly with the increasing concentration of
α-mangostin. The percentages of apoptotic cells following treatment
with 10 and 15 µM α-mangostin were 28.1 (early apoptotic
cells, 10.79%; late apoptotic cells, 17.31%) and 33.65% (early
apoptotic cells, 9.84%; late apoptotic cells, 23.81%), respectively
(Fig. 3A). Flow cytometry was
used to examine the effects of α-mangostin on the YD-15 cell cycle.
The percentage of sub-G1 cells in the control group was 0.28%
(Fig. 3B). The number of cells in
the sub-G1 phase tended to increase in a concentration-dependent
manner (10 µM, 1.38% and 15 µM, 6.08%; Fig. 3B). These results suggest that the
inhibitory effects of α-mangostin on cell viability are caused by
sub-G1 arrest related to apoptosis.
Effects of α-mangostin on the expression
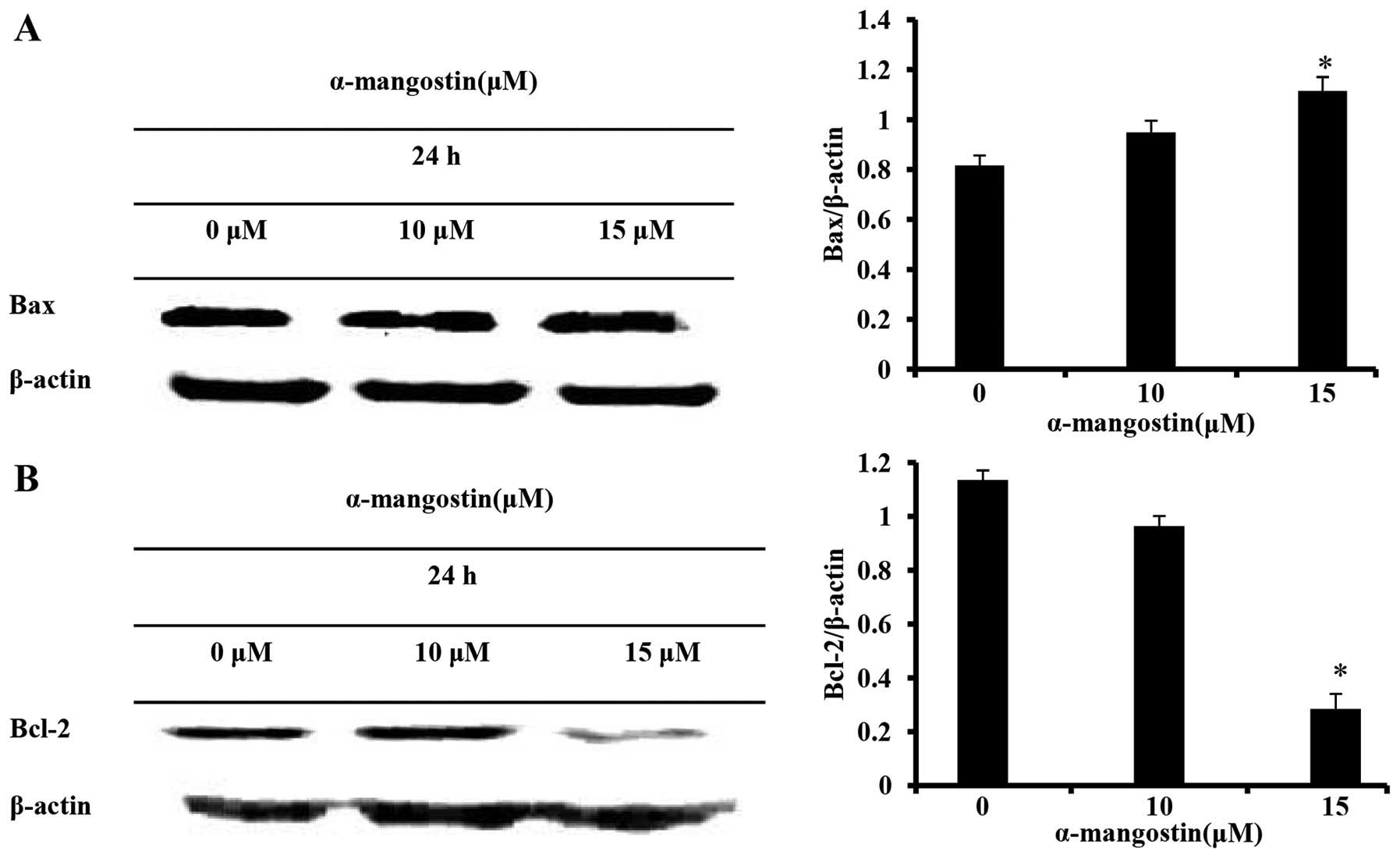
of Bcl-2 family proteins
The expression of Bcl-2 family proteins, responsible
for controlling apoptosis, was determined by western blot analysis
in order to elucidate the mechanisms responsible for the apoptosis
induced by α-mangostin in YD-15 cells. The α-mangostin-treated
group exhibited an increased expression of the pro-apoptotic
factor, Bax, and a decreased expression of the anti-apoptotic
factor, Bcl-2, in a concentration-dependent manner (Fig. 4). These results imply that the
apoptotic mechanism induced by α-mangostin is associated with Bcl-2
family proteins.
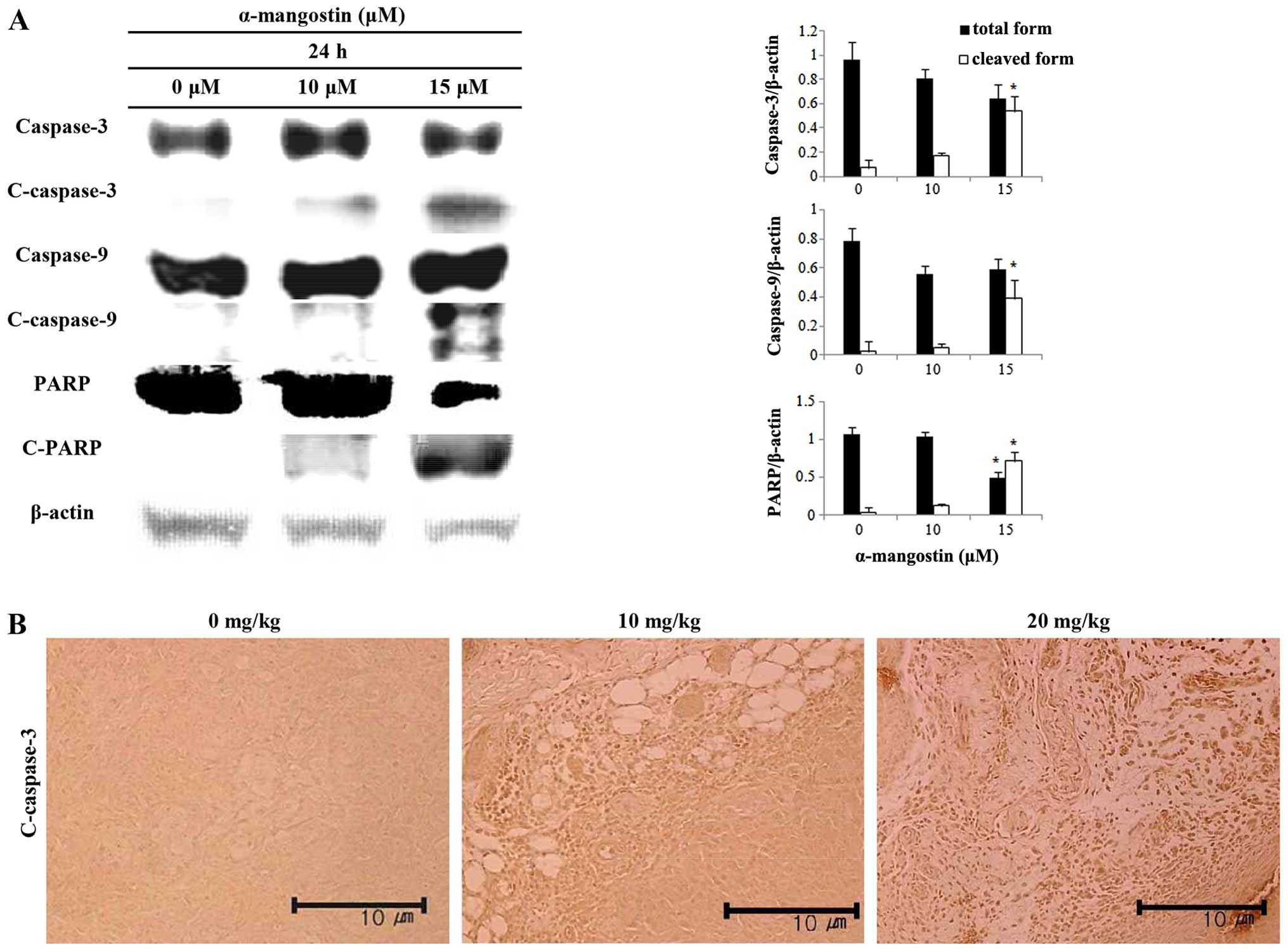
Effects of α-mangostin on caspase
activation
The expression levels of caspase-3, caspase-9 and
PARP were measured by western blot analysis in order to determine
the association between the induction of apoptosis by α-mangostin
and caspase activation in YD-15 cells. The cells treated with
α-mangostin exhibited increased caspase-3 and -9 activity in a
concentration-dependent manner (Fig.
5A). The level of cleaved-PARP increased as well. Cleaved
caspase-3 was visualized by immunohistochemical analysis in order
to examine the effects of α-mangostin on caspase expression in
tumor tissues collected from mice with tumor xenografts (Fig. 5B). The tumor tissue from mice
treated with α-mangostin exhibited increased levels of cleaved
caspase-3 in a concentration-dependent manner, suggesting that the
mechanism of apoptosis induced by α-mangostin is related to caspase
activation, which in turn induces PARP segmentation.
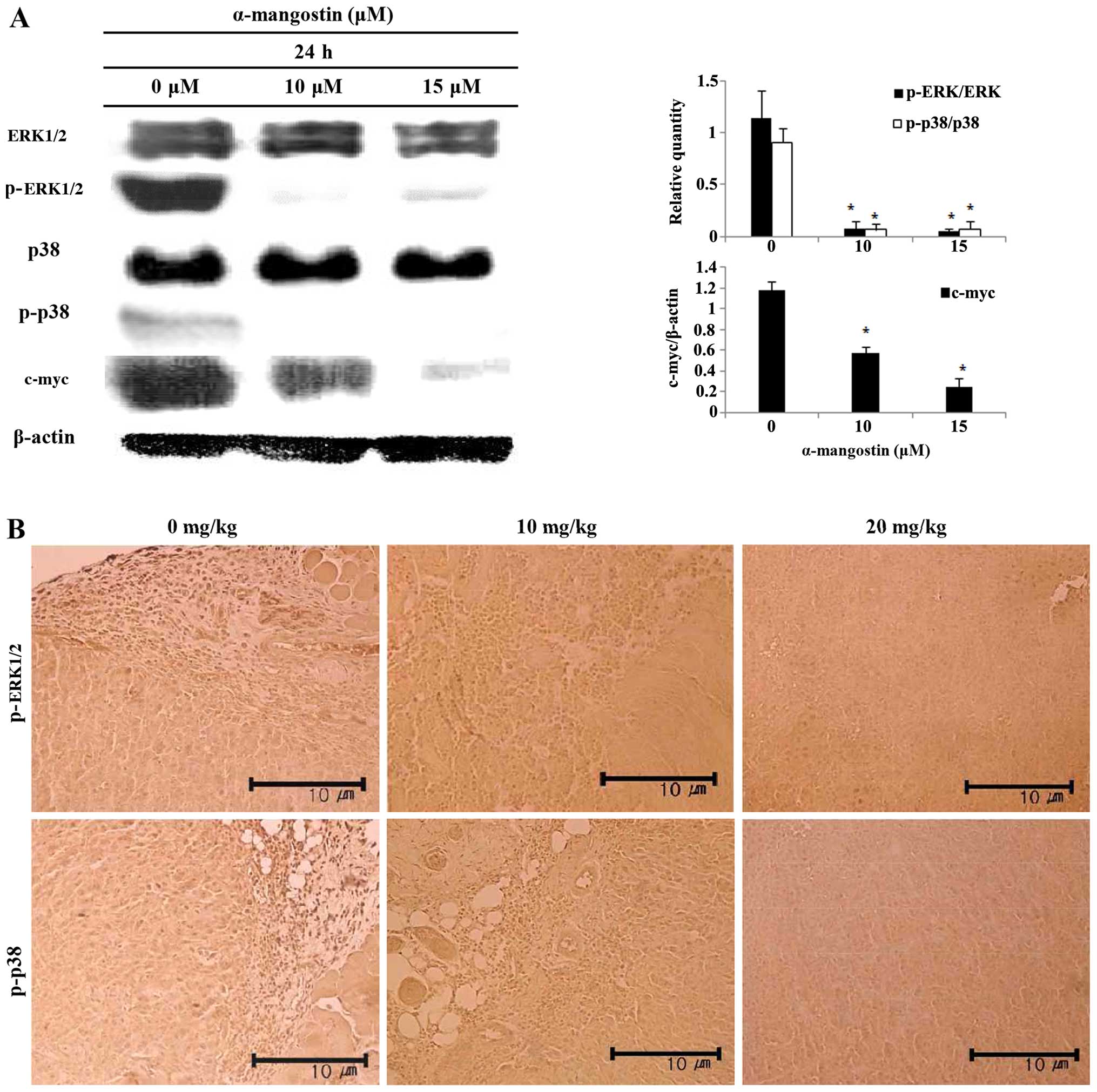
Effects of α-mangostin on ERK1/2 MAPK and
p38 MAPK expression
The levels of ERK1/2, p38 and c-myc were examined by
western blot analysis in order to determine whether the induction
of apoptosis by α-mangostin involves the MAPK pathway. The
inhibition of ERK1/2 and p38 activation, and decreased c-myc
expression were observed in the cells treated with α-mangostin in a
concentration-dependent manner (Fig.
6A). The levels of p-ERK1/2 and p-p38 were determined by
immunohistochemical analysis to determine whether the effects of
α-mangostin involve the MAPK pathway in tumor tissue from mice with
tumor xenografts (Fig. 6B). The
tumor tissue of the mice treated with α-mangostin exhibited
decreased levels of p-ERK1/2 and p-p38 in a concentration-dependent
manner.
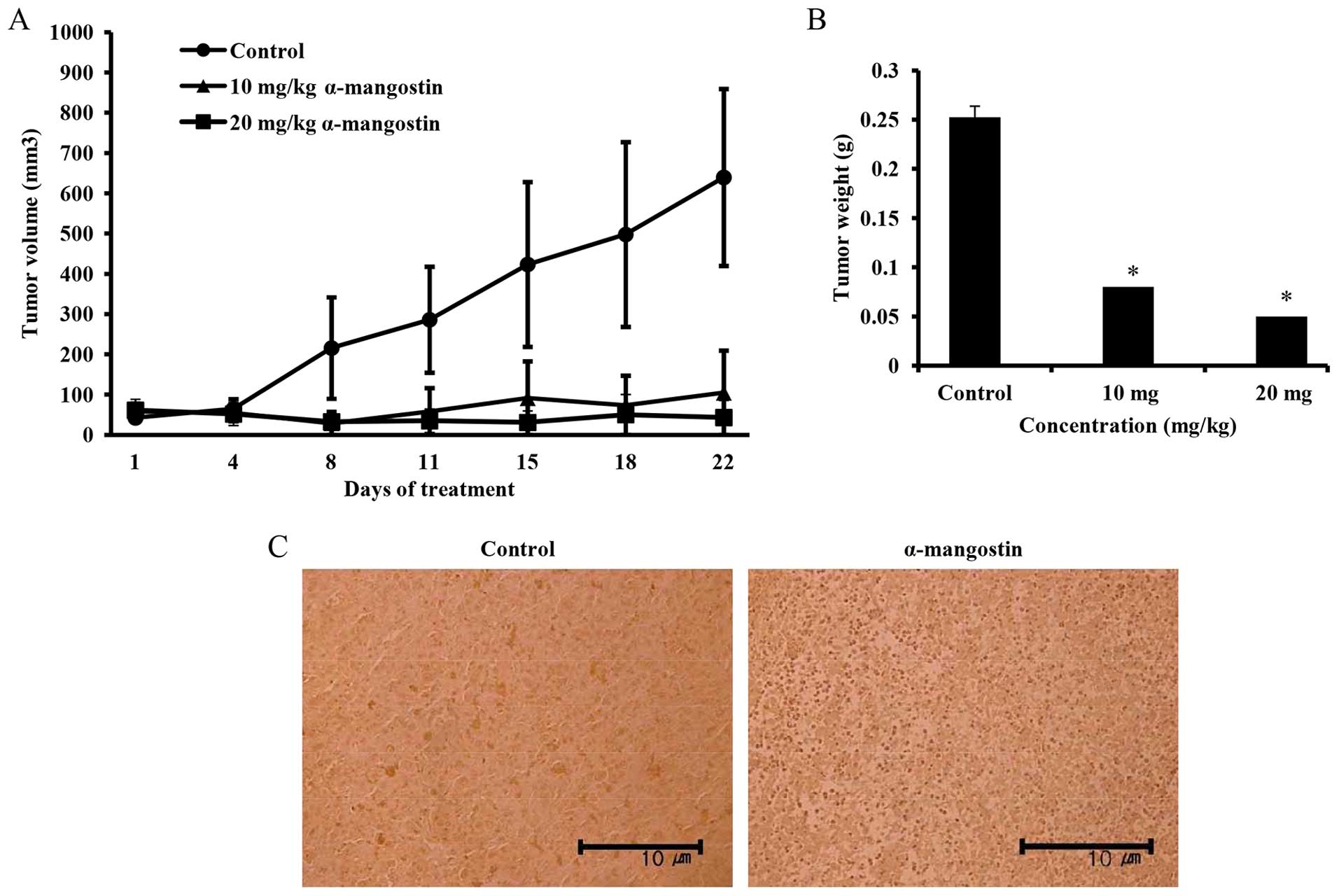
Effects of α-mangostin on mouse tumor
xenografts
The effects of α-mangostin on tumor volume in mice
with YD-15 tumor xenografts were investigated. α-mangostin was
administered by intraperitoneal injection at the dose of 10 mg/kg
(low-dose) and 20 mg/kg (high-dose). The mice in the control group
received the vehicle (0.5% DMSO in PBS) with the same timing and
dosing schedule used for the treatment group. A significant
difference in tumor volume emerged in the control group from day 8
onwards following treatment. The mice treated with 20 mg/kg
α-mangostin exhibited a tumor volume inhibition rate of 89.9%
compared with the control group (Fig.
7A). The sizes of the final tumors in the control and
α-mangostin-treated (10 and 20 mg/kg body weight) mice were 639,
105 and 43 mm3, respectively. Tumor weights in the
experimental nude mice were also determined to be 0.25, 0.08 and
0.05 g in the control, and 10 and 20 mg/kg α-mangostin-treated
groups, respectively, indicating a decreasing trend in tumor weight
upon the administration of α-mangostin (Fig. 7B). A TUNEL assay was performed on
the extracted tumor tissue to examine cell apoptosis. As a result,
many apoptotic cells were confirmed in the mice treated with
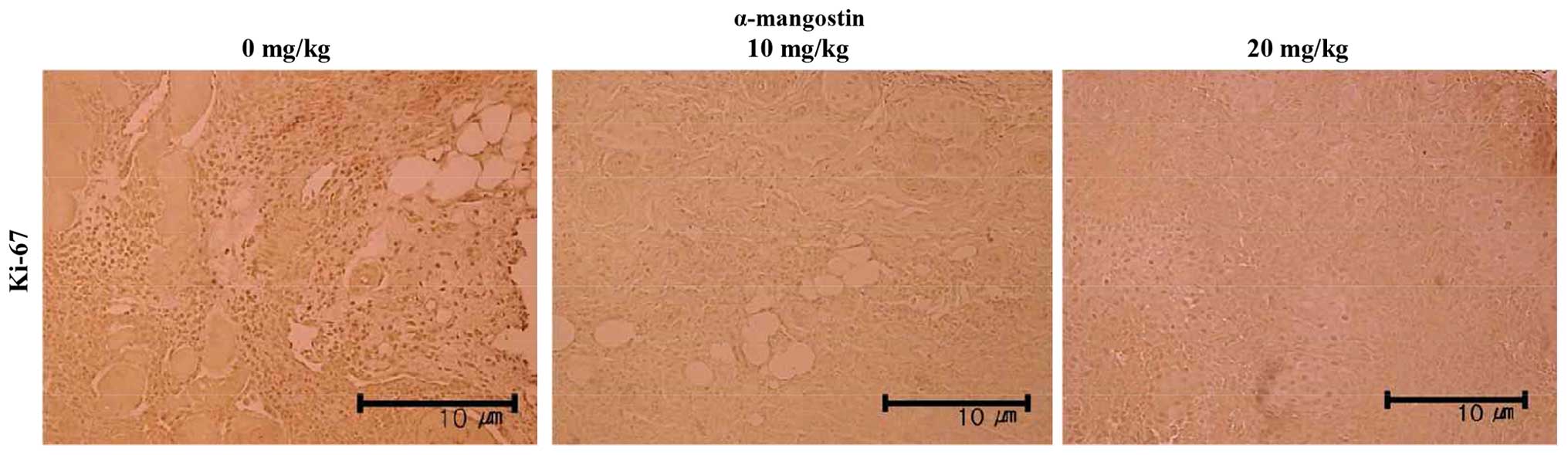
α-mangostin compared with the control group (Fig. 7C). Immunohistochemical analysis
was also performed to examine the expression of Ki-67, the protein
responsible for the rate of tumor growth (Fig. 8). The tumor samples from the mice
treated with α-mangostin exhibited a decreased Ki-67 expression in
a concentration-dependent manner, suggesting that α-mangostin
induced the apoptosis of YD-15 cells (in the tumor xenografts),
thus inhibiting tumor growth.
Discussion
Surgical therapies, radiation therapies, medications
and other current treatments for oral cancer are associated with a
low treatment efficacy and severe adverse effects; thus, in
general, oral cancer has a poor prognosis. Thus, it is important to
develop a treatment strategy that selectively destroys cancer cells
without inducing any toxic effects. In some studies, it was
reported that several naturally derived substances induce apoptosis
and inhibit cancer cell growth (21–24). α-mangostin, a naturally-derived
substance, is extracted from the pericarp of the mangosteen fruit.
α-mangostin was initially reported to be a substance that induces
the apoptosis of cancer cells, particularly that of mammary,
colorectal and liver cancer cells (16,18,19); however, the mechanisms responsible
for the apoptosis induced by α-mangostin in oral cancer cells
remain unknown.
In this study, an MTT assay was performed to confirm
the inhibitory effects of α-mangostin on cell viability (Fig. 2A). The YD-15 cells treated with
α-mangostin exhibited a concentration-dependent decrease in
viability; at a concentration of 15 µM α-mangostin, there
was an approximate inhibition rate of 58%. According to a previous
study by Shibata et al (19), proliferation was significantly
decreased in BJMC3879luc2 cells treated with 12 µM
α-mangostin for 24 and 48 h. In another study by Hsieh et al
(16), an inhibition rate of 50%
was observed in SK-Hep-1 cells treated with 24.8 µM
α-mangostin for 24 h. The cells treated with 19.6 µM
α-mangostin for 48 h also exhibited an inhibition rate of 50%
(16). The findings of these
other studies were similar to those from our study, in which
α-mangostin decreased the viability of YD-15 tongue cancer cells in
a concentration-dependent manner; therefore, α-mangostin is
believed to effectively inhibit cancer cell proliferation.
Apoptosis, otherwise known as programmed cell death,
is characterized by a number of well-defined features, such as
condensation and fragmentation of chromatin, internucleosomal DNA
cleavage, caspase activation and the translocation of
phosphatidylserine from the inner to the outer leaflet of the
plasma membrane (25). In the
present study, these morphological changes were further examined by
DAPI staining, in order to examine the inhibitory effects of
α-mangostin on YD-15 cell viability. Based on DAPI staining, the
number of apoptotic bodies in the cells treated with α-mangostin
increased in a concentration-dependent manner compared to the
control group (Fig. 2B). Thus,
the distinct characteristics of apoptosis (i.e., shrinking of the
cytoplasm, chromosomal condensation and apoptotic body formation)
were observed.
The apoptotic cells were analyzed quantitatively by
flow cytometry to determine whether the morphological changes
observed in the DAPI-stained chromosomes were caused by apoptosis.
In addition, the cell cycle was analyzed to examine the inhibitory
effects of α-mangostin on cell cycle progression. The apoptotic
cells were first analyzed by Annexin V FITC/PI staining. The
percentage of apoptotic cells was 16.06% in the control group and
increased to 28.1 and 33.65% in the groups treated with 10 and 15
µM α-mangostin, respectively (Fig. 3A). Li et al (26) previously examined apoptotic MCF-7
and MDA-MB-231 cells treated with 0, 1, 2 and 4 µM
α-mangostin for 24 h; they detected 4.19, 5.42, 8.89 and 27.96%
apoptotic cells among the MCF-7 cells and 6.69, 7.97, 11.42 and
42.34% among the MDA-MB-231 cells, respectively. Moreover, the
observed increases were concentration-dependent. Based on the
findings of previous studies and those of our study, we concluded
that α-mangostin induced cancer cell apoptosis in a
concentration-dependent manner.
From the perspective of cell proliferation, cancer
cells can be defined as being resistant to cell cycle control
(27). Thus, as regards the
development of particular anticancer drugs and preventive
medications, the extent that a substance affects cancer cell cycle
progression should be identified. In general, if apoptosis is
induced in vitro and in vivo, an increase in the
sub-G1 population is observed along with DNA fragmentation
(28,29). In other words, an increase in the
proportion of sub-G1 cells reflects an increase in apoptosis. In
the present study, flow cytometry was used to analyze the cell
cycle to examine the inhibitory effects of α-mangostin on different
cell cycle phases. No significant differences were observed in the
distribution of G1, S or G2/M cells, whereas the sub-G1 cell
populations in the control group and groups treated with 10 and 15
µM α-mangostin were 0.28, 1.38 and 6.08%, respectively
(Fig. 3B). Based on these
results, we concluded that the inhibitory effects of α-mangostin on
cell proliferation, particularly on the sub-G1 cell cycle arrest,
were induced by apoptosis.
Apoptosis occurs via an organic reaction of various
proteins controlled by internal/external cellular pathways. Bcl-2
family proteins control membrane permeability and are located in
the mitochondrial membrane or move to the mitochondrial membrane to
induce apoptotic cell death (30). Bax and Bad are pro-apoptotic
factors that promote apoptosis, whereas Bcl-2 is an anti-apoptotic
factor (31,32). In this study, the expression
levels of Bax and Bcl-2 were measured by western blot analysis,
which revealed that the expression of the pro-apoptotic factor,
Bax, was increased and the expression of Bcl-2 was decreased in the
α-mangostin-treated cells in a concentration-dependent manner
(Fig. 4). This result implied
that α-mangostin increased Bax expression in the YD-15 cells and
decreased Bcl-2, ultimately leading to apoptosis.
Caspases are key factors that control apoptosis and
are involved in a common pathway of various apoptotic signals.
Caspases are further classified into initiator and effector
caspases. Initiator caspases are activated by death signals to
further activate the effector caspases (33). Caspase-3, which is activated by
caspase-9, can cleave proteins involved in damaged DNA recovery or
PARP. Caspase activation and PARP cleavage are typical
characteristics of apoptosis (34). In this study, the levels of
caspase-9 (initiator caspase), caspase-3 (effector caspase) and
PARP were measured by western blot analysis in order to confirm the
effects of α-mangostin on caspase activity in YD-15 cells. Both
caspase-3 and -9 were activated, as evidenced by the increased
levels of the respective cleaved forms, as well as PARP
segmentation (Fig. 5A). The
results of immunohistochemical analysis of cleaved caspase-3 were
similar to those of western blot analysis (Fig. 5B). Considering these results, it
appears that caspase plays a significant role in the apoptosis
induced by α-mangostin in YD-15 tongue carcinoma cells, and the
activation of caspase-9 and caspase-3 leads to PARP
segmentation.
The MAPK signaling pathway is a core factor
controlling various pathways, including cell growth, proliferation,
segmentation and apoptosis. One of the key players in the MAPK
pathway, ERK1/2, is activated by growth factors, such as those that
promote apoptosis and further control cell growth, survival and
division. Another key component of the MAPK pathway, p38 MAPK, is
activated by chemical and environmental stresses and inflammatory
factors that affect cellular levels (35). ERK1/2 and p38 activation through
various pathways results in their translocation to the nucleus,
where they function as transcription factors for early response
proteins, such as c-myc and c-jun (36). Among the genes activated by the
ERK1/2 signaling pathway, c-myc plays a major role in tumorigenesis
(37). The c-myc protein level is
strictly regulated by ERK1/2 through post-translational mechanisms
(38). In this study, the levels
of ERK1/2, p38 and c-myc were investigated by western blot analysis
in order to verify the involvement of the ERK1/2 and p38 MAPK
pathways in the apoptosis induced by α-mangostin. We determined
that both ERK1/2 and p38 were deactivated via reduced
phosphorylation. The expression of c-myc also decreased. Based on
immunohistochemical analysis, the levels of p-ERK1/2 and p-p38 were
found to decrease in a concentration-dependent manner (Fig. 6). According to a previous study,
no significant differences were observed with respect to p-ERK1/2
and p-JNK1/2 activation in SK-Hep-1 cells treated with 10, 20 and
30 µM α-mangostin for 24 h, compared with the control group,
whereas p-p38 decreased in a concentration-dependent manner
(16). These results confirmed
that the inhibition of p38 MAPK played a crucial role in apoptosis
induced by α-mangostin in cancer cells (16). Moreover, in another study, no
significant changes were observed in SW1353 cells treated with 20
µg/ml α-mangostin for 0, 3 and 6 h with respect to p-p38
activation, whereas p-ERK1/2 tended to increase after 3 h and
decrease after 6 h, along with p-JNK. Thus, the inhibition of
p-ERK1/2 was associated with the apoptosis induced by α-mangostin
(39). Taking into consideration
the findings of previous studies, as well as those from our study,
we hypothesized that α-mangostin inhibited the activation of ERK1/2
and p38 MAPK signaling pathways, which further inhibited the
expression of the c-myc oncogene.
In this study, YD-15 cells were also administered to
nude mice to confirm the in vivo anticancer efficacy of
α-mangostin. Mice were divided into 3 groups: the control
(vehicle-treated) and the 10 and 20 mg/kg α-mangostin treatment
groups. α-mangostin was administered intraperitoneally 5
times/week. A marked difference between the treated and control
groups was observed beginning on day 8 following treatment
(Fig. 7A). On day 22, tumors in
the 20 mg/kg α-mangostin treatment group exhibited an inhibition
rate of 89.9%. A TUNEL assay was performed on tumors extracted from
the experimental nude mice, which revealed a significant increase
in the expression of TUNEL-positive cells in the
α-mangostin-treated group (Fig.
7C).
A Ki-67 antibody was used to differentiate nuclei in
proliferating cells (G1, S, G2 and M phases) from those in resting
cells, as previously described (40). Immunohistochemical analysis
confirmed the expression of Ki-67, a protein used to determine the
rate of proliferation of cancer cells, and demonstrated that its
expression was decreased in the α-mangostin-treated mice (Fig. 8). According to these results,
α-mangostin induced the apoptosis of YD-15 cells and inhibited cell
proliferation.
In conclusion, in the present study, we demonstrated
that treatment with α-mangostin leads to cellular apoptosis through
the inhibition of ERK1/2 and p38 MAPK signaling in YD-15 tongue
carcinoma cells, indicating the potential use of of α-mangostin as
an anticancer treatment, particularly for the treatment of oral
cancer.
Acknowledgments
This study was supported by a research grant of the
Kongju National University in 2014.
References
|
1
|
Jung KW, Won YJ, Kong HJ, Oh CM, Cho HS,
Lee DH and Lee KH: Cancer statistics in Korea: incidence,
mortality, survival, and prevalence in 2012. Cancer Res Treat.
47:127–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neville BW and Day TA: Oral cancer and
precancerous lesions. CA Cancer J Clin. 52:195–215. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silverman S: Early diagnosis of oral
cancer. Cancer. 62:1796–1799. 1998. View Article : Google Scholar
|
|
4
|
Kim MY, Kim CS, Lee SH, Kim JW and Jang
HJ: A clinicostatistical analysis of oral cancer patients for
recent 8 years. J Korean Assoc Oral Maxillofac Surg. 33:660–668.
2007.
|
|
5
|
Lee EJ, Kim MJ and Myoung H: Change of the
invasiveness with selective Cox-2 inhibition in an oral squamous
cell carcinoma cell Line, KB: preliminary in vitro study. J Korean
Assoc Oral Maxillofac Surg. 33:103–108. 2007.
|
|
6
|
Mahabusarakam W, Wiriyachitra P and Taylor
WC: Chemical constituents of Garcinia mangostana. J Nat Prod.
50:474–478. 1987. View Article : Google Scholar
|
|
7
|
Jung HA, Su BN, Keller WJ, Mehta RG and
Kinghorn AD: Antioxidant xanthones from the pericarp of Garcinia
mangostana (Mangosteen). J Agric Food Chem. 54:2077–2082. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akao Y, Nakagawa Y, Iinuma M and Nozawa Y:
Anti-cancer effects of xanthones from pericarps of mangosteen. Int
J Mol Sci. 9:355–370. 2008. View Article : Google Scholar
|
|
9
|
Iinuma M, Tosa H, Tanaka T, Asai F,
Kobayashi Y, Shimano R and Miyauchi K: Antibacterial activity of
xanthones from guttiferaeous plants against methicillin-resistant
Staphylococcus aureus. J Pharm Pharmacol. 48:861–865. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sundaram BM, Gopalakrishnan C, Subramanian
S, Shankaranarayanan D and Kameswaran L: Antimicrobial activities
of Garcinia mangostana. Planta Med. 48:59–60. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen LG, Yang LL and Wang CC:
Anti-inflammatory activity of mangostins from Garcinia mangostana.
Food Chem Toxicol. 46:688–693. 2008. View Article : Google Scholar
|
|
12
|
Shan T, Ma Q, Guo K, Liu J, Li W, Wang F
and Wu E: Xanthones from mangosteen extracts as natural
chemopreventive agents: potential anticancer drugs. Curr Mol Med.
11:666–677. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang DJ, Dai Z and Li YJ: Pharmacological
effects of xanthones as cardiovascular protective agents.
Cardiovasc Drug Rev. 22:91–102. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakatani K, Yamakuni T, Kondo N, Arakawa
T, Oosawa K, Shimura S, Inoue H and Ohizumi Y: γ-Mangostin inhibits
inhibitor-kappaB kinase activity and decreases
lipopolysaccharide-induced cyclooxygenase-2 gene expression in C6
rat glioma cells. Mol Pharmacol. 66:667–674. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pedraza-Chaverri J, Cárdenas-Rodríguez N,
Orozco-Ibarra M and Pérez-Rojas JM: Medicinal properties of
mangosteen (Garcinia mangostana). Food Chem Toxicol. 46:3227–3239.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsieh SC, Huang MH, Cheng CW, Hung JH,
Yang SF and Hsieh YH: α-Mangostin induces mitochondrial dependent
apoptosis in human hepatoma SK-Hep-1 cells through inhibition of
p38 MAPK pathway. Apoptosis. 18:1548–1560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnson JJ, Petiwala SM, Syed DN,
Rasmussen JT, Adhami VM, Siddiqui IA, Kohl AM and Mukhtar H:
α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle
arrest in prostate cancer and decreases xenograft tumor growth.
Carcinogenesis. 33:413–419. 2012. View Article : Google Scholar :
|
|
18
|
Watanapokasin R, Jarinthanan F, Nakamura
Y, Sawasjirakij N, Jaratrungtawee A and Suksamrarn S: Effects of
α-mangostin on apoptosis induction of human colon cancer. World J
Gastroenterol. 17:2086–2095. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibata MA, Iinuma M, Morimoto J, Kurose
H, Akamatsu K, Okuno Y, Akao Y and Otsuki Y: α-Mangostin extracted
from the pericarp of the mangosteen (Garcinia mangostana Linn)
reduces tumor growth and lymph node metastasis in an
immunocompetent xenograft model of metastatic mammary cancer
carrying a p53 mutation. BMC Med. 9:692011. View Article : Google Scholar
|
|
20
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Surh YJ, Hurh YJ, Kang JY, Lee E, Kong G
and Lee SJ: Resveratrol, an antioxidant present in red wine,
induces apoptosis in human promyelocytic leukemia (HL-60) cells.
Cancer Lett. 140:1–10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aisa Y, Miyakawa Y, Nakazato T, Shibata H,
Saito K, Ikeda Y and Kizaki M: Fucoidan induces apoptosis of human
HS-sultan cells accompanied by activation of caspase-3 and
down-regulation of ERK pathways. Am J Hematol. 78:7–14. 2005.
View Article : Google Scholar
|
|
23
|
Lazzè MC, Savio M, Pizzala R, Cazzalini O,
Perucca P, Scovassi AI, Stivala LA and Bianchi L: Anthocyanins
induce cell cycle perturbations and apoptosis in different human
cell lines. Carcinogenesis. 25:1427–1433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shim HY, Park JH, Paik HD, Nah SY, Kim
DSHL and Han YS: Acacetin-induced apoptosis of human breast cancer
MCF-7 cells involves caspase cascade, mitochondria-mediated death
signaling and SAPK/JNK1/2-c-Jun activation. Mol Cells. 24:95–104.
2007.PubMed/NCBI
|
|
25
|
Talib WH and Mahasneh AM:
Antiproliferative activity of plant extracts used against cancer in
traditional medicine. Sci Pharm. 78:33–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li P, Tian W and Ma X: Alpha-mangostin
inhibits intracellular fatty acid synthase and induces apoptosis in
breast cancer cells. Mol Cancer. 13:1382014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Korystov YN, Mosin VA, Shaposhnikova VV,
Levitman MK, Kudryavtsev AA, Kruglyak EB, Sterlina TS, Vik-torov AV
and Drinyaev VA: A comparative study of effects of aversectin C
abamectin and ivermectin on apoptosis of rat thymo-cytes induced by
radiation and dexamethasone. Acta Vet Brno. 68:23–29. 1999.
View Article : Google Scholar
|
|
29
|
Badran A, Iwasaki H, Inoue H and Ueda T:
Atypical nuclear apoptosis downstream to caspase-3 activation in
ara-C treated CCRF-CEM cells. Int J Oncol. 22:517–522.
2003.PubMed/NCBI
|
|
30
|
Willis S, Day CL, Hinds MG and Huang DC:
The Bcl-2-regulated apoptotic pathway. J Cell Sci. 116:4053–4056.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chiarugi V, Magnelli L, Cinelli M and Basi
G: Apoptosis and the cell cycle. Cell Mol Biol Res. 40:603–612.
1994.PubMed/NCBI
|
|
32
|
Donovan M and Cotter TG: Control of
mitochondrial integrity by Bcl-2 family members and
caspase-independent cell death. Biochim Biophys Acta. 1644:133–147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bao Q and Shi Y: Apoptosome: a platform
for the activation of initiator caspases. Cell Death Differ.
14:56–65. 2007. View Article : Google Scholar
|
|
34
|
Galluzzi L, Kepp O, Trojel-Hansen C and
Kroemer G: Mitochondrial control of cellular life, stress, and
death. Circ Res. 111:1198–1207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chuang SM, Wang IC and Yang JL: Roles of
JNK, p38 and ERK mitogen-activated protein kinases in the growth
inhibition and apoptosis induced by cadmium. Carcinogenesis.
21:1423–1432. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bassi R, Heads R, Marber MS and Clark JE:
Targeting p38-MAPK in the ischaemic heart: kill or cure? Curr Opin
Pharmacol. 8:141–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hatano K, Yamaguchi S, Nimura K, Murakami
K, Nagahara A, Fujita K, Uemura M, Nakai Y, Tsuchiya M, Nakayama M,
et al: Residual prostate cancer cells after docetaxel therapy
increase the tumorigenic potential via constitutive signaling of
CXCR4, ERK1/2 and c-Myc. Mol Cancer Res. 11:1088–1100. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sears R, Nuckolls F, Haura E, Taya Y,
Tamai K and Nevins JR: Multiple Ras-dependent phosphorylation
pathways regulate Myc protein stability. Genes Dev. 14:2501–2514.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krajarng A, Nakamura Y, Suksamrarn S and
Watanapokasin R: α-Mangostin induces apoptosis in human
chondrosarcoma cells through downregulation of ERK/JNK and Akt
signaling pathway. J Agric Food Chem. 59:5746–5754. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gerdes J, Lemke H, Baisch H, Wacker HH,
Schwab U and Stein H: Cell cycle analysis of a cell
proliferation-associated human nuclear antigen defined by the
monoclonal antibody Ki-67. J Immunol. 133:1710–1715.
1984.PubMed/NCBI
|