Introduction
Myocardial ischemia-reperfusion (IR) injury
typically occurs when patients are experiencing an ST segment
elevation myocardial infarction (MI) (1). Thrombolytic therapy or primary
percutaneous coronary intervention may be the optimal method to
reduce acute myocardial ischemic injury and limit the size of the
MI (2). However, further
cardiomyocyte death may arise in the process of myocardial
reperfusion, which is termed myocardial IR injury (3). The four recognized forms of
myocardial IR include reversible forms (reperfusion-induced
arrhythmias and myocardial stunning) and irreversible forms
(microvascular obstruction and lethal myocardial reperfusion
injury) (4,5). Several critical factors have been
identified by experimental investigations as potential mediators of
the detrimental effects of myocardial IR, including oxidative
stress, inflammation, intracellular Ca2+ overload, rapid
restoration of physiological pH at the time of reperfusion,
mitochondrial permeability transition pore, and late myocardial
reperfusion injury (6-9). In addition to improvements in
earlier reperfusion and advancements in percutaneous coronary
intervention technology, there are also emerging therapeutic
strategies, including ischemic preconditioning or postconditioning,
remote ischemic preconditioning, therapeutic hyperoxemia and
hypothermia, and pharmacologic agents for preventing myocardial IR
injury (10). Previously, Fan and
Yang suggested that microRNAs (miRs) may be potential therapeutic
targets in IR injury by altering key signaling elements (11).
miRs, a class of endogenous RNAs of ~22 nucleotides
in length, can inhibit the translation of target mRNAs by pairing
to sites in the 3′ untranslated region (3′-UTR) (12,13). He et al detected miR
expression in a myocardial IR model in Sprague-Dawley (SD) rats
following reperfusion and found that miRs had an effect on
myocardial IR (14). It has been
shown that miR-20b is involved in cardiac remodeling and
antagomiR-20b reduces IR-induced vascular endothelial growth factor
(15). Circulating levels of
miR-20b have also been found to be useful as a novel diagnostic
indicator in hypertension-induced heart failure (16). miR-20b-5p has been reported to be
dysregulated in blood malignancies (17). It is known that miR expression is
modulated by small mother against decapentaplegic (Smad)s through
transcriptional and post-transcriptional mechanisms, and miRs
appear to have an important effect on the physiological activity of
transforming growth factor (TGF)-β signaling (18). Smad7 belongs to the third type of
Smad, the inhibitory Smads, with Smad6, however, Smad7 is more
potent than Smad6 at inhibiting TGF-β signaling (19). A previous study demonstrated that
Smad7 has important functions in maintaining cellular homeostasis
in terms of anti-inflammatory and antifibrotic activity under
physiological conditions (20).
Therefore, the present study aimed to investigate whether
miR-20b-5p promotes ventricular remodeling following myocardial IR
injury in rats via inhibiting the expression of Smad7 through
activating the TGF-β/Smad signaling pathway.
Materials and methods
Ethics statement
The present study was approved by the Laboratory
Animal Ethics Committee of The First Affiliated Hospital of Harbin
Medical University (Harbin, China), and was performed in accordance
with the principles of animal protection, welfare and ethics and
the National Laboratory Animal Welfare Ethics regulations.
Dual luciferase reporter gene assay
A search was performed at www.microRNA.org to analyze and predict the target
genes of miR-20b-5p, and the fragment containing the binding sites
was obtained. In strict accordance with the instructions of the
TIANamp Genomic DNA kit (Tiangen Biotech Co., Ltd., Beijing,
China), DNA was extracted from 293T cells (Wuhan Cell Bank, Wuhan,
China). Smad7-3′-UTR-wild-type (wt) and Smad7-3′-UTR-mutant (mut)
without the miR-20b-5p binding site were designed. The recombinant
luciferase reporter vector was constructed to transfect the 293T
cells with the miR-20b-5p mimics and miR-20b-5p inhibitors, and
luciferase activity was measured with a dual luciferase reporter
assay system (Promega Corporation, Madison, WI, USA). Following
transfection for 48 h, the growth medium was removed from the
cultured cells, which were washed twice with PBS. Passive lysis
buffer (100 µl) was added to each well containing the cells,
and the plates were gently rocked at room temperature for 15 min to
collect cell lysates. Subsequently, the program was set to pre-read
the sample for 2 sec and to read its value for 10 sec following
each injection of 100 µl LARIIStop&Glo®
reagent (Promega Corporation). The prepared
LARIIStop&Glo® reagent was added into luminous tubes
or wells containing lysate (20 µl of each sample). Signals
in the luminous tubes or wells were detected with a Modulus™ single
tube multimode reader (TumerBioSystems, Sunnyvale, CA, USA).
Firefly luciferase (LUC) activity and Renilla luciferase (RL)
activity were detected. RL activity reflected the transfection
efficiency of each well, and the ratio of LUC activity to RL
activity (LUC/RL) was calculated as the relative luciferase
expression.
Model generation
A total of 70 healthy male SD rats (250-300 g) of
similar age (7-8 weeks) were purchased from the Experimental Animal
Center of the Military Medical Science Academy of the PLA (Beijing,
China). The rats were fed normal feed and had free access to water
at 20-25°C, and a 12-h light/dark cycle at 22°C, and there was a
72-h adaptation period prior to the experiment. A total of 60 SD
rats were randomly selected to generate a model of IR. Prior to
surgery, the rats were anesthetized with 10% pentobarbital sodium
(90 mg/kg, cat. no. wS20060401, Shanghai Westang Biotechnology Co.,
Ltd., Shanghai, China). Electrocardiographic (ECG) monitoring
electrodes (BeneHearth R3; Mindray, Wuhan, China) were connected to
the rat limbs, and the animals were ventilated with an animal
respirator (R407; RWD Biotech Co., Ltd., Shenzhen, China). The gas
source was room air, the ventilator frequency was 60 breaths/min,
and the tidal volume was 13-15 ml/kg. The heart was exposed via a
left thoracotomy through the fourth intercostal space, and the left
anterior descending (LAD) coronary artery was ligated with a 6/0
atraumatic suture. ST-segment elevation by ECG monitoring indicated
that the vessel was successfully occluded. Following occlusion for
60 min, the coronary artery was recanalized by suture removal, and
ST-segment resolution indicated that the rat model of IR injury was
successfully established.
Animal grouping
The 10 rats that did not undergo IR injury were used
as the sham group. The rats in the sham group were ligated with a
6/0 atraumatic suture in the LAD coronary artery following
thoracotomy without blocking the vessels. The modeled rats were
randomly divided into six groups (n=10 in each group) as follows:
i) IR group: Left coronary artery was ligated and blocked with a
6/0 atraumatic suture; 60 min later, the vessels were opened; ii)
negative control (NC) group: Intramyocardial injection of the
negative control sequence (average of five injections, 2 µg
per injection to a total of 10 µg) was performed 24 h prior
to occlusion of blood vessels, and the remaining procedures were
performed at the same time as in the IR group; iii) miR-20b-5p
mimics group: Intramyocardial injection of miR-20b-5p mimics
(average of five injections, 2 µg per injection to a total
of 10 µg) was performed 24 h prior to occlusion of blood
vessels, and the remaining procedures were conducted at the same
time as in the IR group; iv) miR-20b-5p inhibitors group:
Intramyocardial injection of miR-20b-5p inhibitors (average of five
injections, 2 µg per injection to a total of 10 µg)
was performed 24 h prior to occlusion of blood vessels, and the
remaining procedures were performed at the same time as in the IR
group; v) siRNA-Smad7 (si-Smad7) group: Intramyocardial injection
of si-Smad7 (average of five injections, 2 µg per injection
to a total of 10 µg) was performed 24 h prior to occlusion
of blood vessels, and the remaining procedures were performed at
the same time as in the IR group; vi) miR-20b-5p inhibitors +
si-Smad7 group: Intramyocardial injection of miR-20b-5p inhibitors
and si-Smad7 (average of five injections, 2 µg per injection
to a total of 10 µg) was performed 24 h prior to occlusion
of blood vessels, and the remaining procedures were performed at
the same time as in the IR group. All injection sequences were
purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The
mortality rate of the rats was ~5.9% during the surgical
procedure.
Transthoracic echocardiography
At 28 days post-surgery, the mortality rate of the
rats was ~10%. In accordance with a random number table, five rats
in each group were randomly selected to be anaesthetized with an
intraperitoneal injection of 10% pentobarbital sodium (90 mg/kg,
cat. no. wS20060401, Shanghai Westang Biotechnology Co., Ltd.) and
confirmed with transthoracic echocardiography. Acuson Sequoia 512
color Doppler ultrasonography was performed by professionals;
ultrasonography was performed with a 6C2-S probe at a frequency of
8.5 mHz, and the scanning speed was adjusted to 100 mm/sec. The
detection methods were as follows: Rats were anesthetized with 10%
pentobarbital sodium (90 mg/kg,) and fixed on the testing platform,
following which the papillary muscle level M curve of the left
ventricular long axis and left ventricular short axis were
measured. Left ventricular diastolic diameter (LVEDD; mm), left
ventricular systolic diameter (LVESD; mm), left ventricular
end-diastolic volume (LVEDV) and left ventricular end-systolic
volume (LVESV; µl) were measured continuously in three
cardiac cycles, and the mean value was calculated. According to
Simpson's method, the left ventricular ejection fraction (LVEEF)
was calculated using the following formula: (LVEDV-LVESV)/LVEDV
×100%, and the left ventricular fractional shortening (LVEFS) was
measured using the following formula: (LVEDD-LVESD)/LVEDD ×100%
(21). One end of the catheter
was connected to a pressure sensor, and the other end of the
catheter was inserted into the left ventricle through the right
common carotid artery to measure the left ventricular systolic
pressure (LVESP; mmHg) and the left ventricular end-diastolic
pressure (LVEDP; mmHg). In addition, rat cardiac function was
evaluated by analyzing the association between the above indicators
and cardiac function.
Triphenyltetrazolium chloride (TTC)
staining
Following confirmation with transthoracic ECG, the
heart was isolated for gross examination. The necrotized myocardium
was stained with TTC and visualized under a transmission electron
microscope (TEM). The left ventricular wall was separated into
several sections at a thickness of 5-mm from the apex to the bottom
of the heart, perpendicular to the long axis of the left ventricle;
these sections were subjected to TTC staining, TEM, hematoxylin and
eosin (HE) staining, Masson's staining, immunohistochemistry and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analysis. The apical tissue of the left ventricular wall
was randomly selected from three rats in each group. Ultrathin
sections (50-60 nm) were cut using a vibratome along the long axis
of left ventricle. The myocardium of the left ventricular wall was
stained with TTC, and the ultrastructure was observed. The sections
were stained in 1% TTC phosphate-buffered solution (CAS no.
2530-85-0; Guidechem Shanghai, China). The infarcted myocardium was
off-white, and the surviving myocardium was a normal color. The
infarcted myocardium and the surviving myocardium were separated.
Images of the sections were captured using a camera (LEICA digital
camera 480; Leica Microsystems GmbH, Wetzlar, Germany), and
myocardial infarct size was measured using ImageJ 1.26 image
analysis software (National Institutes of Health, Bethesda, MD,
USA). Myocardial infarct size (mm2) was calculated as
myocardial infarction area (mm2)/left ventricular total
area (mm2) ×100% (22,23).
TEM
Samples were prepared using a conventional TEM
sample preparation technique. The myocardial tissues situated 5 mm
above the apex of the left ventricular wall were randomly selected
from three rats in each group and were cut into small sections of
~1 mm3. These sections were fixed in 4% polyformaldehyde
solution (CAS no. 30525-89-4; Nanjing Guochen Chemicals Co., Ltd.,
Nanjing, China) for 2 h at 4°C, washed in 0.1 mol/l PBS, and fixed
in 1% osmic acid at room temperature (CAS: 20816-12-0, Shanghai
Fusheng Industrial Co., Ltd., Shanghai, China) for 2 h, followed by
standard procedures of dehydration in an ethanol gradient (30% for
5 min, 50% for 5 min, 70% for 10 min, 80% for 15 min, 95% for 15
min, and 100% for 15 min for twice) at room temperature. The
dehydrated myocardial sections were permeabilized, embedded in
epoxy resin (cat. no. NPES-301, Yueyang Liqiang Chemical Co., Ltd.,
Yueyang, China) and cut into ultra-thin sections (50-60 nm) with a
microtome (Leica EM UC7; Leica Microsystems GmbH). Following
staining with uranyl acetate (CAS no. 6159-44-0; Hubei Chushengwei
Chemistry Co., Ltd., Hubei, China) and citrate (CAS no. 512-26-5;
Shanghai Jinjinle Industrial Co., Ltd., Shanghai, China), the
sections were observed with TEM (JSM-840A SEM; JEOL, Ltd., Tokyo,
Japan), and the ultrastructure was recorded by image capture.
HE staining
Myocardial tissue sited 10 mm above the apex of the
left ventricular wall was randomly selected from three rats in each
group, fixed in 4% formaldehyde (volume percentage) for 6 h at 4°C,
embedded in paraffin, and sectioned at 3-µm thickness.
Following heating at 60°C overnight, the sections were successively
dewaxed for 20 min in Xylene I (CAS no. 14936-97-1; Eykits Research
Biological Technology Co., Ltd., Shanghai, China) and Xylene II
(CAS no. 523-67-1; Yuduo Biological Technology Co., Ltd., Shanghai,
China), washed in distilled water for 5 min, and dehydrated in a
100, 95, 80 and 70% ethanol series. The sections were stained in
hematoxylin (CAS no. 474-07-7; Qingdao Jieshikang Biotechnology
Co., Ltd., Qingdao, China) at room temperature for 10 min, washed
in tap water at room temperature for 15 min, counterstained in
eosin (cat. no. RY0648, Qingdao Jieshikang Biotechnology Co., Ltd.)
at room temperature for 30 sec and washed in double distilled water
to remove the red color. The sections were then dehydrated in
alcohol, cleared in xylene, and sealed with neutral balsam. HE
staining and histopathologic examination were used to observe the
color of the myocardial tissues of the rats, the distribution range
and staining intensities. Using the morphological image analysis
system (JD801; Jieda Technology Development Co., Ltd., Nanjing,
China), different groups were selected and analyzed at ×200
magnification. Pathological changes, including necrosis and edema,
were observed in the HE-stained sections. The images were randomly
collected, and the experiment was repeated three times.
Masson's staining
Myocardial tissue sited 15 mm above the apex of the
left ventricular wall was randomly selected from three rats in each
group and fixed in 4% paraformaldehyde at 4°C for 24 h. Following
routine steps of desiccation, clearing, embedding and slicing
(3-µm), the specimens were stained with Picric acid-Sirius
red at room temperature for 30 min and inhibited by hematoxylin at
room temperature for 2 min (CAS no. PT003; Shanghai Bogoo
Biotechnology Co., Ltd., Shanghai, China). Images of the myocardial
sections were obtained under a polarized light microscope (XPT-480;
Shanghai Zhongheng, Co., Ltd., Shanghai, China) and were analyzed
with Image-Pro 6 software (Media Cybernetics Inc., Bethesda, MD,
USA). Five high-magnification fields were randomly selected in each
section, and the myocardial collagen volume fraction (CVF) was
quantitatively analyzed. Five transverse arterioles were randomly
selected in each specimen, and the perivascular collagen area
(PVCA) was measured. The CVF was calculated using the following
formula: CVF (%)=collagen area/entire area ×100%, wherein the
collagen area did not include the area surrounding the vessels. The
PVCA (%) was measured as the collagen area surrounding the
vessel/total vessel wall area ×100% (24).
Immunohistochemistry
Myocardial tissue sited 20 mm above the apex of the
left ventricular wall was randomly selected from three rats in each
group and fixed in 4% paraformaldehyde at 4°C for 24 h. Following
paraffin embedding, a microtome was used to cut 3-µm serial
sections. According to the conventional method of
immunohistochemical staining, following the elimination of
endogenous peroxidase activity in 0.3%
H2O2− methanol solution at room
temperature for 10 min, a 1:100 dilution of rabbit anti-rat Smad7
antibody (cat. no. BA1399; 1:100; Wuhan Boster Biological
Technology Co., Ltd., Wuhan, China) was added to the cardiac tissue
for 16 h at 4°C. The sections were then incubated with rabbit
anti-rat IgG (cat. no. BA1058; 1:100; Wuhan Boster Biological
Technology Co., Ltd.) antibody labeled with horseradish peroxidase
for 2 h at room temperature. At 5 min post-DAB staining (cat. no.
AR1000; Wuhan Boster Biological Technology Co., Ltd.), the sections
were observed and imaged were captured under light microscopy. The
digital images were processed by Image-Pro plus 6.0 software (Media
Cybernetics, Inc.). Five visual fields were randomly selected in
each slice under ×400 magnification, and the percentage of positive
cells in each field was then calculated. PBS, as a control for the
primary antibody, was used as the negative control, and normal
tissues were used as the positive control; yellow or brown staining
in the cytoplasm or cell membrane indicated a positive cell. Four
high-power fields (magnification, ×400) in each slice were
selected, the number of positive cells of 200 cells in each field
was calculated to obtain the percentage of the positive cells:
Positive myocardial cells/total myocardial cells. If the percentage
was >10%, the sample was considered positive (+); otherwise, it
was considered negative (−) (25). The experiment was repeated three
times, and the mean value was calculated.
RT-qPCR analysis
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was used to extract total RNA from the myocardial
tissues in each group. Ultrapure water treated with
diethylpyrocarbonate (Sangon Biotech Co., Ltd., Shanghai, China)
was used to dissolve RNA, and the absorbance at 260 and 280 nm was
detected by ND-1000 ultraviolet-visible spectrophotometry
(NanoDrop; Thermo Fisher Scientific, Inc., Wilmington, DE, USA).
Subsequently, the quality of total RNA was determined, and the RNA
concentration was adjusted. According to the kit (Fermentas; Thermo
Fisher Scientific, Inc.), reverse transcription of the extracted
RNA was completed by the two-step method. The reaction conditions
were as follows: 70°C for 10 min, ice bath for 2 min, 42°C for 60
min, 70°C for 10 min, and temporary hold at −80°C. RT-qPCR was
performed using the TaqMan probe method, the reaction system was
used in accordance with the kit instructions (Fermentas; Thermo
Fisher Scientific, Inc.), and the primer sequences are shown in
Table I. The reaction system
contains 2.0 µl cDNA template, 1.0 µl each of primer
and probe mix, 10.0 µl TaqMan® Fast Advanced
Master Mix (2X), and nuclease-free water was added up to 20
µl. The reaction conditions were as follows:
Pre-denaturation at 95°C for 30 sec; 40 cycles of denaturation at
95°C for 10 sec; annealing at 60°C for 20 sec and extension at 70°C
for 10 sec. The RT-qPCR system (Bio-Rad iQ5; Bio-Rad; Laboratories,
Inc., Hercules, CA, USA) was used for detection, U6 was used as the
internal reference for miR-20b-5p (26), and β-actin was used as the
internal reference for other target genes. The value was calculated
by the relative quantitative method, and the relative expression of
each target gene was determined with the 2−∆∆Cq method
(27) Each experiment was
repeated three times.
 | Table IReverse transcription-quantitative
polymerase chain reaction primer sequences. |
Table I
Reverse transcription-quantitative
polymerase chain reaction primer sequences.
| Gene | Primer
sequence |
|---|
| miRNA-20b-5p | F:
5′-CCTAGTAGTGCCAAAGTGCT-3′ |
| R:
5′-CCAGGAGTACTAGAAGTGATCA-3′ |
| U6 | F:
5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
| R:
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
| Smad3 | F:
5′-CATTACCATCCCCAGGTCAC-3′ |
| R:
5′-AGGCTCTACTGTGTCCAA-3′ |
| Smad7 | F:
5′-ACTCTTGTTGTCCGAATTGA-3′ |
| R:
5′-ACTCTTGTTGTCCGAATTGA-3′ |
| TGF-β1 | F:
5′-TATAGCAACAATTCCTGGCGTTAC-3′ |
| R:
5′-TGTATTCCGTCTCCTTGGTCA-3′ |
| β-actin | F:
5′-CACCCGCGAGTACAACCTTC-3′ |
| R:
5′-CCCATACCCACCATCACACC-3′ |
Western blot analysis
Myocardial tissue sited 25 mm above the apex of the
left ventricular wall was randomly selected from rats of each
group, and 1X SDS lysis buffer was added to the samples (cat. no.
P0013G; Beyotime Institute of Biotechnology, Shanghai, China),
which were homogenized at 1,006 × g at room temperature to achieve
complete cleavage. The samples were then placed on ice for 30 min
at 4°C and centrifuged at 16,099 × g at 4°C for 4 min. The
supernatant was separated and stored at −80°C. The proteins were
extracted using RIPA lysis buffer (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), and the protein concentration was measured
according to the instructions of the BCA kit (cat. no. AR0146,
Wuhan Boshide Company, Wuhan, China); and, the concentration of
each sample was adjusted to 3 µg/µl. Sample buffer
(30 µg per well) was added to the extracted protein, and the
resulting mixture was boiled for 10 min at 95°C and separated by
10% polyacrylamide gel electrophoresis. The proteins were
transferred onto a polyvinylidende difluoride membrane via a
semi-dry transfer method (cat. no. P2438, Sigma-Aldrich; Merck
KGaA). The membranes were blocked with 5% bovine serum albumin
(BSA; Fermentas; Thermo Fisher Scientific, Inc.) at room
temperature for 1 h, following which the blocking solution was
discarded, and the membrane was placed into a plastic container.
Subsequently, 5% BSA with the appropriate concentration of rat
anti-TGF-β1 primary antibody (cat. no. ab2792, Abcam, Cambridge,
MA, USA) was added at a dilution of 1:1,000, rabbit anti-Smad3
primary antibody (cat. no. ab40854, Abcam) was added at a dilution
of 1:1,000, rabbit anti-Smad7 primary antibody (cat. no. ab216428,
Abcam) was added at a dilution of 1:1,000, and rat anti-β-actin
primary antibody (cat. no. ab189146, Abcam) was added at a dilution
of 1:500. Subsequently, with the transfer side up, the samples were
placed in the refrigerator at 4°C overnight. The following day, the
samples were rinsed with 0.05% TBST (three times, 10 min each), and
the goat anti-rat IgG secondary antibody labeled with horseradish
peroxidase was added at a dilution of 1:2,000 (cat. no. ab6789,
Abcam) for incubation at 4°C for 4-6 h and then washed with TBS-T
(three times, 15 min each). The chemiluminescence reagents A and B
(Yanhui Biotechnology Co., Ltd., Shanghai, China) were mixed at a
1:1 ratio, and the mixture was evenly added to the nitrocellulose
membrane. Relative light density analysis was performed on all the
western blot bands using Quantity One 4.6 software (Bio-Rad
Laboratories, Inc.).
Statistical analysis
All the data were analyzed utilizing the Statistical
Package for the Social Sciences version 21.0 (SPSS; IBM Corps.,
Armonk, NY, USA). All experiments were repeated three times.
Continuous data are presented as the mean ± standard deviation.
Multiple groups were compared using one-way analysis of variance
with the least significant difference test. Pairwise comparisons
among multiple groups were performed with the least significant
difference t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
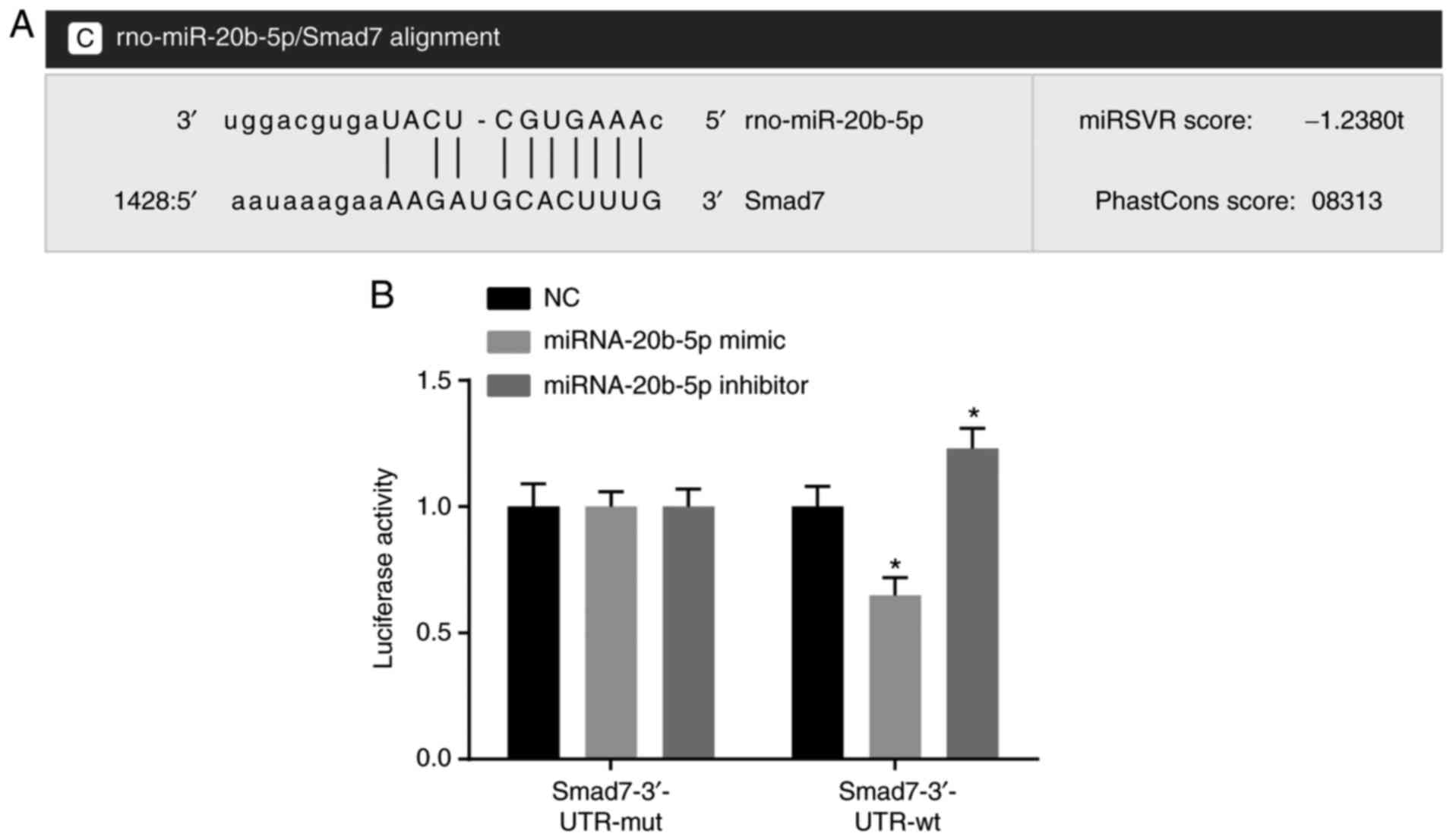
miRNA-20b-5p targets Smad
The results of the statistical analysis showed that
there was a specific binding region between the 3′UTR 1458-1464
sequence and the miR-20b-5p sequence of Smad7, and Smad7 was found
to be a potential target gene of miR-20b-5p (Fig. 1A). Luciferase reporter gene assays
were used to verify this gene as a target of miR-20b-5p (Fig. 1B), and the results indicated that
miR-20b-5p mimics had no significant effect on the luciferase
activity of the Smad7-3′-UTR-mut group (P>0.05), but
significantly decreased the luciferase activity in the
Smad7-3′-UTR-wt group (P<0.05). miR-20b-5p was found to
specifically bind to the Smad7-3′-UTR and downregulated expression
of Smad7 was observed. The effect of the miR-20b-5p inhibitor was
evaluated by dual luciferase reporter gene assays. The results
(Fig. 1B) showed that luciferase
activity was significantly increased in the Smad7-3′-UTR-wt group,
compared with that in the NC group (P<0.05), suggesting that
miR-20b-5p inhibitor upregulated the expression of Smad7. Taken
together, miR-20b-5p was found to specifically bind Smad7 and
negatively regulate the expression of Smad7.
Animal model generation
In establishing the rat model of myocardial IR
injury, the data indicated no significant change in the ST segment
of the ECG in the sham group at the preoperative, intraoperative or
postoperative stages. The ST segment of the ECG of rats of the
model group was significantly higher following ligation than
pre-ligation, and decreased following reperfusion, which indicated
that opening the chest without blocking the blood vessels had no
significant effect on the cardiac electrophysiology of the rats.
The changes in the ST segment were significant following ligation
of the anterior descending coronary artery. Following reperfusion,
cardiac electrophysiology was restored, which indicated that the
rat model of myocardial IR injury was successfully established. All
the experiments were performed in surviving rats without malignant
arrhythmia. The survival rates of the rats in each group following
28 days of modeling are shown in Table II.
 | Table IISurvival rates of rats. |
Table II
Survival rates of rats.
| Group | Pre-modeling
(n) | 28 days
post-modeling (n) | Survival rate
(%) |
|---|
| Sham | 10 | 10 | 100 |
| IR | 10 | 9 | 90 |
| NC | 10 | 9 | 90 |
| miR-20b-5p
mimics | 10 | 10 | 100 |
| miR-20b-5p
inhibitors | 10 | 8 | 80 |
| si-Smad7 | 10 | 9 | 90 |
| miR-20b-5p
inhibitors + si-Smad7 | 10 | 10 | 100 |
Increased expression of miR-20b-5p and
decreased expression of Smad7 are unfavorable for cardiac function
in IR injury model rats
Compared with the sham group, the other six groups
had significantly increased LVEDV, LVEDD, LVESV and LVESD (all
P<0.05). Compared with the IR group, the NC group and the
miR-20b-5p inhibitors + si-Smad7 group showed no significant
differences in LVEDV, LVEDD, LVESV and LVESD (all P>0.05); the
miR-20b-5p inhibitors group exhibited a significant decrease in
LVEDV, LVEDD, LVESV and LVESD (all P<0.05); and the miR-20b-5p
mimics group and the si-Smad7 group exhibited a significant
increase in LVEDV, LVEDD, LVESV and LVESD (all P<0.05). No
significant difference in these values were found between the
miR-20b-5p mimics group and the si-Smad7 group (all P>0.05).
These results are shown in Table
III.
 | Table IIIResults of LVEDV, LVEDD, LVESV and
LVESD by echocardiography (n=5). |
Table III
Results of LVEDV, LVEDD, LVESV and
LVESD by echocardiography (n=5).
| Group | LVEDV
(µl) | LVEDD (mm) | LVESV
(µl) | LVESD (mm) |
|---|
| Sham | 226.22±48.92 | 5.68±0.54 | 81.21±15.12 | 3.25±0.33 |
| IR |
738.17±92.36a | 11.37±2.10a |
266.40±11.22a | 7.83±1.00a |
| NC |
739.10±89.25a | 11.40±2.00a |
267.40±11.12a | 7.84±1.10a |
| miR-20b-5p
mimics |
939.25±111.32a,b,c | 31.40±2.00a,b,c |
366.40±11.22a,b,c | 10.83±1.00a,b,c |
| miR-20b-5p
inhibitors |
414.79±89.25a,b,c | 8.47±2.02a,b,c |
116.45±45.04a,b,c | 5.62±2.23a,b,c |
| si-Smad7 |
938.25±120.02a,b,c | 31.30±2.02a,b,c |
356.40±11.22a,b,c | 9.83±1.00a,b,c |
| miR-20b-5p
inhibitors + si-Smad7 |
733.17±110.23a | 11.39±2.00a |
270.40±11.22a | 7.90±1.00a |
Compared with the sham group, the LVEEF and LVEFS
were significantly decreased in the other six groups (all
P<0.05). Compared with the IR group, no significant differences
in LVEEF or LVEFS were found between the NC group and the
miR-20b-5p inhibitors + si-Smad7 group (P>0.05). The miR-20b-5p
inhibitors group had significantly increased LVEEF and LVEFS
(P<0.05), whereas the miR-20b-5p mimics group and the si-Smad7
group had significantly decrease in LVEEF and LVEFS (P<0.05).
There were no differences in these values between the miR-20b-5p
mimics group and the si-Smad7 group (P>0.05). These results are
presented in Table IV.
 | Table IVEchocardiograph results of LVEEF and
LVEFS (n=5). |
Table IV
Echocardiograph results of LVEEF and
LVEFS (n=5).
| Group | LVEEF (%) | LVEFS (%) |
|---|
| Sham | 79.41±4.78 | 49.65±6.19 |
| IR | 57.41±3.28a | 29.21±5.12a |
| NC | 56.51±3.22a | 28.54±4.56a |
| miR-20b-5p
mimics | 40.23±2.12a,b,c | 19.12±3.34a,b,c |
| miR-20b-5p
inhibitors | 68.23±2.34a,b,c | 37.34±4.01a,b,c |
| si-Smad7 | 39.67±2.45a,b,c | 18.17±3.96a,b,c |
| miR-20b-5p
inhibitors + si-Smad7 | 57.67±1.45a | 28.03±3.96a |
Compared with the sham group, the other six groups
exhibited a decrease in LVESP and an increase in LVEDP (all
P<0.05). Compared with the IR group, no significant differences
in LVESP or LVEDP were found between the NC group and the
miR-20b-5p inhibitors + si-Smad7 group (P>0.05). The miR-20b-5p
inhibitors group exhibited an increase in LVESP and a decrease in
LVEDP (P<0.05), and the miR-20b-5p mimics group and the si-Smad7
group exhibited a decrease in LVESP and an increase in LVEDP
(P<0.05). There were no differences in these values between the
miR-20b-5p mimics group and the si-Smad7 group (P>0.05), as
shown in Table V. Taken together,
the overexpression of miR-20b-5p and downregulated expression of
Smad7 were critical in the cardiac function of rats with IR
injury.
 | Table VEchocardiograph results of LVESP and
LVEDP (n=5). |
Table V
Echocardiograph results of LVESP and
LVEDP (n=5).
| Group | LVESP (mmHg) | LVEDP (mmHg) |
|---|
| Sham | 128.11±4.02 | 3.51± 0.87 |
| IR | 92.42±8.22a | 8.92±1.32a |
| NC | 91.21±8.50a | 9.05±1.25a |
| miR-20b-5p
mimics | 76.58±3.22a,b,c | 13.19±1.48a,b,c |
| miR-20b-5p
inhibitors | 111.45±7.23a,b,c | 6.16±0.92a,b,c |
| si-Smad7 | 74.42±3.33a,b,c | 14.02±1.45a,b,c |
| miR-20b-5p
inhibitors + si-Smad7 | 90.52±8.02a | 8.92±1.13a |
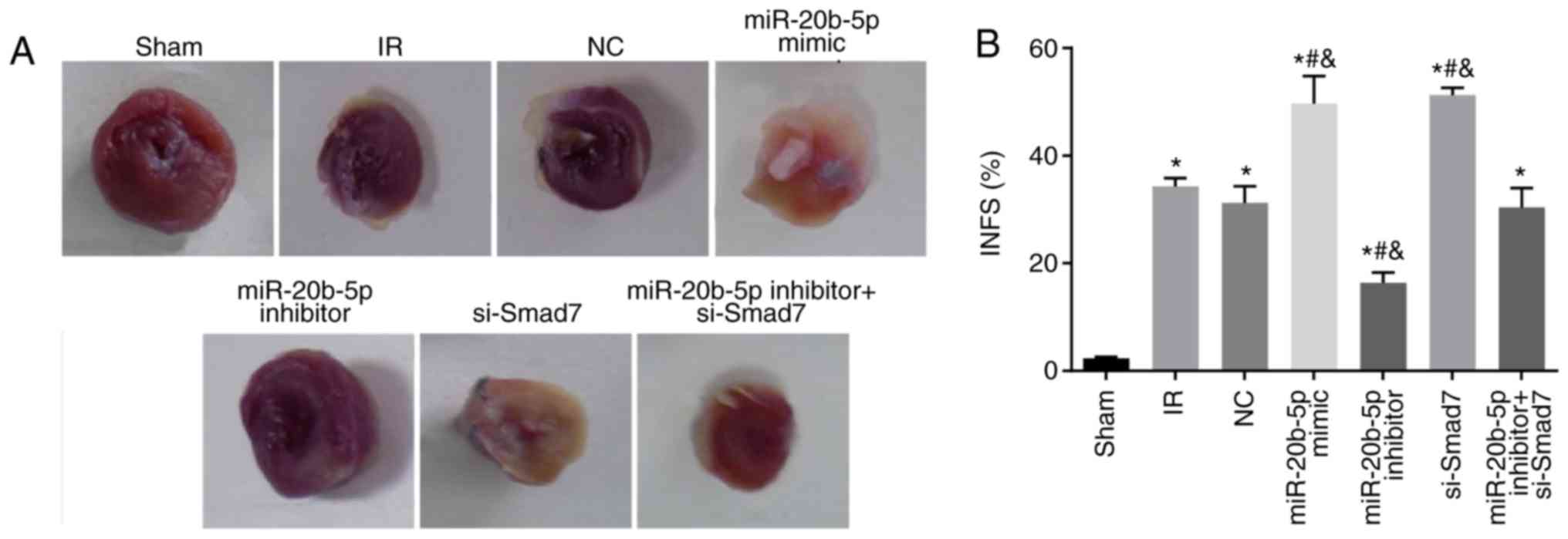
Increased expression of miR-20b-5p and
decreased expression of Smad7 enlarge myocardial infarct size in
rats
Compared with the sham group, the other sex groups
exhibited a significant increase in myocardial infarction area (all
P<0.05). Compared with the IR group, no significant differences
in myocardial infarction area were found in the miR-20b-5p
inhibitors + si-Smad7 group or the NC group (P>0.05), whereas
miR-20b-5p inhibitors group exhibited a significant decrease in
myocardial infarction area (P<0.05), and the miR-20b-5p mimics
group and the si-Smad7 group exhibited significant increases in
myocardial infarction area (P<0.05). There was no significant
difference in this value between the miR-20b-5p mimics group and
the si-Smad7 group (P>0.05), as shown in Fig. 2A and B. The overexpression of
miR-20b-5p and downregulation of Smad7 increased myocardial infarct
size in the rats.
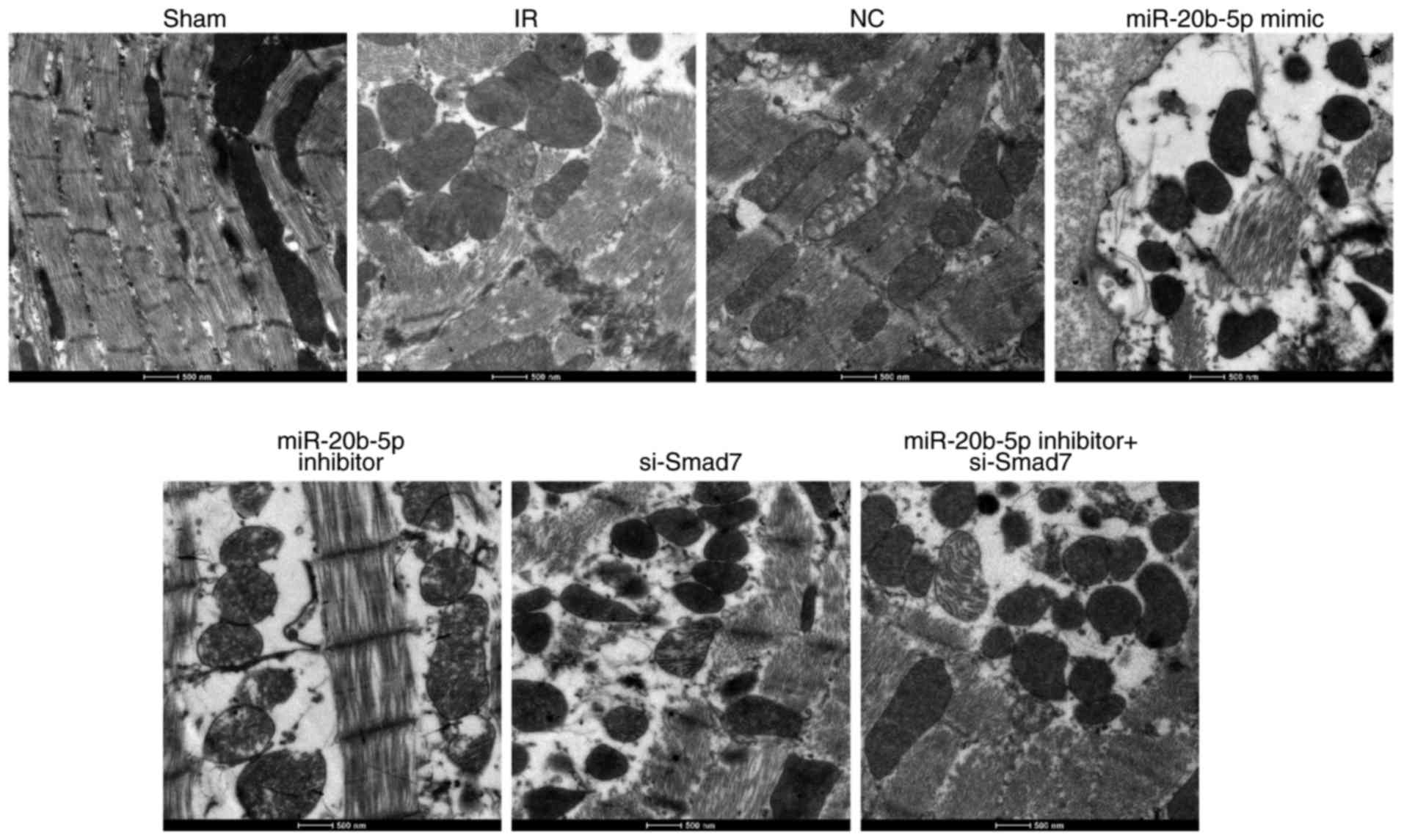
Increased expression of miR-20b-5p and
decreased expression of Smad7 inhibit the growth of cardiomyocytes
in rats with IR injury
The ultrastructural examination of cardiomyocytes in
each group showed the orderly arrangement of myofilaments, normal
shape and structure of mitochondria, and orderly arrangement and
clear structure of cristae in rat cardiomyocytes in the sham group.
In the IR group, the myofilaments of rat cardiomyocytes were broken
and dissolved, and the majority of these were loosely arranged and
displaced. The electron density of mitochondria was increased, and
regions of the cristae membranes were blurred and dissolved. The
myocardial ultrastructure in the NC group and the miR-20b-5p
inhibitors + si-Smad7 group was similar to that in the IR group.
Compared with the IR group, the miR-20b-5p inhibitors group showed
marginal recovery with orderly arrangement of myofilaments and
distinguished mitochondria; in the miR-20b-5p mimics group and the
si-Smad7 group, the myofilament rupture and dissolution were more
severe, and the majority of the filaments were loosely arranged and
displaced. The electron density of mitochondria was higher, and the
majority of the cristae of mitochondria were indistinct, with
evidence of dissolution (Fig. 3).
The low expression of miR-20b-5p and high expression of Smad7
promoted the growth of cardiomyocytes in the rats with IR
injury.
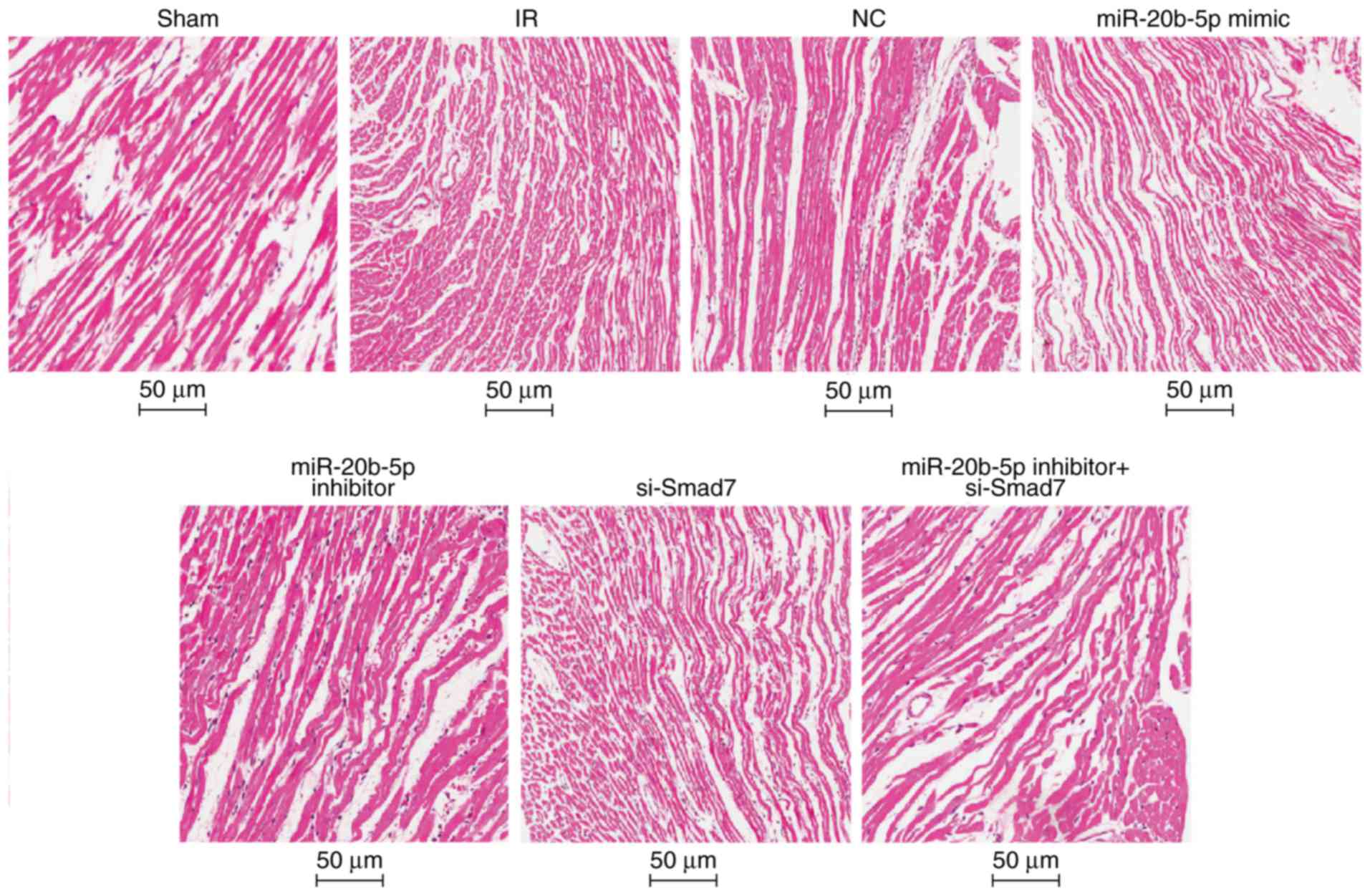
Increased expression of miR-20b-5p and
decreased expression of Smad7 accelerate myocardial necrosis in
rats with IR injury
Under the light microscope, the myocardial fibers
were orderly and tightly arranged with no edema or hemorrhage in
the sham group. In the IR group, the myocardial fibers were
disordered, the cells were swollen, interstitial edema was obvious,
and myocardial cells showed marked necrosis. The results of the HE
staining were similar in the NC group, the miR-20b-5p inhibitors +
si-Smad7 group and the IR group. Compared with the IR group, the
myocardial fiber arrangement was more orderly and myocardial cell
necrosis was significantly reduced in the miR-20b-5p inhibitors
group. In the miR-20b-5p mimics group and the si-Smad7 group, the
myocardial fibers were disordered, and the necrotic area was
significantly increased (Fig. 4).
The high expression of miR-20b-5p and low expression of Smad7
enhanced myocardial necrosis in rats with IR injury.
Increased expression of miR-20b-5p and
decreased expression of Smad7 result in increased CVF and PVCA
The results of the Masson's staining of myocardial
tissue in each group are shown in Fig. 5A and B. Compared with the sham
group, the other six groups exhibited significantly increased CVF
and PVCA (P<0.05). Compared with the IR group, there were no
significant differences in CVF or PVCA in the NC group or the
miR-20b-5p inhibitors + si-Smad7 group (P>0.05), the miR-20b-5p
inhibitors group exhibited decreased CVF and PVCA (P<0.05), and
the miR-20b-5p mimics group and the si-Smad7 group exhibited
increased CVF and PVCA (P<0.05). The CVF and PVCA values did not
differ significantly between the miR-20b-5p mimics group and the
si-Smad7 group (P>0.05). The overexpression of miR-20b-5p and
downregulation of Smad7 increased myocardial collagen in the rats
with IR injury.
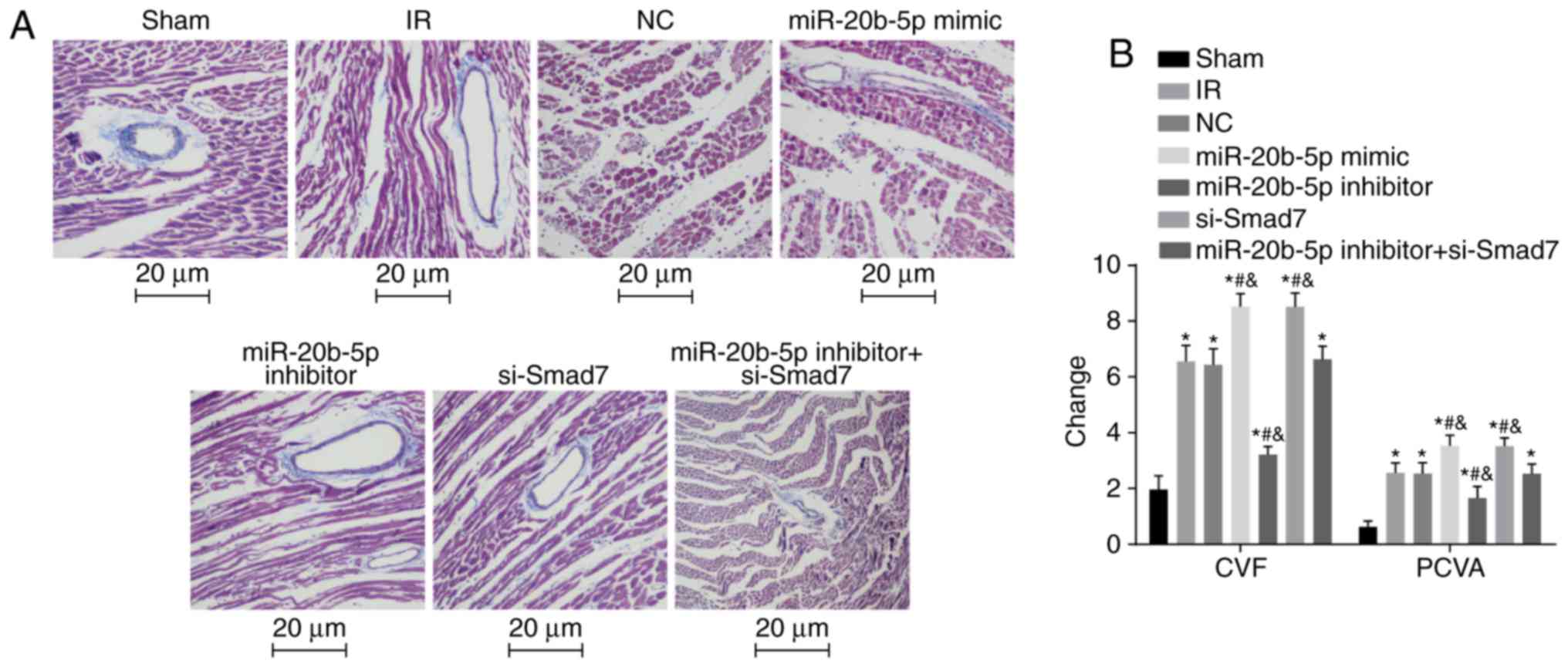 | Figure 5Masson's staining of myocardial
tissue from rats in each group. (A) Masson's staining of myocardial
tissue from rats in each group (magnification, ×400). (B) Changes
in CVF and PVCA in rats in each group are shown in a bar graph.
*P<0.05, compared with the sham group;
#P<0.05, compared with the IR group;
&P<0.05, compared with the miR-20b-5p inhibitors
+ si-Smad7 group. CVF, myocardial collagen volume fraction; PVCA,
perivascular collagen area; miR-20b-5p, microRNA-20b-5p; Smad7,
small mothers against decapentaplegic homolog 7; si-, small
interfering RNA; NC, negative control; IR,
ischemia-reperfusion. |
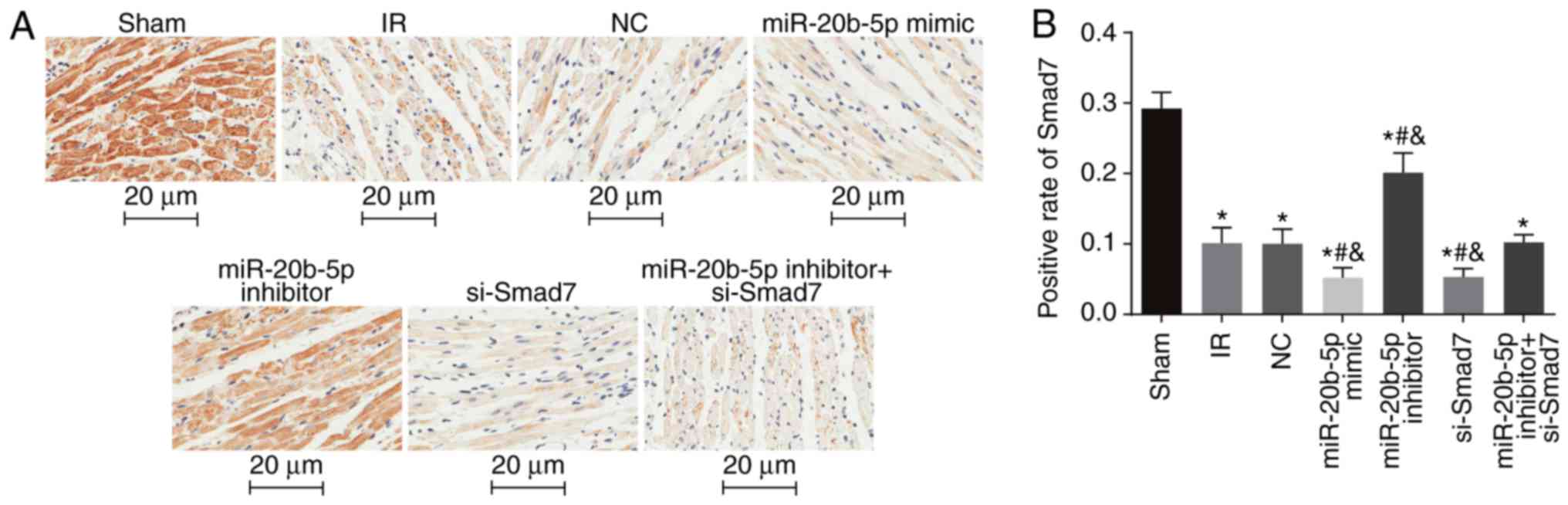
Smad7-positive rate is low in myocardial
tissue of rats with IR injury
The positive expression of Smad7 in the myocardial
cytosol and membrane were indicated by brown staining (Fig. 6A). Compared with the sham group,
the other six groups had a significantly decreased Smad7-positive
rate (P<0.05). Compared with the IR group, there was no
significant difference in the Smad7-positive rate in the NC group
or the miR-20b-5p inhibitors + si-Smad7 group (P>0.05), whereas
the Smad7-positive rate was significantly increased in the
miR-20b-5p inhibitors group, and decreased in the miR-20b-5p mimics
group and the si-Smad7 group (P<0.05). There was no significant
difference between the miR-20b-5p mimics group and the si-Smad7
group (P>0.05), as shown in Fig.
6B. The overexpression of Smad7 had a protective effect in the
rats with IR injury.
Expression of miR-20b-5p and the mRNA
expression of TGF-β1 and Smad3 are upregulated, and the mRNA
expression of Smad7 is decreased in the myocardium of rats with IR
injury
Compared with the sham group, the other six groups
exhibited significantly increased expression of miR-20b-5p and mRNA
expression of TGF-β1 and Smad3, whereas the mRNA expression of
Smad7 was significantly decreased (all P<0.05; Fig. 7). Compared with the IR group,
there was no significant difference in these markers in the NC
group (P>0.05); the miR-20b-5p inhibitors + si-Smad7 group
showed no significant difference in the mRNA expression of TGF-β1,
Smad7 or Smad3 (P>0.05), however, a significant decrease in the
expression of miR-20b-5p was observed (P<0.05); the miR-20b-5p
inhibitors group exhibited a decrease in the expression of
miR-20b-5p and the mRNA expression of TGF-β1 and Smad3, and an
increase in the mRNA expression of Smad7 (all P<0.05); the
miR-20b-5p mimics group exhibited a marked increase in expression
of miR-20b-5p and the mRNA expression of TGF-β1 and Smad3, and a
significant decrease in the mRNA expression of Smad7 (all
P<0.05); the si-Smad7 group showed no significant difference in
the expression of miR-20b-5p (P>0.05), but there were
significant increases in the mRNA expression of TGF-β1 and Smad3,
and a significant decrease in the mRNA expression of Smad7 (all
P<0.05). These results are shown in Fig. 7. Activation of the TGF-β1/Smad
signaling pathway promoted ventricular remodeling in the rats with
IR injury.
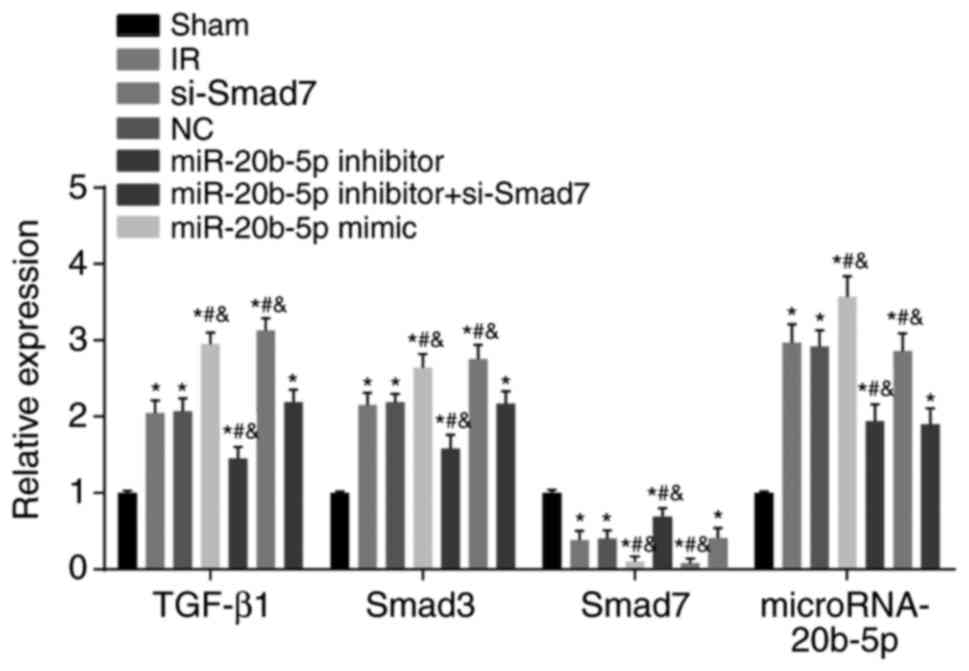 | Figure 7Expression of miR-20b-5p and mRNA
expression of TGF-β1/Smad signaling pathway-related proteins in
myocardial tissue from rats in each group. *P<0.05,
compared with the sham group; #P<0.05, compared with
the IR group; &P<0.05, compared with the
miR-20b-5p inhibitors + si-Smad7 group. miR-20b-5p,
microRNA-20b-5p; Smad3, small mothers against decapentaplegic
homolog 3; Smad7, small mothers against decapentaplegic homolog 7;
si-, small interfering RNA; TGF-β1, transforming growth factor-β1;
NC, negative control; IR, ischemia-reperfusion. |
Protein expression levels of TGF-β1 and
Smad3 are upregulated and the protein expression of Smad7 is
decreased in the myocardium of rats with IR injury
Compared with the sham group, the other six groups
showed a significant increase in the protein expression of TGF-β1
and Smad3, whereas the protein expression of Smad7 decreased (all
P<0.05; Fig. 8A and B).
Compared with the IR group, no significant difference in the
protein expression of TGF-β1 and Smad3 were observed in the NC
group or the miR-20b-5p inhibitors + si-Smad7 group (P>0.05).
The miR-20b-5p inhibitors group exhibited a marked decrease in the
expression of TGF-β1 and Smad3 and a significant increase in the
expression of Smad7 (P<0.05), and the miR-20b-5p mimics group
and the si-Smad7 group exhibited a marked increase in the
expression of TGF-β1 and Smad3 and a decrease in the expression of
Smad7 (P<0.05). No significant differences (P>0.05) in these
values were found between the miR-20b-5p mimics group and the
si-Smad7 group (Fig. 8). The low
expression of miR-20b-5p and high expression of Smad7 inhibited the
TGF-β1/Smad signaling pathway.
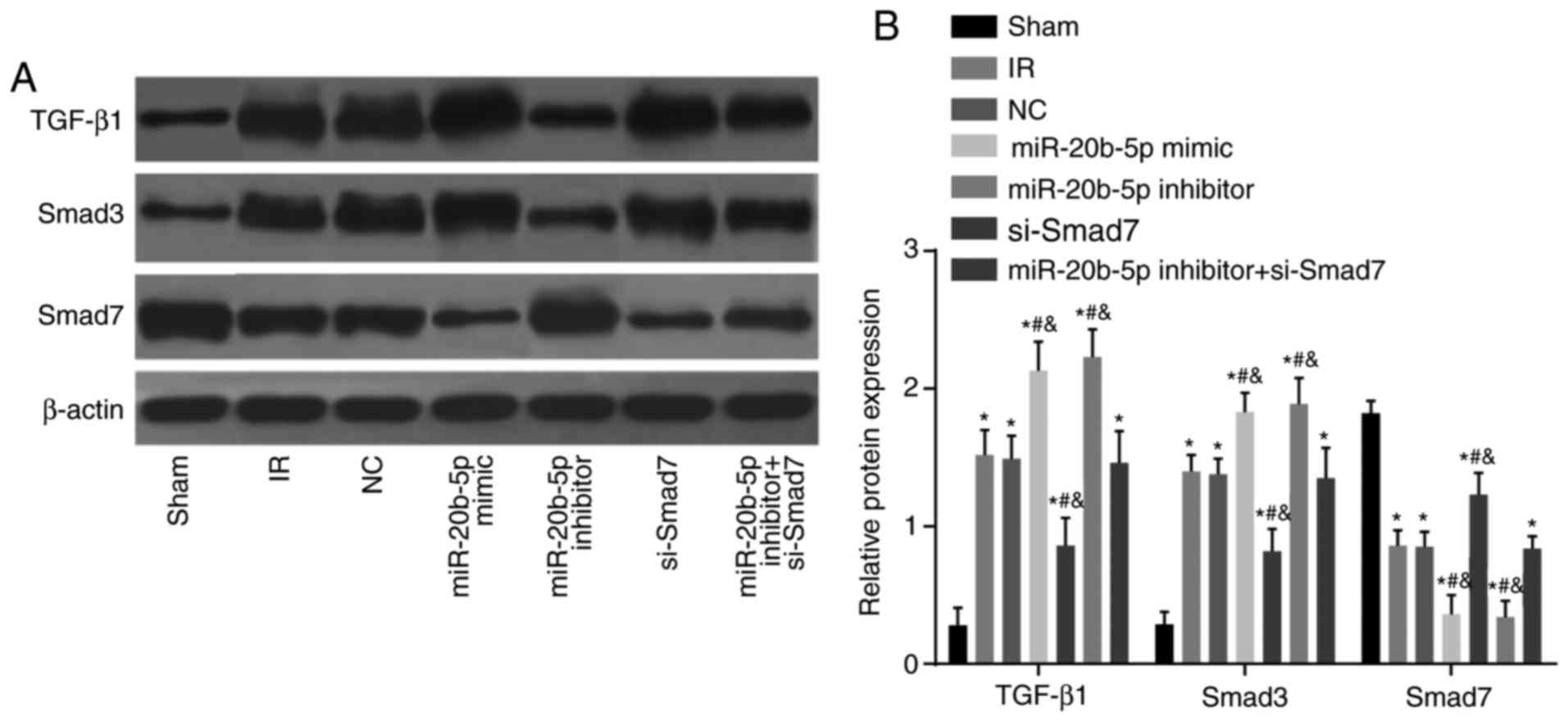 | Figure 8Expression of TGF-β1/Smad signaling
pathway components in myocardial tissue from rats in each group.
(A) Banding diagram of the expression of TGF-β1/Smad signaling
pathway components in myocardial tissue from rats in each group,
(B) Histogram of the expression of TGF-β1/Smad signaling pathway
components in myocardial tissue from rats in each group.
*P<0.05, compared with the sham group;
#P<0.05, compared with the IR group;
&P<0.05, compared with the miR-20b-5p inhibitors
+ si-Smad7 group. miR-20b-5p, microRNA-20b-5p; Smad3, small mothers
against decapentaplegic homolog 3; Smad7, small mothers against
decapentaplegic homolog 7; si-, small interfering RNA; TGF-β1,
transforming growth factor-β1; NC, negative control; IR,
ischemia-reperfusion. |
Acknowledgments
Not applicable.
Discussion
With the development of thrombolysis, percutaneous
transluminal coronary angioplasty and coronary artery bypass
grafting, myocardial IR injury has become the main obstacle in
obtaining the optimal therapeutic effect from ischemic heart
disease during reperfusion treatment (28). Following myocardial IR therapy,
the heart is confronted with further injury through ventricular
remodeling (29,30). Therefore, it is important to
introduce effective myocardial protection measures to reduce
ventricular remodeling following myocardial IR therapy (31). Following myocardial IR therapy in
rats, the present study found that myocardial ischemia was involved
with the process of cardiac remodeling following treatment, and the
infarct size and myocardial collagen content were increased. The
results showed that miR-20b-5p inhibited the expression of Smad7,
activated the TGF-β signaling pathway, and promoted ventricular
remodeling following myocardial IR injury in the rats.
Following myocardial ischemia, the sudden recovery
of myocardial blood flow aggravates ischemic myocardial injury,
which clinically manifests as arrhythmia, myocardial stunning,
heart dysfunction and other symptoms, and is termed myocardial IR
injury (10). Increasing the
concentration of glycolytic substrates in the reperfusion blood can
reduce the damage to ischemic myocardial cells and promote the
recovery of myocardial function, whereas inhibiting the glycolytic
process can increase the overload of myocardial intracellular
calcium and delay the recovery of myocardial function (32). Galang et al (33) reported that, when IR occurred in
the isolated rat heart, cardiomyocyte apoptosis was reduced by
erythrocuprein and catalase. The present study found that, compared
with that in the sham group, the area of myocardial infarction
following reperfusion in the other six groups was significantly
higher. Following the downregulation of miR-20b-5p, the infarct
size decreased significantly. These results showed that myocardial
IR injury led to the apoptosis of myocardial cells, and that
miR-20b-5p was significant in promoting apoptosis in myocardial IR
injury.
Ventricular remodeling is the internal cause of
cardiac insufficiency and heart failure (29). Numerous basic and clinical studies
have shown that ventricular remodeling can be organized or reversed
(34). Ventricular remodeling is
usually associated with fibrosis (35). A number of studies have indicated
that the canonical TGF-β1/Smad3 pathway is critically involved in
the pathogenesis of fibrosis in several tissues (36). Long-term ventricular remodeling
eventually manifests as increased heart rate, LVEDV and LVRDV, and
decreased systolic volume and LVEEF (37). The ventricular remodeling
following myocardial infarction has been verified in a number of
studies, however, ventricular remodeling following myocardial IR is
rare; therefore, the present study established a rat model of
myocardial IR injury model to observe the dynamic process of
ventricular remodeling following myocardial IR injury. The results
of transthoracic echocardiography showed that, following
reperfusion, LVEEF and LVEFS decreased significantly. These results
suggested that the heart underwent a process of ventricular
remodeling following myocardial IR injury in rats, resulting in a
decline in cardiac function.
miRs, by combining with target mRNAs, regulate
target mRNA translation and are involved in the regulation of genes
that are widely involved in cell differentiation, proliferation and
apoptosis (38,39). The expression of miRs has strict
temporal and tissue specificity, and the cardiovascular system has
its specific miR expression profile (40). It has been revealed that miRs
expressed in the cardiovascular system are involved in the
development of the cardiovascular system and the course of disease
(41). Mukhopadhyay et al
(15) suggested that increased
miR-20b (anti-angiogenic) was linked with cardiac remodeling. Smads
are considered to be mediators and regulators of TGF-β signaling
(42). Smad7 has been suggested
as an important inhibitory protein in the TGF-β signaling pathway
(18). In the present study,
RT-qPCR and western blot analyses were used to detect the
expression of miR-20b-5p, TGF-β1, Smad3 and Smad7, and the results
showed that the expression of miR-20b-5p, TGF-β1 and Smad3 were
significantly increased, whereas that of Smad7 was significantly
decreased following myocardial IR. Smad7 was found to be essential
for cardiac development at the embryonic stage and for cardiac
function in adults (43). In
addition, Smad7 can reduce angiotensin II-induced hypertensive
cardiac remodeling, and it may be a therapeutic target for
hypertensive cardiovascular diseases (44). The promoter region of Smad7 is
stimulated by the ectopic expression of Smad3, and active TGF-β and
activin receptors, indicating that the transcription of Smad7 is
regulated by these two receptors (45). Smad3 activates myostatin, thereby
inducing the expression of Smad7, whereas Smad7 mediates the
myostatin signal transduction pathway through a negative feedback
mechanism (46). These results
suggested that miR-20b-5p upregulated the expression of Smad7 in
the process of ventricular remodeling following myocardial IR
injury.
In conclusion, the present in vivo model of
myocardial IR injury in rats verified the process of ventricular
remodeling following myocardial IR, indicating that miR-20b-5p
promoted ventricular remodeling following myocardial IR injury in
rats via inhibiting the expression of Smad7 through activating the
TGF-β/Smad signaling pathway. However, due to limited time and
funds, the present study failed to observe longer term myocardial
remodeling following myocardial IR injury. Verification of these
results is required in future investigations.
Funding
No funding was received.
Availability of data and materials
The datasets generated/analysed during the current
study are available.
Authors' contributions
ZGL and HY contributed in the conception of the
work, conducting the study, preparing the manuscript. RSX collated
the data, designed and developed the database, and CLG been
involved in reviewing the results and discussions. YT analyzed and
interpreted the data, and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Laboratory
Animal Ethics Committee of The First Affiliated Hospital of Harbin
Medical University, and was performed in accordance with the
principles of animal protection, welfare and ethics and the
National Laboratory Animal Welfare Ethics regulations.
Consent for publication
Consent for publication was obtained from the
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
De Roeck L, Vandamme S, Everaert BR,
Hoymans V, Haine S, Vandendriessche T, Bosmans J, Ronsyn MW,
Miljoen H, Van Berendoncks A, et al: Adiponectin and
ischemia-reperfusion injury in ST segment elevation myocardial
infarction. Eur Heart J Acute Cardiovasc Care. 5:71–76. 2016.
View Article : Google Scholar
|
|
2
|
Selmer R, Halvorsen S, Myhre KI, Wisløff
TF and Kristiansen IS: Cost-effectiveness of primary percutaneous
coronary intervention versus thrombolytic therapy for acute
myocardial infarction. Scand Cardiovasc J. 39:276–285. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ong SB, Samangouei P, Kalkhoran SB and
Hausenloy DJ: The mitochondrial permeability transition pore and
its role in myocardial ischemia reperfusion injury. J Mol Cell
Cardiol. 78:23–34. 2015. View Article : Google Scholar
|
|
4
|
Fröhlich GM, Meier P, White SK, Yellon DM
and Hausenloy DJ: Myocardial reperfusion injury: Looking beyond
primary PCI. Eur Heart J. 34:1714–1722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wick G, Grundtman C, Mayerl C,
Wimpissinger TF, Feichtinger J, Zelger B, Sgonc R and Wolfram D:
The immunology of fibrosis. Annu Rev Immunol. 31:107–135. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neri M, Fineschi V, Di Paolo M, Pomara C,
Riezzo I, Turillazzi E and Cerretani D: Cardiac oxidative stress
and inflammatory cytokines response after myocardial infarction.
Curr Vasc Pharmacol. 13:26–36. 2015. View Article : Google Scholar
|
|
7
|
Zhang M, Xu YJ, Saini HK, Turan B, Liu PP
and Dhalla NS: TNF-alpha as a potential mediator of cardiac
dysfunction due to intracellular Ca2+-overload. Biochem
Biophys Res Commun. 327:57–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hausenloy DJ and Yellon DM: The
mitochondrial permeability transition pore: Its fundamental role in
mediating cell death during ischaemia and reperfusion. J Mol Cell
Cardiol. 35:339–341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
hao ZQ, Nakamura M, Wang NP, Velez DA,
Hewan-Lowe KO, Guyton RA and Vinten-Johansen J: Dynamic progression
of contractile and endothelial dysfunction and infarct extension in
the late phase of reperfusion. J Surg Res. 94:133–144. 2000.
View Article : Google Scholar
|
|
10
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan ZX and Yang J: The role of microRNAs
in regulating myocardial ischemia reperfusion injury. Saudi Med J.
36:787–793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki HI and Miyazono K: Emerging
complexity of microRNA generation cascades. J Biochem. 149:15–25.
2011. View Article : Google Scholar
|
|
13
|
Huntzinger E and Izaurralde E: Gene
silencing by microRNAs: Contributions of translational repression
and mRNA decay. Nat Rev Genet. 12:99–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He B, Xiao J, Ren AJ, Zhang YF, Zhang H,
Chen M, Xie B, Gao XG and Wang YW: Role of miR-1 and miR-133a in
myocardial ischemic postconditioning. J Biomed Sci. 18:222011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mukhopadhyay P, Das S, Ahsan MK, Otani H
and Das DK: Modulation of microRNA 20b with resveratrol and
longevinex is linked with their potent anti-angiogenic action in
the ischaemic myocardium and synergestic effects of resveratrol and
γ-tocotrienol. J Cell Mol Med. 16:2504–2517. 2012. View Article : Google Scholar
|
|
16
|
Dickinson BA, Semus HM, Montgomery RL,
Stack C, Latimer PA, Lewton SM, Lynch JM, Hullinger TG, Seto AG and
van Rooij E: Plasma microRNAs serve as biomarkers of therapeutic
efficacy and disease progression in hypertension-induced heart
failure. Eur J Heart Fail. 15:650–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Chen D, Jin L, Liu J, Su Z, Li Y,
Gui Y and Lai Y: MicroRNA-20b-5p functions as a tumor suppressor in
renal cell carcinoma by regulating cellular proliferation,
migration and apoptosis. Mol Med Rep. 13:1895–1901. 2016.
View Article : Google Scholar
|
|
18
|
Blahna MT and Hata A: Smad-mediated
regulation of microRNA biosynthesis. FEBS Lett. 586:1906–1912.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanyu A, Ishidou Y, Ebisawa T, Shimanuki
T, Imamura T and Miyazono K: The N domain of Smad7 is essential for
specific inhibition of transforming growth factor-beta signaling. J
Cell Biol. 155:1017–1027. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zorzi F, Angelucci E, Sedda S, Pallone F
and Monteleone G: Smad7 antisense oligonucleotide-based therapy for
inflammatory bowel diseases. Dig Liver Dis. 45:552–555. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cozzolino D, Sasso FC, Salvatore T,
Torella M, Gentile S, Torella R and Giugliano D: Acute effects of
beta-endorphin on cardiovascular function in patients with mild to
moderate chronic heart failure. Am Heart J. 148:E132004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takagawa J, Zhang Y, Wong ML, Sievers RE,
Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W and Springer
ML: Myocardial infarct size measurement in the mouse chronic
infarction model: Comparison of area- and length-based approaches.
J Appl Physiol. 102:2104–2111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Timmers L, Lim SK, Arslan F, Armstrong JS,
Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, et
al: Reduction of myocardial infarct size by human mesenchymal stem
cell conditioned medium. Stem Cell Res. 1:129–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li XW, Wang XM, Li S and Yang JR: Effects
of chrysin (5,7-dihydroxyf lavone) on vascular remodeling in
hypoxia-induced pulmonary hypertension in rats. Chin Med. 10:42015.
View Article : Google Scholar
|
|
25
|
Brown RS and Wahl RL: Overexpression of
Glut-1 glucose transporter in human breast cancer. An
immunohistochemical study. Cancer. 72:2979–2985. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li R, Yan G, Li Q, Sun H, Hu Y, Sun J and
Xu B: MicroRNA-P145 protects cardiomyocytes against hydrogen
peroxide (H2O2)-induced apoptosis through
targeting the mitochondria apoptotic pathway. PLoS One.
7:e449072012. View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Liu H, Shang J, Chu F, Li A, Wu B, Xie X,
Liu W, Yang H and Tong T: Protective effects of Shen-Yuan-Dan, a
traditional Chinese medicine, against myocardial
ischemia/reperfusion injury in vivo and in vitro. Evid Based
Complement Alternat Med. 2013:9563972013. View Article : Google Scholar
|
|
29
|
Cohn JN, Ferrari R and Sharpe N: Cardiac
remodeling-concepts and clinical implications: A consensus paper
from an international forum on cardiac remodeling. Behalf of an
international forum on cardiac remodeling. J Am Coll Cardiol.
35:569–582. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ito Y, Ito K, Shiroto T, Tsuburaya R, Yi
GJ, Takeda M, Fukumoto Y, Yasuda S and Shimokawa H: Cardiac shock
wave therapy ameliorates left ventricular remodeling after
myocardial ischemia-reperfusion injury in pigs in vivo. Coron
Artery Dis. 21:304–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hagiwara S, Iwasaka H, Shingu C, Matumoto
S, Hasegawa A and Noguchi T: Heat shock protein 47 (HSP47)
antisense oligonucleotides reduce cardiac remodeling and improve
cardiac function in a rat model of myocardial infarction. Thorac
Cardiovasc Surg. 59:386–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Apstein CS: Increased glycolytic substrate
protection improves ischemic cardiac dysfunction and reduces
injury. Am Heart J. 139:S107–S114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Galang N, Sasaki H and Maulik N: Apoptotic
cell death during ischemia/reperfusion and its attenuation by
antioxidant therapy. Toxicology. 148:111–118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fedak PW, Verma S, Weisel RD, Skrtic M and
Li RK: Cardiac remodeling and failure: From molecules to man (Part
III). Cardiovasc Pathol. 14:109–119. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Donekal S, Venkatesh BA, Liu YC, Liu CY,
Yoneyama K, Wu CO, Nacif M, Gomes AS, Hundley WG, Bluemke DA and
Lima JA: Interstitial fibrosis, left ventricular remodeling, and
myocardial mechanical behavior in a population-based multiethnic
cohort: The multi-ethnic study of Atherosclerosis (MESA) study.
Circ Cardiovasc Imaging. 7:292–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu M, Chen J, Huang Y, Ke J, Li L, Huang
D and Wu W: Triptolide alleviates isoprenaline-induced cardiac
remodeling in rats via TGF-β1/Smad3 and p38 MAPK signaling pathway.
Pharmazie. 70:244–250. 2015.PubMed/NCBI
|
|
37
|
Kasama S, Toyama T, Hatori T, Sumino H,
Kumakura H, Takayama Y, Ichikawa S, Suzuki T and Kurabayashi M:
Evaluation of cardiac sympathetic nerve activity and left
ventricular remodelling in patients with dilated cardiomyopathy on
the treatment containing carvedilol. Eur Heart J. 28:989–995. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fazi F and Nervi C: MicroRNA: Basic
mechanisms and transcriptional regulatory networks for cell fate
determination. Cardiovasc Res. 79:553–561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Landgraf P, Rusu M, Sheridan R, Sewer A,
Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M,
et al: A mammalian microRNA expression atlas based on small RNA
library sequencing. Cell. 129:1401–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao Y, Samal E and Srivastava D: Serum
response factor regulates a muscle-specific microRNA that targets
Hand2 during cardiogenesis. Nature. 436:214–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kretzschmar M and Massague J: SMADs:
Mediators and regulators of TGF-beta signaling. Curr Opin Genet
Dev. 8:103–111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Q, Chen H, Zheng D, Kuang C, Fang H,
Zou B, Zhu W, Bu G, Jin T, Wang Z, et al: Smad7 is required for the
development and function of the heart. J Biol Chem. 284:292–300.
2009. View Article : Google Scholar :
|
|
44
|
Wei LH, Huang XR, Zhang Y, Li YQ, Chen HY,
Yan BP, Yu CM and Lan HY: Smad7 inhibits angiotensin II-induced
hypertensive cardiac remodelling. Cardiovasc Res. 99:665–673. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nagarajan RP, Zhang J, Li W and Chen Y:
Regulation of Smad7 promoter by direct association with Smad3 and
Smad4. J Biol Chem. 274:33412–33418. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu X, Topouzis S, Liang LF and Stotish
RL: Myostatin signaling through Smad2, Smad3 and Smad4 is regulated
by the inhibitory Smad7 by a negative feedback mechanism. Cytokine.
26:262–272. 2004. View Article : Google Scholar : PubMed/NCBI
|