Introduction
Colorectal cancer (CRC) is one of the most common
types of cancer and is the third leading cause of cancer-associated
mortality in men and women in the western world (1). Rectal adenocarcinoma comprises up to
30% of these tumors and they are frequently treated with
(neoadjuvant) radiochemotherapy (RCTx) to reduce tumor mass prior
to surgery (2,3). It has been shown that short-term
preoperative RCTx reduces the risk of local recurrence, overall
mortality rate and cancer-associated mortality rate in patients who
undergo a standardized total mesorectal excision (3,4).
In Germany, pre-operative radiation therapy comprises short-term
(5×5 Gy on 5 subsequent days) and long-term radiation protocols
(fractionated scheme of 2 Gy/week to a cumulative dose of 45–50.4
Gy) (5). However, not all types
of rectal carcinoma respond to RCTx equally. Although 15–27% of
patients show pathological complete response (pCR, no remaining
vital tumor cells) associated with an improved long-term outcome, a
significant proportion of tumors exhibit variable degrees of
therapy resistance (6). This
issue has been addressed by pathologists with the introduction of
classification schemes that describe the degree of tumor regression
in response to neoadjuvant therapy (7–9).
However, biomarkers that can reliably predict sensitivity of an
individual tumor to neoadjuvant RCTx have not been identified.
Heterogeneous nuclear ribonucleoprotein K (hnRNP K)
is a ubiquitously expressed 65 kDa-protein containing three
eponymous K homology domains that mediate binding to poly(C)
regions in DNA and RNA to regulate gene transcription, pre-mRNA
maturation processes and translation (10,11). HnRNP K is upregulated and
associated with poor prognosis in multiple malignancies, including
CRC, and it has been shown in previous studies that hnRNP K confers
radioresistance to CRC and malignant melanoma cells in a
mitogen-activated protein kinase (MAPK)-dependent manner (12–15). At present, whether hnRNP K
represents a valuable biomarker for radioresistance and, in
particular, how the downstream effects of hnRNP K protect the tumor
cells from the deleterious effects of ionizing radiation (IR)
remain to be elucidated. It is known that hnRNP K acts as a
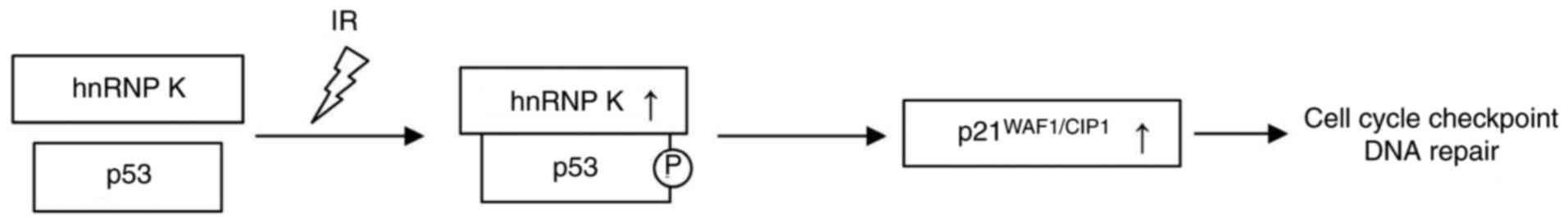
transcriptional cofactor in the induction of p53 target genes
following DNA damage upon protein/protein interaction with p53,
leading to the upregulation of p21 (also known as WAF1/CIP1;
Fig. 1) (16–19). By contrast, p21 acts as a
cyclin-dependent kinase inhibitor and is an important component of
the G1 checkpoint response to DNA damage (20).
Therefore, the aim of the present study was to
investigate whether the expression of hnRNP K is associated with
poor response to RCTx in rectal cancer, and also whether a possible
radioprotective effect is mediated via the induction of p53 target
genes by hnRNP K.
Materials and methods
Tissue samples, histology and
immunohistochemistry (IHC)
A total of 68 consecutive cases of invasive
adenocarcinoma of the rectum, in patients who had undergone prior
radiotherapy and/or chemotherapy, and 14 corresponding
pre-therapeutic biopsies from a total of 68 patients were included
in the present study (Table I).
The tissue specimens were fixed in 4% buffered formaldehyde at room
temperature for 24 h and subsequently embedded in paraffin. For
each post-RCTx case, full histological slides were re-reviewed for
the percentage of vital tumor cells to assess the tumor regression
grade according to Dworak et al (7). For all cases, two representative
regions (×10 field; 4.909 mm2) per case were selected
for the creation of a tissue microarray (TMA). Immunohistochemistry
was performed on a Ventana Autostainer (Ventana, Tucson, AZ, USA)
following heat-induced epitope retrieval (HIER; pH 8.0; 32 min for
hnRNP K and p21, and 60 min for p53, respectively) using the
following antibodies: Anti-hnRNP K (cat. no. LS-C30312-50; rabbit
polyclonal; 1:250, Biozol, Eching, Germany; incubation at 37°C for
24 min), anti-p53 (clone DO7; cat. no. 790-2912; mouse monoclonal;
prediluted to ~0.5 µg/ml; Ventana; incubation at 37°C for 16
min) and anti-p21 (cat. no. 760-4453; mouse monoclonal; prediluted
to ~4.83 µg/ml; Ventana; incubation at 37°C for 24 min). The
OptiView Diaminobenzidine IHC detection kit (Ventana), which
eliminates the requirement for blocking reagents by the use of HRP
multimer technology, was used for detection, following the
manufacturer’s protocol. The IHC staining intensities were graded
as absent (0), weak (1) and
strong (2) using ×10
magnification on a Leica DM6000B light microscope (Leica
Microsystems GmbH, Wetzlar, Germany).
 | Table IClinicopathological data. |
Table I
Clinicopathological data.
| Clinicopathological
factor | n (%) | |
|---|
| Patients | 68 (100) | |
| Samples | 82 | |
| Resected
specimens | 68 | |
| Pre-therapeutic
biopsies | 14 | |
| Sex | | |
| Male | 49 (72) | |
| Female | 19 (28) | |
| Age, years [range
(median)] | 32–88 (62.5) | |
| Therapy
(n=64) | | |
| Radiotherapy | 5 (8) | |
| Radiotherapy +
chemotherapy | 59 (92) | |
| ypT stage
(n=68) | | |
| ypT0 | 10 (15) | |
| ypT1 | 5 (7) | |
| ypT2 | 17 (25) | |
| ypT3 | 33 (49) | |
| ypT4 | 3 (4) | |
| ypN stage
(n=66) | | |
| ypN0 | 42 (64) | |
| ypN+ | 24 (36) | |
| Dworak regression
grade (n=68) | | |
| 0 (no
regression) | 0 (0) | |
| 1 (predominance of
tumor over fibrosis) | 21 (31) | |
| 2 (predominance of
fibrosis, tumor visible) | 24 (35) | |
| 3 (fibrosis, few
nests of tumor cells) | 13 (19) | |
| 4 (no visible
tumor cells) | 10 (15) | |
| Expression of hnRNP
K (cytoplasmic; n=68) | Pre-therapeutic
(n=14) | Post-therapeutic
(n=54) |
| Absent (0) | 12 (86) | 20 (37) |
| Weak (1) | 2 (14) | 16 (30) |
| Strong (2) | 0 (0) | 18 (33) |
| Expression of p53
(n=59) | Pre-therapeutic
(n=13) | Post-therapeutic
(n=46) |
| Absent (0) | 1 (8) | 9 (19) |
| Weak (1) | 5 (38) | 16 (35) |
| Strong (2) | 7 (54) | 21 (46) |
| Expression of p21
(n=55) | Pre-therapeutic
(n=13) | Post-therapeutic
(n=43) |
| Absent (0) | 9 (70) | 35 (82) |
| Weak (1) | 2 (15) | 4 (9) |
| Strong (2) | 2 (15) | 4 (9) |
| KRAS codon
12/13 mutation (n=59)a | | |
| Codon 12/13
mutation | 9 (15) | |
| Codon 12/13
wild-type | 50 (85) | |
Sanger sequencing
DNA from FFPE specimens was extracted with the
Maxwell® 16 FFPE Tissue LEV DNA Purification kit with
the use of the Maxwell® 16 AS3000 instrument (both
Promega Corporation, Madison, WI, USA). Amplification of the KRAS
Exon 2 polymerase chain reaction (PCR) products was performed with
the following PCR primers: Forward,
5′-GTCACATTTTCATTATTTTTATTATAAGGCCTG-3′ and reverse,
5′-CCTCTATTGTTGGATCATATTCGTCCAC-3′. PCR was performed with 10
µl Gene Amp™ Fast PCR Master mix (Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 2.8 µl each of the forward and
reverse primers (1 pmol/µl), 2.8 µl nuclease-free
water and 2 µl DNA (undiluted and 1:10 diluted without prior
quantification), with an initial denaturation at 95°C for 10 sec,
and 40 cycles of denaturation at 94°C for 10 sec and annealing at
63°C for 20 sec. The sequencing PCR was performed with an initial
denaturation at 96°C for 1 min (25 cycles), at 96°C for 10 sec,
50°C for 5 sec and 60°C for 1.15 min using the BigDye®
Terminator v3.1 Mix Cycle Sequencing kit (Thermo Fisher Scientific,
Inc.) and the gene specific primers mentioned above. Following
purification with MultiScreen®-HV plates (Merck
Millipore, Darmstadt, Germany) and Sephardi G-50 (GE Healthcare
Life Sciences, Chalfont, UK) the PCR products were sequenced using
a 96-capillary 3730×l DNA analyzer (Thermo Fisher Scientific,
Inc.).
Next-generation sequencing
In addition to KRAS mutation analysis by Sanger
sequencing, samples with low tumor cell content were analyzed by
more sensitive next-generation sequencing with the use of a Custom
GeneRead DNASeq Panel (Qiagen GmbH, Hilden, Germany) consisting of
189 amplicons for mutation analysis of 19 cancer-related genes,
(NRAS, H3F3A, RET, KRAS, AKT1, TP53, ERBB2, H3F3B, GNA11, ALK,
GNAS, CTNNB1, PIK3CA, PDGFRA, KIT, EGFR, MET, BRAF, GNAQ),
according to the manufacturer’s protocol. In brief, genomic DNA was
quantified and target enrichment was processed with the GeneRead
DNAseq Panel PCR V2 kit (Qiagen GmbH) applying 40 ng genomic DNA
quantified with a Qubit® dsDNA HS kit and a
Qubit® 2.0 Fluorometer (Life Technologies; Thermo Fisher
Scientific, Inc.). An initial activation step (95°C; 15 min) was
followed by 25 cycles of denaturing at 95°C (15 sec) and annealing
at 60°C (4 min), followed by extension at 72°C for 10 min. All
purification and size selection steps were performed using
Agencourt AMPure XP magnetic beads (Beckman Coulter, Inc., Brea,
CA, USA). End repair, A-addition and ligation to NEXTflex-96 DNA
barcodes (Bioo Scientific, Austin, TX, USA) were performed using
the GeneRead DNA Library I Core kit (Qiagen GmbH). Amplification of
adapter-ligated DNA was conducted using NEXTflex primers (Bioo
Scientific) and the HiFi PCR Master mix (GeneRead DNA I Amp kit;
Qiagen GmbH) with an initial activation step of 98°C (2 min), five
cycles of denaturation at 98°C (20 sec), annealing at 60°C (30 sec)
and extension at 72°C (30 sec), followed by a final extension at
72°C (10 min). Following a final purification with Agencourt AMPure
XP Beads (Beckman Coulter, Inc.), the library PCR products were
quantified, diluted, pooled and sequenced using the MiSeq™ V2
reagent kit on an MiSeq™ instrument (Illumina, Inc., San Diego, CA,
USA). The data were exported as FASTQ files and analyzed via the
CLC Biomedical Genomic Workbench version 3.0.1 (Qiagen GmbH).
Cell culture, cell transfection
experiments and in vitro X-ray irradiation
The SW480 and Colo320 CRC cell lines were newly
purchased from the Leibniz Institute DSMZ (Braunschweig, Germany).
The cells were maintained in RPMI-1640 medium (Gibco, Thermo Fisher
Scientific, Inc.) supplemented with 10% FCS (Boehringer Mannheim,
Mannheim, Germany) under standard cell culture conditions (37°C, 5%
CO2). The reagents for transfection experiments included
Lipofectamine 2000 transfection reagent (Invitrogen, Thermo Fisher
Scientific, Inc.), Silencer® Select hnRNP K (s e quenc
e: 3′-AUAAUCAUAGGUUUCAUCGta; 5′-CGAUGAAACCUAUGAUUAUtt) and
Silencer® Select negative control siRNA #1 (Thermo
Fisher Scientific, Inc.). The transfected cells were harvested at
48 h or underwent treatment according to the experimental protocol.
Exposure of the cells to 240 kV X-rays was performed using the
YXLON Maxishot system (Hamburg, Germany) with a 3-mm beryllium
filter. The dose rate rose to a plateau at 1 Gy/min at 13 mA. The
absorbed dose was determined using a PTW Unidose dosimeter (PTW
Freiburg GmbH, Freiburg, Germany). To guarantee equal surrounding
conditions, non-irradiated SW480 and Colo320 control cells were
stored under equivalent conditions at room temperature during the
irradiation experiments.
PathScan stress and apoptosis signaling
array
To analyze the activation of cellular stress
reactions, the PathScan Stress and Apoptosis Signaling Antibody
Array kit (Cell Signaling Technology, Inc., Danvers, MA, USA) was
used following the manufacturer’s protocol.
Western immunoblotting and protein
immunoprecipitation
Immunoblotting was performed according to standard
methods using the XCell Sure Lock™ Mini-Cell Electrophoresis
system. For equalization of protein concentrations of whole cell
lysates, a BCA Protein Assay kit was used according to the
manufacturer’s protocol (Thermo Fisher Scientific, Inc.) following
protein extraction using radioimmunoprecipitation assay (RIPA)
buffer (Cell Signaling Technology, Inc.). A total of 20 µg
protein per lane was loaded on pre-cast 10% NuPAGE Bis-Tris Gels
(Thermo Fisher Scientific, Inc.). Anti-GAPDH (Cell Signaling
Technology, Inc.; cat. no. 3683; dilution, 1:10,000) was used as a
loading control. Following protein transfer to a polyvinylidene
difluoride membrane (Biozol) and blocking with 5% non-fat dry milk
in PBS (Biozol), primary antibodies were incubated at 4°C overnight
and secondary antibodies were incubated at room temperature for 60
min. The primary antibodies and concentrations were as follows:
Rabbit anti-hnRNP K (Biozol; cat. no. LS-C30312-50; 1:1,000); mouse
anti-phospho-p53 (Ser15; Cell Signaling Technology, Inc.; cat. no.
9286; 1:1,000); mouse anti-p53 (Cell Signaling Technology, Inc.;
cat. no. 48818; 1:1,000); rabbit anti-CDKN1A
(p21WAF1/CIP1; LifeSpan Biosciences; Biozol; cat. no.
LS-B9242; 1:1,000); rabbit GAPDH (HRP-conjugated; Cell Signaling
Technology, Inc.; cat. no. 3683; 1:10,000). The secondary
antibodies were as follows: Goat anti-rabbit (HRP-conjugated; Dako,
Glostrup, Denmark; cat. no. P044801-2; 1:10,000); rabbit anti-mouse
(HRP-conjugated; Dako; P016102-2; 1:10,000). Digital image
acquisition was performed using the myECL™ Imager system (Thermo
Fisher Scientific, Inc.). For protein co-immunoprecipitation, the
MultiMACS™ Protein A/G kit (Miltenyi Biotech, Bergisch Gladbach,
Germany) was used according to the manufacturer’s protocol.
Briefly, the cells were lysed with RIPA buffer. Following
incubation of the whole cell lysate with 2 µg of anti-p53
antibody, the antibody-p53-complex was labeled with 50 µl
µMACS Protein G MicroBeads for 30 min on ice. The
µMACS columns were placed in the magnetic field of the
µMACS separator. The non-bound protein fraction was
collected prior to washing the columns four times using the
µMACS washing buffer. Subsequently, the
co-immunoprecipitated proteins were eluted with pre-heated (95°C)
1X SDS gel loading buffer. For the analysis of non-specific protein
binding, the cell lysates were incubated with microbeads only. The
protein fractions were analyzed by SDS-PAGE and western blot
analysis as described above.
Immunofluorescence (IF) microscopy
The IF staining was performed as previously
described (13) using a rabbit
monoclonal antibody targeting hnRNP K (1:250, Biozol; cat. no.
LS-C30312-50)/mouse monoclonal antibody targeting Ser15-phospho-p53
(1:250, Cell Signaling Technology, Inc.; cat. no. 9286).
Fluorescence-labeled secondary antibody (Alexa Fluor®
488-conjugate; donkey polyclonal anti-rabbit, 1:500; Thermo Fisher
Scientific, Inc.; cat. no. A-21206) and Texas-Red-X-conjugate (goat
polyclonal anti-mouse, 1:500; Thermo Fisher Scientific, Inc.; cat.
no. T-6390). Primary and secondary antibodies were incubated for 1
h at room temperature in the dark. For image acquisition, a Zeiss
Axioimager 2i fluorescence microscope and the ISIS fluorescence
imaging system (MetaSystems, Altlussheim, Germany) were used.
Statistical analysis
Differences in IHC expression levels and between
fractions of vital tumor cells (comparisons between two groups)
were calculated by Student’s t-test (two-tailed) using GraphPad
software (v.6; GraphPad Software, Inc., La Jolla, CA, USA).
Contingency analyses (correlations between protein expression
levels) were performed by χ2 test in GraphPad. P<0.05
was considered to indicate a statistically significant
difference.
Results
Clinicopathological characteristics
There were 68 patients (49 men, 19 women) and 82
tissue samples (68 resection specimens and 14 corresponding
pre-therapeutic biopsies) included in the present study. The
detailed clinicopathological characteristics are summarized in
Table I. The median patient age
was 62.5 years (range, 32–88 years). In 10 patients (15%), there
was complete pathological remission following neoadjuvant RCTx
(ypT0, Dworak grade 4), whereas in 58 patients (85%), there were
variable levels of residual tumor cells. A total of 24 patients
(36%) had positive lymph nodes following therapy (ypN+)
sand nine patients (15%) had KRAS codon 12/13 mutations.
However, more detailed analysis of the data revealed that
KRAS codon 12/13 mutations were detectable in 7/23 (30%) of
pre-therapeutic tissue samples, but in only 2/36 (6%) of
post-therapy resection specimens (Table II).
 | Table IIDetailed results from KRAS
mutation analysis. |
Table II
Detailed results from KRAS
mutation analysis.
| Codon | n (%) |
|---|
| Samples for
mutation testing | 59 (100) |
| KRAS codon
12/13 mutation (n=59) | |
| Codon 12/13
mutation | 9 (15) |
| Codon 12/13
wild-type | 50 (85) |
| Analysis from
pre-treatment material (n=23) | |
| Codon 12/13
mutation | 7 (30) |
| Codon 12/13
wild-type | 16 (70) |
| Analysis from
resection specimen (n=36) | |
| Codon 12/13
mutation | 2 (6) |
| Codon 12/13
wild-type | 34 (94) |
Expression of hnRNP K, p53 and p21 in
rectal adenocarcinoma samples
The staining results for hnRNP K, p53 and p21 are
summarized in Table I.
Representative microphotographs of absent (0), moderate (1) and strong (2) cytoplasmic staining intensities are
shown in Fig. 2A. The healthy
mucosa showed immunoreactivity for hnRNP K restricted to cell
nuclei, whereas p53 was expressed only in the nuclei of a
subpopulation of basal crypt cells; there was no detectable p21
immunostaining in healthy colonic mucosa (left panel). Uniform
cytoplasmic hnRNP K expression was only detectable in tumor tissue,
and RCTx led to further upregulation of cytoplasmic hnRNP K;
however, this result was not statistically significant (Fig. 2Ba; P=0.2669). No significant
differences in the expression levels of p53 and p21 were identified
when comparing post-therapeutic resection specimens to correlating
pre-therapeutic biopsies (Fig. 2Bb
and c; P=0.6267 and 0.4653, respectively). The fraction of
vital tumor cells following RCTx was significantly higher in hnRNP
K/p21-positive cases (P=0.0047 and 0.0223, respectively) and there
was a significant correlation between the expression levels of
hnRNP K and p53 (P=0.0125), but not p21 (P=0.2255) (Fig. 2Ca-c). No correlation was found
between the fraction of vital tumor cells following RCTx and the
expression of p53 (Fig. 2Cb;
P=0.4821). There was also no significant correlation between pre-
or post-therapeutic hnRNP K, the expression of p53 or p21 and any
clinicopathological characteristic (age, sex, ypT/N stage or
KRAS mutation status) or between hnRNP K/p21 and p53/p21
staining intensities (P>0.05; data not shown).
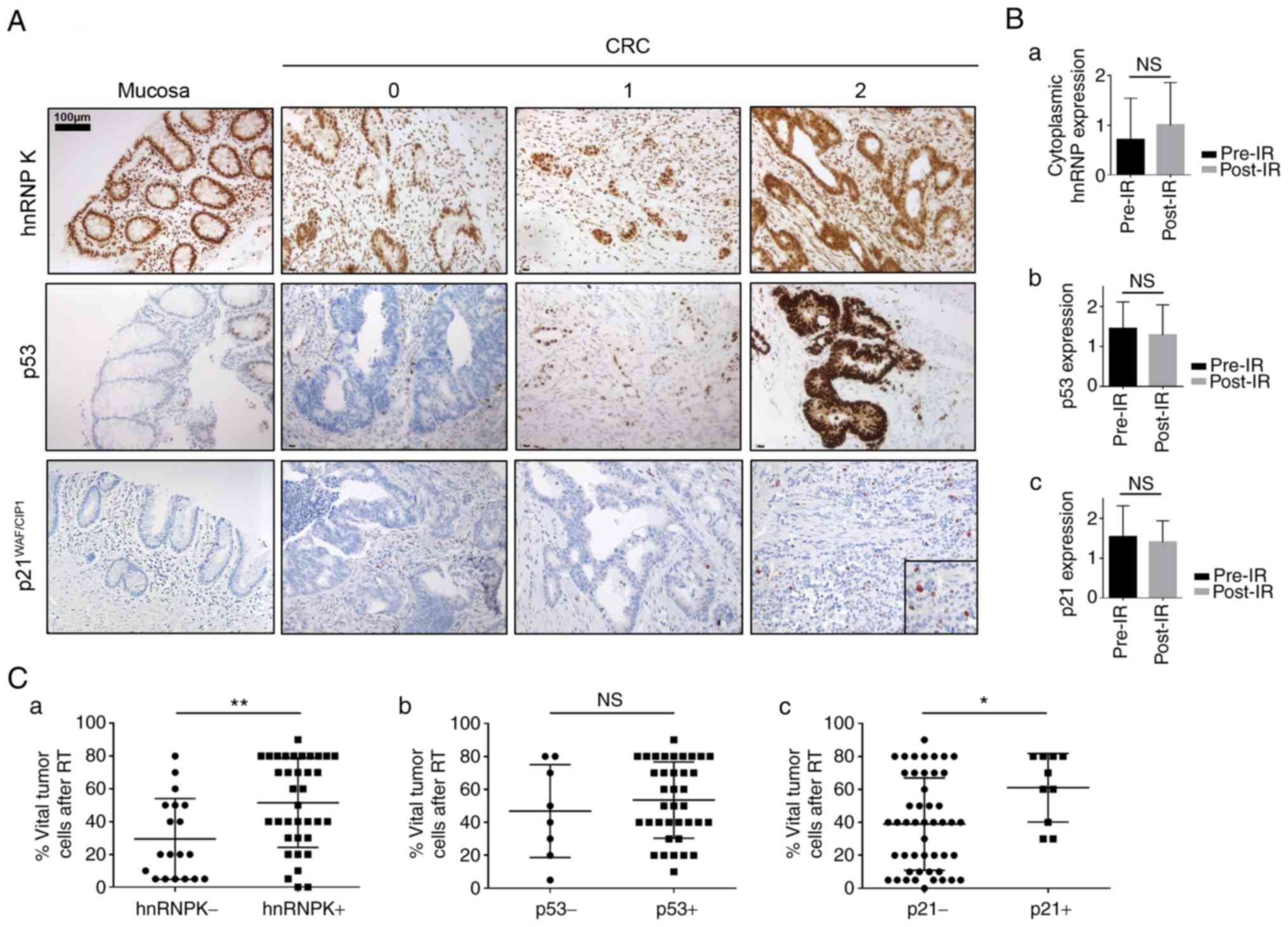 | Figure 2IHC staining for hnRNP K, p53 and p21
in rectal adenocarcinoma samples. (A) representative
microphotographs of absent/weak (0), moderate (1) and strong (3) staining intensities; the left panel
shows expression patterns of hnRNP K, p53 and p21 in healthy
colonic mucosa. Scale bar=100 µm. (B) Comparison
between immunostaining intensities for (a) hnRNP K, (b) p53 and (c)
p21 pre- and post-IR. (C) Fraction of vital tumor cells (tumor
regression) in post-therapeutic surgical specimens with respect to
expression levels of (a) hnRNP K, (b) p53 and (c) p21.
*P<0.05; **P<0.01. hnRNP K,
heterogeneous nuclear ribonucleoprotein K; CRC, colorectal cancer;
IHC, immunohistochemistry; IR, ionizing radiation; RT,
radiochemotherapy; NS, not significant. |
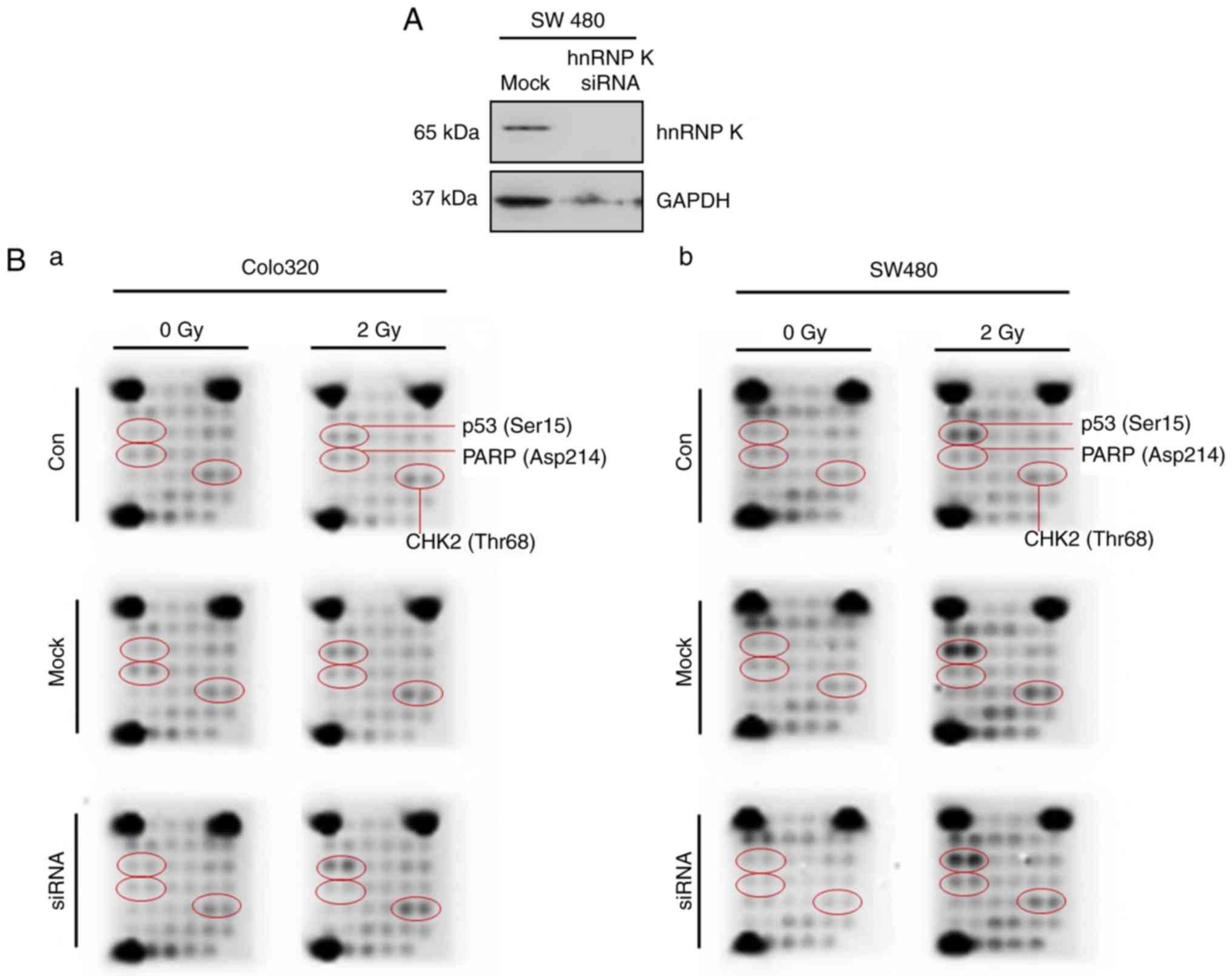
PathScan intracellular signaling array
upon IR and hnRNP K siRNA knockdown in vitro
To assess the effect of IR on tyrosine kinase
signaling and the role of hnRNP K in the radioresistance of human
CRC cells in vitro, Colo320 and SW480 cells were subjected
to 2 Gy gammairradiation. Successful siRNA knockdown of hnRNP K was
confirmed by western immunoblotting compared with mock-transfected
cells (Fig. 3A). In the untreated
Colo320 cells, IR led to a marginal increase in p53 phosphorylation
(Ser15) and a marginal increase in poly (ADP-ribose) polymerase
(PARP) cleavage and CHK2 (Thr68) phosphorylation (Fig. 3Ba). However, these alterations
were neither affected by mock-siRNA nor by hnRNP K-siRNA
transfection. There was marked Ser15 phosphorylation in response to
IR in SW480 cells, however, this effect was not altered by hnRNP K
siRNA knockdown (Fig. 3Bb). Only
a marginal increase was found in PARP cleavage and CHK2 (Thr68)
phosphorylation in response to IR.
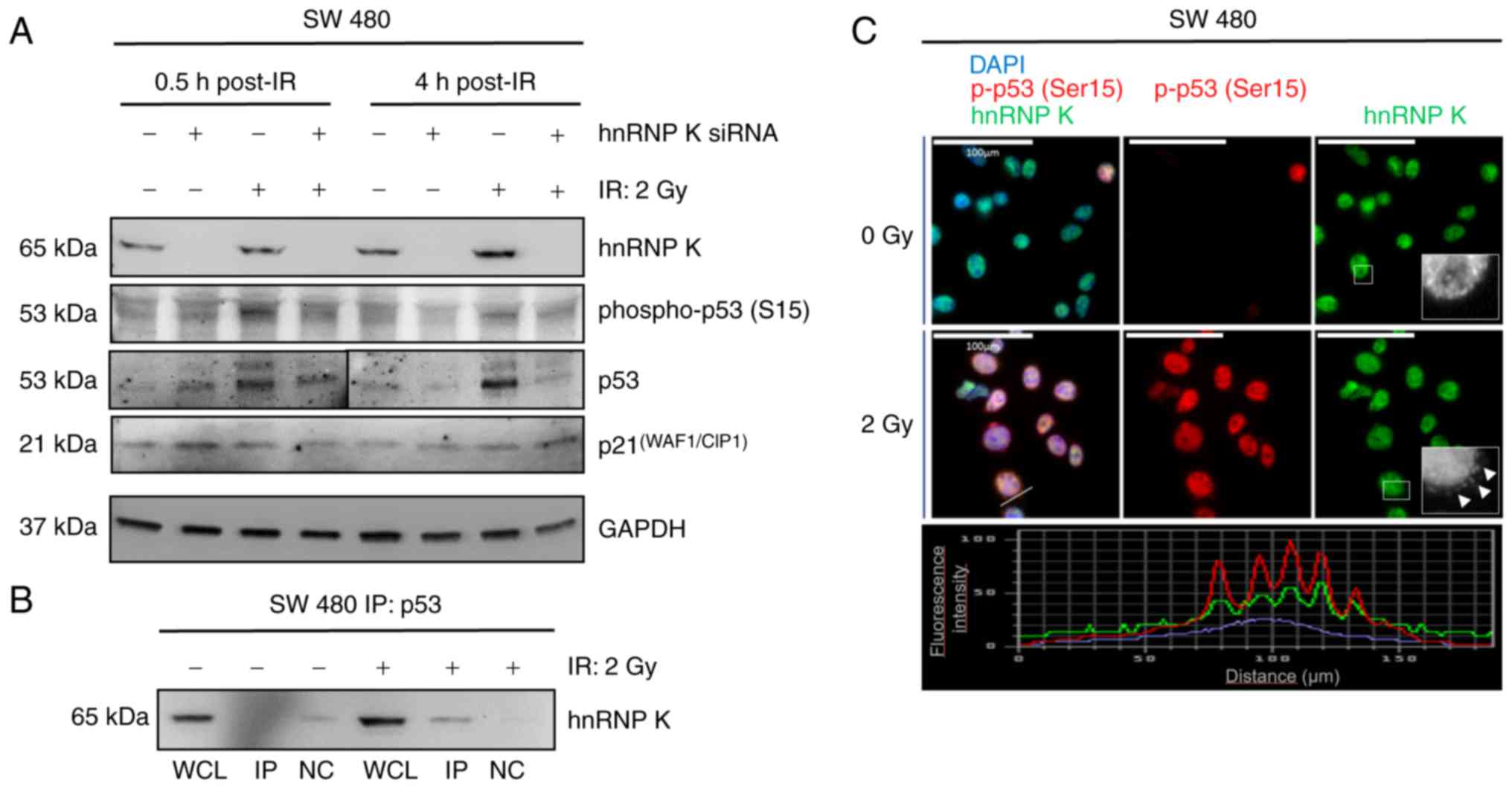
Effect of hnRNP K siRNA knockdown on the
expression of p53, p53 Ser15-phosphorylation, p53/hnRNP K
interaction and expression of p21 in vitro
To assess early and late effects of IR and hnRNP K
on p53 phosphorylation and the expression level of p21, western
immunoblotting was performed for hnRNP K, p53, phospho-p53 (Ser15),
and p21 in the mock-transfected and hnRNP K siRNA-transfected cells
0.5 and 4 h following exposure to 2 Gy gamma-irradiation (Fig. 4A). Successful siRNA-knockdown was
again shown through western immunoblotting for hnRNP K. An increase
in p53 Ser15-phosphorylation was detected 0.5 h and, to a lesser
extent, 4 h post-IR. This change was mirrored by an increase in the
expression of p53, however, these effects were eliminated by siRNA
knockdown of hnRNP K at 0.5 and 4 h following IR. p53
Ser15-phosphorylation remained detectable upon hnRNP K knockdown,
and no changes in the expression of p21were observed in response to
IR in the presence or absence of hnRNP K. The
co-immunoprecipitation experiments verified enhanced hnRNP K/p53
complex formation upon 2 Gy gamma-irradiation (Fig. 4B), and immunofluorescence
microscopy confirmed the enhanced p53 Ser15-phosphorylation and
cytoplasmic co-localization of hnRNP K and Ser-15 phosphorylated
p53 in the 2 Gy-irradiated SW480 cells 0.5 h following exposure to
IR (Fig. 4C).
Discussion
hnRNP K is upregulated in several malignancies and
is involved in the regulation of transcription, mRNA maturation and
translation (10,12-14). The protein has also been shown to
be a key element of the DNA damage response pathway through
interaction with p53 and induction of p53 target genes, including
p21, as a transcriptional cofactor for p53 (16,18). In the present study, the aim was
to evaluate whether hnRNP K is involved in the radioresistance of
rectal adenocarcinomas and, if this is the case, to elucidate the
molecular mechanisms underlying hnRNP K-mediated resistance to
RCTx. The sample cohort comprised 68 patients with a median age of
62.5 years, representing the typical age group for (colo) rectal
carcinoma, with a preponderance of males (1). There was complete tumor regression
upon neoadjuvant therapy (ypT0; complete pathological remission,
Dworak grade 4) in 15% of patients, reflecting data from literature
(6). KRAS codon 12/13
mutations were only detected in 15% of patients. The literature
suggests that a proportion of 30-52% of CRCs harbor mutations in
the RAS oncogene, depending on which RAS isoforms
(KRAS or NRAS) and hotspots (codon 12/13, 59, 61, 117
and 146) are taken into account (21,22). A closer look at the results of the
present study revealed that mutation analyses in pre-therapeutic
tissue resulted in 30% KRAS-mutant tumors, which was in line
with published data (21),
whereas analyses in post-therapeutic specimens yielded only 6%
mutation-positive tumors. This may be due to the fact that, in
certain specimens, a good response to neoadjuvant therapy led to
extensive scarring with minimal residual tumor cells, which led to
the diagnostic limit for reliable detection of RAS mutation
not being reached in these cases. In the present study, Sanger and
next-generation sequencing were used for KRAS codon 12/13
mutation analyses. Sanger sequencing has a reported limit of
detection of 10-20% allele frequency, whereas next-generation
sequencing can detect mutations down to an allele frequency of 0.2%
depending on the DNA quality of the FFPE specimens (23,24). However, in certain ypT1/Dworak 3
tumors, the actual mutated allele frequency may have been below
that diagnostic threshold. Together with the limitation that only
KRAS codons 12 and 13 mutations were analyzed, the results
have to be interpreted with caution regarding the impact of
RAS mutations, and the limitations may explain why it was
not possible to verify the association between RAS mutations
and radioresistance that has been previously reported (25,26).
No significant differences were observed between
pre- and post-therapeutic levels of hnRNP K, p53 or p21 when
comparing pre-therapeutic biopsies to post-therapeutic resection
specimens. For hnRNP K, this observation is in line with our
previous findings from malignant melanoma and from CRC, where IR
induced a rapid, but temporal increase in the expression of hnRNP K
(13,15). High expression levels of hnRNP K
and p21 in the resection specimens correlated with poor response to
prior RCTx, supporting a role for these proteins in
radioresistance, and between expression levels of hnRNP K and p53.
As IR-induced upregulation of p53 in intestinal epithelium has been
previously described, and the hnRNP K/p53 interaction is a key
element in the cellular response to IR in U2OS osteosarcoma cells,
this result suggests a possible role for the interaction of hnRNP K
and p53 in radioresistance of rectal adenocarcinoma, which may be
mediated via p21 in CRC (16,27).
To elucidate the molecular mechanisms underlying
hnRNP K-mediated radioresistance, the present study used an in
vitro irradiation model using SW480 and Colo320 CRC cell lines.
In line with published data, PathScan intracellular signaling array
revealed the increased phosphorylation of p53 Ser15, PARP cleavage
and phosphorylation of CHK2 (Thr68) (28-30). However, these effects were not
affected by the siRNA-mediated knockdown of hnRNP K, and thus
appeared to occur independently of the presence of hnRNP K. This
result is supported by data from Moumen et al (16), having previously shown IR-induced
p53 Ser15-phosphorylation in hnRNP K-depleted cells. The SW480 line
was used for further hnRNP K knockdown experiments due to its
individual molecular profile. This cell line harbors two p53 point
mutations that are frequently observed in CRC
(Arg273His/Pro309Ser), however, p53 still binds its consensus DNA
sequences with wild-type affinity and constitutively activates p21
in these cells (19,31). In addition, SW480 cells carry a
KRAS G12V mutation, and our previous study identified hnRNP
K to be a key factor in RAS-mediated radioresistance in CRC
(15). Western immunoblotting of
cell lysates was performed, and it was found that, in line with the
results from PathScan, radiation had increased p53
Ser15-phosphorylation and the expression of p53 at 0.5 and 4 h
post-IR. The marginal decrease in Ser15-phosphorylation following
transfection with siRNA targeting hnRNP K may be due to the
observed downregulation of the protein level of p53 upon hnRNP K
knockdown; this is supported by the correlation between the
expression of hnRNP K and p53 found in post-radiation patient
samples. There were no detectable changes in expression levels of
p21 upon IR. However, 2 Gy irradiation induced hnRNP K/p53 complex
formation, as demonstrated by protein co-immunoprecipitation of
hnRNP K via p53 and cytoplasmic co-localization of hnRNP K and
Ser15-phosphorylated p53 in immunofluorescence microscopy. These
results confirmed that, although IR upregulated hnRNP K and p53 and
enhanced the interaction of hnRNP K with (phospho-)p53 in the
cytoplasm, p53 Ser15-phosphorylation occurred independently from
the presence of hnRNP K. There were no changes in the expression of
p21 in response to IR, and basal levels of p21 remained detectable
following hnRNP K siRNA knockdown.
Taken together, the results of the present study add
to the evidence that hnRNP K is important in the radioresistance of
CRC, comparable to our previous results and findings in other
tumors (13,15). The radioprotective effect of hnRNP
K appeared to be independent of the hnRNP K-mediated caspase
inhibition that has been described previously (32), as the knockdown of hnRNP K had no
effect on PARP cleavage in the in vitro irradiation model.
The effect may instead be mediated via cytoplasmic interaction of
hnRNP K with phosphorylated p53, as the expression levels of the
two proteins in post-therapy tissue specimens were significantly
correlated, and gamma-irradiation enhanced cytoplasmic hnRNP K/p53
complex formation and hnRNP K/phospho-p53 co-localization in
vitro. However, it is unlikely to be mediated via the induction
of p21 downstream in the p53 pathway, which is known to be
activated upon IR in human osteosarcoma (U2OS) cells (18). Instead, as hnRNP K has been shown
to regulate the maturation and translation of multiple target mRNAs
relevant to oncogenic transformation and proliferation, it may be
that the hnRNP K/(phospho-)p53 interaction has an impact on hnRNP
K-mediated mRNA processing (11,34). For example, hnRNP K binds to a
CU-rich element in thymidine phosphorylase (TP) mRNA, resulting in
the upregulation of TP and resistance to apoptosis in
nasopharyngeal carcinoma (34).
In addition, hnRNP Q has been shown to bind to the 5′-untranslated
region of mouse p53 mRNA and thus regulates the translation of p53,
which suggests the possibility of a similar regulatory circuit
involving hnRNP K and p53 in CRC cells (35). Further investigations are required
to clarify whether the IR-induced hnRNP K/p53 interaction in the
cytoplasm modulates the binding of hnRNP K to its mRNA targets, and
whether hnRNP K is involved in regulating the protein expression of
p53. Elucidating these mechanisms may ultimately assist in the
identification of radiosensitizing compounds to improve biological
response to radiotherapy applied to rectal adenocarcinomas in a
neoadjuvant or palliative setting.
In conclusion, the results of the present study
support a radioprotective role for hnRNP K in rectal
adenocarcinoma, which may be mediated through its interaction with
p53. However, this effect appears to be independent of the hnRNP
K/p53-induced upregulation of p21.
Funding
The Manfred Stolte-Stiftung für Gastroenterologische
Pathologie provided financial support for KSte, EW and SE. The
Medizinerkolleg Münster provided financial support for WD. The
Deutsche Forschungsgemeinschaft) provided financial support for
KSte (grant no. STE 2467/1-1). The Medical Faculty of the
University of Münster Münster provided financial support for KSte,
JS and KSto (grant no. I-SP111504). The study sponsors were not
involved in the study design, in the collection, analysis and
interpretation of data, in the writing of the manuscript, or in the
decision to submit the manuscript for publication.
Availability of data and materials
All data generated or analyzed in the present study
are included in this published article.
Authors’ contributions
KSteinestel and WD performed (immuno-)histological
analyses; WD, KStock and JS performed KRAS mutation
analyses. SE performed in vitro analyses. KSteinestel, EW,
MP, SH and SE conceived the study, were involved in its design and
coordination, and assisted in drafting the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All tissue samples were collected for histologic
examination and diagnostic purposes and anonymized for use in the
study in accordance with The Code of Ethics of the World Medical
Association (Declaration of Helsinki). Informed consent was
therefore not required. The study received institutional review
board approval from the Ethics Committee of the University of
Münster (no. 2015-628-f-S).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
CRC
|
colorectal carcinoma
|
|
hnRNP K
|
heterogeneous nuclear
ribonucleoprotein K
|
|
IR
|
ionizing radiation
|
|
pCR
|
pathological complete remission
|
|
RCTx
|
radiochemotherapy
|
Acknowledgments
The authors would like to thank Ms. Inka Buchroth
and Ms. Claudia Schlosser for their technical assistance.
References
|
1
|
Siegel R, DeSantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei EK, Giovannucci E, Wu K, Rosner B,
Fuchs CS, Willett WC and Colditz GA: Comparison of risk factors for
colon and rectal cancer. Int J Cancer. 108:433–442. 2004.
View Article : Google Scholar
|
|
3
|
Kapiteijn E, Marijnen CA, Nagtegaal ID,
Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B,
van Krieken JHJ, et al: Preoperative radiotherapy combined with
total mesorectal excision for resectable rectal cancer. N Engl J
Med. 345:638–646. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cammà C, Giunta M, Fiorica F, Pagliaro L,
Craxì A and Cottone M: Preoperative radiotherapy for resectable
rectal cancer: A meta-analysis. JAMA. 284:1008–1015. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmiegel W, Pox C, Reinacher-Schick A,
Adler G, Fleig W, Fçlsch U, Frühmorgen P, Graeven U, Hohenberger W,
Holstege A, et al: S3-Leitlinie, Kolorektales Karzinom Ergebnisse
evidenzbasierter Konsensuskonferenzen am 6./7. Februar 2004 und am
8./9. Juni 2007 (für die Themenkomplexe IV, VI und VII). Z
Gastroenterol. 46:1–73. 2008.In German.
|
|
6
|
Maas M, Nelemans PJ, Valentini V, Das P,
Rödel C, Kuo L-J, Calvo FA, García-Aguilar J, Glynne-Jones R and
Haustermans K: Long-term outcome in patients with a pathological
complete response after chemoradiation for rectal cancer: A pooled
analysis of individual patient data. Lancet Oncol. 11:835–844.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dworak O, Keilholz L and Hoffmann A:
Pathological features of rectal cancer after preoperative
radiochemotherapy. Int J colorectal dis. 12:19–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Becker K, Mueller JD, Schulmacher C, Ott
K, Fink U, Busch R, Böttcher K, Siewert JR and Höfler H:
Histomorphology and grading of regression in gastric carcinoma
treated with neoadjuvant chemotherapy. Cancer. 98:1521–1530. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wittekind C and Tannapfel A:
Regressionsgrading des präoperativ-radiochemotherapierten
rektumkarzinoms. Der Pathologe. 24:61–65. 2003.In German.
|
|
10
|
Bomsztyk K, Denisenko O and Ostrowski J:
hnRNP K: One protein multiple processes. Bioessays. 26:629–638.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ostareck-Lederer A, Ostareck DH, Cans C,
Neubauer G, Bomsztyk K, Superti-Furga G and Hentze MW:
c-Src-mediated phosphorylation of hnRNP K drives translational
activation of specifically silenced mRNAs. Mol Cell Biol.
22:4535–4543. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carpenter B, McKay M, Dundas S, Lawrie L,
Telfer C and Murray G: Heterogeneous nuclear ribonucleoprotein K is
over expressed, aberrantly localised and is associated with poor
prognosis in colorectal cancer. Br J Cancer. 95:921–927. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eder S, Lamkowski A, Priller M, Port M and
Steinestel K: Radiosensitization and downregulation of
heterogeneous nuclear ribonucleoprotein K (hnRNP K) upon inhibition
of mitogen/extracellular signal-regulated kinase (MEK) in malignant
melanoma cells. Oncotarget. 6:17178–17191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barboro P, Ferrari N and Balbi C: Emerging
roles of heterogeneous nuclear ribonucleoprotein K (hnRNP K) in
cancer progression. Cancer Lett. 352:152–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eder S, Arndt A, Lamkowski A, Daskalaki W,
Rump A, Priller M, Genze F, Wardelmann E, Port M and Steinestel K:
Baseline MAPK signaling activity confers intrinsic radioresistance
to KRAS-mutant colorectal carcinoma cells by rapid upregulation of
heterogeneous nuclear ribonucleoprotein K (hnRNP K). Cancer Lett.
385:160–167. 2017. View Article : Google Scholar
|
|
16
|
Moumen A, Masterson P, O’Connor MJ and
Jackson SP: hnRNP K: An HDM2 target and transcriptional coactivator
of p53 in response to DNA damage. Cell. 123:1065–1078. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Enge M, Bao W, Hedström E, Jackson SP,
Moumen A and Selivanova G: MDM2-dependent downregulation of p21 and
hnRNP K provides a switch between apoptosis and growth arrest
induced by pharmacologically activated p53. Cancer cell.
15:171–183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moumen A, Magill C, Dry KL and Jackson SP:
ATM-dependent phosphorylation of heterogeneous nuclear
ribonucleoprotein K promotes p53 transcriptional activation in
response to DNA damage. Cell Cycle. 12:698–704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimura T, Kakuda S, Ochiai Y, Nakagawa H,
Kuwahara Y, Takai Y, Kobayashi J, Komatsu K and Fukumoto M:
Acquired radiore-sistance of human tumor cells by
DNA-PK/AKT/GSK3β-mediated cyclin D1 overexpression. Oncogene.
29:4826–4837. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bae I, Fan S, Bhatia K, Kohn KW, Fornace
AJ and O’Connor PM: Relationships between G1 arrest and
stability of the p53 and p21Cip1/Waf1 proteins following
γ-irradiation of human lymphoma cells. Cancer Res. 55:2387–2393.
1995.PubMed/NCBI
|
|
21
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D
and Jassem J: Panitumumab-FOLFOX4 treatment and RAS mutations in
colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fernández-Medarde A and Santos E: Ras in
cancer and developmental diseases. Genes Cancer. 2:344–358. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moskalev EA, Stöhr R, Rieker R, Hebele S,
Fuchs F, Sirbu H, Mastitsky SE, Boltze C, König H and Agaimy A:
Increased detection rates of EGFR and KRAS mutations in NSCLC
specimens with low tumour cell content by 454 deep sequencing.
Virchows Arch. 462:409–419. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsiatis AC, Norris-Kirby A, Rich RG, Hafez
MJ, Gocke CD, Eshleman JR and Murphy KM: Comparison of sanger
sequencing, pyrosequencing, and melting curve analysis for the
detection of KRAS mutations: diagnostic and clinical implications.
J Mol Diagn. 12:425–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang M, Kern AM, Hulskotter M, Greninger
P, Singh A, Pan Y, Chowdhury D, Krause M, Baumann M, Benes CH, et
al: EGFR-mediated chromatin condensation protects KRAS-mutant
cancer cells against ionizing radiation. Cancer Res. 74:2825–2834.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gupta AK, Bakanauskas VJ, Cerniglia GJ,
Cheng Y, Bernhard EJ, Muschel RJ and McKenna WG: The Ras radiation
resistance pathway. Cancer Res. 61:4278–4282. 2010.
|
|
27
|
Qiu W, Leibowitz B, Zhang L and Yu J:
Growth factors protect intestinal stem cells from radiation-induced
apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis.
Oncogene. 29:1622–1632. 2010. View Article : Google Scholar
|
|
28
|
Canman CE, Lim DS, Cimprich KA, Taya Y,
Tamai K, Sakaguchi K, Appella E, Kastan MB and Siliciano JD:
Activation of the ATM kinase by ionizing radiation and
phosphorylation of p53. Science. 281:1677–1679. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ward IM, Wu X and Chen J: Threonine 68 of
Chk2 is phosphorylated at sites of DNA strand breaks. J Biol Chem.
276:47755–47758. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chinnaiyan P, Vallabhaneni G, Armstrong E,
Huang SM and Harari PM: Modulation of radiation response by histone
deacetylase inhibition. Int J Radiat Oncol Biol Phys. 62:223–229.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rochette PJ, Bastien N, Lavoie J, Guérin
SL and Drouin R: SW480, a p53 double-mutant cell line retains
proficiency for some p53 functions. J Mol Biol. 352:44–57. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiao Z, Ko HL, Goh EH, Wang B and Ren EC:
hnRNP K suppresses apoptosis independent of p53 status by
maintaining high levels of endogenous caspase inhibitors.
Carcinogenesis. 34:1458–1467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Evans JR, Mitchell SA, Spriggs KA,
Ostrowski J, Bomsztyk K, Ostarek D and Willis AE: Members of the
poly (rC) binding protein family stimulate the activity of the
c-myc internal ribosome entry segment in vitro and in vivo.
Oncogene. 22:8012–8020. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen LC, Liu HP, Li HP, Hsueh C, Yu JS,
Liang CL and Chang YS: Thymidine phosphorylase mRNA stability and
protein levels are increased through ERK-mediated cytoplasmic
accumulation of hnRNP K in nasopharyngeal carcinoma cells.
Oncogene. 28:1904–1915. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim D, Kim W, Lee K, Kim S, Lee H, Kim H,
Jung Y, Choi J and Kim K: hnRNP Q regulates translation of p53 in
normal and stress conditions. Cell Death Differ. 20:226–234. 2013.
View Article : Google Scholar :
|