Introduction
Neuromyelitis optica (NMO) is a severely disabling
inflammatory disease of the central nervous system that
predominantly affects the optic nerves and the spinal cord
(1). The prevalence of NMO
worldwide ranges from 0.5-4.4 cases per 100,000 (2,3)
and the mortality rate directly associated with NMO attacks ranges
from 7 to 30%, predominantly attributable to neurogenic respiratory
failure (4-6). Most of the treatments for acute
attacks and NMO relapses are targeted to controlling the symptoms,
although large-scale randomized trials have been too scarce to
allow optimization of the treatment strategies for NMO (7). Furthermore, immune modulatory and
anti-inflammatory agents have not been demonstrated to influence
the course of NMO progression and therefore are not suitable for
curing chronic neurological disability. Therefore, novel treatment
modalities to alleviate the disability resulting from NMO have
evoked considerable interest. Much attention has been focused on
restorative therapy, particularly the use of stem cells.
Mesenchymal stem cells (MSCs) are adult
non-hematopoietic pluripotent cells with a high capacity to be
induced to differentiate into other cell lines, including
adipocytes, chondrocytes and stromal cells (8). Certain characteristics make MSCs
attractive as therapeutic candidates, including innate
pluripotency, the capacity to facilitate the repair of lesions
through multiple avenues in immune regulation, angiogenesis and
neurogenesis, and their immune privileged status (9). MSCs may be isolated from various
sources, including bone marrow, adipose tissue (10), umbilical cord (11) and peripheral blood, among which
the major and most frequently studied source is the bone marrow.
Furthermore, a growing body of pre-clinical studies has
consistently demonstrated that bone marrow-derived MSCs (BM-MSCs)
are capable of safely ameliorating clinical outcomes of certain
neurological degenerative diseases, including stroke (12) and multiple sclerosis (13,14).
A recent paper published by the authors' laboratory
revealed that the transplantation of autologous BM-MSCs exerted
positive effects in the treatment of NMO (15). However, little is known about the
cytological features of BM-MSCs from patients with NMO. In the
present study, a comprehensive comparison of the cytological
features of BM-MSCs from patients with NMO and healthy subjects was
conducted. The results obtained will provide a general
understanding of BM-MSCs derived from patients with NMO and may
assist in the optimization of culture and cell proliferation
protocols, thereby leading to improved MSC transplantation
results.
Materials and methods
Patients
Informed consent was obtained from all participants
in the present study and the study was approved by the Tianjin
Medical University General Hospital Institutional Review Board and
Ethics Committee (Tianjin, China). Patients with NMO were recruited
between January 2016 and December 2017 from Tianjin Medical
University General Hospital. The five patients (all female; patient
characteristics are presented in Table SI) were enrolled on the
basis of the following criteria: i) They conformed with the
diagnostic criteria proposed by Wingerchuk et al (3) in 2007; ii) exclusion criteria
included a history of diabetes, cardio- or cerebrovascular diseases
and inflammatory, and autoimmune diseases other than NMO; iii) none
of the patients had received corticosteroid or other
immunosuppressant therapy within the last 4 weeks; and iv) all
patients exhibited no other autoimmune diseases and all patients
with NMO were monitored over a one year period (detailed
information are described in Table SI). A total of five age-matched
healthy female volunteers were enrolled as health controls.
Isolation and identification of
BM-MSCs
Aliquots of 5-10 ml adult donor bone marrow
aspirates were provided by the Department of Hematology of Tianjin
Medical University General Hospital. Bone marrow-derived
mononuclear cells (BM-MNCs) were isolated and cultured in
Gibco® Dulbecco's Modified Eagle Medium/Nutrient Mixture
F-12 (D-MEM/F-12 medium; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone,
Chicago, IL, USA), 100 U/ml penicillin/streptomycin, 2 mM
L-glutamine (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 2
ng/ml human basic fibroblast growth factor and 10 ng/ml human
epidermal growth factor (PeproTech, Inc., Rocky Hill, NJ, USA).
Platelet-derived growth factor-BB (PDGF-BB) was also purchased from
PeproTech, Inc. Following culture at 37°C in a humid atmosphere
with 5% CO2 for 3 days, the culture medium was
completely replaced and non-adherent cells were removed. When the
cells had reached ~80-85% confluence, the adherent cells were
detached by treatment with 0.125% trypsin and 0.1% EDTA
(Sigma-Aldrich; Merck KGaA), and put back into the medium at a 1:2
dilution under the same culture conditions.
At passage 3, adherent cells were identified by
surface markers with monoclonal antibodies cluster of
differentiation (CD)29, CD166, CD44, CD73, CD34 and CD90
(BioLegend, Inc., San Diego, CA, USA), CD45 (BD Pharmingen; Becton,
Dickinson and Company, Franklin Lakes, NJ, USA), CD105 (Abcam,
Cambridge, MA, USA) using a FACS flow cytometer (BD Biosciences,
Becton, Dickinson and Company; the details of all the antibodies
are described in Table I).
 | Table IPhenotype characteristics of bone
marrow-mesen-chymal stem cells. |
Table I
Phenotype characteristics of bone
marrow-mesen-chymal stem cells.
| Surface marker | Patients with NMO
(%) | Healthy control
(%) | P-value |
|---|
| CD29 | 98.35 | 99.01 | NS |
| CD34 | – | – | – |
| CD44 | 96.27 | 95.98 | NS |
| CD45 | – | – | – |
| CD73 | 99.15 | 98.79 | NS |
| CD90 | 95.30 | 96.12 | NS |
| CD105 | 92.28 | 93.19 | NS |
| CD166 | 94.25 | 95.42 | NS |
Morphology of BM-MSCs
BM-MSCs were cultured in DMEM/F-12 medium and
observed under an inverted light microscope. At the same time,
BM-MSCs were stained for β-tubulin for the morphology examination.
BM-MSCs were grown on glass chamber slides at 90% confluence and
fixed with 4% paraformaldehyde at room temperature for 30 min.
Subsequently, the BM-MSCs were first permeated with cold acetone
for 10 min and washed with ice-cold PBS. The BM-MSCs were then
blocked with 5% bovine serum albumin (BSA; Sigma-Aldrich, Merck
KGaA) in PBS at room temperature for 30 min, followed by incubation
with rabbit antihuman β-tubulin antibody (Thermo Fisher Scientific,
Inc.) overnight at 4°C. After three washes with ice-cold PBS, the
BM-MSCs were incubated with goat tetramethylrhodamine-conjugated
goat anti-rabbit immunoglobulin G secondary antibody (Thermo Fisher
Scientific, Inc.; details of the antibodies are described in the
Table II) at room temperature
for 30 min and their nuclei were stained with 1 µg/µl
4′,6-diamidino-2-phenylindole (DAPI) at room temperature for 10
min. The cells were then washed twice with ice-cold PBS and the
images were visualized using a fluorescence microscope (Nikon
Corporation, Tokyo, Japan).
 | Table IIAntibody information for fluorescence
activated cell sorting. |
Table II
Antibody information for fluorescence
activated cell sorting.
| Name | Clone ID | Manufacturer; cat.
no.; RRID | Dilution |
| APC anti-human CD29
antibody | TS2/16 | BioLegend, Inc.
303007, RRID:AB_314323; mouse; monoclonal antibody | 5 µl per
million cells in 100 µl staining volume |
| PE anti-human CD34
antibody | 561 | BioLegend, Inc.;
343605; RRID:AB_1732033; mouse; monoclonal antibody |
| FITC anti-human
CD44 antibody | BJ18 | BioLegend, Inc.;
338803; RRID:AB_1501204; mouse; monoclonal antibody |
| PE anti-human CD166
antibody | 3A6 | BioLegend, Inc.;
343903; RRID:AB_2289303; mouse; monoclonal antibody |
| PerCP/Cyanine 5.5
anti-human CD73 | AD2 | BioLegend, Inc.;
344013; RRID:AB_2561756; mouse; monoclonal antibody |
| FITC anti-human
CD90 antibody | 5.00E+10 | BioLegend, Inc.;
328107; RRID:AB_893438; mouse; monoclonal antibody |
| PerCP/Cyanine 5.5
anti-human CD105 antibody | MEM-229 | Abcam; ab234265;
mouse; monoclonal antibody |
| APC anti-human CD45
antibody | HI30 | BD Biosciences;
560973; RRID:AB_10565969; mouse; monoclonal antibody |
Cell proliferation assay
To assess the proliferation state of the BM-MSCs
cells following the various treatments an MTT (Sigma-Aldrich; Merck
KGaA) proliferation assay was performed according to the
manufacturer's protocol. In brief, BM-MSCs cells were seeded into
96-well plates at a density of 2×104 cells/ml for 1-4
days. An aliquot of 20 µl MTT labeling reagent (5 mg/ml) was
added daily to each well and the plates were incubated at 37°C for
4 h. The resulting formazan crystals were solubilized by adding 100
µl DMSO per well and the plates were incubated at room
temperature for 10 min. The absorbance of formazan was measured at
575 nm and this was taken as the measure for the proliferation
state of the cells. Trypan blue cell exclusion was also used at
room temperature for 5 min to assess cell viability and the cell
number with a light microscope.
Apoptosis of BM-MSCs
The apoptotic rate of the BM-MSCs was evaluated
using a commercial fluorescein thiocyanate (FITC)-Annexin V
apoptosis detection kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China), as recommended by the manufacturer. BM-MSCs
(5×105 cells) were harvested, washed and incubated in
the Annexin V binding buffer. Subsequently, the BM-MSCs were
stained with Annexin V-FITC and propidium iodide (PI), and flow
cytometric analysis was then performed using a flow cytometer (BD
FACSAria™ III) and the data were analyzed with FlowJo 7.6 software
(FlowJo LLC, Ashland, OR, USA). Apoptotic cells were identified in
the early (FITC-Annexin V positive, PI negative) and the late
(FITC-Annexin V and PI double-positive) stages.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to isolate total RNA, following
the manufacturer's protocol and the concentration of total RNA was
measured using a spectrophotometer following treatment with DNase I
(Invitrogen; Thermo Fisher Scientific, Inc.). A sample (2
µg) of RNA was used for reverse transcription in a 20
µl reaction containing EasyScript® reverse
transcriptase kit (TransGen Biotech Co., Ltd., Beijing, China.).
The thermal program consisted of 42°C for 30 min, and then 85°C for
5 sec to inactivate enzymes. RT-qPCR was performed using a Bio-Rad
RT-PCR system instrument (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) with an SYBR®-Green PCR kit (Roche Diagnostics,
Indianapolis, IN, USA). Genes involved in the cell cycle,
proliferation and cell apoptosis were selected for investigation.
Details of all the primers are described in Table III. The thermal cycling program
consisted of 95°C for 10 min, followed by 40 cycles of 15 sec at
95°C and 60 sec at 60°C. Relative quantities of target genes were
determined for unknown samples using the comparative threshold
cycle (ΔΔCq) method (16) and
normalized against GAPDH as the control. Each test sample was
amplified in three different wells with a 20 µl reaction
volume, following the manufacturer's protocol for each specific
experiment and each test was repeated at least in triplicate.
 | Table IIIAntibody information for western
blotting and immunofluorescence. |
Table III
Antibody information for western
blotting and immunofluorescence.
| Name | Immunogen | Manufacturer; cat.
no.; RRID; species | Dilution |
|---|
| CCNA2 | Synthetic peptide
within Human cyclin A2 aa 100-200 (N-terminal) | Abcam; ab32498;
RRID:AB_731777; rabbit; monoclonal antibody | 1:1,000 |
| CDKN2A | Recombinant
full-length protein corresponding to Human CDKN2A/p16INK4a aa
1-156 | Abcam; ab16123;
RRID:AB_302274; mouse; monoclonal antibody | 1:100 |
| AREG | A peptide mapping
near the N-terminus of amphiregulin of human origin | Santa Cruz
Biotechnology, Inc.; sc-27156; RRID:AB_2227600; goat; polyclonal
antibody | 1:500 |
| Bcl-xl | Full length Bcl-xL
of human origin | Santa Cruz
Biotechnology, Inc.; sc-56021; RRID:AB_781603; mouse; monoclonal
antibody | 1:500 |
| Fas | Amino acids 1-335
representing full length FAS of human origin | Santa Cruz
Biotechnology, Inc.; sc-74540; RRID: AB_1121387; mouse; monoclonal
antibody | 1:500 |
| β-actin | A synthetic peptide
corresponding to amino-terminal residues of human β-actin | Cell Signaling
Technology, Inc.; 3700, RRID: AB_2242334; mouse; monoclonal
antibody | 1:2,000 |
| B-tubulin
polyclonal antibody | – | Thermo Fisher
Scientific, Inc.; PA5-16863, RRID:AB_10986058; rabbit; polyclonal
antibody | 1:100 |
| Goat anti-rabbit
IgG, TRITC | – | Thermo Fisher
Scientific, Inc.; A16101, RRID:AB_2534775; goat; polyclonal
antibody | 1:100 |
Differentiation capacity of BM-MSCs
BM-MSCs were induced to differentiate into
osteoblasts and adipocytes using the following procedure. The
induction medium for osteogenesis was Gibco® Iscove's
modified Dulbecco's medium (IMDM; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS, 0.1 µM dexamethasone, 0.2 mM
ascorbic acid 2-phosphate and 10 mM glycerol 2-phosphate
(Sigma-Aldrich; Merck KGaA). The induction medium for adipogenesis
was IMDM supplemented with 10% FBS, 1 µM dexamethasone, 0.5
mM 3-isobutyl-1-methylxanthine, 10 µg/ml insulin and 60
µM indomethacin (Sigma-Aldrich; Merck KGaA). After 3 days,
the culture medium was completely replaced and the medium was
subsequently changed twice weekly thereafter. After the
predetermined culture time had elapsed, adipocytes were stained
with Oil Red O at room temperature for 30 min; the osteoblasts were
identified using alkaline phosphatase (ALP) and Alizarin Red S
assays were performed at room temperature in the dark for 50 min
(Sigma-Aldrich; Merck KGaA).
Western blot analysis
The total proteins of the BM-MSCs were extracted
with Pierce™ IP Lysis buffer (Thermo Fisher Scientific, Inc.) and
the concentration was determined with bicinchoninic acid (BCA)
methods with a BCA protein assay kit (Beijing Solarbio Science
& Technology Co., Lt., Beijing, China). The proteins were
separated using 10% SDS-PAGE and 15 µg protein were loaded
in each lane. Subsequently, the proteins were transferred
electrophoretically to a polyvinylidene difluoride membranes (Merck
KGaA). The membranes were blocked with 5% defatted milk at room
temperature for 1 h and then incubated with the primary antibodies
overnight. The next day, the membranes were incubated with the
HRP-conjugated secondary antibodies for 1 h. Protein bands were
detected using an Amersham ECL Western blotting detection kit (GE
Healthcare Life Sciences, Little Chalfont, UK). The protein bands
were statistically analyzed with ImageJ 1.4 software (National
Institutes of Health, Bethesda, MD, USA). For western blotting
analysis, the β-actin antibody (used as the loading control) was
purchased from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA);
the antibodies against cyclin A2 (CCNA2), cyclin-dependent kinase
inhibitor 2A (CDKN2A), amphiregulin (AREG) and Bcl-xl were from
Cell Signaling Technology, Inc., (Danvers, MA, USA); and the
anti-Fas antibody came from R&D Systems, Inc., (Minneapolis,
MN, USA). All antibodies are described in further detail in
Table II.
Statistical analysis
Each experiment was repeated at least three times.
All data are presented as the mean ± standard deviation. The
difference between means was statistically analyzed using a t-test.
All statistical analyses were performed using GraphPad Prism 6
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
NMO patient-derived BM-MSCs exhibit
normal differentiation capacity and a similar morphology compared
with BM-MSCs derived from healthy controls
Aliquots of 5-10 ml adult donor bone marrow
aspirates (five from patients with NMO and five from age- and
sex-matched healthy subjects) were provided by the Department of
Hematology of Tianjin Medical University General Hospital. After
isolation, BM-MSCs at passage 3 were harvested to analyze their
immunophenotype using flow cytometric analysis. BM-MSCs from
patients with NMO and healthy controls were revealed to express
CD105, CD73, CD90, CD29, CD44 and CD166, although they lacked
expression of CD34 and CD45 (Table
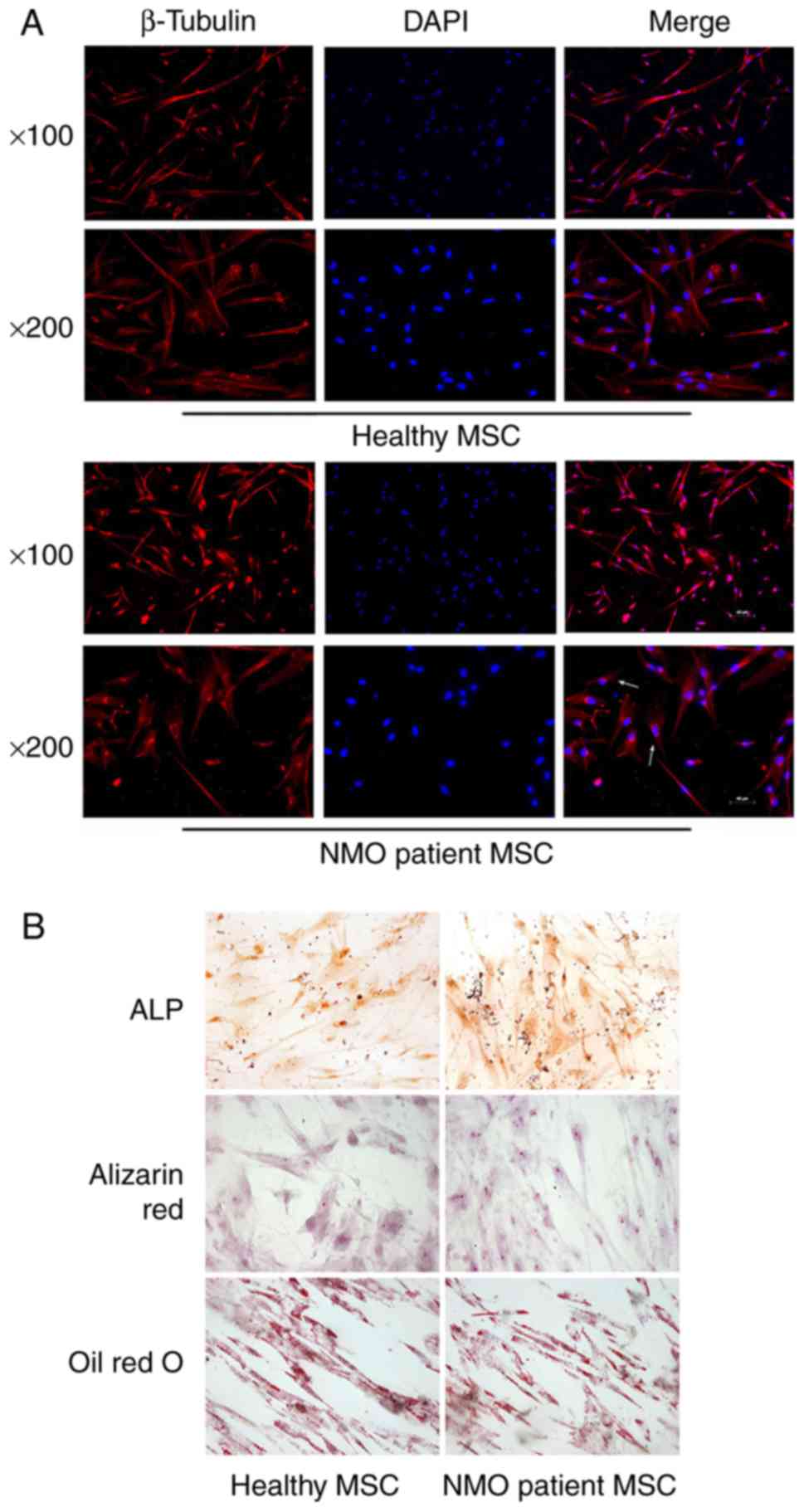
IV). All the BM-MSCs formed a monolayer of bipolar spindle-like
cells, with a 'whirlpool-like' array. However, certain BM-MSCs from
the patients with NMO were observed to be aberrant, with an
irregular and ragged appearance following staining with β-tubulin
under a fluorescence confocal microscope. Compared with the
elongated morphology of the BM-MSCs from the healthy controls,
certain BM-MSCs from patients with NMO also exhibited a
'cobblestone'-like' appearance (Fig.
1A).
 | Table IVQuantitative-polymerase chain
reaction primer sequences. |
Table IV
Quantitative-polymerase chain
reaction primer sequences.
| Genes | Sequence
(5′-3′) |
|---|
| AIF | F:
ATCTACAGGTGCTTCTGG |
| R:
GGCCTTTTTCTGTTTCTG |
| ATM | F:
GTGCCAGAATGATAAAGC |
| R:
GAAGCCAATACTGGACTG |
| BAX | F:
GACGGCAACTTCAACTGG |
| R:
GGAGGAAGTCCAATGTCC |
| Bcl-xl | F:
GCTGGTGGTTGACTTTCT |
| R:
TCCATCTCCGATTCAGTC |
| Caspase 3 | F:
TCTGGAATATCCCTGGAC |
| R:
ATGTTTCCCTGAGGTTTG |
| Caspase 8 | F:
CCTGAGCCTGGACTACAT |
| R:
TCAGGAAGGACAGATTGC |
| CYCS | F:
GCCCCTGGATACTCTTAC |
| R:
GCCCTTTCTTCCTTCTTC |
| FADD | F:
GGTGTCGTCCAGCCTGTC |
| R:
TCCAGGTCGTTCTGCTCC |
| Fas | F:
GATGACCGTCGCTGGAAG |
| R:
CCATCGTGTGTGCCTGCT |
| FasL | F:
CTCCAGGCACAGTTCTTC |
| R:
GTGGTTCCCTCTCTTCTT |
| TNFSF10 | F:
ATGGCTATGATGGAGGTC |
| R:
AGAAACAAGCAATGCCAC |
| TRADD | F:
CAAAATGGGCACGAAGAG |
| R:
CGGTGGATCTTCAGCATC |
| CCNA2 | F:
ACAGTAAACAGCCTGCGTTC |
| R:
CAGGGCATCTTCACGCTCTA |
| CCNB1 | F:
GTGCCAGTGCCAGTGTCTGA |
| R:
CCATTGGGCTTGGAGAGGCA |
| CCNE2 | F:
TGTAACAATCATCTCCTGGC |
| R:
GGAGGTAAAATGGCACAAGG |
| CDC2 | F:
TAGAAAGTGAAGAGGAAGGG |
| R:
TACTGACCAGGAGGGATAGA |
| CDC20 | F:
CAAAGCCAAGGAAGCCGCAG |
| R:
TCACCGCCAGGTTTGCTAGG |
| CDK4 | F:
CGGTGCCTATGGGACAGTGT |
| R:
CAGTCGCCTCAGTAAAGCCA |
| CDK6 | F:
TGCTGAGGCACCTGGAGACC |
| R:
TCAGTGGGCACTCCAGGCTC |
| CDKN2A | F:
TTCCTGGACACGCTGGTGGT |
| R:
GGTTACTGCCTCTGGTGCCC |
| ORC5L | F:
ATACTGGATGCTTTGAGCCG |
| R:
TAGGCAGCATAGAAATCAGC |
| SMAD3 | F:
GCGGTCAAGAGCCTGGTCAA |
| R:
CACAGGCGGCAGTAGATGAC |
| YWHAZ | F:
ATGTTGTAGGAGCCCGTAGG |
| R:
TTTGCTCTCTGCTTGTGAAG |
| AREG | F:
GGTGCTGTCGCTCTTGAT |
| R:
GCATTTCACTCACAGGGG |
| CXCL1 | F:
CCCAAACCGAAGTCATAG |
| R:
CCTTCTGGTCAGTTGGAT |
| DLG7 | F:
ACCTGGTCCAAGACAAAC |
| R:
CTGCTTTGCTGCTTGAGT |
| DLGAP5 | F:
CTACTCAAGCAGCAAAGC |
| R:
GGCAGGTCTTCCTTTACT |
| KITLG | F:
GTCATTGTTGGATAAGCG |
| R:
TCTGGGCTCTTGAATGAT |
The differentiation capabilities of BM-MSCs were
subsequently investigated following induction with the differently
conditioned media. BM-MSCs were able to differentiate into
osteoblasts and adipocytes, as tested by positive staining of ALP
and Alizarin Red S (for osteoblasts), and Oil Red O (for
adipocytes). The results revealed that the BM-MSCs from the healthy
controls and the patients with NMO were easily induced to
differentiate into osteoblasts and the adipocyte lineage (Fig. 1B). There appeared to be no obvious
differences in the differentiation capability between BM-MSCs from
either the healthy controls or the patients with NMO. These data
indicated that there was little discrepancy in morphology between
BM-MSCs from healthy control subjects and the patients with NMO,
since all the BM-MSCs had very similar differentiation
capabilities.
NMO patient-derived BM-MSCs exhibit
decreased proliferative capability in vitro
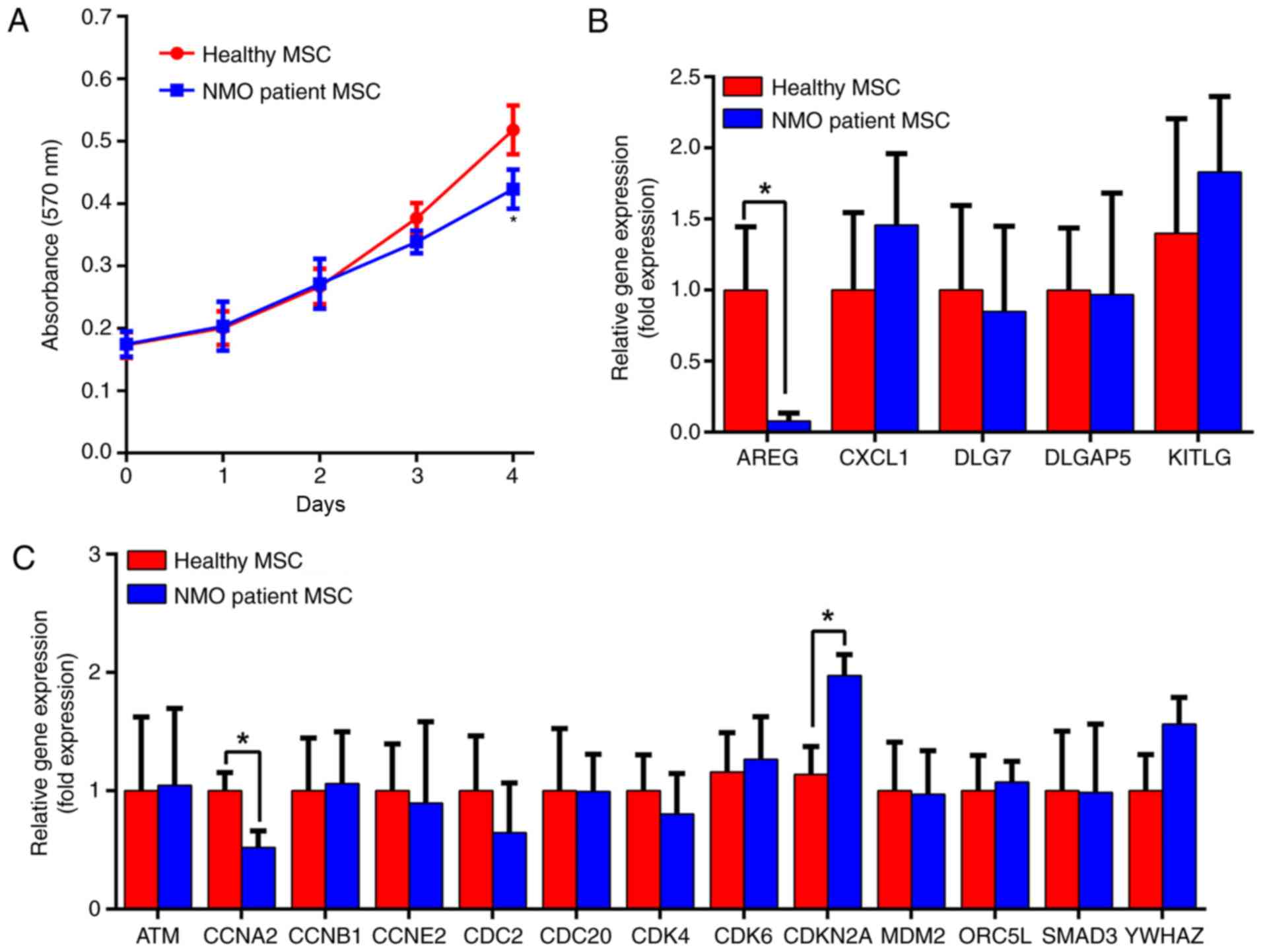
The proliferative capability of the BM-MSCs was
measured using a non-radioactive MTT assay method. The extent of
cell proliferation was measured following culture for 0, 1, 2, 3
and 4 days, in a regular MSC culture medium. As presented in
Fig. 2A, the proliferation rate
of BM-MSCs from the patients with NMO was decreased compared with
the healthy controls from day 3. However, following 4 days of
culture, the proliferation of the BM-MSCs from the patients with
NMO was significantly decreased (P<0.05). In addition, along
with the proliferation assay, the mRNA expression levels of an
array of cell cycle- and proliferation-associated genes in the
BM-MSCs from the patients with NMO and healthy controls were
detected by RT-qPCR (Fig. 2B and
C). These experiments revealed that the expression levels of
AREG and CCNA2 were significantly decreased in BM-MSCs from the
patients with NMO compared with the healthy controls (P<0.05),
whereas the expression of CDKN2A significantly increased in the
patient group (P<0.05). Changes in the protein levels of the
above genes revealed a similar tendency. Three experimental
subjects were randomly selected from the NMO patient group and the
healthy subject group, respectively, and these exhibited the same
tendency (Fig. 3A and B). These
data suggested that the proliferative capabilities of BM-MSCs from
the patients with NMO were lower compared with those of BM-MSCs
from the healthy controls.
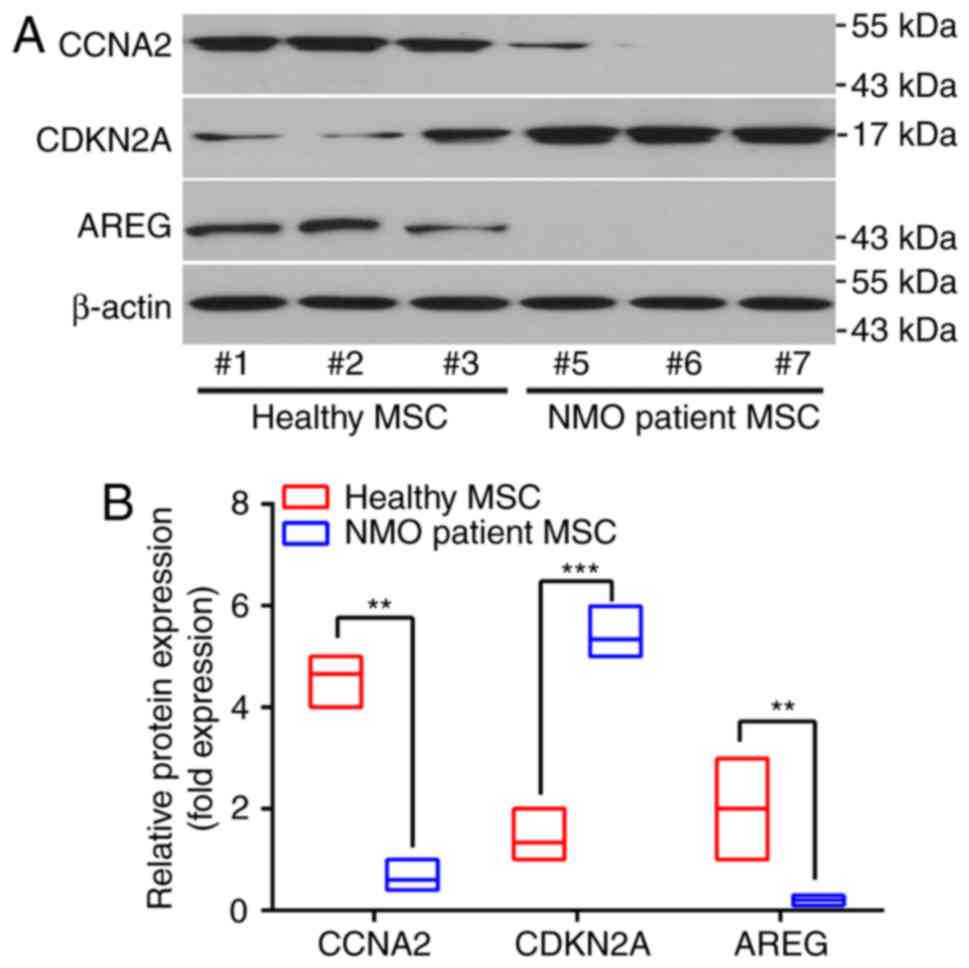 | Figure 3Different protein expression patterns
of BM-MSCs from patients with NMO and healthy controls. (A)
Representative results of the protein expression patterns of AREG,
CCNA2 and CDKN2A, as determined by western blot analysis, are
presented. Samples #1, #2 and #3 were taken from the healthy
volunteers, whereas samples #5, #6, #7 were from the NMO patient
group. (B) The relative protein expression levels of AREG, CCNA2
and CDKN2A. For the western blot analysis, the protein bands were
statistically analyzed with ImageJ software and β-actin was used as
an internal control. Results are presented as the mean ± standard
deviation of three independent experiments and each experiment was
performed in triplicate. **P<0.01 and
***P<0.001 vs. the healthy control. BM-MSC, bone
marrow-derived mesenchymal stem cell; NMO, neuromyelitis optica;
AREG, amphiregulin; CCNA2, cyclin A2; CDKN2, cyclin-dependent
kinase inhibitor 2A. |
NMO patient-derived BM-MSCs exhibit
increased senescence in vitro
The viability of BM-MSCs was subsequently compared
between the patients with NMO and the healthy controls, as
indicated by the apoptotic rate of BM-MSCs under normal culture
conditions. As presented in Fig.
4, the apoptotic rate of BM-MSCs from the patients with NMO was
three times higher compared with the healthy controls. In addition,
the expression levels of an array of cell apoptosis and cell
death-associated genes were compared between the two groups. Of the
genes assessed, the expression of Fas was revealed to be
significantly increased (P<0.05), whereas that of the
pro-survival gene Bcl-xl was significantly decreased in BM-MSCs
from the patients with NMO at the mRNA and the protein levels
(P<0.01; Fig. 4B-D). These
data suggested that senescence may be increased in the BM-MSCs from
patients with NMO compared with the healthy controls. Furthermore,
the BM-MSCs from patients with NMO were at a disadvantage in terms
of their self-renewal capability.
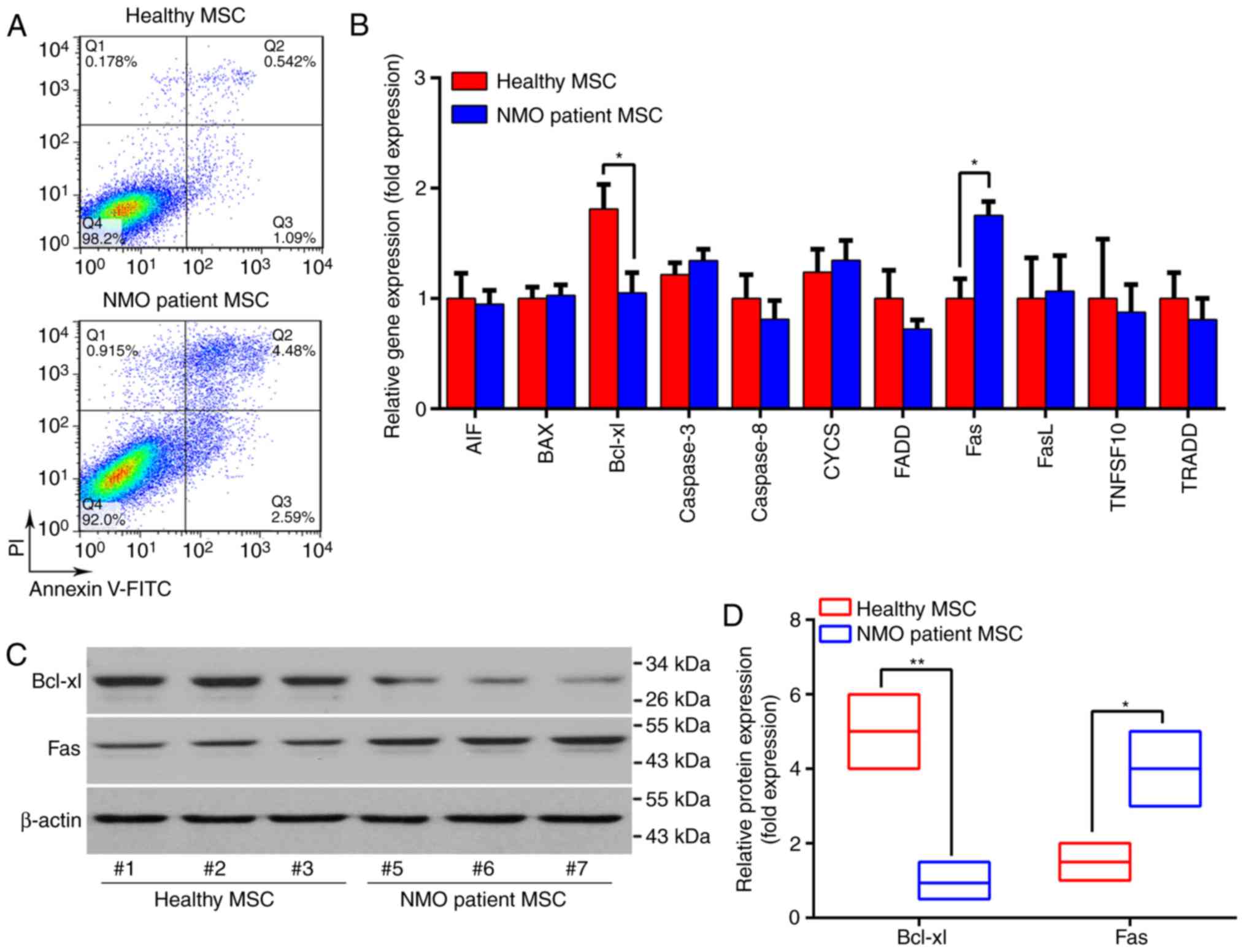 | Figure 4The apoptotic rate and
apoptosis-associated gene expression patterns of BM-MSCs from
patients with NMO and healthy controls. (A) Representative dot
plots of the apoptotic rate of BM-MSCs are presented. (B) The
global apoptosis-associated gene expression profile of BM-MSCs was
determined using RT-qPCR. (C) The protein expression levels of
Bcl-xl and Fas were determined by western blotting and
representative results are presented. Samples #1, #2 and #3 were
taken from the healthy volunteers, whereas samples #5, #6, #7 were
from the NMO patient group. (D) Relative protein expression levels
of Bcl-xl and Fas. For the western blot analysis, the protein bands
were statistically analyzed with ImageJ software and β-actin was
used as an internal control. Results are presented as the mean ±
standard deviation of three independent experiments and each
experiment was performed in triplicate. *P<0.05 and
**P<0.01 vs. the healthy controls. BM-MSC, bone
marrow-derived mesenchymal stem cell; NMO, neuromyelitis optica;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; FITC, fluorescein isothiocyanate; PI, propidium
iodide. |
PDGF-BB can stimulate cell proliferation
and overcome the senescence disadvantage, of BM-MSCs from patients
with NMO
Several growth factors and cytokines have been
considered to be critical factors regulating MSC proliferation,
migration and differentiation, among which PDGF is regarded as the
most potent mitotic stimulatory factor (17,18). PDGFs are disulfide-linked
homodimers or heterodimers, consisting of several 12.0-13.5 kDa
polypeptide chains designated as the PDGF-A, -B, -C and -D chains
and the five naturally occurring PDGFs are PDGF-AA, PDGF-BB,
PDGF-AB, PDGF-CC and PDGF-DD (18). Considering that PDGF-BB is the
major growth factor in bone matrix and its safety and effectiveness
have been verified in a number of clinical trials (19,20), it seemed logical during these
experiments to supplement the NMO BM-MSC culture medium with
PDGF-BB in order to optimize the BM-MSCs' biological properties.
The MTT assay experiment revealed that 20 ng/ml PDGF-BB was able to
significantly promote the proliferation rate of BM-MSCs from
patients with NMO on days 3 and 4 (P<0.05; Fig. 5A). Furthermore, the cytometric
images indicated that the apoptotic rate of the NMO patient group
was decreased from 9.745±0.245% to 3.672±0.128% (P<0.01;
Fig. 5B). Additionally,
improvements at the molecular level were also confirmed by RT-qPCR.
As presented in Fig. 5C, the
expression levels of AREG, CCNA2 and Bcl-xl were significantly
increased (P<0.01); conversely, the expression levels of CDKN2A
and Fas were significantly decreased (all P<0.05). The protein
expression levels following PDGF-BB treatment in MSC cells from the
identical patients were also examined. Three patients were selected
and the protein expression levels of AREG, CCNA2 and Bcl-xl were
revealed to be significantly increased in the patients with NMO MSC
with PDGF (P<0.05), whereas the expression levels of Fas and
CDKN2A were significantly decreased (P<0.01; Fig. 6A and B).
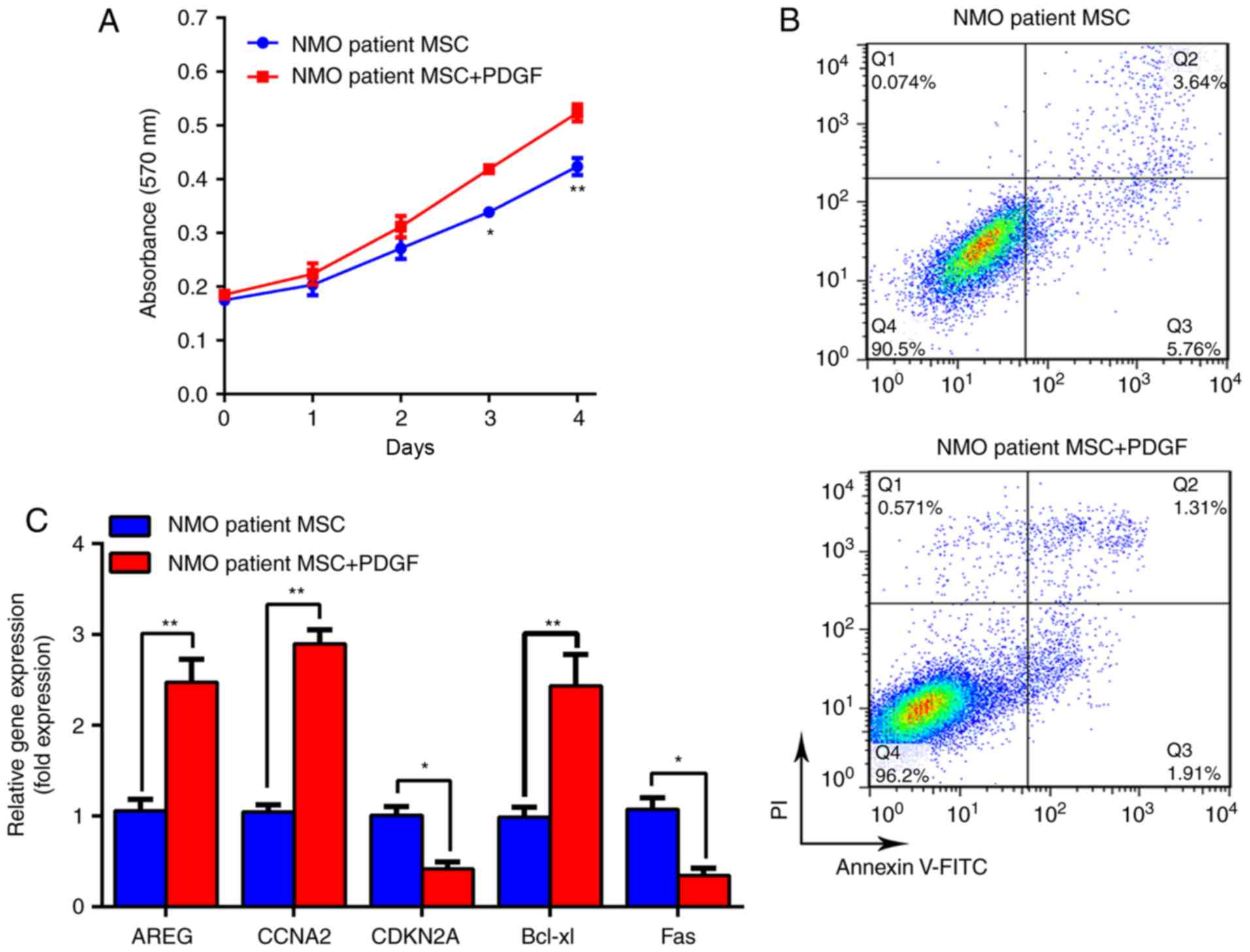 | Figure 5PDGF-BB can stimulate proliferation
and overcome the senescence disadvantage, of BM-MSCs from patients
with NMO. (A) The proliferation curve of BM-MSCs from patients with
NMO treated with PDGF-BB (20 ng/ml) is presented. (B)
Representative dot plots demonstrating the apoptotic rate of
BM-MSCs from patients with NMO treated with PDGF-BB (20 ng/ml). (C)
mRNA levels of AREG, CCNA2, CDKN2A and Fas genes in BM-MSCs from
patients with NMO treated with PDGF-BB (20 ng/ml), as determined
using RT-qPCR are presented. *P<0.05 and
**P<0.01 vs. the controls. BM-MSC, bone
marrow-derived mesenchymal stem cell; NMO, neuromyelitis optica;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; AREG, amphiregulin; CCNA2, cyclin A2; CDKN2,
cyclin-dependent kinase inhibitor 2A; PGDF-BB, platelet-derived
growth factor-BB; FITC, fluorescein isothiocyanate; PI, propidium
iodide. |
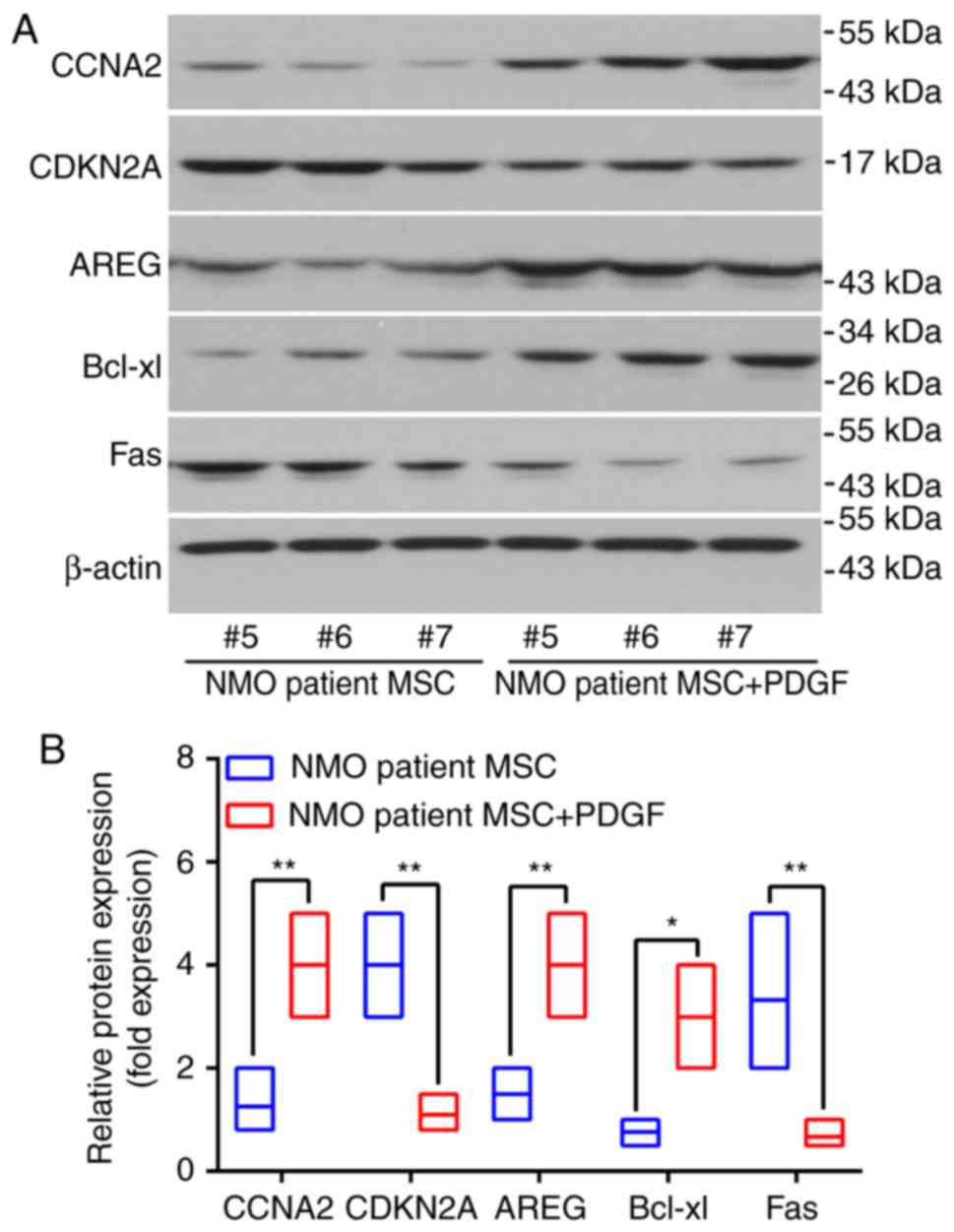 | Figure 6PDGF-BB is able to rescue the protein
expression levels of the proliferation and proliferation-associated
genes of interest studied in the BM-MSCs from patients with NMO.
(A) The protein levels of AREG, CCNA2, CDKN2A and Fas in BM-MSCs
from patients with NMO treated with PDGF-BB (20 ng/ml) were
detected using western blot analysis. Representative results are
presented. The protein levels of the BM-MSCs from samples #5, #6
and #7 (pertaining to the patients with NMO) were assessing by
western blotting following treatment with PDGF-BB. (B) Relative
protein expression levels of AREG, CCNA2, CDKN2A, Bcl-xl and Fas.
For the western blot analysis, the protein bands were statistically
analyzed with ImageJ software and β-actin was used as an internal
control. Results are presented as the mean ± standard deviation of
three independent experiments and each experiment was performed in
triplicate. *P<0.05 and **P<0.01 vs.
the healthy controls. PGDF-BB, platelet-derived growth factor-BB;
BM-MSC, bone marrow-derived mesenchymal stem cell; NMO,
neuromyelitis optica; AREG, amphiregulin; CCNA2, cyclin A2; CDKN2,
cyclin-dependent kinase inhibitor 2A; PDGF, platelet-derived growth
factor. |
Discussion
NMO is a humoral immunity-mediated inflammatory
demyelinating disease that preferentially targets the spinal cord
and optic nerves (21). NMO is
associated with a generally poor prognosis and the permanent myelin
losses that precede and characterize the progressive stage of NMO
remain untreatable (22). Stem
cell transplantation has been considered to be a potential
treatment method for neurological disorders due to the multifaceted
roles of stem cells. Greco et al (23) applied hematopoietic stem cells to
treat NMO and discovered that the treatment was able to reduce
neuroinflammation and halt the progression of disability. By
contrast, Matiello et al (24) demonstrated that autologous
hematopoietic stem cell transplantation was unable to prevent the
relapse of NMO (24). These
results provide the foresight that caution is necessary in terms of
selecting an appropriate type of stem cells to treat NMO.
MSCs have been extensively studied as cellular
therapies due to their various modes of action. MSCs exhibit
numerous characteristics, including immune modulatory, neurotrophic
(25) and repair-promoting
properties, which make them attractive candidates for treating
various degenerative neurological diseases, including stroke
(26,27), multiple sclerosis (13,28) and Parkinson's disease (29,30). In 2012, Lu et al (31) reported that four out of five
patients with NMO demonstrated therapeutic improvements following
receiving human umbilical cord MSC therapy (31). However, the use of these
allogeneic cells is associated with ethical concerns and the
possibility of immunological rejection. Although the authors'
recent clinical pilot observation revealed that the infusion of
autologous MSCs provided beneficial effects in terms of lowering
the relapse rate and disability in patients with NMO (15), further studies are required in
order to focus on the cytological features of patient-derived
BM-MSCs that may influence the therapeutic effects on patients with
NMO. Therefore, the present study aimed to demonstrate whether the
biological properties of BM-MSCs from patients with NMO are
different from those of BM-MSCs taken from healthy control
subjects. The results obtained revealed that there was no
difference in the immune phenotype markers of BM-MSCs, comparing
between the patients with NMO and healthy controls. The appearances
of certain of the BM-MSCs from the patients with NMO were revealed
to be slightly aberrant, even though they exhibited a normal
differentiation capability; however, the BM-MSCs from patients did
reveal decreased proliferative capacity and increased
senescence.
In addition to the observed biological phenomena, it
was determined that an array of cell cycle, proliferation and
apoptosis-associated gene expression profiles varied between the
BM-MSCs derived from the patients with NMO and the healthy
controls. Decreased expression levels of AREG have been
demonstrated to induce abnormalities of homeostasis and to decrease
cell proliferation (32). In
addition, CDKN2A is a gene that can induce arrested cell growth.
The activation of CDKN2A may inhibit the cell cycle transition from
G1 to the S phase by inhibiting cyclin-dependent kinases (CDKs).
CCNA2 belongs to the cyclin family that regulates the S phase of
cell cycle progression by interacting with CDKs and decreased CCNA2
may arrest the cell cycle at S phase. Therefore, aberrant
expression of CKDN2A and CCNA2 may lead to maladjusted cell growth.
In the present study, the expression levels of Fas in the patients
with NMO were increased compared with those of healthy controls.
The Fas receptor is a death receptor on the surface of cells that
initiates programmed cell death when bound to its ligand, Fas-L and
an increasing number of reports have demonstrated that Fas
overexpression is associated with apoptosis and cell senescence
(33-35). Bcl-xL is a canonical
anti-apoptotic protein in the Bcl-2 family. Bcl-xL inhibits Bak or
Bax activation, which is an essential event for apoptosis and
senescence execution (36,37).
PDGF signaling pathways have long been demonstrated
to be involved in pre- and post-natal vasculogenesis and/or
angiogenesis. PDGFs are potent mitogens for MSCs and are involved
in their growth and differentiation. PDGF isoforms exert their
biological effects via the activation of two tyrosine kinase
receptors, PDGF receptor-α (PDGFR)-α and PDGFR-β, which are
abundantly expressed on MSCs (17). Studies have been published on a
large number of successful applications of PDGF-BB-based therapies
for various types of maladies, including tendon and bone fracture
repairs, and demyelinating disease (20,38). For example, Chen et al
(19) performed PDGF-BB-based
stem cell gene therapy to improve bone strength in hematopoietic
stem cell transplantation subjects and in their in vivo
model the proliferation of BM-MSCs increased significantly compared
with the control group (19).
In order to find the potential mechanism of
different cytological features of BM MSCs from patients with NMO
and healthy subjects, 28 genes involved in the cell cycle,
proliferation and cell apoptosis were screened with the RT-qPCR
method and found the genes, which were differentially expressed in
two kinds of BM-MSCs, could be divided into two groups: Cell cycle
associated genes like AREG, CCNA2 and CDKN2A, and cell program
death associated genes like Bcl-xl and Fas. The results of the
present study demonstrated the expression of CCNA2 and Fas
increased, and the expression of CDKN2A and Bcl-xl was decreased in
BM-MSCs from patients with NMO. A wide range of evidence indicates
that CCNA2 is implicated in the initiation and progression of DNA
synthesis and involved in the G2/M transition, and CDKN2A could
inhibit the cell cycle through blocking transition from G1 to S
phase. In addition, Bcl-xl acts as an anti-apoptotic protein by
preventing the release of cytochrome c. Fas, a death receptor on
the surface of cells, can lead to programmed cell death (32-37). In addition, the results of the
present study are consistent with the above reports and the
cytological features observed. Furthermore, the results suggest
that these proteins may act as promising targets for the defected
cytological features of BM-MSCs from patients with NMO. Moreover,
the synergetic effects from two different proteins on the same cell
signaling pathway may serve critical roles in that progress.
However, whether there is a regulatory network or interaction
between these proteins needs to be further investigated.
In conclusion, it has been demonstrated in the
present study that, although the BM-MSCs from patients with NMO
exhibited a similar cell morphology and differentiation ability
when compared with the healthy controls, the cells grew more slowly
and tended to be a little more inclined towards senescence. These
aberrant changes in cytological features of patient-derived BM-MSCs
could influence the therapeutic effects of autologous BM-MSC
infusion treatment in NMO. Promising approaches to overcome the
above-mentioned defects were to routinely add PDGF in vitro
during the expansion phase of the BM-MSCs, or to combine PDGF
cytokine therapy with BM-MSC infusion in refractory patients with
NMO. Although the present study has provided a theoretical basis
for treatment of NMO, further studies are required in view of the
limited number of patients with NMO. In addition, the immune
modulatory and neurotrophic effects of these cells that were not
included in this study also require further investigation.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81371372, 81501031 and
81571172), the National Key Clinical Specialty Construction Program
of China and the Natural Science Foundation of Tianjin (grant no.
15JCQNJC44800).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YYan, FDS and CY formulated the study concept and
designed the project, GC, YYang and LM acquired the data, GC and CY
analyzed and interpreted the data, GC, GXZ and FDS wrote and edited
the paper. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all participants
in the present study and the study was approved by the Tianjin
Medical University General Hospital Institutional Review Board and
Ethics Committee (Tianjin, China).
Patient consent for publication
Each participant provided written informed
consent.
Competing interests
The authors declare they have no competing
interests.
Acknowledgments
The authors would like to thank Mr. Katherine Regan
for editorial assistance.
Abbreviations:
|
BM-MNCs
|
bone marrow-derived mononuclear
cells
|
|
MSCs
|
mesenchymal stem cells
|
|
NMO
|
neuromyelitis optica
|
|
PDGF
|
platelet-derived growth factor
|
References
|
1
|
Jarius S and Wildemann B: Aquaporin-4
antibodies (NMO-IgG) as a serological marker of neuromyelitis
optica: A critical review of the literature. Brain Pathol.
23:661–683. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vodopivec I, Matiello M and Prasad S:
Treatment of neuromyelitis optica. Curr Opin Ophthalmol.
26:476–483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wingerchuk DM, Pittock SJ, Lucchinetti CF,
Lennon VA and Weinshenker BG: A secondary progressive clinical
course is uncommon in neuromyelitis optica. Neurology. 68:603–605.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kitley J, Leite MI, Nakashima I, Waters P,
McNeillis B, Brown R, Takai Y, Takahashi T, Misu T, Elsone L, et
al: Prognostic factors and disease course in aquaporin-4
antibody-positive patients with neuromyelitis optica spectrum
disorder from the United Kingdom and Japan. Brain. 135:1834–1849.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wingerchuk DM and Weinshenker BG:
Neuromyelitis optica: Clinical predictors of a relapsing course and
survival. Neurology. 60:848–853. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cabre P, González-Quevedo A, Bonnan M,
Saiz A, Olindo S, Graus F, Smadja D, Merle H, Thomas L and
Cabrera-Gomez JA: Relapsing neuromyelitis optica: Long term history
and clinical predictors of death. J Neurol Neurosurg Psychiatry.
80:1162–1164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watanabe S, Nakashima I, Misu T, Miyazawa
I, Shiga Y, Fujihara K and Itoyama Y: Therapeutic efficacy of
plasma exchange in NMO-IgG-positive patients with neuromyelitis
optica. Mult Scler. 13:128–132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Miguel MP, Fuentes-Julián S,
Blázquez-Martínez A, Pascual CY, Aller MA, Arias J and
Arnalich-Montiel F: Immunosuppressive properties of mesenchymal
stem cells: Advances and applications. Curr Mol Med. 12:574–591.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eckert MA, Vu Q, Xie K, Yu J, Liao W,
Cramer SC and Zhao W: Evidence for high translational potential of
mesenchymal stromal cell therapy to improve recovery from ischemic
stroke. J Cereb Blood Flow Metab. 33:1322–1334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Bowles AC, Semon JA, Scruggs BA,
Zhang S, Strong AL, Gimble JM and Bunnell BA: Transplantation of
autologous adipose stem cells lacks therapeutic efficacy in the
experimental autoimmune encephalomyelitis model. PLoS One.
9:e850072014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li D, Chai J, Shen C, Han Y and Sun T:
Human umbilical cord-derived mesenchymal stem cells differentiate
into epidermal-like cells using a novel co-culture technique.
Cytotechnology. 66:699–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zacharek A, Shehadah A, Chen J, Cui X,
Roberts C, Lu M and Chopp M: Comparison of bone marrow stromal
cells derived from stroke and normal rats for stroke treatment.
Stroke. 41:524–530. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cohen JA: Mesenchymal stem cell
transplantation in multiple sclerosis. J Neurol Sci. 333:43–49.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El-Akabawy G and Rashed LA: Beneficial
effects of bone marrow-derived mesenchymal stem cell
transplantation in a non-immune model of demyelination. Ann Anat.
198:11–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu Y, Yan Y, Qi Y, Yang L, Li T, Zhang N,
Yu C, Su L, Zhang R, Shen Y, et al: Impact of autologous
mesenchymal stem cell infusion on neuromyelitis optica spectrum
disorder: A pilot, 2-year observation study. CNS Neurosci Ther.
22:677–685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Belotti D, Capelli C, Resovi A, Introna M
and Taraboletti G: Thrombospondin-1 promotes mesenchymal stromal
cell functions via TGFβ and in cooperation with PDGF. Matrix Biol.
55:106–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Papadopoulos N and Lennartsson J: The
PDGF/PDGFR pathway as a drug target. Mol Aspects Med. 62:75–88.
2018. View Article : Google Scholar
|
|
19
|
Chen W, Baylink DJ, Brier-Jones J, Neises
A, Kiroyan JB, Rundle CH, Lau KH and Zhang XB: PDGFB-based stem
cell gene therapy increases bone strength in the mouse. Proc Natl
Acad Sci USA. 112:E3893–E3900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Solchaga LA, Bendele A, Shah V, Snel LB,
Kestler HK, Dines JS and Hee CK: Comparison of the effect of
intra-tendon applications of recombinant human platelet-derived
growth factor-BB, platelet-rich plasma, steroids in a rat achilles
tendon collagenase model. J Orthop Res. 32:145–150. 2014.
View Article : Google Scholar
|
|
21
|
Lennon VA, Wingerchuk DM, Kryzer TJ,
Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I and Weinshenker
BG: A serum autoantibody marker of neuromyelitis optica:
Distinction from multiple sclerosis. Lancet. 364:2106–2112. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao J, Yang R, Biswas S, Qin X, Zhang M
and Deng W: Mesenchymal stem cells and induced pluripotent stem
cells as therapies for multiple sclerosis. Int J Mol Sci.
16:9283–9302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Greco R, Bondanza A, Vago L, Moiola L,
Rossi P, Furlan R, Martino G, Radaelli M, Martinelli V, Carbone MR,
et al: Allogeneic hematopoietic stem cell transplantation for
neuromyelitis optica. Ann Neurol. 75:447–453. 2014. View Article : Google Scholar
|
|
24
|
Matiello M, Pittock SJ, Porrata L and
Weinshenker BG: Failure of autologous hematopoietic stem cell
transplantation to prevent relapse of neuromyelitis optica. Arch
Neurol. 68:953–955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilkins A, Kemp K, Ginty M, Hares K,
Mallam E and Scolding N: Human bone marrow-derived mesenchymal stem
cells secrete brain-derived neurotrophic factor which promotes
neuronal survival in vitro. Stem Cell Res. 3:63–70. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cramer SC, Sur M, Dobkin BH, O'Brien C,
Sanger TD, Trojanowski JQ, Rumsey JM, Hicks R, Cameron J, Chen D,
et al: Harnessing neuroplasticity for clinical applications. Brain.
134:1591–1609. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH
and Bang OY: STARTING collaborators: A long-term follow-up study of
intravenous autologous mesenchymal stem cell transplantation in
patients with ischemic stroke. Stem Cells. 28:1099–1106. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Connick P, Kolappan M, Crawley C, Webber
DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ,
et al: Autologous mesenchymal stem cells for the treatment of
secondary progressive multiple sclerosis: An open-label phase 2a
proof-of-concept study. Lancet Neurol. 11:150–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park HJ, Shin JY, Lee BR, Kim HO and Lee
PH: Mesenchymal stem cells augment neurogenesis in the
subventricular zone and enhance differentiation of neural precursor
cells into dopaminergic neurons in the substantia nigra of a
parkinsonian model. Cell Transplant. 21:1629–1640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Danielyan L, Schäfer R, von
Ameln-Mayerhofer A, Bernhard F, Verleysdonk S, Buadze M, Lourhmati
A, Klopfer T, Schaumann F, Schmid B, et al: Therapeutic efficacy of
intranasally delivered mesenchymal stem cells in a rat model of
Parkinson disease. Rejuvenation Res. 14:3–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu Z, Ye D, Qian L, Zhu L, Wang C, Guan D,
Zhang X and Xu Y: Human umbilical cord mesenchymal stem cell
therapy on neuromyelitis optica. Curr Neurovasc Res. 9:250–255.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zaiss DMW, Gause WC, Osborne LC and Artis
D: Emerging functions of amphiregulin in orchestrating immunity,
inflammation, and tissue repair. Immunity. 42:216–226. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jeon H and Boo YC: Senescent endothelial
cells are prone to TNF-α-induced cell death due to expression of
FAS receptor. Biochem Biophys Res Commun. 438:277–282. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ols ML, Cullen JL, Turqueti-Neves A, Giles
J and Shlomchik MJ: Dendritic cells regulate extrafollicular
autoreactive B cells via T cells expressing Fas and Fas ligand.
Immunity. 45:1052–1065. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cruz AC, Ramaswamy M, Ouyang C, Klebanoff
CA, Sengupta P, Yamamoto TN, Meylan F, Thomas SK, Richoz N, Eil R,
et al: Fas/CD95 prevents autoimmunity independently of lipid raft
localization and efficient apoptosis induction. Nat Commun.
7:138952016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ikezawa K, Hikita H, Shigekawa M, Iwahashi
K, Eguchi H, Sakamori R, Tatsumi T and Takehara T: Increased Bcl-xL
expression in pancreatic neoplasia promotes carcinogenesis by
inhibiting senescence and apoptosis. Cell Mol Gastroenterol
Hepatol. 4:185–200.e181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aouacheria A, Baghdiguian S, Lamb HM,
Huska JD, Pineda FJ and Hardwick JM: Connecting mitochondrial
dynamics and life-or-death events via Bcl-2 family proteins.
Neurochem Int. 109:141–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang Y and Dreyfus CF: The role of growth
factors as a therapeutic approach to demyelinating disease. Exp
Neurol. 283:531–540. 2016. View Article : Google Scholar : PubMed/NCBI
|