Introduction
Osteoarthritis (OA) is the most common degenerative
joint disease, afflicting mainly the weight-bearing joints. It is
estimated that >50 million individuals in the USA will be
affected by the year 2020 (1).
The clinical symptoms of OA include chronic joint pain, limited
movement and irreversible joint dysfunction, all of which are
caused by synovitis, articular cartilage degeneration, osteophyte
formation and subchondral bone sclerosis (2). Data from the Global Burden of
Disease study in 2010 revealed that OA of the hip and knee was
ranked as the 11th highest contributor to global disability and the
38th highest contributor to disability-adjusted life years
(3). Despite the identification
of risk factors, such as ageing, obesity and metabolic disorders,
no effective interventions for preventing the progression of OA are
currently available. Therefore, there is an urgent need for more
effective and safe therapies for OA.
Chondrocytes, the unique cells in the articular
cartilage, are responsible for synthesizing and regenerating
extracellular matrix (ECM), which is primarily composed of type II
collagen and proteoglycan (4).
During the course of OA, the overproduction of pro-inflammatory
cytokines, such as interleukin (IL)-1β and tumor necrosis factor
(TNF)-α, induces chondrocytes to secrete proteolytic enzymes, such
as matrix metalloproteinases (MMPs) and a disintegrin and
metalloproteinase with thrombospondin motifs (ADAMTs), resulting in
the loss of the major components of the ECM (5,6)
and even the occurrence of apoptosis (7). Additionally, IL-1β has been
confirmed to stimulate the activation of the nuclear factor (NF)-κB
signaling pathway in OA chondrocytes (8), which is implicated in inflammatory
response and cell apoptosis (9).
Therefore, the inhibition of both inflammatory cytokines and NF-κB
molecules may be considered as a therapeutic target for attenuating
the progression of OA.
Artesunate (ART), a semi-synthetic derivative of
arte-misinin derived from Artemisia annua, is one of the
most effective clinical treatments for malaria in China (10). This drug has received widespread
attention due to its pharmacological properties beyond being an
anti-malarial drug. ART was demonstrated to inhibit the expression
of TNF-α-induced pro-inflammatory cytokines by repressing the NF-κB
pathway in fibroblast-like synoviocytes (11). In addition, ART was effective in
suppressing multiple pathogenic factors and inflammation through
inhibiting the LPS/TLR4/NF-κB pathway to alleviate hepatic fibrosis
(12). Of note, ART was able to
decrease the levels of nitric oxide, maintain oxidative homeostasis
and inhibit cyclooxygenase (COX)-2 expression and cell apoptosis in
rats with rheumatoid arthritis (RA) (13). A recent study also demonstrated
that ART attenuated the progression of experimental OA by
suppressing the expression of osteoclast-specific and
angiogenesis-related genes in the serum and synovium (14). However, the effect of ART on OA
chondrocytes remains elusive.
The aim of the present study was to investigate the
anti-inflammatory and anti-apoptotic effects and the molecular
mechanisms underlying the effects of ART on IL-1β-induced
chondrocyte-like ATDC5 cells, as well as the role of ART in a mouse
model of OA.
Materials and methods
Materials
ART was purchased from WanXiangHengYuan Technology
Co., Ltd. ATDC5 cells were purchased from Riken Cell Bank. Fetal
bovine serum (FBS), Dulbecco's modified Eagle's minimum essential
medium/Ham's F12 medium (DMEM/F12), penicillin/streptomycin,
trypsin and insulin-transferrin-selenite (ITS) were purchased from
Invitrogen; Thermo Fisher Scientific, Inc. Alcian Blue 8GX was
purchased from Sigma-Aldrich; Merck KGaA. The Cell Counting Kit-8
(CCK-8) was purchased from Dojindo Molecular Technologies, Inc. The
primary antibodies against Bax, Bcl-2, cleaved caspase-3, cleaved
caspase-7, IκBα, p-IκBα, p65, p-p65, lamin B and β-tubulin were
obtained from Cell Signaling Technology, Inc.; MMP-3, MMP-13,
ADAMTS-5, COX-2 and β-actin were purchased from Abcam.
Three-month-old male C57BL/6 mouse (n=60) were purchased from Vital
River.
Cell differentiation and culture
ATDC5 is a murine teratocarcinoma cell line. The
cells were cultured in DMEM/F12 with 5% FBS and 1%
penicillin/streptomycin in a humidified incubator with 5%
CO2 at 37°C. Once the cells were 70-80% confluent, the
medium was supplemented with 1% ITS. The differentiation medium was
changed every 2 days to induce differentiation of the cells into
chondrocyte-like cells. To evaluate the level of glycosaminoglycan
production, 1% Alcian blue staining was performed, and the mRNA
expression levels of collagen (COL) II and COL X were analyzed to
further confirm the differentiation level of the ATDC5 cells. The
primers used to amplify COL II and COL X in mice are listed in
Table I. Finally, ATDC5 cells
were used for the experiments after 2 weeks of differentiation in
culture.
 | Table ISequences of primers used in
quantitative polymerase chain reaction analysis. |
Table I
Sequences of primers used in
quantitative polymerase chain reaction analysis.
| Gene | Primer sequences
(5′-3′) |
|---|
| COL II | |
| Forward |
ACGAAGCGGCTGGCAACCTCA |
| Reverse |
CCCTCGGCCCTCATCTCTACATCA |
| COL X | |
| Forward |
TGCCCGTGTCTGCTTTTACTGTCA |
| Reverse |
TCAAATGGGATGGGGGCACCTACT |
| MMP-3 | |
| Forward |
ACATGGAGACTTTGTCCCTTTTG |
| Reverse |
TTGGCTGAGTGGTAGAGTCCC |
| MMP-13 | |
| Forward |
TGTTTGCAGAGCACTACTTGAA |
| Reverse |
CAGTCACCTCTAAGCCAAAGAAA |
| ADAMTS-5 | |
| Forward |
GGAGCGAGGCCATTTACAAC |
| Reverse |
CGTAGACAAGGTAGCCCACTTT |
| COX-2 | |
| Forward |
TTCCAATCCATGTCAAAACCGT |
| Reverse | AGTCCGGG
TACAGTCACACTT |
| β-actin | |
| Forward |
GGCTGTATTCCCCTCCATCG |
| Reverse |
CCAGTTGGTAACAATGCCATGT |
Cell viability
Cell viability was determined using the CCK-8 assay,
according to the manufacturer's instructions. The chondrocyte-like
ATDC5 cells were divided into two groups: In group 1, the cells
were seeded in 96-well plates at a density of 4,000 cells/well and
cultured with or without ART (3.125, 6.25, 12.5, 25 and 50
µM) for 24 h; in group 2, the cells were pretreated with ART
(3.125, 6.25, 12.5, 25 and 50 µM) for 24 h, then
co-incubated with IL-1β (10 ng/ml) for a further 24 h.
Subsequently, 10 µl of CCK-8 solution was added to each well
and incubated at 37°C for 2 h. The absorbance at 450 nm was
measured using a Multiskan GO microplate reader (Thermo Fisher
Scientific, Inc.).
Flow cytometric analysis of cell
apoptosis
Cell apoptosis was monitored using a flow cytometry
apoptosis detection kit [phycoerythrin (PE)-Annexin
V/7-aminoactinomycin (7-ADD) double-fluorescence labelling]. After
re-suspending the cells in Annexin V binding buffer, the harvested
chondrocyte-like ATDC5 cells (1×105) were stained with 5
µl PE-Annexin V and 5 µl 7-ADD for 15 min at room
temperature in the dark. The stained cells were analyzed with a
FACSAria™ II flow cytometer (BD Biosciences) within 1 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Chondrocyte-like ATDC5 cells were washed with cold
PBS and incubated with TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) to extract total RNA. The total RNA was
quantified with a spectrophotometer at 260 nm (Thermo Scientific
NanoDrop 2000), and the ratio of the absorbance at A260/A280 was
used to evaluate the purity of the RNA. cDNA was synthesized using
2 µg RNA with a PrimeScript™ RT Master Mix (Takara Bio,
Inc.). cDNA was then subjected to RT-qPCR analysis with
SYBR® Fast qPCR Mix (Takara Bio, Inc.) using the CFX96
Real-Time PCR system (Bio-Rad Laboratories, Inc.) under conditions
of 94°C for 30 sec, followed by 40 cycles at 95°C for 5 sec and
60°C for 10 sec, and finally the dissociation curve of each primer
pair was analyzed to determine primer specificity. The reaction was
performed in a total volume of 20 µl (2 µl diluted
cDNA, 10 µl SYBR Green Master Mix, 1 µl forward
primer, 1 µl reverse primer and 6 µl RNase-free
water). Target mRNA levels were normalized to the β-actin level,
which was used as a control. Data were analyzed using the
2−ΔΔCq method (15).
All the PCRs were performed in triplicate for each gene. The
primers used to amplify MMP-3, MMP-13, ADAMTS-5, COX-2 and β-actin
in mice are shown in Table
II.
 | Table IIChanges in cartilage thickness in
different groups and at different time-points. |
Table II
Changes in cartilage thickness in
different groups and at different time-points.
| Time (days) | HC (mm) | CC (mm) |
|---|
| Sham | Vehicle | ART | Sham | Vehicle | ART |
|---|
| 30 | 0.79±0.032 | 0.74±0.041 | 0.78±0.040 | 0.34±0.038 | 0.36±0.041 | 0.35±0.038 |
| 60 | 0.76±0.092 |
0.44±0.143a |
0.75±0.103b | 0.33±0.110 |
0.68±0.127a |
0.36±0.100b |
Isolation of cytosol and nucleus
fractions
To determine the redistribution of p65, a nuclear
and cytoplasmic extraction kit (Thermo Fisher Scientific, Inc.) was
used. Following ART treatment, the cells were collected, washed
twice with cold PBS and then air-dried. Next, the cells were
incubated with Cytoplasmic Extraction Reagent (CER)I on ice for 10
min prior to the addition of CER II. After incubation together for
1 min, the cells were centrifuged at 16,000 × g and 4°C for 5 min,
and then the supernatant (cytoplasm extract) was collected. The
insoluble fraction was re-suspended in cold NER for 40 min,
centrifuged at 16,000 × g and 4°C for 10 min, and the supernatant
(nuclear extract) was then collected. The supernatants were frozen
in liquid nitrogen and stored at -80°C until analysis.
Western blot analysis
The collected chondrocyte-like ATDC5 cells were
washed three times with cold PBS, re-suspended in RIPA buffer and
incubated on ice for 30 min. The protein concentration was
determined using the bicinchoninic acid assay (Bio-Rad
Laboratories, Inc.). Equal amounts of protein were subjected to 10%
SDS-PAGE and subsequently transferred to PVDF membranes. The
membranes were blocked with 5% non-fat milk for 1 h at room
temperature and incubated at 4°C overnight with the primary
antibodies against MMP-3 (1:2,000, cat. no. ab52915), MMP-13
(1:3,000, cat. no. ab39012), ADAMTS-5 (1:1,000, cat. no. ab182795),
COX-2 (1:1,000, cat. no. ab62331), β-actin (1:2500, cat. no.
ab8226) (all from Abcam); Bcl-2 (1:1,000, cat. no. 3498), Bax
(1:1,000, cat. no. 14796), cleaved caspase-3 (1:1,000, cat. no.
9654), cleaved caspase-7 (1:1,000, cat. no. 8438), IκBα (1:1,000,
cat. no. 4814), p-IκBα (1:1,000, cat. no. 2859), p65 (1:1,000, cat.
no. 6956), p-p65 (1:1,000, cat. no. 3036) lamin B (1:5,000, cat.
no. 12255), and β-tubulin (1:1,000, cat. no. 2146) (all from Cell
Signaling Technology, Inc.). After washing three times with TBST
for 5 min, the membranes were incubated with secondary antibodies
[peroxidase-conjugated AffiniPure goat anti-rabbit IgG (H + L),
1:800, cat. no. ZB-2301, OriGene Technologies, Inc.] for 2 h.
Finally, the immunoreactive bands were detected with the AP
chromogenic substrate (Thermo Fisher Scientific, Inc.).
Animal experiments
All experimental procedures were approved by the
Institutional Animal Care and Use Committee of First Affiliated
Hospital of Xinjiang Medical University (protocol no.
IACUC20171129-01). The mice were housed under controlled conditions
(temperature, 25±2°C; light/dark cycle, 12/12 h; relative humidity,
70%). Prior to the anterior cruciate ligament transection (ACLT)
surgery, the mice were anesthetized with intravenous injection of
1% pentobarbitone (40 mg/kg). Following a parapatellar incision,
the ACL of the right knee was transected to establish the OA model.
Then, the joint capsule and skin were sutured layer-by-layer. For
the sham group, a parapatellar incision was performed in the right
knee joint to expose the ACL, after which time the joint capsule
and skin were sutured separately. To identify the optimal dose (100
mg/kg), a preliminary experiment was first performed by using
multiple concentrations of ART (50, 100 and 200 mg/kg) injected for
8 weeks postoperatively (Fig.
S1). At 50 mg/kg, ART exerted minimal chondroprotective
effects, and 200 mg/kg ART induced proteoglycan loss in articular
cartilage. Therefore, in the formal experiment, all mice were
randomly assigned into the sham, vehicle-treated ACLT and ART (100
mg/kg)-treated ACLT groups (n=20 per group). Either ART (100 mg/kg)
or an equivalent volume of 5% NaHCO3 was administered
intraperitoneally for 4 and 8 weeks starting on the second
postoperative day. A total of 10 mice from each group were
sacrificed at 4 and 8 weeks after experiment completion.
Histological analysis
The right knee joints were dissected and fixed in
10% buffered formalin for 24 h and then decalcified in 10% EDTA (pH
7.3) for 3 weeks. The specimens were embedded in paraffin and cut
into 4-µm sections for hematoxylin and eosin (H&E) and
safranin O staining. The thickness of the hyaline cartilage (HC)
and the calcified cartilage (CC) were measured by H&E staining
(thickness of HC, distance from the articular cartilage surface to
the tidemark; thickness of CC, distance from the tidemark to the
subchondral bone plate). Osteoarthritis Research Society
International-modified Mankin criteria (OARSI) scores were
calculated for evaluating the state of articular cartilage in each
group. All counting was conducted blindly by an author who had not
been involved in the experiments.
Statistical analysis
Data are expressed as the means ± standard deviation
of the representative experiment performed in triplicate. One-way
analysis of variance followed by the Least Significant Difference
post hoc test was used to determine whether the differences among
groups were statistically significant. SPSS 22.0 (IBM Corp.) was
used for all data analyses. P<0.05 was considered to indicate
statistically significant differences.
Results
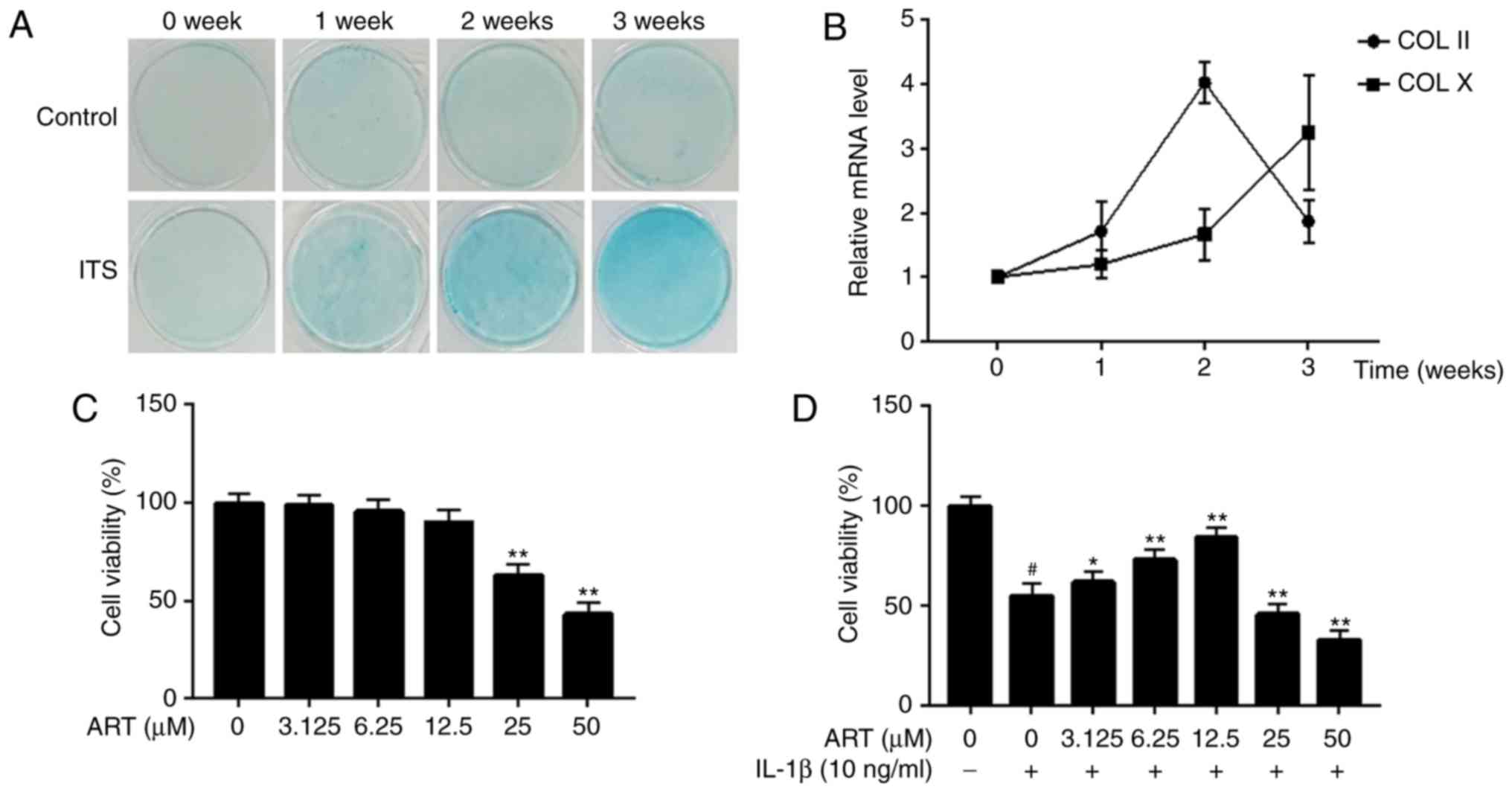
Differentiation of ATDC5 cells
To determine whether ITS induces ATDC5 cells to form
cartilage nodules, the cells were treated with ITS for 3 weeks, and
1% Alcian blue staining was performed at 0, 1, 2 and 3 weeks. As
shown in Fig. 1A, ITS treatment
resulted in a gradual increase in the staining intensity in the
ATDC5 cells in a time-dependent manner. Next, the expression of
chondrogenic differentiation markers (COL II and COL X) was
assessed using RT-qPCR. The data revealed that the mRNA level of
COL II increased significantly after 1 week of induction of the
chondrocytes, reaching a peak at 2 weeks, suggesting early-stage
differentiation of the chondrocytes. The mRNA level of COL X also
increased between 0 and 2 weeks, but exceeded the expression level
of COL II at 3 weeks, indicating late-stage differentiation of the
chondrocytes (Fig. 1B). These
results demonstrated that the ATDC5 cells differentiated from
proliferative to hypertrophic chondrocytes. Therefore, ATDC5 cells
that had been induced for 2 weeks were selected for the following
in vitro experiments.
Effects of ART on chondrocyte-like ATDC5
cell viability
A CCK-8 assay was performed to investigate the
effect of ART on the viability of chondrocyte-like ATDC5 cells and
the cells incubated with IL-1β. The results revealed that 25 and 50
µM ART significantly reduced cell viability, whereas
concentrations of ≤12.5 µM ART had no harmful effects on
cellular viability after treatment for 24 h (Fig. 1C). Furthermore, compared with the
control group, a significant decrease in IL-1β-induced cell
viability was reversed by ART at lower concentrations (3.125, 6.25
and 12.5 µM) in a dose-dependent manner. However, higher
concentrations of ART (25 and 50 µM) significantly reduced
cell viability (Fig. 1D). As a
result, 3.125, 6.25 and 12.5 µM ART were selected as the
low, medium and high concentrations, respectively.
ART reduces the production of
inflammatory cytokines in IL-1β-induced chondrocyte-like ATDC5
cells
The anti-inflammatory effect of ART on
chondrocyte-like ATDC5 cells induced by IL-1β was next analyzed.
The mRNA and protein expression levels of inflammatory factors were
evaluated by RT-qPCR and western blot analysis, respectively. The
results revealed that MMP-3, MMP-13, ADAMTS-5 and COX-2 were
prominently upregulated in the chondrocyte-like ATDC5 cells exposed
to 10 ng/ml IL-1β for 24 h. Both the gene and protein levels of
MMP-3, MMP-13 and COX-2 were reduced by ART treatment in a
dose-dependent manner. Although 3.125 µM ART failed to
inhibit the protein expression of ADAMTS-5, ART at concentrations
of 6.25 µM and 12.5 µM reduced the expression of
ADAMTS-5 at the gene and protein levels (Fig. 2A-C). Taken together, these results
indicated that ART suppressed the inflammatory response by
downregulating the expression of inflammatory cytokines at both the
gene and protein levels.
ART inhibits IL-1β-induced apoptosis in
chondrocyte-like ATDC5 cells
To assess the effect of ART on cell apoptosis
induced by IL-1β, flow cytometry and western blot analysis were
performed. First, the chondrocyte-like ATDC5 cells were treated
with ART at different concentrations for 24 h to assess the drug
cytotoxicity. The results demonstrated that ART was only mildly
cytotoxic at lower concentrations (3.125, 6.25 and 12.5 µM),
but highly cytotoxic at higher concentrations (25 and 50
µM), which was consistent with the results of the CCK-8
assay (Fig. 3). Furthermore,
compared with the control group, the percentage of apoptotic cells
was found to be markedly increased in the IL-1β-treated group,
while this increase was attenuated by ART treatment in a
dose-independent manner (Fig.
4).
To further investigate the effect of ART on the
mitochondrial apoptosis pathway, the protein levels of the
anti-apoptotic factor Bcl-2 and the pro-apoptotic factors Bax,
cleaved caspase-3 and cleaved caspase-7 were detected by western
blotting. The results demonstrated that IL-1β significantly
decreased the expression of Bcl-2 and increased the expression of
Bax, cleaved caspase-3 and cleaved caspase-7, while these effects
were partially reversed by ART (Fig.
5). Taken together, these results suggested that ART played an
anti-apoptotic role in IL-1β-induced chondrocyte-like ATDC5
cells.
ART represses the NF-κB signaling pathway
in IL-1β-induced chondrocyte-like ATDC5 cells
To explore the molecular mechanism through which ART
exerts anti-inflammatory and anti-apoptotic effects on
IL-1β-induced chondrocyte-like ATDC5 cells, western blot analysis
was performed to detect changes in the NF-κB signaling pathway. The
results demonstrated that the expression levels of p-IκBα and p-p65
were markedly increased in the IL-1β-induced group compared with
the control group. Moreover, stimulation of chondrocyte-like ATDC5
cells with IL-1β resulted in marked degradation of IκBα. However,
ART significantly repressed the IL-1β-induced phosphorylation of
IκBα and p65 and degradation of IκBα (Fig. 6A and B). In addition, as NF-κB
activation requires the nuclear translocation of p65, we further
investigated the effect of ART on the redistribution of p65 in the
cytoplasm and nucleus. The results demonstrated that IL-1β
significantly increased the nuclear translocation of p65. By
contrast, ART treatment effectively upregulated the cytosolic
levels and downregulated the nuclear levels of the p65 protein
(Fig. 6C and D). Moreover, the
use of ART alone failed to affect the expression of p65 in the
cytoplasm and the nucleus (Fig. 6C
and D). Taken together, these findings demonstrated that
treatment with ART significantly inhibited NF-κB signaling.
ART attenuates the progression of
ACLT-induced OA in mice
Finally, ART was administered intraperitoneally to
mice after the ACLT procedure to investigate its chondroprotective
effects. H&E staining revealed an increase in CC thickness in
the vehicle-treated group relative to the sham group at
postoperative week 8, which was delayed by ART treatment (Fig. 7A and Table II). Safranin O staining
demonstrated that the loss of proteoglycan was significantly
attenuated in the ART-treated group compared with the
vehicle-treated group at postoperative weeks 4 and 8 (Fig. 7B), which was supported by the
OARSI scores (Fig. 7C). These
results indicated that ART exerted strong protective effects on
articular cartilage in OA.
Discussion
Currently available pharmacological treatments have
failed to halt or reverse the progression of OA, and are
accompanied by a variety of side effects (16). Thus, bioactive small molecules
from natural herbage that may be suitable for OA treatment have
recently been drawing attention, particularly those with minimal or
no side effects (17-19). ART, a bioactive small molecule,
has been used to treat various ailments ranging from malaria to
tumors and RA (20). To the best
of our knowledge, the present study was the first to demonstrate
that ART treatment suppressed the expression of inflammatory
mediators at both the gene and protein levels, and inhibited
apoptosis in IL-1β-induced chondrocyte-like ATDC5 cells, which was
associated with the inactivation of the NF-κB signaling pathway. In
addition, ART treatment exerted protective effects on articular
cartilage in an ACLT mouse model.
In healthy chondrocytes, the synthesis and
degradation of ECM are in dynamic balance. However, this balance is
disrupted by reduced anabolic and elevated catabolic capacities of
OA chondrocytes (21). Previous
studies have indicated that MMPs and ADAMTs are responsible for
degrading type II collagen and proteoglycan in ECM, and the
suppression of these enzymes has the ability to attenuate articular
cartilage degeneration (22,23). According to the findings of the
present study, in the presence of IL-β, ART treatment inhibited the
catabolism of ECM components by downregulating MMP-3, MMP-13 and
ADAMTS-5.
COX-2 is an important inflammatory mediator that
contributes to prostaglandin E2 (PGE2) generation (24). Increased PGE2 levels lead to
activation of MMPs and other inflammatory cytokines (25), thereby perpetuating a pathogenic
circle in the OA cartilage. In the present study, COX-2 was
markedly elevated following IL-1β stimulation and was reduced by
ART treatment in a dose-dependent manner. Moreover, the
downregulation of COX-2 may alleviate the catabolism and
inflammatory response, thus delaying the progression of OA.
Additionally, ART has been reported to suppress the expression of
other catabolic genes, including MMP-2 and MMP-9 (26), and angiogenesis-related cytokines,
such as VEGF and HIF-1α (27).
Taken together, these findings indicate that ART exerts strong
anti-inflammatory protective effects in OA chondrocytes.
The apoptosis of chondrocytes is closely associated
with OA development. It is widely accepted that increased IL-1β
induces apoptosis of chondrocytes through upregulating
pro-apoptotic and downregulating anti-apoptotic proteins, thereby
accelerating cartilage degradation (7). In OA chon-drocytes, the expression
of the pro-apoptotic factors Bax, cleaved caspase-3 and cleaved
caspase-7 are higher than normal, while the expression of the
anti-apoptotic factor Bcl-2 is lower (28,29). Our findings were in agreement with
those of previous reports. Moreover, we found that ART reduced the
occurrence of apoptosis in a dose-dependent manner. In addition,
ART not only decreased the expression of Bax, cleaved caspase-3 and
cleaved caspase-7, but also enhanced the expression of Bcl-2. Taken
together, these observations confirm that ART exerts an
anti-apoptotic effect on OA chon-drocytes. However, several studies
have reported that ART induces apoptosis in some tumor cell lines
(30,31). It may be hypothesized that these
differences are due to the different doses or action times of ART
treatment and the different cell types used in the experiments.
The NF-κB family of transcription factors plays a
key role in the regulation of inflammation, immune response, and
cell proliferation and apoptosis (32). Inappropriate NF-κB activity not
only exaggerates the inflammation of chondrocytes via promoting the
overexpression of inflammatory genes, but also accelerates the
apoptosis of chondrocytes by disrupting the expression of
apoptosis-related proteins (33).
Therefore, targeted inhibition of the NF-κB pathway may be
beneficial for the treatment of OA. The present study demonstrated
that ART inhibited the IL-1β-induced phosphorylation of IκBα and
p65, as well as the degradation of IκBα. Importantly, ART treatment
repressed the translocation of p65 from the cytoplasm to the
nucleus and improved the redistribution of p65, supporting that ART
had no obvious effect on the total expression of p65 in the cells.
Collectively, these findings suggest that inactivation of NF-κB
signaling is one of the mechanisms by which ART plays a protective
role in OA chondrocytes.
In this study, an unstable and mechanical-loading OA
model was established by transecting the ACL in mice. Histological
analysis revealed an increase in CC thickness in the vehicle group
at postoperative week 8, while this change was not synchronized
with the loss of proteoglycan, which began on postoperative week 4.
This finding is consistent with a previous study (34) and warrants further investigation
by exploring potential cell signaling mechanisms in subchondral
bone. Moreover, the histological scoring of OA increased over time
in the vehicle-treated group. However, ATR administration not only
inhibited the increase in CC thickness and the loss of
proteoglycan, but also lowered the histological scoring of OA.
These findings indicate that ART delays the progression of OA.
In summary, the present study revealed that ART
protected chondrocytes against inflammation and apoptosis in
vitro and attenuated articular cartilage degeneration in
vivo, indicating that ART may be a promising potential
preventive therapy for OA.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (nos. U1503221 and
81860746).
Availability of data and materials
The datasets generated and analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
All the authors have read and approved the final
version of this manuscript. YL, WM and LC designed the research and
wrote the paper. YL, WM, JR and SW performed the experiments. TW
and BJ analyzed the data and edited the paper. HM and AA
contributed the materials and reagents. YL, KZ and LC revised the
manuscript and guided the research.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Institutional Animal Care and Use Committee of First Affiliated
Hospital of Xinjiang Medical University (protocol no.
IACUC20171129-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Helmick CG, Felson DT, Lawrence RC,
Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD,
Merkel PA, et al: Estimates of the prevalence of arthritis and
other rheumatic conditions in the United States. Part I. Arthritis
Rheum. 58:15–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen D, Shen J, Zhao W, Wang T, Han L,
Hamilton JL and Im HJ: Osteoarthritis: Toward a comprehensive
understanding of pathological mechanism. Bone Res. 5:160442017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cross M, Smith E, Hoy D, Nolte S, Ackerman
I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, et al:
The global burden of hip and knee osteoarthritis: Estimates from
the global burden of disease 2010 study. Ann Rheum Dis.
73:1323–1330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poole AR, Kobayashi M, Yasuda T, Laverty
S, Mwale F, Kojima T, Sakai T, Wahl C, El-Maadawy S, Webb G, et al:
Type II collagen degradation and its regulation in articular
cartilage in osteoarthritis. Ann Rheum Dis. 61(Suppl 2): ii78–ii81.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi M, Squires GR, Mousa A, Tanzer
M, Zukor DJ, Antoniou J, Feige U and Poole AR: Role of
interleukin-1 and tumor necrosis factor alpha in matrix degradation
of human osteoarthritic cartilage. Arthritis Rheum. 52:128–135.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang M, Shen J, Jin H, Im HJ, Sandy J and
Chen D: Recent progress in understanding molecular mechanisms of
cartilage degeneration during osteoarthritis. Ann N Y Acad Sci.
1240:61–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang F, Wu L, Li L and Chen S: Monotropein
exerts protective effects against IL-1β-induced apoptosis and
catabolic responses on osteoarthritis chondrocytes. Int
Immunopharmacol. 23:575–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rigoglou S and Papavassiliou AG: The NF-κB
signalling pathway in osteoarthritis. Int J Biochem Cell Biol.
45:2580–2584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan T, Chen R, Wu D, Cai N, Shi X, Li B
and Pan J: Alpha-Mangostin suppresses interleukin-1β-induced
apoptosis in rat chondrocytes by inhibiting the NF-κB signaling
pathway and delays the progression of osteoarthritis in a rat
model. Int Immunopharmacol. 52:156–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du Y, Chen G, Zhang X, Yu C, Cao Y and Cui
L: Artesunate and erythropoietin synergistically improve the
outcome of experimental cerebral malaria. Int Immunopharmacol.
48:219–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu H, He Y, Yang X, Liang L, Zhan Z, Ye Y,
Yang X, Lian F and Sun L: Anti-malarial agent artesunate inhibits
TNF-alpha-induced production of proinflammatory cytokines via
inhibition of NF-kappaB and PI3 kinase/Akt signal pathway in human
rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology
(Oxford). 46:920–926. 2007. View Article : Google Scholar
|
|
12
|
Lai L, Chen Y, Tian X, Li X, Zhang X, Lei
J, Bi Y, Fang B and Song X: Artesunate alleviates hepatic fibrosis
induced by multiple pathogenic factors and inflammation through the
inhibition of LPS/TLR4/NF-κB signaling pathway in rats. Eur J
Pharmacol. 765:234–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guruprasad B, Chaudhary P, Choedon T and
Kumar VL: Artesunate ameliorates functional limitations in Freund's
complete adjuvant-induced monoarthritis in rat by maintaining
oxidative homeostasis and inhibiting COX-2 expression.
Inflammation. 38:1028–1035. 2015. View Article : Google Scholar
|
|
14
|
Zhao C, Liu Q and Wang K: Artesunate
attenuates ACLT-induced osteoarthritis by suppressing
osteoclastogenesis and aberrant angiogenesis. Biomed Pharmacother.
96:410–416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Le Graverand-Gastineau MP: Disease
modifying osteoarthritis drugs: Facing development challenges and
choosing molecular targets. Curr Drug Targets. 11:528–535. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu C, Li Y, Hu S, Cai Y, Yang Z and Peng
K: Scoparone prevents IL-1β-induced inflammatory response in human
osteoarthritis chondrocytes through the PI3K/Akt/NF-κB pathway.
Biomed Pharmacother. 106:1169–1174. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu M, Zhong S, Kong R, Shao H, Wang C,
Piao H, Lv W, Chu X and Zhao Y: Paeonol alleviates
interleukin-1β-induced inflammatory responses in chondrocytes
during osteoarthritis. Biomed Pharmacother. 95:914–921. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng Z, Zheng W, Li X, Lin J, Xie C, Li H,
Cheng L, Wu A and Ni W: Cryptotanshinone protects against
IL-1β-induced inflammation in human osteoarthritis chondrocytes and
ameliorates the progression of osteoarthritis in mice. Int
Immunopharmacol. 50:161–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ho WE, Peh HY, Chan TK and Wong WS:
Artemisinins: Pharmacological actions beyond anti-malarial.
Pharmacol Ther. 142:126–139. 2014. View Article : Google Scholar
|
|
21
|
Pereira D, Ramos E and Branco J:
Osteoarthritis. Acta Med Port. 28:99–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tetlow LC, Adlam DJ and Woolley DE: Matrix
metalloproteinase and proinflammatory cytokine production by
chondrocytes of human osteoarthritic cartilage: Associations with
degenerative changes. Arthritis Rheum. 44:585–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Verma P and Dalal K: ADAMTS-4 and
ADAMTS-5: Key enzymes in osteoarthritis. J Cell Biochem.
112:3507–3514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakao S, Ogtata Y, Shimizu E, Yamazaki M,
Furuyama S and Sugiya H: Tumor necrosis factor alpha
(TNF-alpha)-induced prostaglandin E2 release is mediated by the
activation of cyclooxygenase-2 (COX-2) transcription via NFkappaB
in human gingival fibroblasts. Mol Cell Biochem. 238:11–18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu B, Goode AP, Carter TE, Utturkar GM,
Huebner JL, Taylor DC, Moorman CT III, Garrett WE, Kraus VB, Guilak
F, et al: Matrix metalloproteinase activity and prosta-glandin E2
are elevated in the synovial fluid of meniscus tear patients.
Connect Tissue Res. 58:305–316. 2017. View Article : Google Scholar
|
|
26
|
Li Y, Wang S, Wang Y, Zhou C, Chen G, Shen
W, Li C, Lin W, Lin S, Huang H, et al: Inhibitory effect of the
antimalarial agent artesunate on collagen-induced arthritis in rats
through nuclear factor kappa B and mitogen-activated protein kinase
signaling pathway. Transl Res. 161:89–98. 2013. View Article : Google Scholar
|
|
27
|
He Y, Fan J, Lin H, Yang X, Ye Y, Liang L,
Zhan Z, Dong X, Sun L and Xu H: The anti-malaria agent artesunate
inhibits expression of vascular endothelial growth factor and
hypoxia-inducible factor-1α in human rheumatoid arthritis
fibroblast-like synoviocyte. Rheumatol Int. 31:53–60. 2011.
View Article : Google Scholar
|
|
28
|
Na JY, Kim S, Song K, Lim KH, Shin GW, Kim
JH, Kim B, Kwon YB and Kwon J: Anti-apoptotic activity of
Ginsenoside Rb1 in hydrogen peroxide-treated chondrocytes:
Stabilization of mitochondria and the inhibition of caspase-3. J
Ginseng Res. 36:242–247. 2012. View Article : Google Scholar
|
|
29
|
Musumeci G, Castrogiovanni P, Mazzone V,
Szychlinska MA, Castorina S and Loreto C: Histochemistry as a
unique approach for investigating normal and osteoarthritic
cartilage. Eur J Histochem. 58:23712014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qin G, Wu L, Liu H, Pang Y, Zhao C, Wu S,
Wang X and Chen T: Artesunate induces apoptosis via a
ROS-independent and Bax-mediated intrinsic pathway in HepG2 cells.
Exp Cell Res. 336:308–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang P, Luo HS, Li M and Tan SY:
Artesunate inhibits the growth and induces apoptosis of human
gastric cancer cells by downregulating COX-2. Onco Targets Ther.
8:845–854. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marcu KB, Otero M, Olivotto E, Borzi RM
and Goldring MB: NF-kappaB signaling: Multiple angles to target OA.
Curr Drug Targets. 11:599–613. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pan T, Shi X, Chen H, Chen R, Wu D, Lin Z,
Zhang J and Pan J: Geniposide suppresses interleukin-1β-induced
inflammation and apoptosis in rat chondrocytes via the
PI3K/Akt/NF-κB signaling pathway. Inflammation. 41:390–399. 2018.
View Article : Google Scholar
|
|
34
|
Cui Z, Crane J, Xie H, Jin X, Zhen G, Li
C, Xie L, Wang L, Bian Q, Qiu T, et al: Halofuginone attenuates
osteoarthritis by inhibition of TGF-β activity and H-type vessel
formation in subchondral bone. Ann Rheum Dis. 75:1714–1721. 2016.
View Article : Google Scholar
|