Introduction
Oxidative stress (OS), which was initially
conceptualized in 1985 (1), is
regarded as an important phenomenon in redox biology and medicine.
OS was initially defined as an imbalance between oxidants and
antioxidants, which leads to a disruption of redox signaling and/or
molecular damage (2). Reactive
oxygen species (ROS) were considered to be damaging agents in
living organisms; however, they were subsequently also determined
to serve positive roles in living organisms (3). Thus, the definition of OS was
revised to refer to an imbalance between ROS generation and
elimination (4). OS can be
classified as basal, low intensity, intermediate intensity OS or
high intensity OS (4).
The eyes are susceptible to OS injury due to the
production of ROS, as eyes are exposed to various adverse
environments that are able to shift the cell redox status towards
oxidizing conditions, including ionizing radiation, light exposure,
ultraviolet rays, chemical pollutants and pathogenic microbes
(5). Additionally, the retina is
vulnerable to OS due to its high oxygen consumption (6,7).
OS can induce peroxidation of nucleic acids, bases, lipids,
proteins and carbohydrates, resulting in a number of eye conditions
[dry eye syndrome, diabetic retinopathy, autoimmune and
inflammatory uveitis, corneal and conjunctive diseases, cataracts,
glaucoma, age-related macular degeneration (AMD) and retinitis
pigmentosa (RP)], as well as chronic inflammation (5,8).
Amyloid β (Aβ), which promotes the progression of AMD (9), can induce OS; Aβ has been used in
animal or cell models of OS and AMD (10-12). In the present study, ROS were
investigated in ARPE-19 cells treated with Aβ1-40.
Hexokinases (HKs) catalyze the first step of glucose
metabolism; glucose, which is transported through glucose
transporters in the mitochondrial membrane, is phosphorylated by
HKs, producing glucose-6-phosphate (G6P) (13-16); G6P also provides feedback
regulation of HK activity. HKs serve important roles in the
regulation of metabolic process, as G6P is a precursor of ATP,
glycogenesis, and pentose phosphate and hexosamine biosynthetic
pathways (13,14,16,17). HK has four isomers (I, II, III and
IV) in mammalian cells; hexokinase II (HKII) serves important roles
in insulin-sensitive tissues, such as skeletal muscle, heart and
adipose tissue (18).
HKII has been reported to induce important effects
on mitochondrial function in myocardial cells (19). HKII, which binds to mitochondria,
can suppress the mitochondrial translo-cation of Bax and the
release of cytochrome c (Cyt c) (20,21), therefore preventing cell apoptosis
(22). It has been reported that
ischemia or glucose deprivation in adult hearts or isolated
cardiomyocytes can result in the dissociation of HKII from
mitochondria, thereby releasing mitochondrial Cyt c to
induce apoptosis (23,24).
N-acetylcysteine (NAC), an antioxidant, is used as a
mucolytic agent for treating various disorders, including
paracetamol intoxication, doxorubicin cardiotoxicity and
ischemia-reperfusion cardiac injury in clinical settings (25,26). The effects of HKII on
Aβ1-40-induced OS injury were studied in retinal pigment epithelial
(RPE) cells, using NAC as a control.
Materials and methods
Cell culture, oxidative stress model and
morphological observation
The human RPE cell line (ARPE-19/HPV-16) was
purchased from the American Type Culture Collection. The cell line
was cultured at 37°C with 5% CO2 in an incubator (Thermo
Fisher Scientific, Inc.) with DMEM/F-12 medium (cat. no. 11330057;
Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1% 10,000
U/ml penicillin/10,000 µg/ml streptomycin (Gibco; Thermo
Fisher Scientific, Inc,.). The cells were subcultured every 3 days
in a 35-mm culture flask (Corning Inc.). After the cells
(2×104 cells/well) were seeded in 96-well plates
(Corning Inc.), and different concentrations of Aβ1-40 (0, 0.01,
0.1, 0.5, 1, 5 and 10 µmol/ml; Sigma-Aldrich; Merck KGaA)
mixed with culture medium were added to the cells in order to
establish OS, the cells were incubated at 37°C for 24 h. For
subsequent experiments, 0.5 µmol/ml Aβ1-40 was used.
Following culture for 24 h, the morphology of cells in the control
and 0.5 µmol/ml Aβ1-40 groups was observed under an inverted
phase contrast microscope (magnification, ×200; Olympus
Corporation).
Cell viability
Cell viability was measured via a Cell Counting
Kit-8 (CCK-8) assay. ARPE-19 cells (2×104 cells/well)
were seeded in a 96-well plates (Corning Inc.) and cultured in an
incubator for 24 h at 37°C. Cells were cultured with various
concentrations (0.1, 1 or 10 µmol/ml) of NAC (Shanghai
Aladdin Bio-Chem Technology Co., Ltd.); for subsequent experiments,
1 µmol/ml NAC was used. A CCK-8 kit (Sigma-Aldrich; Merck
KGaA) was diluted with serum-free DMEM (1:9). The culture medium
was then replaced, and the cells were washed three times with PBS
(Gibco; Thermo Fisher Scientific, Inc.). CCK-8 working solution (10
µl) was added to each well, and plates were incubated in an
incubator for a further 2 h. The optical density at 490 nm was then
detected using a microplate reader (Thermo Fisher Scientific,
Inc.).
Cell transfection
Small interfering RNA specific for HKII (siHKII;
5′-GACCCTCTACAAGCTACAT-3′) and negative control (NC) siRNA
(5′-GGTAAGCAAGGGAGATCAA-3′) were synthesized by Orbigen, Inc.
(Allele Biotechnology). Serum-free DMEM was diluted (1:1) with
Lipofectamine® (Invitrogen; Thermo Fisher Scientific,
Inc.). Then, 50 nmol/l siHKII or NC mixed with the Lipofectamine
solution was added to the cells for 1-2 h, following which the
solution was replaced with normal culture medium. Subsequent
experiments were performed 72 h following transfection.
Apoptosis and ROS analysis
After the cells had been treated with Aβ1-40 (0.5
µmol/ml), NAC (1 µmol/ml), Aβ1-40 (0.5
µmol/ml) + NAC (1 µmol/ml) or Aβ1-40 (0.5
µmol/ml) + NAC (1 µmol/ml) + siHKII (50 nM) for 24 h,
respectively, they were collected by trypsin (Gibco; Thermo Fisher
Scientific, Inc.) via centrifugation at 800 × g for 5 min at 4°C.
An Annexin V-FITC/propidium iodide (PI) apoptosis detection kit (BD
Biosciences) and a fluorometric intracellular ROS kit (cat. no.
MAK143; Sigma-Aldrich; Merck KGaA) were applied to detect the
apoptosis and ROS contents of ARPE cells using a flow cytometer
(FACSCalibur; BD Biosciences). The cells were incubated with
Annexin V-FITC and PI in the dark for 20 min at room temperature;
the same temperature and duration were used for ROS assays. The
fluorescence intensity was analyzed using CellQuest software
(version 3.3; BD Biosciences). Experiments were conducted according
to the manufacturers' protocols. The apoptosis rate was calculated
as the percentage of early + late apoptotic cells.
Mitochondrial membrane potential (MMP)
analysis
The JC-1 fluorescent probe can be used to detect
changes in the MMP; the color of fluorescence is altered when the
MMP changes (27). Cells were
seeded in a 6-well plate at 1×105 cells/well for 24 h,
and the cells were treated as aforementioned for 24 h; untreated
cells were regarded as a control group. The cells were collected
with 0.25% trypsin for 5 min at 37°C and via centrifugation at 800
× g for 5 min at 4°C, and 105 cells were resuspended in
0.5 ml DMEM. A JC-1 MMP assay kit (Beijing Leagene Biotech Co.,
Ltd.) was used to analyze the MMP. The experimental procedure was
performed according to the manufacturer's protocols. The
fluorescence was detected using a flow cytometer (FACSCalibur) and
CellQuest version 3.3 software at wavelengths of 530 and 590
nm.
Western blotting
The cells were seeded in 90-mm petri dishes (Corning
Inc.) at 106 cells/dish, and were treated as
aforementioned for 24 h. The medium was discarded, and PBS was used
to wash the cells three times. Total protein was extracted by a
cell scraper (Thermo Fisher Scientific, Inc.) with 300 µl
cell lysis buffer (cat. no. RABLYSIS1; Sigma-Aldrich; Merck KGaA)
on ice, and the cells were centrifuged at 4°C and 12,000 × g for 15
min. Mitochondria in ARPE-19 cells were extracted with a
mitochondria isolation kit (Sigma-Aldrich; Merck KGaA). Cell lysis
buffer (Sigma-Aldrich; Merck KGaA) was used to extract protein from
mitochondria via centrifugation at 12,000 × g for 15 min at 4°C. A
BCA assay kit (Sigma-Aldrich; Merck KGaA) was used for determining
the amount of protein. Then, 40 µg protein was separated via
12% SDS-PAGE and transferred to PVDF membranes (Sigma-Aldrich;
Merck KGaA). Protein membranes were blocked with 3% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) for 2 h at room temperature.
Primary antibodies were diluted in TBS with 0.1% Tween-20 (TBST).
Cleaved caspase-3 (1:1,000; cat. no. 9661; Cell Signaling
Technology, Inc.), Bcl-2 (1:1,000; cat. no. 4223; Cell Signaling
Technology, Inc.), Bax (1:1,000; cat. no. 5023; Cell Signaling
Technology, Inc.), manganese superoxide dismutase (MnSOD; 1:1,000;
cat. no. 13141; Cell Signaling Technology, Inc.), copper/zinc
superoxide dismutase (CuZnSOD; 1:1,000; cat. no. 2770; Cell
Signaling Technology, Inc.), Cyt c (1:1,000; cat. no. 11940;
Cell Signaling Technology, Inc.), HKII (1:1,000; cat. no. 2867;
Cell Signaling Technology, Inc.), GAPDH (1:1,000; cat. no. 5174;
Cell Signaling Technology, Inc.) and mitochondrial marker Cyt
c oxidase subunit IV (1:1,000; cat. no. 4850; Cell Signaling
Technology, Inc.) antibodies were used to incubate the protein
membranes for 12 h at 4°C. Following three washes with TBST,
horseradish peroxidase-conjugated anti-rabbit secondary antibodies
(1:10,000; cat. no. 7074; Cell Signaling Technology, Inc.) were
added to the membranes, and the protein membranes were incubated
for 1.5 h at room temperature. Bands were visualized using ECL
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The protein
bands were analyzed using ImageJ v1.45s (National Institutes of
Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols. cDNA was
synthesized from mRNA by using a PrimeScript First Strand cDNA
synthesis kit (Takara Bio, Inc.); the RT reaction was performed at
45°C for 20 min and 95°C for 5 min. qPCR was performed using an
SYBR Premix Ex Taq kit (Takara Biotechnology Co., Ltd.) under the
following conditions: 94°C for 75 sec, then 50 cycles of 55°C for
45 sec and 72°C for 10 min. The primers (Sangon Biotech Co., Ltd.)
used for qPCR were as follows: HKII, forward 5′-AGA CTG TCC TTT CCA
CAT GG-3′, reverse 5′-TTC CAG GTG CAT TCG ACA AG-3′; GAPDH, forward
5′-ACG GAT TTG GTC GTA TTG GG-3′, reverse 5′-CGC TCC TGG AAG ATG
GTG AT-3′. The 2−∆∆Cq method was employed to analyze the
relative levels of gene expression (28).
Statistical analysis
All values were presented as the mean ± SD. All
experiments were repeated three times. For comparison, one-way
ANOVA followed by a Tukey's post hoc test was performed with
GraphPad Prism 5.0 software (GraphPad Software, Inc.). The
untreated experimental groups were regarded as the control group.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of Aβ1-40 on ARPE-19 cells
ARPE-19 cells were treated with different
concentrations of Aβ1-40 for 24 h (0, 0.01, 0.1, 0.5, 1, 5, 10
µmol/ml). Aβ1-40 reduced ARPE-19 cell viability; the extent
of inhibition increased with increasing concentrations of Aβ1-40
(Fig. 1) increased. Then, 0.5
µmol/ml Aβ1-40 was selected to treat ARPE-19 cell; the
extent of inhibition increased with prolonged duration of treatment
(Fig. 2A). As observed via
microscopy, the cell morphology was altered by Aβ1-40 treatment;
cells shrunk, and cell fragments were detected in the 0.5
µmol/ml Aβ1-40 group (Fig.
2B). In addition, the number of cells notably decreased.
Additionally, 0.5 µmol/ml Aβ1-40 significantly increased the
apoptosis and ROS content of ARPE-19 cells (Fig. 2C-E). Notably, the expression of
HKII in ARPE-19 cells was not significantly altered in the 0.5
µmol/ml Aβ1-40 group compared with the control group;
however, HKII expression in ARPE-19 cell mitochondria was
significantly decreased following Aβ1-40 treatment (Fig. 2F-H).
 | Figure 2Effects of Aβ1-40 on ARPE-19 retinal
pigment epithelial cells. ARPE-19 cells were treated with 0.5
µmol/ml Aβ1-40; untreated cells were used as a control
group. (A) Aβ1-40 (0.5 µmol/ml) were added to cells for 12,
24, 48 and 72 h; cell viability was determined using a Cell
Counting Kit-8 assay. (B) Images of ARPE-19 cells following
treatment with 0.5 µmol/ml Aβ1-40 for 24 h. Scale bar, 100
µm. After 0.5 µmol/ml Aβ1-40 was added to cells for
24 h, (C and D) apoptosis and (E) ROS levels were determined via
flow cytometry. Effects of Aβ1-40 (0.5 µmol/ml) on (F) HKII
mRNA levels as determined by a reverse transcription-quantitative
PCR assay, and (G) cellular and (H) mitochondrial HKII protein
levels as determined by western blotting. Data are presented as the
mean ± standard deviation. **P<0.01 vs. control.
Aβ1-40, amyloid β1-40; COX IV, cytochrome c oxidase subunit
IV; HKII, hexokinase II; OD, optical density; PI, propidium iodide;
ROS, reactive oxygen species. |
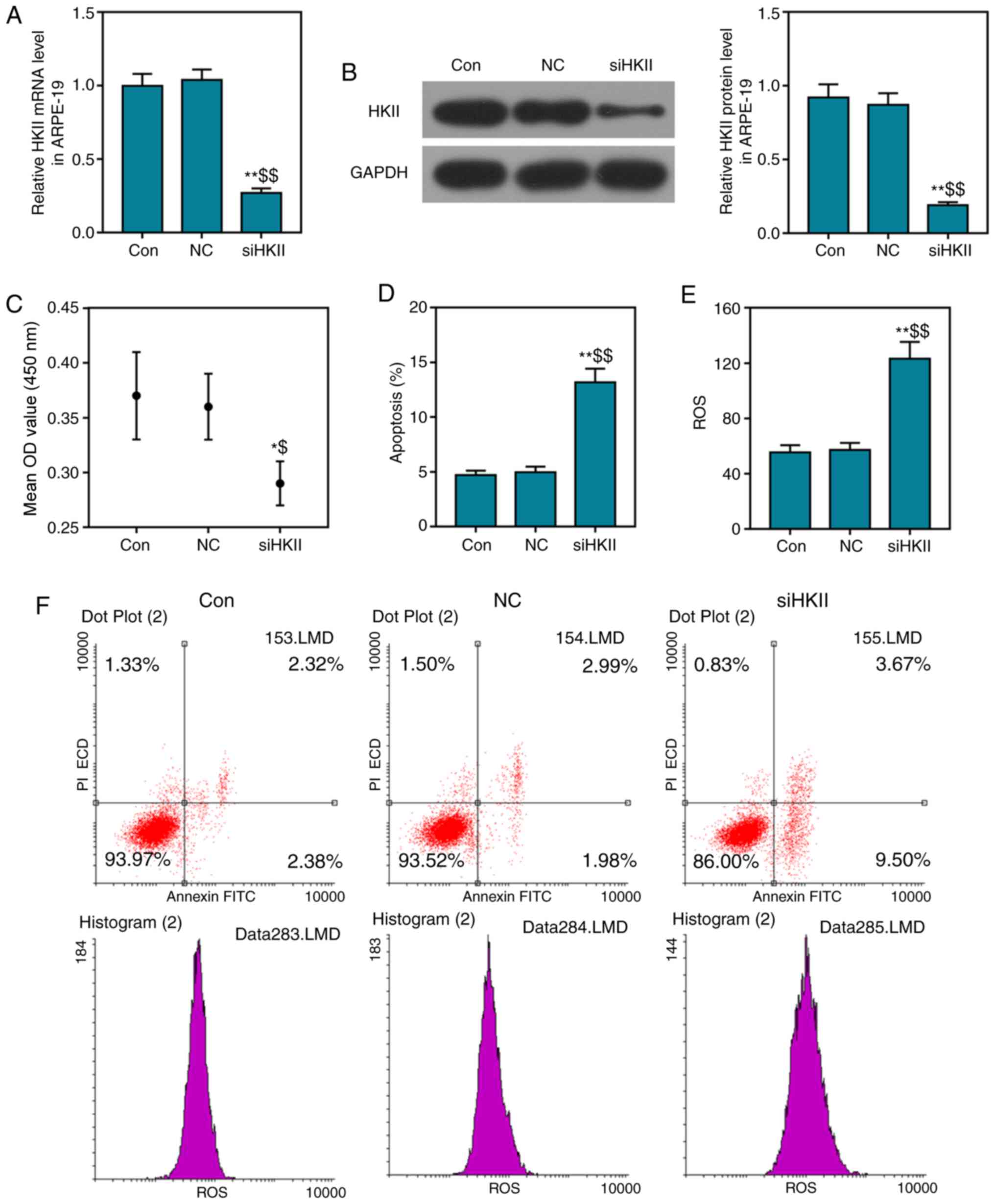
Effects of siHKII on ARPE-19 cells
siHKII significantly suppressed HKII expression in
ARPE-19 cells, and significantly decreased the viability and
increased the apoptosis of cells (Fig. 3A-D). Additionally, the ROS content
of ARPE-19 cells was also significantly increased (Fig. 3E). The percentage of early and
late apoptotic cells was notably increased in the siHKII group
compared with the two control groups (Fig. 3F). These findings indicated that
inhibition of HKII damaged RPE cells.
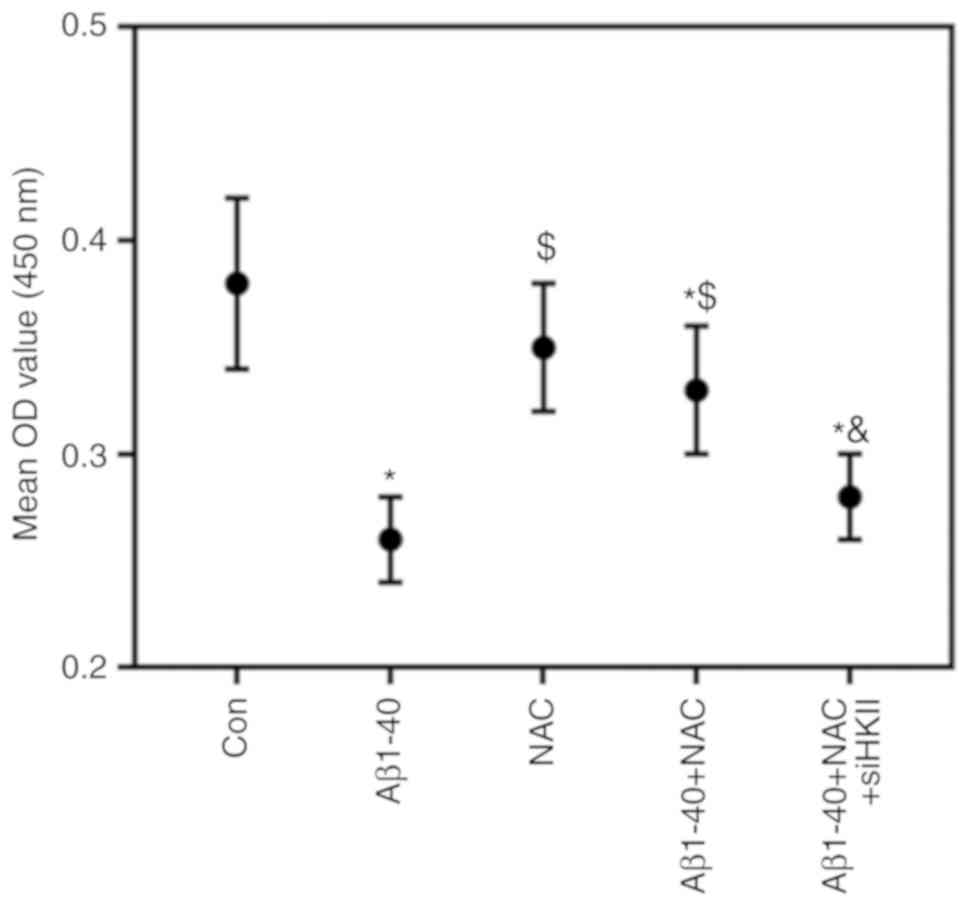
Knockdown of HKII alleviates the
protective role of NAC in Aβ1-40-induced OS injury
High concentrations of NAC damaged ARPE-19 cells
(Fig. 4); therefore, 1
µmol/ml NAC was selected for subsequent experiments. NAC
attenuated the effects of Aβ1-40 on ARPE-19 cell viability; this
was reversed by knockdown of HKII (Fig. 5). NAC reduced the rate of
apop-tosis and ROS content in ARPE-19 cells treated with Aβ1-40
(Fig. 6A-C). Conversely, siHKII
induced ARPE-19 cell apoptosis in the Aβ1-40 + NAC group.
Furthermore, Aβ1-40 treatment significantly decreased the MMP in
ARPE-19 cells (Fig. 6D); NAC
attenuated the effects of Aβ1-40 treatment, but siHKII reversed the
effects of NAC, reducing the MMP in Aβ1-40 + NAC-treated cells.
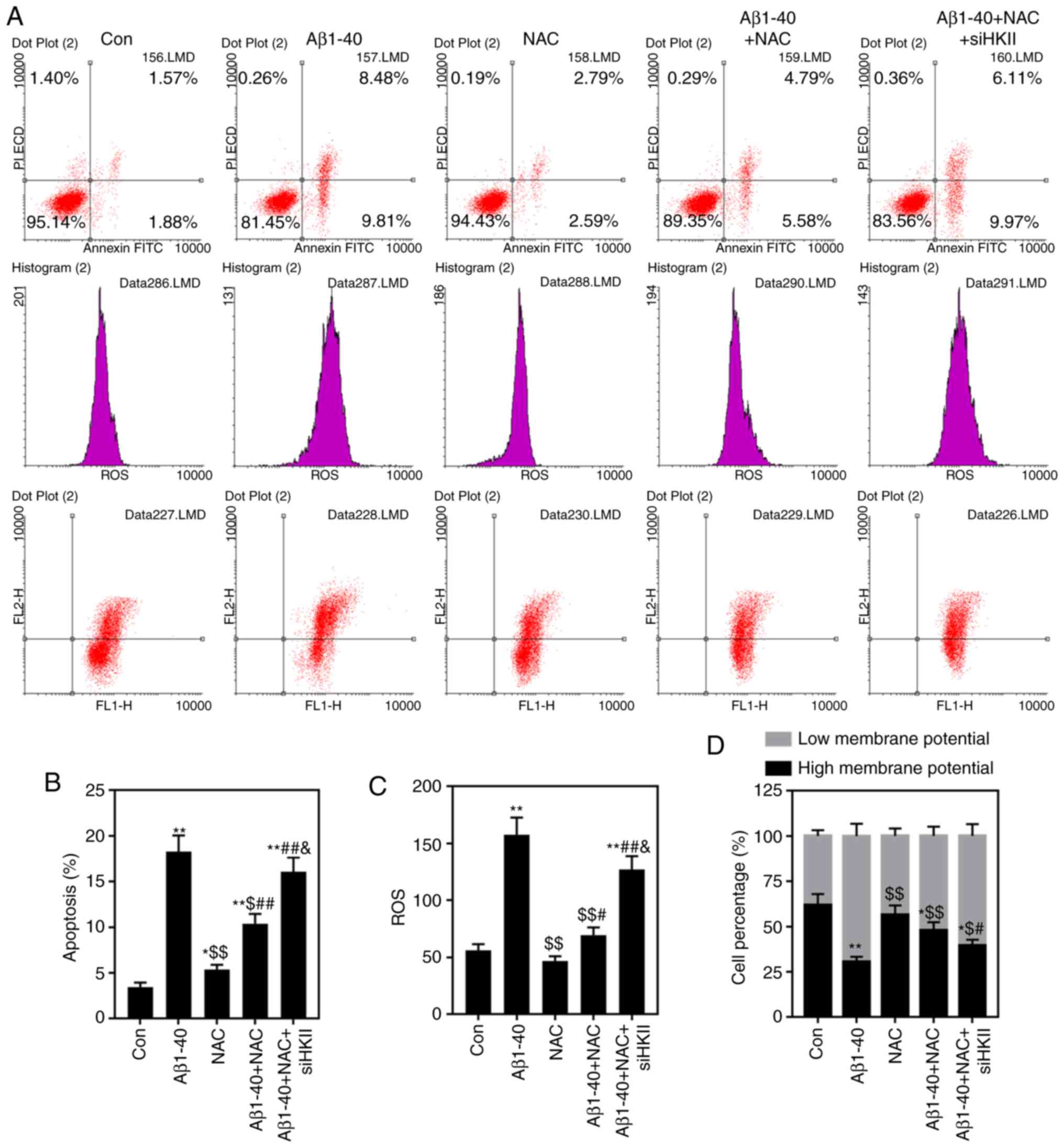 | Figure 6Effects of Aβ1-40, NAC and siHKII
treatment on ARPE-19 retinal pigment epithelial cells. ARPE-19
cells transfected with siHKII or negative control siRNA were
treated for 24 h with Aβ1-40 (0.5 µmol/ml) and/or NAC (1
µmol/ml). (A and B) Apoptosis and (C) ROS levels were
determined via flow cytometry. (D) Analysis of the MMP in treated
ARPE-19 cells. Data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01 vs. Con;
$P<0.05, $$P<0.01 vs. Aβ1-40;
#P<0.05, ##P<0.01 vs. NAC;
&P<0.05 vs. Aβ1-40 + NAC. Aβ1-40, amyloid β1-40;
Con, control; HKII, hexokinase II; MMP, mitochondrial membrane
potential; NAC, N-acetylcysteine; PI, propidium iodide; ROS,
reactive oxygen species; si(RNA), small interfering (RNA). |
Effects of Aβ1-40, NAC and siHKII
treatments on the levels of apoptosis-associated and ROS-associated
proteins in ARPE-19 cells
As presented in Fig.
7, it was demonstrated that Aβ1-40 treatment significantly
downregulated Bcl-2, MnSOD and CuZnSOD protein expression levels,
and significantly upregulated cleaved caspase-3, Bax and Cyt
c protein expression. NAC significantly attenuated the
effects of Aβ1-40 on the expression of these proteins in ARPE-19
cells; however, siHKII significantly decreased Bcl-2, MnSOD and
CuZnSOD expression, and increased cleaved caspase-3, Bax and Cyt
c levels in ARPE-19 cells treated with Aβ1-40 and NAC.
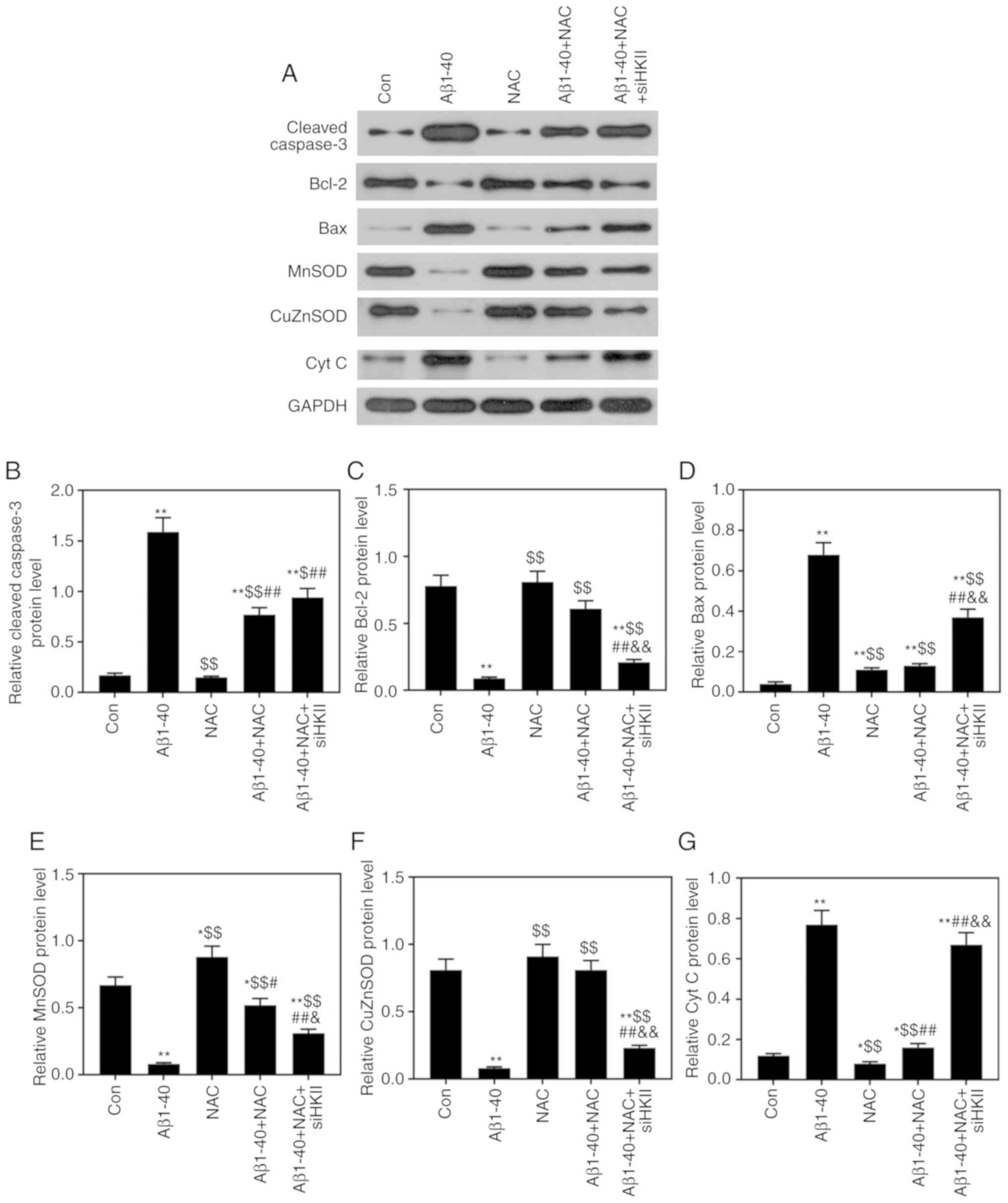 | Figure 7Effects of Aβ1-40, NAC and siHKII
treatment on the levels of apoptosis- and reactive oxygen
species-associated proteins in ARPE-19 retinal pigment epithelial
cells. ARPE-19 cells transfected with siHKII or negative control
siRNA were treated for 24 h with Aβ1-40 (0.5 µmol/ml) and/or
NAC (1 µmol/ml). (A) Western blotting was performed to
determine the protein expression levels of (B) caspase-3, (C)
Bcl-2, (D) Bax, (E) MnSOD, (F) CuZnSOD and (G) Cyt c.
Expression was quantified using ImageJ. Data are presented as the
mean ± standard deviation. *P<0.05,
**P<0.01 vs. Con; $P<0.05,
$$P<0.01 vs. Aβ1-40; #P<0.05,
##P<0.01 vs. NAC; &P<0.05,
&&P<0.01 vs. Aβ1-40 + NAC. Aβ1-40, amyloid
β1-40; Con, control; Cyt c, cytochrome c; CuZnSod,
copper-zinc-SOD; HKII, hexokinase II; MnSOD, manganese-SOD; NAC,
N-acetylcysteine; si(RNA), small interfering (RNA); SOD, superoxide
dismutase. |
Effects of Aβ1-40, NAC and siHKII
treatments on the expression of HKII in ARPE-19 cells and
mitochondria
NAC treatment markedly increased HKII mRNA levels in
ARPE-19 cells; however, siHKII resulted in a significant reduction
in HKII mRNA expression compared with all other groups (Fig. 8A). Furthermore, siHKII also
signifi-cantly reduced HKII protein levels in ARPE-19 cells treated
with Aβ1-40 and NAC; HKII protein levels were not significantly
altered by Aβ1-40 or NAC (Fig.
8B). Conversely, Aβ1-40 significantly downregulated HKII
protein levels in mitochondria, whereas NAC attenuated the effects
of Aβ1-40 on mitochondrial HKII protein levels (Fig. 8C). siHKII significantly reduced
HKII protein levels in the mitochondria of ARPE-19 cells treated
with Aβ1-40 and NAC (Fig. 8C),
indicating that the changes in HKII induced by siHKII mainly
occurred in the mitochondria.
 | Figure 8Effects of Aβ1-40, NAC and siHKII
treatment on the expression of HKII in ARPE-19 retinal pigment
epithelial cells and mitochondria. ARPE-19 cells transfected with
siHKII or negative control siRNA were treated for 24 h with Aβ1-40
(0.5 µmol/ml) and/or NAC (1 µmol/ml). (A) HKII mRNA
levels in ARPE-19 cells, as determined via reverse
transcription-quantitative PCR analysis. (B) Whole cell and (C)
mitochondrial levels of HKII in ARPE-19 cells, as determined by
western blotting. The stains were analyzed using Image J system.
Expression was quantified using ImageJ. Data are presented as the
mean ± standard deviation. *P<0.05,
**P<0.01 vs. Con; $P<0.05,
$$P<0.01 vs. Aβ1-40; #P<0.05,
##P<0.01 vs. NAC; &P<0.05,
&&P<0.01 vs. Aβ1-40 + NAC. Aβ1-40, amyloid
β1-40; Con, control; COX IV, cytochrome c oxidase subunit
IV; HKII, hexokinase II; NAC, N-acetylcysteine; si(RNA), small
interfering (RNA). |
Discussion
Retinal degeneration, including AMD and RP, is one
of the most common neurodegenerative diseases globally (29). AMD and RP are characterized by
gradual faded central vision due to photoreceptor cell
degeneration; RPE cells, which are a layer of cells in the retina,
support the photoreceptors function, thus serving an important role
in maintaining retinal homeostasis (30,31). Patients with RP exhibit ocular OS
and damage from the absence of the ability to overcome systemic OS;
an imbalance between the generation and elimination of ROS leads to
OS, oxidation of macromolecules and eventually retinal disease
(32,33). ARPE-19 cell viability was
inhibited, and apoptosis and ROS contents were increased following
induction of OS via Aβ1-40 treatment.
OS, inflammation and endoplasmic reticulum stress
are involved in glutamate excitotoxicity, contributing to
mitochondrial dysfunction (34).
The mechanism via which dissociation of HKII induces mitochondrial
dysfunction and apoptosis in the retina remains unclear. It was
previously reported that when HKII dissociates from the
mitochondrial membrane, Bax translocates into the mitochondria and
binds to unoccupied voltage-dependent anion channels (VDACs) to
form a large pore, which exhibits 4- and 10-fold higher conductance levels
than VDACs and Bax channels, respectively; however, the large pore
lacks the ion selectivity of individual channels (21,35). Furthermore, the VDAC-Bax pore can
result in the release of Cyt c to the cytosol (36). Additionally, others have also
suggested that disaggregation of the VDAC-HKII interaction could
open the Bax-independent mitochondrial permeability transition pore
(PTP) (37,38), a multiprotein complex including
cyclophilin D in the matrix, adenine nucleotide translocator in the
inner membrane, and VDACs in the outer membrane (39,40). The opening of the PTP can lead to
rapid MMP depolarization and matrix swelling, therefore resulting
in the unfolding of cristae and breaches in the outer mitochondrial
membrane, rendering it permeable to proteins (41). In the present study, the results
showed that Aβ1-40 treatment decreased the MMP, which is consistent
with the research of Moreira et al (42). It has been suggested that MMP
depolarization is associated with apoptosis. Conversely, it has
been reported that Aβ can potentiate Ca2+-induced PTP
formation in liver mitochondria (42). In the present study, Aβ1-40 may
reduce the transmembrane potential by reducing the concentration of
Ca2+, thereby causing changes in mitochondrial membrane
permeability, leading to the release of proapoptotic substances and
activation of the caspase family, and promoting cell apoptosis.
Mitochondria are not only the primary cellular energy source under
aerobic conditions, but also an important an important component in
apoptotic cell death (43). A
previous study reported that the MMP regulates matrix configuration
and Cyt c release during apoptosis (44). Additionally, outer mitochondrial
membrane permeabilization is a crucial signal for apoptosis,
resulting in the liberation of proapoptotic molecules such as
cytochrome c and procaspase activation (45). Regardless of the mechanism, the
findings from the present study suggested that the dissociation of
HKII from the mitochondrial membrane inhibits proliferation,
induces apoptosis, increases the ROS levels and decreases the MMP
in RPE cells.
NAC, an antioxidant, has been reported to reduce
retinal superoxide radicals and promote cone cell survival in mouse
models (46). The present study
further revealed that NAC improved the viability and reduced the
apoptosis of HPE cells under OS induced by Aβ1-40 treatment.
Previous studies reported that increased expression of HKII
provided protection (47,48), and that decreased expression of
HKII promoted cell apoptosis (21). For example, overexpression of HKII
provided protection against peroxide in cardiomyocytes (19,37). The present study demonstrated that
the inhibition of HKII mRNA expression decreased ARPE-19 cell
viability, and promoted cell apoptosis and ROS, indicating that
decreased expression of HKII promoted cell damage. However, a
potential association between NAC and HKII, and the mechanisms via
which altered HKII expression induces cell damage remain
unclear.
Akt, a member of the AGC kinase group, is important
in various cell functions, including proliferation, apoptosis and
metabolism (49). Previous
studies reported that Akt was upregulated in tumors or following
insulin treatment; HKII levels were also increased under these
conditions (19,50), suggesting a potential association
between the Akt pathway and HKII. A number of studies have
demonstrated that the Akt pathway was associated with apoptosis
pathways, and that apoptosis was induced by inhibiting the Akt
pathway (51,52). The present study suggested that
NAC reduced Aβ1-40-induced damage by upregulating HKII levels in
the mitochondria, and that the downregulation of HKII promoted
apoptosis pathways, including upregulated cleaved caspase-3, Bax
and Cyt c, and decreased Bcl-2 expression, all of which are
involved in the mitochondrial apoptosis pathway (53-55).
A previous study observed that activation of Akt
increased HK activity in mitochondria, and that mitochondrial HK
was required for the antiapoptotic properties of Akt signaling
(56). The function of living
cells and mechanisms of energy can be assessed via the MMP
(57); a study indicated that
antiproliferative and proapoptotic effects occurred following loss
of the MMP (58). Downregulation
of mitochondrial HKII, either by Aβ1-40-induced OS or
siHKII-mediated knockdown, resulted in a lower MMP, increased
apoptosis and downregulation of SOD. NAC treatment attenuated the
proapoptotic and antiproliferative effects of Aβ1-40-induced OS in
ARPE-19 cells, which was accompanied with restoration of the normal
MMP; however, knockdown HKII reversed these effects and again
decreased the MMP. CuZnSOD and MnSOD eliminate ROS and maintain
redox balance in the immune system (59). NAC reduced ROS levels in
Aβ1-40-induced ARPE-19 cells; HKII knockdown enhanced ROS levels,
potentially via the down-regulation of CuZnSOD and MnSOD.
The aims of the present study were to investigate
the roles of HKII in OS-induced injury in RPE cells. The present
findings suggested that NAC reduced OS-associated damage, and
increased the viability of RPE cells subjected to Aβ1-40-induced
OS. It was further suggested that the effects of NAC on OS involved
upregulation of mitochondrial HKII levels, as HKII serves roles in
regulating mitochondrial apoptotic pathways. It was also observed
that decreased expression of HKII promoted the expression of
proapoptotic proteins associated with the mitochondrial apoptosis
pathway, and reduced the levels of MnSOD and CuZnSOD in RPE cells
under OS. However, there are certain limitations to the present
study; for example, the levels of VDACs, and the expression and
activation Akt were not detected. These and other limitations will
be resolved in future investigations.
Acknowledgments
Not applicable.
Funding
This study was supported by the Beijing Shijitan
Hospital Fund (grant no. 2016-c08), the Beijing Talents Training
Fund (grant no. 2010D003034000006) and the Beijing High-level
Talents Training Fund for Health System (grant no. 2014-3-049).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
LC made substantial contributions to the conception
and design of the study. LX, BX and JX were involved in data
acquisition, analysis and interpretation. LC drafted the article
and critically revised it for important intellectual content. All
authors approved the final version of the manuscript to be
published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wei EP, Christman CW, Kontos HA and
Povlishock JT: Effects of oxygen radicals on cerebral arterioles.
Am J Physiol. 248:H157–H162. 1985.PubMed/NCBI
|
|
2
|
Sies H: Oxidative stress: A concept in
redox biology and medicine. Redox Biol. 4:180–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Russell EG and Cotter TG: New insight into
the role of reactive oxygen species (ROS) in cellular
signal-transduction processes. Int Rev Cell Mol Biol. 319:221–254.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lushchak VI: Free radicals, reactive
oxygen species, oxidative stress and its classification. Chem Biol
Interact. 224:164–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kruk J, Kubasik-Kladna K and Aboul-Enein
HY: The role oxidative stress in the pathogenesis of eye diseases:
Current status and a dual role of physical activity. Mini Rev Med
Chem. 16:241–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ames A III: Energy requirements of CNS
cells as related to their function and to their vulnerability to
ischemia: A commentary based on studies on retina. Can J Physiol
Pharmacol. (70 Suppl): S158–S164. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo X, Dason ES, Zanon-Moreno V, Jiang Q,
Nahirnyj A, Chan D, Flanagan JG and Sivak JM: PGC-1α signaling
coordinates susceptibility to metabolic and oxidative injury in the
inner retina. Am J Pathol. 184:1017–1029. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moreno ML, Mérida S, Bosch-Morell F,
Miranda M and Villar VM: Autophagy dysfunction and oxidative
stress, two related mechanisms implicated in retinitis pigmentosa.
Front Physiol. 9:10082018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang K, Zhu X, Zhang K, Yao Y, Zhuang M,
Tan C, Zhou F and Zhu L: Puerarin inhibits amyloid β-induced NLRP3
inflammasome activation in retinal pigment epithelial cells via
suppressing ROS-dependent oxidative and endoplasmic reticulum
stresses. Exp Cell Res. 357:335–340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Briyal S, Shepard C and Gulati A:
Endothelin receptor type B agonist, IRL-1620, prevents beta amyloid
(Aβ) induced oxidative stress and cognitive impairment in normal
and diabetic rats. Pharmacol Biochem Behav. 120:65–72. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang R, Wang Q, Min L, Sui R, Li J and Liu
X: Monosialoanglioside improves memory deficits and relieves
oxidative stress in the hippocampus of rat model of Alzheimer's
disease. Neurol Sci. 34:1447–1451. 2013. View Article : Google Scholar
|
|
12
|
Yoshida T, Ohno-Matsui K, Ichinose S, Sato
T, Iwata N, Saido TC, Hisatomi T, Mochizuki M and Morita I: The
potential role of amyloid beta in the pathogenesis of age-related
macular degeneration. J Clin Invest. 115:2793–2800. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wilson JE: Hexokinases. Rev Physiol
Biochem Pharmacol. 126:65–198. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wilson JE: Isozymes of mammalian
hexokinase: Structure, subcellular localization and metabolic
function. J Exp Biol. 206:2049–2057. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ardehali H, Printz RL, Whitesell RR, May
JM and Granner DK: Functional interaction between the N- and
C-terminal halves of human hexokinase II. J Biol Chem.
274:15986–15989. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robey RB and Hay N: Mitochondrial
hexokinases, novel mediators of the antiapoptotic effects of growth
factors and Akt. Oncogene. 25:4683–4696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pedersen PL: Warburg, me and Hexokinase 2:
Multiple discoveries of key molecular events underlying one of
cancers' most common phenotypes, the 'Warburg Effect', i.e.,
elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr.
39:211–222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heikkinen S, Suppola S, Malkki M, Deeb SS,
Jänne J and Laakso M: Mouse hexokinase II gene: Structure, cDNA,
promoter analysis, and expression pattern. Mamm Genome. 11:91–96.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roberts DJ, Tan-Sah VP, Smith JM and
Miyamoto S: Akt phosphorylates HK-II at Thr-473 and increases
mitochondrial HK-II association to protect cardiomyocytes. J Biol
Chem. 288:23798–23806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Majewski N, Nogueira V, Bhaskar P, Coy PE,
Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB and Hay N:
Hexokinase-mitochondria interaction mediated by Akt is required to
inhibit apoptosis in the presence or absence of Bax and Bak. Mol
Cell. 16:819–830. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pastorino JG, Shulga N and Hoek JB:
Mitochondrial binding of hexokinase II inhibits Bax-induced
cytochrome c release and apoptosis. J Biol Chem. 277:7610–7618.
2002. View Article : Google Scholar
|
|
22
|
Das S, Steenbergen C and Murphy E: Does
the voltage dependent anion channel modulate cardiac
ischemia-reperfusion injury? Biochim Biophys Acta. 1818:1451–1456.
2012. View Article : Google Scholar :
|
|
23
|
Pasdois P, Parker JE and Halestrap AP:
Extent of mitochondrial hexokinase II dissociation during ischemia
correlates with mitochondrial cytochrome c release, reactive oxygen
species production, and infarct size on reperfusion. J Am Heart
Assoc. 2:e0056452012.PubMed/NCBI
|
|
24
|
Calmettes G, John SA, Weiss JN and Ribalet
B: Hexokinase-mitochondrial interactions regulate glucose
metabolism differentially in adult and neonatal cardiac myocytes. J
Gen Physiol. 142:425–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Samuni Y, Goldstein S, Dean OM and Berk M:
The chemistry and biological activities of N-acetylcysteine.
Biochim Biophys Acta. 1830:4117–4129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rushworth GF and Megson IL: Existing and
potential therapeutic uses for N-acetylcysteine: The need for
conversion to intracellular glutathione for antioxidant benefits.
Pharmacol Ther. 141:150–159. 2014. View Article : Google Scholar
|
|
27
|
Garner DL and Thomas CA:
Organelle-specific probe JC-1 identifies membrane potential
differences in the mitochondrial function of bovine sperm. Mol
Reprod Dev. 53:222–229. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Wert KJ, Lin JH and Tsang SH: General
pathophysiology in retinal degeneration. Dev Ophthalmol. 53:33–43.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Geerlings MJ, de Jong EK and den Hollander
AI: The complement system in age-related macular degeneration: A
review of rare genetic variants and implications for personalized
treatment. Mol Immunol. 84:65–76. 2017. View Article : Google Scholar :
|
|
31
|
Petit L and Punzo C: mTORC1 sustains
vision in retinitis pigmentosa. Oncotarget. 6:16786–16787. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Campochiaro PA, Strauss RW, Lu L, Hafiz G,
Wolfson Y, Shah SM, Sophie R, Mir TA and Scholl HP: Is there excess
oxidative stress and damage in eyes of patients with retinitis
pigmentosa? Antioxid Redox Signal. 23:643–648. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mitra RN, Conley SM and Naash MI:
Therapeutic approach of nanotechnology for oxidative stress induced
ocular neurodegenerative diseases. Adv Exp Med Biol. 854:463–469.
2016. View Article : Google Scholar
|
|
34
|
Li Y, Li J, Li S, Li Y, Wang X, Liu B, Fu
Q and Ma S: Curcumin attenuates glutamate neurotoxicity in the
hippocampus by suppression of ER stress-associated TXNIP/NLRP3
inflammasome activation in a manner dependent on AMPK. Toxicol Appl
Pharmacol. 286:53–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shimizu S, Ide T, Yanagida T and Tsujimoto
Y: Electrophysiological study of a novel large pore formed by Bax
and the voltage-dependent anion channel that is permeable to
cytochrome c. J Biol Chem. 275:12321–12325. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Banerjee J and Ghosh S: Phosphorylation of
rat brain mitochondrial voltage-dependent anion as a potential tool
to control leakage of cytochrome c. J Neurochem. 98:670–676. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun L, Shukair S, Naik TJ, Moazed F and
Ardehali H: Glucose phosphorylation and mitochondrial binding are
required for the protective effects of hexokinases I and II. Mol
Cell Biol. 28:1007–1017. 2008. View Article : Google Scholar :
|
|
38
|
Chiara F, Castellaro D, Marin O,
Petronilli V, Brusilow WS, Juhaszova M, Sollott SJ, Forte M,
Bernardi P and Rasola A: Hexokinase II detachment from mitochondria
triggers apoptosis through the permeability transition pore
independent of voltage-dependent anion channels. PLoS One.
3:e18522008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase II: Cancer's double-edged sword acting as both
facilitator and gatekeeper of malignancy when bound to
mitochondria. Oncogene. 25:4777–4786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Halestrap AP, McStay GP and Clarke SJ: The
permeability transition pore complex: Another view. Biochimie.
84:153–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Woldetsadik AD, Vogel MC, Rabeh WM and
Magzoub M: Hexokinase II-derived cell-penetrating peptide targets
mitochondria and triggers apoptosis in cancer cells. FASEB J.
31:2168–2184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moreira PI, Santos MS, Moreno A, Rego AC
and Oliveira C: Effect of amyloid beta-peptide on permeability
transition pore: A comparative study. J Neurosci Res. 69:257–267.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gottlieb RA: Mitochondria: Execution
central. FEBS Lett. 482:6–12. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gottlieb E, Armour SM, Harris MH and
Thompson CB: Mitochondrial membrane potential regulates matrix
configuration and cytochrome c release during apoptosis. Cell Death
Differ. 10:709–717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Birkinshaw RW and Czabotar PE: The BCL-2
family of proteins and mitochondrial outer membrane
permeabilisation. Semin Cell Dev Biol. 72:152–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee SY, Usui S, Zafar AB, Oveson BC, Jo
YJ, Lu L, Masoudi S and Campochiaro PA: N-Acetylcysteine promotes
long-term survival of cones in a model of retinitis pigmentosa. J
Cell Physiol. 226:1843–1849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
McCommis KS, Douglas DL, Krenz M and
Baines CP: Cardiac-specific hexokinase 2 overexpression attenuates
hypertrophy by increasing pentose phosphate pathway flux. J Am
Heart Assoc. 2:e0003552013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Corona JC, Gimenez-Cassina A, Lim F and
Diaz-Nido J: Hexokinase II gene transfer protects against
neurodegeneration in the rotenone and MPTP mouse models of
Parkinson's disease. J Neurosci Res. 88:1943–1950. 2010.PubMed/NCBI
|
|
49
|
Abeyrathna P and Su Y: The critical role
of Akt in cardiovascular function. Vascul Pharmacol. 74:38–48.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhuo B, Li Y, Li Z, Qin H, Sun Q, Zhang F,
Shen Y, Shi Y and Wang R: PI3K/Akt signaling mediated Hexokinase-2
expression inhibits cell apoptosis and promotes tumor growth in
pediatric osteosarcoma. Biochem Biophys Res Commun. 464:401–406.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hyun S, Kim MS, Song YS, Bak Y, Ham SY,
Lee DH, Hong J and Yoon DY: Peroxisome proliferator-activated
receptor-gamma agonist 4-O-methylhonokiol induces apoptosis by
triggering the intrinsic apoptosis pathway and inhibiting the
PI3K/Akt survival pathway in SiHa human cervical cancer cells. J
Microbiol Biotechnol. 25:334–342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun D, Sawada A, Nakashima M, Kobayashi T,
Ogawa O and Matsui Y: MK2206 potentiates cisplatin-induced
cytotoxicity and apoptosis through an interaction of inactivated
Akt signaling pathway. Urol Oncol. 33:111.e17–e26. 2015. View Article : Google Scholar
|
|
53
|
Duan WR, Garner DS, Williams SD,
Funckes-Shippy CL, Spath IS and Blomme EA: Comparison of
immunohistochemistry for activated caspase-3 and cleaved
cytokeratin 18 with the TUNEL method for quantification of
apoptosis in histological sections of PC-3 subcutaneous xenografts.
J Pathol. 199:221–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu YQ, Liu YF, Ma XM, Xiao YD, Wang YB,
Zhang MZ, Cheng AX, Wang TT, Li JL, Zhao PX, et al: Hydrogen-rich
saline attenuates skin ischemia/reperfusion induced apoptosis via
regulating Bax/Bcl-2 ratio and ASK-1/JNK pathway. J Plast Reconstr
Aesthet Surg. 68:e147–e156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liang S, Sun K, Wang Y, Dong S, Wang C,
Liu L and Wu Y: Role of Cyt-C/caspases-9,3, Bax/Bcl-2 and the FAS
death receptor pathway in apoptosis induced by zinc oxide
nanoparticles in human aortic endothelial cells and the protective
effect by alpha-lipoic acid. Chem Biol Interact. 258:40–51. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gottlob K, Majewski N, Kennedy S, Kandel
E, Robey RB and Hay N: Inhibition of early apoptotic events by
Akt/PKB is dependent on the first committed step of glycolysis and
mitochondrial hexokinase. Genes Dev. 15:1406–1418. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lobanova EG and Kondrat'eva EV:
Measurement of mitochondrial membrane potential in leukocyte
suspension by fluorescent spectroscopy. Bull Exp Biol Med.
157:288–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tian Q and Zang YH: Antiproliferative and
apoptotic effects of the ethanolic herbal extract of Achillea
falcata in human cervical cancer cells are mediated via cell cycle
arrest and mitochondrial membrane potential loss. J BUON.
20:1487–1496. 2015.
|
|
59
|
Lu X, Wang C and Liu B: The role of
Cu/Zn-SOD and Mn-SOD in the immune response to oxidative stress and
pathogen challenge in the clam Meretrix meretrix. Fish Shellfish
Immunol. 42:58–65. 2015. View Article : Google Scholar
|