Introduction
Diabetes mellitus is a major risk factor of
cardiovascular diseases, and diabetic patients develop more serious
neointimal hyperplasia following coronary arterial interventions
compared with non-diabetic patients (1,2).
Vascular remodeling is the most important pathological basis of
diabetic vascular complications, which is the major cause of
mortality among patients with diabetes (3). Neointimal hyperplasia plays a
crucial role in the process of diabetic vascular remodeling
(4). It is commonly accepted that
the contractile-synthetic phenotype transformation of vascular
smooth muscle cells (VSMCs) is responsible for neointimal formation
(5). A large number of
experimental and clinical studies have been undertaken to
investigate how to attenuate vascular remodeling by improving the
function of VSMCs (6–8). However, no breakthrough progress has
been made, and there are currently no promising strategies for
alleviating diabetic vascular remodeling.
MicroRNAs (miRNAs/miRs) control diverse cellular
functions by negatively modulating the expression of genes. A large
number of studies have confirmed that dysregulated miRNAs are
involved in the development of neointimal hyperplasia (9–12).
Evidence has demonstrated that the expression of miR-24 differs
significantly between patients with atherosclerosis and age-matched
controls, whereas overexpression of miR-24 can suppress the
development of atherosclerosis (13). Moreover, previously published data
by the authors of the current study also indicated that miR-24 may
regulate diabetic vascular remodeling by affecting the function of
VSMCs (14–17). However, the potential molecular
mechanisms have not yet been fully elucidated.
The NOD-like receptor family pyrin domain-containing
3 (NLRP3) inflammasome is a macromolecular complex that consists of
NLRP3, an adaptor protein apoptosis-associated speck-like protein
(ASC) and caspase-1. The NLRP3 inflammasome is activated to produce
inflammatory factors, including interleukin (IL)-1β, IL-18 and
tumor necrosis factor (TNF)-α (18). Inflammatory response is closely
implicated in vascular proliferative diseases (19,20). It has been confirmed that the
NLRP3-related inflammatory signaling pathway is involved in the
proliferation and phenotype transformation of VSMCs (21,22). Moreover, NLRP3 is one of the
miR-24 target genes predicted by bioinformatics and confirmed by
previous experiments (23).
However, whether miR-24 can attenuate diabetic vascular remodeling
through targeting the NLRP3 signaling pathway remains largely
unknown.
In the present study, a carotid artery balloon
injury diabetic rat model was established in order to investigate
whether the downregulation of NLRP3 by overexpression of miR-24 can
attenuate diabetic vascular remodeling by reducing proliferation,
phenotype transformation and inflammation in VSMCs. The aim of the
study was to provide novel insights into the benefits and potential
mechanisms of action of miR-24 against diabetic vascular
remodeling.
Materials and methods
Ethics statement
All experimental procedures and animal care were
approved by the Institutional Animal Care and Use Committee of
China Three Gorges University, and conformed to the Guide for the
Care and Use of Laboratory Animals by the National Institutes of
Health (24).
Preparation of adenoviral vectors
The custom AdMax system (Microbix Biosystems Inc.)
was used to generate adenoviral vectors according to the
manufacturer’s protocols, which encoded miR-24 and GFP.
Subsequently, the adenoviral vectors (500 μl) were
transfected into 293 cells (cat. no. CRL-1573; American Type
Culture Collection) using Lipofectamine™ 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) to amplifythe generated adenoviral vectors
until the viral titer reached 1×109 PFU/ml.
Establishment and evaluation of a rat
model of diabetes
A total of 60 adult male Sprague Dawley rats (aged
~8 weeks; weight, ~270 g) housed at 23±2°C with 12-h light/dark
cycles were on a high-fat and high-sugar diet (20% lard stearin,
10% sucrose, and 0.1% bile salt were added to the normal diet) and
had free access to water for 4 weeks. Then, the rats were
administered streptozotocin (~35 mg/kg) by intraperitoneal
injection and had free access to food and water for 1 week. The
blood sugar level in the tail vein was measured, and once that
exceeded 15.0 mmol/l the diabetic rat model was considered to be
successfully established (10).
Establishment of the carotid balloon
injury diabetic rat model and viral infection
A total of 48 well-established diabetic rat models
(weighing ~400 g) were assigned into the sham, saline, injury and
GFP viral infection (Ad-NC), and injury and miR-24 viral infection
(Ad-miR-24) groups (Fig. 1). The
procedure for establishing a diabetic rat model of carotid balloon
injury was as follows: 3% sodium pentobarbital (30 mg/kg) was used
to anesthetize the rats, followed by exposing the left external
carotid artery. Subsequently, a balloon catheter (diameter, 1.25
mm) was inserted into the left common carotid artery through the
exposed external carotid artery, and the balloon was inflated and
passed three times with rotation. In the sham group, left external
carotid artery was exposed but a balloon was not inserted. For
viral infection, equal volume (50 μl) of Ad-miR-24, Ad-NC
and saline were incubated with the common carotid arteries. After
~30 min, the blood flow in the carotid artery was restored. Two
weeks after viral infection, rats were re-anesthetized with 3%
sodium pentobarbital (30 mg/kg) and the left common carotid artery
and jugular vein were exposed. The left common carotid artery
segments were harvested for subsequent examination. At this time,
the rats were still under anesthesia. Finally, 10% potassium
chloride (75 mg/kg) was injected via the jugular vein for
euthanasia.
Histomorphological and
immunohistochemical analysis
Paraformaldehyde (4.0%) was used to fix the
harvested arteries for 48 h at room temperature and the specimens
were then embedded in paraffin and cut into 4-μm-thick
sections. Next, the tissue sections were heated at 60°C for 1 h
then dewaxed and rehydrated by immersion in dimethylbenzene and
ethanol series. Hematoxylin and eosin (H&E) staining was
subsequently performed at room temperature (hematoxylin staining, 5
min; eosin staining, 2 min). A light microscope (magnification,
×200) was used for the analysis of neointima formation.
Immunohistochemical staining of PCNA, CD45 and CD31 were performed
to analyze the proliferative activity of VSMCs, infiltration of
neutrophils and reendothelialization, respectively (16,25). Briefly, for immunohistochemical
staining, the sections was de-waxed, and the antigen retrieval was
performed by autoclaving at 121°C for 6 min. The sections were
incubated with 1% goat serum (cat. no. 31873; Thermo Fisher
Scientific, Inc.) for 30 min at room temperature. After blocking
for nonspecific staining, the sections were incubated with
anti-proliferating cell nuclear antigen (PCNA; dilution, 1:100;
cat. no. ab18197; Abcam), anti-CD45 (dilution, 1:100; cat. no.
ab10558; Abcam) or anti-CD31 (dilution 1:50; cat. no. ab24590;
Abcam) antibodies overnight at 4°C. The sections were then
incubated with a horseradish peroxidase-conjugated secondary
antibody (dilution 1:3,000; cat. no. sc-2005; Santa Cruz
Biotechnology, Inc.) for another 1 h at room temperature. Finally,
3,3′-diaminobenzidine and hematoxylin were separately added to the
sections for 1 min at room temperature. A light microscope was also
used for immunohistochemical analysis (magnification: PCNA, ×100;
CD45, ×400; CD31, ×400). Masson’s trichrome staining was also
performed to evaluate collagen deposition in the vessels, as
previously described (26).
Image-Pro Plus 5.0 software (Media Cybernetics, Inc.) was used to
calculate the percentage of PCNA-positive cells and the areas of
collagen, intima and media in the neointima.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
The gene expression of miR-24, apoptosis-associated
speck-like protein (ASC) and caspase-1 was assessed. Total RNA was
extracted from the harvested artery specimens with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Obtained RNA (~4.0 μg) was then reverse-transcribed
into cDNA using SuperScript IV Reverse Transcriptase (Thermo Fisher
Scientific, Inc.) at 37°C for 60 min. RT-qPCR was performed using
the ABI Prism 7500 system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with luminaris color hiGreen qPCR master mix
(Fermentas; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for the qPCR: 50°C for 2 min and
95°C for 10 min; 40 cycles of 95°C for 30 sec and 60°C for 30 sec.
Data were analyzed using the 2−ΔΔCq method (27). The following primers were used:
miR-24 forward, 5′-TGCGCTGGCTCAGTTCAGCAGG-3′ and reverse,
5′-CCAGTGCAGGGTCCGAGGTATT-3′; U6 forward,
5′-CGCTTCGGCAGCACATATAC-3′ and reverse, 5′-AAATATGGAACGCTTCACGA-3′;
caspase-1 forward, 5′-ACTCGTACACGTCTTGCCCTC-3′ and reverse,
5′-CTGGGCAGGCAGCAAATTC-3′; ASC forward, 5′-TGGAGTCGTATGGCTTGGAG-3′
and reverse, 5′-TGTCCTTCAGTCAGCACACT-3′; and GAPDH forward,
5′-TGGCCTTCCGTGTTCCTAC-3′ and reverse,
5′-GAGTTGCTGTTGAAGTCGCA-3′.
Western blotting
The protein expression levels of NLRP3, ASC,
caspase-1 and GAPDH in the harvested artery specimens were also
determined. Briefly, the proteins were extracted from the specimens
using the protein extraction kit (cat. no. P0028; Beyotime
Institute of Biotechnology) and the concentration was measured by
bicinchoninic acid protein assay kit. The obtained proteins (40
μg) were separated by electrophoresis with NuPAGE™ Novex
4–12% Bis-Tris Protein Gel (Invitrogen; Thermo Fisher Scientific,
Inc.) and transferred onto a polyvinylidene fluoride membrane. The
membrane was blocked with 5% non-fat dry milk in PBS with 0.05%
Tween-20 for 2 h at room temperature. Subsequently, the blocked
membranes were incubated with primary antibodies against NLRP3
(cat. no. 13158), ASC (cat. no. 67824) and caspase-1 (cat. no.
3866) overnight at 4°C (dilution, 1:1,000; Cell Signaling
Technology, Inc.). Finally, the membranes were incubated with
horseradish peroxidase-conjugated rabbit anti-rat IgG secondary
antibodies (dilution, 1:2,000; cat. no. GB-10041; Pierce; Thermo
Fisher Scientific, Inc.) for another 2 h at room temperature. An
enhanced chemiluminescence detection kit (Thermo Fisher Scientific,
Inc.) was used for visualization and GAPDH served as a loading
control. An Odyssey® Infrared Imaging system (model
9120; LI-COR Biosciences) was used to capture images of the
membranes and Quantity One 1-D software (version 4.6.9; Bio-Rad
Laboratories, Inc.) was used to quantify the protein bands.
ELISA
The ELISA method was used to determine the levels of
TNF-α (cat. no. SRTA00; R&D Systems, Inc.), IL-1β (cat. no.
SRLB00; R&D Systems, Inc.) and IL-18 (cat. no. CSB-E04610r;
Cusabio Technology LLC) in the harvested artery specimens. Briefly,
the harvested artery specimens were homogenized at 4°C, centrifuged
at 500 × g and 4°C for 20 min, and then the supernatant was taken
for ELISA detection following the manufacturer’s instructions.
Statistical analysis
All statistical analyses were performed with SPSS
software (version 21.0; IBM Corp.). All values are presented as the
mean ± standard deviation. Statistical comparisons between mean
values of groups were assessed using one-way analysis of variance
followed by Tukey’s post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Relative expression of miR-24 and NLRP3
in diabetic rats with balloon-injured arteries
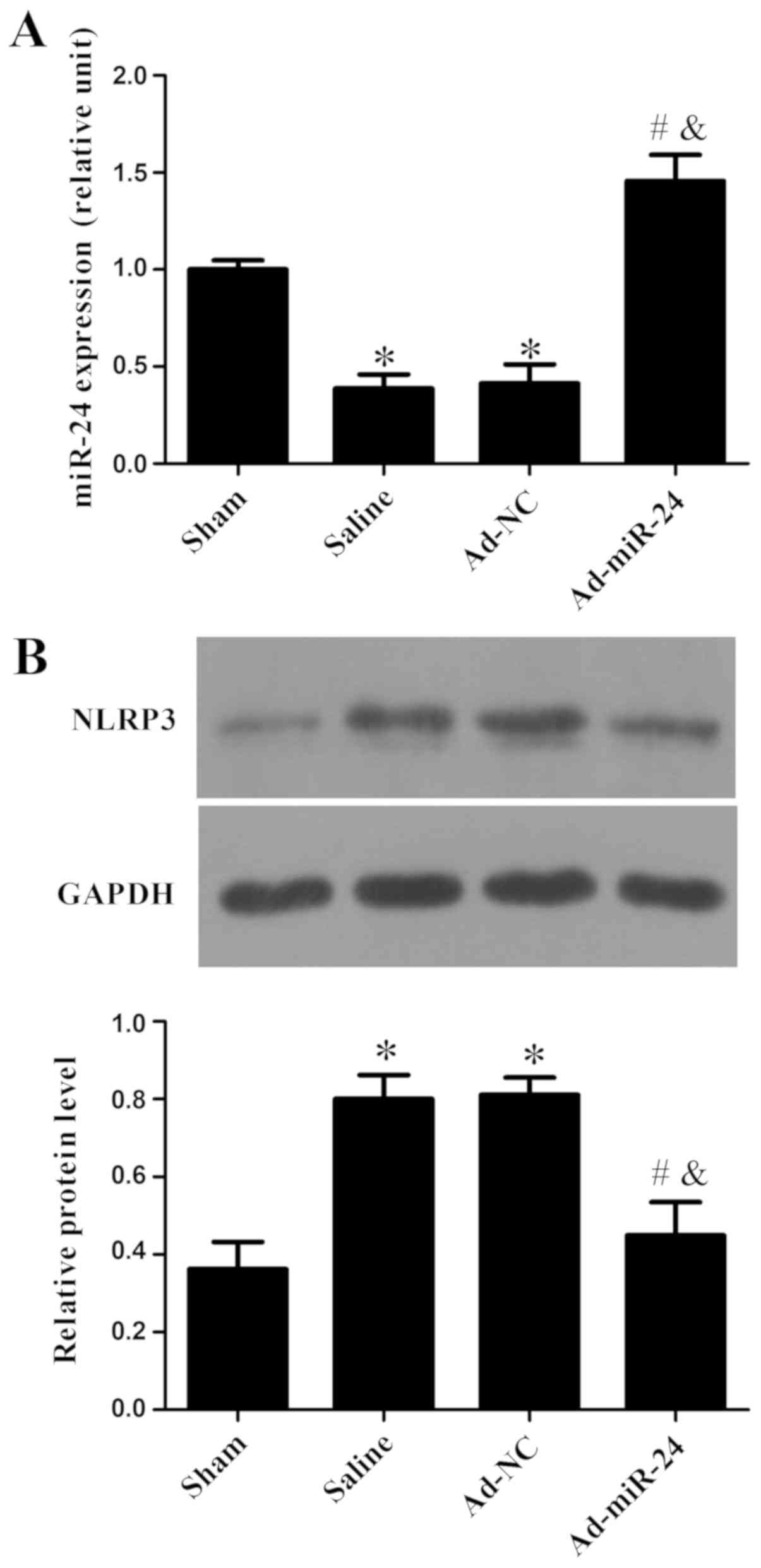
As shown in Fig.
2A, miR-24 was markedly reduced in the carotid arteries of
diabetic rats after injury compared with the sham group
(P<0.05), and adenovirus-mediated miR-24 viral infection
significantly increased miR-24 expression compared with the Ad-NC
group (P<0.05). Western blotting was used to detect the
expression of NLRP3. As shown in Fig.
2B, compared with the sham group, protein expression level of
NLRP3 increased markedly in saline and Ad-NC groups. However,
miR-24 viral infection significantly reduced NLRP3 protein levels
compared with the saline or Ad-NC groups (P<0.05).
Overexpression of miR-24 inhibits
neointimal hyperplasia
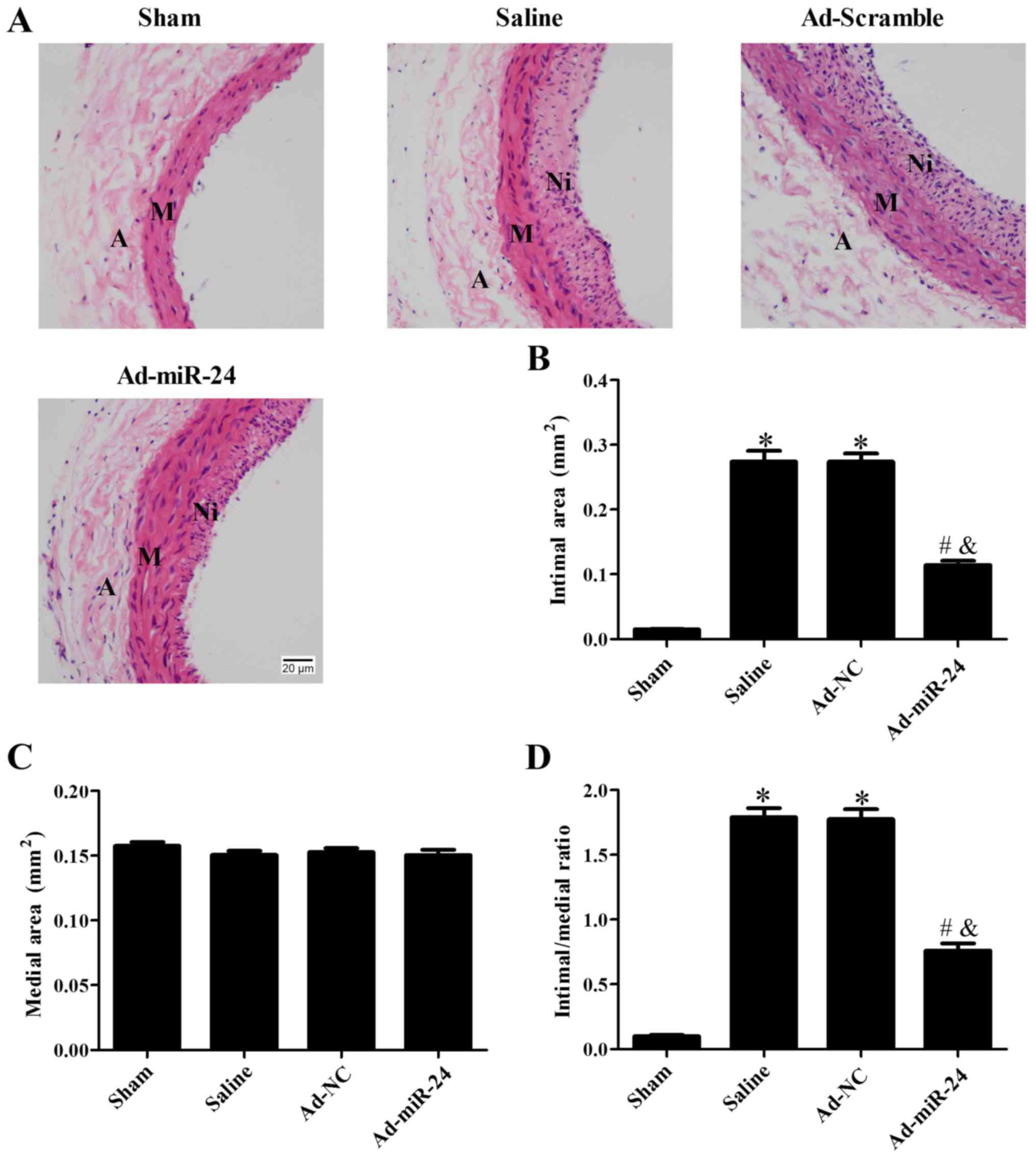
The intimal hyperplasia was assessed by H&E
staining (Fig. 3). As shown in
Fig. 3A and B,
adenovirus-mediated miR-24 viral infection significantly reduced
the neointimal area compared with saline and Ad-NC groups
(P<0.05) and the changes in the ratio of intima/media were
consistent with this observation (P<0.05; Fig. 3D). There were no marked changes in
the total area of the medial layer between the four groups included
in the current study (P>0.05; Fig.
3C).
Overexpression of miR-24 suppresses VSMC
proliferation and phenotype transformation, and decreases collagen
deposition
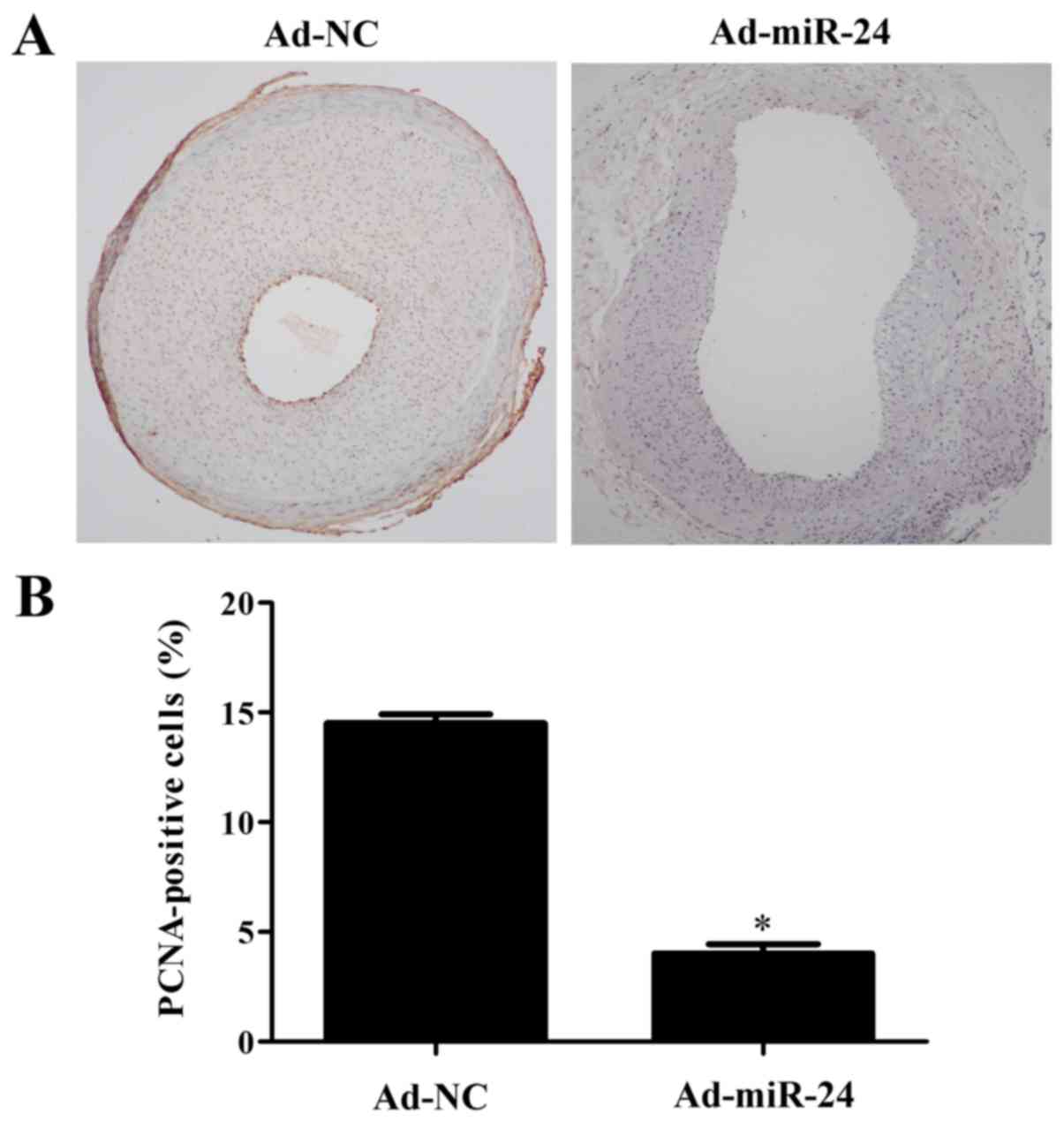
As shown in Fig. 4A
and B, PCNA expression was markedly increased in the injured
arteries, but miR-24 overexpression was able to reduce the level of
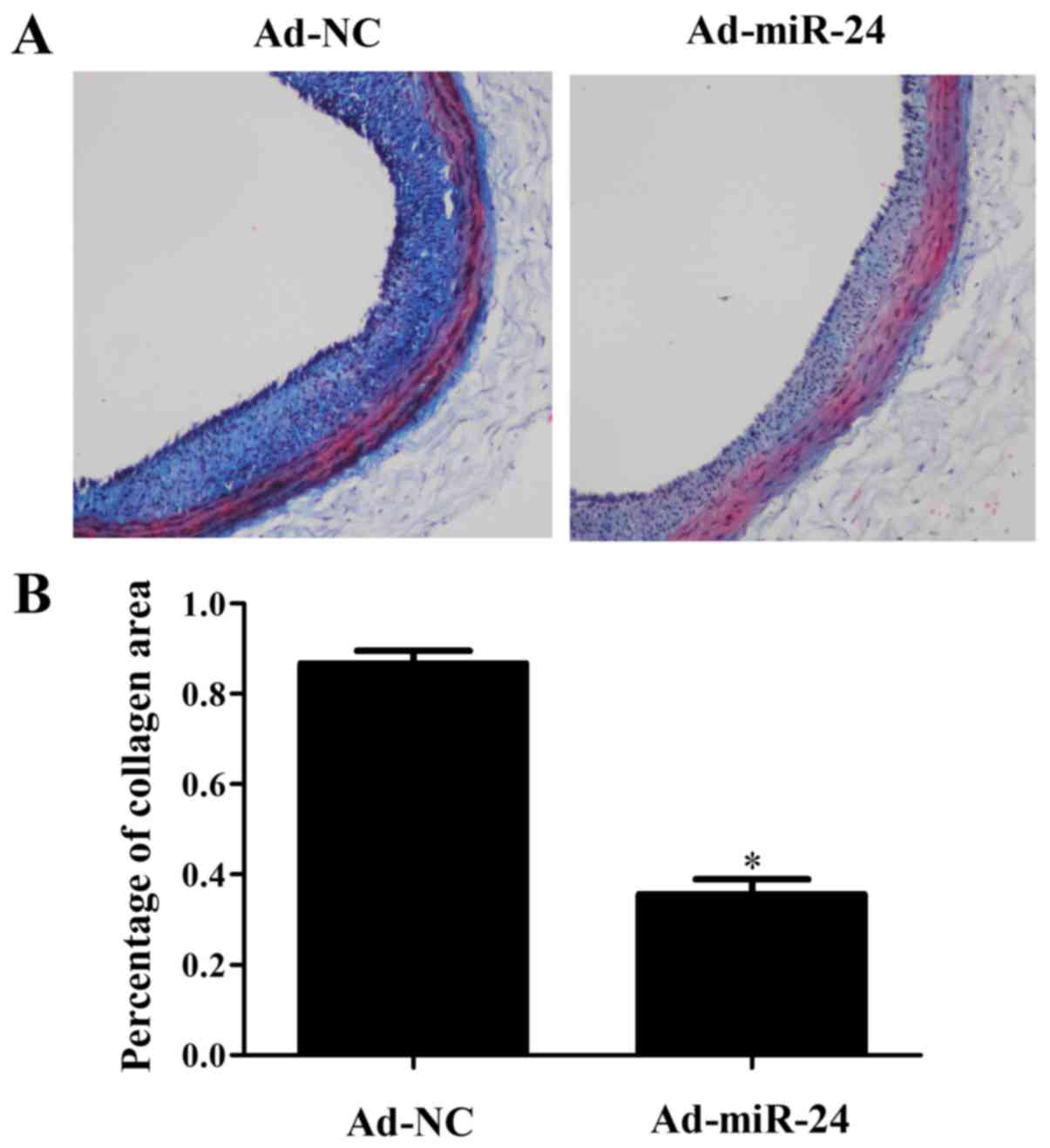
PCNA by 68% (P<0.05). In addition, the collagen deposition in
the injured artery was assessed by Masson’s trichrome staining. As
shown in Fig. 5, compared with
the Ad-NC group, the level of collagen in injured arteries was
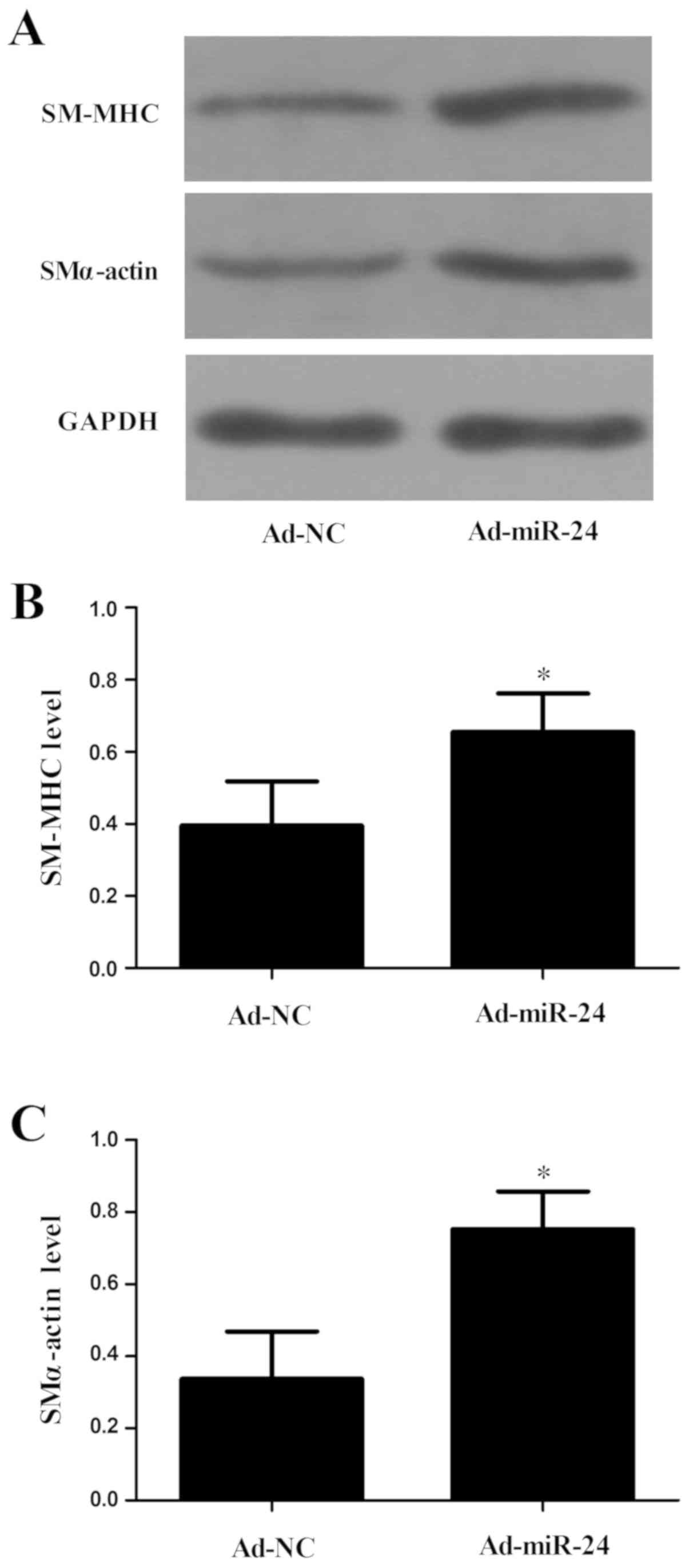
lower in the Ad-miR-24 group (P<0.05). Compared with the Ad-NC
group, expression levels of contractile markers, including smooth
muscle (SM) α-actin and SM-myosin heavy chain (MHC), were
upregulated significantly in the Ad-miR-24 group, as assessed by
western blotting (both P<0.05; Fig. 6).
Overexpression of miR-24 promotes
reendothelialization following balloon injury
Immunostaining was performed to investigate whether
overexpression of miR-24 could promote reendothelialization
following balloon injury in diabetic rats. As shown in Fig. 7, the number of CD31-positive cells
along the luminal surface increased following adenovirus-mediated
miR-24 viral infection.
Overexpression of miR-24 ameliorates
inflammatory response
The ELISA method was used to determine the
expression of TNF-α, IL-1β and IL-18 in the harvested artery
specimens. It was observed that the levels of IL-1β, IL-18 and
TNF-α were significantly upregulated in the injured arteries;
however, miR-24 overexpression was able to markedly reduce their
expression (all P<0.05; Fig.
8B–D). Consistent with the above findings, immunostaining
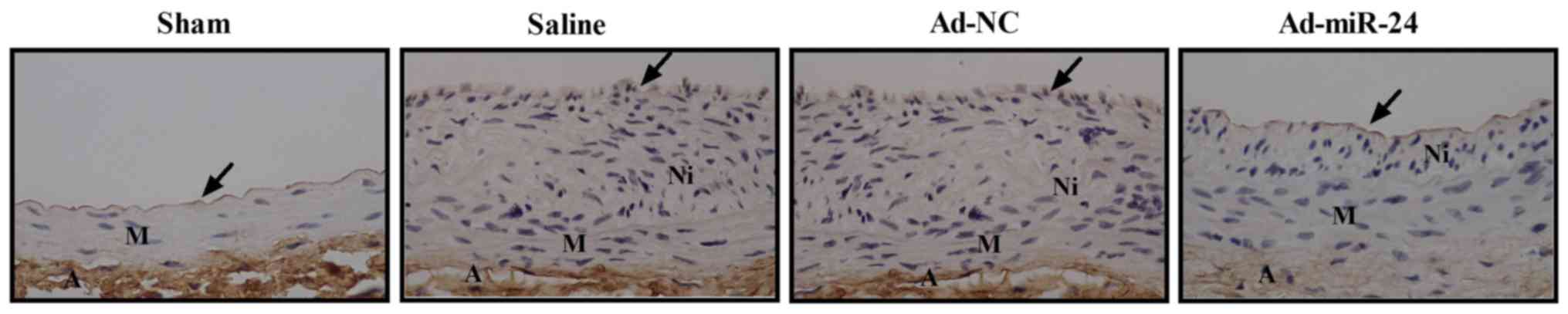
results also demonstrated that the expression levels of neutrophil
cell marker CD45 in the neointima markedly decreased following
miR-24 viral infection (Fig.
8A).
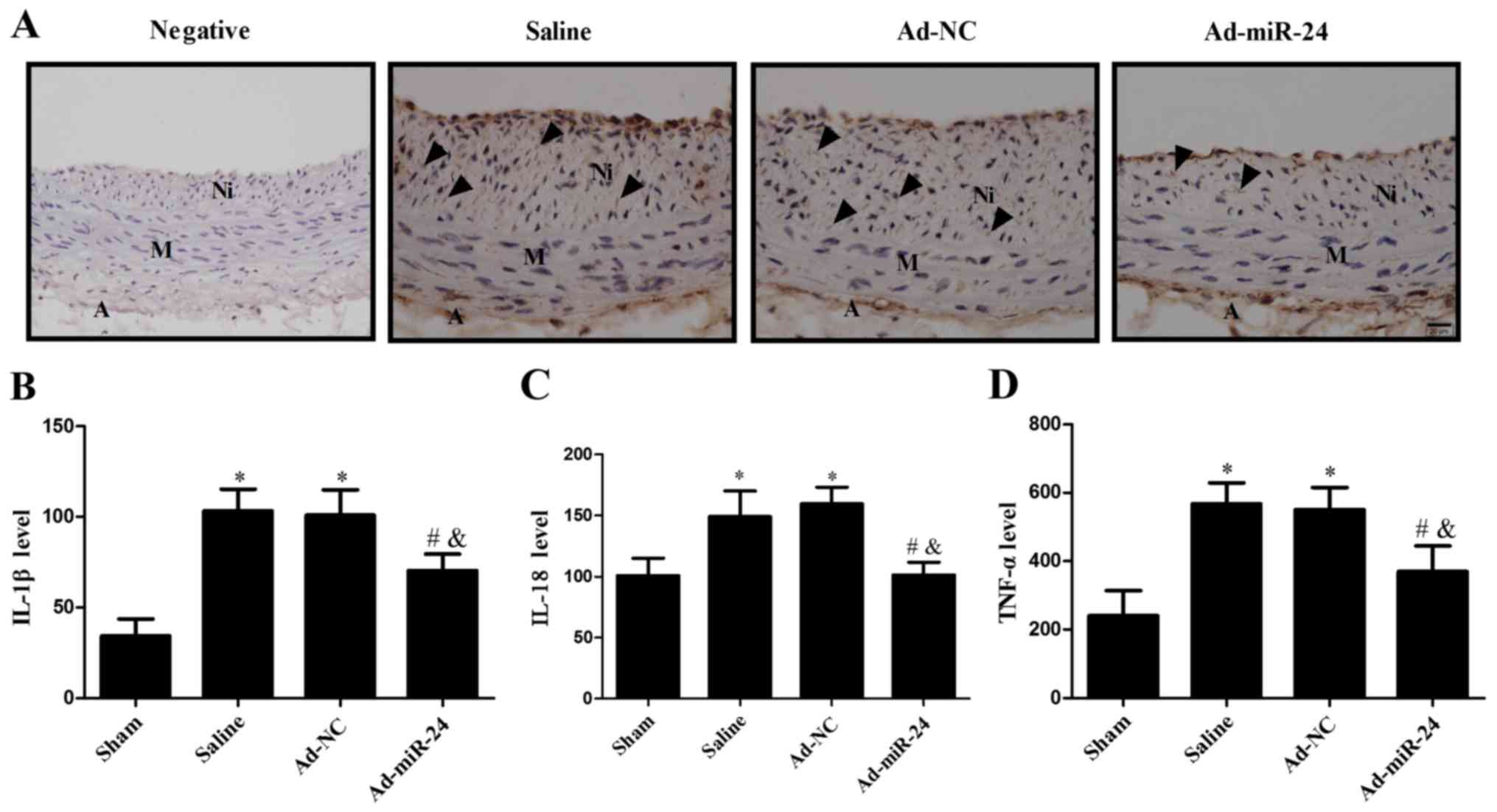 | Figure 8Overexpression of miR-24 reduces the
inflammatory response. (A) Immunohistochemistry was used to detect
neutrophil cell marker CD45-positive cells in the neointima
(arrows). Magnification, ×400. Expression levels of (B) IL-1β, (C)
IL-18 and (D) TNF-α were detected by ELISA. Values are presented as
the mean ± standard deviation (n=6 per group).
*P<0.05 vs. sham group; #P<0.05 vs.
saline; and &P<0.05 vs. Ad-NC group. A,
adventitia; M, media; Ni, neointima. IL, interleukin; TNF, tumor
necrosis factor; miR, microRNA; Ad, adenovirus; NC, GFP
sequence. |
Overexpression of miR-24 represses the
NLRP3 signaling pathway
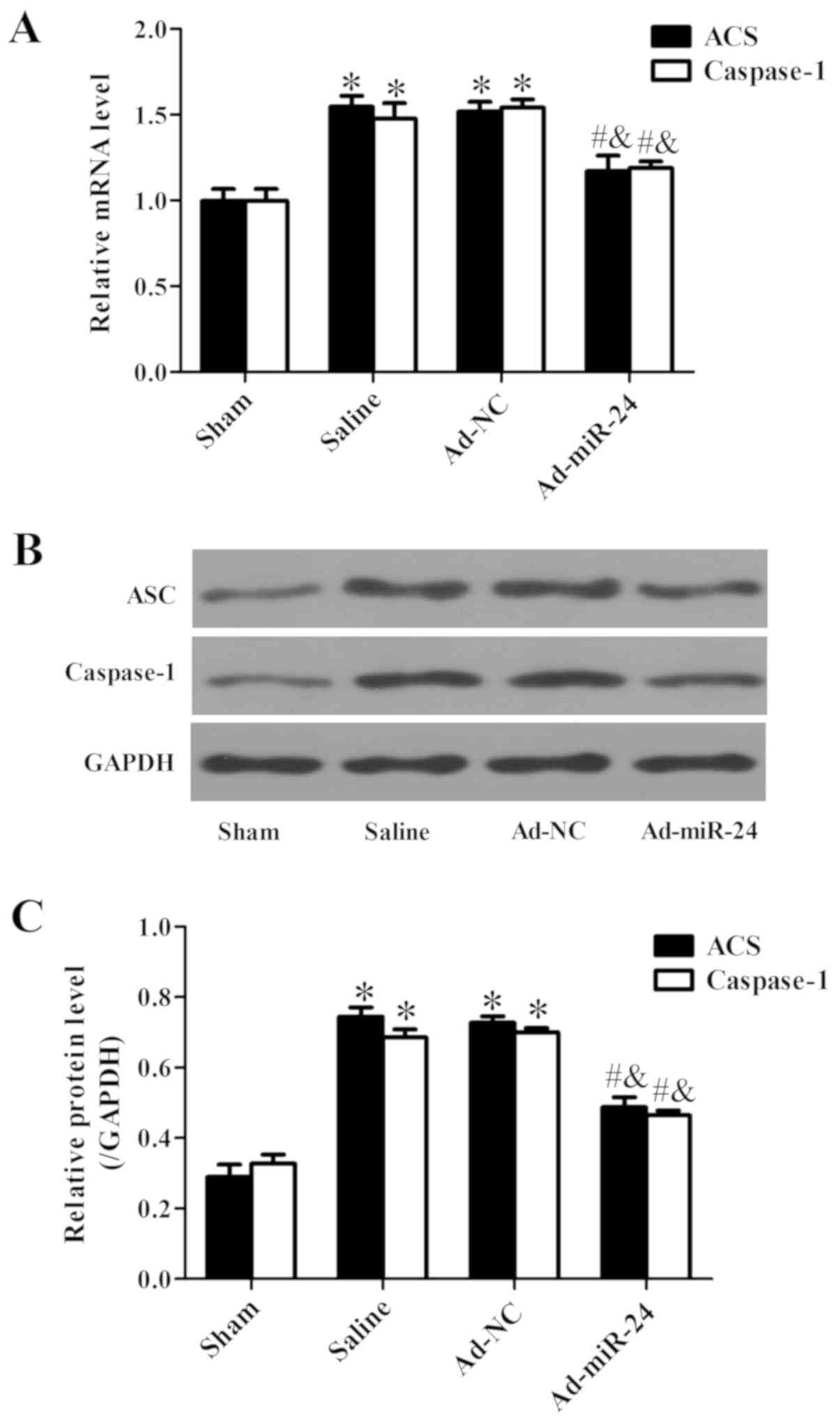
ASC and caspase-1 are the main downstream molecules
of the NLRP3 signaling pathway (23). The mRNA and protein expression of
ASC and caspase-1 was measured by RT-qPCR and western blotting,
respectively. The mRNA (Fig. 9A)
and protein (Fig. 9B and C)
expression levels of ASC and caspase-1 were both significantly
upregulated in the injured arteries; however, their expression was
attenuated following miR-24 viral infection (all P<0.05;
Fig. 9).
Discussion
Diabetic patients are at a markedly increased risk
of developing neointimal hyperplasia and may require repeated
coronary interventions (28).
Although drug-eluting stents are currently widely used, the
incidence of restenosis and late thrombosis is higher among
diabetic patients (29). Previous
research by the authors of the current study demonstrated that
miR-24 can attenuate vascular remodeling in diabetic rats (14–17). However, miR-24 regulates the
expression of hundreds of target genes, and multiple signaling
pathways are involved in the development of diabetic vascular
remodeling (30–32). Further research is warranted to
further elucidate the complex regulatory interconnections between
miR-24 and diabetic-related vascular proliferative diseases. As the
dominant cellular constituent of arteries, VSMCs is a critical
determinant of vascular disease (33). In the present study, it was
observed that overexpression of miR-24 markedly attenuated intimal
hyperplasia caused by vascular injury in diabetic rats. Moreover,
VSMC proliferation and phenotype transformation were associated
with an increase in the expression of NLRP3, ASC and caspase-1, and
the secretion of pro-inflammatory factors, including IL-1β, IL-18
and TNF-α. The afore-mentioned trends notably changed following
miR-24 viral infection. The data of the present study suggested
that miR-24 may attenuate vascular remodeling in diabetic rats by
suppressing the NLRP3/caspase-1/IL-1β-related inflammatory
signaling pathway.
miRNAs are non-coding RNAs, ~22 nucleotides in
length, that control diverse cellular functions by degrading target
mRNAs or inhibiting translation post-transcriptionally (12). It has been confirmed that miRNAs
play a crucial role in the mechanisms underlying vascular
remodeling (12,34). The proliferation, migration and
inflammation of VSMCs may be markedly ameliorated by targeting
phosphatidylinositol 3-kinase regulatory subunit α,
platelet-derived growth factor subunit B, Wnt4 and high mobility
group protein B1 via miR-24 overexpression (14–17). According to TargetScan
bioinformatics analysis, miR-24 has multiple intervention targets,
one of which is NLRP3 (23). The
data of the present study demonstrated that the expression of NLRP3
was regulated by miR-24 in vivo.
The NLRP3 inflammasome is a multi-protein signaling
complex, which is composed of NLRP3, ASC and caspase-1 (35). When the NLRP3 inflammasome is
activated by an external stimulus, NLRP3 and ASC immediately form a
complex and then activate caspase-1 to promote the maturation and
release of IL-1β and IL-18 (18).
It was previously reported that excessive activation of the NLRP3
inflammasome is associated with a variety of diseases (36). VSMCs play a key role in the
development of diabetic vascular remodeling. Briefly, in the
healthy vasculature, VSMCs display a low proliferative rate with a
quiescent and contractile phenotype. However, under an external
stimulus, VSMCs migrate from the medial layer into the intimal
layer, accompanied by a switch to a proliferative synthetic
phenotype (37). This phenotypic
transformation in associated with reduced expression of
contractility markers SMα-actin and SM-MHC; however, the production
of inflammatory cytokines is increased (19,20). Phenotypic transformation of VSMCs
plays a crucial role in vascular proliferative diseases by
facilitating VSMC migration to the intima from the medial layer and
secretion of extracellular matrix to promote neointima formation
(38). In addition, accumulating
evidence indicates that the NLRP3 inflammasome is also involved in
VSMC phenotypic transformation, proliferation and vascular
remodeling by mediating inflammatory response (21,22). Consistent with the aforementioned
findings, the present study also indicated that the NLRP3-related
inflammatory signaling pathway was activated in injured diabetic
rat arteries, and inhibiting NLRP3 by miR-24 upregulation decreased
the production of IL-1β, IL-18 and TNF-α, the proliferation of
VSMCs, and infiltration by neutrophils, as demonstrated by CD45
staining results. Inhibition of the NLRP3 pathway also reduced
collagen generation and increased the expression of SMα-actin and
SM-MHC.
The dysfunction of endothelial cells (ECs) caused by
inflammation or other external stimuli is another key factor
promoting diabetic vascular remodeling. Jansen et al
(39) reported that endothelial
microparticle-promoted inhibition of vascular remodeling is
abrogated under hyperglycemic conditions. The current research in
this field mainly focuses on improving the function of VSMCs
(14–17,21); however, ECs have not been
extensively investigated. The delay in endothelialization severely
compromises the therapeutic effect of ECs (40). Previously published data by the
authors of the current study demonstrated that miR-24 was enriched
in VSMCs rather than ECs (41).
The present study demonstrated that miR-24 gene transfer increased
CD31 expression and accelerated reendothelialization following
arterial injury in diabetic rats.
In conclusion, miR-24 not only attenuated the
phenotypic transformation and proliferation of VSMCs, but also
inhibited intimal hyperplasia and collagen deposition, and
accelerated reendothelialization in diabetic rats in a carotid
artery balloon injury model. These effects may be mediated by the
downregulation of the NLRP3/caspase-1/IL-1β-related inflammatory
signaling pathway. However, direct downregulation of NLRP3
signaling molecules may provide further supporting evidence. The
effect of specific inhibitors of ASC, caspase-1 and IL-1β on high
glucose-induced VSMC inflammation will be studied in-depth in
future research. The data obtained in the present study further
elucidated the role of miR-24 in diabetic vascular remodeling and
indicated that miR-24 may be a potential intervention target in
vascular proliferative diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81800258 and
81470387), and the Natural Science Foundation of Yichang city,
China (grant no. A18-301-10).
Availability of data and materials
The data used and/or analyzed in this study are
available from the corresponding author with reasonable
request.
Authors’ contributions
ZXF and WYC wrote the manuscript, interpreted the
data and performed the experiments. CJY and JZ acquired and
analyzed the data. JY and CXH performed the literature search,
designed the study and revised the manuscript. All authors have
read and approval the final manuscript.
Ethics approval and consent to
participate
All experimental procedures and animal care were
approved by the Institutional Animal Care and Use Committee of
China Three Gorges University, and conformed to the Guide for the
Care and Use of Laboratory Animals by the National Institutes of
Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Naito R and Miyauchi K: Coronary artery
disease and type 2 diabetes mellitus. Int Heart J. 58:475–480.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yahagi K, Kolodgie FD, Lutter C, Mori H,
Romero ME, Finn AV and Virmani R: Pathology of human coronary and
carotid artery atherosclerosis and vascular calcification in
diabetes mellitus. Arterioscler Thromb Vasc Biol. 37:191–204. 2017.
View Article : Google Scholar
|
|
3
|
Beckman JA and Creager MA: Vascular
complications of diabetes. Circ Res. 118:1771–1785. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang WX, Tai GJ, Li XX and Xu M:
Inhibition of neointima hyperplasia by the combined therapy of
linagliptin and metformin via AMPK/Nox4 signaling in diabetic rats.
Free Radic Biol Med. 143:153–163. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen J, Zhang J, Yang J, Xu L, Hu Q, Xu C,
Yang S and Jiang H: Histone demethylase KDM3a, a novel regulator of
vascular smooth muscle cells, controls vascular neointimal
hyperplasia in diabetic rats. Atherosclerosis. 257:152–163. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzuki J, Tezuka D, Morishita R and Isobe
M: An initial case of suppressed restenosis with nuclear
factor-kappa B decoy transfection after percutaneous coronary
intervention. J Gene Med. 11:89–91. 2009. View Article : Google Scholar
|
|
7
|
Wang Y, Zhang X, Gao L, Li J, Chen W, Chi
J, Zhang X, Fu Y, Zhao M, Liu N, et al: Cortistatin exerts
antiproliferation and antimigration effects in vascular smooth
muscle cells stimulated by Ang II through suppressing ERK1/2, p38
MAPK, JNK and ERK5 signaling pathways. Ann Transl Med. 7:5612019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hao H, Gabbiani G and Bochaton-Piallat ML:
Arterial smooth muscle cell heterogeneity: Implications for
atherosclerosis and restenosis development. Arterioscler Thromb
Vasc Biol. 23:1510–1520. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng S, Gao L, Zhang D, Tian X, Kong L,
Shi H, Wu L, Huang Z, Du B and Liang C: MiR-93 regulates vascular
smooth muscle cell proliferation, and neointimal formation through
targeting Mfn2. Int J Biol Sci. 15:2615–2626. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li H, Zhao J, Liu B, Luo J, Li Z, Qin X
and Wei Y: MicroRNA-320 targeting neuropilin 1 inhibits
proliferation and migration of vascular smooth muscle cells and
neointimal formation. Int J Med Sci. 16:106–114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lightell DJ Jr, Moss SC and Woods TC:
Upregulation of miR-221 and -222 in response to increased
extracellular signal-regulated kinases 1/2 activity exacerbates
neointimal hyperplasia in diabetes mellitus. Atherosclerosis.
269:71–78. 2018. View Article : Google Scholar :
|
|
12
|
Fan ZX and Yang J: Microribonucleic acids
and vascular restenosis. Saudi Med J. 35:796–801. 2014.PubMed/NCBI
|
|
13
|
Ren K, Zhu X, Zheng Z, Mo ZC, Peng XS,
Zeng YZ, Ou HX, Zhang QH, Qi HZ, Zhao GJ and Yi GH: MicroRNA-24
aggravates atherosclerosis by inhibiting selective lipid uptake
from HDL cholesterol via the post-transcriptional repression of
scavenger receptor class B type I. Atherosclerosis. 270:57–67.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai W, Zhang J and Yang J, Fan Z, Liu X,
Gao W, Zeng P, Xiong M, Ma C and Yang J: MicroRNA-24 attenuates
vascular remodeling in diabetic rats through PI3K/Akt signaling
pathway. Nutr Metab Cardiovasc Dis. 29:621–632. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang J, Zeng P, Yang J, Liu X, Ding J,
Wang H and Chen L: MicroRNA-24 regulates vascular remodeling via
inhibiting PDGF-BB pathway in diabetic rat model. Gene. 659:67–76.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang J, Fan Z, Yang J, Ding J, Yang C and
Chen L: MicroRNA-24 attenuates neointimal hyperplasia in the
diabetic rat carotid artery injury model by inhibiting wnt4
signaling pathway. Int J Mol Sci. 17:pii: E765. 2016. View Article : Google Scholar
|
|
17
|
Yang J, Chen L, Ding J, Fan Z, Li S, Wu H,
Zhang J, Yang C, Wang H, Zeng P and Yang J: MicroRNA-24 inhibits
high glucose-induced vascular smooth muscle cell proliferation and
migration by targeting HMGB1. Gene. 586:268–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim EJ, Park SY, Baek SE, Jang MA, Lee WS,
Bae SS, Kim K and Kim CD: HMGB1 increases IL-1β production in
vascular smooth muscle cells via NLRP3 Inflammasome. Front Physiol.
9:3132018. View Article : Google Scholar
|
|
19
|
Pasqua T, Pagliaro P, Rocca C, Angelone T
and Penna C: Role of NLRP-3 inflammasome in hypertension: A
potential therapeutic target. Curr Pharm Biotechnol. 19:708–714.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang R, Wu W, Li W, Huang S, Li Z, Liu R,
Shan Z, Zhang C, Li W and Wang S: Activation of NLRP3 inflammasome
promotes foam cell formation in vascular smooth muscle cells and
atherogenesis Via HMGB. J Am Heart Assoc. 7:e0085962018. View Article : Google Scholar
|
|
21
|
Ren XS, Tong Y, Ling L, Chen D, Sun HJ,
Zhou H, Qi XH, Chen Q, Li YH, Kang YM and Zhu GQ: NLRP3 gene
deletion attenuates angiotensin II-Induced phenotypic
transformation of vascular smooth muscle cells and vascular
remodeling. Cell Physiol Biochem. 44:2269–2280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun HJ, Ren XS, Xiong XQ, Chen YZ, Zhao
MX, Wang JJ, Zhou YB, Han Y, Chen Q, Li YH, et al: NLRP3
inflammasome activation contributes to VSMC phenotypic
transformation and proliferation in hypertension. Cell Death Dis.
8:e30742017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin Y and Yang Y: miR-24 inhibits
inflammatory responses in LPS-induced acute lung injury of neonatal
rats through targeting NLRP3. Pathol Res Pract. 215:683–688. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bayne K: Revised guide for the care and
use of laboratory animals available american physiological society.
Physiologist. 39:199208–211. 1996.
|
|
25
|
Yang J, Jiang H, Chen SS, Chen J, Li WQ,
Xu SK and Wang JC: Lentivirus-mediated RNAi targeting CREB binding
protein attenuates neointimal formation and promotes
re-endothelialization in balloon injured rat carotid artery. Cell
Physiol Biochem. 26:441–448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khuman MW, Harikumar SK, Sadam A, Kesavan
M, Susanth VS, Parida S, Singh KP and Sarkar SN: Candesartan
ameliorates arsenic-induced hypertensive vascular remodeling by
regularizing angiotensin II and TGF-beta signaling in rats.
Toxicology. 374:29–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Bednarska J, Bednarska-Chabowska D and
Adamiec-Mroczek J: Coronary artery disease: New insights into
revascularization treatment of diabetic patients. Adv Clin Exp Med.
26:1163–1167. 2017.PubMed/NCBI
|
|
29
|
Wang JL, Qin Z, Wang ZJ, Shi DM, Liu YY,
Zhao YX, Yang LX, Cheng WJ and Zhou YJ: New predictors of in-stent
restenosis in patients with diabetes mellitus undergoing
percutaneous coronary intervention with drug-eluting stent. J
Geriatr Cardiol. 15:137–145. 2018.PubMed/NCBI
|
|
30
|
Zheng Y, Li Y, Liu G, Qi X and Cao X:
MicroRNA-24 inhibits the proliferation and migration of endothelial
cells in patients with atherosclerosis by targeting importin-α3 and
regulating inflammatory responses. Exp Ther Med. 15:338–344.
2018.
|
|
31
|
Fiedler J, Stöhr A, Gupta SK, Hartmann D,
Holzmann A, Just A, Hansen A, Hilfiker-Kleiner D, Eschenhagen T and
Thum T: Functional microRNA library screening identifies the
hypoxamir miR-24 as a potent regulator of smooth muscle cell
proliferation and vascularization. Antioxid Redox Signal.
21:1167–1176. 2014. View Article : Google Scholar :
|
|
32
|
Chan MC, Hilyard AC, Wu C, Davis BN, Hill
NS, Lal A, Lieberman J, Lagna G and Hata A: Molecular basis for
antagonism between PDGF and the TGFbeta family of signalling
pathways by control of miR-24 expression. EMBO J. 29:559–573. 2010.
View Article : Google Scholar
|
|
33
|
Durham AL, Speer MY, Scatena M, Giachelli
CM and Shanahan CM: Role of smooth muscle cells in vascular
calcification: Implications in atherosclerosis and arterial
stiffness. Cardiovasc Res. 114:590–600. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang D and Atanasov AG: The microRNAs
regulating vascular smooth muscle cell proliferation: A Minireview.
Int J Mol Sci. 20:Pii: E324. 2019.
|
|
35
|
Jo EK, Kim JK, Shin DM and Sasakawa C:
Molecular mechanisms regulating NLRP3 inflammasome activation. Cell
Mol Immunol. 13:148–159. 2016. View Article : Google Scholar :
|
|
36
|
Whiteford JR, De Rossi G and Woodfin A:
Mutually supportive mechanisms of inflammation and vascular
remodeling. Int Rev Cell Mol Biol. 326:201–278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu QB, Wan MY, Wang PY, Zhang CX, Xu DY,
Liao X and Sun HJ: Chicoric acid prevents PDGF-BB-induced VSMC
dedifferentiation, proliferation and migration by suppressing
ROS/NFκB/mTOR/P70S6K signaling cascade. Redox Bio. 114:656–668.
2018. View Article : Google Scholar
|
|
38
|
Li FJ, Zhang CL, Luo XJ, Peng J and Yang
TL: Involvement of the MiR-181b-5p/HMGB1 pathway in ang II-induced
phenotypic transformation of smooth muscle cells in hypertension.
Aging Dis. 10:231–248. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jansen F, Zietzer A, Stumpf T, Flender A,
Schmitz T, Nickenig G and Werner N: Endothelial
microparticle-promoted inhibition of vascular remodeling is
abrogated under hyperglycaemic conditions. J Mol Cell Cardiol.
112:91–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang HK, Kim PH, Kim DW, Cho HM, Jeong
MJ, Kim DH, Joung YK, Lim KS, Kim HB, Lim HC, et al: Coronary
stents with inducible VEGF/HGF-secreting UCB-MSCs reduced
restenosis and increased re-endothelialization in a swine model.
Exp Mol Med. 50:1142018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Cai W, Fan Z, Yang C, Wang W,
Xiong M, Ma C and Yang J: MicroRNA-24 inhibits the oxidative stress
induced by vascular injury by activating the Nrf2/Ho-1 signaling
pathway. Atherosclerosis. 290:9–18. 2019. View Article : Google Scholar : PubMed/NCBI
|