Introduction
Inflammaging refers to chronic sterile inflammation
that is characterized by cell senescence and aging (1). Periodontitis, a form of
inflammaging, is the most common and widespread oral disease that
causes tooth mobility and loss (2). The process of periodontitis destroys
tooth-supporting connective tissues and alveolar bone (3). Although dysbiotic microbiological
burden may play a role in disease development (4), dysregulated immune responses to the
microbial insults ultimately destroy the periodontium (5). Equally, inflammaging induced by host
cell pathogens plays a crucial role in the deterioration of the
immune system (6,7). Senescent cells are characterized by
haphazard secretion of inflammatory cytokines, such as interleukin
(IL)-1β, IL-6 and tumor necrosis factor-alpha (TNF-α), which is
referred to as the senescence-associated secretory phenotype (SASP)
(2,8). Therapeutic agents capable of
eliminating inflammatory damage, as well as slowing down or
reversing the inflammaging process to maintain health in the
periodontium, would provide novel avenues for the treatment of
periodontitis.
Toll-like receptor 4 (TLR4) is the main receptor for
lipo-polysaccharides (LPS) present in gram-negative bacteria
(9). The TLR4 protein functions
as a key regulator of periodontitis by modulating cell signal
transduction, apoptosis, and the quality and magnitude of immune
responses (10,11). Polymorphisms in the TLR4 gene are
associated with increased risk of periodontitis (12). Studies have evaluated the effect
of TLR4 in senescence (13-15). Both in vivo and in
vitro studies have shown that increased expression of TLR4
enhances aging (14,15). Additionally, activation of the
TLR4-mediated ERK pathway accelerates skin aging by elevating
expression of SASP factors such as IL-6 or IL-8 (16). Senescent fibro-blasts have been
hypothesized to express high levels of SASP factors, and exhibit
higher expression of biologically active TLR4 compared with other
cells in diseased periodontal tissues (17,18). However, there is less focus on the
fundamental biological mechanisms underlying the roles of TLR4 and
senescent gingival fibroblasts in periodontitis.
It has been demonstrated that B cell-specific
Moloney murine leukemia virus integration site 1 (Bmi-1) can reduce
cell senescence and SASP (19).
Bmi-1 plays important roles in regulating cell cycle and senescence
via the inhibition of p16/retinoblastoma protein (Rb) and p19/p53
pathways (19,20). The nucleotide-binding and
oligomerization domain-like receptor 3 (NLRP3) regulates
age-related inflammation and acts as a positive modulator of aging
(21,22). Activation of the TLR4 pathway
promotes the priming of the NLRP3 inflammasome, which is composed
of the receptor protein NLRP3, Caspase-1 and apoptosis-associated
speck-like protein containing a CARD domain (ASC) (23,24). The activated NLRP3 assembles at
the inflammasome, leading to the activation of Caspase-1 and
secretion of IL-1β, which are key factors in periodontal disease
(25). Thus, it was hypothesized
that the TLR4 signaling pathway affects Bmi-1 and NLRP3 in the
pathogenesis of periodontal inflammaging.
The present study aimed to investigate the
regulatory effect of TLR4 signaling pathway on inflammaging
processes in the periodontium. A ligature-induced periodontitis
model in mice was to study whether TLR4 signaling pathway
aggravated the inflammaging process in the periodontium via
regulation of Bmi-1 expression and the NLRP3 pathway.
Additionallly, Bmi-1 protein expression was evaluated in patients
with periodontitis compared with periodontally healthy individuals.
These results indicated the anti-inflammaging effects of the
TLR4/Bmi-1/NLRP3 pathway in periodontitisl thus, targeting the
TLR4/Bmi-1/NLRP3 pathway may serve as a novel anti-inflammaging
strategy for therapeutic management.
Materials and methods
Animals and treatment schedule
A completely randomized study design was used. A
total of 24 wild-type (WT) C57BL/10 and TLR4 knockout (KO) male and
female mice (age, 6-8 weeks old; weight, 20.0-22.0 g) were
purchased from the Model Animal Research Center of Nanjing
University. They were reared in a room with a controlled
environment (22-24°C, 50±5% relative humidity) in 12:12-h
dark/light cycles with access to food and water ad libitum.
All mice were divided randomly into three groups (8 mice/group):
The control group that was not subjected to treatment; and the WT
and TLR4 KO groups in which a model of periodontitis was induced.
To establish a mouse model of periodontitis, mice were anesthetized
via an intraperitoneal injection of 4% chloral hydrate (350 mg/kg).
Then, the gingival sulcus of left maxillary second molars was
ligated with a 9-0 sterile silk for 21 days. All experiments were
carried out according to the guidelines of the Experimental Animal
Research Institute of Nanjing Medical University. Approval for the
study was obtained from the Nanjing Animal Experimental Ethics
Committee (permit no. IACUC-1901052).
Subjects and human gingiva samples
A total of 20 volunteers (age, 20-40 years) were
recruited at the Affiliated Hospital of Stomatology at Nanjing
Medical University from January 2019 to December 2019. The patient
group (n=12) was comprised of 6 males and 6 females, while the
control group (n=8) consisted of 4 males and 4 females. The program
was approved by the Committee on the Ethics of Nanjing Medical
University (permit no. PJ2018-050-001) and complied with the
Declaration of Helsinki. All volunteers were required to sign an
informed consent form prior to enrolment in the study. Before the
study, a detailed medical history was obtained using a
questionnaire, while clinical examinations were performed to assess
the health of the volunteers. Exclusion criteria included:
Pregnancy; the use of any antibiotic medicine in the last 3 months;
and any systemic disease that could influence the periodontal
status. The clinical periodontal measurements taken included the
gingival index, probing pocket depth (PD), gingival recession,
clinical attachment level (CAL) and the percentage of sites
bleeding on probing (BOP). These measurements were performed by a
single calibrated investigator. PD and CAL were measured to the
nearest mm at six surfaces of all teeth using a straight
periodontal probe. Subjects were divided into two groups based on
their clinical periodontal measurements: Healthy, the absence of
periodontal disease, PD ≤3 mm, and BOP <10%; and periodontitis,
PD >3 mm, BOP ≥10% and presence of bone resorption in the
radiographic evaluation. Gingival tissue of healthy donors was
obtained surgically from the third molars. Gingival tissue of
patients with periodontitis was isolated from the inner wall of the
periodontal pocket around the affected tooth with PD ≥5 mm and BOP
(+) during periodontal surgery.
Cell cultures and treatment
Mice gingiva tissues were extracted from 5-week-old
mice after CO2 inhalation euthanasia (100%
CO2 at a fill rate of 20% volume displaced/min) and
cervical dislocation. The tissues were washed with sterile PBS
(cat. no. 10010049; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 100 U/l penicillin-streptomycin (cat. no.
15070063; Gibco; Thermo Fisher Scientific, Inc.) three times. These
tissues were sectioned into pieces of 1-2 mm3, placed
into a 35-mm culture dish, and incubated with DMEM (cat. no.
11965092; Thermo Fisher Scientific, Inc.) supplemented with 15% FBS
(cat. no. 16140071; Gibco; Thermo Fisher Scientific, Inc.) and 100
U/l penicillin-streptomycin. All cells were cultured under a
humidified atmosphere of 5% CO2 at 37°C and were also
used after 3-6 passages to reduce phenotypic drift. The culture
medium was changed every 2 days. Human gingival fibroblasts were
cultured using human gingival samples from donors during
periodontal surgery or extracted from the third molar according to
the same protocol as cells isolated from mice gingiva as described
previously. During in vitro experiments using mouse gingival
fibroblasts, the control group was treated with PBS as a control,
and the WT and TLR4 KO groups were treated with 100 ng/ml LPS
(MedChemExpress LLC) for 24 h at 37°C; the TLR4 + PTC209 group was
treated with 100 ng/ml LPS and 500 nM PTC209 (MedChemExpress LLC)
simultaneously for 24 h at 37°C. The cells were collected for the
different assays after treatment. For in vitro experiments
using human gingival fibroblasts, the healthy group was treated
with PBS as a control; the periodontitis group was treated with 100
ng/ml LPS from Porphyromonas gingivalis (P.
gingivalis; Invitrogen; Thermo Fisher Scientific, Inc.) for 24
h at 37°C. The cells were collected for the different assays after
treatment.
Micro-computed tomography (CT)
Mouse maxillae were fixed in 4% paraformaldehyde
solution overnight at 4°C and scanned on a SkyScan1072 scanner
(Bruker Corporation) operated at 45 kV and 253 µA with 0.98°
rotation between frames used at a detection pixel size of 9
µm. The samples were tightly wrapped in plastic to prevent
movement and dehydration during scanning. Mimics17 software
supplied with the instrument was used to reconstruct
three-dimensional images. Multiplanar reconstruction was performed
using reconstruction software NRecon (version 1.6.6.0; Bruker
Corporation) and CT-analyzer software CTAn (version 1.13.2.1;
Bruker Corporation). All images were reoriented so that the
cement-enamel junction (CEJ) and the root apex of the maxillary
second molar could simultaneously appear in the same micro-CT
slice. The distance of CEJ to the alveolar bone crest (CEJ-ABC) was
measured to evaluate bone loss around six sites on the ligated
side. The alveolar ridge around the maxillary second molar was
selected as the trabecular volume of interest and the bone volume
to total volume ratio (BV/TV) was assessed in this region.
Preparation of tissue samples and
histochemical staining
The excised left maxillae separated from mice after
euthanasia were fixed in 4% paraformaldehyde solution overnight at
4°C, and then decalcified in EDTA glycerol solution for a month on
a rotating table at 4°C. After fixation, 5-µm sections in
the mesiodistal plane were cut from each demineralized specimen
embedded in paraffin wax. For hematoxylin-eosin staining, the
sections were incubated in hematoxylin for 2 min and eosin for 1
min at room temperature after paraformaldehyde fixation. Five
random slides per sample were analyzed using a light microscope
(magnification, ×200) to visualize sections.
Human gingival samples from donors were fixed in 4%
paraformaldehyde solution overnight at 4°C and embedded in
paraffin. They were sectioned into slices with a thickness of ~5
µm. Masson's trichrome staining was performed using a Masson
Stain kit at room temperature and the duration of Masson staining
steps were according to the manufacturer's instructions (cat. no.
D026; Nanjing Jiancheng Bioengineering Institute). Five random
fields per sample were analyzed using a light microscope
(magnification, ×200) to visualize sections.
Tartrate-resistant acid phosphatase
(TRAP) staining
TRAP staining was conducted using a TRAP Stain kit
(cat. no. D023-1-1; Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer's instructions. The nuclei were
counterstained with methyl green for 5 min at room temperature.
Then, TRAP-positive cells were counted in five random fields per
sample under a light microscope (magnification, ×400). The images
were analyzed and quantified using Image-Pro Plus 6 software (Media
Cybernetics, Inc.).
Immunohistochemical staining
Maxilla were dissected, and the maxillary second
molar and surrounding tissue were retained to perform
immunohistochemistry staining with primary antibodies against TNF-α
(cat. no. ab6671; Abcam), IL-6 (cat. no. a0286; ABclonal Biotech
Co., Ltd.), IL-1β (cat. no. ab9722; Abcam), p16 (cat. no. ab211542;
Abcam), p21 (cat. no. ab188224; Abcam), p53 (cat. no. ab131442;
Abcam), p19 (cat. no. sc-1665; Santa Cruz Biotechnology, Inc.),
NLRP3 (cat. no. 15101s; Cell Signaling Technology, Inc.), ASC (cat.
no. ab175449; Abcam) and Caspase-1 (cat. no. 22915-1-AP;
ProteinTech Group, Inc.). Briefly, dewaxed and rehydrated
paraffin-embedded sections were boiled for antigen retrieval in
citrate-EDTA antigen retrieval solution in a steamer for 30 min.
Endogenous peroxidase activity was then blocked with methanol:
Hydrogen peroxide (1:10) for 10 min at room temperature. After
washing with TBS (pH 7.6), the slides were blocked with 10% normal
goat serum (Beyotime Institute of Biotechnology) for 1 h at room
temperature and then incubated with the primary antibodies
overnight at 4°C. The dilutions of all antibodies were determined
were as follows: TNF-α (1:100), IL-6 (1:200), IL-1β (1:100), p16
(1:100), p21 (1:100), p53 (1:100), p19 (1:100), NLRP3 (1:100), ASC
(1:100) and Caspase-1 (1:100). Incubation with a secondary antibody
(biotinylated goat anti-rabbit or anti-mouse IgG; 1:200; cat. nos.
SA00004-2 and SA00004-1; ProteinTech Group, Inc.) for 1 h at room
temperature was then performed. After rinsing with TBS for 15 min,
sections were incubated with Vectastain Elite ABC reagent for 45
min at 37°C. Then, sections were developed with
3,3-diaminobenzidine (2.5 mg/ml) and subsequently counterstained
with hematoxylin for 1 min at room temperature. The slides were
dehydrated, cleared and mounted. Positive cells were counted under
a light microscope (magnification, ×400) in five random slides per
sample. Images were analyzed and quantified by Image-Pro Plus 6
software (26).
Western blot analysis
Western blot analysis was performed using primary
antibodies against TNF-α (cat. no. ab6671; Abcam), IL-6 (cat. no.
a0286; ABclonal Biotech Co., Ltd.), IL-1β (cat. no. ab9722; Abcam),
p16 (cat. no. ab211542; Abcam), p21 (cat. no. ab188224; Abcam), p53
(cat. no. ab131442; Abcam), p19 (cat. no. sc-1665; Santa Cruz
Biotechnology, Inc.), NLRP3 (cat. no. 15101s; Cell Signaling
Technology, Inc.), ASC (cat. no. ab175449; Abcam), Caspase-1 (cat.
no. 22915-1-AP; ProteinTech Group, Inc.) and GAPDH (cat. no.
AP0063; Bioworld Technology, Inc.). Cells were lysed in RIPA
protein extraction regent (Beyotime Institute of Biotechnology)
after washing with PBS three times to extract total proteins. The
homogenate was centrifuged at 13,000 × g for 15 min at 4°C and the
supernatant was collected. BCA protein assays (Beyotime Institute
of Biotechnology) were performed to determine protein
concentrations. For most blots, proteins (~10 µg/lane) were
resolved by SDS-PAGE using 8-15% gradient gels and then transferred
to PVDF membranes. Membranes were blocked with 5% non-fat milk for
60 min at room temperature and then incubated with the
corresponding primary antibodies overnight at 4°C. The dilutions of
antibodies were determined as follows: TNF-α (1:1,000), IL-6
(1:1,000), IL-1β (1:1,000), p16 (1:1,000), p21 (1:1,000), p53
(1:1,000), p19 (1:500), NLRP3 (1:1,000), ASC (1:1,000) and
Caspase-1 (1:1,000). After washing, the membrane was incubated with
HRP-conjugated goat anti-mouse or anti-rabbit secondary antibodies
(1:2,000; cat. nos. SA00001-1 and SA00001-2; ProteinTech Group,
Inc.) for 60 min at room temperature. The membrane was washed three
times in TBS with 0.1% Tween-20 (Beyotime Institute of
Biotechnology) and then visualized with HRP Substrate Luminol
Reagent (EMD Millipore). Quantification and analysis of blots was
performed using Image J (version 1.44; National Institutes of
Health) (27).
β-galactosidase activity analysis
β-galactosidase activity was evaluated using a
FluoReporter lacZ Flow Cytometry kit (cat. no. F-1930; Thermo
Fisher Scientific, Inc.) according to standard procedures. Briefly,
cells were resuspended at a concentration of 107
cells/ml in staining medium and 100 µl was aspirated into a
flow cytometer tube. Fluorodeoxyglucose (FDG) loading was initiated
by the addition of 100 µl FDG working solution (2 mM), which
was prewarmed at 37°C in the aforementioned flow cytometer tube.
Subsequently, 1.8 ml ice-cold staining medium containing 1.5
µM propidium iodide was added into the flow cytometer tube
for 1 min to stop the FDG loading. A DxFLEX flow cytometer (Beckman
Coulter, Inc.) was set up and calibrated to detect fluorescein,
propidium iodide and forward scatter according to the
manufacturer's instructions. All cells were analyzed and processed
with CytExpert software 1.1 (Beckman Coulter, Inc.).
Immunofluorescent staining
Paraffinized tissue sections were stained with
primary antibodies against Bmi-1 (1:100; cat. no. 5856s; Cell
Signaling Technology, Inc.) and vimentin (1:100; cat. no. ab8978;
Abcam). After fixation and antigen retrieval, 5-µm-thick
paraffinized sections were incubated with 10% with 10% normal goat
serum (Beyotime) for 1 h at room temperature to block nonspecific
binding. The slides were incubated with the primary antibodies
overnight, and then with the corresponding secondary antibodies
[Alexa Fluor® 594-conjugated goat anti-mouse (1:200;
cat. no. ab150120; Abcam) and Alexa Fluor 488-conjugated goat
anti-rabbit (1:200; cat. no. ab150077; Abcam)] for 1 h at room
temperature. The dilutions of all antibodies were determined
according to the manufacturer's instructions. Additionally, cell
nuclei were counterstained with DAPI for 5 min at room temperature
(cat. no. D9542; Sigma-Aldrich; Merck KGaA) and sealed with
mounting medium to prevent fluorescent quenching. The positive
cells were counted under a laser confocal microscope. The
fluorescent-positive area was quantified using Image-Pro Plus 6
software (26).
Statistical analysis
Data were presented as the mean ± standard deviation
(SD) from three separate experiments and Student's t-tests were
used to compare data between the two groups. All data were analyzed
using GraphPad Prism 6 (version 6.07; GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
TLR4 KO relieves the destruction of
periodontal soft and hard tissue
To evaluate the effect of TLR4 KO on the
pathogenesis of periodontitis, the maxillary second molar was
ligated in 8-week-old WT or TLR4 knockout mice for 21 days.
Micro-CT was used to detect the extent of alveolar bone destruction
and absorption. Compared with the WT group, the TLR4 KO group
exhibited reduced bone loss and lesser reduction of the alveolar
bone volume (Fig. 1A, B, E and
F). HE staining revealed that the depth of the periodontal
pocket and attachment loss in the TLR4 KO group were decreased
compared with in the WT group (Fig.
1C). Furthermore, the percentage of TRAP-positive osteoclasts
in the alveolar bone of TLR4 KO mice was significantly decreased
(Fig. 1D and G). Therefore, the
results indicated that TLR4 played a role in the progressive
destruction of periodontal tissue.
 | Figure 1TLR4 KO attenuates the destruction of
periodontal tissue in a mouse model of periodontitis. Periodontitis
was induced by a 9-0 sterile silk ligature placed inside the
gingival sulcus in 8-week-old mice for 21 days. (A)
Three-dimensional volume reconstruction images of the left maxilla
of mice analyzed by micro-CT scans showed that TLR4 KO could
attenuate bone loss in the alveolar ridge. (B) Multiplanar
reconstructions on the mesiodistal plane of the left maxilla of
mice. The length from buccal to lingual was 500 µm. (C) HE
staining showing pathological alterations in mouse periodontium
samples derived from different groups (magnification, ×100; scale
bar, 50 µm). (D) Osteoclasts were visualized by staining for
TRAP activity (magnification, ×400). (E) Bone volume in the
alveolar ridge of the left maxillary second molars was analyzed
using the micro-CT scans. (F) Distance between the CEJ and ABC on
the distal side of the second maxillary molar was determined using
HE-stained frontal sections. (G) Percentage of TRAP-positive cells
in the bone surface was calculated. Data are presented as the mean
± SD (n=3/group). ##P<0.01, ###P<0.001
vs. control; *P<0.05, **P<0.01 vs.
TLR4, Toll-like receptor 4; WT, wild-type; KO, knockout; CT,
computed tomography; HE, hematoxylin-eosin; TARP,
tartrate-resistant acid phosphatase; CEJ, cementum-enamel junction;
ABC, alveolar bone crest; BV/TV, bone volume-to-total volume
ratio. |
Deletion of TLR4 slows down the onset of
cell senescence and SASP in gingiva
To investigate the downstream roles of TLR4 gene KO
in suppressing cell senescence and SASP, the expression levels of
cell senescence-associated proteins were examined using
immunohistochemical staining. Compared with the WT mice, the
expression levels of proteins involved in senescence pathways such
as p16, p19, p21 and p53 were significantly upregulated in the
periodontal soft tissues of TLR4 KO mice (Fig. 2). When stimulated with LPS in
vitro, mouse gingival fibroblasts exhibited decreased levels of
p16, p19, p21 or p53 proteins in the TLR4 KO group compared with
the WT group (Fig. 3A and B).
Flow cytometry was used to determine the activity of
β-galactosidase, a molecular marker of senescent cells, in gingival
fzibroblasts. Significantly reduced β-galactosidase activity was
observed in the TLR4 KO group compared with the WT group (Fig. 3C and D).
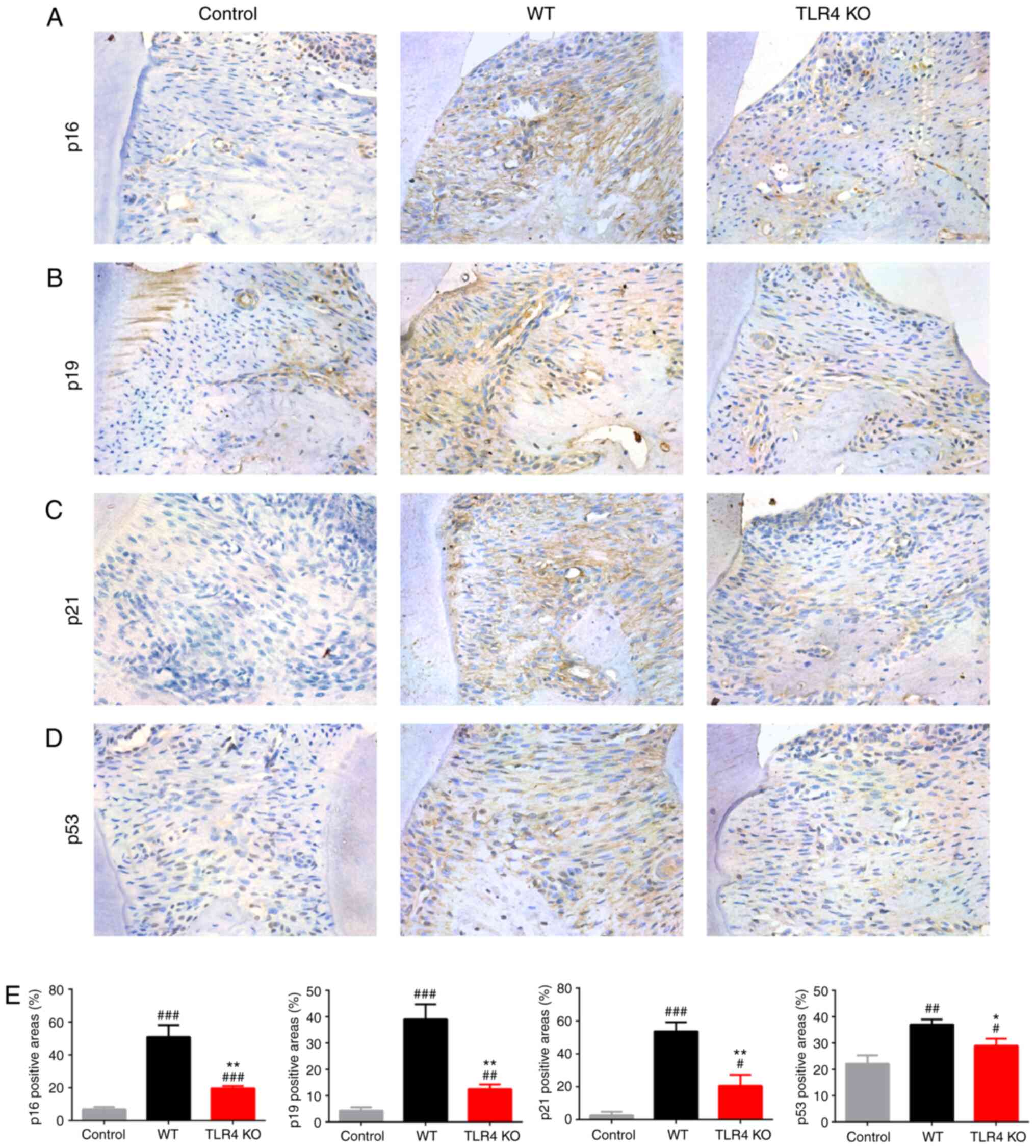 | Figure 2TLR4 KO attenuates senescence in
gingiva in a mouse model of periodontitis. Immunohistochemical
staining of maxillary paraffin sections was used to evaluate the
expression of (A) p16, (B) p19, (C) p21 and (D) p53 (magnification,
×400). (E) Histograms showing the percentage of tissue area
positive for p16, p19, p21 and p53 in the gingiva. Data are
presented as the mean ± SD (n=3/group). #P<0.05,
##P<0.01, ###P<0.001 vs. control;
*P<0.05, **P<0.01 vs. WT. TLR4,
Toll-like receptor 4; WT, wild-type; KO, knockout. |
 | Figure 3TLR4 KO attenuates senescence in
mouse gingival fibroblasts following LPS stimulation. (A) Western
blot analysis of p16, p19, p21 and p53 expression in gingival
fibroblasts treated with LPS. (B) Relative protein levels of p16,
p19, p21 and p53 normalized to GAPDH. (C) β-gal activity was
determined via flow cytometry. (D) Relative β-gal levels
represented as bar histograms. Data are presented as the mean ± SD
(n=3/group). #P<0.05, ##P<0.01 vs.
control; *P<0.05, **P<0.01 vs. WT.
TLR4, Toll-like receptor 4; WT, wild-type; KO, knockout; LPS,
lipopolysaccharide; β-gal, β-galactosidase; FDG,
fluorodeoxyglucose. |
To assess whether TLR4 KO reduces SASP factors in
periodontal tissue, the expression levels of SASP-associated
proteins such as IL-1β, IL-6 and TNF-α were examined. Compared with
the WT group, the levels of IL-1β, IL-6 and TNF-α were
significantly reduced in positive periodontium regions in the TLR4
KO mice (Fig. 4A-F). Similarly,
the expression levels of IL-1β, IL-6 and TNF-α in gingival
fibroblasts were significantly reduced in the TLR4 KO group
compared with in the WT group after stimulation with LPS in
vitro (Fig. 4G and H). The
data indicated that TLR4 exacerbated cellular senescence and
intensified SASP factors during the loss of alveolar bone.
 | Figure 4TLR4 KO reduces SASP factor levels in
gingival tissues and fibroblasts in a mouse model of periodontitis.
Immunohistochemical staining of maxillary paraffin sections was
used to evaluate the expression of (A) IL-1β, (B) IL-6 and (C)
TNF-α (magnification, ×400). Statistical analysis of the percentage
of tissue area positive for (D) IL-1β, (E) IL-6 and (F) TNF-α in
the gingiva. (G) Western blot analysis of IL-1β, IL-6 and TNF-α
expression in gingival fibroblasts following lipopolysaccharide
treatment. (H) Relative protein levels of IL-1β, IL-6 and TNF-α
normalized to GAPDH. Data are presented as the mean ± SD
(n=3/group). #P<0.05, ##P<0.01,
###P<0.001 vs. control; *P<0.05,
**P<0.01, ***P<0.001 vs. WT. TLR4,
Toll-like receptor 4; WT, wild-type; KO, knockout; IL, interleukin;
TNF, tumor necrosis factor. |
TLR4 gene KO upregulates Bmi-1 expression
and inhibits the NLRP3 pathway in periodontal soft tissue and
gingival fibroblasts
Whereas previous data indicates that Bmi-1 regulates
SASP in multiple cells via suppression of p16/Rb and p19/p53/p21
signaling pathways (20), Bmi-1
mechanisms in the aging process have not been established. Data
from immunohistochemical staining assays revealed that
Bmi-1-positive regions in periodontal soft tissue were
significantly higher in the TLR4 KO group compared to the WT group
(Fig. 5A and E). Furthermore, the
expression of Bmi-1 in gingival fibroblasts was increased in the
TLR4 KO group compared with the WT group (Fig. 5F and G).
 | Figure 5TLR4 KO leads to increased expression
of Bmi-1 and suppression of the NLRP3 signaling pathway in gingiva
and gingival fibroblasts in a mouse model of periodontitis.
Immunohistochemical staining of maxillary paraffinized tissue
sections showing the expression of (A) Bmi-1, (B) NLRP3, (C)
Caspase-1 and (D) ASC (magnification, ×400). (E) Statistical
analysis of the percentage of tissue area positive for NLRP3, ASC,
Caspase-1 and Bmi-1 in the gingiva. (F) Western blot analysis of
NLRP3, ASC, Caspase-1 and Bmi-1 expression in gingival fibroblasts
treated with lipopolysaccharide. (G) Relative protein levels of
NLRP3, ASC, Caspase-1 and Bmi-1 normalized to GAPDH. Data are
presented as the mean ± SD (n=3/group). #P<0.05,
##P<0.01, ###P<0.001 vs. control;
*P<0.05, **P<0.01,
***P<0.001 vs. WT. TLR4, Toll-like receptor 4; WT,
wild-type; KO, knockout; Bmi-1, B cell-specific Moloney murine
leukemia virus integration site 1; NLRP3, nucleotide-binding and
oligomerization domain-like receptor 3; ASC, apoptosis-associated
speck-like protein containing a CARD domain. |
The NLRP3 inflammasome, a commonly studied
inflammasome complex, is considered to be a classical upstream
regulator of age-related inflammation, which may activate Caspase-1
and IL-1β (28).
Immunohistochemical staining was performed to evaluate the
expression of NLRP3 and Caspase-1 in gingiva. The results revealed
that expression of NLRP3 and Caspase-1 was reduced in the TLR4 KO
group compared with the WT group (Fig. 5B, C and E). In addition, the
expression of ASC, a crucial adaptor protein for successful
inflammasome activation by the NLRPs and AIM2 (23), was found to be significantly
reduced in the TLR4 KO group compared with the WT group (Fig. 5D and E). In gingival fibroblasts,
the expression levels of NLRP3, Caspase-1 and ASC were also
significantly reduced in the TLR4 KO group (Fig. 5F and G). Therefore, these findings
indicated that the Bmi-1 and NLRP3 pathways were involved in
TLR4-induced periodontal inflammaging.
Bmi-1 inhibitor PTC209 reverses the
suppression of NLRP3 pathway and SASP in TLR4 KO gingival
fibroblasts
To further explore the role of Bmi-1, the Bmi-1
inhibitor PTC209 was used to investigate the activation ofNLRP3
pathway and the expression of SASP. After stimulating mouse
gingival fibroblasts with LPS and 500 nM PTC209 simultaneously for
24 h, downregulation of Bmi-1 was observed in the TLR4 KO + PTC209
group compared with the TLR4 KO group (Fig. 6A and B). Conversely, the
expression of NLRP3, Caspase-1 and ASC were increased in the TLR4
KO + PTC209 group compared with the TLR4 KO fibroblasts (Fig. 6A and B). These findings suggested
a feedback regulation between Bmi-1 and the NLRP3 pathway.
 | Figure 6Bmi-1 inhibitor PTC209 attenuates the
protective effects of TLR4 KO against senescence and activation of
NLRP3 pathway in mouse gingival fibro-blasts after LPS stimulation.
(A) Western blot analysis of the effect of PTC209 on the expression
of Bmi-1, NLRP3, ASC and Caspase-1. (B) Relative protein levels of
Bmi-1, NLRP3, ASC and Caspase-1 normalized to GAPDH. (C) Activity
of β-gal determined via flow cytometry. (D) Western blot analysis
of p16, p19, p21 and p53 protein levels following treatment with
PTC209. (E) Western blot analysis of IL-1β, IL-6 and TNF-α protein
levels following treatment with PTC209. (F) Histograms showing
relative β-gal levels. (G) Relative protein levels of p16, p19, p21
and p53 normalized to GAPDH. (H) Relative protein levels of IL-1β,
IL-6 and TNF-α normalized to GAPDH. Data are presented as the mean
± SD (n=3/group). #P<0.05, ##P<0.01,
###P<0.001 vs. control; *P<0.05,
**P<0.01, ***P<0.001 vs. WT;
@P<0.05, @@P<0.01,
@@@P<0.001 vs. TLR4 KO. TLR4, Toll-like receptor 4;
WT, wild-type; KO, knockout; Bmi-1, B cell-specific Moloney murine
leukemia virus integration site 1; NLRP3, nucleotide-binding and
oligomerization domain-like receptor 3; ASC, apoptosis-associated
speck-like protein containing a CARD domain; β-gal,
β-galactosidase; IL, interleukin; TNF, tumor necrosis factor; FDG,
fluorodeoxyglucose. |
In addition, the use of PTC209 enhanced the
expression of the cell cycle-associated proteins p16, p19, p21 and
p53, as well as SASP factors such as IL-1β, IL-6 and TNF-α, in the
TLR4 KO group (Fig. 6D, E, G and
H). Moreover, the activity of β-galactosidase was increased in
the TLR4 KO + PTC209 group compared with the TLR4 KO group
(Fig. 6C and F). Together, these
data indicated that suppression of Bmi-1 attenuated the
anti-inflammaging effect of TLR4 KO in LPS-aged gingival
fibroblasts.
Expression of Bmi-1 decreases in the
gingival tissues from patients with chronic periodontitis
It was previously observed that there were increased
levels of SASP factors such as IL-1β, IL-6 and TNF-α in the
gingival crevicular fluid of patients with periodontal disease
(29,30). The findings of the present study
suggested that Bmi-1 downregulated SASP factor secretion in mouse
periodontitis. Therefore, we compared the expression of Bmi-1 in
the gingiva of periodontitis patients and healthy individuals.
Masson-trichrome staining showed higher infiltration of
inflammatory cells and the destruction of collagen fibers in
patients with periodontitis compared with healthy individuals
(Fig. 7A). Immunofluorescent
assays showed that, compared with the healthy group, the percentage
of Bmi-1-positive regions in the gingiva of patients with
periodontitis was significantly reduced, particularly in gingival
fibroblasts positive for the fibroblast marker vimentin (Fig. 7B and E). Primary human gingival
fibroblasts were extracted from subjects in the two groups.
Stimulation of the gingival fibroblasts with 100 ng/ml LPS from
P. gingivalis for 24 h was conducted to imitate an
inflammatory microenvironment in periodontitis. Bmi-1 protein
expression in gingival fibroblasts was significantly decreased in
the periodontitis group compared with the healthy group (Fig. 7C and D). These findings suggested
that Bmi-1 was involved in the development of periodontitis.
Discussion
Periodontitis triggered by bacterial infection is a
persistent chronic inflammation state of gums and the surrounding
structures (3). The innate immune
system protects the body from bacterial invasion; however,
dysfunction of the immune system may lead to age-related
inflammation (31,32). Previous reports have indicated
that age-related inflammation, also referred to as inflammaging,
accelerates gingival senescence in gingival tissues (33,34). The persistent presence of
senescent cells can foster chronic inflammation and modify the
periodontal microenvironment via changes in the levels of SASP
factors (34,35). However, the precise mechanism
underlying the development of inflammaging in chronic periodontitis
has remained largely unclear. The present study indicated that the
TLR4 pathway, mobilized by bacterial infection, may suppress Bmi-1
expression and promote activation of the NLRP3 pathway, as well as
increasing levels of SASP factors. These events resulted in
gingival senescence, which is characterized by excessive secretion
of aging biomarkers such as p16, p53 and p21.
LPS, a component of gram-negative bacteria,
stimulates inflammation and promotes the resorption of alveolar
bone in chronic periodontitis (36). There has been considerable focus
on cellular senescence induced by repeated LPS stimulation
(37). It has been reported that
bacterial-derived LPS evokes premature alveolar osteocyte
senescence and perturbation of SASP factors, promoting the onset of
alveolar bone loss (38). The
present study observed that LPS stimulated the senescence process
and SASP factors in gingival fibroblasts; following LPS
stimulation, gingival fibroblasts exhibited a significant increase
in the expression of p16, p53 and p21, as well as the secretion of
IL-1β, IL-6 and TNF-α. These observations were consistent with a
recent study, which showed that LPS increased secretion of IL-6 and
TNF-α through p53-dependent activation (39).
TLR4 is a type I integral membrane glycoprotein
which can be stimulated by lipopolysaccharide (LPS) (40). The TLR4 pathway affects how innate
immune cells sense and respond to infectious stimuli; as a response
mechanism, the cells release toxic factors that aggravate
periodontitis (41). Results from
TLR4 KO mice and gingival fibroblasts demonstrated that TLR4
deletion attenuated the destruction of periodontal tissues by
suppressing the expression of senescence-associated proteins and
SASP. A previous study found that the activation of the TLR4
pathway regulates the senescence of mouse lung endothelial cells
(13). The present study
suggested that TLR4 functions as a regulator of cellular senescence
in fibroblasts aged in response to LPS stimulation, resulting in
persistent chronic low-grade inflammation.
The NLRP3 inflammasome, a commonly studied
inflammasome complex, affects patients with chronic periodontitis
by catalyzing the initiation and progression of inflammation
(42,43). Previous studies suggested that
activation of the NLRP3 inflammasome is associated with age-related
innate immune activation and may be involved in the onset of
periodontitis (21,28). The present study indicated that
TLR4 gene KO downregulated the NLRP3 inflammasome and its
down-stream molecules, ASC and Caspase-1. Whereas other studies
have provided evidence that the NLRP3 inflammasome is a key
upstream regulator of SASP associated with cellular senescence and
inflammatory disease (28,42),
the present study suggested that it is TLR4 that regulates the
NLRP3 inflammasome and its effector molecules. However, further
studies are required to examine whether TLR4 regulates SASP in
periodontitis through NLRP3 activation.
It has been reported that Bmi-1 regulates tissue
homeostasis and the development of degenerative diseases, and
prevents stem cell aging via the Bmi-1/p16 pathway (20,44). There is, however, less data
concerning the function of Bmi-1 in the development of
periodontitis. The present study showed elevated expression of
Bmi-1 in TLR4 KO mice. To further explore the function of Bmi-1 in
the pathogenesis of periodontitis, PTC209 was used in TLR4 KO
gingival fibroblasts. It was demonstrated that after inhibition of
Bmi-1, the reduced activity of the NLRP3 pathway in TLR4 KO
fibroblasts and resulting reduction in gingival senescence was
attenuated. Thus, Bmi-1 is an important regulator of senescence and
inflammation in gingiva, information that could be explored to
develop improved therapies for periodontitis.
The present findings suggest that in a mouse model
of periodontitis, Bmi-1 negatively regulates TLR4-specific
activation of the NLRP3 pathway and SASP secretion in the gingiva.
As perturbation of cells in the periodontium contributes to
inflammaging in the periodontium (2), the role of Bmi-1 in the development
of periodontitis in humans is unknown. The present data indicated
that the expression of Bmi-1 in the gingiva was decreased in
patients with periodontitis compared with the healthy group,
suggesting a close association between Bmi-1 and periodontitis.
These results may aid early detection of inflammaging, thus
preventing the development of chronic periodontitis. This method
may overcome the limitations associated with the evaluation of
clinical manifestations of periodontitis or the expression of SASP
factors (45). Thus, Bmi-1 may be
a reliable predictor of a patient's periodontal status.
Longitudinal studies with larger sample sizes are required to
validate the hypothesis that Bmi-1 could be a biomarker for
periodontitis.
In conclusion, the present study indicated that
periodontitis activated the TLR4 pathway in gingival fibroblasts
and down-regulated Bmi-1. Suppression of Bmi-1 expression
accelerated the priming of NLRP3 inflammasome and the accumulation
of senescent cells. The increased secretion of age-related
inflammatory markers, such as IL-1β, IL-6 and TNF-α, promoted the
inflammatory bone destruction in periodontitis. The present study
reported an important pathway that potentially mediates the
occurrence and development of chronic periodontitis, proposed a
novel perspective regarding immune mechanisms involved in
periodontitis, and provided a novel future direction for the
clinical management of periodontitis.
Funding
This study was supported by grants from the Youth
Program of National Natural Science Foundation of China (grant no.
81901416) and the project funded by the Priority Academic Program
Development of Jiangsu Higher Education Institutions (grant no.
2018-87).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WC and HYM conceived the study and participated in
its design, as well as revising and editing the manuscript. ZYQ and
XG designed the study, drafted the manuscript and contributed
equally to the study. ZYQ, SYL, NNH and YL performed the
experiments and collected the data. YLC, CXH and JBL helped collect
clinical samples and performed data analysis. All authors had full
access to all the data in the study, and take responsibility for
the integrity of the data and the accuracy of the data analysis.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Nanjing
Animal Experimental Ethics Committee (permit no. IACUC-1901052) and
human studies were approved by the Ethical Committee Department,
Affiliated Hospital of Stomatology, Nanjing Medical University
(permit no. PJ2018-050-001). Written informed consent was obtained
from patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Franceschi C, Garagnani P, Parini P,
Giuliani C and Santoro A: Inflammaging: A new immunemetabolic
viewpoint for age-related diseases. Nat Rev Endocrinol. 14:576–590.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ebersole JL, Dawson DA III, Emecen Huja P,
Pandruvada S, Basu A, Nguyen L, Zhang Y and Gonzalez OA: Age and
periodontal health-immunological view. Curr Oral Health Rep.
5:229–241. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:1809–1820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mombelli A: Microbial colonization of the
periodontal pocket and its significance for periodontal therapy.
Periodontol. 76:85–96. 2018. View Article : Google Scholar
|
|
5
|
Hajishengallis G: Immunomicrobial
pathogenesis of periodontitis: Keystones, pathobionts, and host
response. Trends Immunol. 35:3–11. 2014. View Article : Google Scholar
|
|
6
|
Franceschi C, Bonafè M, Valensin S,
Olivieri F, De Luca M, Ottaviani E and De Benedictis G:
Inflamm-aging. An evolutionary perspective on immunosenescence. Ann
N Y Acad Sci. 908:244–254. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim KA, Jeong JJ, Yoo SY and Kim DH: Gut
microbiota lipopolysaccharide accelerates inflamm-aging in mice.
BMC Microbiol. 16:92016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prattichizzo F, De Nigris V, La Sala L,
Procopio AD, Olivieri F and Ceriello A: 'Inflammaging' as a
Druggable Target: A senescence-associated secretory
phenotype-centered view of type 2 diabetes. Oxid Med Cell Longev.
2016:18103272016. View Article : Google Scholar
|
|
9
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Naseery NI, Mousa HSE, Noreldin AE,
El-Far AH and Elewa YHA: Aging-associated immunosenescence via
alterations in splenic immune cell populations in rat. Life Sci.
241:1171682020. View Article : Google Scholar
|
|
11
|
Watanabe K, Iizuka T, Adeleke A, Pham L,
Shlimon AE, Yasin M, Horvath P and Unterman TG: Involvement of
toll-like receptor 4 in alveolar bone loss and glucose homeo-stasis
in experimental periodontitis. J Periodontal Res. 46:21–30. 2011.
View Article : Google Scholar
|
|
12
|
Jin SH, Guan XY, Liang WH, Bai GH and Liu
JG: TLR4 polymorphism and periodontitis susceptibility: A
meta-analysis. Medicine (Baltimore). 95:e48452016. View Article : Google Scholar
|
|
13
|
Kim SJ, Shan P, Hwangbo C, Zhang Y, Min
JN, Zhang X, Ardito T, Li A, Peng T, Sauler M and Lee PJ:
Endothelial toll-like receptor 4 maintains lung integrity via
epigenetic suppression of p16INK4a. Aging Cell.
18:e129142019. View Article : Google Scholar
|
|
14
|
Asquith M, Haberthur K, Brown M, Engelmann
F, Murphy A, Al-Mahdi Z and Messaoudi I: Age-dependent changes in
innate immune phenotype and function in rhesus macaques (Macaca
mulatta). Pathobiol Aging Age Relat Dis. 2:2012.PubMed/NCBI
|
|
15
|
Calvo-Rodriguez M, de la Fuente C,
Garcia-Durillo M, Garcia-Rodriguez C, Villalobos C and Nunez L:
Aging and amyloid β oligomers enhance TLR4 expression, LPS-induced
Ca2+ responses, and neuron cell death in cultured rat
hippocampal neurons. J Neuroinflammation. 14:242017. View Article : Google Scholar
|
|
16
|
Seo SW, Park SK, Oh SJ and Shin OS:
TLR4-mediated activation of the ERK pathway following UVA
irradiation contributes to increased cytokine and MMP expression in
senescent human dermal fibroblasts. PLoS One. 13:e02023232018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coppe JP, Patil CK, Rodier F, Sun Y, Muñoz
DP, Goldstein J, Nelson PS, Desprez PY and Campisi J:
Senescence-associated secretory phenotypes reveal
cell-nonautonomous functions of oncogenic RAS and the p53 tumor
suppressor. PLoS biology. 6:2853–2868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li JP, Chen Y, Ng CH, Fung ML, Xu A, Cheng
B, Tsao SW and Leung WK: Differential expression of Toll-like
receptor 4 in healthy and diseased human gingiva. J Periodontal
Res. 49:845–854. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin X, Ojo D, Wei F, Wong N, Gu Y and Tang
D: A novel aspect of tumorigenesis-BMI1 functions in regulating DNA
damage response. Biomolecules. 5:3396–3415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen H, Chen H, Liang J, Gu X, Zhou J, Xie
C, Lv X, Wang R, Li Q, Mao Z, et al: TGF-β1/IL-11/MEK/ERK signaling
mediates senescence-associated pulmonary fibrosis in a
stress-induced premature senescence model of Bmi-1 deficiency. Exp
Mol Med. 52:130–151. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ebersole JL, Kirakodu S, Novak MJ, Exposto
CR, Stromberg AJ, Shen S, Orraca L, Gonzalez-Martinez J and
Gonzalez OA: Effects of aging in the expression of NOD-like
receptors and inflammasome-related genes in oral mucosa. Mol Oral
Microbiol. 31:18–32. 2016. View Article : Google Scholar :
|
|
22
|
Liston A and Masters SL:
Homeostasis-altering molecular processes as mechanisms of
inflammasome activation. Nat Rev Immunol. 17:208–214. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schroder K and Tschopp J: The
inflammasomes. Cell. 140:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qu J, Tao XY, Teng P, Zhang Y, Guo CL, Hu
L, Qian YN, Jiang CY and Liu WT: Blocking ATP-sensitive potassium
channel alleviates morphine tolerance by inhibiting
HSP70-TLR4-NLRP3-mediated neuroinf lammation. J Neuroinflammation.
14:2282017. View Article : Google Scholar
|
|
25
|
Kawahara Y, Kaneko T, Yoshinaga Y, Arita
Y, Nakamura K, Koga C, Yoshimura A and Sakagami R: Effects of
sulfonylureas on periodontopathic bacteria-induced inflammation. J
Dent Res. 99:830–838. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fathi E and Farahzadi R: Zinc sulphate
mediates the stimulation of cell proliferation of rat adipose
tissue-derived mesenchymal stem cells under high intensity of EMF
exposure. Biol Trace Elem Res. 184:529–535. 2018. View Article : Google Scholar
|
|
27
|
Fathi E, Farahzadi R, Valipour B and
Sanaat Z: Cytokines secreted from bone marrow derived mesenchymal
stem cells promote apoptosis and change cell cycle distribution of
K562 cell line as clinical agent in cell transplantation. PLoS One.
14:e02156782019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Youm YH, Grant RW, McCabe LR, Albarado DC,
Nguyen KY, Ravussin A, Pistell P, Newman S, Carter R, Laque A, et
al: Canonical Nlrp3 inflammasome links systemic low-grade
inflammation to functional decline in aging. Cell Metab.
18:519–532. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Offenbacher S, Barros SP, Singer RE, Moss
K, Williams RC and Beck JD: Periodontal disease at the
biofilm-gingival interface. J Periodontol. 78:1911–1925. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Romero-Castro NS, Vazquez-Villamar M,
Munoz-Valle JF, Reyes-Fernández S, Serna-Radilla VO,
García-Arellano S and Castro-Alarcón N: Relationship between TNF-α,
MMP-8, and MMP-9 levels in gingival crevicular fluid and the
subgingival microbiota in periodontal disease. Odontology.
108:25–33. 2020. View Article : Google Scholar
|
|
31
|
Knight ET, Liu J, Seymour GJ, Faggion CM
and Cullinan MP: Risk factors that may modify the innate and
adaptive immune responses in periodontal diseases. Periodontol.
71:22–51. 2016. View Article : Google Scholar
|
|
32
|
Salminen A, Kaarniranta K and Kauppinen A:
Immunosenescence: The potential role of myeloid-derived suppressor
cells (MDSC) in age-related immune deficiency. Cell Mol Life Sci.
76:1901–1918. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang P and Wang Q, Nie L, Zhu R, Zhou X,
Zhao P, Ji N, Liang X, Ding Y, Yuan Q and Wang Q:
Hyperglycemia-induced inflamm-aging accelerates gingival senescence
via NLRC4 phosphorylation. J Biol Chem. 294:18807–18819. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Q, Zhou X, Zhang P, Zhao P, Nie L, Ji
N, Ding Y and Wang Q: 25-Hydroxyvitamin D3 positively
regulates periodontal inflammaging via SOCS3/STAT signaling in
diabetic mice. Steroids. 156:1085702020. View Article : Google Scholar
|
|
35
|
Kuang Y, Hu B, Feng G, Xiang M, Deng Y,
Tan M, Li J and Song J: Metformin prevents against oxidative
stress-induced senescence in human periodontal ligament cells.
Biogerontology. 21:13–27. 2020. View Article : Google Scholar
|
|
36
|
Taubman MA, Valverde P, Han X and Kawai T:
Immune response: the key to bone resorption in periodontal disease.
J Periodontol. 76:2033–2041. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xia ML, Xie XH, Ding JH, Du RH and Hu G:
Astragaloside IV inhibits astrocyte senescence: Implication in
Parkinson's disease. Immune response: the key to bone resorption in
periodontal disease. J Neuroinflammation. 17:1052020. View Article : Google Scholar
|
|
38
|
Aquino-Martinez R, Rowsey JL, Fraser DG,
Eckhardt BA, Khosla S, Farr JN and Monroe DG: LPS-induced premature
osteocyte senescence: Implications in inflammatory alveolar bone
loss and periodontal disease pathogenesis. Bone. 132:1152202020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu J, Zeng J, Wang X, Zheng M and Luan Q:
P53 mediates lipopolysaccharide-induced inflammation in human
gingival fibroblasts. J Periodontol. 89:1142–1151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takeuchi O and Akira S: Pattern
recognition receptors and inflammation. Cell. 140:805–820. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marchesan J, Jiao Y, Schaff RA, Hao J,
Morelli T, Kinney JS, Gerow E, Sheridan R, Rodrigues V, Paster BJ,
et al: TLR4, NOD1 and NOD2 mediate immune recognition of putative
newly identified periodontal pathogens. Mol Oral Microbiol.
31:243–258. 2016. View Article : Google Scholar
|
|
42
|
Isaza-Guzman DM, Medina-Piedrahita VM,
Gutierrez-Henao C and Tobon-Arroyave SI: Salivary Levels of NLRP3
inflammasome-related proteins as potential biomarkers of
periodontal clinical status. J Periodontol. 88:1329–1338. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marchesan JT, Girnary MS, Moss K, Monaghan
ET, Egnatz GJ, Jiao Y, Zhang S, Beck J and Swanson KV: Role of
inflammasomes in the pathogenesis of periodontal disease and
therapeutics. Periodontol. 82:93–114. 2020. View Article : Google Scholar
|
|
44
|
Chen G, Zhang Y, Yu S, Sun W and Miao D:
Bmi1 overexpression in mesenchymal stem cells exerts antiaging and
antiosteoporosis effects by inactivating p16/p19 signaling and
inhibiting oxidative stress. Stem Cells. 37:1200–1211. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kinney JS, Morelli T, Oh M, Braun TM,
Ramseier CA, Sugai JV and Giannobile WV: Crevicular fluid
biomarkers and periodontal disease progression. J Clin Periodontol.
41:113–120. 2014. View Article : Google Scholar :
|





















