Introduction
Steroid-induced osteonecrosis of the femoral head
(SONFH) is a common and progressive musculoskeletal disorder
characterized by severe pain and dysfunction of the hip joint,
caused by the excessive use of glucocorticoids (1-3).
Although the explicit mechanisms responsible for the development of
SONFH remain unclear, a growing body of evidence has recognized an
association between abnormal cellular behaviors of bone
marrow-derived mesenchymal stem cells (BMSCs) and the development
of SONFH (4-6). Emerging evidence has also
demonstrated that long-term exposure to high doses of
glucocorticoids, such as dexamethasone (Dex), can inhibit the
proliferation and induce the apoptosis of BMSCs (7,8),
which is considered a potential mechanism for the pathogenesis of
SONFH.
Long noncoding RNAs (lncRNAs) are RNA transcripts
that are >200 nucleotides in length, which are implicated in
various pathophysiological processes by regulating the expression
of critical genes to influence cell differentiation, proliferation
and apoptosis, although they have no coding potential (9-11).
LINC00473, a lncRNA located on human chromosome 6q27 (12), has been shown to be significantly
downregulated in human BMSCs (hBMSCs) obtained from patients with
SONFH, based on a previous microarray analysis by the authors
(13). Several studies have
confirmed the role of LINC00473 in the proliferation and apoptosis
of various tumor cells (14-17); however, the underlying effects and
molecular mechanisms of LINC00473 on Dex-stimulated hBMSCs remain
unknown.
Protein kinase B (Akt) is a primary mediator of the
phosphoinositide 3-kinase (PI3K)/Akt signaling pathway, which has
been shown to affect cell apoptosis, proliferation and migration
(18-20). Increases in the levels of B-cell
lymphoma 2 (Bcl-2), an anti-apoptotic protein, can lead to the
inhibition of apoptosis through the decrease of cleaved caspase-3.
Moreover, the phosphorylation of Akt increases the expression of
Bcl-2 through the inactivation of the Bcl-2-associated death
promoter (Bad) (21,22). Taken together, these results
indicate that the Akt/Bad/Bcl-2 signaling cascade plays a key role
in cell apoptosis, proliferation and survival. Moreover, Dex has
been reported to regulate cellular behavior, including the
inhibition of cancer cell migration and osteoblast osteogenesis, by
suppressing the Akt signaling pathway (23,24), which prob-ably is the molecular
mechanism responsible for the apoptosis of BMSCs induced by
Dex.
Phosphatidylethanolamine-binding protein 1 (PEBP1),
which is also referred to as Raf1 kinase inhibitory protein (RKIP),
has been found to be potentially involved in diverse biological
functions through the regulation of various protein kinases in
several signaling pathways, such as the RAF/MEK/mitogen-activated
protein kinase (MAPK)/extra-cellular regulated kinase (ERK),
nuclear factor (NF)-κB and PI3K/Akt/mammalian target of rapamycin
(mTOR) pathways (25,26). In addition, the influence of PEBP1
on cell apoptosis, proliferation, migration and autophagy has been
demonstrated. For instance, PEBP1 has been reported to inhibit
starvation-induced autophagy through the activation of the
Akt/MTORC1 pathway (27).
Surprisingly, it was found that PEBP1 is involved in
the competitive endogenous RNA (ceRNA) network of LINC00473, and
there may be a potential correlation between LINC00473 and PEBP1,
based on the statistics obtained from the Encyclopedia of RNA
Interactomes (ENCORI; http://starbase.sysu.edu.cn/index.php) (28). Notably, a majority of these data
have been obtained from cancer-related studies, but almost none are
from studies on BMSCs. Due to this disparity, the present study
aimed to investigate whether LINC00473 can rescue hBMSC from
apoptosis induced by Dex via the Akt/Bad/Bcl-2 signaling pathway.
In addition, the present study aimed to further determine whether
the activation of the Akt/Bad/Bcl-2 signaling pathway induced by
LINC00473 is mediated by PEBP1, in order to obtain evidence to
identify potential targets of LINC00473.
Materials and methods
Clinical tissue sample extraction
The present study was approved by the Ethics
Committee of Qingdao University Affiliated Hospital (Qingdao,
China). Bone marrow tissue of the proximal femur was obtained from
6 patients with SONFH (4 males and 2 females, aged 52 to 67 years)
and 6 patients (3 males and 3 females, aged 61 to 71 years) with
femoral neck fractures as the controls, during total hip
arthroplasty (THA) surgery at the Department of Orthopedics at
Qingdao University Affiliated Hospital. All donors were Chinese and
provided informed consent for participation.
hBMSC isolation and culture
Based on previously described methods, hBMSCs were
isolated from the bone marrow tissue and cultured (29). Briefly, the bone marrow was mixed
with an equal volume of phosphate-buffered saline (PBS), and then
spread on lymphocyte separation medium (LSM; Beijing Solarbio
Science & Technology Co., Ltd.; 1:1, v/v). After washing with
PBS, the mononuclear cell layer was collected following
centrifugation at 500 × g for 30 min at room temperature. The cells
were then cultured in low-glucose DMEM (Beijing Solarbio Science
& Technology Co., Ltd.) containing 10% (v/v) fetal bovine serum
(FBS) (Gibco; Thermo Fisher Scientific, Inc.) and 100 units/ml
penicillin-streptomycin (Beijing Solarbio Science & Technology
Co., Ltd.), and maintained in a humidified incubator (HERAcell vios
160i, Thermo Fisher Scientific, Inc.) at 5% CO2 and
37°C. When 80-90% confluency was reached, the hBMSCs were detached
using 0.05% trypsin-EDTA (Beijing Solarbio Science & Technology
Co., Ltd.) and sub-cultured at a ratio of 1:2 in new culture
flasks. The cells at passage 3 were used in subsequent
experiments.
Phenotyping of hBMSCs
Flow cytometric analysis for surface markers was
performed following each extraction of primary cells, and 3
replicates were made in the experiment. In brief, after the 3rd
passage hBMSCs were collected, rinsed and blocked with pre-cold PBS
containing 1% FBS 3 times, the cell suspension was incubated for 30
min in the dark at 37°C with the following 2 pairs of mouse
anti-human antibodies (30): 20
µl of CD34PE (cat. no. 560941) and 20 µl of CD45-FITC
(cat. no. 555482); 20 µl of CD73PE (cat. no. 561014) and 2
µl of CD90FITC (cat. no. 555595), through double-staining
based on previous descriptions (31). The cells were incu-bated with
isotype control antibodies for PE Mouse IgG1, κ (cat. no. 555749)
and FITC Mouse IgG1, κ (cat. no. 554679) served as a negative
control as recommended by the manufacturer. The expression levels
of hBMSC surface markers were investigated using an Apogee
A50-MICRO flow cytometer (Apogee Corporation). All antibodies were
purchased from BD Biosciences.
Osteogenic and adipogenic differentiation
of hBMSCs
The capacity of the hBMSCs to undergo osteogenic and
adipogenic differentiation was assessed using a differentiation
medium (Biogene Biotechnology, Inc.), as per the manufacturer's
instructions. Osteogenic induction medium was composed of complete
medium supplemented with 1% glutamine, 1% penicillin-streptomycin,
1% β-glycerophosphate, 0.2% ascorbate acid and 0.01% Dex.
Adipogenic induction medium was composed of complete medium
supplemented with 0.1% insulin, 0.1% isobutylmethylxanthine, 0.1%
rosiglitazone and 0.1% dexamethasone.
For osteogenic differentiation, the cells were
treated with the osteogenic differentiation medium after the
confluency reached 60%, and were fixed with 0.2 ml of 4%
paraformaldehyde solution for 15 min, and incubated with alkaline
phosphatase (Beijing Solarbio Science & Technology Co., Ltd.)
for 20 min in dark at 37°C after 14 days. For adipogenic
differentiation, the cells were treated with the adipogenic
differentiation medium after confluency reached 90%, and were then
fixed with 0.2 ml of 4% paraformaldehyde solution for 15 min, and
stained with Oil Red O (Beijing Solarbio Science & Technology
Co., Ltd.) for 15 min after 21 days. The activity part of ALP and
lipid droplet formation in cells were observed under an inverted
phase-contrast microscope (CKX41, Olympus Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cultured cells
using a RNAiso plus kit (Takara Bio Inc.), and was then reverse
transcribed into cDNA using a PrimeScript RT reagent kit (Takara
Bio Inc.). qPCR was performed using a SYBR Premix Ex Taq II kit
(Takara Bio Inc.) along with a Roche LightCycler 480 Detection
System (Roche Diagnostics) as instructed by the manufacturer. The
RT-qPCR thermocycling conditions were as follows: Denaturation at
95°C for 30 sec; 40 cycles at 95°C for 5 sec and 60°C for 30 sec;
dissociation at 95°C for 5 sec and 60°C for 1 min; and cooling at
50°C for 30 sec. The primers used in the present study were
provided by Guangzhou RiboBio Co., Ltd. The sequences of the
primers were as follows: Human LINC00473 forward, 5′-GGC AGC CTC
AGG TTA CAA AT-3′ and reverse, 5′-AGG AGC AGG TAG GGA AAT GA-3′;
human PEBP1 forward, 5′-CTC GCG ATG CTG GTG TAC C-3′ and reverse,
5′-GGA TCC CTG CTT CCC ACA CAG C-3′; and human glyceraldehyde
3-phosphate dehydrogenase (GAPDH) forward, 5′-CCC ACG CCT CCT CCG
TTG AC-3′ and reverse, 5′-ATA CCT GGA AAT CAG CTT TAC AA-3′. The
relative expression levels of LINC00473 and PEBP1 were evaluated
using the 2−ΔΔCq method (32), and were normalized to the GAPDH
levels. The experiment was performed in triplicate.
Lentivirus vector construction and
infection of hBMSCs
The pRLenti-EF1a-EGFP-CMV-LINC00473-up lentivirus
(H12785) and negative control lentivirus (GL103) were provided by
OBiO Technology (Shanghai) Corp., Ltd. The hBMSCs were infected
with the LINC00473-up lentivirus at a final multiplicity of
infection (MOI) of 100 containing 5 µg/ml polybrene, and
were observed for the expression of green fluorescent protein (GFP)
under an inverted fluorescence microscope (EVOS FL, Invitrogen;
Thermo Fisher Scientific, Inc.) after 24 h. The efficiency of
LINC00473-up and the negative control were determined by RT-qPCR
after 3 days.
Treatment of hBMSCs
The hBMSCs obtained from the patients with femoral
neck fractures were used in the following experiments. The cells
treated with 1 µM Dex were named as the Dex group (Dex),
while the cells infected with LINC00473-up lentivirus following
exposure to 1 µM Dex were named as the Dex + LINC00473-up
group (Dex + LINC00473-up), and cells infected with a random
sequence following exposure to 1 µM Dex were named as the
Dex + Vector group (Dex + Vector). Cells cultured in complete
medium were regarded as the normal group (Normal).
In addition, MK-2206 (Selleckchem), an Akt
inhibitor, and SC79 (Selleckchem), an Akt activator, were used in
the antagonist and activation experiments, respectively. Cells in
the Dex + LINC00473-up group were treated with MK-2206 (5
µmol/l) to create the Dex + LINC00473-up + MK-2206 group
(Dex + LINC00473-up + MK-2206), while cells in the Dex group were
treated with SC79 (8 µg/ml) to create the Dex + SC79 group
(Dex + SC79).
Cell proliferation analysis
In brief, 5×103 cells/well were seeded
into 96-well plates, and were treated according to their grouping
after 24 h. Subsequently, a Cell Counting Kit-8 (CCK-8 assay kit;
Beijing Solarbio Science & Technology Co., Ltd.) was used to
analyze cell proliferation from day 1 to 7, as recommended by the
manufacturer. A microplate reader (5082, Tecan Austria GmbH) was
used to measure the absorbance at 450 nm. The experiment was
performed in triplicate.
Assessment of the morphology of apoptotic
cells
In brief, 5×104 cells/well were seeded
into 24-well plates, and were then treated based on their grouping.
After 7 days, the morphology of the apoptotic cells was assessed
using a chromatin dye Hoechst 33342 kit (Beijing Solarbio Science
& Technology Co., Ltd.), as recommended by the manufacturer.
Apoptotic cells were observed through fluorescence microscopy (EVOS
FL, Invitrogen; Thermo Fisher Scientific, Inc.) and identified as
the cells with blue hyper-fluorescence, and with one of the
following morphological alterations: Chromatic agglutination,
karyopyknosis, nuclear fragmentation (33,34), and were then counted through 6
randomly selected fields. The experiment was performed in
triplicate.
Flow cytometric analysis of
apoptosis
In brief, 2×105 cells/well were seeded
into 6-well plates, and were then treated based on their grouping.
After 7 days, the apoptotic cells were analyzed using an Apogee
A50-MICRO flow cytometer (Apogee Corporation) along with an Annexin
V-PE/7-AAD apoptosis detection kit (BD Biosciences) as instructed
by the manufacturer, and at least 104 cells in each
sample were analyzed. The emission max wavelength of PE and 7-AAD
was 578 nm and 655 nm, respectively. Both of their excitation
wavelengths were 488 nm (35).
The experiment was performed in triplicate.
Western blot analysis
A pre-cold RIPA Lysis Buffer (Beijing Solarbio
Science & Technology Co., Ltd.) containing 1% protease
inhibitor cocktail (MedChemExpress) was used to extract total
protein from the hBMSCs treated based on their grouping. After the
protein concentration was determined by a bicinchoninic acid (BCA)
protein assay kit (Beijing Solarbio Science & Technology Co.,
Ltd.), the cell lysates were mixed with a protein loading buffer
(EpiZyme) at a ratio of 1:5, and heated to 95°C for 5 min. The
separation of the protein sample (50 µg) in each group was
conducted by 12.5% SDS-PAGE (EpiZyme), and the protein samples were
electrotransferred onto a PVDF membrane (MilliporeSigma) following
separation. Subsequently, 5% non-fat milk in TBST (Beijing Solarbio
Science & Technology Co., Ltd.) was used to block the PVDF
membranes for 2 h at 37°C. Following incubation with primary
antibodies at 4°C overnight, the PVDF membranes were incubated with
corresponding secondary antibodies (HRP-conjugated) at 37°C for 1
h. The target bands were visualized using ECL-PLUS reagents
(MilliporeSigma), and were scanned using a BioSpectrum Imaging
System (UVP, Thermo Fisher Scientific, Inc.). The results of
western blot analysis were quantified through integrated density
using ImageJ software (version 1.52u), and were normalized to GAPDH
levels.
The primary antibodies, including rabbit anti-human
Akt (cat. no. 4685S), phosphorylated (p-)Akt (cat. no. 13038S), Bad
(cat. no. 9239S), p-Bad (cat. no. 4366S), Bcl-2 (cat. no. 4223S),
cleaved caspase-3 (cat. no. 9664S), caspase-3 (cat. no. 14220S),
PEBP1 (cat. no. 13006S) antibodies, were provided by Cell Signaling
Technology, Inc. and were diluted in antibody dilution buffer
(Boster Biological Technology, Inc.) at ratio of 1:1,000 as
recommended by the manufacturer. The primary antibody for GAPDH
(cat. no. E-AB-20072) and all the secondary antibodies (cat. nos. :
E-AB-1003) were purchased from Elabscience Biotechnology Inc. and
were diluted in antibody dilution buffer (Boster Biological
Technology, Inc.) at ratio of 1:5,000 and 1:1,000,
respectively.
ceRNA interation network of LINC00473 in
humans
The data of the ceRNA interation network of
LINC00473 in humans were obtained from ENCORI. Briefly, on the home
page of the ENCORI website (http://starbase.sysu.edu.cn/index.php),
'ceRNA-Network' was selected from the navigation bar, and
'lncRNA-ceRNA' was then selected. Subsequently, 'human' was
selected in the section of 'Genome', and a search for 'LINC00473'
was made in the section of 'ceRNA Gene' to complete the ceRNA
interaction network of LINC00473 in humans. 'Pan-Cancer' was then
selected to obtain the coefficient-R value in various types of
cancer, which were created as a statistics chart using GraphPad
Prism 8 software (GraphPad, Inc.).
PEBP1 recombinant plasmid and
transfection of hBMSCs with shRNA
To further determine whether the activation of the
Akt/Bad/Bcl-2 signaling pathway by LINC00473 is mediated by PEBP1,
the shRNA and overexpression plasmid of PEBP1 were transfected into
Dex-induced hBMSCs using a Lipofectamine 2000 system (Thermo Fisher
Scientific, Inc.), as recommended by the manufacturer. Three shRNAs
of PEBP1, including sh-PEBP1-1 (target sequence, tgGGA TGA CTA TGT
GCC CAA A), sh-PEBP1-2 (target sequence, caAAT TCA AGG TGG CGT CCT
T) and sh-PEBP1-3 (target sequence, cgAGC AGG ACA GGC CGC TAA A),
and the overexpression plasmid (vector name, GV657) for PEBP1
(PEBP1-up) were provided by Genechem Corporation. Moreover, a
random sequence (TTC TCC GAA CGT GTC ACG T) was selected as the
negative control of shRNA (sh-Control), and a blank vector was used
as the negative control of the overexpression plasmid for PEBP1
(PEBP1-Control).
Following infection with the LINC00473-up
lentivirus, the Dex-induced hBMSCs were transfected with a shRNA of
PEBP1 to create the Dex + LINC00473-up + sh-PEBP1 group (Dex +
LINC00473-up + sh-PEBP1). The Dex-stimulated hBMSCs were
transfected with the recombinant plasmid of PEBP1 to create the Dex
+ PEBP1-up group (Dex + PEBP1-up). Following transfection for 24 h,
the transfection efficiency of PEBP1 was detected by RT-qPCR and
western blot analysis.
Statistical analysis
SPSS 19.0 software (IBM Corporation) was used to
perform one-way analysis of variance (ANOVA) to compare the results
between >3 groups, and an unpaired t-test to compare the data of
2 groups. Parametric data are presented as the means ± standard
deviation (SD). Furthermore, the homogeneity test for variance was
also performed. When the statistics exhibited heteroscedasticity,
Tamhane's T2 test was used. A P-value <0.05 was considered to
indicate a statistically significant result. GraphPad Prism 8
software (GraphPad, Inc.) was used to create the statistics
charts.
Results
Identification of hBMSCs
The third passage of cells was observed to have a
homogeneous fibroblast-like, spindle-shaped morphology (Fig. 1A). Furthermore, their ability to
differentiate into osteogenic and adipogenic lineages was confirmed
using ALP and Oil Red O staining (Fig. 1B-D). The phenotyping of the hBMSCs
detected using flow cytometry further suggested that the majority
of the isolated cells expressed the two typical surface markers of
marrow-derived stem cells: CD73 (98.45%) and CD90 (98.78%)
(Fig. 1Eb), and few cells
expressed the two specific cell surface markers of hematopoietic
cells: CD34 (0%) and CD45 (0.67%) (Fig. 1Ec).
 | Figure 1Identification of hBMSCs. (A)
Representative images showing morphology of hBMSCs under an
inverted phase contrast microscope (scale bar, 1,000 µm).
(B) ALP staining (scale bar, 1,000 µm). (C) Lipid droplets
formation (scale bar, 400 µm). (D) Oil Red O staining (scale
bar, 400 µm). (E) Phenotypic analysis of hBMSCs by flow
cytometry (CD34, CD45, CD73 and CD90): (E-a) Cells were incubated
with isotype control antibodies for PE mouse IgG1, κ and FITC mouse
IgG1, κ served as a control; (E-b) Most of the isolated cells
expressed the two typical surface markers of MSCs: CD73 (98.45%)
and CD90 (98.78%); (E-c) Few cells expressed the two specific cell
surface markers of hematopoietic cells: CD34 (0%) and CD45 (0.67%).
hBMSCs, human bone marrow-derived mesenchymal stem cells; ALP,
alkaline phosphatase. |
LINC00473 is downregulated in the hBMSCs
of patients with SONFH
To determine the role of LINC00473 in SONFH, the
expression of LINC00473 in hBMSCs of the proximal femur obtained
from patients with SONFH and femoral neck fractures were assessed
by RT-qPCR. The relative LINC00473 expression in hBMSCs obtained
from the patients with SONFH was significantly lower than that of
the cells from the control patients (Fig. 2), which is in line with the
previous microarray analysis (13) that indicated that this may be
caused by glucocorticoids.
Efficiency of transfection of LINC00473
and PEBP1 into hBMSCs
Following infection with LINC00473-up lentivirus,
>90% of the hBMSCs were positive for GFP (Fig. 3Aa and b). Moreover, the relative
expression of LINC00473 in the LINC00473-up group was significantly
upregulated by LINC00473-up lentivirus (494%), compared with the
level observed in the normal group and vector group (Fig. 3Ac).
In addition, the relative expression of PEBP1 mRNA
in the transfected hBMSCs was downregulated by all 3 types of
sh-PEBP1 at varying degrees (37.7% by sh-PEBP1-1, 55.9% by
sh-PEBP1-2 and 74% by sh-PEBP1-3), compared with the normal and
sh-Control cells (Fig. 3Ba). The
relative mRNA expression of PEBP1 in the hBMSCs was significantly
upregulated by PEBP1-up (1,169%), compared with the normal and
PEBP1-control cells (Fig. 3Bb).
Correspondingly, the protein level of PEBP1 in the transfected
hBMSCs was significantly downregulated by sh-PEBP1-1 (48.7%),
sh-PEBP1-2 (73.1%) and sh-PEBP1-3 (93.5%) (Fig. 3Ca), and upregulated by PEBP1-up
(441%) (Fig. 3Cb), compared with
the normal, sh-Control and PEBP1-control cells.
The above-mentioned results demonstrated that the
hBMSCs were successfully transfected with the LINC00473-up
lentivirus, shRNA and recombinant plasmids of PEBP1 using the
present method. sh-PEBP1-3 was found to exert the most significant
effect on the knockdown of PEBP1 mRNA and protein expression levels
and was thus selected for use in the following experiments.
LINC00473 reverses the inhibitory effect
induced by Dex on the proliferation of hBMSCs through the
activation of the Akt signaling pathway
The effect of LINC00473 on the proliferation of
hBMSCs in the presence of Dex (1 µM) was evaluated by CCK-8
assay. As also previously reported (13), the proliferation of hBMSCs was
markedly inhibited following exposure to 1 µM Dex, whereas
the inhibitory effect of Dex on hBMSCs was significantly reversed
by the upregulation of LINC00473. Notably, the effect of LINC00473
on the proliferation of hBMSCs was similar to that of SC79 (a
unique specific activator of Akt signaling pathway). However, this
effect was weakened by MK-2206 (a selective inhibitor of Akt
signaling pathway) (Fig. 4A). The
results revealed that LINC00473 attenuated the inhibitory effects
exerted by a high concentration of Dex (1 µM) on the
proliferation of hBMSCs through the activation of the Akt signaling
pathway.
 | Figure 4LINC00473 attenuates the Dex-induced
inhibition of the viability of hBMSCs by activating the Akt
signaling pathway. (A) The viability of hBMSCs in each group was
evaluated by CCK-8 assay from 1 to 7 days. (B-a) The apoptotic
cells were investigated by a fluorescence microscope through
Hoechst 33342 staining (scale bar, 200 µm). White arrows
indicate the cells with karyopyknosis. (B-b) The percentage of
apoptotic cells was calculated by cell count. A total of 6 randomly
selected fields were quantified, and a total of 300 cells in each
group were counted. (C-a) Flow cytometric analysis revealed the
apoptotic cells with Annexin V-PE and 7-AAD staining: Cells in Q1
represent necrotic cells, cells in Q2 represent late apoptotic
cells, cells in Q3 represent early apoptotic cells, cells in Q4
represent normal cells. (C-b) Percentage of Annexin+
cells in Q2 and Q3 in each group. All data are presented as the
mean value ± standard deviation of 3 independent experiments.
*P<0.05 compared with the normal group,
#P<0.05 compared with the Dex group and the Dex +
Vector group, &P<0.05 compared with the Dex +
LINC00473-up group. LINC00473, lncCRNA-00473; hBMSCs, human bone
marrow-derived mesenchymal stem cells; Dex, dexamethasone; Akt,
protein kinase B; CCK-8, Cell Counting Kit-8; Q, quadrant. |
LINC00473 attenuates the Dex-induced
apoptosis of hBMSCs through the activation of the Akt signaling
pathway
From the assessment of apoptosis morphology,
characteristics of apoptotic cells, such as chromatic
agglutination, karyopyknosis and nuclear fragmentation, were
observed in the hBMSCs stained with Hoechst 33342 following
continuous exposure to 1 µM Dex for 7 days, as reported in a
previous study (13). By
contrast, the upregulation of LINC00473 significantly decreased the
number of apoptotic hBMSCs observed in response to Dex, which
resembled the effects of SC79 under the same conditions.
Nevertheless, the effect of LINC00473 on Dex-induced hBMSCs were
inhibited by MK-2206 (Fig. 4Ba and
b).
Likewise, the flow cytometry assay conducted through
Annexin V-PE/7-AAD double-staining revealed that both LINC00473 and
SC79 markedly reversed the inhibitory effects on hBMSC viability
induced by Dex. Moreover, supplementation with MK-2206 suppressed
the anti-apoptotic effects of LINC00473 on Dex-stimulated hBMSCs
(Fig. 4Ca and b). Taken together,
these data demonstrated that LINC00473 reversed the Dex-induced
apoptosis of hBMSCs through the activation of the Akt signaling
pathway.
LINC00473 activates the Akt/Bad/Bcl-2
signaling pathway in Dex-stimulated hBMSCs
To further explore the role of LINC00473 in the
Akt/Bad/Bcl-2 signaling cascade, western blot analysis was
conducted to detect the phosphorylation of Akt and Bad, in addition
to the protein expression of Bcl-2 and cleaved caspase-3, after the
cells were treated based on their grouping for 24 h. The results
demonstrated that expo-sure to 1 µM Dex not only inhibited
the phosphorylation of Akt and Bad, but also decreased the protein
expression of Bcl-2 and increased the cleavage of caspase-3, which
is in line with the results of previous research studies (23,24). However, the upregulation of
LINC00473 significantly attenuated the negative effects of Dex by
promoting the protein level of p-Akt, p-Bad and Bcl-2, while
inhibiting the cleavage of caspase-3, which is consistent with the
effect observed with SC79 (an Akt activator). Notably, the
regulatory effects of LINC00473 on p-Akt, p-Bad, Bcl-2 levels and
the cleavage of caspase-3 in the Dex-stimulated hBMSCs were
antagonized by MK-2206 (an Akt inhibitor) (Fig. 5A and B). Collectively, these
findings demonstrated that LINC00473 attenuated the negative
effects on hBMSCs induced Dex by activating the Akt/Bad/Bcl-2
signaling pathway.
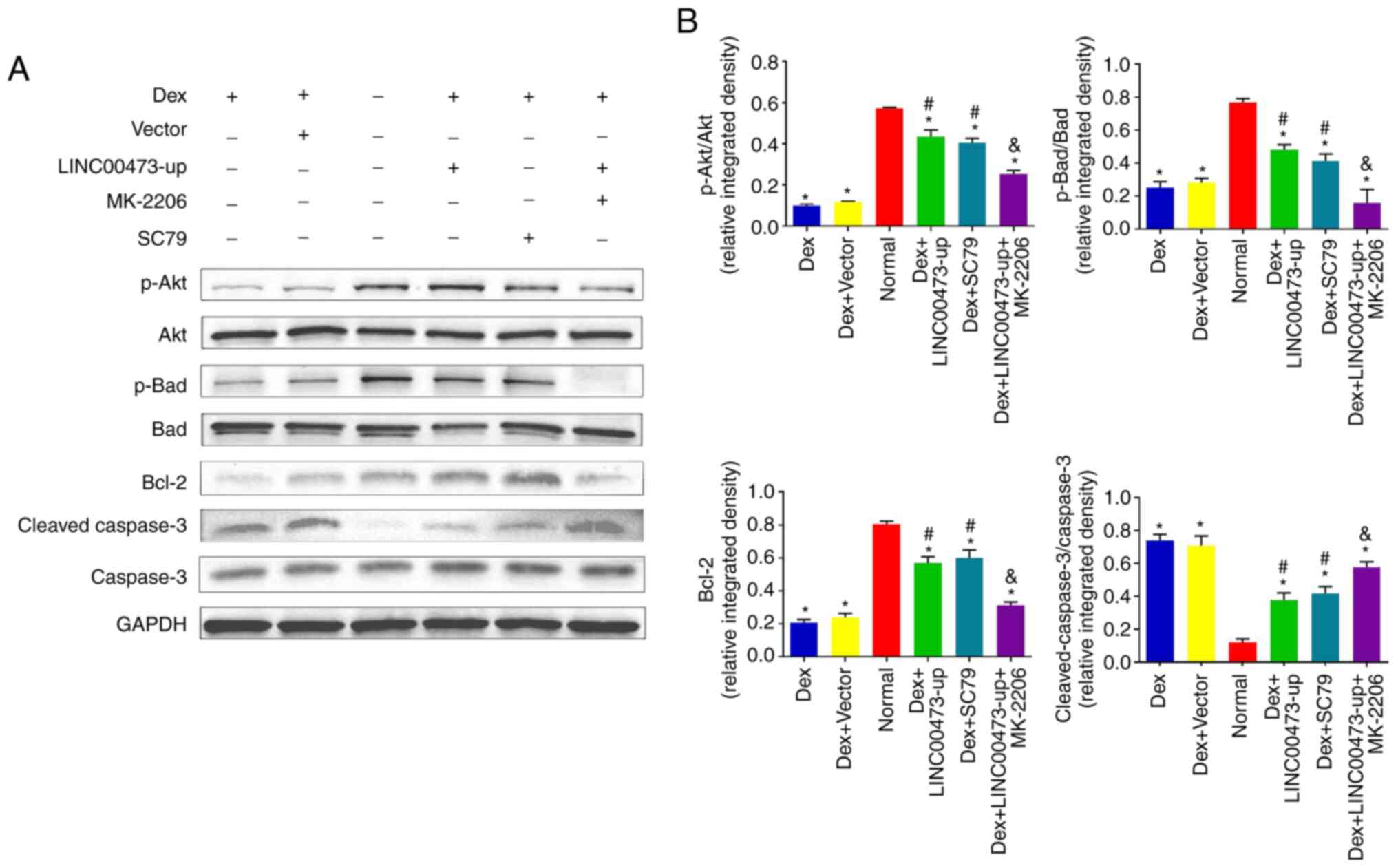 | Figure 5LINC00473 activates the Akt/Bad/Bcl-2
signaling pathway in Dex-stimulated hBMSCs. The results of western
blot analysis revealed (A) the protein expression levels of p-Akt,
Akt, p-Bad, Bad, Bcl-2 and cleaved caspase-3 in each group, and (B)
the quantification of integrated density in target bands by
normalization to GAPDH. All data are presented as the mean value ±
standard deviation of 3 independent experiments.
*P<0.05 compared with the normal group,
#P<0.05 compared with the Dex group and the Dex +
Vector group, &P<0.05 compared with the Dex +
LINC00473-up group. LINC00473, lncRNA-00473; Akt, protein kinase B;
Bad, Bcl-2-associated death promoter; Bcl-2, B-cell lymphoma 2;
hBMSCs, human bone marrow-derived mesenchymal stem cells; Dex,
dexamethasone. |
PEBP1 mediates the activation of the
Akt/Bad/Bcl-2 signaling pathway through LINC00473 in Dex-stimulated
hBMSCs
To further reveal the underlying mechanisms of
LINC00473 in the regulation of the Akt/Bad/Bcl-2 signaling pathway,
ENCORI (http://starbase.sysu.edu.cn/index.php) was searched to
deter-mine the ceRNA network of LINC00473 (28), and it was found that there was a
potential correlation between LINC00473 and PEBP1 in 32 types of
cancer (Fig. 6). Furthermore,
PEBP1 has been reported to inhibit starvation-induced autophagy by
promoting the phosphorylation of Akt (27). Therefore, the present study
investigated the role of PEBP1 in the activation of the
Akt/Bad/Bcl-2 signaling pathway by LINC00473 in Dex-stimulated
hBMSCs. As shown in Fig. 7Aa and
b, the upregulation of LINC00473 markedly promoted the
phosphorylation of Akt in Dex-stimulated hBMSCs, while increasing
the protein level of PEBP1. However, the elevation of Akt
phosphorylation by LINC00473 was significantly suppressed following
the knockdown of PEBP1 (Fig. 7Ba and
b). Furthermore, the upregulation of LINC00473 and PEBP1
resulted in an analogous and marked increase in Akt phosphorylation
in the Dex-stimulated hBMSCs (Fig.
7Ca and b). In addition, the results of RT-qPCR suggested that
the changes in the PEBP1 mRNA expression levels were consistent
with those observed at the protein level for PEBP1 (Fig. 7D). Taken together, these results
demonstrated that PEBP1 mediated the activation of the
Akt/Bad/Bcl-2 signaling pathway induced by LINC00473 in
Dex-stimulated hBMSCs.
 | Figure 7PEBP1 mediates the activation of the
Akt/Bad/Bcl-2 signaling pathway by LINC00473 in Dex-stimulated
hBMSCs. The result of western blot analysis revealed (Aa and b) the
protein expression levels of PEBP1, p-Akt and Akt in each groups
following infection with LINC00473-up lentivirus in Dex-induced
hBMSCs (*P<0.05 compared with the Dex group and Dex +
Vector group), and (Ba and b) the protein expression levels of
PEBP1 and p-Akt in each groups following co-transfection with
LINC00473 and PEBP1 in Dex-stimulated hBMSCs (*P<0.05
compared with the Dex + LINC00473-up group and Dex + LINC00473-up +
sh-Control group), and (Ca and b) the protein expression levels of
PEBP1 and p-Akt in each group following transfection with LINC00473
and PEBP1 respectively in Dex-induced hBMSCs (*P<0.05
compared with the Dex group, Dex + Vector group and Dex +
PEBP1-Control group). (D) The results of RT-qPCR revaled the PEBP1
mRNA expression in each groups (*P<0.05 compared with
the Dex group and Dex + Vector group, #P<0.05 compared with the
Dex + LINC00473-up group, &P<0.05 compared with
the Dex group and Dex + PEBP1-Control group). All data are
presented as the mean values ± standard deviation 3 three
independent experiments. PEBP1, phosphatidylethanolamine-binding
protein 1; Akt, protein kinase B; Bad, Bcl-2-associated death
promoter; Bcl-2, B-cell lymphoma 2; LINC00473, lncRNA-00473;
hBMSCs, human bone marrow-derived mesenchymal stem cells; Dex,
dexamethasone; p-Akt, phosphorylated Akt; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
Discussion
BMSCs have the ability of self-renewal, excellent
proliferation and multipotential differentiation into an osteogenic
lineage, adipogenic lineage and chondrogenic lineage, as well as
other embryonic lineages (36).
Therefore, the dysfunction of BMSCs can lead to a variety of
skeletal muscle diseases (6,37).
As a typical representative, the development of osteonecrosis of
the femoral head (ONFH) is closely associated with the abnormal
cellular behavior of BMSCs (6).
The excessive use of glucocorticoids is the primary cause of the
pathogeny of SONFH, and to a certain extent, is known to be due to
glucocorticoid-induced inhibition of the proliferation and
apoptosis of BMSCs (6,24).
It has been confirmed that lncRNAs play important
roles in cell proliferation, apoptosis and differentiation by
regulating the expression levels of critical genes (9-11).
In the present study, the expression of LINC00473 was detected in
hBMSCs obtained from patients with SONFH, and it was found that
LINC00473 was significantly downregulated in these hBMSCs, compared
with the controls, which was in agreement with the results of our
previous microarray analysis (13). LINC00473 is an intergenic lncRNA
located on human chromosome 6q27 that consists of 2 exons and 2
annotated transcript isoforms (12). The role of LINC00473 on the
proliferation and apoptosis of various tumor cells has been
elucidated in several studies (14-17); however, the effect of LINC00473 on
BMSCs has not yet been fully elucidated.
Dex is a common glucocorticoid drug, and emerging
studies have proven that Dex exerts variable effects on BMSCs based
on concentrations and exposure times (7,8,38).
Recent evidence has indicated that long-term exposure to a high
concentration of Dex (1 µM) can significantly inhibit the
proliferation and induce the apoptosis of BMSCs (8,13).
In the present study, based on previous findings, the concentration
of 1 µM Dex was selected for use as the condition that
induces the apoptosis of hBMSCs (8,13),
to mimic the cellular micro-environments of SONFH. Moreover, hBMSCs
were infected with the LINC00473-up lentivirus in consideration of
the decrease in LINC00473 expression in hBMSCs obtained from
patients with SONFH to investigate the role of LINC00473 on hBMSCs
induced by 1 µM Dex. As previously reported (8,13),
1 µM Dex significantly inhibited the proliferation and
induced the apoptosis of hBMSCs. However, it was found that the
upregulation of LINC00473 attenuated the inhibitory effects induced
by 1 µM Dex on the proliferation and apoptosis of the
hBMSCs, indicating the anti-apoptotic effects of LINC00473.
Akt is a primary mediator of the PI3K/Akt signaling
pathway, which has been shown to be involved in cell apoptosis,
proliferation and migration (18-20). In terms of the anti-apoptotic
effect, the Akt/Bad/Bcl-2 signaling cascade is the focus of current
research. In general, the phosphorylation-mediated activation of
Akt leads to the inactivation of Bad, which increases the
expression of Bcl-2 protein, which then inhibits apoptosis of cells
by suppressing the cleavage of caspase-3 (a downstream key factor
of apoptosis) (21,22). Although the explicit mechanisms of
the Dex-induced apoptosis of BMSCs remain unclear, they may
potentially involve the suppression of the Akt signaling pathway
(23,24).
Therefore, the present investigated the effects of
LINC00473 on the protein levels of the Akt/Bad/Bcl-2 signaling
pathway, and performed antagonist and activation experiments to
further elucidate the molecular mechanisms through which LINC00473
inhibits the Dex-induced apoptosis of hBMSCs. The results
demonstrated that 1 µM Dex suppressed the phosphorylation of
Akt and Bad, decreased the protein expression of Bcl-2, and
increased the expression of cleaved caspase-3, leading to the
apoptosis of hBMSCs, which was in agreement with previous research
results (23,24). Moreover, the upregulation of
LINC00473 significantly attenuated the negative effects induced by
Dex by promoting the phosphorylation of Akt and Bad, and increasing
the protein level of Bcl-2, while inhibiting the cleavage of
caspase-3. Notably, MK-2206, a selective inhibitor of Akt,
partially inhibited the upregulation of the protein expression
levels of the Akt/Bad/Bcl-2 signaling pathway triggered by
LINC00473, while attenuating the anti-apoptotic effect of LINC00473
on Dex-induced hBMSCs. Furthermore, the results of the activation
experiment revealed that the anti-apoptotic effect of LINC00473 and
the regulatory effect induced by LINC00473 on the Akt/Bad/Bcl-2
signaling pathway in Dex-stimulated hBMSCs, was similar to that of
SC79 (a unique specific activator of Akt). Taken together, it is
reasonable to speculate that LINC00473 rescued the hBMSCs from
Dex-induced apoptosis by activating the Akt/Bad/Bcl-2 signaling
pathway. However, the explicit mechanisms through which LINC00473
regulates the Akt/Bad/Bcl-2 signaling pathway warrant further
investigation.
To the best of our knowledge, certain lncRNAs act as
ceRNAs to regulate the expression of mRNAs that affect the
biological function of cells (39). Surprisingly, ENCORI we searched to
identify the ceRNA network of LINC00473 (28), and a potential correlation was
found between LINC00473 and PEBP1 in 32 types of cancer. PEBP1 is
also known as RKIP and is involved in diverse biological functions
through its regulation of various signaling pathways, and has been
reported to inhibit starvation-induced autophagy through the
activation of the Akt/MTORC1 pathway. Consequently, PEBP1 was
selected to explore the mechanisms through which LINC00473
regulates the Akt/Bad/Bcl-2 signaling pathway.
In further experiments, it was found that the
upregulation of LINC00473 significantly promoted the
phosphorylation of Akt in Dex-stimulated hBMSCs and increased the
protein level of PEBP1. However, the promotion of Akt
phosphorylation by LINC00473 was significantly suppressed following
the knockdown of PEBP1. Furthermore, the upregulation of PEBP1
triggered a significant increase in Akt phosphorylation in
Dex-stimulated hBMSCs, which corresponds with the results of the
upregulation of LINC00473. Taken together, the results demonstrated
that PEBP1 mediated the activation of the Akt/Bad/Bcl-2 signaling
pathway induced by LINC00473 in Dex-stimulated hBMSCs.
In conclusion, the present study confirmed the
downregulation of LINC00473 in the hBMSCs of patients with SONFH,
compared with the controls. In addition, LINC00473 reserved the
inhibitory effects induced by Dex on the proliferation of hBMSCs
and attenuated the Dex-induced apoptosis of hBMSCs by activating
the Akt/Bad/Bcl-2 signaling pathway. It is noteworthy that PEBP1
mediated this effect of LINC00473. Collectively, LINC00473 rescued
the hBMSCs from apoptosis induced by Dex through the PEBP1-mediated
Akt/Bad/Bcl-2 signaling pathway.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81802151),
the Shandong Province Natural Science Foundation (grant nos.
ZR2016HQ05, ZR2017BH089 and ZR2019MH012), the China Postdoctoral
Science Foundation (grant no. 2018M642616), and the Qingdao Applied
Foundational Research Youth Project (grant no. 19-6-2-55-cg).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX, YJ and ZZ performed the experiments and analyzed
the results. YX and YJ wrote and drafted the manuscript. TL wrote,
reviewed and edited the manuscript. TL and YW conceived the
methodology, while TL and YW designed the research study and were
major contributors in recruiting the donors. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Qingdao University Affiliated Hospital (Qingdao,
China). All donors were Chinese and provided informed consent for
participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Wang XS, Zhuang QY, Weng XS, Lin J, Jin J
and Qian WW: Etiological and clinical analysis of osteonecrosis of
the femoral head in Chinese patients. Chin Med J (Engl).
126:290–295. 2013.
|
|
2
|
Hao C, Yang S, Xu W, Shen JK, Ye S, Liu X,
Dong Z, Xiao B and Feng Y: MiR-708 promotes steroid-induced
osteonecrosis of femoral head, suppresses osteogenic
differentiation by targeting SMAD3. Sci Rep. 6:225992016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang YL, Yin JH, Ding H, Zhang W, Zhang
CQ and Gao YS: Vitamin K2 prevents glucocorticoid-induced
osteonecrosis of the femoral head in rats. Int J Biol Sci.
12:347–358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han L, Wang B, Wang R, Gong S, Chen G and
Xu W: The shift in the balance between osteoblastogenesis and
adipogenesis of mesenchymal stem cells mediated by glucocorticoid
receptor. Stem Cell Res Ther. 10:3772019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao X, Wei Z, Li D, Yang Z, Tian M and
Kang P: Glucocorticoid enhanced the expression of ski in
osteonecrosis of femoral head: The effect on adipogenesis of rabbit
BMSCs. Calcif Tissue Int. 105:506–517. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Houdek MT, Wyles CC, Packard BD, Terzic A,
Behfar A and Sierra RJ: Decreased osteogenic activity of
mesenchymal stem cells in patients with corticosteroid-induced
osteonecrosis of the femoral head. J Arthroplasty. 31:893–898.
2016. View Article : Google Scholar
|
|
7
|
Song IH, Caplan AI and Dennis JE:
Dexamethasone inhibition of confluence-induced apoptosis in human
mesenchymal stem cells. J Orthop Res. 27:216–221. 2009. View Article : Google Scholar
|
|
8
|
Fan Q, Zhan X, Li X, Zhao J and Chen Y:
Vanadate inhibits dexamethasone-induced apoptosis of rat bone
marrow-derived mesenchymal stem cells. Ann Clin Lab Sci.
45:173–180. 2015.PubMed/NCBI
|
|
9
|
Patil VS, Zhou R and Rana TM: Gene
regulation by non-coding RNAs. Crit Rev Biochem Mol Biol. 49:16–32.
2014. View Article : Google Scholar
|
|
10
|
Wei B, Wei W, Zhao B, Guo X and Liu S:
Long non-coding RNA HOTAIR inhibits miR-17-5p to regulate
osteogenic differentiation and proliferation in non-traumatic
osteonecrosis of the femoral head. PLoS One. 12:e01690972017.
View Article : Google Scholar
|
|
11
|
Huang Y, Zheng Y, Jia L and Li W: Long
noncoding RNA H19 promotes osteoblast differentiation via
TGF-β1/smad3/HDAC signalinging pathway by deriving miR-675. Stem
Cells. 33:3481–3492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pruunsild P, Bengtson CP and Bading H:
Networks of cultured ipsc-derived neurons reveal the human synaptic
activity-regulated adaptive gene program. Cell Rep. 18:122–135.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Y, Jiang Y, Wang Y, Ren Y, Zhao Z, Wang
T and Li T: LINC00473 regulated apoptosis, proliferation and
migration but could not reverse cell cycle arrest of human bone
marrow mesenchymal stem cells induced by a high-dosage of
dexamethasone. Stem Cell Res. 48:1019542020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen H, Yang F, Li X, Gong ZJ and Wang LW:
Long noncoding RNA lnc473 inhibits the ubiquitination of survivin
via association with usp9x and enhances cell proliferation and
invasion in hepatocellular carcinoma cells. Biochem Biophys Res
Commun. 99:702–710. 2018. View Article : Google Scholar
|
|
15
|
Zhang W and Song Y: LINC00473 predicts
poor prognosis and regulates cell migration and invasion in gastric
cancer. Biomed Pharmacother. 107:1–6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi C, Yang Y, Yu J, Meng F, Zhang T and
Gao Y: The long noncoding rna linc00473, a target of microRNA 34a,
promotes tumorigenesis by inhibiting ilf2 degradation in cervical
cancer. Am J Cancer Res. 7:2157–2168. 2017.PubMed/NCBI
|
|
17
|
Zhu SB, Fu W, Zhang L, Fu K, Hu JH, Jia W
and Liu G: LINC00473 antagonizes the tumour suppressor miR-195 to
mediate the pathogenesis of wilms tumour via IKKα. Cell Prolif.
51:e124162018. View Article : Google Scholar
|
|
18
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li L, Xia Y, Wang Z, Cao X, Da Z, Guo G,
Qian J, Liu X, Fan Y, Sun L, et al: Suppression of the PI3K-akt
pathway is involved in the decreased adhesion and migration of bone
marrow-derived mesenchymal stem cells from non-obese diabetic mice.
Cell Biol Int. 35:961–966. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu YX, Du J, Si MS, Mo JJ, Qiao SC and Lai
HC: The roles of PI3K/akt signalinging pathway in regulating
MC3T3-E1 preosteo-blast proliferation and differentiation on SLA
and SLActive titanium surfaces. J Biomed Mater Res A. 101:748–754.
2013. View Article : Google Scholar
|
|
21
|
Llambi F, Wang YM, Victor B, Yang M,
Schneider DM, Gingras S, Parsons MJ, Zheng JH, Brown SA, Pelletier
S, et al: BOK is a non-canonical BCL-2 family effector of apoptosis
regulated by ER-associated degradation. Cell. 165:421–433. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu Y, Wu G, Zhu G, Ma C and Zhao H:
Chronic sleep restriction induces changes in the mandibular
condylar cartilage of rats: Roles of akt, bad and caspase-3. BOK is
a non-canonical BCL-2 family effector of apoptosis regulated by
ER-associated degradation. Int J Clin Exp Med. 7:2585–2592.
2014.
|
|
23
|
Pan JM, Wu LG, Cai JW, Wu LT and Liang M:
Dexamethasone suppresses osteogenesis of osteoblast via the
PI3K/akt signalinging pathway in vitro and in vivo. J Recept Signal
Transduct Res. 39:80–86. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tao SC, Yuan T, Rui BY, Zhu ZZ, Guo SC and
Zhang CQ: Exosomes derived from human platelet-rich plasma prevent
apoptosis induced by glucocorticoid-associated endoplasmic
reticulum stress in rat osteonecrosis of the femoral head via the
akt/bad/bcl-2 signaling pathway. Theranostics. 7:733–750. 2017.
View Article : Google Scholar :
|
|
25
|
Schoentgen F and Jonic S: PEBP1/RKIP
behavior: A mirror of actin-membrane organization. Cell Mol Life
Sci. 77:859–874. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim W, Cho SB, Jung HY, Yoo DY, Oh JK,
Choi GM, Cho TG, Kim DW, Hwang IK, Choi SY and Moon SM:
Phosphatidylethanolamine-Binding protein 1 ameliorates
ischemia-induced inflammation and neuronal damage in the rabbit
spinal cord. Cells. 8:13702019. View Article : Google Scholar
|
|
27
|
Noh HS, Hah YS, Zada S, Ha JH, Sim G,
Hwang JS, Lai TH, Nguyen HQ, Park JY, Kim HJ, et al: PEBP1, a RAF
kinase inhibitory protein, negatively regulates starvation induced
autophagy by direct interaction with LC3. Autophagy. 12:2183–2196.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
StarBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar
|
|
29
|
Otsuru S, Hofmann TJ, Olson TS, Dominici M
and Horwitz EM: Improved isolation and expansion of bone marrow
mesenchymal stromal cells using a novel marrow filter device.
StarBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Cytotherapy.
15:146–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A,
Prockop DJ and Horwitz EM: Minimal criteria for defining
multipotent mesenchymal stromal cells. The international society
for cellular therapy position statement Cytotherapy. 8:315–317.
2006.
|
|
31
|
Fu L, Tang T, Miao Y, Zhang S, Qu Z and
Dai K: Stimulation of osteogenic differentiation and inhibition of
adipogenic differentiation in bone marrow stromal cells by
alendronate via ERK and JNK activation. Bone. 43:40–47. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Zhu W, Chen J, Cong X, Hu S and Chen X:
Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem
cells. Stem Cells. 24:416–425. 2006. View Article : Google Scholar
|
|
34
|
Wang XY, Fan XS, Cai L, Liu S, Cong XF and
Chen X: Lysophosphatidic acid rescues bone mesenchymal stem cells
from hydrogen peroxide-induced apoptosis. Apoptosis. 20:273–284.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schmid I, Krall WJ, Uittenbogaart CH,
Braun J and Giorgi JV: Dead cell discrimination with
7-amino-actinomycin D in combination with dual color
immunofluorescence in single laser flow cytometry. Cytometry.
13:204–208. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Uccelli A, Moretta L and Pistoia V:
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
8:726–736. 2008. View Article : Google Scholar
|
|
37
|
Yeung DK, Griffith JF, Antonio GE, Lee FK,
Woo J and Leung PC: Osteoporosis is associated with increased
marrow fat content and decreased marrow fat unsaturation: A proton
MR spectroscopy study. Mesenchymal stem cells in health and
disease. J Magn Reson Imaging. 22:279–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang H, Pang B, Li Y, Zhu D, Pang T and
Liu Y: Dexamethasone has variable effects on mesenchymal stromal
cells. Cytotherapy. 14:423–430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song X, Cao G, Jing L, Lin S, Wang X,
Zhang J, Wang M, Liu W and Lv C: Analysing the relationship between
lncRNA and protein-coding gene and the role of lncRNA as ceRNA in
pulmonary fibrosis. J Cell Mol Med. 18:991–1003. 2014. View Article : Google Scholar : PubMed/NCBI
|




















