Introduction
Nasal polyps (NPs) are characterized by the
inflammatory overgrowth of sinonasal tissue (1). Although NPs occur in various
diseases, such as malignancy and cystic fibrosis, they are more
likely to be associated with chronic rhinosinusitis (CRS), which is
also known as chronic rhinosinusitis with nasal polyps (CRSwNP)
(2). The prevalence of CRSwNP in
European populations is between 2.1% (France) (3) and 4.4% (Finland) (4) and is 4.2% in the United States
(5). The prevalence of diagnosis
based on endoscopic results in the general population was 2.5 or
2.6% in Korea (6) and 1.1% in
China (7).
The pathological mechanisms that result in chronic
nasal inflammation observed in CRSwNP are not completely
understood. Studies have focused on defining the roles of sinonasal
epithelial cells, the host immune system and pathogens in CRSwNP
pathogenesis (8-10). It was hypothesized that a damaged
epithelial barrier could contribute to enhanced exposure to
pathogens, antigens and particulates that, in the context of a
dysregulated host immune response, may promote chronic inflammation
(11). NP tissue is characterized
by the predominant infiltration of inflammatory cells, structural
fibrosis, stromal tissue edema and tissue remodeling (12). Much of the NP stroma is dense with
fibroblasts that produce numerous cytokines, such as transforming
growth factor, interleukin 6 and matrix metalloproteinase, thereby
causing the infiltration of inflammatory cells (13,14). Moreover, fibroblasts produce
extracellular matrix proteins such as collagen I and aggrecan that
play critical roles in tissue remodeling (15).
Currently, topical corticosteroids are regarded as
the most effective treatment for nasal polyposis (16); however, studies have shown that
nasal polyp-derived fibroblasts (NPDFs) are less sensitive to the
inhibitory effects of corticosteroids compared with other cell
types (17-19). Surgical excision of NPs in
patients with significant tissue remodeling may not be beneficial
as the NPs can either recur or the surgery can cause severe
complications such as adhesion and scar formation (20,21).
Recent studies showed that the treatment of NPs with
intralesional bleomycin A5 (BLE-A5) injections is safe and
effective, although its underlying mechanism remains unclear
(22-24). Studies on the mechanism of BLE in
the treatment of tumors have shown that BLE can induce cancer cell
apoptosis by increasing the levels of reactive oxygen species (ROS)
(25,26). In a previous study, we found that
BLE could effectively induce NPDF apoptosis via a
Bax/Bcl-2-mediated, mitochondria-dependent pathway (27). Moreover, we tested genotoxic and
cytotoxic effects in BLE-A5-treated NPDF in our previous
researches, including DNA smear testing, cell cytotoxicity assay
and cell immigration assay. We found that BLE-A5 can induce DNA
fragmentation, reduce cell viability and suppress proliferation in
NPDFs (24,27). We also found that NPDF is more
sensitive to BLE-A5 administration compared with normal nasal
mucosa derived fibroblasts (NMDF) (28). Hence, the present study focused on
the detailed mechanism underlying the pro-apoptotic effects of
BLE-A5 in NPDFs rather than compare the differences in sensitivity
to BLE-A5 treatment in NPDFs and NMDFs.
Mitochondria are highly dynamic organelles that
undergo continuous cycles of fusion and fission. Fission is
involved in the elimination of damaged mitochondria, which is also
known as mitophagy (29).
Dynamin-related protein 1 (Drp1) is a highly conserved gene that
plays a key role in mitochondrial fission. It was reported that
activation of Drp1 protein occurs by phosphorylation of the serine
616 residue [p-Drp1(S616)] and is mediated by the cyclin B1-CDK1
complex, which then causes mitochondrial fission (30-32). Some studies have shown that Drp1
is necessary for eliminating dysfunctional mitochondria via
mitophagy (33-35). Moreover, Drp1 is also associated
with the translocation of Bax to the mitochondrial outer membrane
(36). However, whether BLE can
affect Drp1 expression and function in NPDFs is still unclear.
Mitophagy is a catabolic process conserved from
yeast to mammals that provides a self-protective mechanism by which
cells endure stress. Under stress conditions, dysfunctional
mitochondria activate the serine/threonine-protein kinase PINK1
(PINK1)-Parkin-dependent ubiquitination response that involves the
remodeling and recycling of mitochondria by mitophagy (37). PINK1 is involved in the
degradation of dysfunctional mitochondria, accumulates in
depolarized mitochondria and recruits Parkin, which triggers
mitochondrial engulfment by the autophagosome (38).
The aim of the present study was to reveal the
underlying mechanisms of BLE treatment on NPs and to determine the
association between mitophagy and apoptosis in BLE-treated
NPDFs.
Materials and methods
Immunofluorescence analysis of NP
fibroblasts and epithelial cell colocalization with TUNEL
In the present study, 12 patients (6 females and 6
males; mean age, 42.3±8.5 years,) were recruited from the
Department of Otorhinolaryngology at Sun Yat-sen Memorial Hospital
(Guangzhou, China) between April 2017 to May 2018. All patients
were nonsmokers and had either not been treated with
glucocorticoids (systemic or topical), antihistamines,
non-steroidal anti-inflammatory drugs, or macrolide antibiotics for
at least 1 month or who had ceased treatment at least 1 month prior
due to lack of alleviation or even exacerbation of symptoms. All
participants provided written informed consent in advance, and NP
tissues were obtained during surgery. The study was approved by the
Ethics Committee of Sun Yat-sen Memorial Hospital (approval no.
SYSU81500773).
NP tissue preparation and treatment were performed
as described in our previous study (24). After BLE-A5 treatment, a
midsagittal section of formalin-fixed (4% at room temperature for 1
h), paraffin-embedded nasal polyp tissue was permeabilized with
0.1% Triton X-100. Subsequently, the slides were blocked with 10%
goat serum (Sigma-Aldrich; Merck KGaA) at room temperature for 1 h,
followed by incubation with primary antibodies against vimentin
(mouse anti-human; 1:200; cat. no. sc-32322; Santa Cruz
Biotechnology, Inc.) and pancytokeratin (CK-pan; rabbit anti-human;
cat. no. sc-15367, 1:200; Santa Cruz Biotechnology, Inc.) overnight
at 4°C. The next day, the slides were incubated with Alexa Fluor
594 anti-mouse (goat anti-mouse; 1:200; cat. no. ab150116; Abcam)
and Alexa Fluor 647 anti-rabbit secondary antibodies (goat
anti-rabbit; 1:200; cat. no. ab150079; Abcam) for 1 h at room
temperature and were washed three times with PBS for 5 min. TUNEL
staining was performed to label the 3′-end of the fragmented DNA in
apoptotic cells using a FITC-TUNEL cell apoptosis detection kit
(Beyotime Institute of Biotechnology). Slides were mounted on
SlowFade mounting media (cat. no. S36937; Thermo Fisher Scientific,
Inc.). Cells were observed under a fluorescence microscope at 200
magnification. Green cells were regarded as apoptotic cells, red as
fibroblasts and yellow as epithelial cells. Four slides were used
to calculate the percentage of apoptotic cells.
Fibroblast isolation and
identification
Fibroblasts were isolated from NPs and identified as
previously reported (27).
Briefly, NP tissues (~4×4 mm) were cultured in a 6-well dish in
DMEM/F-12 (Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 mg/ml streptomycin in a humidified atmosphere at 37°C with
5% CO2. Fibroblasts were isolated from the tissue via
adhesion and migration in a culture dish. When the fibroblast
cultures reached 60% confluence, the remaining tissue was
discarded, and adherent cells were digested (in 0.05% trypsin-0.02%
EDTA) and seeded in a 150 cm2 dish. Fibroblasts were
identified by immunofluorescent staining with CK-pan and vimentin
antibodies. Briefly, fibroblasts were cultured on 4-well culture
slides, washed with PBS and fixed in 4% paraformaldehyde at room
temperature for 20 min. Subsequently, the cells were permeabilized
with 0.1% Triton X-100 at room temperature for 15 min and blocked
in 5% non-fat milk-PBS at room temperature for 1 h, followed by
incubation with primary antibodies against vimentin (1:200; Santa
Cruz Biotechnology, Inc.) or CK-pan (1:200; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. The next day, the slides
were incubated with FITC-conjugated secondary antibodies (goat
anti-mouse; 1:200; cat. no. ab6785; Abcam and goat anti-rabbit;
1:200; cat. no. ab6717; Abcam) at room temperature for 30 min and
washed three times with PBS for 5 min. The cells were incubated
with DAPI (1:1,000) at room temperature for 5 min to label the
nuclei, and the stained slides were examined under a fluorescence
microscope (Olympus Corporation) at ×40 magnification.
Cell treatments
To examine the effect of BLE dose and exposure time
on the expression of mitochondrial apoptotic pathway-associated
proteins in NPDFs, cells were treated with various concentrations
of BLE-A5 (0, 50, 100, 200 or 400 µM; cat. no. 19692; Cayman
Chemical Company) for 48 h or with 200 µM BLE-A5 for various
durations (0, 6, 12, 24, 48 or 72 h). In a previous study, we found
that treatment of NPDFs with 200 µM BLE-A5 for 48 h could
effectively induce NPDF apoptosis, and hence this treatment
protocol (200 µM BLE-A5 for 48 h) was used for most of the
experiments in the present study. Aliquoted BLE was dissolved in
PBS at a concentration of 20 mM and stored at -30°C before use.
Validated small interfering RNA (siRNA) that specifically targeted
Drp1 (50 nM; target sequence, 5′-CAA GGA GCC AGT CAA ATT A-3′;
Shanghai GenePharma Co., Ltd.) was used to knock down Drp1. Cells
were transfected using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The control groups consisted of untransfected NPDFs
and NPDFs transfected with scrambled siRNA (50 nM; Si-Ctrl; 5′-UUC
UCC GAA CGU GUC ACG U-′; Shanghai GenePharma Co., Ltd.).
Henceforth, Si-Drp1 refers to siRNA targeting Drp1, Si-Ctrl refers
to nonspecific siRNA and untransfected cells are referred to as the
negative control (NC). Both si-Drp1 and si-Ctrl were transfected
for 72 h before subsequent treatment. Knockdown efficiency was
determined by PCR and western blotting. To show the effects of the
Cyclin B1-CDK1 complex on p-Drp1 levels, NPDFs were pretreated with
the CDK1 inhibitor RO-3306 (20 µM; cat. no. 872573-93-8;
Sigma-Aldrich; Merck KGaA) for 24 h before p-Drp1(S616) (1:1,000;
cat. no. 3455; Cell Signaling Technology, Inc.) levels were
analyzed by western blotting. RO-3306 was diluted in DMSO; thus,
the control group was treated with an equal dose of DMSO
(vehicle).
Reverse transcription-PCR (RT-PCR)
Untransfected (NC), Si-Ctrl-transfected, or
Si-Drp1-transfected NPDFs were homogenized in 1 ml
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was extracted, and 2 µg of RNA/sample was
reverse transcribed into cDNA using a reverse transcription kit
according to the manufacturer's protocol (cat. no. FSQ-101; Toyobo
Life Science). Subsequently, using the Roche PCR system (Roche
Diagnostics), PCR was performed on a reaction mixture containing 2
µl cDNA, 0.2 µl of each primer (forward and reverse),
5 µl 2X PCR master mix reaction buffer (cat. no. F1696K;
Toyobo Life Science) and deionized water to a final volume of 10
µl. The following thermocycling conditions were used for the
PCR: Initial denaturation at 90°C for 30 sec, followed by 40 cycles
of 94°C for 30 sec, 60°C for 30 sec and 72°C for 1 min. Equal
volumes of PCR products were evaluated by 5% agarose gel
electrophoresis and visualized using the Bio-Rad Gel Doc XR
documentation system (Bio-Rad Laboratories, Inc.). DNA bands were
measured using ImageJ (version 1.6; National Institutes of Health)
The primer sequences used for PCR are listed as follows: Drp1
forward, 5′-ATA TGC CGA GTT CCT GCA CTG -3′ and reverse, 5′-AGT AGA
CGC GGA GGT TGA TAG- 3′ (39) and
GAPDH forward, 5′-CAG TGC CAG CCT CTG CTC AT-3′ and reverse, 5′-ATA
CTC AGC ACC AGC AC AT-3′ (40).
Flow cytometry with Annexin V and
propidium iodide (PI)
An Annexin V/PI apoptosis kit (Thermo Fisher
Scientific, Inc.) was used to assess BLE-induced NPDF apoptosis
according to the manufacturer's instructions. Briefly, Si-Ctrl- or
Si-Drp1-transfected NPDFs were incubated in 6-well plates at a
density of 5×106 cells/well with DMEM/F12 supplemented
with 10% FBS and various concentrations of BLE (0, 50, or 200
µM) for 24 or 48 h. The cultured fibroblasts were gently
suspended in binding buffer and incubated in the dark at room
temperature for 15 min with 5 µl Annexin V-FITC and 10
µl PI. Annexin V-FITC- and PI-labeled cells were analyzed
using a flow cytometer (BD Biosciences). Dot plots (with PI on the
y-axis and Annexin V-FITC on the x-axis) were used to identify
viable cells, which appear negative for PI and Annexin V-FITC
staining. Cells in the early stages of apoptosis appear as Annexin
V-positive and PI-negative, while cells in late apoptosis or full
necrosis show both Annexin V-FITC-positive and PI-positive
staining.
Mitochondrial and cytosolic
fractionation
Cells were lysed with Buffer A [0.25 M sucrose, 10
mM Tris-HCl (pH 7.5), 10 mM KCl, 1.5 mM MgCl2, 1 mM
EDTA, 1 mM dithiothreitol, and 0.1 mM PMSF] with a homogenizer. The
homogenate was centrifuged at 750 × g for 10 min at 4°C, and the
supernatant was collected and centrifuged at 10,000 × g for 30 min
at 4°C. After the second centrifugation, the supernatant was
collected as the cytosolic fraction, and the pellet was collected
as the mitochondrial fraction. The pellets were resuspended in
Buffer B [0.25 M sucrose, 10 mM Tris-HCl (pH 7.5), 10 mM KCl, 1.5
mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, 0.1 mM PMSF
and 1% NP40].
Western blotting
Cells from different groups were homogenized in RIPA
buffer (Sigma Aldrich; Merck KGaA) containing 1 mM PMSF, protease
inhibitor cocktail and phosphatase inhibitor cocktail (Roche
Diagnostics) on ice for 30 min. Protein concentration was
determined using a BCA protein assay kit (Beyotime Institute of
Biotechnology). Equal amounts of protein (10 µg/lane) were
separated using 10% SDS-PAGE and transferred onto PVDF membranes
(EMD Millipore). The membranes were blocked in 5% non-fat milk in
TBS for 1 h at room temperature. Subsequently, the membranes were
incubated overnight at 4°C with primary antibodies against Drp1
(1:500), p-Drp1 (S616) (1:500; cat. no. 3455), Bax (1:1,000; cat.
no. 5023), Bcl-2 (1:1,000; cat. no. 15071), cleaved caspase-9
(1:1,000; cat. no. 20750), PINK1 (1:1,000; cat no. 6946), Parkin
(1:1,000; cat. no. 4211), LC3B (1:1,000; cat. no. 3868),
cyclooxygenase (COX) IV (1:1,000; cat. no. 4850), α-tubulin
(1:1,000; cat. no. 2125), β-actin (1:3,000; cat. no. 4970) or GAPDH
(1:10,000; cat. no. 5174). After washing TBS with 5% Tween-20, the
membranes were incubated with horseradish peroxidase-conjugated
anti-rabbit (1:2,000; cat. no. 7074) or anti-mouse (1:2,000; cat.
no. 7076) secondary antibodies for 1 h at room temperature and then
visualized using an enhanced chemiluminescence kit (EMD Millipore).
All aforementioned antibodies were purchased from Cell Signaling
Technology, Inc. Band images were captured using the Bio-Rad Gel
Doc XR documentation system (Bio-Rad Laboratories, Inc.). Relative
protein expression levels were determined by ImageJ (version 1.6;
National Institutes of Health) and standardized to GAPDH or
β-actin. The protein levels of α-tubulin were used to confirm equal
loading of cytosolic proteins, and the protein levels of COX IV
were used to confirm equal loading of mitochondrial proteins.
Mitochondrial morphology analysis
To examine the effects of Drp1 on the mitochondrial
morphology of BLE-treated NPDFs, Si-Ctrl- or Si-Drp1-transfected
cells were cultured in 24-well culture plates at a density of
1×105 cells/well and treated with 200 µM BLE-A5
for 48 h. Subsequently, the cells were incubated with 500 nM
MitoTracker Red (MitoRed; Thermo Fisher Scientific, Inc.)
mitochondrial dye in a humidity-controlled environmental chamber at
37°C with 5% CO2 for 30 min. After fixation in 4%
paraformaldehyde at room temperature for 20 min, cells were
permeabilized with 0.1% Triton X-100 at room temperature for 15 min
and blocked in 5% non-fat milk-PBS at room temperature for 1 h. The
cells were then incubated with primary antibodies against p-Drp1
(1:200) or LC3B (1:200) overnight at 4°C. The next day, the slides
were incubated with FITC-conjugated secondary antibodies (1:200;
cat. no. ab6717; Abcam) for 30 min and washed three times with PBS
for 5 min. The cells were incubated with DAPI (1:1,000) at room
temperature for 5 min to label the nuclei. The stained slides were
examined under a fluorescence microscope (Olympus Corporation) at
×40 magnification.
Superoxide analysis
Cells were stained with MitoSOX Red (Thermo Fisher
Scientific, Inc.), and ROS production was measured by flow
cytometry. Si-Ctrl- or Si-Drp1-transfected NPDFs were treated with
or without 200 µM BLE-A5 for 48 h and then incubated with 5
µM MitoSOX Red for 30 min. The cells were collected and
washed with PBS, and fluorescence was analyzed using FACSAria III
(BD Biosciences).
Mitochondrial DNA (mtDNA) analysis
After BLE-A5 treatment, total cellular DNA of
Si-Ctrl- or Si-Drp1-transfected NPDFs was extracted using
DNAzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The relative mtDNA copy number was defined as the ratio of
mtDNA [represented by the NADH-ubiquinone oxidoreductase chain 1
(ND1) gene] to nuclear DNA (nDNA, represented by the β-actin gene),
as assessed by quantitative PCR (qPCR). SYBR green was used as a
fluorophore (Toyobo Life Science). The following thermocycling
conditions were used for the qPCR: Initial denaturation at 95°C for
5 min; followed by 45 cycles of 95°C for 10 sec, 60°C for 10 sec
and 72°C for 20 sec. Finally, a melting curve was plotted to
confirm the presence of a single PCR product at the following
conditions: 95°C for 5 sec, 66°C for 1 min and gradual increase in
temperature up to 97°C for fluorescence acquisition. Analysis of
the mtDNA/nDNA ratio was calculated using the 2-ΔΔCq
method (41). The following
primer pairs were used for the qPCR: ND1 forward, 5′-CCC TAA AAC
CCG CCA CAT CT-3′ and reverse, 5′-GAG CGA TGG TGA GAG CTA AGG T-3′
and β-actin forward, 5′-ATG AAG ATC AAG ATC ATT GCT CCT C-3′ and
reverse, 5′-ACA TCT GCT GGA AGG TGG ACA -3′. Two independent PCRs
were performed for mitochondrial and nuclear DNA in each
sample.
Mitochondrial membrane potential
analysis
The mitochondrial membrane potential was analyzed
using the radiometric cationic fluorescent dye JC-1. The cells were
stained with 10 µM JC-1 at 37°C for 10 min. The change in
fluorescence intensity of the dye was measured in the red and green
channels by flow cytometry and normalized to that of the control
group.
ATP analysis
ATP was measured by a bioluminescence assay using an
ATP determination kit (Molecular Probes; Thermo Fisher Scientific,
Inc.). Briefly, Si-Ctrl- or Si-Drp1-transfected NPDFs were treated
with 200 µM BLE-A5 for 48 h and resuspended in reaction
buffer containing 1 mM dithiothreitol, 0.5 mM D-luciferin and 12.5
µg/ml firefly luciferase. After 15 min of incubation,
cellular ATP was measured using a microplate luminometer (Molecular
Devices, LLC).
Co-immunoprecipitation
In brief, NPDFs were treated without or with 200
µM BLE-A5 for 48 h, collected, washed with ice-cold PBS, and
lysed with lysis buffer containing 50 mmol/l Tris (pH 8.0), 150
mmol/l NaCl and 1% NP40. Lysates containing 300 µg protein
was incubated overnight at 4°C with 10 µg anti-cyclin B1
antibody (cat. no. ab32053; Abcam). Protein A/G PLUS-agarose (50
µl; Santa Cruz Biotechnology, Inc.) was subsequently added
to each sample, and the incubation was continued for an additional
3 h at 4°C with gentle shaking. The immunoprecipitates were
subjected to SDS-PAGE followed by immunoblotting using anti-CDK1
(1:1,000) or anti-cyclin B1 (1:1,000; Santa Cruz Biotechnology,
Inc.) antibodies. Electrophoresis, western blotting and imaging
procedures were performed as described in under the Western
blotting subsection.
Statistical analysis
All experiments were repeated three times or more to
provide sufficient data for statistical analysis. The differences
were analyzed with Student's t-test or one-way ANOVA followed by
Dunnett's test or Sidak's multiple comparisons test. Statistical
tests were conducted using GraphPad Prism version 5 (GraphPad
Software, Inc.). Statistical significance was determined at the 95%
confidence level. P<0.05 was considered to indicate a
statistically significant difference.
Results
BLE-A5 mainly induces fibroblast
apoptosis in nasal polyps
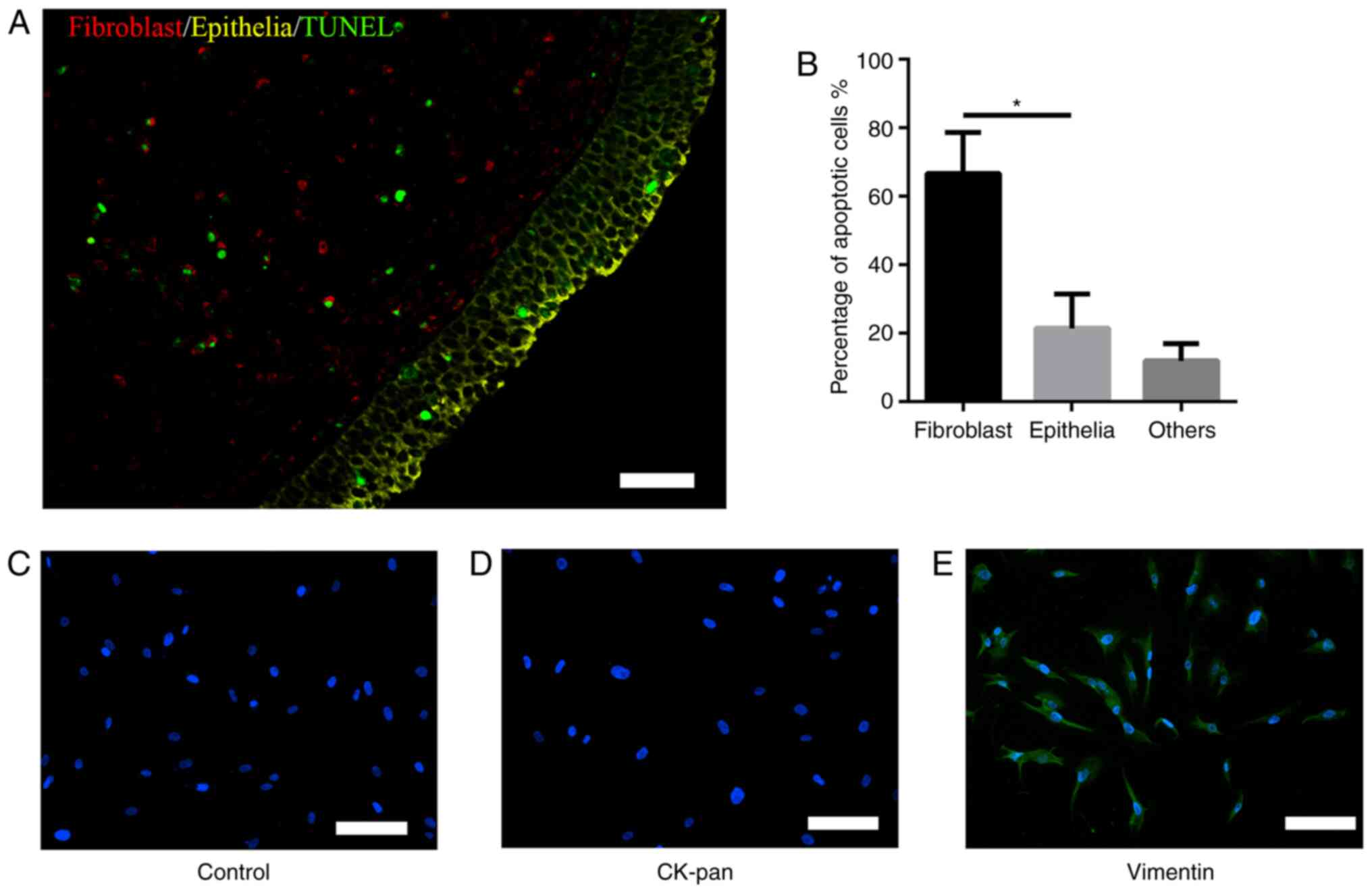
As shown in Fig. 1A
and B, immunofluorescence analysis of TUNEL co-localization
showed that BLE-A5 induced apoptosis (green) mainly in fibroblasts
(red, 66±12%) but not epithelial cells (yellow, 21±10%). Based on
these results, the present study focused on the mechanism by which
BLE-A5 triggered NPDF apoptosis in subsequent experiments.
Identification of NPDFs
Isolated fibroblasts showed vimentin-positive and
CK-pan-negative immunofluorescence staining, with a spindle- and
nest-like distribution. These features were consistent with
fibroblast characteristics (Fig.
1C-E), which showed the positive identification of NPDFs.
BLE-A5 treatment induces apoptosis in
human NPDFs and alters Drp1 expression
In a previous study (25), we found that BLE-A5 could induce
NPDF apoptosis in a time- and dose-dependent manner. The present
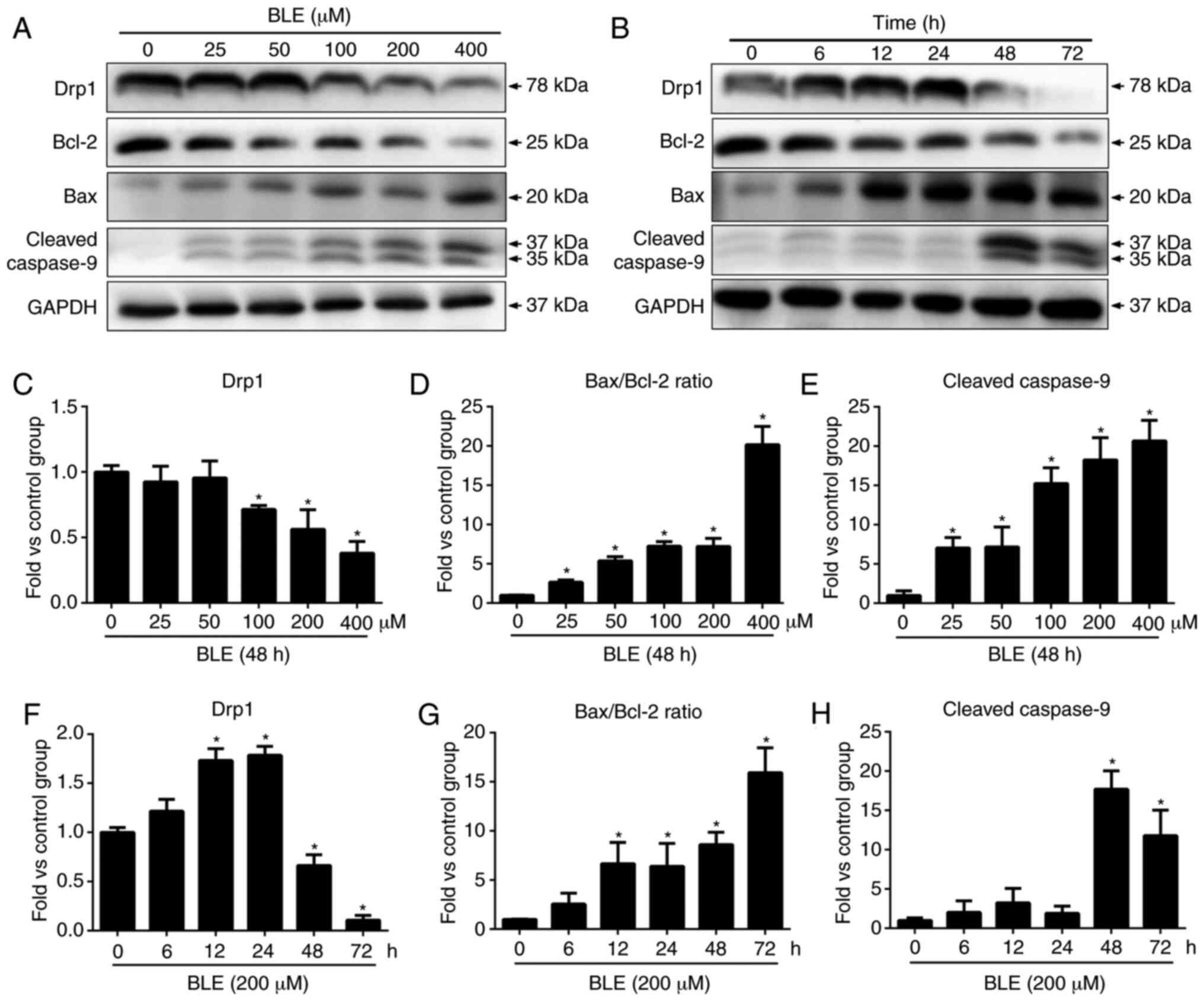
study confirmed the pro-apoptotic effects of BLE-A5 by western blot
analysis of Bax, Bcl-2 and cleaved caspase-9. BLE-A5 increased the
levels of cleaved caspase-9 and decreased the levels of Bcl-2
(Fig. 2), suggesting the possible
involvement of a mitochondria-mediated apoptosis pathway. Even a
low dose of BLE-A5 (25 µM) altered the Bax/Bcl-2 ratio and
the expression of cleaved caspase-9 (Fig. 1D and F). Compared to the control
group, the ratio of Bax/Bcl-2 and cleaved caspase-9 expression
increased significantly after 12 and 48 h BLE-A5 treatment,
respectively. These results are consistent with our previous
findings that exposure to 200 µM BLE-A5 for 48 h can
effectively induce apoptosis in NPDFs.
To examine the effects of BLE-A5 on mitochondrial
fusion, the protein levels of Drp1 in NPDFs were measured by
western blotting. As shown in Fig.
2A, BLE-A5 treatment for 48 h showed a dose-dependent decrease
in Drp1 protein in NPDFs. When testing the effect of the duration
of BLE exposure on NPDFs, it was found that Drp1 expression
increased at the 12-24 h time points and then sharply decreased
after 48 h.
Drp1 knockdown increases the sensitivity
of NPDFs to BLE
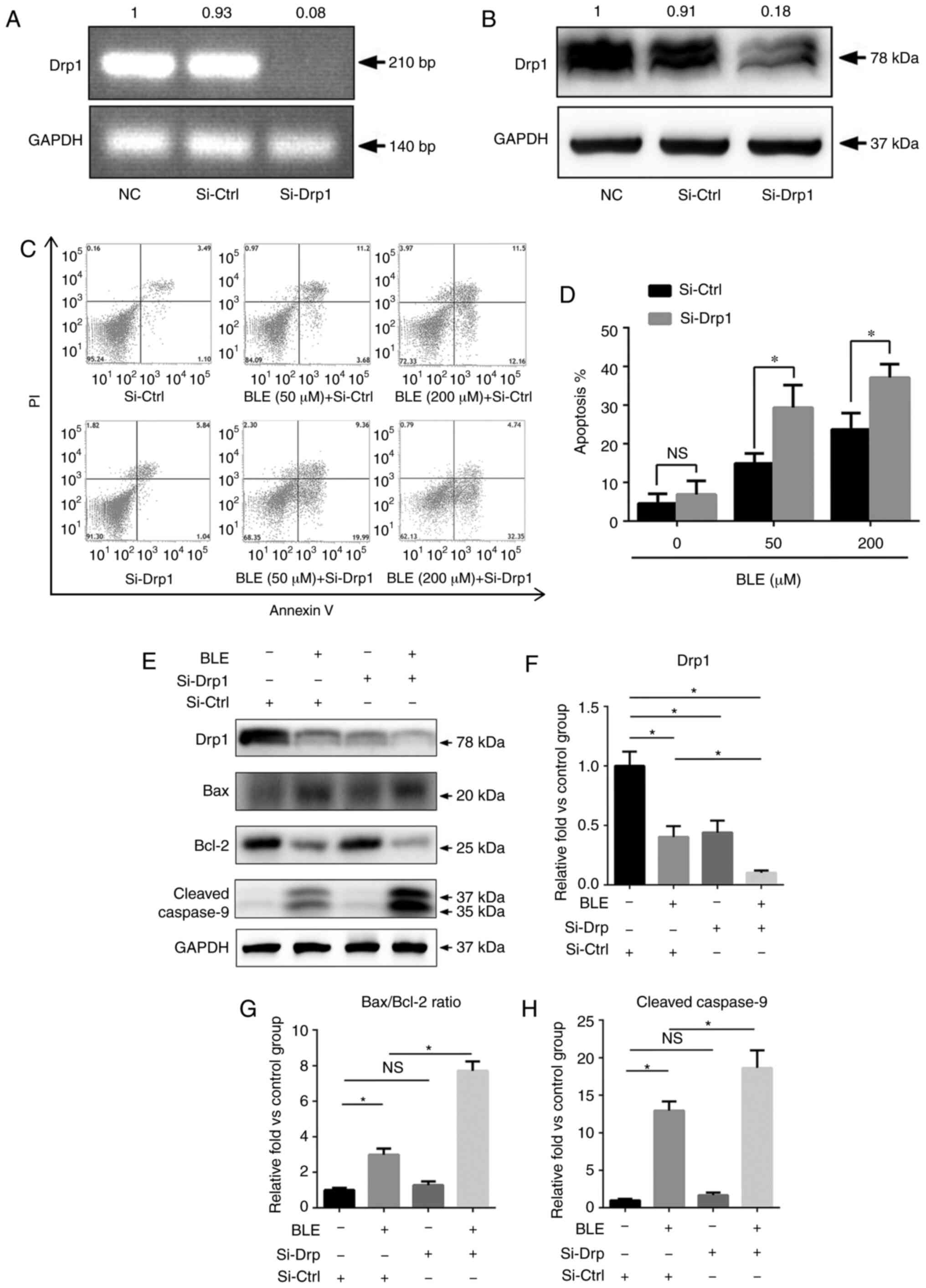
To study the functional role of Drp1 in BLE-treated
NPDFs, NPDFs were transiently transfected with siRNA to
down-regulate the expression of Drp1 (Si-Drp1) or with nonspecific
siRNA (Si-Ctrl) as a control. Untransfected NPDFs were also used as
NCs. Transfection of cells with Si-Drp1 significantly decreased
Drp1 mRNA (Fig. 3A) and protein
expression (Fig. 3B).
Following siRNA transfection, NPDFs were exposed to
50 or 200 µM BLE-A5 for 48 h. The levels of apoptosis were
then assessed using an Annexin V/PI apoptosis kit (Fig. 3C). The results showed that the
percentage of apoptotic NPDFs was significantly increased after
Drp1 knockdown and exposure to both 50 and 200 µM BLE-A5
(Fig. 3D).
Next, the present study analyzed proteins associated
with the mitochondria-mediated apoptotic pathway by western
blotting (Fig. 3E). As shown in
Fig. 3F, the expression levels of
Drp1 in NPDFs after siRNA transfection was similar to that of NPDFs
treated with 200 µM BLE for 48 h. Additionally,
Si-Drp1-transfected NPDFs that were treated with the same dose of
BLE showed a significant decrease in Drp1 expression (Fig. 3F). The Bax/Bcl-2 ratio and cleaved
caspase-9 expression were increased by BLE-A5 treatment but did not
change in the Si-Drp1-transfected group compared to the control
group. Furthermore, Si-Drp1-transfected NPDFs exposed to BLE-A5
exhibited significantly increased Bax/Bcl-2 ratios and cleaved
caspase-9 expression compared with the Si-Ctrl group treated with
the same dose of BLE (Fig. 3G and
H).
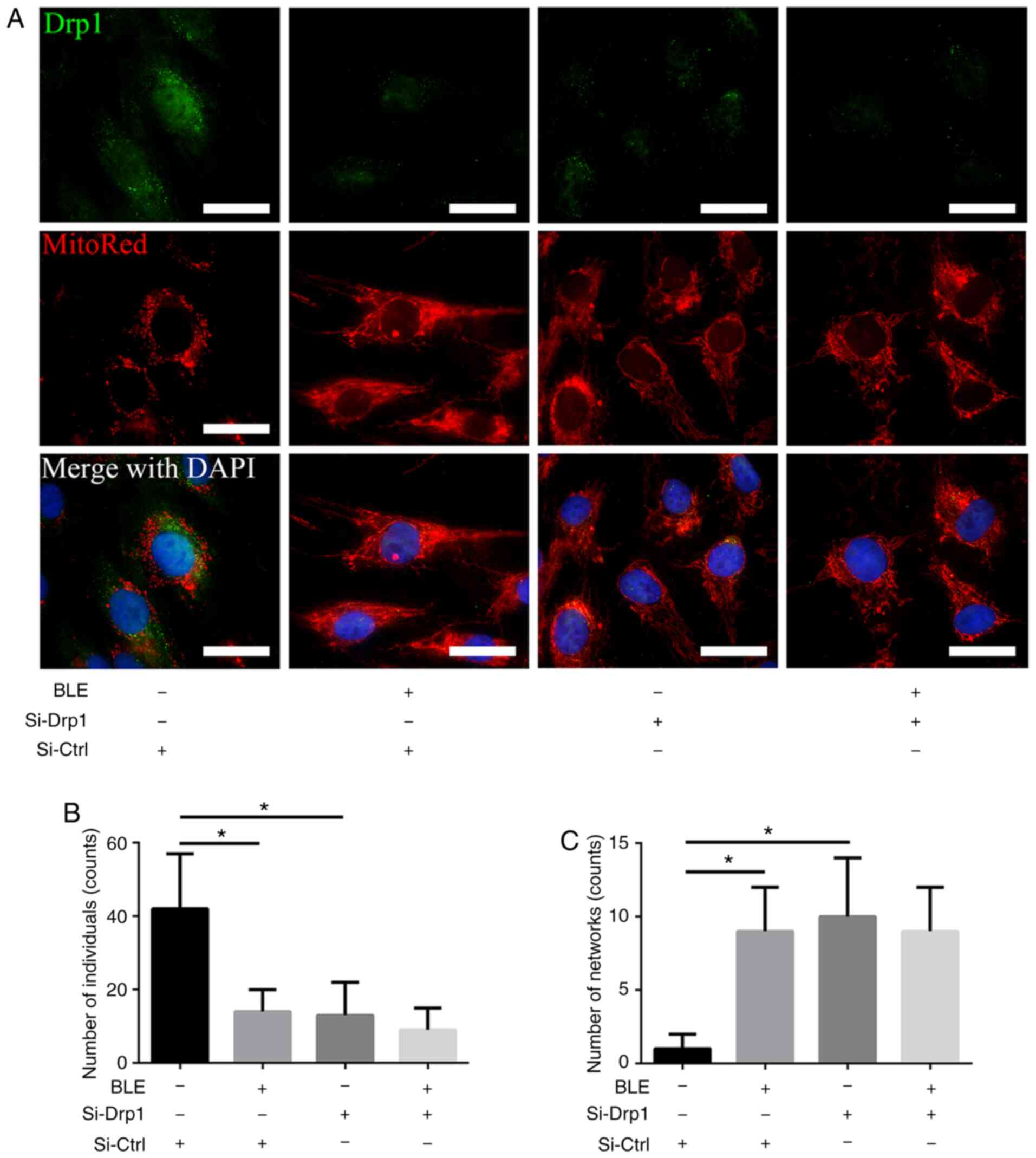
BLE-A5 inhibits Drp1-mediated
mitochondrial fission in NPDFs
Fluorescence microscopy was performed to directly
visualize the changes in mitochondrial morphology in NPDFs. MitoRed
was used to identify mitochondria, and a Drp1 primary antibody
linked to a FITC-tagged secondary antibody was used to detect Drp1.
As shown in Fig. 4, the Si-Ctrl
group showed robust green fluorescence in the cytoplasm, which
co-localized with MitoRed fluorescence in the form of short tubules
or round fragments. By contrast, Si-Drp1 transfection markedly
decreased the green (Drp1) fluorescence, and the mitochondrial
morphology was altered to long, interconnected tubular networks.
Similar phenotypes were observed in the Si-Ctrl group treated with
BLE-A5. However, when Si-Drp1-transfected NPDFs were treated with
BLE-A5, Drp1 fluorescence was extremely weak and the mitochondria
appeared as long tubular networks. These results indicated that BLE
treatment changed the mitochondrial morphology by decreasing Drp1
expression.
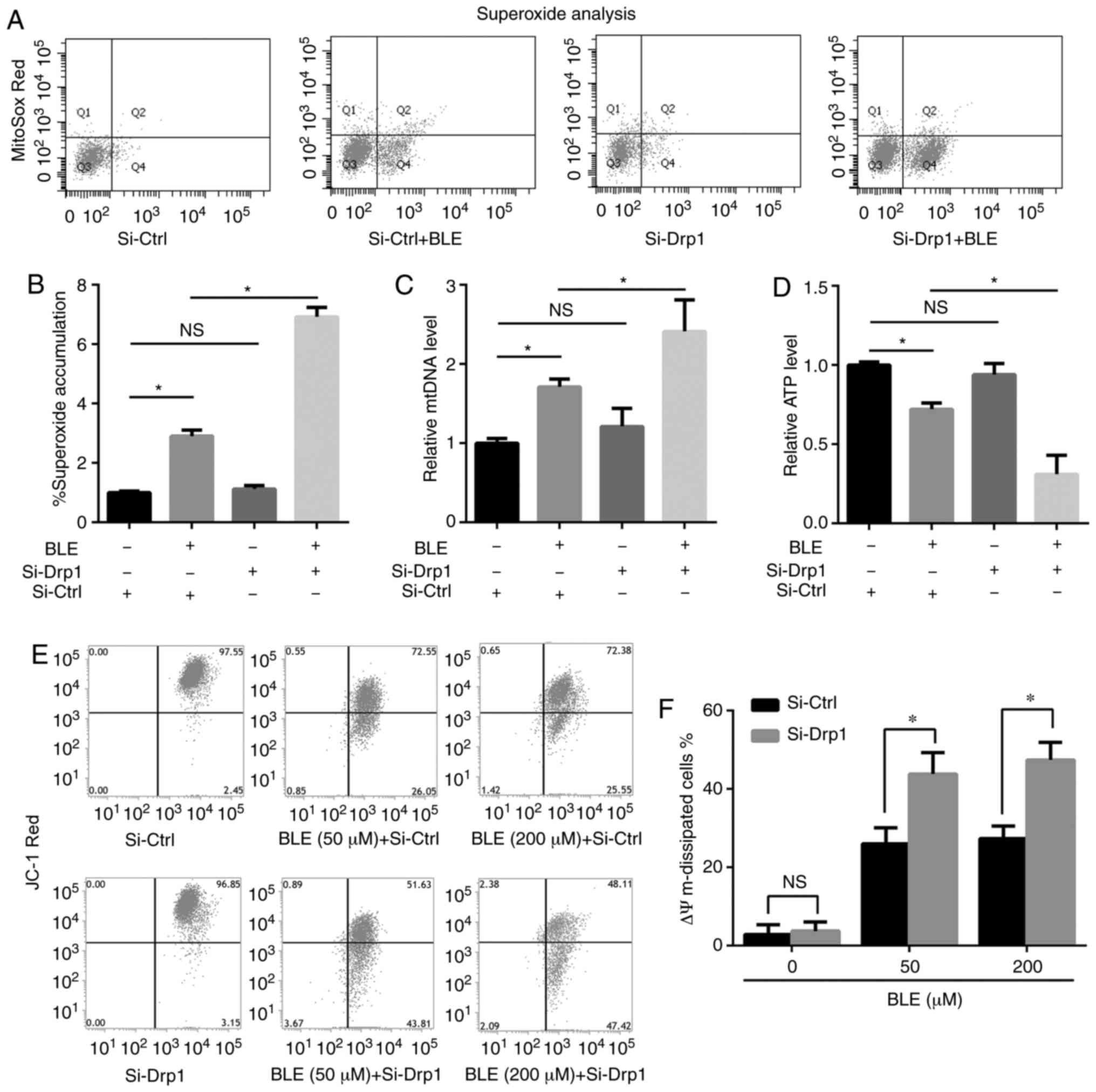
Drp1 knockdown aggravates BLE-induced
mitochondrial dysfunction in NPDFs
To determine the effects of Drp1 on BLE-induced
mitochondrial dysfunction, the present study measured exogenous ROS
levels, mtDNA levels, ATP levels and mitochondrial membrane
potential (Δψm) in Si-Ctrl- or Si-Drp1-transfected NPDFs treated
with or without BLE.
As shown in Fig. 5A
and B, the levels of ROS in Si-Ctrl- and Si-Drp1-transfected
NPDFs were enhanced by BLE-A5 treatment, but Drp1 knockdown alone
did not change ROS levels compared with controls.
Si-Drp1-transfected NPDFs showed higher levels of superoxide
accumulation compared with Si-Ctrl-transfected cells when exposed
to the same dose of BLE-A5 (200 µM for 48 h). Similar
results were observed for mtDNA levels; mtDNA was increased by
BLE-A5 exposure in Si-Ctrl- and Si-Drp1-transfected NPDFs but
showed no significant change in response to Drp1 knockdown alone.
Si-Drp1-transfected NPDFs were more likely to undergo mtDNA
duplication compared with the Si-Ctrl group when treated with
BLE-A5 (Fig. 5C). These changes
were consistent with the decrease in mitochondrial ATP production
(Fig. 5D). Si-Drp1 NPDFs
generated less ATP compared with the Si-Ctrl group when exposed to
BLE-A5, indicating increased impairments in the oxidative
phosphorylation ability of mitochondria. When JC-1 was used to
measure the Δψm levels (Fig. 5E and
F), it was found that the mitochondrial potential of
Si-Drp1-transfected NPDFs was similar to Si-Ctrl-transfected cells
but decreased below that of the Si-Ctrl group in the presence of 50
or 200 µM BLE-A5. Taken together, these results suggested
that Drp1 knockdown can aggravate BLE-induced mitochondrial
dysfunction in NPDFs.
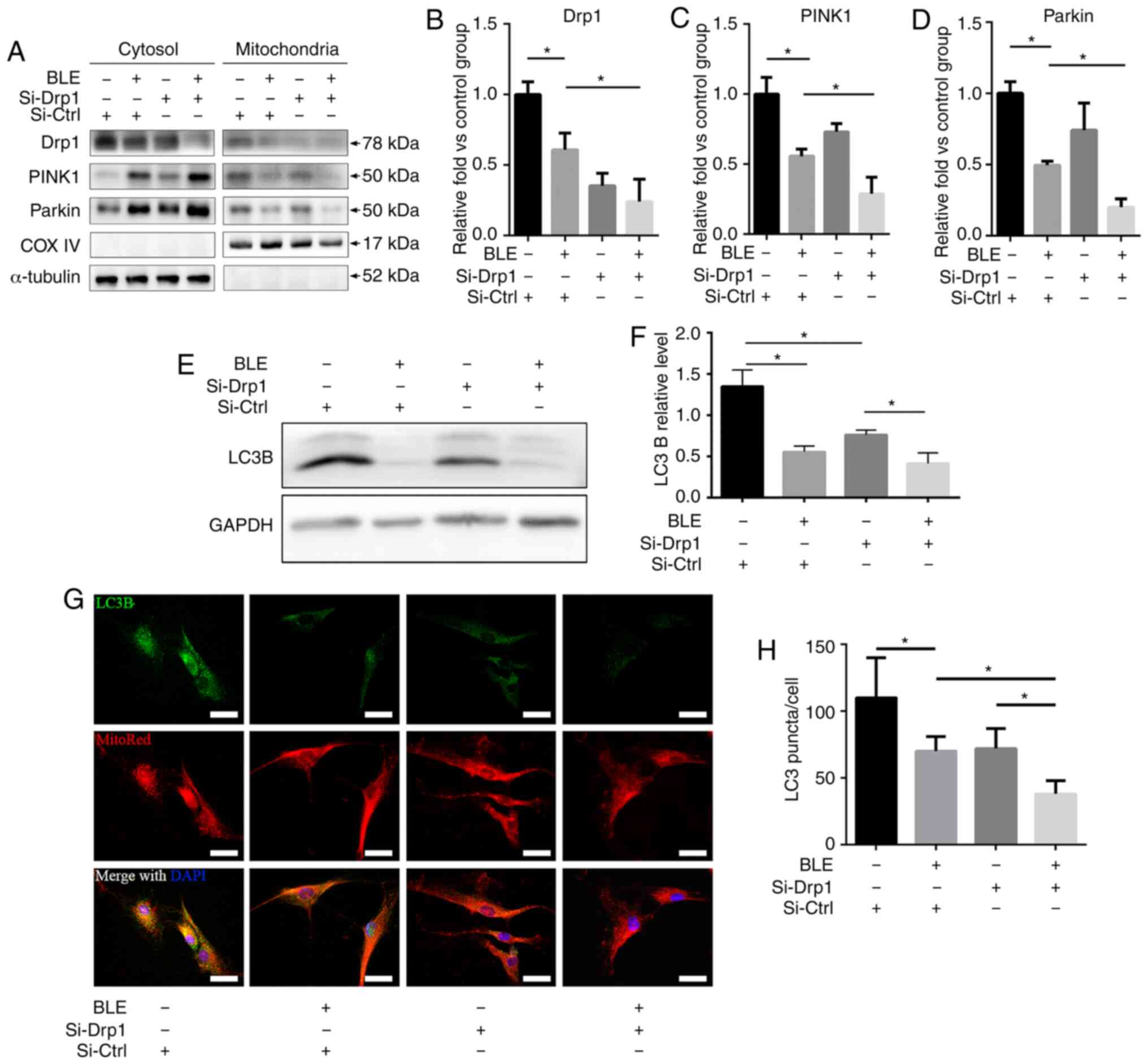
BLE-A5 treatment inhibits Drp1-mediated
mitophagy in NPDFs
The localization of Drp1 and mitophagy-related
proteins in mitochondrial and cytosolic fractions of NPDFs were
assessed following BLE-A5 treatment (200 µM for 48 h)
(Fig. 6A). There was a decrease
in Drp1 expression in both the mitochondrial and cytosolic
fractions of NPDFs treated with BLE-A5, regardless of siRNA
transfection (Fig. 6B). Moreover,
PINK1 and Parkin, two proteins that are essential for the
ubiquitination of dysfunctional mitochondria and subsequent
activation of mitophagy, were significantly decreased in the
mitochondrial fraction of Si-Drp1-transfected NPDFs compared to
Si-Ctrl NPDFs in the presence of BLE-A5 (Fig. 6C and D). These results indicated
that BLE can inhibit mitophagy in NPDFs, and Drp1 knockdown can
increase BLE-mediated mitophagy blockade in NPDFs.
Autophagy was also assessed by measuring LC3B
expression by western blotting and immunofluorescence
co-localization with MitoRed (Fig.
6E-H). The results showed that both BLE treatment and Drp1
knockdown decreased LC3B expression, which further confirmed that
mitophagy is inhibited by BLE treatment and Drp1 plays a role in
BLE-mediated mitophagy inhibition in NPDFs.
BLE-A5 decreases cyclin B1-CDK1
complex-mediated phosphorylation of Drp1 in NPDFs
Western blotting showed that BLE-A5 significantly
decreased the phosphorylation of Drp1 at serine 616 (Fig. 7A and B), which is required for
Drp1 translocation into the mitochondrial membrane.
Si-Drp1-transfected NPDFs also showed decreases in p-Drp1(S616)
levels, which decreased further when the cells were treated with
BLE-A5 (200 µM for 48 h). Quantification of the p-Drp1/Drp1
ratio confirmed a significant decrease in either siDrp1 or BLE-A5
treated NPDFs.
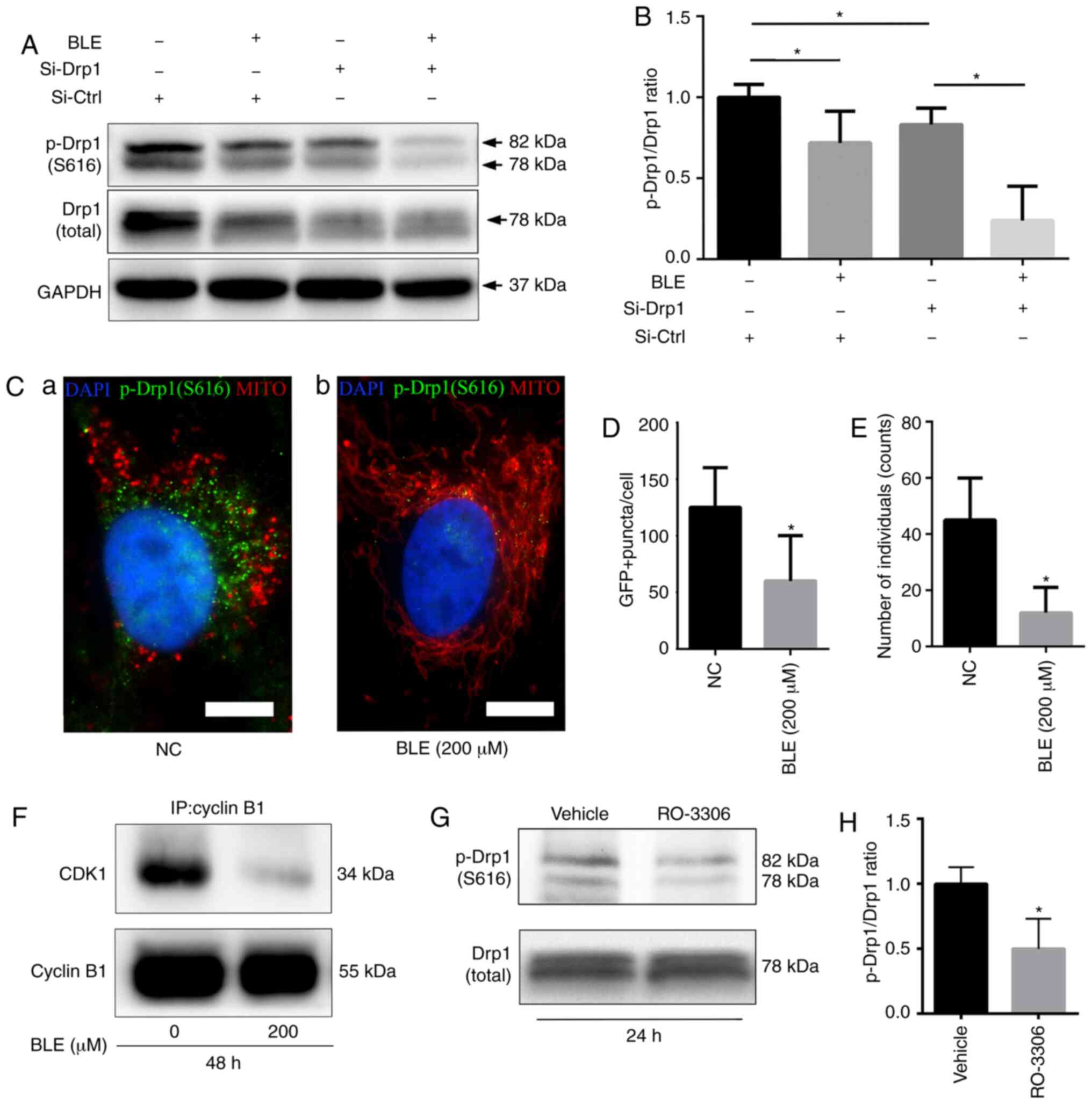 | Figure 7BLE treatment decreases S616
phosphorylation of Drp1 in NPDFs. (A) Cell lysates from NPDFs under
different conditions were immunoblotted for p-Drp1 (S616). (B)
Quantification of p-Drp1 levels shown in (A). (C)
Immunofluorescence analysis of p-Drp1(S616) co-staining with
MitoRed. (Ca) Untreated NPDFs. (Cb) NPDFs treated with 200
µM BLE-A5. DAPI is shown in blue, p-Drp1(S616) is shown in
green and mitochondria are shown in red. Scale bar, 5 µm.
Quantification of (D) GFP-positive puncta and (E) the number of
individual mitochondria. (F) The effects of BLE treatment on the
expression of the cyclin B1-CDK1 complex in NPDFs was determined by
co-immunoprecipitation with anti-cyclin-B1, followed by
immunoblotting for CDK1 and cyclin B1. (G) Treatment with the CDK1
inhibitor RO-3306 decreased the levels of p-Drp1 in NPDFs as shown
by western blotting. (H) Quantification of p-Drp1 levels shown in
(G). Values are presented as the mean ± standard deviation. n=3.
*P<0.05. Data were analyzed by one-way ANOVA with
Sidak's multiple comparisons test (B) or Student's t-test (D, E and
H). MITO, mitochondria; IP, immunoprecipitation; NPDF, nasal
polyp-derived fibroblast; BLE-A5, bleomycin A5; NC, negative
control; GFP, green fluorescent protein; p, phosphorylated; Drp1,
dynamin-related protein 1; Si, small interfering RNA; Ctrl,
control. |
To confirm the association between the decreased
p-Drp1(S616) levels and the changes in mitochondrial morphology, a
FITC-conjugated secondary antibody (green fluorescence) was used to
examine p-Drp1(S616) localization in NPDFs co-stained with MitoRed.
Fluorescence microscopy showed that when NPDFs were treated with
BLE-A5, the decrease in green fluorescence was consistent with
mitochondrial fusion (indicated by long tubular networks) (Fig. 7C-E).
The cyclin B1-CDK1 complex is essential for the
phosphorylation of Drp1 [p-Drp1(S616)] during mitochondrial fission
(42). Co-immunoprecipitation was
performed to examine the effects of BLE-A5 treatment on cyclin
B1-CDK1 complex formation in NPDFs. It was found that BLE-A5
reduced the levels of cyclin B1-CDK1 complex formation (Fig. 7F), thereby suppressing Drp1
phosphorylation and subsequent mitochondrial translocation. RO-3306
is a well-known CDK1 inhibitor. When treated with RO-3306, NPDFs
showed a significant decrease in p-Drp1 expression (Fig. 7G), but no obvious change in total
Drp1 levels. Thus, a significant decrease in the p-Drp1/Drp1 ratio
was observed in RO-3306-treated cells (Fig. 7H).
Discussion
As a traditional antitumor drug, the clinical
application of BLE-A5 has been limited by severe complications,
such as lung fibrosis and scleroderma (43). However, intralesional injection of
BLE-A5 into keloids, hypertrophic scars (44) and maxillofacial hemangiomas
(45) have proven to be effective
and safe treatment alternatives. In addition, BLE-A5 injections
have already been used to treat NPs in China, especially in
difficult-to-treat, glucocorticoid-insensitive and recurrent cases
(22,23). One important stage in the
pathology of NPs is irreversible tissue remodeling, which is mainly
driven by fibroblasts (NPDFs) that respond to inflammation and
produce extracellular matrix proteins (46). The present study first performed
immunofluorescence TUNEL co-localization analysis. The results
showed that BLE-A5-induced apoptosis mainly occurred in
fibroblasts. This result was consistent with the clinical effects
showing that BLE-A5 injection can effectively reduce polyp volume
(22). It is well documented that
nasal polyps are reconstructed tissues which are initiated by the
immune response, including inflammatory cell infiltration and
inflammatory factor secretion. However, the present study mainly
focused on the pro-apoptotic effects of BLE-A5 on NPDFs. Further
studies still need to be performed to assess the effects of BLA-A5
in nasal polyp inflammation response.
The present study revealed that BLE-A5 could
decrease the expression of Drp1 and induce mitochondrial
pathway-mediated apoptosis in NPDFs in a time- and dose-dependent
manner. BLE-A5-treated NPDFs exhibited an early phase increase in
Drp1 expression at the 12-24 h time points, which then sharply
decreased after 48 h, and the increase in cleaved caspase-9 levels
occurred at 48-72 h. These results match our previous findings
showing that 200 µM BLE-A5 induced NPDF apoptosis mainly
after 48 h (27). In the early
phase (between 12-24 h), BLE-A5-treated NPDFs did not undergo
obvious apoptosis; however, cellular ROS were increased (28). Therefore, the increase in Drp1
expression may be due to the ROS stress response. It was reported
that ROS generation can effectively increase Drp1 expression
(47). However, ROS accumulation
in BLE-A5-treated NPDFs only occurs in the early phase (28). After 24 h, ROS levels decreased to
normal levels, and so Drp1 decreased accordingly. When the cells
were treated for 48 h, the suppressive effects of BLE-A5 on Drp1
expression dominated, and hence a sharp decrease in Drp1 expression
was observed. Additionally, Si-Drp1-transfected NPDFs were more
sensitive to BLE-induced apoptosis compared with control cells.
Theoretically, Drp1 overexpression should be able to abolish the
pro-apoptotic effects of BLE-A5 in NPDFs. However, the present
study attempted to overexpress Drp1 in NPDFs using viral vectors in
preliminary experiments. However, the results showed that Drp1
overexpression in NPDFs cannot increase p-Drp1 accordingly and did
not show protective effects against BLE-A5 administration. p-Drp1
is the activated form of Drp-1 that performs mitochondrial
pro-fission functions (48).
Because no commercial specific Drp-1 activator is available, the
present study was unable to perform rescue experiments. Moreover,
it was found that BLE-A5 treatment could change the morphology of
mitochondria in NPDFs, transforming them from short tubules or
round fragments into long tubular networks. Furthermore,
Si-Drp1-transfected NPDFs showed similar mitochondrial morphology
as that of Si-Ctrl-transfected cells treated with BLE-A5. However,
simultaneous Drp1 silencing and BLE-A5 treatment showed no further
changes in the mitochondrial structure compared to Si-Drp1 or
BLE-A5-treated NPDFs. This phenomenon may due to either Si-Drp1 or
BLE-A5 treatment under these conditions have hit the limit of
mitochondrial fusion; hence, the overall structure showed no
further change by co-administration. It was also found that Drp1
knockdown in NPDFs increased mitochondrial dysfunction when the
cells were exposed to BLE-A5 and that the PINK1-Parkin-mediated
mitophagy pathway was inhibited more severely in
Si-Drp1-transfected NPDFs compared with Si-Ctrl-transfected cells
when exposed to BLE. These findings were further supported by
showing that BLE-A5 could suppress the formation of the cyclin
B1-CDK1 complex, thereby decreasing Drp1 phosphorylation.
Bcl-2 and Bax are two essential proteins that
regulate mitochondria-mediated apoptosis. Pro-apoptotic Bax can
form a complex with the anti-apoptotic Bcl-2; thus, the Bax/Bcl-2
ratio determines whether a cell will undergo apoptosis (49). Caspase-9 is the protease that
initiates the mitochondria-mediated apoptotic pathway and is
activated by multiprotein activation platforms (50). The present study showed that
BLE-A5 treatment altered the Bax/Bcl-2 ratio and activated
caspase-9, thus initiating mitochondria-mediated apoptosis.
Furthermore, the Bax/Bcl-2 ratio and cleaved caspase-9 expression
was higher in Si-Drp1-transfected NPDFs than in Si-Ctrl-transfected
cells exposed to a clinical dose of BLE-A5 (200 µM),
indicating that Drp1 could reduce the activation of
mitochondria-mediated apoptosis in BLE-treated NPDFs.
Mitochondria are double membrane-bound, subcellular
organelles that regulate a host of metabolic functions and are
closely associated with the intrinsic apoptosis pathway (51). When a cell undergoes internal or
external stress, its mitochondria take quality-control steps to
eliminate damaged proteins, lipids and DNA by isolating damaged
factors and transferring them to the lysosome for degradation
(52). This mitochondrial fission
process is mediated by large GTPases in the dynamin family
(53). Among these GTPases, Drp1
is known to play a critical role in regulating mitochondrial
fission in cells under stress (54). The present study found that a low
dose of BLE-A5 could enhance Drp1 expression NPDFs, but a clinical
dose could decrease Drp1 expression and change the morphology of
mitochondria from fragments into networks. To examine whether such
changes play a protective or pro-apoptotic role during BLE
exposure, Drp1 expression in NPDFs was knocked down via siRNA
transfection. The results showed that Si-Drp1-transfected NPDFs
were more sensitive to BLE-A5-induced apoptosis than
Si-Ctrl-transfected cells, indicating that the BLE-induced decrease
in Drp1 expression may shut down the quality-control mechanisms of
mitochondria in NPDFs, rendering these cells unable to eliminate
cytotoxic factors.
ATP, the 'energy currency' of the cell, is essential
for cellular metabolism and is primarily produced by mitochondria.
However, mitochondria also continually produce harmful ROS as a
byproduct of electron transport during oxidative phosphorylation
(55). Mitochondria contain their
own self-replicating genomes (mtDNA) that encode essential protein
components of the electron transport chain (56). The mitochondrial membrane
potential (Δψm) can reflect the functional status of mitochondria
(57). ROS-induced stress can
cause the accumulation of mutated mtDNA in human cells (58,59). A study showed that BLE could
induce lung epithelial cell apoptosis via activation of the ROS/Akt
signaling pathway and damage mtDNA (60,61). Consistent with these previous
studies, the present results showed that BLE exposure in NPDFs
enhanced ROS and mtDNA levels, inhibited ATP generation and caused
the mitochondrial membrane potential to depolarize. Moreover, these
indications of BLE-induced mitochondrial dysfunction were more
significant in Si-Drp1-transfected NPDFs than in control cells,
indicating that the self-protective mechanism of Drp1-mediated
mitochondrial fission was further suppressed by BLE-A5
treatment.
When stress is not sufficient to induce apoptosis,
an important mechanism that protects cells from the harmful
accumulation of mtDNA caused by excessive ROS is the activation of
mitophagy (62). Studies of
PINK1-Parkin-mediated mitophagy have yielded insight into a
molecular quality control mechanism via the elimination of damaged
mitochondria (63-65). When a mitochondrion becomes
damaged, PINK1 accumulates in the mitochondrial outer membrane,
which then recruits Parkin from the cytosol (66). Parkin conjugates ubiquitin to a
variety of proteins on the outer mitochondrial membrane to generate
an autophagosome, which is later degraded by lysosomal hydrolases
(67). The present study found
that BLE treatment could suppress such protective mechanisms in
NPDFs by blocking the mitochondrial accumulation of PINK1-Parkin.
Moreover, this suppression was enhanced by Drp1 knockdown,
indicating the necessity of Drp1 in the activation of
PINK1-Parkin-mediated mitophagy. LC3B was measured by western
blotting and immunofluorescence, and the findings also supported
this theory.
It was reported that Drp1 activation depends on the
cyclin B1-CDK1 complex-mediated phosphorylation of Drp1 at S616 and
subsequent localization on the outer mitochondrial membrane
(68). The present results showed
that BLE treatment reduced the phosphorylation of Drp1 at S616 and
its localization in mitochondria. Si-Drp1-transfected NPDFs
expressed lower levels of p-Drp1(S616), indicating that knockdown
of total Drp1 expression could also decrease p-Drp1(S616) levels
and thereby suppress its function in mitochondrial fission. The
co-immuno-precipitation results showed that BLE suppressed the
formation of the cyclin B1-CDK1 complex and thus decreased the
phosphorylation of Drp1. CDK1 inhibition by RO-3306 decreased
p-Drp1(S616) expression in NPDFs, which also provided direct
evidence that CDK1 is required for the activation of Drp1.
In summary, the present findings suggested that BLE
inhibits the interaction of cyclin B1 with CDK1 and decreases the
expression of total Drp1 in NPDFs, thus decreasing the levels of
p-Drp1 (S616). This decreased expression blocks the protective
mechanisms of mitochondrial fission and PINK1-Parkin-mediated
mitophagy to eliminate dysfunctional mitochondria. In this manner,
BLE can effectively induce NPDF apoptosis (Fig. 8). Further studies are still needed
to evaluate the safety and effectiveness of such treatments.
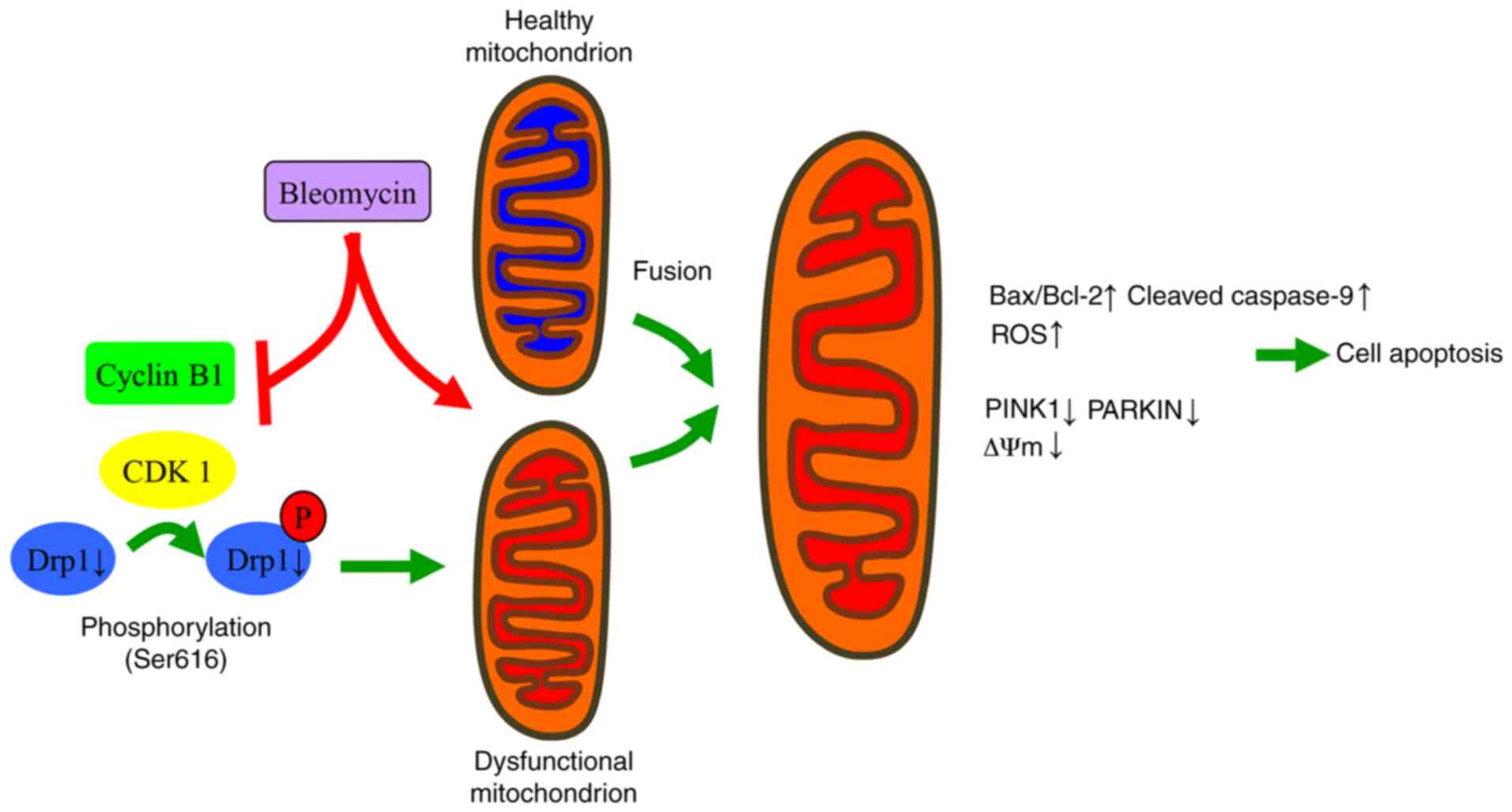 | Figure 8Proposed mechanisms by which Drp1
mediates the changes in mitochondrial morphology and function in
response to BLE treatment in NPDFs. BLE-A5 treatment can lead to
mitochondrial dysfunction and suppress the formation of active
cyclin B1-CDK1 complexes. This suppression inhibits the
phosphorylation of Drp1 and its translocation to the mitochondrial
membrane, thus increasing the fusion of dysfunctional and healthy
mitochondria. Subsequently, ROS production increases, mitochondrial
membrane potential dissipates, and apoptosis is induced in NPDFs.
NPDF, nasal polyp-derived fibroblast; Drp1, dynamin-related protein
1; ROS, reactive oxygen species; P, phosphorylation; PINK1,
serine/threonine kinase PINK1; C-Cas9, cleaved caspase-9. |
Acknowledgments
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (grant no. 81500773) and the Natural
Science Foundation of Guangdong Province of China (grant no.
2015A030310125).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW, YM, JW and HO performed the experiments. FW
analyzed the data and wrote the manuscript. HD, YZ, PT and HZ
conceptualized the study design, and contributed to data analysis
and experimental materials. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Sun Yat-sen Memorial Hospital (approval no. SYSU81500773;
Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hulse KE, Stevens WW, Tan BK and Schleimer
RP: Pathogenesis of nasal polyposis. Clin Exp Allergy. 45:328–346.
2015. View Article : Google Scholar :
|
|
2
|
Schleimer RP: Immunopathogenesis of
chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol.
12:331–357. 2017. View Article : Google Scholar :
|
|
3
|
Klossek JM, Neukirch F, Pribil C,
Jankowski R, Serrano E, Chanal I and El Hasnaoui A: Prevalence of
nasal polyposis in France: A cross-sectional, case-control study.
Allergy. 60:233–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hedman J, Kaprio J, Poussa T and Nieminen
MM: Prevalence of asthma, aspirin intolerance, nasal polyposis and
chronic obstructive pulmonary disease in a population-based study.
Int J Epidemiol. 28:717–722. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Settipane GA and Chafee FH: Nasal polyps
in asthma and rhinitis. A review of 6,037 patients. J Allergy Clin
Immunol. 59:17–21. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahn JC, Kim JW, Lee CH and Rhee CS:
Prevalence and risk factors of chronic rhinosinusitus, allergic
rhinitis, and nasal septal deviation: Results of the Korean
national health and nutrition survey 2008-2012. AMA Otolaryngol
Head Neck Surg. 142:162–167. 2016.
|
|
7
|
Stevens WW, Schleimer RP and Kern RC:
Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol
Pract. 4:565–572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kato A: Immunopathology of chronic
rhinosinusitis. Allergol Int. 64:121–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dobzanski A, Khalil SM and Lane AP: Nasal
polyp fibroblasts modulate epithelial characteristics via Wnt
signaling. Int Forum Allergy Rhinol. 8:1412–1420. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Veloso-Teles R, Cerejeira R, Roque-Farinha
R and Buchwald CV: Systemic immune profile in patients with CRSwNP.
Ear Nose Throat J. Dec 4–2019.Epub Ahead of Print. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Gevaert E, Lou H, Wang X, Zhang
L, Bachert C and Zhang N: Chronic rhinosinusitis in Asia. J Allergy
Clin Immunol. 140:1230–1239. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng J, Zhou P, Liu Y, Liu F, Yi X, Liu S,
Holtappels G, Bachert C and Zhang N: The development of nasal polyp
disease involves early nasal mucosal inflammation and remodelling.
PLoS One. 8:e823732013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho JS, Kang JH, Um JY, Han IH, Park IH
and Lee HM: Lipopolysaccharide induces pro-inflammatory cytokines
and MMP production via TLR4 in nasal polyp-derived fibroblast and
organ culture. PLoS One. 9:e906832014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cho JS, Han IH, Lee HR and Lee HM:
Prostaglandin E2 induces IL-6 and IL-8 production by the EP
receptors/Akt/NF-κB pathways in nasal polyp-derived fibroblasts.
Allergy Asthma Immunol Res. 6:449–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park IH, Park SJ, Cho JS, Moon YM, Kim TH,
Lee SH and Lee HM: Role of reactive oxygen species in transforming
growth factor beta1-induced alpha smooth-muscle actin and collagen
production in nasal polyp-derived fibroblasts. Int Arch Allergy
Immunol. 159:278–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thomas M, Yawn BP, Price D, Lund V, Mullol
J and Fokkens W; European Position Paper on Rhinosinusitis and
Nasal Polyps Group: EPOS primary care guidelines: European position
paper on the primary care diagnosis and management of
rhinosinusitis and nasal polyps 2007-a summary. Prim Care Respir J.
17:79–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fernandez-Bertolin L, Mullol J,
Fuentes-Prado M, Roca-Ferrer J, Alobid I, Picado C and Pujols L:
Effect of lipopolysaccharide on glucocorticoid receptor function in
control nasal mucosa fibroblasts and in fibroblasts from patients
with chronic rhinosinusitis with nasal polyps and asthma. PLoS One.
10:e1254432015. View Article : Google Scholar
|
|
18
|
Embid C, Fernández-Bertolin L, Pujols L,
Alobid I, Mullol J and Picado C: Nuclear translocation of the
glucocorticoid receptor in fibroblasts of asthmatic patients with
nasal polyposis insensitive to glucocorticoid treatment. Arch
Bronconeumol. 47:115–121. 2011.In English, Spanish. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pujols L, Fuentes-Prado M,
Fernández-Bertolin L, Alobid I, Roca-Ferrer J, Mullol J and Picado
C: Lower sensitivity of nasal polyp fibroblasts to glucocorticoid
anti-proliferative effects. Respir Med. 105:218–225. 2011.
View Article : Google Scholar
|
|
20
|
Bhattacharyya N: Influence of polyps on
outcomes after endoscopic sinus surgery. Laryngoscope.
117:1834–1838. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wynn R and Har-El G: Recurrence rates
after endoscopic sinus surgery for massive sinus polyposis.
Laryngoscope. 114:811–813. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Zou J, Sun B, Liang J, Zhao L and
Li B: Study on the treatment of nasal polyposis with bleomycin A5
local injection. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 20:51–53.
2006.In Chinese. PubMed/NCBI
|
|
23
|
Zhang X, Zou J, Li B, Ren X and Shi J:
Eosinophil apoptosis in nasal polyposis tissue after bleomycin A5
local injection. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 18:279–281.
2004.In Chinese. PubMed/NCBI
|
|
24
|
Tian P, Wu F, Wang J, Ou H, Liu X, Chen Q,
Dang H, Zheng Y, Zhang X and Zou H: Intralesional bleomycin A5
injection for the treatment of nasal polyps through inducing
apoptosis. Acta Otolaryngol. 138:475–482. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Patel PR, Hegde ML, Theruvathu J, Mitra
SA, Boldogh I and Sowers L: Norepinephrine reduces reactive oxygen
species (ROS) and DNA damage in ovarian surface epithelial cells. J
Bioanal Biomed. 7:75–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kucuksayan E, Cort A, Timur M, Ozdemir E,
Yucel SG and Ozben T: N-acetyl-L-cysteine inhibits bleomycin
induced apoptosis in malignant testicular germ cell tumors. J Cell
Biochem. 114:1685–1694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu F, Tian P, Ma Y, Wang J, Ou H and Zou
H: Induction of apoptosis in nasal polyp-derived fibroblasts by
bleomycin A5 in vitro. Mol Med Rep. 17:5384–5389. 2018.PubMed/NCBI
|
|
28
|
Wu F, Tian P, Ma Y, Wang J, Ou H and Zou
H: Reactive oxygen species are necessary for bleomycin A5-induced
apoptosis and extracellular matrix elimination of nasal
polyp-derived fibroblasts. Ann Otol Rhinol Laryngol. 128:135–144.
2019. View Article : Google Scholar
|
|
29
|
Nasrallah CM and Horvath TL: Mitochondrial
dynamics in the central regulation of metabolism. Nat Rev
Endocrinol. 10:650–658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu
X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, et al:
Dynamin-related protein 1-mediated mitochondrial mitotic fission
permits hyper-proliferation of vascular smooth muscle cells and
offers a novel therapeutic target in pulmonary hypertension. Circ
Res. 110:1484–1497. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang CR and Blackstone C: Cyclic
AMP-dependent protein kinase phosphorylation of Drp1 regulates its
GTPase activity and mitochondrial morphology. J Biol Chem.
282:21583–21587. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cribbs JT and Strack S: Reversible
phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and
calcineurin regulates mitochondrial fission and cell death. Embo
Rep. 8:939–944. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song M, Gong G, Burelle Y, Gustafsson ÅB,
Kitsis RN, Matkovich SJ and Dorn GW II: Interdependence of parkin-
mediated mitophagy and mitochondrial fission in adult mouse hearts.
Circ Res. 117:346–351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zuo W, Zhang S, Xia CY, Guo XF, He WB and
Chen NH: Mitochondria autophagy is induced after hypoxic/ischemic
stress in a Drp1 dependent manner: The role of inhibition of Drp1
in ischemic brain damage. Neuropharmacology. 86:103–115. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kageyama Y, Hoshijima M, Seo K, Bedja D,
Sysa-Shah P, Andrabi SA, Chen W, Höke A, Dawson VL, Dawson TM, et
al: Parkin-independent mitophagy requires Drp1 and maintains the
integrity of mammalian heart and brain. EMBO J. 33:2798–2813. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suen DF, Norris KL and Youle RJ:
Mitochondrial dynamics and apoptosis. Genes Dev. 22:1577–1590.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sosulski ML, Gongora R, Danchuk S, Dong C,
Luo F and Sanchez CG: Deregulation of selective autophagy during
aging and pulmonary fibrosis: The role of TGFβ1. Aging Cell.
14:774–783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eiyama A and Okamoto K:
PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell
Biol. 33:95–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang H, Zhang C, Tang Y, Zhao J, Wang T,
Liu H and Sun X: The regulator of calcineurin 1 increases adenine
nucleotide translocator 1 and leads to mitochondrial dysfunctions.
J Neurochem. 140:307–319. 2017. View Article : Google Scholar :
|
|
40
|
Zhang YC, Zuo WQ, Rong QF, Teng GL and
Zhang YM: Glucocorticoid receptor expression on acute lung injury
induced by endotoxin in rats. World J Emerg Med. 1:65–69.
2010.PubMed/NCBI
|
|
41
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
42
|
Yamano K and Youle RJ: Coupling
mitochondrial and cell division. Nat Cell Biol. 13:1026–1027. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Martin WG, Ristow KM, Habermann TM, Colgan
JP, Witzig TE and Ansell SM: Bleomycin pulmonary toxicity has a
negative impact on the outcome of patients with Hodgkin's lymphoma.
J Clin Oncol. 23:7614–7620. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Manca G, Pandolfi P, Gregorelli C, Cadossi
M and de Terlizzi F: Treatment of keloids and hypertrophic scars
with bleomycin and electroporation. Plast Reconstr Surg.
132:e621–e630. 2013. View Article : Google Scholar
|
|
45
|
Luo QF and Zhao FY: The effects of
Bleomycin A5 on infantile maxillofacial haemangioma. Head Face Med.
7:112011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Carroll WW, O'Connell BP, Schlosser RJ,
Gudis DA, Karnezis TT, Lawrence LA, Soler ZM and Mulligan JK:
Fibroblast levels are increased in chronic rhinosinusitis with
nasal polyps and are associated with worse subjective disease
severity. Int Forum Allergy Rhinol. 6:162–168. 2016. View Article : Google Scholar
|
|
47
|
Hu J, Zhang Y, Jiang X, Zhang H, Gao Z, Li
Y, Fu R, Li L, Li J, Cui H and Gao N: ROS-mediated activation and
mitochondrial translocation of CaMKII contributes to Drp1-dependent
mitochondrial fission and apoptosis in triple-negative breast
cancer cells by isorhamnetin and chloroquine. J Exp Clin Cancer
Res. 38:2252019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ausman J, Abbade J, Ermini L, Farrell A,
Tagliaferro A, Post M and Caniggia I: Ceramide-induced BOK promotes
mitochondrial fission in preeclampsia. Cell Death Dis. 9:2982018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sharifi S, Barar J, Hejazi MS and Samadi
N: Roles of the Bcl-2/Bax ratio, caspase-8 and 9 in resistance of
breast cancer cells to paclitaxel. Asian Pac J Cancer Prev.
15:8617–8622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim B, Srivastava SK and Kim SH: Caspase-9
as a therapeutic target for treating cancer. Expert Opin Ther
Targets. 19:113–127. 2015. View Article : Google Scholar
|
|
51
|
Youle RJ and van der Bliek AM:
Mitochondrial fission, fusion, and stress. Science. 337:1062–1065.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hoppins S, Lackner L and Nunnari J: The
machines that divide and fuse mitochondria. Annu Rev Biochem.
76:751–780. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tilokani L, Nagashima S, Paupe V and
Prudent J: Mitochondrial dynamics: Overview of molecular
mechanisms. Essays Biochem. 62:341–360. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Westermann B: Mitochondrial fusion and
fission in cell life and death. Nat Rev Mol Cell Biol. 11:872–884.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Balaban RS, Nemoto S and Finkel T:
Mitochondria, oxidants, and aging. Cell. 120:483–495. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dorn GN II, Vega RB and Kelly DP:
Mitochondrial biogenesis and dynamics in the developing and
diseased heart. Genes Dev. 29:1981–1991. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang BB, Wang DG, Guo FF and Xuan C:
Mitochondrial membrane potential and reactive oxygen species in
cancer stem cells. Fam Cancer. 14:19–23. 2015. View Article : Google Scholar
|
|
58
|
Eaton JS, Lin ZP, Sartorelli AC, Bonawitz
ND and Shadel GS: Ataxia-telangiectasia mutated kinase regulates
ribonucleotide reductase and mitochondrial homeostasis. J Clin
Invest. 117:2723–2734. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lee HC, Yin PH, Lu CY, Chi CW and Wei YH:
Increase of mitochondria and mitochondrial DNA in response to
oxidative stress in human cells. Biochem J. 348:425–432. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kim SJ, Cheresh P, Jablonski RP,
Morales-Nebreda L, Cheng Y, Hogan E, Yeldandi A, Chi M, Piseaux R,
Ridge K, et al: Mitochondrial catalase overexpressed transgenic
mice are protected against lung fibrosis in part via preventing
alveolar epithelial cell mitochondrial DNA damage. Free Radic Biol
Med. 101:482–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang L, Lin Z, Wang Y, Li C, Xu W, Li Q,
Yao W, Song Z and Liu G: Nickle(II) ions exacerbate
bleomycin-induced pulmonary inflammation and fibrosis by activating
the ROS/Akt signaling pathway. Environ Sci Pollut Res Int.
25:4406–4418. 2018. View Article : Google Scholar
|
|
62
|
Kowald A and Kirkwood TB: The evolution
and role of mitochondrial fusion and fission in aging and disease.
Commun Integr Biol. 4:627–629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Clark IE, Dodson MW, Jiang C, Cao JH, Huh
JR, Seol JH, Yoo SJ, Hay BA and Guo M: Drosophila pink1 is required
for mitochondrial function and interacts genetically with parkin.
Nature. 441:1162–1166. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Park J, Lee SB, Lee S, Kim Y, Song S, Kim
S, Bae E, Kim J, Shong M, Kim JM and Chung J: Mitochondrial
dysfunction in Drosophila PINK1 mutants is complemented by parkin.
Nature. 441:1157–1161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Greene JC, Whitworth AJ, Kuo I, Andrews
LA, Feany MB and Pallanck LJ: Mitochondrial pathology and apoptotic
muscle degeneration in Drosophila parkin mutants. Proc Natl Acad
Sci USA. 100:4078–4083. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Barodia SK, Creed RB and Goldberg MS:
Parkin and PINK1 functions in oxidative stress and
neurodegeneration. Brain Res Bull. 133:51–59. 2017. View Article : Google Scholar :
|
|
67
|
Lazarou M, Sliter DA, Kane LA, Sarraf SA,
Wang C, Burman JL, Sideris DP, Fogel AI and Youle RJ: The ubiquitin
kinase PINK1 recruits autophagy receptors to induce mitophagy.
Nature. 524:309–314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Taguchi N, Ishihara N, Jofuku A, Oka T and
Mihara K: Mitotic phosphorylation of dynamin-related GTPase Drp1
participates in mitochondrial fission. J Biol Chem.
282:11521–11529. 2007. View Article : Google Scholar : PubMed/NCBI
|