Introduction
Ovarian teratocarcinoma is a rare type of tumor,
accounting for ~30% of all types of ovarian cancer and originating
from germ cell in the ovary (1).
Histologically, ovarian teratocarcinoma can be classified into two
subtypes, namely mature and immature types. Immature ovarian
teratocarcinoma (IOT) is rare but aggressive, representing 1% of
all teratomas and 1% of all ovarian neoplasms (2). It is also one of the most common
histologic subtypes of malignant ovarian germ cell tumors (MOGCTs),
representing ~36.5% of MOGCT cases (3). Ovarian teratocarcinomas are mainly
benign and can be cured by surgery, whereas IOT is a serious threat
to the health of females at reproductive age (4). Further, the etiology of IOT remains
elusive. Due to the rarity of IOT, relevant data and studies are
limited and restricted to survival analyses of cases with rare
experimental evidence.
Next-generation sequencing technology has revealed
that majority of the human genome is transcribed into non-coding
RNAs (ncRNAs), including microRNA (miRNA), circular RNA (circRNA),
long noncoding RNA (lncRNA) and pseudogenes (5). These ncRNAs serve an essential role
in gene regulation at the transcriptional and post-transcriptional
levels (6), and their anomalous
expression patterns are implicated in pathogenesis of multiple
diseases, including cancer (7).
The emergence of the 'competing endogenous RNA (ceRNA) hypothesis',
which suggests that lncRNAs release target genes repressed by
miRNAs via sequestering miRNAs, has attracted attentions from
multiple researchers (8). To
function as an effective miRNA decoy, the lncRNA usually primarily
steadily expresses in cytoplasm and coexists in the RNA-induced
silencing complex (RISC) with miRNA (9). For example, abnormally highly
expressed lncRNA insulin growth factor 2 antisense in gastric
cancer (GC) is predominantly localized in cytoplasm and serves as a
ceRNA of miR-503 to promote the GC progression (10). Whether a similar regulatory
pattern is implicated in IOT requires further exploration.
The present study aimed to identify key molecules
that participate in the malignant phenotype of IOT and elucidate
the underlying mechanism, and to provide new therapeutic targets
for IOT.
Materials and methods
Clinical samples
A total of 45 paired IOT tissues and mature ovarian
teratocarcinoma (MOT) (benign) tissues were obtained from patients
(age range, 10-20 years) who underwent surgical resection at The
First Hospital of Fuzhou Fujian between January 2014 and February
2019. Written consent to the usage of ovarian teratocarcinoma
tissues in the present study were obtained from all patients
(patients ≥18 years old) or their legal guardians (<18 years
old) prior to testing. The present study was approved by the Ethics
Committee of The First Hospital of Fuzhou Fujian.
Cell culture and transfection
The human ovarian teratoma Hs 38.T cell line, 293T
cell line and ovarian teratocarcinoma PA-1 cell line were
commercially obtained from the American Type Culture Collection and
cultured in Dulbecco's Modified Eagle Medium (DMEM; Sigma-Aldrich;
Merck KGaA), followed by supplementation with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin mix in a humidified atmosphere of 5%
CO2 at 37°C. The full-length sequences of murine double
minute homolog 2 (MDM2), mouse double minute 4 (MDM4), cyclin
dependent kinase 6 (CDK6) and cyclin D1 (CCND1) were subcloned into
a pcDNA3.1 vector (Addgene, Inc.) with an empty plasmid was used as
a negative control, which was referred to as 'Vector'. miR-214-5p
mimic and negative control (NC mimic) were purchased from Shanghai
GenePharma Co., Ltd. A total of 50 nM miR-214-5p mimic and 50 nM NC
mimic were separately transfected into cells. The short hairpin
RNAs (shRNAs) were synthesized by Guangzhou Ribobio Co., Ltd.
Similarly, shRNAs targeting LINC00324 (sh-LINC00324#1, 5′-CCG GTA
ACC TAT TCC TTG AAG ACA CCT CGA GGT GTC TTC AAG GAA TAG GTT ATT TTT
G-3′; sh-LINC00324#2, 5′-CCG GTC ACA TAA TGT TGA AAG TCT GCT CGA
GCA GAC TTT CAA CAT TAT GTG ATT TTT G-3′; and sh-LINC00324#3,
5′-CCG GAC AAA TCT TAG ACG TAA TCC CCT CGA GGG GAT TAC GTC TAA GAT
TTG TTT TTG-3′) and negative control shRNA (sh-NC, 5′-CCG GTG CCA
CCT AAA TTT ACC AAG TCT CGA GAC TTG GTA AAT TTA GGT GGC ATT TTT
G-3′) were also synthesized and obtained from Shanghai GenePharma
Co., Ltd. The cells were transfected with 20 nM shRNAs. The
plasmids were transfected into cells in 6-well plates at a
concentration of 2 µg per well using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.).
RNA extraction and reverse
transcription-quantitative (RT-qPCR)
Total RNA in the treated cells was extracted using a
TRIzol™ Plus RNA Purification kit (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was transcribed using PrimeScriptTM II
Reverse Transcriptase (Takara Biotechnology, Inc.). The temperature
protocol for RT was as follows: 37°C for 15 min and 85°C for 5 sec.
qPCR was conducted with SYBR® Premix Ex Taq™ II (Takara
Bio, Inc.) in triplicate via iQ5 Real-time PCR detection system
(Bio-Rad Laboratories, Inc.) following the manufacturer's protocol.
The thermocycling conditions were as follows: 5 min at 95°C,
followed by 40 cycles of 95°C for 30 sec, annealing at 65°C for 45
sec, extension at 72°C for 30 sec, and a final extension of 72°C
for 5 min. U2 and GAPDH were used as the endogenous controls.
Relative expression of genes was analyzed using 2−ΔΔCq
method (11). Primer sequences
were as follows: miR-199a-5p forward, 5′-GCC GAG CCC AGT GTT CAG
ACT-3′ and reverse, 5′-CCC AGT GTT CAG ACT ACC TGT TC-3′;
miR-513c-5p forward, 5′-CCG AGT TCT CAA GGA GGT GTC-3′ and reverse,
5′-TTC TCA AGG AGG TGT CGT TTA T-3′; miR-214-5p forward, 5′-TGA GTG
CCT GTC TAC ACT TG-3′ and reverse, 5′-TGC CTG TCT ACA CTT GCT GTG
C-3′; miR-202-3p forward, 5′-TTA TAG AGA GGT ATA GGG CA-3′ and
reverse, 5′-AGA GGT ATA GGG CAT GGG AA-3′; LINC00324 forward,
5′-GGC CCC ACA AAT CAC ACA AC-3′ and reverse, 5′-TAC CGA CTT GGT
GCC ATT CC-3′; CDK6 forward, 5′-GCA GGG AAA GAA AAG TGC AAT GA-3′
and reverse, 5′-CCC GGA GAT CGG TCT AGC TT-3′; MDM2 forward, 5′-GCG
AGC TTG GCT GCT TCT-3′ and reverse, 5′-TCC CTC AAG ACT CCC CAG
TT-3′; MDM4 forward, 5′-ATG ATC AGC AGG AGC AGC AT-3′ and reverse,
5′-GCT CTG AGG TAG GCA GTG TG-3′; CCND1 forward, 5′-AGG CTG TGT CCC
TCT TCT CT-3′ and reverse, 5′-GGT GGC ACG TAA GAC ACA CT-3′; GAPDH
forward, 5′-GGA GCG AGA TCC CTC CAA AAT-3′ and reverse, 5′-GGC TGT
TGT CAT ACT TCT CAT GG-3′; and U6 forward, 5′-CTC GCT TCG GCA GCA
CA-3′ and reverse, 5′-AAC GCT TCA CGA ATT TGC GT-3′.
Cell proliferation assays
For the Cell Counting Kit-8 (CCK-8) assay, the CCK-8
kit [Yeasen Biotechnology (Shanghai) Co., Ltd.] was utilized
following the manufacturer's protocol. Treated PA-1 or Hs 38.T
cells were seeded in the 96-well culture plates (1,000 cells/well).
Absorbance at 450 nm was measured by a microplate reader
(Multiskan™ MK3; Thermo Fisher Scientific, Inc.) every 24 h during
a 4-day period. A colony formation assay was performed as described
previ-ously (12). An
5′-ethynyl-2′-deoxyuridine (EdU) assay was conducted using the
Click-iT EdU Alexa Fluor 488 Imaging kit (Guangzhou RiboBio Co.,
Ltd.) according to manufacturer's protocol.
Flow cytometry analysis
For apoptotic analyses, treated PA-1 or Hs 38.T
cells were double-stained with Annexin V-FITC (5 µl) for 15
min at room temperature and propidium iodide (PI; 5 µg/ml)
for 15 min at 4°C and analyzed with a FACScan flow cytometer (BD
Biosciences, Inc.). For cell cycle analyses, PA-1 cells were
collected and fixed in 70% ethanol at 4°C overnight, then stained
with 0.5 ml FxCycle™ PI/RNase staining solution for 15 min at room
temperature (Thermo Fisher Scientific, Inc.) and analyzed using a
FACScan flow cytometer (Thermo Fisher Scientific, Inc.). Data were
assessed with FlowJo software version 10.5.3 (FlowJo LLC).
Terminal-deoxynucleotidyl transferase
mediated nick end labeling (TUNEL) assay
Cells were fixed with 4% paraformaldehyde for 15 min
at room temperature. Subsequently, cells were treated with 1X TUNEL
reagent (Clontech Laboratories, Inc.) for 1 h at room temperature,
and 10 mM PBS was used as the washing buffer. The nuclei were
stained with 1X DAPI for 10 min at room temperature. Images were
captured using a fluorescence microscope (Olympus Corporation;
magnification, ×100).
Subcellular fractionation assay
Cytoplasmic and nuclear RNA of the PA-1 cells were
isolated by utilizing a Cytoplasmic & Nuclear RNA Purification
kit (Norgen) following the manufacturer's guidelines. 18S and U2
served as the cytoplasmic and nuclear controls, respectively.
Bioinformatics analysis
For differential miRNA analyses, miRNA sequence raw
data were downloaded from Gene Expression Omnibus database
(accession no. GSE98536; https://www.ncbi.nlm.nih.gov/geo/) and converted to
fastaq format using SRAtoolkit. Raw data were processed according
to previous study (13).
Differentially expressed miRNAs between 2 MOGCT samples and 7
benign OGCT samples were analyzed by R-Language Limma Package
(Gordon Smyth; v3.44.1; http://bioconductor.org/pack-ages/release/bioc/html/limma.html)
(14) with default setting and
identified with |log2FC| >1.0 and P<0.05 as the criteria. As
for Kyoto Encyclopedia of Genes and Genomes pathway analyses, the
signaling pathway analysis was performed using the DAVID database
(v6.8; https://david.ncifcrf.gov/summary.jsp). lncRNAs that
can bind with miR-214-5p were predicted using StarBase V3.0
(http://starbase.sysu.edu.cn/) by
searching the miRNA-lncRNA column.
RNA fluorescence in situ hybridization
(FISH)
Fluorescein-labeled probes against long-chain
intergenic non-coding RNA324 (LINC00324) and miR-214-5p were
synthesized by Guangzhou RiboBio Co., Ltd. FISH was performed using
the Fluorescence in situ Hybridization kit (Guangzhou
RiboBio Co., Ltd.) following the manufacturer's protocol. Cells
were stained with 1X DAPI for 10 min at room temperature, and
fluorescence signals were captured using the LSM 900 laser scanning
microscope (Zeiss AG; magnification, ×1,000).
RNA-binding protein immunoprecipitation
(RIP)
An RIP assay was performed using the Magna RIP™
RNA-binding Protein Immunoprecipitation kit (EMD Millipore).
Anti-Ago2 (1:200; cat. no. MABE253; EMD Millipore) and anti-IgG
anti-bodies (1:200; cat. no. AB21-KC; EMD Millipore) were used for
immunoprecipitation. The co-precipitated RNAs were purified with 1X
phenol-chloroformisoamyl-alcohol mixture (125:24:1; pH<4),
followed by qPCR to evaluate the enrichment of LINC00324 and
miR-214-5p to Ago2.
RNA pull-down analysis
RNA pull-down was conducted as described previously
(15). The biotinylated DNA probe
complementary to LINC00324 was synthesized by Guangzhou RiboBio
Co., Ltd. The level of miR-214-5p was analyzed by qPCR.
Luciferase reporter assay
The whole sequences of LINC00324, CDK6 3′UTR, CCND1
3′UTR, MDM2 3′UTR or MDM4 3′UTR were cloned into the pmirGLO vector
(Promega Corporation). The mutant sequences were obtained using the
KOD enzyme (Toyobo) and were cloned into pmirGLO vector (Promega
Corporation) using T4 ligase (New England Biolabs, Inc.). 293T
(Amercian Type Culture Collection) and PA-1 cells
(2×104) were seeded into 24-well plates, then
transfected with plasmids, miR-214-5p mimic and controls using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). A total of 2 days after transfection cells were
harvested and subjected to luciferase activity analysis using DLR™
assay system (Promega Corporation), and the luciferase activity of
each reporter was measured by normalizing to Renilla
luciferase activity.
Western blot analysis
M-PER Mammalian Protein Extraction Reagent (Thermo
Fisher Scientific, Inc.) was utilized to extract the total proteins
from PA-1 or Hs 38.T cells. Protein concentration was measured by
using a BCA kit (cat. no. 23227; Pierce; Thermo Fisher Scientific,
Inc.). Following separation using 10% SDS-PAGE with 20 µg
protein per lane, PVDF membranes were blocked with 3% BSA in 1X
TBST (Sigma-Aldrich; Merck KGaA) at 37°C for 1 h and incubated
overnight at 4°C with the following primary antibodies purchased
from Abcam: Anti-Bcl-2 (1,000; cat. no. ab32124); anti-Bax (1,000;
cat. no. ab32503); anti-Caspase-3 (1:1,000; cat. no. ab13847);
anti-Cleaved Caspase-3 (1,000; cat. no. ab2302); anti-Caspase-9
(1,000; cat. no. ab32539); anti-Cleaved Caspase-9 (1,000; cat. no.
ab2324); and anti-GAPDH (1,000; cat. no. ab9485). The membranes
were then incubated with goat anti-mouse IgG H&L conjugated to
HRP (1:5,000; Abcam; cat. no. ab97040) for 4 h at 37°C. An ECL
detection kit (cat. no. 32134; Pierce; Thermo Fisher Scientific,
Inc was adopted to detect the bands. The densitometric analysis was
performed using Image J (v1.8.0; National Institutes of
Health).
Statistical analysis
All data were analyzed using SPSS 22.0 software (IBM
Corp.). Data obtained from at least 3 independent experiments are
expressed as mean ± standard deviation. A student's t-test and
one-way analysis of variance were used to calculate significance.
The Kaplan-Meier method was used to analyze of prognosis of
patients with IOT. A Pearson's correlation test was used to analyze
the correlation between LINC00324 and miR-214-5p, and between
miR-214-5p and CDK6/CCND1/MDM2/MDM4 expression levels in IOT
tissues. P<0.05 was considered to indicate a statistically
significant difference.
Results
miRNA-214-5p is downregulated in
malignant ovarian teratocarcinoma and involved in the regulation of
IOT cell viability and functions
Through analyzing miRNA expression profiling data
(GSE98536) examined in a previous study (13), 4 miRNAs (miR-199a-5p, miR-513c-5p,
miR-214-5p and miR-202-3p) were downregulated in 2 MOGCT samples
obtained from two patients respectively diagnosed with dysgerma and
primitive germ cell tumor compared with 7 benign ovarian germ cell
tumor (OGCT) samples collected from 7 mature teratoma patients. As
IOT is a common subtype of MOGCT, we hypothesized that these 4
miRNAs may have similar expression pattern in IOT tissues. Through
conducting RT-qPCR in 45 paired MOT and IOT tissues, it was
identified that that lower expression levels of miR-214-5p
(0.231-fold change) and miR-202-3p (0.548-fold change) were
exhibited in IOT tissues compared with MOT tissues (Fig. 1A). Moreover, low miR-214-5p
expression was closely associated with shorter survival times in
patients with ovarian teratocarcinoma (Fig. S1A). These 2 candidate miRNAs were
selected for subsequent RT-qPCR validations in ovarian
teratocarcinoma cells. As presented in Fig. 1B, miR-202-3p failed to exhibit
differential expression between ovarian teratoma cells (Hs 38.T)
and teratocarcinoma cells (PA-1), whereas that miR-214-5p was
significantly downregulated in the PA-1 cells compared with the Hs
38.T cells. The functional differences of Hs 38.T and PA-1 cells
containing different expressions of miR-214-5p were also verified.
As shown in Fig. S1B-D, CCK-8,
colony formation and EdU assay data demonstrated that the
proliferation ability of Hs 38.T cells was inhibited compared with
that of PA-1 cells, as demonstrated by lower OD values, smaller
colony numbers and a lower percentage of EdU-positive cells.
Concomitantly, flow cytometry and TUNEL assays demonstrated that
the apoptosis ability of Hs 38.T cells was improved compared with
that of PA-1 cells, as demonstrated by the higher apoptotic cell
rate and percentage of TUNEL-positive cells in Hs 38.T cells
(Fig. S1E and F). In addition,
the western blot analysis further examined the apoptosis ability of
Hs 38.T and PA-1 cells. The expression level of anti-apoptosis
protein (Bcl-2) was decreased in the Hs 38.T cells compared with
the PA-1 cells. However, the pro-apoptosis proteins (Bax, Cleaved
Caspase-3 and Cleaved Caspase-9) exhibited higher expression levels
in Hs 38.T cells compared with the PA-1 cells (Fig. S1G). All these data supported the
hypothesis that the downregulation of miR-214-5p may contribute to
the malignancy of IOT.
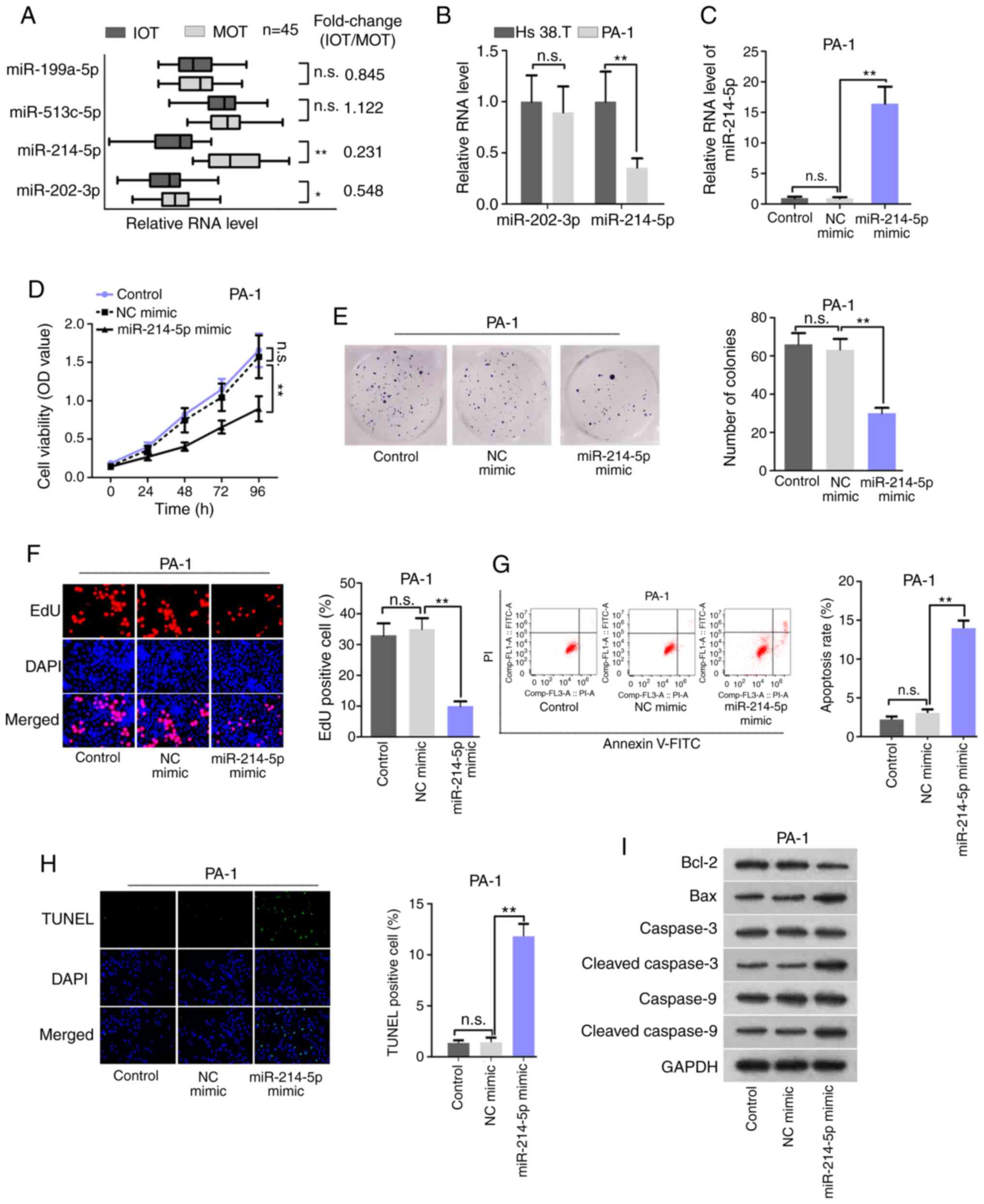 | Figure 1Expression profile and function of
miR-214-5p in immature ovarian teratocarcinoma. (A) RT-qPCR
analyzed the expression patterns of miR-199a-5p, miR-513c-5p,
miR-214-5p, and miR-202-3p in 45 pairs of IOT and MOT tissues. (B)
RT-qPCR determined the expression levels of miR-202-3p and
miR-214-5p in Hs 38.T and PA-1 cells. (C) RT-qPCR determined the
overexpression efficiency of miR-214-5p in PA-1 cells. (D) Cell
Counting Kit-8 examined the cell viability in PA-1 cells. (E)
Colony formation assay determined the clonogenic activity of PA-1
cells. (F) EdU assay determined PA-1 cell proliferation.
Magnification, ×200. (G) Flow cytometry analyzed the levels of
apoptosis in PA-1 cells. (H) TUNEL assay determined the PA-1 cell
apoptosis. Magnification, ×200. (I) Western blot analysis examined
changes in expression of the apoptosis-associated proteins. Control
referred to untreated condition. *P<0.05 and
**P<0.01. n.s., no significance; miR, microRNA; RT-qPCR, reverse
transcription-quantitative PCR; IOT, immature ovarian
teratocarcinoma; MOT, mature ovarian teratocarcinoma; n.s., not
significant; NC, negative control; OD, optical density; PI,
propidium iodide; TUNEL, terminal-deoxynucleotidyl transferase
mediated nick end labeling. |
Subsequently, gain-of-function assays were performed
in PA-1 cells using a miR-214-5p mimic. A satisfactory
over-expression efficiency of miR-214-5p was confirmed through
RT-qPCR, which detected that the expression of miR-214-5p was
increased in miR-214-5p mimic-transfected PA-1 cells compared with
the NC mimic group (Fig. 1C). In
a similar way, the results of CCK-8 assay demonstrated that
miR-214-5p upregulation markedly attenuated PA-1 cell viability
(Fig. 1D). In addition, the
colony formation and EdU assays revealed that the colony number and
percentages of the EdU-positive PA-1 cells decreased in response to
miR-214-5p overexpression (Fig. 1E
and F), further demonstrating that cell proliferation was
inhibited by miR-214-5p upregulation. Moreover, the flow cytometry
and TUNEL assay data suggested that overexpressing miR-214-5p
induced the increased the apoptotic cell rate and the number of
TUNEL-positive cells (Fig. 1G and
H). In concordance with the flow cytometry analyses data, the
levels of Bax, cleaved caspase-3 and cleaved caspase-9 were
upregulated upon overexpressing miR-214-5p, yet that of Bcl-2 was
decreased in PA-1 cells (Fig.
1I). These data indicated that miR-214-5p overexpression
inhibited cell proliferation and promoted cell apoptosis in ovarian
teratocarcinoma.
Aberrantly expressed LINC00324 interacts
with miR-214-5p and facilitates the malignant phenotype of IOT
cells
Multiple studies have revealed that lncRNAs can
interact with miRNAs via their miRNA response elements through
ceRNA network (16). miR-214-5p
was predicted to contain binding sites for 83 lncRNAs by StarBase
V3.0 (http://starbase.sysu.edu.cn/). The
present study then employed RT-qPCR to assess the expression
profile of these 83 lncRNAs in randomly selected 3 paired IOT and
MOT clinical samples. Among 29 aberrantly-expressed lncRNAs in the
paired IOT and MOT tissue samples, 15 lncRNAs were upregulated in
IOT tissues (Fig. 2A). LINC00324
was selected for subsequent analysis due to its most significant
upregulation tendency (logFC=5.77; P=0.002). Moreover, Kaplan-Meier
curve analysis indicated that high LINC00324 levels may be
associated with unfavorable prognosis in patients with IOT
(Fig. S2A). Then, RT-qPCR data
demonstrated that LINC00324 was predominantly distributed in
cytoplasmic fraction of PA-1 cells (Fig. S2B), indicating that LINC00324 may
exert its function at post-transcriptional levels via ceRNA
regulatory pattern. As miRNAs bind to their targets in an
Ago2-dependent manner, an RIP assay was performed to verify their
interaction using Ago2 antibody. It was identified that both
LINC00324 and miR-214-5p could be co-immunoprecipitated by Ago2
antibody (Fig. 2B), indirectly
suggesting the binding association between LINC00324 and
miR-214-5p. The RNA pull-down assay measured the enrichment of
miR-214-5p in the biotinylated LINC00324 wide-type
(bio-LINC00324-Wt) group or the mutant form (bio-LINC00324-Mut)
(Fig. 2C, upper), demonstrating
that LINC00324 bound with miR-214-5p in a sequence-specific manner.
Therefore, StarBase was used to predict the binding site between
LINC00324 and miR-214-5p (Fig.
2C, lower panel). miR-214-5p was overexpressed in 293T cells
(Fig. 2D). Additionally, the
luciferase reporter assay indicated that the luciferase activity of
LINC00324-Wt was repressed by the miR-214-5p mimic, and that of
LINC00324-Mut was not affected (Fig.
2E), further confirming the binding association between
LINC00324 and miR-214-5p. RNA-FISH demonstrated that LINC00324 and
miR-214-5p co-localized in cytoplasm of PA-1 cells (Fig. S2C). The Pearson's correlation
analysis data showed that LINC00324 was negatively correlated with
miR-214-5p in IOT tissues (Fig.
S2D). These data provided evidence that LINC00324 acted as a
miR-214-5p sponge.
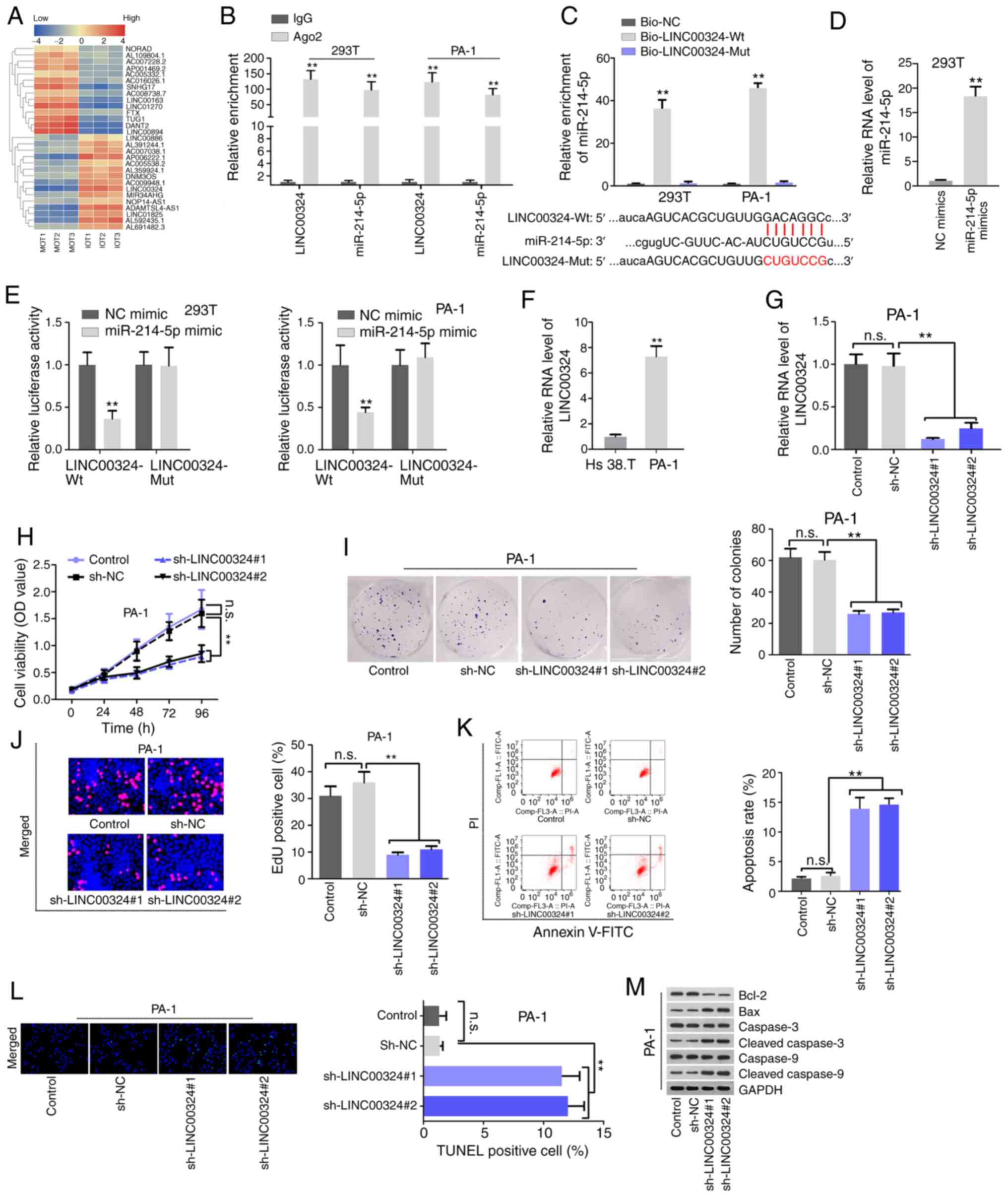 | Figure 2Aberrantly expressed LINC00324
functions as a miR-214-5p sponge and contributes to the malignant
phenotype of PA-1 cells. (A) Hierarchical clus-tered heat map
delineated the differentially expressed lncRNAs that possibly bind
with miR-214-5p in 3 sets of paired MOT and IOT tissues, as
examined by RT-qPCR. (B) RNA-binding protein immunoprecipitation
with qPCR measured the enrichment of LINC00324 and miR-214-5p in
Anti-Ago2 of 293T and PA-1 cells. Anti-IgG served as a negative
control. (C) RNA pull-down measured the enrichment of miR-214-5p in
Bio-LINC00324-Wt and Bio-LINC00324-Mut groups (upper panel). The
binding sequence between LINC00324-Wt/LINC00324-Mut and miR-214-5p
were indicated (lower panel). (D) RT-qPCR measured the
overexpression efficiency of miR-214-5p in 293T cells. (E) A
luciferase reporter assay examined the luciferase activity of
LINC00324-Wt reporter and LINC00324-Mut reporter when
overexpressing miR-214-5p. (F) RT-qPCR revealed the differential
expression of LINC00324 in Hs 38.T and PA-1 cells. (G) RT-qPCR
examined the knockdown efficiency of LINC00324 in PA-1 cells. (H-J)
The effect of LINC00324 knockdown on cell proliferation was
analyzed by CCK-8, colony formation, and EdU assays. Magnification,
×200. (K-M) The effect of LINC00324 knockdown on cell apoptosis was
determined by (K) flow cytometry, (L) TUNEL assays and (M) western
blot analysis. Magnification, ×200. **P<0.01.
LINC00324, long-chain intergenic non-coding RNA324; miR, microRNA;
RT-qPCR, reverse transcription-quantitative PCR; Wt, wild-type;
Mut, mutant; NC, negative control; n.s., not significant; sh, short
hairpin; PI, propidium iodide; OD, optical density. |
The role of LINC00324 in IOT cells was then
investigated. The RT-qPCR data verified that LINC00324 was
abnormally upregulated in ovarian teratocarcinoma PA-1 cells
compared with the benign ovarian teratoma Hs 38.T cells (Fig. 2F). The efficiency of LINC00324
knockdown was examined for subsequent loss-of function assays.
LINC00324 expression was decreased in
sh-LINC00324#1/2/3-transfected PA-1 cells (Fig. 2G). CCK-8, colony formation and EdU
assays demonstrated that depleted LINC00324 inhibited PA-1 cell
proliferation. When knocking down LINC00324, cell viability, colony
number and EdU positive cells were all decreased (Fig. 2H-J). Furthermore, LINC00324
deficiency increased apoptosis in the PA-1 cells, which was
verified by flow cytometry, TUNEL and western blot assays.
Downregulating LINC00324 increased apoptotic cell rate and the
number of TUNEL-positive cells. Additionally, pro-apoptosis protein
expression was also elevated, and anti-apoptosis protein expression
was suppressed in response to LINC00324 knock-down (Fig. 2K-M). Taken together, these data
indicated that LINC00324 acted as a miR-214-5p sponge and promoted
the malignant phenotype of IOT cells.
Tumor protein p53 (p53) signaling pathway
is a downstream pathway of miR-214-5p
We further investigated whether the
LINC00324/miR-214-5p axis affected IOT development via modulating
signaling pathway. Through Kyoto Encyclopedia of Genes and Genomes
pathway analyses, it was observed that the possible target genes of
miR-214-5p, predicted by StarBase v3.0, were predominantly enriched
in the p53 signaling pathway (Fig.
3A). Considering that lncRNAs could sponge miRNAs, thereby
relieving the suppression of miRNAs on target genes in tumor cells,
RT-qPCR was conducted to screen miR-214-5p target genes involved in
the p53 pathway via knocking down LINC00324. As shown in Fig. 3B, 4 target genes (CDK6, MDM2, MDM4 and
CCND1) were significantly downregulated in cells transfected with
sh-LINC00324#1/#2/#3 compared with controls. RIP suggested that the
aforementioned mRNAs were immunoprecipitated by Ago2 antibody
together with miR-214-5p, indicating that they co-existed in the
RISC (Fig. 3C). The putative
binding sites of miR-214-5p on 3′ untranslated regions (UTRs) of 4
mRNAs were indicated in Fig.
S2E. The luciferase reporter assay data further confirmed that
miR-214-5p was able to bind at sequence-specific sites within the
3′UTR of CDK6, MDM2, MDM4 and CCND1. The luciferase activity of the
indicated mRNA wild types was markedly decreased compared with that
of mRNA mutant types when overexpressing miR-214-5p (Fig. 3D). RT-qPCR analyses revealed that
CDK6, MDM2, MDM4 and CCND1 mRNA levels were upregulated in
malignant ovarian teratocarcinoma tissues and cells (Fig. 3E and F), and were suppressed by
introduction of exogenous miR-214-5p (Fig. 3G). Moreover, Kaplan-Meier analyses
demonstrated that the high levels of CDK6, MDM2, MDM4 and CCND1
were associated with the poorer prognosis of patients with IOT
(Fig. S2F). Pearson's
correlation analyses demonstrated that miR-214-5p was negatively
correlated with CDK6, MDM2, MDM4 and CCND1 (Fig. 3H). To explore the cellular
functions of these mRNAs, RT-qPCR and western blot analysis were
conducted to knock down them using 2 shRNAs, respectively, and
selected ones that demonstrated the best knockdown efficiency. The
results indicated that the mRNA and protein levels of CDK6, MDM2,
MDM4 and CCND1 were suppressed. Besides, the knockdown efficiency
of sh-CDK6#1, sh-MDM2#1, sh-MDM4#1 and sh-CCND1#1 was detected by
comparing with sh-NC (Fig.
S3A-E). The CCK-8 and colony formation assays subsequently
revealed that depletion of CDK6, MDM2, MDM4 and CCND1 could
decrease the rate of cell proliferation (Fig. 3I and J). A previous study
identified that CCND1 could regulate the cell cycle together with
CDK6 (17). It has been also
demonstrated that MDM2/MDM4 proteins exacerbate the interaction
with p53 in tumor cells to attenuate apoptotic activity of p53
(18,19). The effects of CCND1/CDK6
deficiency on cell cycle and MDM2/MDM4 deficiency on cell apoptosis
were then explored. Flow cytometry analysis indicated that
depletion of CCND1 or CDK6 resulted in G1 cell cycle arrest
(Fig. 3K). In addition, MDM2 or
MDM4 insufficiency accelerated cell apoptosis, which was validated
by flow cytometry and western blot analysis data (Fig. 3L and M). Taken together, the
results suggest that the downstream targets (CDK6/CCND1/MDM2/MDM4)
of miR-214-5p may promote cell proliferation via regulation of cell
cycle and apoptosis.
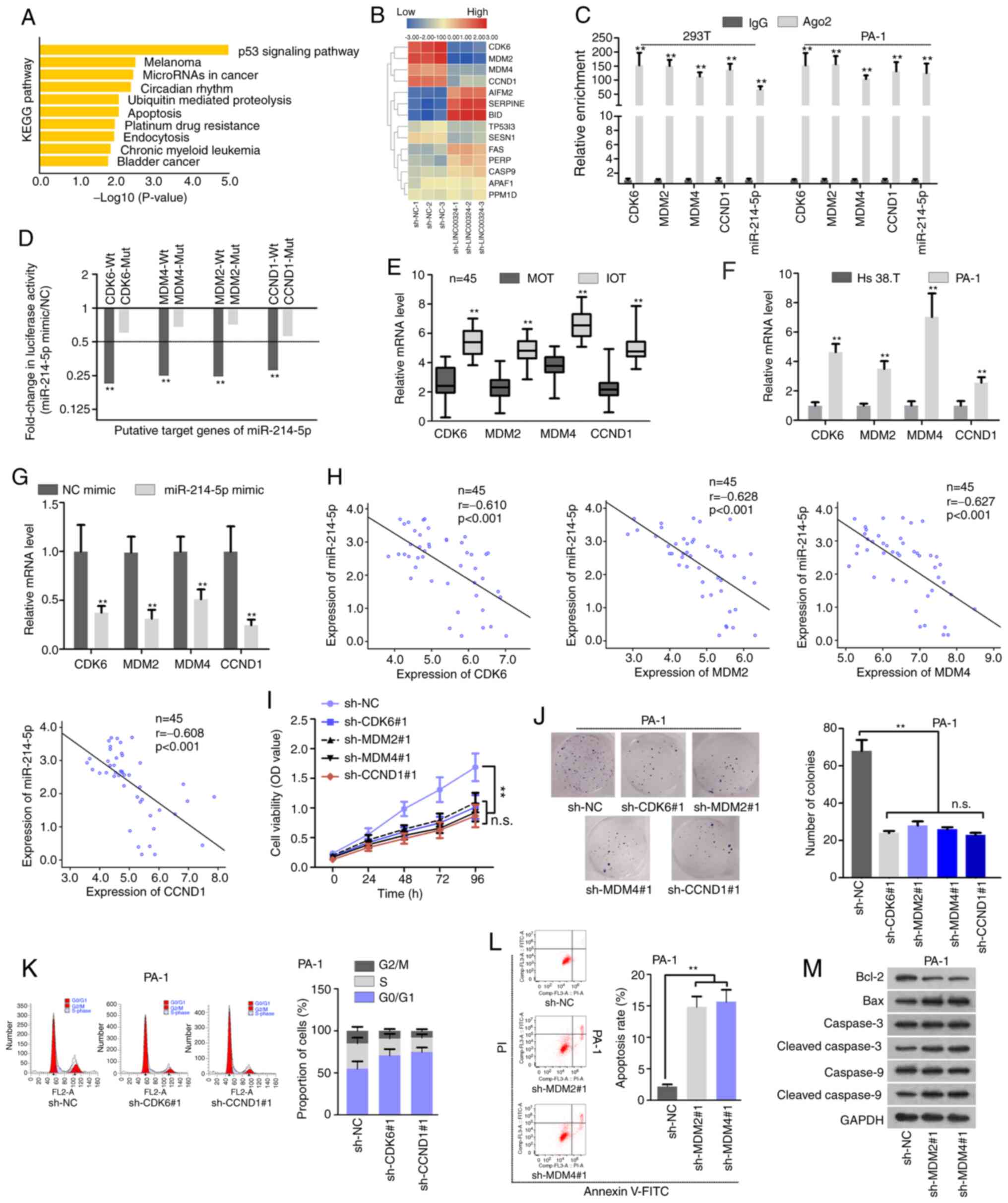 | Figure 3Downstream pathway of miR-214-5p was
enriched in the p53 signaling pathway. (A) Kyoto Encyclopedia of
Genes and Genomes pathway analyses predicted the potential target
genes of miR-214-5p involved in several signaling pathways. (B)
Hierarchical clustered heat map demonstrated the expression profile
of miR-214-5p targets involved in the p53 pathway when knocking
down LINC00324, as examined by RT-qPCR. (C) The RIP assay indicated
that CDK6, MDM2, MDM4, CCND1 and miR-214-5p were immunoprecipitated
by anti-Ago2 in 293T and PA-1 cells. (D) The luciferase reporter
assay demon-strated a >2-fold change in the luciferase activity
of CDK6/MDM4/MDM2/CCND1-Wt/Mut reporter following the
overexpression miR-214-5p in PA-1 cells. (E) The expression
patterns of CDK6, MDM2, MDM4 and CCND1 in 45 paired MOT and IOT
tissues, as determined by RT-qPCR. (F) RT-qPCR determined the
difference in expression levels of CDK6, MDM2, MDM4, and CCND1 in
Hs 38.T and PA-1 cells. (G) RT-qPCR detected the effect of
miR-214-5p overexpression on mRNA level of CDK6, MDM2, MDM4 and
CCND1 in PA-1 cells. (H) Pearson's correlation analyses determined
the correlations between CDK6/MDM2/MDM4/CCND1 and miR-214-5p in 45
IOT tissues. (I and J) The effect of interfering with CDK6, MDM2,
MDM4 or CCND1 expression on PA-1 cell proliferation was assessed by
(I) Cell Counting Kit-8 and (J) colony formation assays. (K) The
effect of CDK6 or CCND1 knockdown on PA-1 cell cycle was analyzed
by flow cytometry. (L and M) The effect of MDM2 or MDM4 knockdown
on PA-1 cell apoptosis was analyzed by (L) flow cytometry and (M)
western blot analysis. **P<0.01. miR, microRNA; p53,
tumor protein p53; LINC00324, long-chain intergenic non-coding
RNA324; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; CDK6, cyclin dependent kinase 6; MDM4, mouse double
minute 4; MDM2, murine double minute homolog 2; CCND1, cyclin D1;
Wt, wild-type; Mut, mutant; IOT, immature ovarian teratocarcinoma;
MOT, mature ovarian teratocarcinoma; NC, negative control; OD,
optical density; sh, short hairpin; n.s, not significant. |
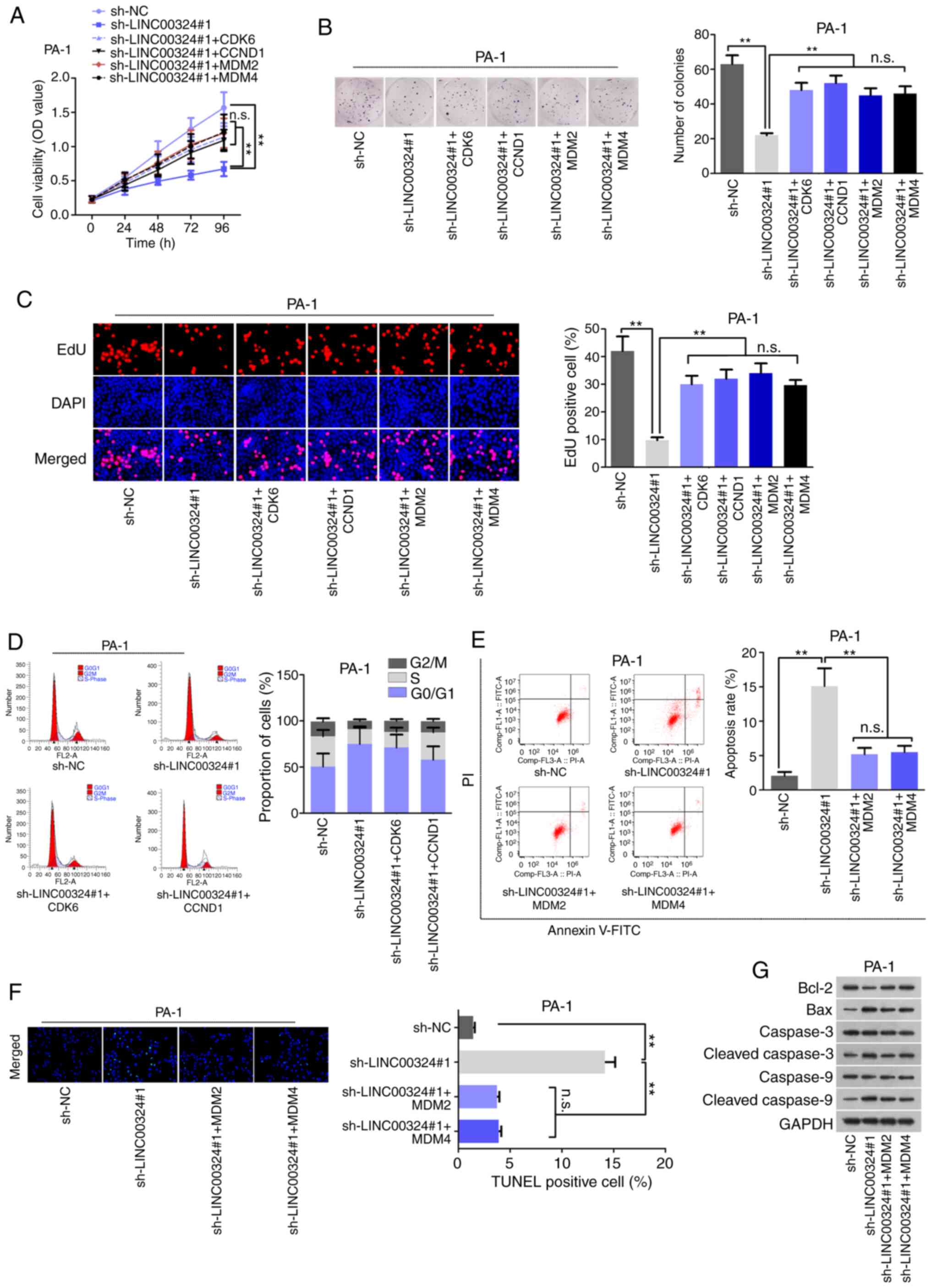 | Figure 4Abnormal proliferation of IOT cells
was regulated by LINC00324-miR-214-5p-CDK6/CCND1/MDM2/MDM4 ceRNA
network. (A) CCK-8, (B) colony formation and (C) EdU assays
measured cell proliferation in different transfection groups.
Magnification, ×200. (D) Flow cytometry analyses measured G1 cell
cycle situation in different transfection groups. (E) Flow
cytometry, (F) TUNEL and (G) western blot analysis evaluated the
levels of cell apoptosis in differently transfected cells.
Magnification, ×200. **P<0.01. IOT, immature ovarian
teratocarcinoma; LINC00324, long-chain intergenic non-coding
RNA324; miR, microRNA; CDK6, cyclin dependent kinase 6; MDM4, mouse
double minute 4; MDM2, murine double minute homolog 2; CCND1,
cyclin D1; ceRNA, competing endogenous RNA; sh, short hairpin; NC,
negative control; OD optical density; PI, propidium iodide; n.s,
not significant. |
Abnormal proliferation of IOT cells is
regulated by the LINC00324-miR-214-5p-CDK6/CCND1/MDM2/MDM4 ceRNA
network
Whether LINC00324 affected biological function of
IOT cells via the miR-214-5p-CDK6/CCND1/MDM2/MDM4 axis remains
unknown. Therefore, a series of rescue assays were conducted using
overexpression CDK6, CCND1, MDM2 and MDM4. Prior to that, the
overexpression efficiency of these mRNAs was analyzed by RT-qPCR
and western blot analysis, and vector was used as the negative
control (Fig. S3F-J). In the
rescue assays, the upregulation of CDK6, CCND1, MDM2 or MDM4
counteracted the suppressive effects of LINC00324 knockdown on cell
proliferation (Fig. 4A-C). In
addition, CDK6 or CCND1 overexpression reversed the LINC00324
knockdown-mediated function on cell cycle arrest in PA-1 cells
(Fig. 4D). Additionally, MDM2 or
MDM4 overexpression repressed cell apoptosis, originally activated
by LINC00324 downregulation (Fig. 4E
and F). Finally, the western blot assay data further confirmed
the adverse role of MDM2 or MDM4 overexpression on
sh-LINC00324-stimulated cell apoptosis (Fig. 4G). These data demonstrated that
LINC00324 accelerated cell proliferation and inhibited cell
apoptosis in IOT via sponging miR-214-5p to upregulate
CDK6/CCND1/MDM2/MDM4 expression (Fig. S4).
Discussion
Over the previous decade, an increasing number of
studies have elucidated that dysregulated miRNAs are implicated in
the initiation and progression of human malignancies. Liu et
al (20) uncovered that
miR-34a was expressed at low levels in CD44+ prostate
cancer and its ectopic expression suppressed clonogenic activity
and metastasis of tumor cells (20). miRNA sequencing (miRNA-seq) is a
useful method for the identification of differentially expressed
miRNAs. Zhu et al (15)
performed miRNA-seq analyses in non-epithelial ovarian tumors and
identified that miR-199a-5p, miR-214-5p, miR-513c-5p and miR-202-3p
were downregulated in malignant ovarian germ cell tumors. Although
the MOGCT samples analyzed by Zhu et al (15) did not involve IOT samples, we
hypothesized that expression of these miRNAs was also dysregulated
in IOT samples. Using an RT-qPCR assay, the present study
identified that only miR-214-5p was downregulated in the IOT
tissues and cells. The high level of miR-214-5p contributed to the
prognosis of the patients of IOT. miR-214-5p is one of the mature
isoforms of miR-214, which are implicated in the pathogenesis of
multiple cardiovascular diseases (21). Previously published studies have
also revealed that miR-214-5p serves a tumor-inhibitor role in
various types of cancer, such as prostate cancer (22), hepatocellular carcinoma (23) and pancreatic cancer (24). To the best of our knowledge, the
present study is the first to reveal the functions of miR-214-5p in
IOT, which may provide a promising therapeutic target for IOT.
LINC00324, also known as C17orf44, a 2,115-bp long
intervening/intergenic noncoding RNA located on human chromosome
17p13.1, has been demonstrated to serve an oncogenic role in
various types of cancer. Wu et al (25) and Pan et al (26) identified that LINC00324 inhibited
cell apoptosis and facilitated cell proliferation and migration in
osteosarcoma and lung adenocarcinoma (25,26). In addition, Zou et al
(27) demonstrated that LINC00324
exerted carcinogenic functions in gastric cancer. In the present
study, it was first verified that LINC00324 was upregulated in IOT
tissues and cells. Besides, high expression of LINC00324 was
closely associated with poor prognosis in patients with IOT.
LINC00324 served as the miR-214-5p sponge in IOT cells. In
addition, LINC00324 silencing suppressed cell proliferation and
encouraged cell apoptosis in IOT, indicating that LINC00324 was an
oncogenic gene in IOT and may be a potential therapeutic target of
IOT.
The present study also suggested that LINC00324
knock-down apparently led to a decrease in CDK6, CCND1, MDM2 and
MDM4 expression levels. CDK6, CCND1, MDM2 and MDM4 expression
levels were upregulated in IOT tissues, and were negatively
correlated with miR-214-5p. Dysregulation of these mRNAs was also
closely associated with poor prognosis in patients with IOT.
Moreover, CDK6 can form cyclin D-CDK4/6 complexes in the control of
cell cycle G1 phase progression and G1/S transition (28). CCND1 encodes cyclin D1 and forms a
complex with CDK6 to regulate CDK6 activity (17,29). The homologs MDM2 and MDM4 also
form a MDM2/MDM4 complex that binds with p53 to suppressing p53
transcriptional activity, ultimately resulting in p53 proteasomal
degradation and an inhibition of cell apoptosis (30). In the present study, knockdown of
CDK6 or CCND1 induced G1 cell cycle arrest in IOT. CDK6 or CCND1
overexpression may mitigate the LINC00324 knockdown induced cell
cycle arrest. Furthermore, the depletion of MDM2 or MDM4 promoted
cell apoptosis, and ectopic upregulation of MDM2 or MDM4 may offset
the stimulating function of LINC00324 efficiency on cell apoptosis.
However, whether LINC00324 regulates IOT malignant phenotype
through affecting the formation of CDK6/CCND1 or MDM2/MDM4 requires
further exploration.
In summary, LINC00324 facilitates cell proliferation
through competitively binding with miR-214-5p to upregulate CDK6,
CCND1, MDM2 and MDM4 expression levels under the suppression of
miR-214-5p in IOT cells.
Supplementary Data
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MC conceived the study, analyzed the data and
drafted the manuscript. YZ and MZ helped design the experiments. LX
and SW helped collect data and design the study. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Written informed consent for the use of ovarian
teratocarcinoma tissues in the present study were obtained from all
patients or their legal guardians prior to testing. The study was
approved by the Ethics Committee of The First Hospital of Fuzhou
Fujian (Fuzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Outwater EK, Siegelman ES and Hunt J: L:
Ovarian teratomas: Tumor types and imaging characteristics.
Radiographics. 21:475–490. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jorge S, Jones NL, Chen L, Hou JY, Tergas
AI, Burke WM, Ananth CV, Neugut AI, Herhshman DL and Wright JD:
Characteristics, treatment and outcomes of women with immature
ovarian teratoma, 1998-2012. Gynecol Oncol. 142:261–266. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hung YC, Chang WC, Chen LM, Chang YY, Wu
LY, Chung WM, Lin TY, Chen LC and Ma WL: Non-genomic
estrogen/estrogen receptor α promotes cellular malignancy of
immature ovarian teratoma in vitro. J Cell Physiol. 229:752–761.
2014. View Article : Google Scholar
|
|
4
|
Chan JK, Gardner AB, Chan JE, Guan A,
Alshak M and Kapp DS: The influence of age and other prognostic
factors associated with survival of ovarian immature teratoma-A
study of 1307 patients. Gynecol Oncol. 142:446–451. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eidem TM, Kugel JF and Goodrich JA:
Noncoding RNAs: Regulators of the mammalian transcription
machinery. J Mol Biol. 428:2652–2659. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet. 15(Spec No 1): R17–R29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu D, Li Y, Luo G, Xiao X, Tao D, Wu X,
Wang M, Huang C, Wang L, Zeng F and Jiang G: LncRNA SPRY4-IT1
sponges miR-101-3p to promote proliferation and metastasis of
bladder cancer cells through up-regulating EZH2. Cancer Lett.
388:281–291. 2017. View Article : Google Scholar
|
|
10
|
Huang J, Chen YX and Zhang B: IGF2-AS
affects the prognosis and metastasis of gastric adenocarcinoma via
acting as a ceRNA of miR-503 to regulate SHOX2. Gastric Cancer.
23:23–38. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
12
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar
|
|
13
|
Chang RK, Li X, Mu N, Hrydziuszko O,
Garcia-Majano B, Larsson C and Lui WO: MicroRNA expression profiles
in non-epithelial ovarian tumors. Int J Oncol. 52:55–66. 2018.
|
|
14
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu P, Wang Y, Wu J, Huang G, Liu B, Ye B,
Du Y, Gao G, Tian Y, He L and Fan Z: LncBRM initiates YAP1
signalling activation to drive self-renewal of liver cancer stem
cells. Nat Commun. 7:136082016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The rosetta stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li N, Zeng J, Sun F, Tong X, Meng G, Wu C,
Ding X, Liu L, Han M, Lu C and Dai F: p27 inhibits CDK6/CCND1
complex formation resulting in cell cycle arrest and inhibition of
cell proliferation. Cell Cycle. 17:2335–2348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Q and Lozano G: Molecular pathways:
Targeting Mdm2 and Mdm4 in cancer therapy. Clin Cancer Res.
19:34–41. 2013. View Article : Google Scholar :
|
|
19
|
Siddik ZH: Chapter 12-Apoptosis in cancer:
Mechanisms, deregulation, and therapeutic targeting. Cancer Drug
Design and Discovery. Neidle S: 2nd edition. Academic Press; San
Diego, CA: pp. 357–390. 2014, View Article : Google Scholar
|
|
20
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 17:211–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Y, Ponnusamy M, Zhang L, Zhang Y, Liu
C, Yu W, Wang K and Li P: The role of miR-214 in cardiovascular
diseases. Eur J Pharmacol. 816:138–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng C, Guo K, Chen B, Wen Y and Xu Y:
MiR-214-5p inhibits human prostate cancer proliferation and
migration through regulating CRMP5. Cancer Biomark. 26:193–202.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pang J, Li Z, Wang G, Li N, Gao Y and Wang
S: MiR-214-5p targets KLF5 and suppresses proliferation of human
hepatocellular carcinoma cells. J Cell Biochem. Sep 11–2018.Epub
ahead of print.
|
|
24
|
Cao TH, Ling X, Chen C, Tang W, Hu DM and
Yin GJ: Role of miR-214-5p in the migration and invasion of
pancreatic cancer cells. Eur Rev Med Pharmacol Sci. 22:7214–7221.
2018.PubMed/NCBI
|
|
25
|
Wu S, Gu Z, Wu Y, Wu W, Mao B and Zhao S:
LINC00324 accelerates the proliferation and migration of
osteosarcoma through regulating WDR66. J Cell Physiol. 235:339–348.
2020. View Article : Google Scholar
|
|
26
|
Pan ZH, Guo XQ, Shan J and Luo SX:
LINC00324 exerts tumor-promoting functions in lung adenocarcinoma
via targeting miR-615-5p/AKT1 axis. Eur Rev Med Pharmacol Sci.
22:8333–8342. 2018.PubMed/NCBI
|
|
27
|
Zou Z, Ma T, He X, Zhou J, Ma H, Xie M,
Liu Y, Lu D, Di S and Zhang Z: Long intergenic non-coding RNA 00324
promotes gastric cancer cell proliferation via binding with HuR and
stabilizing FAM83B expression. Cell Death Dis. 9:7172018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
VanArsdale T, Boshoff C, Arndt KT and
Abraham RT: Molecular pathways: Targeting the cyclin D-CDK4/6 axis
for cancer treatment. Clin Cancer Res. 21:2905–2910. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jirawatnotai S, Hu Y, Livingston DM and
Sicinski P: Proteomic identification of a direct role for cyclin d1
in DNA damage repair. Cancer Res. 72:4289–4293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shadfan M, Lopez-Pajares V and Yuan ZM:
MDM2 and MDMX: Alone and together in regulation of p53. Transl
Cancer Res. 1:88–89. 2012.PubMed/NCBI
|


















