Introduction
Dendritic cells (DCs) serve a critical role in
coordinating immune responses. The main function of DCs is to
search their environment for foreign antigens and present these
antigens on their cell surface to T cells. They also act as
messengers between the innate and the adaptive immune system
(1). Once activated by injury or
inflammatory stimuli, DCs migrate to the lymph nodes and stimulate
T cells to differentiate and commence a robust immune response
(2). Therefore, maintaining the
normal migratory ability of DCs is crucial for the normal function
of the body's immune system. However, the pathogenesis of numerous
types of diseases, including chronic myelocytic leukemia and
several other types of cancers, has been attributed to the
deteriorated migratory ability of DCs (3,4).
For example, a previous study reported that endometrial and
cervical tumors were capable of inhibiting DC migration from the
lesion tissue to the draining lymph node, which subsequently
promoted the immune evasion of the tumors (5). Therefore, it is important to
determine the factors that may affect the migration of DCs.
Prostaglandin E2 (PGE2) is generated by
the cyclooxygenase conversion of arachidonic acid, which is
released from membrane phospholipids, modulating various
pathological and physiological processes (6). Moreover, PGE2 has been
demonstrated to be a key modulator of DC function, including the
ability to regulate the migration of DCs (7). However, the existing data regarding
the role of PGE2 are controversial. Several reports have
suggested that PGE2 may improve the migratory ability of
DCs (8-10). On the other hand, a few studies,
including our previous study, have provided contradictory results,
suggesting an inhibitory role of PGE2 in DC migration
(11,12). Therefore, it remains a priority to
clarify the regulatory mechanism of PGE2 on the
migratory ability of DCs.
The present study used murine bone marrow-derived
DCs (BMDCs) to clarify the modulatory mechanism of PGE2
on the migratory ability of DCs. The results of the current study
may provide an improved understanding on the mechanism of DC
migration under both pathological and physiological conditions.
Furthermore, the biological implications of these findings may
provide a different perspective of the immunological surveillance
in the progression of several types of diseases.
Materials and methods
Cell culture
The animal studies were approved by the Research
Council and Animal Care and Use Committee of the Research Institute
of Surgery, Daping Hospital, Third Military Medical University
(Chongqing, China). All experiments conformed to the guidelines of
ethical use of animals, and all efforts were made to minimize
animal suffering and reduce the number of animals used. DCs were
isolated from mouse bone marrow as previously described with slight
modifications (13,14). C57BL/6 mice (male; age, 6-8 weeks;
weight, 20-25 g; n=40) were provided by the Experimental Animal
Center of Daping Hospital, Third Military Medical University. Mice
were maintained in a specific pathogen-free environment at 22±2°C
with 55±5% humidity under a 12-h light/dark cycle. Food and water
were provided ad libitum. The health and behavior of the
animals were monitored once every morning and afternoon. The mice
were sacrificed by cervical dislo cation. Death was confirmed by
the lack of pulse, breathing, corneal reflex, response to toe
pinch, respiratory sounds and heartbeat. Briefly, bone marrow cells
were flushed out from the femurs and tibias of mice with a 1-ml
syringe filled with RPMI-1640, and red cells were subsequently
removed using erythrocyte lysis fluid (Beyotime Institute of
Biotechnology). The remaining cells were cultured in RPMI-1640
supplemented with 10% FBS (both from Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin, 100 µg/ml
streptomycin, 20 ng/ml recombinant granulocyte-macrophage
colony-stimulating factor and IL-4 (both from PeproTech, Inc.).
Cells were cultured at 37°C in an atmosphere containing 5%
CO2. On day 3, non-adherent granulocytes were gently
removed, and fresh media were added. On day 7, the immature DCs
were stimulated with 1 µg/ml LPS (MilliporeSigma) for 24 h.
On day 8, mature DCs were collected. In subsequent experiments,
mature DCs were treated with different concentrations of
PGE2 for 24 h.
Transwell migration assay
The lower chambers of 24-well Transwell plates (8.0
µm pore size; MilliporeSigma) were filled with 600 µl
serum-free RPMI-1640 medium including C-C motif chemokine 19
(CCL19; 100 ng/ml; PeproTech, Inc.). DCs (1×105 cells in
0.1 ml) resuspended in serum-free RPMI-1640 medium were seeded in
the upper chambers of the Transwell plates and allowed to migrate
for 3 h at 37°C in 5% CO2. The numbers of migrated DCs
that were harvested from the medium of the lower chambers were
counted with a hemocytometer under an inverted microscope (DMi8;
Leica Microsystems GmbH; magnification, ×100).
3D migration assay
DCs were mixed with a collagen matrix (BD Matrigel;
BD Biosciences; final concentration 1.7 mg/ml; 4.5×105
cells/ml; 45 µl cells/well) in migration chambers (cat. no.
70326-10; CoverWell™ Perfusion Chamber; Electron Microscopy
Sciences). The remaining space of the chambers was filled with
RPMI-1640 medium containing 200 ng/ml CCL19. Migration of DCs was
recorded by bright-field time-lapse video microscopy at 37°C, which
started 10 min after cell injection, using inverted microscopes
(Observer Z1; Zeiss GmbH; magnification, ×100) fitted with 10×
objectives and Axiocam cameras (Zeiss GmbH). Cells were imaged at a
frame rate of 2 min up to 61 frames. Computer-assisted cell
tracking was performed with custom software (ImageJ v1.46r bundled
with 64-bit Java v1.6.0_20; National Institutes of Health). The
average speed was calculated as the step length per minute for each
cell. A total of 30 randomly selected cells were included in one
experiment.
Flow cytometry
DCs (1×106) were blocked for 15 min at
4°C with PBS containing 0.5% BSA (MilliporeSigma), and subsequently
incubated with the respective antibodies for 30 min at 4°C. After
being washed twice with PBS, the cells were resuspended in 200
µl PBS. The antibodies used included
phycoerythrin-conjugated anti-mouse CD40 (1:100; cat. no. 12-0401),
CD80 (1:100; cat. no. 12-0801), CD86 (1:100; cat. no. 12-0862),
major histocompatibility complex II (MHCII; 1:100; cat. no.
12-5321) and C-C chemokine receptor type 7 (CCR7; 1:100; cat. no.
12-1971; all from eBioscience; Thermo Fisher Scientific, Inc.).
FACS analysis was performed on a FACSCalibur flow cytometer using
CellQuest Pro software (version 6.0; BD Biosciences).
Western blotting
Western blotting was used to determine the protein
expression level. After treatment with different concentrations of
PGE2, cell lysates were prepared by collecting DCs in
the cell lysis buffer for Western or IP (cat. no. P0013; Beyotime
Institute of Biotechnology). Protein concentration was determined
using BCA Protein Assay kit (cat. no. 23227; Thermo Fisher
Scientific, Inc.). Cell lysates containing equal amounts of protein
(30-50 µg) were subjected to a 10% SDS-PAGE. Proteins were
transferred to nitrocellulose membranes and subsequently blocked in
TBS-0.05% Tween-20 buffer containing 5% BSA (MilliporeSigma). The
membranes were incubated with antibodies against phosphopaxillin
(Tyr118) (1:1,000; cat. no. orb14813; Biorbyt Ltd.), paxillin
(1:1,000; cat. no. orb89537; Biorbyt Ltd.) and GAPDH (1:2,000; cat.
no. 60004-1-Ig; ProteinTech Group, Inc.) overnight at 4°C.
Following incubation with HRP-conjugated goat anti-mouse (1:1,000;
cat. no. SA00001-1; ProteinTech Group, Inc.) or goat anti-rabbit
(1:1,000; cat. no. A0208; Beyotime Institute of Biotechnology)
secondary antibody at room temperature for 1 h, immunoreactivities
were detected using SuperSignal™ West Pico PLUS Chemiluminescent
Substrate (cat. no. 34577; Thermo Fisher Scientific, Inc.).
Densitometric analysis was performed using ImageJ software (ImageJ
v1.46r bundled with 64-bit Java v1.6.0_20; National Institutes of
Health) with protein expression levels normalized to GAPDH.
Immunofluorescence detection of F-actin
cytoskeleton
DCs (1×106) were seeded on the coverslips
coated with poly-L-Lysine and incubated at 37°C overnight. The
cells were subsequently fixed with 4% paraformaldehyde at 4°C for
15 min, permeabilized with 0.1% Triton X-100 at room temperature
for 5 min and incubated with 1% BSA at room temperature for 30 min.
To detect F-actin, the cells were stained with a FITC-phalloidin
solution (5 µg/ml in 1% BSA-PBS; MilliporeSigma) for 45 min
at room temperature. Subsequently, the cells were counterstained
with DAPI for 5 min at room temperature. Fluorescent images were
acquired using confocal microscopy (magnification, ×400).
In vivo migration assay
C57BL/6 mice (n=5 per group; male; age, 4-6 weeks;
weight, 20-25 g) provided by the Experimental Animal Center of
Daping Hospital, Third Military Medical University, were maintained
as aforementioned. The mice were injected in the left footpad with
1×106 labeled DCs. DCs were labeled with Qtracker™ 705
cell labeling kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Animals injected with PBS were used as
controls. The experimental protocol lasted 48 h and no mice died
during the protocol. The mice were sacrificed by cervical
dislocation as aforementioned. The numbers of labeled DCs collected
from inguinal and popliteal lymph nodes were determined by FACS.
FACS analysis was performed on a FACSCalibur flow cytometer using
CellQuest Pro software (version 6.0; BD Biosciences). For the
detection of labeled DCs in dissected tissues, the lymph nodes of
mice were dissected 48 h after injection of DCs, embedded in
Tissue-Tek OCT compound (Sakura Finetek USA, Inc.) and frozen in
liquid nitrogen. Cryosections (8 µm) were cut using a
cryostat (Leica Microsystems GmbH). The sections were dried and
frozen at -20°C before use. The slides were fixed with acetone (15
min; 4°C) and counterstained with DAPI (5 min; room temperature).
Following washing with PBS, the slides were mounted in 50% glycerol
(in PBS) and examined using fluorescence microscopy (magnification,
×400).
RNA-sequencing (RNA-seq) analysis
Total RNAs of DCs treated with 0, 2.5 and 10
µg/ml PGE2 for 24 h were extracted using
TRIzol® Reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Qubit RNA Assay Kit
and Qubit 2.0 Fluorometer (Thermo Fisher Scientific, Inc.) were
used to quantify the extracted RNA. Sequencing libraries of mRNA
were constructed using Hieff NGS™ MaxUp Dual-mode mRNA Library Prep
Kits for Illumina® (Shanghai Yeasen Biotechnology Co.,
Ltd.). mRNA was purified from total RNA using Oligo dT magnetic
beads. The mRNA was reversely transcribed to double-stranded cDNA
(dscDNA). The dscDNA was repaired with phosphate and stickiness A
at the two ends (5′ and 3′), and then ligated with a DNA adaptor at
the 3′-end. The amplification products were purified using Hieff
NGS™ DNA Selection Beads (Shanghai Yeasen Biotechnology Co., Ltd).
The obtained library products were sequenced with a pair end 150
base pair strategy using the Illumina NovaSeq6000 platform
(Illumina, Inc.). Gene expression profiles were analyzed by Sangon
Biotech Co., Ltd. Genes with >1.2-fold changes in their
expression were considered as differentially expressed genes. All
unique genes were functionally annotated by searching against the
National Center for Biotechnology Information NR or NT database
(http://www.ncbi.nlm.nih.gov/), Gene
Ontology (GO) database (http://www.geneontology.org/) and Kyoto Encyclopedia
of Genes and Genomes (KEGG) database (http://www.genome.jp/). The Q-values of the GO and
KEGG analysis were calculated, and Q<0.05 was considered to
indicate a statistically significant difference. The mouse
reference genome data from the Ensembl database (http://www.ensembl.org/) were used.
Statistical analysis
Histogram and scatter graphs were generated using
GraphPad Prism v5 software (GraphPad Software, Inc.) and data are
presented as the mean ± SEM. Differences between multiple groups
were analyzed by one-way ANOVA followed by Dunnett's post hoc test
using IBM SPSS Statistics v19 software (IBM Corp.) P<0.05 was
considered to indicate a statistically significant difference.
Results
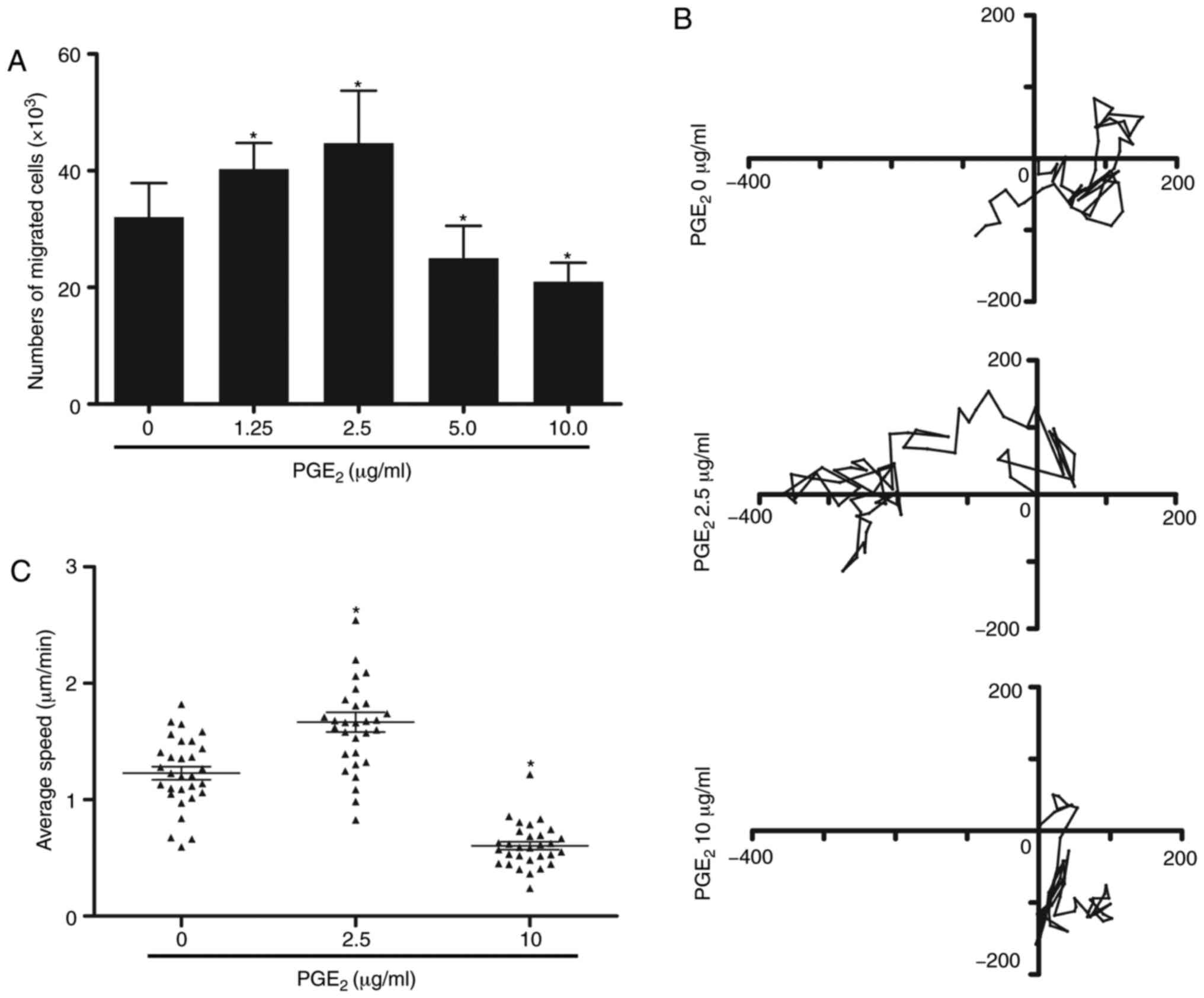
PGE2 serves a dual role in the
migration of DCs
To determine the effect of PGE2 on cell
migration, gradient concentrations of PGE2 were added to
the culture medium of DCs. The migratory ability of DCs was
determined by a Transwell migration assay. According to the
results, the concentrations of PGE2 were divided into
two different groups. As depicted in Fig. 1A, lower concentrations of
PGE2 (1.25-2.5 µg/ml) promoted cell migration,
while the migratory ability of DCs was significantly inhibited
following treatment with higher concentrations of PGE2
(5-10 µg/ml) compared with cells treated with 0 µg/ml
PGE2. As 2.5 and 10 µg/ml PGE2
demonstrated the highest effect on the migratory ability, these
doses were selected to treat DCs in subsequent experiments. A 3D
migration assay was subsequently used to validate these findings.
Time-lapse video analysis was applied to observe the migration of
individual DCs. High concentration of PGE2 significantly
inhibited DC migration, whereas low concentration exhibited the
opposite effect compared with cells treated with 0 µg/ml
PGE2 (Fig. 1B and C).
These findings suggested that PGE2 may serve a dual role
in modulating the migration of DCs.
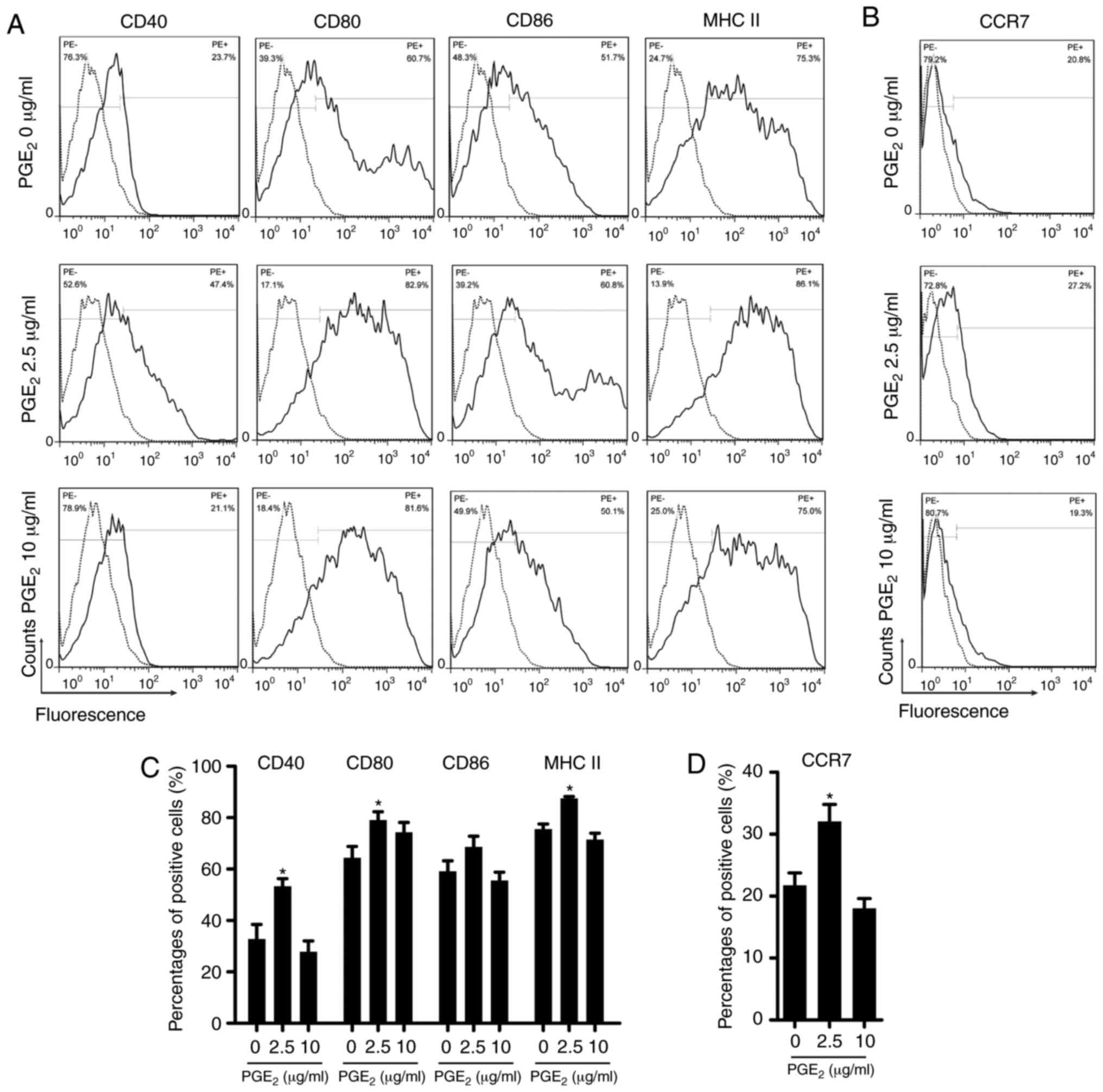
PGE2 affects the expression of
surface molecules on DCs
Following stimulation with 2.5 µg/ml
PGE2, the expression of several co-stimulatory molecules
on the surface of the DCs, such as CD40, CD80 and MHC II, was
significantly increased (Fig. 2A and
C). The expression of CD86 was also increased, but it was not
statistically significant. Treatment with 10 µg/ml
PGE2 decreased the expression of all molecules except
for that of CD80, which was unexpectedly increased, although not
statistically significant. Since CCR7 is required for the migration
of DCs (9), the surface
expression of CCR7 was also analyzed (Fig. 2B and D). The expression of CCR7
was significantly upregulated following treatment with 2.5
µg/ml PGE2 and slightly downregulated after
incubation with 10 µg/ml PGE2 compared with cells
treated with 0 µg/ml PGE2.
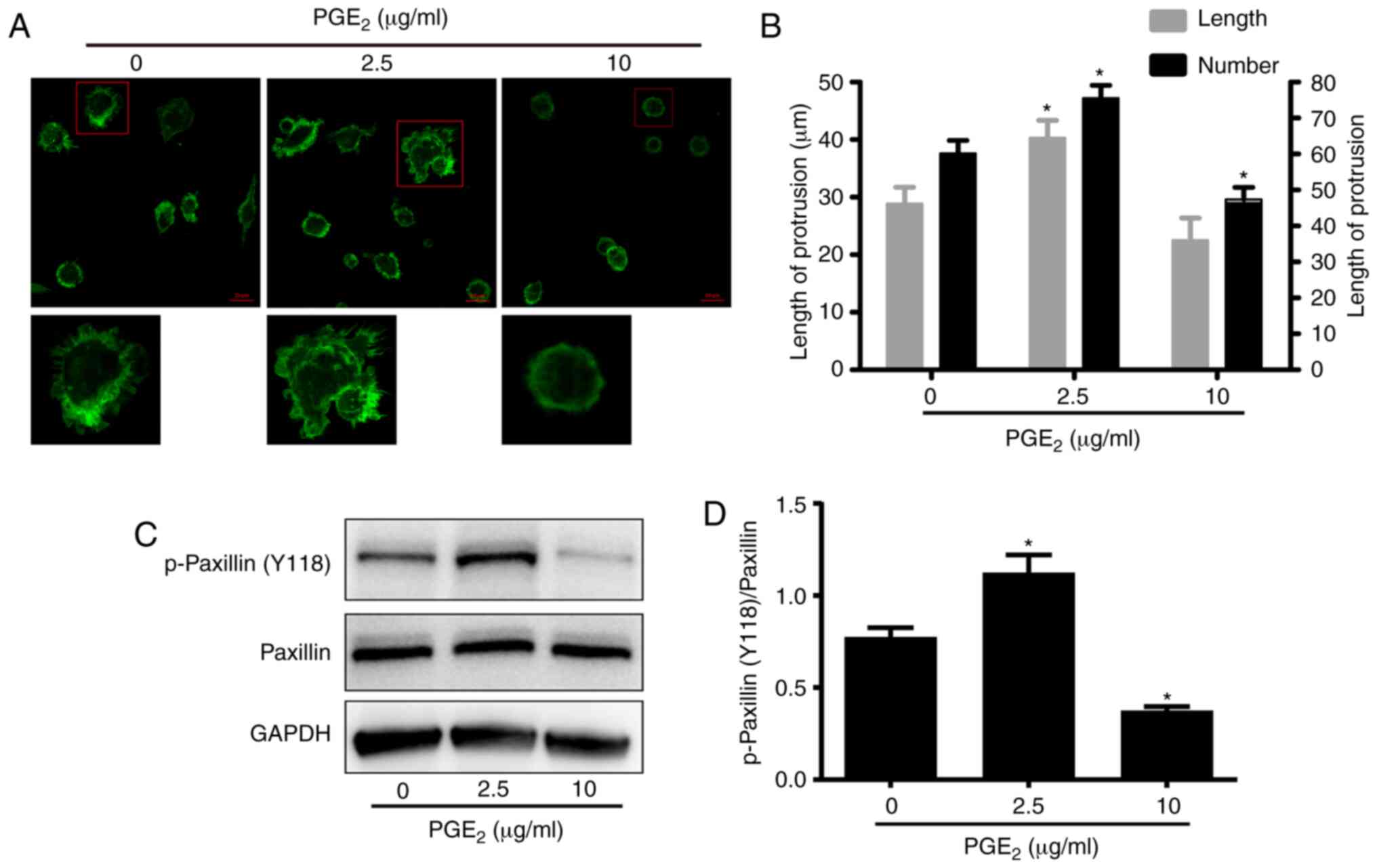
PGE2 exerts its effect on DC
migration by reorganizing the F-actin cytoskeleton
Cell migration is dependent on cytoskeletal
rearrangements, including the reorganization of the F-actin
cytoskeleton (15). Therefore, it
was further investigated whether the F-actin cytoskeleton of DCs
was affected by PGE2 treatment. The F-actin cytoskeleton
was analyzed by immunofluorescence staining using confocal
microscopy. The F-actin cytoskeleton in DCs has been reported to
attach to the internal surface of the cell membrane (16). As illustrated in Fig. 3A, compared with the control
samples, following treatment with low concentration of
PGE2 (2.5 µg/ml), the F-actin cytoskeleton was
irregularly organized. However, treatment with high concentration
of PGE2 (10 µg/ml) induced the arrangement of the
F-actin cytoskeleton into regular circles. The method of Hu et
al (16) was used to detect
the formation of filopodia in DCs, according to which the number of
cell protrusions was calculated. As depicted in Fig. 3B, the number of filopodia on the
surface of DCs was decreased following culture of DCs in media
containing 10 µg/ml PGE2. By contrast, treatment
with 2.5 µg/ml PGE2 increased the number of
filopodia on the surface of DCs compared with cells treated with 0
µg/ml PGE2. Via recruiting structural and
signaling molecules, paxillin is a multifunctional focal adhesion
adaptor protein, which has been reported to serve an important role
in cell migration (17). Western
blotting was used to analyze the phosphorylation levels of paxillin
at Tyr118. As illustrated in Fig. 3C
and D, treatment with 10 µg/ml PGE2 reduced
the phosphorylation levels of paxillin at Tyr118 in DCs compared
with cells treated with 0 µg/ml PGE2. By
contrast, treatment with 2.5 µg/ml PGE2 enhanced
the phosphorylation levels of paxillin at Tyr118. However, the
total protein expression levels of paxillin were not affected by
neither of the treatments. Therefore, these results suggested that
PGE2 may exert its effect on DC migration by
reorganizing the F-actin cytoskeleton.
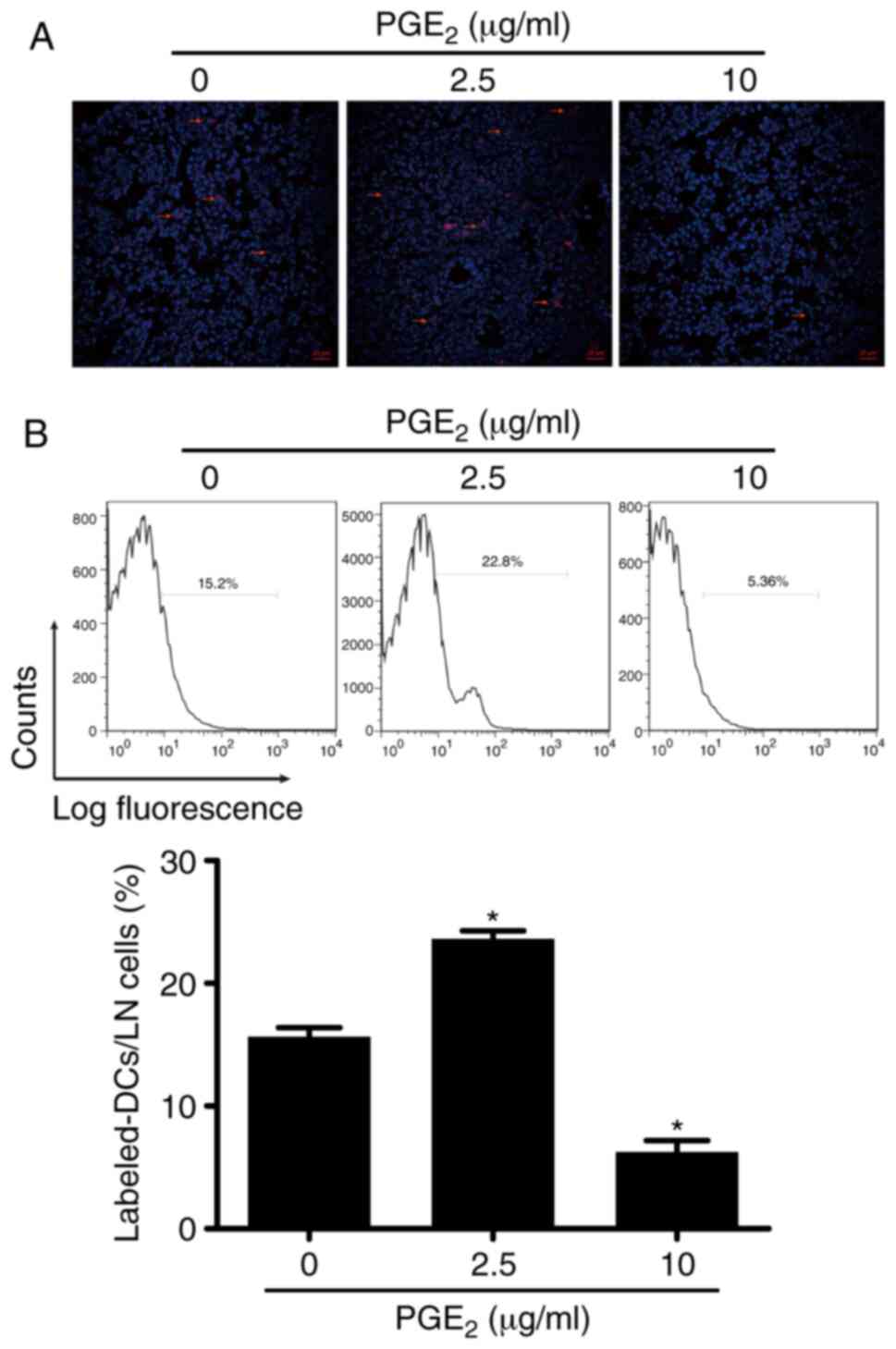
Validation of the effect of
PGE2 on DC migration in vivo
The aforementioned experiments demonstrated the
modulatory effect of PGE2 on DC migration in
vitro. This mechanism was subsequently validated with in
vivo experiments using C57BL/6 mice. Both the results from FACS
and immunofluorescence analyses revealed that high concentration of
PGE2 (10 µg/ml) inhibited DC migration in
vivo, which was evidenced by the lower number of labeled DCs
observed in the lymph nodes compared with mice injected with 0
µg/ml PGE2-treated DCs (Fig. 4A and B). On the other hand, the
higher number of labeled cells obtained following treatment with
2.5 µg/ml PGE2 indicated that DC migration was
facilitated by the low concentration of PGE2 in
vivo. These results indicated that PGE2 exhibited
the same modulatory effect on DC migration both in vivo and
in vitro.
Transcriptome analysis of DCs treated
with PGE2
A transcrip-tome analysis using RNA-seq was
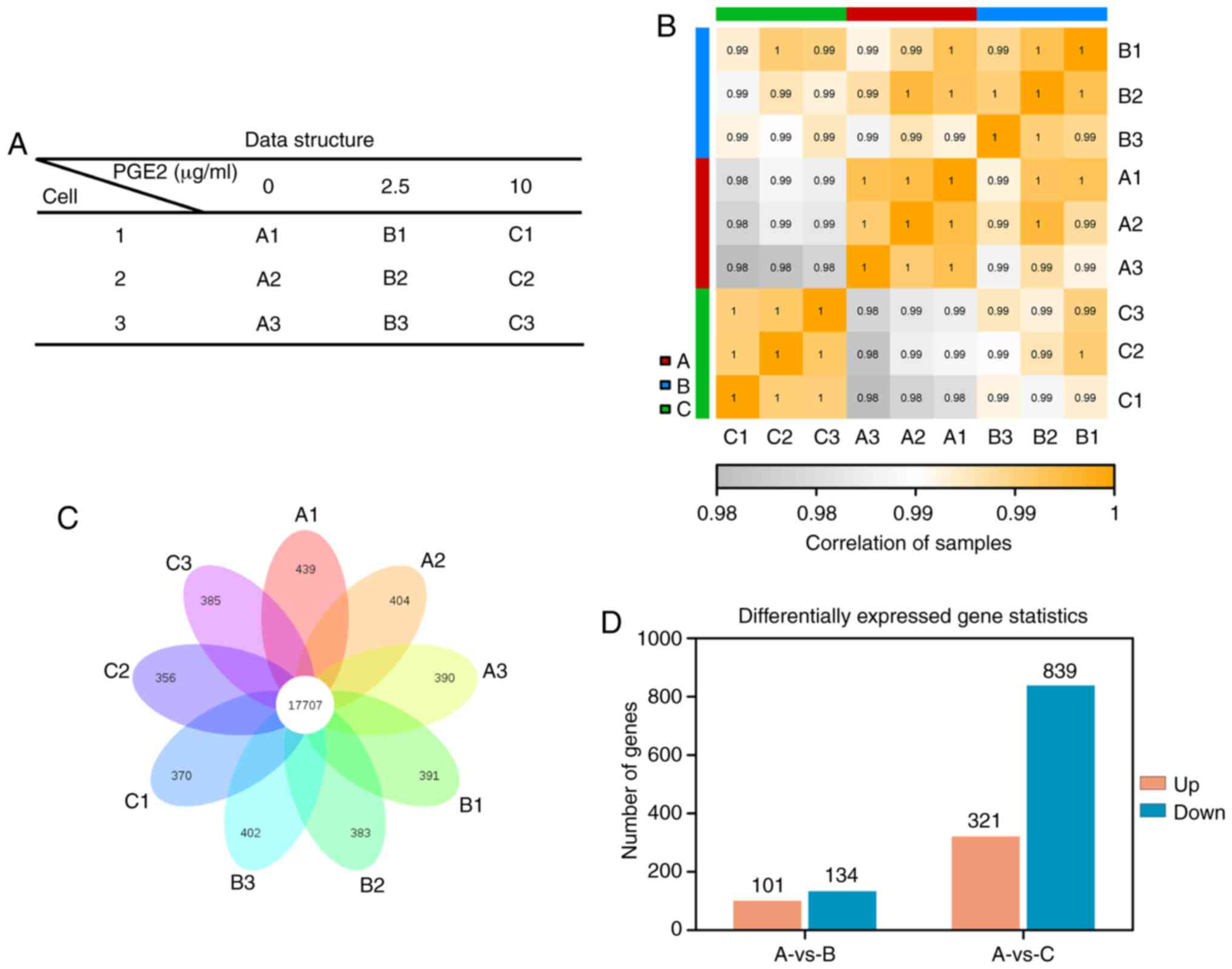
performed, and the datasets analyzed are presented in Fig. 5A. A total of 9 samples divided
into three groups, which represented three different concentrations
of PGE2, were included. The correlation between samples
is demonstrated in Fig. 5B.
Between any two samples, a higher correlation indicated higher
similarity of the expression patterns and better biological
repeatability. As illustrated in Fig.
5B, the sample correlations within groups were higher compared
with those between groups, indicating that the repeatability of
samples within each group was satisfactory. A total of 17,707 genes
were identified to be co-expressed among all samples (Fig. 5C). In addition, >300 uniquely
expressed genes were detected in each sample. The gene expression
profiles were considered to be differentially expressed when a
>1.2-fold change was obtained. The analysis identified 321
upregulated genes and 839 downregulated genes following treatment
with 10 µg/ml PGE2 (Fig. 5D). Conversely, treatment with 2.5
µg/ml PGE2 resulted in 101 upregulated genes and
134 downregulated genes compared with the 0 µg/ml
PGE2 group. As presented in Tables I and II, GO functional term enrichment
analysis illustrated that these genes were related to cellular
functions, such as 'cell motility', 'cell migration', 'cell
chemotaxis' and 'cell adhesion', among others. Furthermore, KEGG
signaling pathway enrichment analysis was performed to analyze the
relevant signaling pathways of the differentially expressed genes.
The results revealed that signaling path-ways, including 'cell
adhesion molecules', 'MAPK signaling pathway', 'focal adhesion',
'cytokine-cytokine receptor interaction', 'regulation of actin
cytoskeleton', 'leukocyte transendothelial migration', 'chemokine
signaling pathway' and 'PI3K-Akt signaling pathway', among others,
were associated with the differentially expressed genes (Tables III and IV).
 | Table IGO term analysis of the
differentially expressed genes induced by high concentration of
prostaglandin E2. |
Table I
GO term analysis of the
differentially expressed genes induced by high concentration of
prostaglandin E2.
A, Upregulated
genes
|
|---|
| GO term | Gene number | Q-value |
|---|
| GO:0048870 'cell
motility' | 33 | 0.031953 |
| GO:0016477 'cell
migration' | 32 | 0.018743 |
| GO:0006935
'chemotaxis' | 20 | 0.002641 |
| GO:0030335
'positive regulation of cell migration' | 17 | 0.011600 |
| GO:2000147
'positive regulation of cell motility' | 17 | 0.015399 |
| GO:0060326 'cell
chemotaxis' | 14 | 0.001859 |
| GO:0030595
'leukocyte chemotaxis' | 8 | 0.049438 |
| GO:1990266
'neutrophil migration' | 6 | 0.042027 |
| GO:0038089
'positive regulation of cell migration by vascular endothelial
growth factor signaling pathway' | 2 | 0.033365 |
| GO:0090063
'positive regulation of microtubule nucleation' | 2 | 0.021358 |
| GO:0010968
'regulation of microtubule nucleation' | 2 | 0.033365 |
|
B, Downregulated
genes
|
| GO term | Gene number | Q-value |
|
| GO:0030334
'regulation of cell migration' | 69 |
3.79×10−9 |
| GO:2000145
'regulation of cell motility' | 69 |
3.27×10−8 |
| GO:0048870 'cell
motility' | 33 | 0.031953 |
| GO:0060326 'cell
chemotaxis' | 33 |
1.80×10−7 |
| GO:0016477 'cell
migration' | 32 | 0.018743 |
| GO:0030595
'leukocyte chemotaxis' | 28 |
3.84×10−8 |
| GO:0050839 'cell
adhesion molecule binding' | 21 | 0.001122 |
| GO:0006935
'chemotaxis' | 20 | 0.002641 |
| GO:0005096 'GTPase
activator activity' | 19 | 0.009055 |
| GO:0030335
'positive regulation of cell migration' | 17 | 0.011600 |
| GO:2000147
'positive regulation of cell motility' | 17 | 0.015399 |
| GO:0004896
'cytokine receptor activity' | 13 | 0.001157 |
| GO:0003777
'microtubule motor activity' | 9 | 0.028882 |
| GO:0048020 'CCR
chemokine receptor binding' | 7 | 0.022002 |
| GO:0090063
'positive regulation of microtubule nucleation' | 2 | 0.021358 |
 | Table IIGO term analysis of the
differentially expressed genes induced by low concentration of
prostaglandin E2. |
Table II
GO term analysis of the
differentially expressed genes induced by low concentration of
prostaglandin E2.
A, Upregulated
genes
|
|---|
| GO term | Gene number | Q-value |
|---|
| GO:0044281 'small
molecule metabolic process' | 17 | 0.043246 |
| GO:0030335
'positive regulation of cell migration' | 8 | 0.067606 |
| GO:2000147
'positive regulation of cell motility' | 8 | 0.075799 |
| GO:0051272
'positive regulation of cellular component movement' | 8 | 0.078608 |
| GO:0042572 'retinol
metabolic process' | 4 | 0.017945 |
| GO:0006720
'isoprenoid metabolic process' | 4 | 0.043305 |
| GO:0016101
'diterpenoid metabolic process' | 4 | 0.030277 |
| GO:0032620
'interleukin-17 production' | 3 | 0.043246 |
| GO:0046460 'neutral
lipid biosynthetic process' | 3 | 0.043246 |
| GO:0051973
'positive regulation of telomerase activity' | 3 | 0.043246 |
|
B, Downregulated
genes
|
| GO term | Gene number | Q-value |
|
| GO:0007155 'cell
adhesion' | 23 | 0.000066 |
| GO:0022610
'biological adhesion' | 23 | 0.000071 |
| GO:0048870 'cell
motility' | 19 | 0.010072 |
| GO:0016477 'cell
migration' | 18 | 0.008858 |
| GO:0030334
'regulation of cell migration' | 16 | 0.000929 |
| GO:2000145
'regulation of cell motility' | 16 | 0.001466 |
| GO:0030155
'regulation of cell adhesion' | 15 | 0.000305 |
| GO:0043062
'extracellular structure organization' | 12 | 0.000018 |
| GO:2000147
'positive regulation of cell motility' | 10 | 0.012432 |
| GO:0060326 'cell
chemotaxis' | 8 | 0.005049 |
| GO:0050921
'positive regulation of chemotaxis' | 6 | 0.002927 |
 | Table IIIKEGG pathway analysis of the
differentially expressed genes induced by high concentration of
prostaglandin E2. |
Table III
KEGG pathway analysis of the
differentially expressed genes induced by high concentration of
prostaglandin E2.
A, Upregulated
genes
|
|---|
| KEGG pathway | Gene number | Q-value |
|---|
| 'Cytokine-cytokine
receptor interaction' | 12 | 0.115422 |
| 'PI3K-Akt signaling
pathway' | 11 | 0.440419 |
| 'MAPK signaling
pathway' | 10 | 0.414593 |
| 'Focal
adhesion' | 7 | 0.463380 |
| 'Rap1 signaling
pathway' | 5 | 0.756316 |
| 'Chemokine
signaling pathway' | 5 | 0.751124 |
| 'Cell adhesion
molecules (CAMs)' | 4 | 0.756316 |
| 'Ras signaling
pathway' | 4 | 0.886593 |
| 'Antigen processing
and presentation' | 3 | 0.729945 |
| 'Leukocyte
transendothelial migration' | 2 | 0.886593 |
| 'Regulation of
actin cytoskeleton' | 2 | 0.958670 |
|
B, Downregulated
genes
|
| KEGG pathway | Gene number | Q-value |
|
| 'Cell adhesion
molecules (CAMs)' | 20 | 0.000566 |
| 'Chemokine
signaling pathway' | 20 | 0.003940 |
| 'MAPK signaling
pathway' | 17 | 0.523913 |
| 'Focal
adhesion' | 16 | 0.103853 |
| 'Ras signaling
pathway' | 15 | 0.344020 |
| 'Rap1 signaling
pathway' | 14 | 0.338164 |
| 'Antigen processing
and presentation' | 14 | 0.000502 |
| 'Regulation of
actin cytoskeleton' | 12 | 0.592493 |
| 'cAMP signaling
pathway' | 7 | 0.988183 |
| 'Leukocyte
transendothelial migration' | 6 | 0.794709 |
| 'T cell receptor
signaling pathway' | 3 | 1.000000 |
 | Table IVKEGG pathway analysis of the
differentially expressed genes induced by low concentration of
prostaglandin E2. |
Table IV
KEGG pathway analysis of the
differentially expressed genes induced by low concentration of
prostaglandin E2.
A, Upregulated
genes
|
|---|
| KEGG pathway | Gene number | Q-value |
|---|
| 'PI3K-Akt signaling
pathway' | 5 | 0.170644 |
| 'Cytokine-cytokine
receptor interaction' | 4 | 0.223721 |
| 'Arginine and
proline metabolism' | 4 | 0.007759 |
| 'Glycerolipid
metabolism' | 4 | 0.007759 |
| 'Focal
adhesion' | 3 | 0.282260 |
| 'Rap1 signaling
pathway' | 2 | 0.453930 |
| 'Ras signaling
pathway' | 2 | 0.453930 |
| 'Cell adhesion
molecules (CAMs)' | 1 | 0.536388 |
|
B, Downregulated
genes
|
| KEGG pathway | Gene number | Q-value |
|
| 'PI3K-Akt signaling
pathway' | 7 | 0.217307 |
| 'AGE-RAGE signaling
pathway in diabetic complications' | 7 | 0.003353 |
| 'Chemokine
signaling pathway' | 5 | 0.209988 |
| 'Focal
adhesion' | 4 | 0.321484 |
| 'cAMP signaling
pathway' | 3 | 0.516267 |
| 'Regulation of
actin cytoskeleton' | 3 | 0.542349 |
| 'Cell adhesion
molecules (CAMs)' | 2 | 0.611224 |
| 'Antigen processing
and presentation' | 2 | 0.467692 |
Discussion
PGE2 has been associated with numerous
processes resulting in the induction of inflammation (18). PGE2 is usually
considered to be a classical pro-inflammatory mediator. For
example, Hooper et al (19) described novel pro-inflammatory
functions of PGE2 in murine BMDCs, including its ability
to inhibit the production of IL-27. However, accumulating evidence
has indicated that PGE2 may also exert anti-inflammatory
effects. For instance, a previous study has reported that
PGE2 exhibited an anti-inflammatory effect by inhibiting
cytokine production in human lung macrophages (20). Therefore, it is not surprising
that PGE2 has been discovered to serve a dual role in
certain modulatory processes. In experimental autoimmune
encephalomyelitis, PGE2 has been indicated to facilitate
the generation of Th1 and Th17 cells during immunization, but
attenuate the invasion of these cells into the brain, thereby
protecting the blood brain barrier (21). Moreover, Poloso et al
(22) demonstrated that
physiologically relevant concentrations of PGE2
suppressed IL-23 production in DCs, while lower concentrations of
PGE2 promoted IL-23 production. Notably, PGE2
has been identified to regulate macrophage migration in a
concentration-dependent manner (23). Low concentrations of
PGE2 have been observed to promote migration of
macrophages, while high doses of PGE2 have been
indicated to inhibit migration and promote adhesion of macrophages
(23). These effects occurred via
a similar mechanism as those observed in the present study, except
for the fact that a different cell type was used.
As both DCs and macrophages are central cells in the
immune system, it is helpful to understand the dual function of
PGE2 in immune regulation. More importantly, these
findings have suggested that the applied concentration of
PGE2 may be the key in clarifying its actual role.
Therefore, a large range of PGE2 concentrations was
initially used in the present study to determine their effect on DC
migration. In particular, 5 µg/ml PGE2 exhibited
an inhibitory effect on DC migration, which was also reported by
Baratelli et al (12). To
further validate these findings, a higher concentration of PGE2 (10
µg/ml) was used in the experiments of the current study. As
expected, 10 µg/ml PGE2 exerted a stronger
inhibitory effect on DC migration compared with of 5 µg/ml.
These results strengthened the hypothesis that DC migration may be
inhibited by high concentration of PGE2. In addition,
the data of the present study were not only obtained with in
vitro experiments, but were also validated in vivo using
an animal model, which further supported the hypothesized
mechanism.
Numerous molecules and substances that serve
important roles in physiological and pathological processes have
been demonstrated to exert a dual role (24-31). For example, as a key factor
regulating cellular hypoxia, hypoxia inducible factor-1α (HIF-1α)
has been reported to upregulate the expression levels of forkhead
box P3 (FOXP3) to positively regulate the differentiation function
of regulatory T cells (24,25). On the other hand, other previous
studies have indicated that HIF-1α negatively regulated the
differentiation of regulatory T cells by promoting the degradation
of FOXP3 (26,27). In cancer progression, numerous
molecules have been indicated to simultaneously exhibit both tumor
suppressive and oncogenic effects, such as yes-associated protein 1
and p21 (RAC1)-activated kinase 6 (28-31). Moreover, PGE2 was
previously identified to serve a dual role in regulating the
migratory ability of macrophages (23).
In our previous study, it was demonstrated that
compared with normal cervical tissues, the expression of
PGE2 was gradually upregulated in samples of low-grade
squamous intraepithelial lesion, high-grade squamous
intraepithelial lesion and squamous carcinoma, which was
accompanied by the progression of the disease (11). Therefore, an association between
the regulation of DC migration by PGE2 and the
progression of cervical cancer was hypothesized to exist, based on
the results of the current study. In normal cervical tissues and
during the early stages of the development of cervical lesions,
PGE2 was considered to serve a tumor suppressive role.
The low concentration of PGE2 promoted DC migration,
which maintained a normal function of the immune response. However,
when the disease further developed, the lesion tissues began to
continuously synthesize and release PGE2, which resulted
in high levels of PGE2. DC migration was subsequently
inhibited, thereby altering the normal function of the immune
response, which further promoted the development of the disease.
The highest concentration of PGE2 detected in the
cervical lesion tissues was a 4-fold increase compared with the
normal tissues. This may explain why a high concentration of
PGE2 is required to inhibit DC migration.
To the best of our knowledge, the present results
may provide a novel model of DC migration in response to
chemokines, where the presence of a gradient concentration of
PGE2 may modulate cytoskeletal reorganization. A number
of molecules are considered to serve a role in mediating the
effects of PGE2. For example, it has been revealed that
when low doses of PGE2 were present during
differentiation of DCs, the migration of DCs towards CCL19 and
CCL21 was favored (32,33). This process has been suggested to
be regulated via the prostaglandin E receptor (EP)4/cAMP/protein
kinase A pathway in a PGE2-dependent manner (34-36). The regulation of cytoskeletal
remodeling by PGE2 has been indicated to be mediated by
the increase of intracellular cAMP levels following signaling via
the EP2 and EP4 receptors, which has been identified by the use of
EP receptor agonists. Moreover, both EP2 and EP4 have been
suggested to be required for podosome disassembly and focal
adhesion formation in DCs following treatment with high
concentration of PGE2 (37). However, EP2 has been suggested to
mediate the effects of PGE2 in inhibiting the migration
of DCs (12). Therefore, future
experiments will aim to determine the currently controversial
molecular mechanism of PGE2 function.
RNA-seq technology can simultaneously detect the
expression of thousands of genes, and profile gene expression in
the context of a sample's entire transcriptome. In the present
study, to determine the downstream effects of PGE2 on
DCs, RNA-seq technology was applied. When analyzing the data of
RNA-seq, previous studies have used a threshold value of 2 for the
fold change of the differential expression (38,39). However, the current study used a
threshold value of 1.2 to screen the differentially expressed genes
to the greatest possible extent. It must be noted that the present
study presents certain limitations. Firstly, the mRNA and also
possibly the protein expression levels of several genes that affect
the remodeling of the cytoskeletal reorganization will not change
(40). Therefore, these genes
cannot be detected and identified as differentially expressed genes
using RNA-seq. Examples of such genes include paxillin, vinculin
and actin (40). Secondly, the
DCs used in the present study were primary cultured cells, which
may contain a certain proportion of impure cells (41,42). This may lead to partial
interference with the obtained RNA-seq results. Thirdly, the
samples used for RNA-seq analysis were collected at a fixed time
point following treatment with PGE2. However, it is
possible that different genes require distinct treatment times to
exhibit differential expression levels, which may exclude certain
key molecules from being identified.
In conclusion, the present study used DCs as a model
to study the role of PGE2 in cell migration. The
findings provided novel insights into the dose-dependent effects of
PGE2 as a modulator of actin cytoskeletal
reorganization, which is essential for cell migration. Moreover,
the results may provide an improved understanding of the mechanisms
in different types of malignant disease, such as gynecological
tumors, where PGE2 demonstrates pleiotropic functions
associated with proliferation, metastasis and invasion.
Funding
The present study was funded by National Natural
Science Foundation of China (grant no. 81272864) and Natural
Science Foundation of Chongqing (grant nos. cstc-2017shms-zdyfX0043
and cstc2019jcyj-msxmX0445).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JG and JH designed the study. GD and JH performed
the experiments and were major contributors in writing the
manuscript. XZ, XS and MT assisted with the experiments and data
analysis. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Daping Hospital, Army Medical University (Third
Military Medical University; Chongqing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Professor Jiongyu
Hu and Professor Yizhi Peng (Institute of Burn Research, Southwest
Hospital, State Key Laboratory of Trauma, Burns and Combined
Injury, Third Military Medical University, Chongqing, China) for
technical assistance in preparing the manuscript.
References
|
1
|
Qian C and Cao X: Dendritic cells in the
regulation of immunity and inflammation. Semin Immunol. 35:3–11.
2018. View Article : Google Scholar
|
|
2
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dong R, Cwynarski K, Entwistle A,
Marelli-Berg F, Dazzi F, Simpson E, Goldman JM, Melo JV, Lechler
RI, Bellantuono I, et al: Dendritic cells from CML patients have
altered actin organization, reduced antigen processing, and
impaired migration. Blood. 101:3560–3567. 2003. View Article : Google Scholar
|
|
4
|
Worbs T, Hammerschmidt SI and Förster R:
Dendritic cell migration in health and disease. Nat Rev Immunol.
17:30–48. 2017. View Article : Google Scholar
|
|
5
|
Kara PP, Ayhan A, Caner B, Gultekin M,
Ugur O, Bozkurt MF, Usubutun A and Uner A: Analysis of dendritic
cells in sentinel lymph nodes of patients with endometrial and
patients with cervical cancers. Int J Gynecol Cancer. 19:1239–1243.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saalbach A, Janik T, Busch M, Herbert D,
Anderegg U and Simon JC: Fibroblasts support migration of
monocyte-derived dendritic cells by secretion of PGE2
and MMP-1. Exp Dermatol. 24:598–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harizi H: Reciprocal crosstalk between
dendritic cells and natural killer cells under the effects of
PGE2 in immunity and immunopathology. Cell Mol Immunol.
10:213–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yen JH, Khayrullina T and Ganea D:
PGE2-induced metallo-proteinase-9 is essential for
dendritic cell migration. Blood. 111:260–270. 2008. View Article : Google Scholar
|
|
9
|
Scandella E, Men Y, Legler DF, Gillessen
S, Prikler L, Ludewig B and Groettrup M: CCL19/CCL21-triggered
signal transduction and migration of dendritic cells requires
prostaglandin E2. Blood. 103:1595–1601. 2004. View Article : Google Scholar
|
|
10
|
Rubio MT, Means TK, Chakraverty R, Shaffer
J, Fudaba Y, Chittenden M, Luster AD and Sykes M: Maturation of
human monocyte-derived dendritic cells (MoDCs) in the presence of
prostaglandin E2 optimizes CD4 and CD8 T cell-mediated responses to
protein antigens: Role of PGE2 in chemokine and cytokine
expression by MoDCs. Int Immunol. 17:1561–1572. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang J, Diao G, Zhang Q, Chen Y, Han J
and Guo J: E6-regulated overproduction of prostaglandin E2 may
inhibit migration of dendritic cells in human papillomavirus
16-positive cervical lesions. Int J Oncol. 56:921–931.
2020.PubMed/NCBI
|
|
12
|
Baratelli FE, Heuzé-Vourc'h N, Krysan K,
Dohadwala M, Riedl K, Sharma S and Dubinett SM: Prostaglandin
E2-dependent enhancement of tissue inhibitors of
metalloproteinases-1 production limits dendritic cell migration
through extracellular matrix. J Immunol. 173:5458–5466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lutz MB, Kukutsch N, Ogilvie AL, Rössner
S, Koch F, Romani N and Schuler G: An advanced culture method for
generating large quantities of highly pure dendritic cells from
mouse bone marrow. J Immunol Methods. 223:77–92. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han P, Hanlon D, Sobolev O, Chaudhury R
and Edelson RL: Ex vivo dendritic cell generation-A critical
comparison of current approaches. Int Rev Cell Mol Biol.
349:251–307. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burns S, Thrasher AJ, Blundell MP,
Machesky L and Jones GE: Configuration of human dendritic cell
cytoskeleton by Rho GTPases, the WAS protein, and differentiation.
Blood. 98:1142–1149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu ZQ, Xue H, Long JH, Wang Y, Jia Y, Qiu
W, Zhou J, Wen ZY, Yao WJ and Zeng Z: Biophysical properties and
motility of human mature dendritic cells deteriorated by vascular
endothelial growth factor through cytoskeleton remodeling. Int J
Mol Sci. 17:17562016. View Article : Google Scholar :
|
|
17
|
López-Colomé AM, Lee-Rivera I,
Benavides-Hidalgo R and López E: Paxillin: A crossroad in
pathological cell migration. J Hematol Oncol. 10:502017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakanishi M and Rosenberg DW: Multifaceted
roles of PGE2 in inflammation and cancer. Semin
Immunopathol. 35:123–137. 2013. View Article : Google Scholar
|
|
19
|
Hooper KM, Yen JH, Kong W, Rahbari KM, Kuo
PC, Gamero AM and Ganea D: Prostaglandin E2 inhibition of IL-27
production in murine dendritic cells: A novel mechanism that
involves IRF1. J Immunol. 198:1521–1530. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gill SK, Yao Y, Kay LJ, Bewley MA,
Marriott HM and Peachell PT: The anti-inflammatory effects of
PGE2 on human lung macrophages are mediated by the
EP4 receptor. Br J Pharmacol. 173:3099–3109. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Esaki Y, Li Y, Sakata D, Yao C,
Segi-Nishida E, Matsuoka T, Fukuda K and Narumiya S: Dual roles of
PGE2-EP4 signaling in mouse experimental
autoimmune encephalomyelitis. Proc Natl Acad Sci USA.
107:12233–12238. 2010. View Article : Google Scholar
|
|
22
|
Poloso NJ, Urquhart P, Nicolaou A, Wang J
and Woodward DF: PGE2 differentially regulates
monocyte-derived dendritic cell cytokine responses depending on
receptor usage (EP2/EP4). Mol Immunol.
54:284–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Osma-Garcia IC, Punzón C, Fresno M and
Díaz-Muñoz MD: Dose-dependent effects of prostaglandin E2 in
macrophage adhesion and migration. Eur J Immunol. 46:677–688. 2016.
View Article : Google Scholar
|
|
24
|
Clambey ET, McNamee EN, Westrich JA,
Glover LE, Campbell EL, Jedlicka P, de Zoeten EF, Cambier JC,
Stenmark KR, Colgan SP and Eltzschig HK: Hypoxia-inducible factor-1
alpha-dependent induction of FoxP3 drives regulatory T-cell
abundance and function during inflammatory hypoxia of the mucosa.
Proc Natl Acad Sci USA. 109:E2784–E2793. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Flück K, Breves G, Fandrey J and Winning
S: Hypoxia-inducible factor 1 in dendritic cells is crucial for the
activation of protective regulatory T cells in murine colitis.
Mucosal Immunol. 9:379–390. 2016. View Article : Google Scholar
|
|
26
|
Dang EV, Barbi J, Yang HY, Jinasena D, Yu
H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al: Control of
T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell.
146:772–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JH, Elly C, Park Y and Liu YC: E3
ubiquitin ligase VHL regulates hypoxia-inducible factor-1α to
maintain regulatory T cell stability and suppressive capacity.
Immunity. 42:1062–1074. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shibata M, Ham K and Hoque MO: A time for
YAP1: Tumorigenesis, immunosuppression and targeted therapy. Int J
Cancer. 143:2133–2144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang
S, Fang Z, Zhao K, Konaparthi R, Hua S, et al: Targeting
YAP-dependent MDSC infiltration impairs tumor progression. Cancer
Discov. 6:80–95. 2016. View Article : Google Scholar :
|
|
30
|
Hodgson MC, Deryugina EI, Suarez E, Lopez
SM, Lin D, Xue H, Gorlov IP, Wang Y and Agoulnik IU: INPP4B
suppresses prostate cancer cell invasion. Cell Commun Signal.
12:612014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin H, Rothe K, Chen M, Wu A, Babaian A,
Yen R, Zeng J, Ruschmann J, Petriv OI, O'Neill K, et al: The
miR-185/PAK6 axis predicts therapy response and regulates survival
of drug-resistant leukemic stem cells in CML. Blood. 136:596–609.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De Laere M, Berneman ZN and Cools N: To
the brain and back: Migratory paths of dendritic cells in multiple
sclerosis. J Neuropathol Exp Neurol. 77:178–192. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seyfizadeh N, Muthuswamy R, Mitchell DA,
Nierkens S and Seyfizadeh N: Migration of dendritic cells to the
lymph nodes and its enhancement to drive anti-tumor responses. Crit
Rev Oncol Hematol. 107:100–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weinlich R, Bortoluci KR, Chehab CF,
Serezani CH, Ulbrich AG, Peters-Golden M, Russo M and
Amarante-Mendes GP: TLR4/MYD88-dependent, LPS-induced synthesis of
PGE2 by macrophages or dendritic cells prevents
anti-CD3-mediated CD95L upregulation in T
cells. Cell Death Differ. 15:1901–1909. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yokoyama U, Iwatsubo K, Umemura M, Fujita
T and Ishikawa Y: The prostanoid EP4 receptor and its signaling
pathway. Pharmacol Rev. 65:1010–1052. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Regan JW: EP2 and EP4 prostanoid receptor
signaling. Life Sci. 74:143–153. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van Helden SF, Oud MM, Joosten B, Peterse
N, Figdor CG and van Leeuwen FN: PGE2-mediated podosome
loss in dendritic cells is dependent on actomyosin contraction
downstream of the RhoA-Rho-kinase axis. J Cell Sci. 121:1096–1106.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schurch NJ, Schofield P, Gierliński M,
Cole C, Sherstnev A, Singh V, Wrobel N, Gharbi K, Simpson GG,
Owen-Hughes T, et al: How many biological replicates are needed in
an RNA-seq experiment and which differential expression tool should
you use? RNA. 22:839–851. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xi WD, Liu YJ, Sun XB, Shan J, Yi L and
Zhang TT: Bioinformatics analysis of RNA-seq data revealed critical
genes in colon adenocarcinoma. Eur Rev Med Pharmacol Sci.
21:3012–3020. 2017.PubMed/NCBI
|
|
40
|
Witteck A, Yao Y, Fechir M, Förstermann U
and Kleinert H: Rho protein-mediated changes in the structure of
the actin cytoskeleton regulate human inducible NO synthase gene
expression. Exp Cell Res. 287:106–115. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Meyer-Wentrup F and Burdach S: Efficacy of
dendritic cell generation for clinical use: Recovery and purity of
monocytes and mature dendritic cells after immunomagnetic sorting
or adherence selection of CD14+ starting populations. J
Hematother Stem Cell Res. 12:289–299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Marques GS, Silva Z and Videira PA:
Antitumor efficacy of human monocyte-derived dendritic cells:
Comparing effects of two monocyte isolation methods. Biol Proced
Online. 20:42018. View Article : Google Scholar : PubMed/NCBI
|