Introduction
Hereditary hemochromatosis (HH) is a heterogeneous
group of genetic disorders characterized by the deficiency or
dysregulation of the liver hormone hepcidin, a key regulator of
systemic iron homeostasis (1).
Hepcidin acts via binding to the iron exporter ferroportin,
inducing its degradation and subsequently inhibiting intestinal
iron absorption and macrophage iron release (2). Insufficient hepcidin production
results in excessive iron accumulation in the parenchymal cells of
the liver, heart, pancreas and other organs, leading to tissue
damage and fibrosis (3). HH is
caused by mutations in the genes encoding hemochromatosis protein
(HFE), transferrin receptor 2 (Tfr2), hemojuvelin (HJV),
ferroportin (SLC40A1) and hepcidin (Hamp); however, for a limited
subset of patients with HH-like phenotypes, mutations have been
identified in bone morphogenetic protein 6 (BMP6) (4,5).
Bone morphogenetic proteins (BMPs) belong to the
transforming growth factor-β (TGF-β) superfamily (6,7)
and serve distinct roles in various biological processes, ranging
from embryogenesis and development to adult tissue homeostasis
(8,9). Our previous studies have reported
that the loss of endogenous BMP6 in animal models leads to iron
overload and hemochromatosis with low levels of serum hepcidin,
suggesting a key role of BMP6 in iron metabolism (10,11). The administration of BMP6
increases hepcidin expression and consequently reduces serum iron
levels, whereas BMP inhibitors inhibit hepcidin synthesis, mobilize
reticuloendothelial iron cell stores and increase the circulating
iron levels (10,12,13).
Although the pathogenesis of diabetes associated
with hemochromatosis has not been fully elucidated, it is
considered to be multifactorial; it has been suggested that both
insulin deficiency and resistance are contributing factors for
glucose intolerance and diabetes, which have a high prevalence
among patients with hemochromatosis (14-16). Previous studies on mouse models
of HH have demonstrated excessive iron accumulation predominantly
in the exocrine pancreas in Hamp−/−,
HJV−/−, Bmp6−/− and
Trf−/− mice (10,17-21). In aging Hamp−/−
and SLC40A1C326S/C326S mice, iron overload in the
pancreatic acinar cells leads to chronic pancreatitis and exocrine
pancreatic failure without an effect on glucose homeostasis
(22,23). In HFE−/− mice,
which is another mouse model of hemochromatosis, excess iron in β
cells results in pancreatic islet apoptosis, leading to a decrease
in insulin secretory capacity and an age-dependent decrease in
glucose tolerance, without developing diabetes (24).
Considering the numerous studies on the association
between iron metabolism and glucose homeostasis in multiple
transgenic mouse lines, the present study aimed to further
characterize another mouse model of hemochromatosis. Since
Bmp6−/− mice exhibit an iron overload phenotype
with increased iron accumulation in the liver and pancreas, the
present study aimed to analyze glucose homeostasis in
Bmp6−/− mice and characterize the pathogenic
consequences of iron overload on the pancreatic tissue of aging
Bmp6−/− mice.
Materials and methods
Animals
The use and care of animals used in the present
study was in compliance with the standard operating procedures of
the animal facility and the European Convention for the Protection
of Vertebrate Animals used for Experimental and other Scientific
Purposes (ETS 123) (25).
Animals were housed in conventional laboratory conditions with
standard good laboratory practice diet (mucedola S.R.L.) and water
ad libitum. Bmp6−/− mice with a mixed
129Sv/C57 background were obtained by courtesy of Professor
Elisabeth Robertson (University of Oxford, Oxford, UK) (26). Animals were monitored daily for
general health and signs of distress or pain, as evidenced by
decreased or no appetite, weight loss, little or no movement, or
lethargy. Male Bmp6−/− mice and background
strain-matched wild-type (WT) mice were subjected to analyses at 3
and 10 months of age (n=6 mice/group). After blood sampling, all
animals were re-anesthetized and sacrificed by cervical
dislocation, and pancreatic tissue samples were collected. The sera
and pancreatic tissues were subjected to biochemical and
histological analysis, respectively. The present study was approved
by the Ethical Committee of The University of Zagreb, Faculty of
Sciences (Zagreb, Croatia; approval no. 251-58-508-12-49).
Biochemical parameters
Mice were anesthetized intraperitoneally with a
ketamine/xylazine solution (200 mg/kg ketamine and 10 mg/kg
xylazine). Blood samples (200-500 µl) were collected from
the retro-orbital sinus of the mice using capillary tubes following
overnight (16 h) fasting. Within 1 h of collection, the blood
samples were centrifuged at 1,000 × g for 15 min at 4°C for serum
separation. The serum was frozen at −80°C until analysis within 1
week of collection. Blood glucose levels were measured using an
Accu-Chek® glucose assay (Roche Diabetes Care, Ltd.).
Serum alanine transaminase and aspartate transaminase levels were
determined using the Roche Cobas® 6000 clinical chemical
analysis machine (F. Hoffmann-La Roche, Ltd.). All original
reagents were obtained from Roche Diagnostics. Serum amylase and
lipase activity levels were measured as previously described
(27).
Histology and immunohistochemistry
Pancreatic tissues from Bmp6−/−
and WT mice were fixed in 10% formalin at room temperature for 24 h
and embedded in paraffin. Sections were cut at 5 µm,
deparaffinized in xylene and hydrated in distilled water. To
identify morphological changes, the sections were stained with
hematoxylin and eosin according to standard methods. To determine
the iron levels, the sections were placed in Perl's solution (5%
potassium ferrocyanide and 5% HCl) for 30 min at room temperature
and counterstained with nuclear fast red (Sigma-Aldrich; Merck
KGaA) according to the manufacturer's instructions. For the
measurement of collagen deposition, the sections were placed in
0.1% Sirius red solution (Fluka; Honeywell International, Inc.)
dissolved in aqueous saturated 1.2% picric acid, pH 2.0, for 1 h,
washed twice with acidified water (0.5% acetic acid) and passed
through 100% ethanol thrice using standard procedures. Quantitative
analysis of collagen deposition was performed using ImageJ software
(version 1.51r; National Institutes of Health) as previously
described (28). The amount of
collagen was expressed as a percentage of the total pancreatic
surface. The pancreatic islet diameter was measured using ImageJ
software. For immunohistochemistry, rabbit anti-insulin (cat. no.
ab181547; dilution, 1:64,000; Abcam), mouse anti-glucagon (cat. no.
sc-71152; dilution 1:25; Santa Cruz Biotechnology, Inc.), mouse
anti- macrophage (Clone Kim2r; cat. no. ABIN284638; dilution, 1:40;
Antibodies online) and rabbit anti-CD15 (Clone FuT4/1478r; Novusbio
NBP2-53367, dilution 1:10) antibodies were added, and the samples
were incubated at 4°C overnight in a humidified chamber.
Micro-polymer IHC Detection kit (cat. no. ab236467; Abcam) was used
according to manufacturer's instructions with a goat anti-rabbit
secondary antibody incubation for 1 h at room temperature. Images
were captured using an Olympus Bx51 light microscope (Olympus
Corporation) under ×10 and ×20 magnification. A minimum of five
unique fields of view were analyzed per sample of pancreatic tissue
obtained from four mice per group.
Small-animal positron emission tomography
(PET) study
PET studies were performed with a small animal PET
scanner (Raytest ClearPET; Elysia-Raytest GmbH) (29). Briefly, 3-month-old WT and
Bmp6−/− mice (n=4 mice/group) were injected
intraperitoneally with 10-18 MBq 18F-fluorodeoxyglucose
(18-FDG) following a short anesthesia period with 200 mg/kg
ketamine and 10 mg/kg xylazine. PET was started 60 min after 18-FDG
injection. The biodistribution of 18-FDG in the target tissues
(heart, quadriceps, liver, kidney, urinary bladder and adipose
tissue) was compared between non-fasted WT and
Bmp−/− mice. After the experiment, the mice were
allowed to recover from anesthesia and returned to their cages.
Quantitative image analysis
Regions of interest (ROIs) were manually drawn over
the following organs: Liver, heart, kidney, quadriceps, bladder and
adipose tissue. The ROIs were applied to the automatically
co-registered PET images to measure the corresponding 18-FDG
standard uptake value (SUV; mean and maximum). 18-FDG uptake was
quantified using the following formula: SUV=tissue activity
concentration (Bq/ml)/injected dose (Bq) × body weight (g).
Statistical analysis
The data are presented as the mean ± standard
deviation. Changes in gene expression and serum parameter levels
were evaluated using the unpaired two-tailed Student's t-test in
Microsoft Office Excel 2016 (Microsoft Corporation). P<0.05 was
considered to indicate a statistically significant difference.
Results
Young Bmp6−/− mice present
with normal pancreatic parenchyma with iron deposits in the
exocrine pancreas
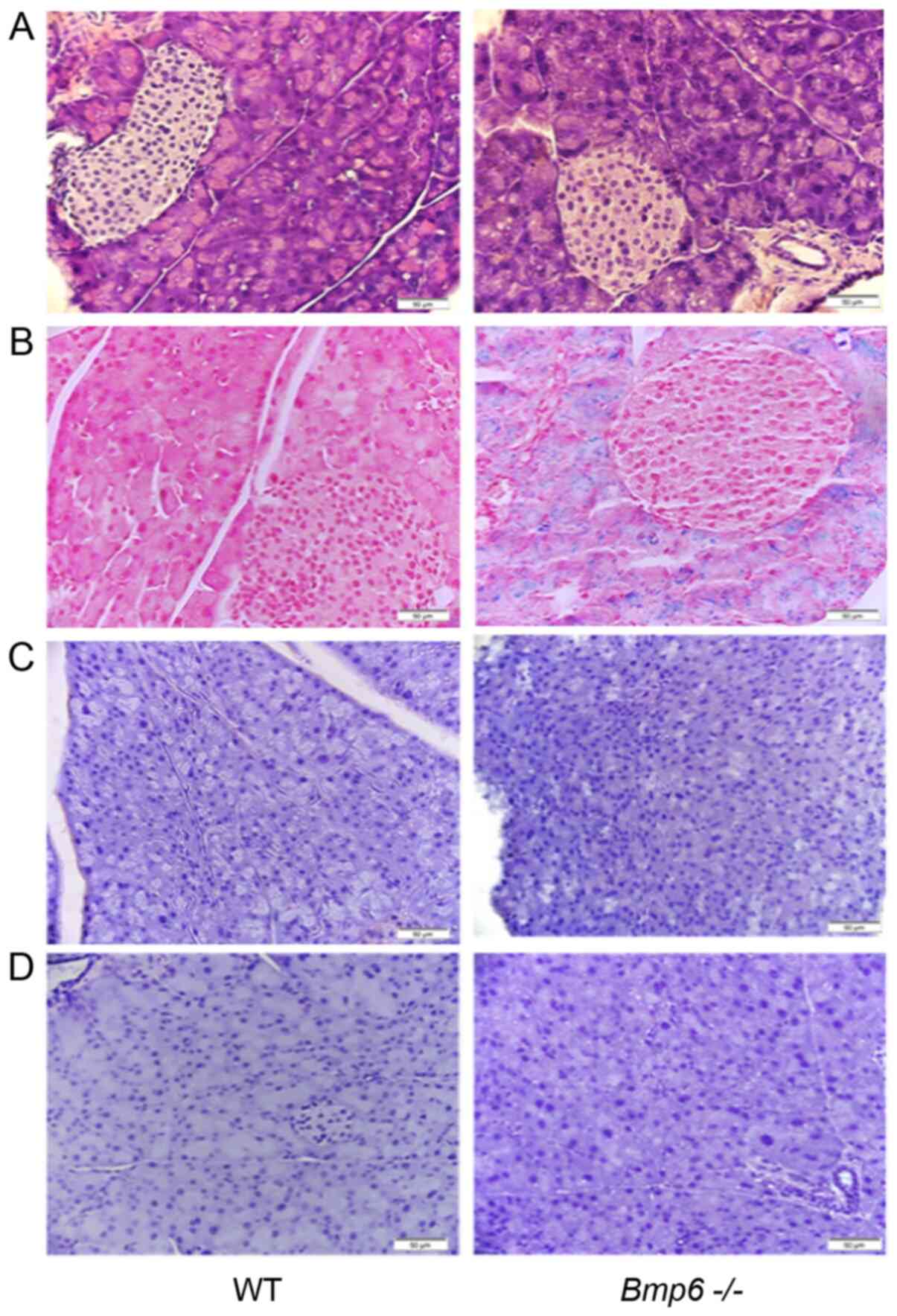
Pancreatic sections of 3-month-old
Bmp6−/− mice exhibited normal pancreatic cell
morphology (Fig. 1A) and iron
deposits in the acinar cells of the exocrine pancreas, whereas no
iron accumulation was observed in the pancreatic islets (Fig. 1B). WT and
Bmp6−/− mice exhibited no signs of inflammatory
processes in the pancreas, as the accumulation of the macrophage
marker KiM2R and the neutrophil marker CD15 was not observed by
immunohistochemistry (Fig. 1C and
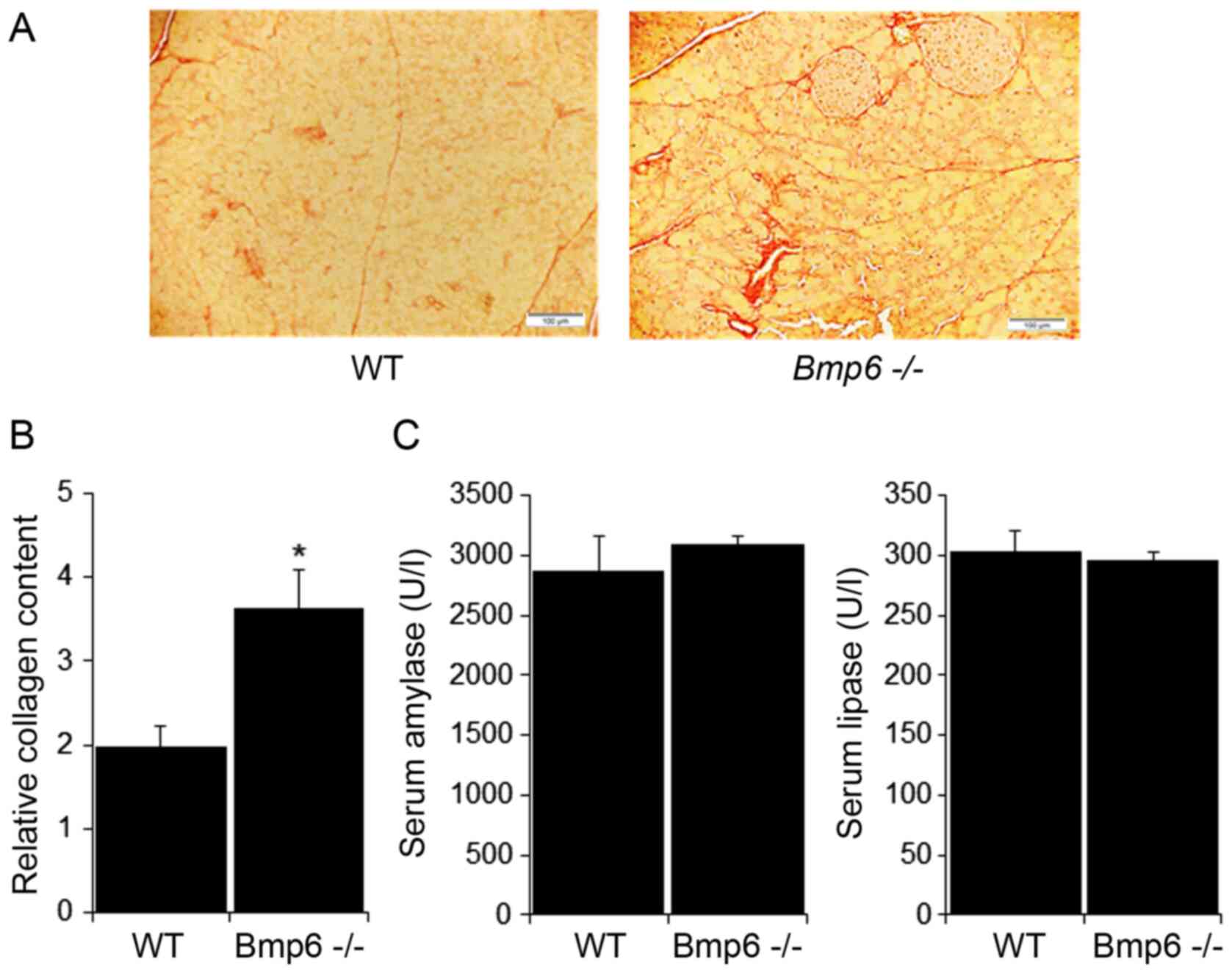
D). Further examination of the pancreatic tissues in
Bmp6−/− mice revealed no signs of fibrosis, as
demonstrated by the absence of collagen fibers and similar levels
of the pancreatic enzymes amylase and lipase compared with those in
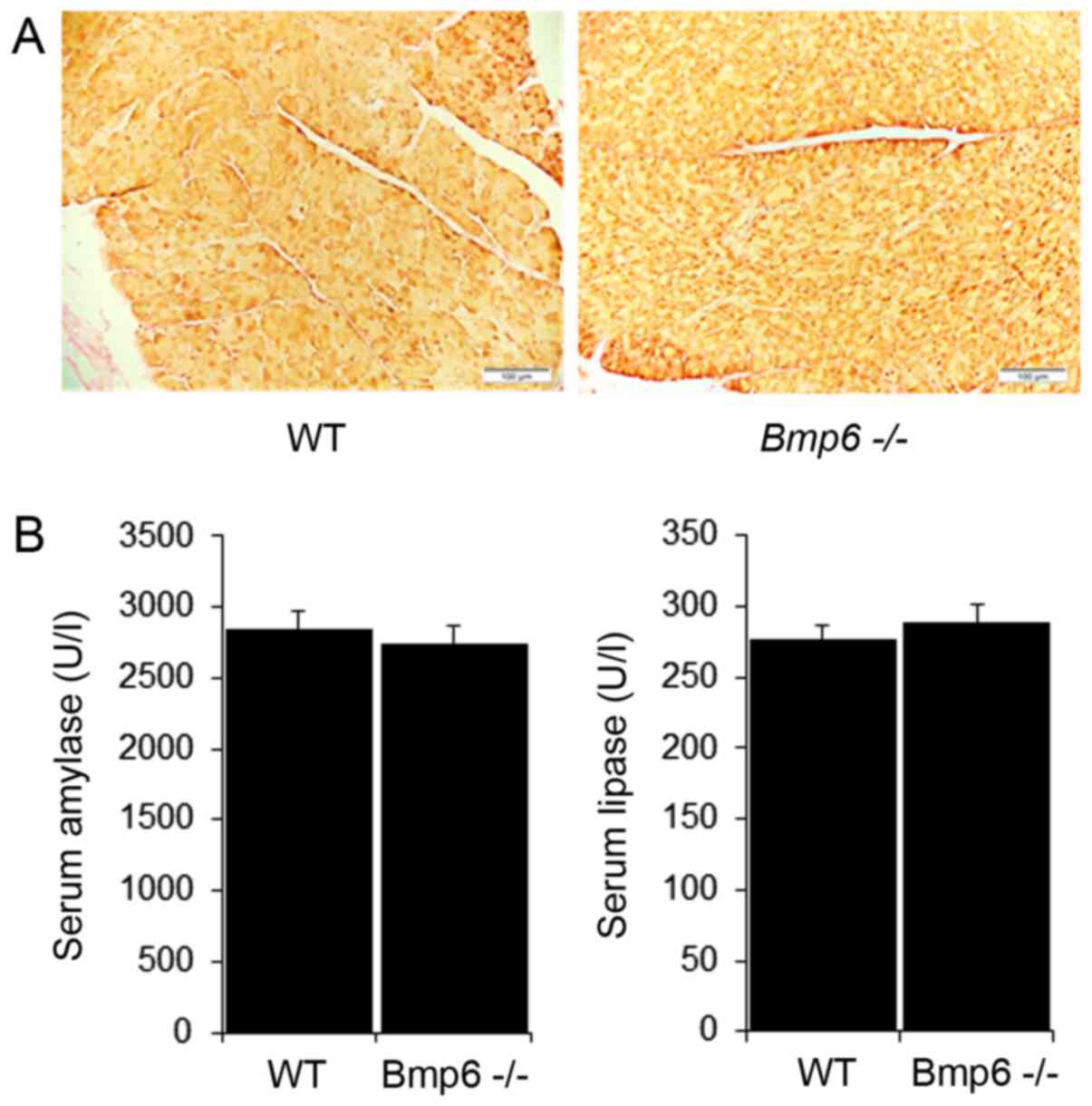
WT mice (Fig. 2A and B). Taken
together, these results demonstrated that iron overload did not
induce any morphological alterations of the exocrine pancreas in
3-month-old Bmp6−/− mice.
Young Bmp6−/− mice exhibit
normal morphology and function of the endocrine pancreas
The effects of iron overload on glucose homeostasis
were next analyzed in 3-month-old Bmp6−/− mice.
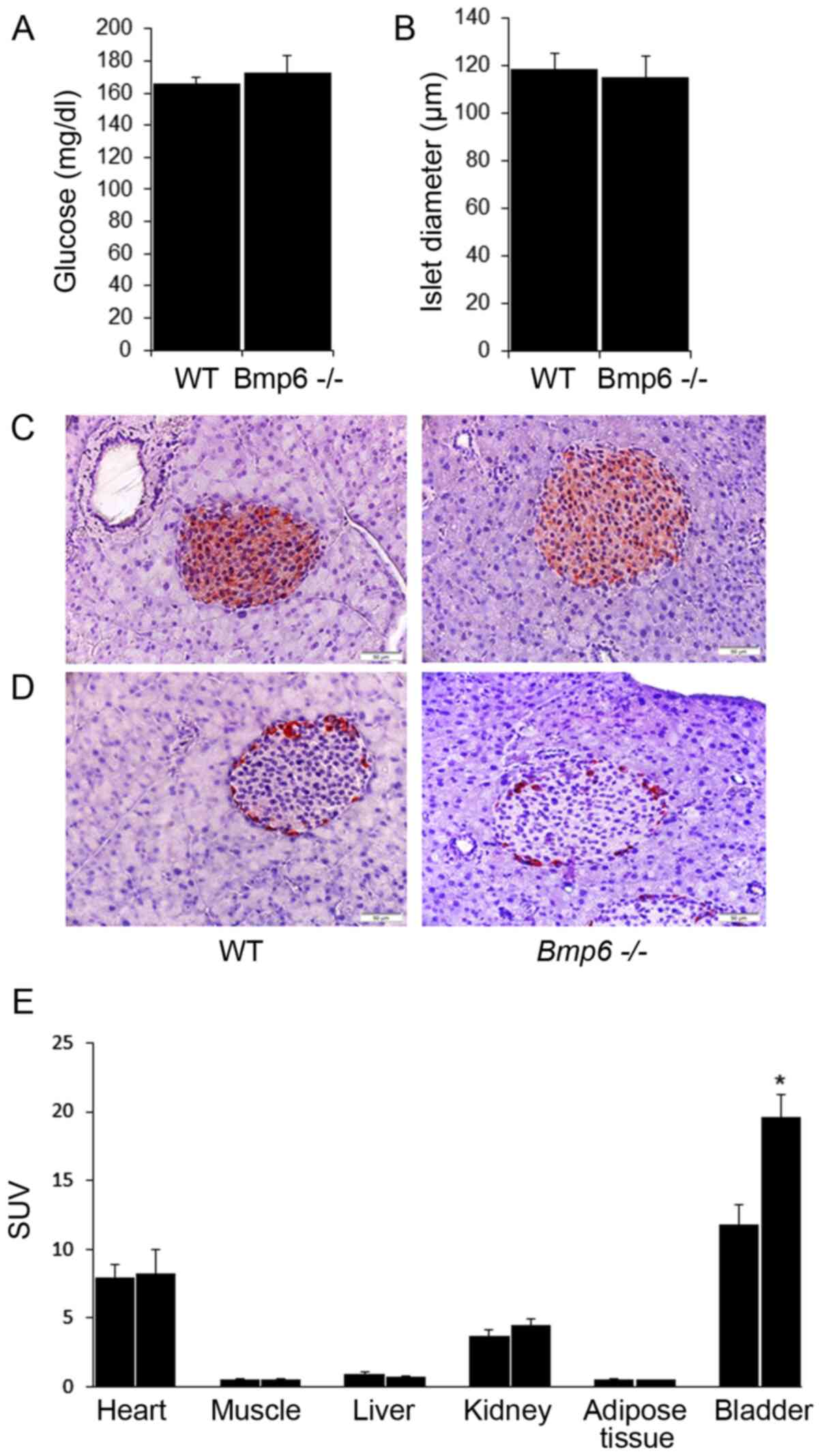
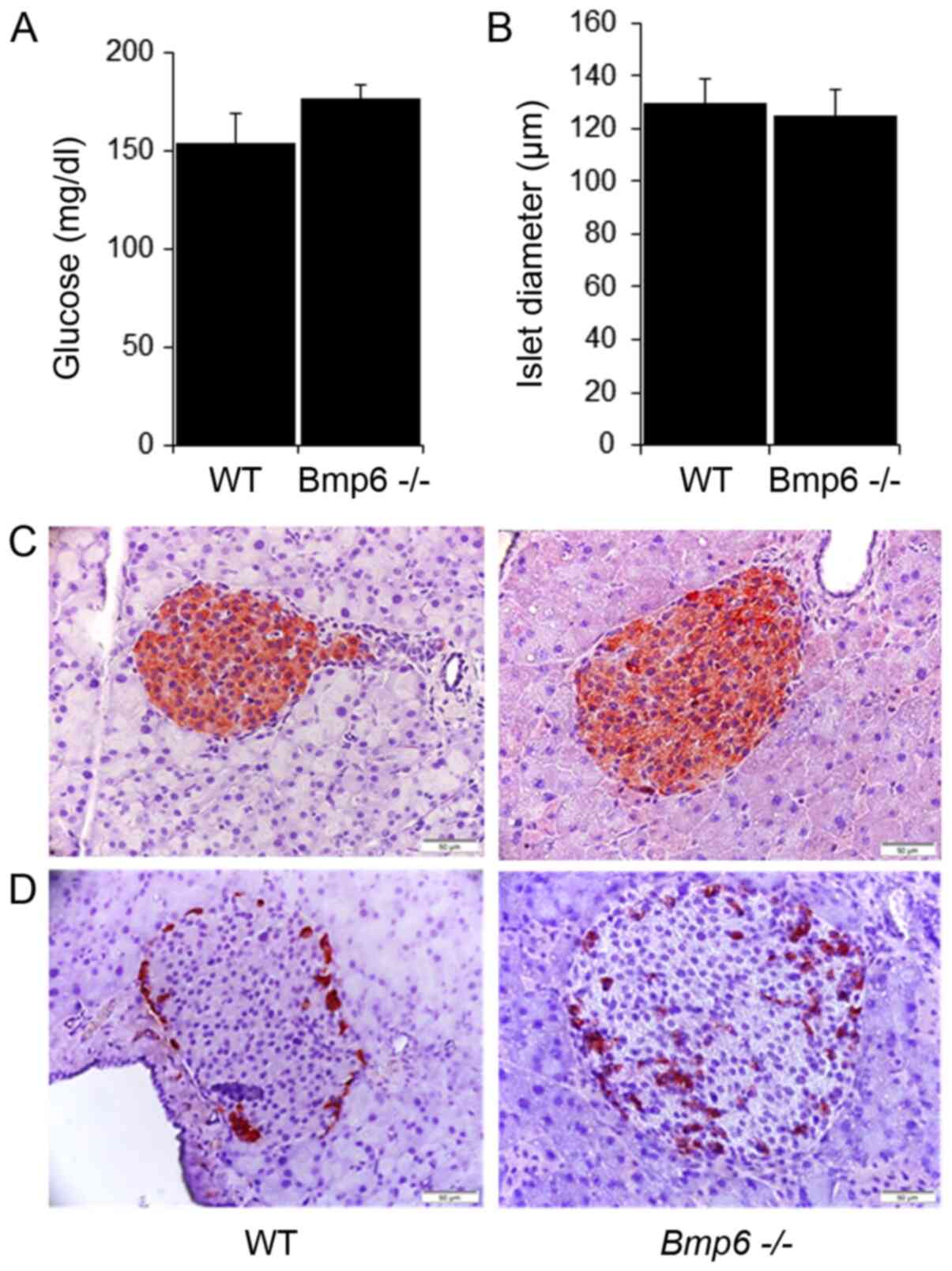
The blood glucose levels did not differ between
Bmp6−/− and WT mice (Fig. 3A). Morphometric analysis of
pancreatic sections revealed no significant differences in the mean
diameter of pancreatic islets compared with that in the WT mice
(Fig. 3B). Immunohistochemical
staining also demonstrated no changes in the distribution of β
insulin cells and insulin content (Fig. 3C). In addition, glucagon-stained
sections revealed no significant changes in the number or
distribution of α-cells located in the periphery of the islets in
Bmp6−/− and WT mice (Fig. 3D). To determine the potential
differences in the biodistribution of glucose, tissue uptake of
18-FDG was evaluated in Bmp6−/− and WT mice. With
the exception of increased 18-FDG uptake in the bladders of
Bmp6−/− mice compared with that in WT mice, no
significant differences in 18-FDG uptake were observed in the
liver, muscle, heart, kidney and adipose tissues between the two
groups (Fig. 3E). These results
suggested that iron overload in the exocrine pancreas did not
affect the viability and function of the endocrine pancreas in
3-month-old Bmp6−/− mice.
Aging Bmp6−/− mice develop
morphologic alterations in the exocrine pancreas due to iron
overload
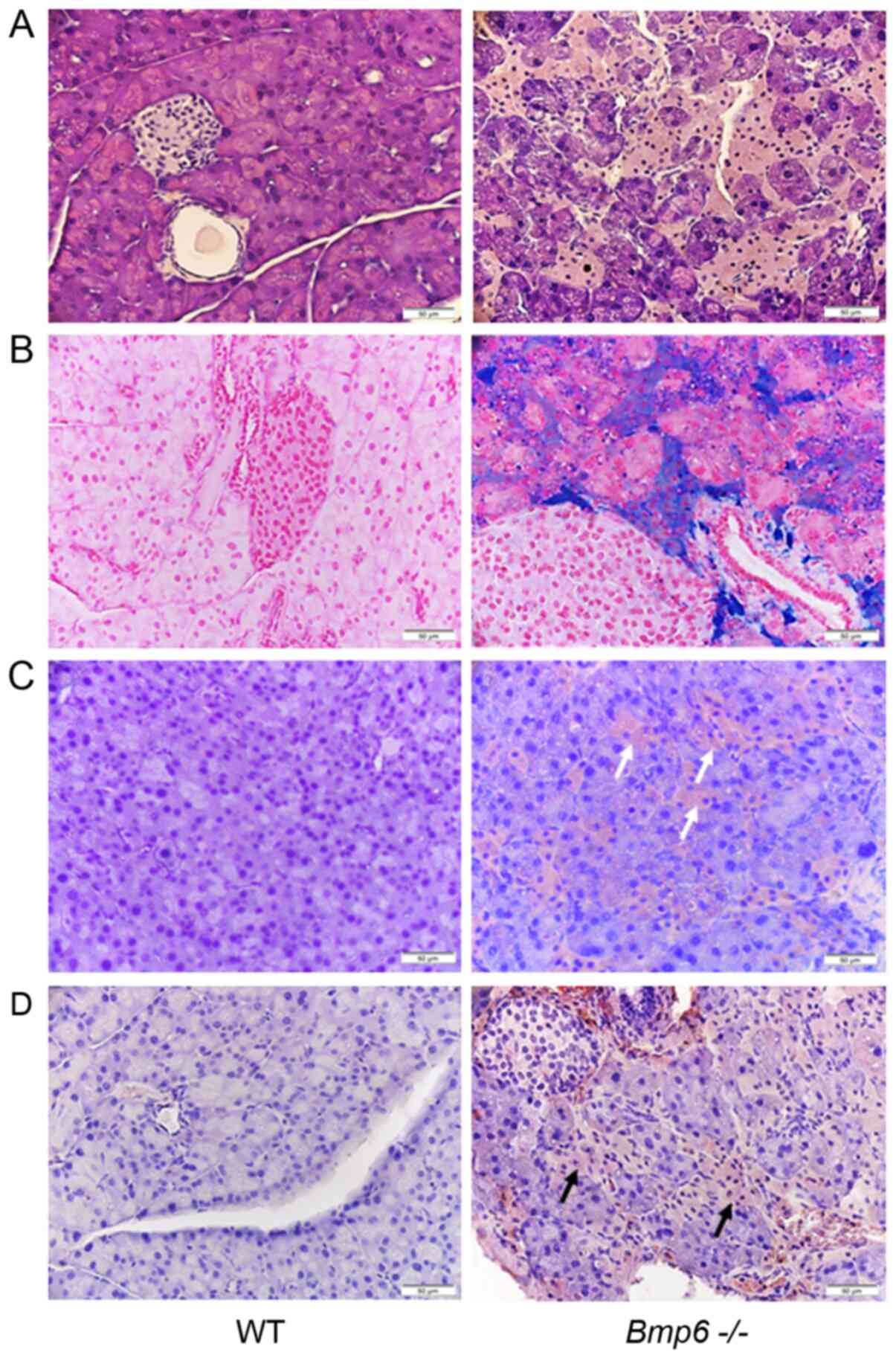
Pancreatic sections from aging WT mice presented
with morphologically normal islets surrounded by exocrine
pancreatic tissue without iron deposits (Fig. 4A and B). By contrast,
histological analysis of 10-month-old Bmp6−/−
mice demonstrated marked iron deposits in the exocrine pancreas,
which were associated with pancreatic atrophy due to pronounced
degeneration of the pancreatic acini (Fig. 4A and B). Morphological
alterations included the shrinkage of numerous acinar cells with
cytoplasmic loss of zymogen granules compared with the WT controls.
In addition, the acinar cells were randomly distributed across the
entire pancreas without a particular pattern. Consistent with the
acinar cell loss, immunohistochemical staining revealed that the
neutrophil marker CD15 (Fig. 4C)
and the macrophage marker KiM2R (Fig. 4D) were present in the pancreatic
tissues of Bmp6−/− mice. In addition, aging
Bmp6−/− mice developed pancreatic fibrosis with
collagen distribution in the interlobular and periacinar areas and
around the pancreatic ducts (Fig.
5A). In aging WT mice, collagen was observed around the
pancreatic ducts and blood vessels. These fibrogenic changes were
quantified by morphometric analysis, which confirmed a marked
increase in the pancreatic collagen deposition in aging
Bmp6−/− mice compared with that in the WT mice
(Fig. 5B). However, no
significant differences were observed in serum amylase and lipase
levels between aging WT and Bmp6−/− mice
(Fig. 5C). Serum alanine
transaminase and aspartate transaminase, blood markers of liver
function, also did not differ between WT and
Bmp6−/− mice at 3 and 10 months (Fig. S1). Iron overload and consequent
exocrine pancreatic damage were not observed in aging WT mice.
These results suggested that iron loading in aging
Bmp6−/− mice led to exocrine pancreatic injury
and fibrosis, as revealed by acinar cell loss, inflammatory cell
infiltration and collagen deposition in the pancreas.
Aging Bmp6−/− mice display
normal morphology and function of the endocrine pancreas
The effects of exocrine pancreatic damage on the
endocrine pancreas were further evaluated in aging
Bmp6−/− mice. No significant differences were
observed in the blood glucose levels between aging WT and
Bmp6−/− mice (Fig.
6A). The mean diameter of pancreatic islets in
Bmp6−/− mice was comparable with that in the WT
control (Fig. 6B). In addition,
pancreatic sections from aging Bmp6−/− mice
appeared to exhibit normal islet architecture and a similar
staining pattern for insulin (Fig.
6C), with the exception of moderately increased
glucagon-positive area (Fig. 6D)
compared with that in the WT mice. Collectively, despite acinar
cell death and atrophy of the exocrine pancreas, no morphological
neither functional impairment was observed on the endocrine
pancreas in aging Bmp6−/− mice.
Discussion
Over the past 10 years, there has been a focus on
understanding the role of iron in the pathogenesis of diabetes
(30). The mechanisms by which
iron contributes to diabetes have not been fully elucidated. Excess
iron in the liver is considered to interfere with glucose
metabolism leading to insulin resistance, whereas pancreatic iron
loading results in β-cell damage and reduced insulin secretion
(14-16). The prevalence of diabetes in
patients with HH has been reported to be 7-40% (14,31), with abnormal glucose tolerance
detected in 30% of patients with HH compared with that in healthy
subjects (14,32). To further understand the role of
iron in diabetes, the present study characterized an animal model
of hemochromatosis using Bmp6−/− mice.
Misexpression of BMP6 has been previously reported
to lead to agenesis of the pancreas and a reduction in the size of
the stomach and the spleen, causing fusion of the liver and
duodenum (33). Our previous
studies have demonstrated that Bmp6−/− mice
present with a phenotype resembling HH, with low levels of hepcidin
expression and iron overload, suggesting a key role of BMP6 in iron
metabolism (10,11,20). The results of the present study
demonstrated that iron overload in Bmp6−/− mice
led to a marked increase of iron content in the exocrine pancreatic
tissue and induced morphologic alterations of the pancreas during
aging. Although these animals exhibited iron accumulation in
pancreatic acinar cells at 3 months, progressive iron deposits
associated with severe tissue degeneration were noticeable at 10
months. Aging Bmp6−/− mice developed severe
pancreatic fibrosis, which was revealed by increased deposition of
collagen compared with that in the age-matched WT mice. This was
accompanied with pancreatic atrophy and macrophage recruitment,
suggesting that macrophages may be involved in clearing apoptotic
cells following injury. By contrast, iron overload, inflammatory
reaction and pancreatic damage were not observed in aging WT
mice.
In the present study, Bmp6−/− mice
did not exhibit any signs of liver damage, as serum levels of
alanine transaminase and aspartate transaminase, which are markers
of liver metabolism (34), did
not differ between the WT and Bmp6−/− mice in
either age group. Previous studies in Bmp6−/−
mice have reported the existence of distinct regulatory mechanisms
that sense hepatic iron in order to stimulate expression of
hepcidin, the main regulator of iron metabolism (20,35). BMP2 has also been demonstrated to
be able to replace BMP6, activate Smad1/5/8 phosphorylation and
significantly induce hepcidin expression in vivo (18,36). In addition to BMP6, another
suitable candidate for hepcidin regulation is BMP2, the dominant
BMP ligand expressed in liver endothelial cells that stimulates
hepcidin in vivo (36,37). The important role of BMP2 in
hepcidin regulation has been confirmed in mice with a conditional
knockout of BMP2, which exhibit a hemochromatosis phenotype similar
to that observed in Bmp6−/− mice (36). Furthermore, similar
hepatocellular iron overload without developing liver fibrosis has
also been reported in other mouse models of HH such as
HFE−/−, SLC40A1C326S/C326S,
Hamp−/− and Hjv−/− mice
(22,23,38,39). Hjv−/− mice have
been demonstrated to be resistant to liver fibrosis even after
consuming a high-fat diet supplemented with iron (40). The livers of
Hamp−/− mice exhibit low mRNA levels of divalent
metal transporter 1 (DMT1) and Tfr1, which mediate
the uptake of non-transferrin-bound and transferrin-bound iron,
respectively (23). By contrast,
the DMT1 mRNA levels are slightly increased in
Hamp-knockout pancreata compared with those in pancreatic
tissues from WT animals, suggesting that Hamp−/−
mice have a transcriptional response promoting iron uptake in the
pancreas. Other studies on HH mouse models, such as
HFE−/−, SLC40A1C326S/C326S and
Hjv−/− mice, have concluded that despite severe
tissue iron overload, these mice are protected from liver damage by
yet unknown mechanisms (22,38-40). Further studies should clarify
whether hepatic iron regulation in Bmp6−/− mice
is modulated by other BMP ligands or other mechanisms, including
transcriptional responses of iron transporters. The results of the
present study were in accordance with those of previous studies
(10,17-20), in which malfunctions of the
hepcidin-ferroportin regulatory axis contributed to iron
accumulation in the exocrine pancreas. Similar sensitivity of the
exocrine pancreas to iron overload has been observed in mice with a
ferroportin mutation (SLC40A1C326S/C326S), where
pancreatic failure leads to premature death between 7 and 14 months
of age (22). These mice display
profound weight loss attributed to malabsorption as a result of
exocrine pancreatic insufficiency and a lack of digestive enzymes
(22). Compared with these mice,
Hamp−/− mice exhibit iron overload-induced
chronic pancreatitis, but the pancreatic damage is not associated
with any changes in serum lipase levels or premature lethality
(23). Chronic pancreatitis is
characterized by inflammatory cell infiltration, acinar cell
degeneration and development of fibrosis, which may lead to the
impairment of exocrine and endocrine pancreatic function (41). In the present study, following
the progression of acinar cell damage, 10-month-old
Bmp6−/− mice exhibited no changes in serum
amylase and lipase levels compared with those in the WT mice,
suggesting limited acinar damage that was not reflected by
histological changes. In addition, these mice had a normal lifespan
without exhibiting any weight loss or diarrhea (data not shown),
suggesting that the extent of pancreatic injury and acinar cell
loss was not sufficient for iron-mediated lethality. Further
studies are needed to investigate why these differences in
mortality occur among mouse models of hemochromatosis with similar
patterns of iron deposition and consequent exocrine pancreatic
insufficiency.
As demonstrated by acinar cell loss, fibrosis and
infiltration of inflammatory cells, the exocrine pancreas in
Bmp6−/− mice was severely affected in the present
study. Our recent study indicated the possible role of BMP6 in
glucose homeostasis (42). The
role of BMP6 in development of diabetes, although reported in the
literature, is still not fully understood. Recently, delayed
fracture healing due to the BMP6 downregulation has been reported
in a streptozotocin-induced rat diabetes model (43). The low Bmp6 expression
levels in smooth muscle progenitor cells in a mouse diabetes model
(44) and in myofibroblast
progenitor cells of patients with diabetes (45) has suggested the role of BMP6 in
vascular tissue remodeling, which may promote the generation of
cells with antiangiogenic and profibrotic properties (46). The present study aimed to
investigate whether exocrine pancreatic damage may impact glucose
metabolism in aging Bmp6−/− mice. The islet
diameters in Bmp6−/− and WT mice were similar
during aging, suggesting no changes in β-cell mass. In addition, no
changes were observed in the insulin content by
immunohistochemistry in the pancreatic tissues during aging in both
animal groups. Blood glucose levels and 18-FDG uptake in the liver,
muscle and adipose tissues were comparable in
Bmp6−/− and WT mice, suggesting normal glucose
metabolism. By contrast, aging Bmp6−/− mice
exhibited moderately increased islet glucagon content compared with
that in the WT mice, indicating increased α-cell mass. The role of
altered glucagon content in aging Bmp6−/− mice
should be additionally studied in animals >10 months.
HFE−/− mice exhibit iron
accumulation in β cells, resulting in decreased insulin secretion
compared with that in WT animals, secondary to β cell oxidant
stress and apoptosis without developing diabetes (24). However, other mouse models of HH
such as Hamp−/− and Hjv−/− mice
present with preferential iron loading in the exocrine pancreas
without impacting β cells and glucose homeostasis (17,38).
SLC40A1C326S/C326S mice also display excessive
iron accumulation in the pancreatic acinar cells but differ from
the other models by failure of the exocrine pancreas (22). Despite degeneration of the
pancreatic acini, aged Hamp−/− mice exhibit
normal glucose homeostasis (17). A previous study has suggested an
important role of zinc transporter ZIP14, a member of the ZIP
family of metal ion transporters, in contributing to
non-transferrin-bound iron uptake and iron accumulation by
hepatocytes and pancreatic acinar cells in iron overload disorders
(47). Since DMT1 and
Tfr1 expression levels are low in
SLC40A1C326S/C326S mice, iron accumulation in the
exocrine pancreas may be attributed to the increased uptake of
non-transferrin-bound iron via ZIP14 (22). In addition,
SLC40A1C326S/C326S mice present with a subset of
acinar cells that lack ferroportin expression, which may be prone
to extensive iron accumulation and degeneration (22). Additionally,
Hamp−/− mice exhibit the same pattern of
ferroportin expression in the pancreas (23). Although the endocrine pancreas of
Hamp−/− mice contains high levels of ferroportin,
a limited number of acinar cells that undergo severe iron overload
have relatively low ferroportin levels; however, it remains unclear
why a number of the acinar cells do not express ferroportin, and
whether this may be the reason for preferential iron accumulation
in the exocrine pancreas in these animals (23). Notably,
SLC40A1C326S/C326S and Hamp−/−
mice exhibit high hepatic levels of Bmp6 mRNA and a
functional BmP/SmAD signaling pathway (48,49), indicating that acinar cell loss
leading to exocrine pancreatic injury in these mice is a direct
effect of iron loading and is not attributed to any effects of
BMP6.
The present study had certain limitations. The
results of the study are preliminary, as only two age groups of
mice were evaluated. To further understand the glucose metabolism
of Bmp6−/− mice, glucose and insulin tolerance
tests, as well as analysis of serum insulin and glucagon levels
will be performed in future studies. In addition, more age groups,
in particular mice >10 months, may provide further insight into
the changes in the pancreas and other organs in this animal model
during aging.
In conclusion, the results of the present study
demonstrated that Bmp6−/− mice exhibited features
of chronic pancreatitis due to age-dependent iron accumulation in
the exocrine pancreas. However, acinar cell atrophy and exocrine
pancreatic injury did not induce diabetes in
Bmp6−/− mice, as these animals exhibited normal
islet structure with unaltered levels of insulin production and
blood glucose. Future studies are needed to determine why iron
predominately accumulated in the exocrine pancreas and thereby
protected pancreatic islets against iron accumulation and oxidative
damage in Bmp6−/− mice.
Supplementary Data
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
MP and SV conceived the study and designed the
methodology. MP, VK, VR, IDC, VF, MM and TBN performed the
experiments and analyzed the data. MP wrote the original draft. MP,
VK, TBN and SV revised the manuscript. SV acquired the funding and
supervised the study. MP, TBN and SV confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All applicable international, national, and/or
institutional guidelines for the care and use of animals were
followed. The present study was approved by the Ethical Committee
of The University of Zagreb, Faculty of Sciences (Zagreb, Croatia;
approval no. 251-58-508-12-49).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Mrs. Djurdjica Car
and Mrs. Mirjana Marija Renic (University of Zagreb, Zagreb,
Croatia) for their technical support in animal experiments.
Abbreviations:
|
BmP
|
bone morphogenetic protein
|
|
HH
|
hereditary hemochromatosis
|
|
HFE
|
hemochromatosis protein
|
|
Hamp
|
hepcidin
|
|
WT
|
wild-type
|
|
18-FDG
|
18F-fluorodeoxyglucose
|
References
|
1
|
Fleming RE and Ponka P: Iron overload in
human disease. N Engl J Med. 366:348–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nemeth E, Tuttle MS, Powelson J, Vaughn
MB, Donovan A, Ward DM, Ganz T and Kaplan J: Hepcidin regulates
cellular iron efflux by binding to ferroportin and inducing its
internalization. Science. 306:2090–2093. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pietrangelo A: Hereditary hemochromatosis:
Pathogenesis, diagnosis, and treatment. Gastroenterology.
139:393–408. 408.e1–e2. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Piubelli C, Castagna A, Marchi G, Rizzi M,
Busti F, Badar S, Marchetti M, De Gobbi M, Roetto A, Xumerle L, et
al: Identification of new BMP6 pro-peptide mutations in patients
with iron overload. Am J Hematol. 92:562–568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Daher R, Kannengiesser C, Houamel D,
Lefebvre T, Bardou-Jacquet E, Ducrot N, de Kerguenec C, Jouanolle
AM, Robreau AM, Oudin C, et al: Heterozygous mutations in BmP6
pro-peptide lead to inappropriate hepcidin synthesis and moderate
iron overload in humans. Gastroenterology. 150:672–683,e4. 2016.
View Article : Google Scholar
|
|
6
|
Urist MR: Bone: Formation by
autoinduction. Science. 150:893–899. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reddi AH: Role of morphogenetic proteins
in skeletal tissue engineering and regeneration. Nat Biotechnol.
16:247–252. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wagner Do, Sieber C, Bhushan R, Börgermann
JH, Graf D and Knaus P: BMPs: From bone to body morphogenetic
proteins. Sci Signal. 3:mr12010.PubMed/NCBI
|
|
9
|
Sampath KT: The systems biology of bone
morphogenetic proteins. Bone Morphogenetic Proteins: Systems
Biology Regulators. Vukicevic S and Sampath KT: Springer
International Publishing; pp. 15–38. 2017
|
|
10
|
Andriopoulos B Jr, Corradini E, Xia Y,
Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A,
Vukicevic S, Lin Hy and Babitt JL: BMP6 is a key endogenous
regulator of hepcidin expression and iron metabolism. Nat Genet.
41:482–487. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meynard D, Kautz L, Darnaud V,
Canonne-Hergaux F, Coppin H and Roth MP: Lack of the bone
morphogenetic protein BMP6 induces massive iron overload. Nat
Genet. 41:478–481. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Corradini E, Schmidt PJ, Meynard D, Garuti
C, Montosi G, Chen S, Vukicevic S, Pietrangelo A, Lin HY and Babitt
JL: BMP6 treatment compensates for the molecular defect and
ameliorates hemochromatosis in Hfe knockout mice. Gastroenterology.
139:1721–1729. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu PB, Hong CC, Sachidanandan C, Babitt
JL, Deng DY, Hoyng SA, Lin HY, Bloch KD and Peterson RT:
Dorsomorphin inhibits BMP signals required for embryogenesis and
iron metabolism. Nat Chem Biol. 4:33–41. 2008. View Article : Google Scholar
|
|
14
|
McClain DA, Abraham D, Rogers J, Brady R,
Gault P, Ajioka R and Kushner JP: High prevalence of abnormal
glucose homeostasis secondary to decreased insulin secretion in
individuals with hereditary haemochromatosis. Diabetologia.
49:1661–1669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mendler MH, Turlin B, Moirand R, Jouanolle
AM, Sapey T, Guyader D, Le Gall JY, Brissot P, David V and Deugnier
Y: Insulin resistance-associated hepatic iron overload.
Gastroenterology. 117:1155–1163. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hramiak IM, Finegood DT and Adams PC:
Factors affecting glucose tolerance in hereditary hemochromatosis.
Clin Invest Med. 20:110–118. 1997.PubMed/NCBI
|
|
17
|
Ramey G, Faye A, Durel B, Viollet B and
Vaulont S: Iron overload in Hepc1(-/-) mice is not impairing
glucose homeostasis. FEBS Lett. 581:1053–1057. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Latour C, Besson-Fournier C, Meynard D,
Silvestri L, Gourbeyre O, Aguilar-Martinez P, Schmidt PJ, Fleming
MD, Roth MP and Coppin H: Differing impact of the deletion of
hemochromatosis-associated molecules HFE and transferrin receptor-2
on the iron phenotype of mice lacking bone morphogenetic protein 6
or hemojuvelin. Hepatology. 63:126–137. 2016. View Article : Google Scholar
|
|
19
|
Latour C, Besson-Fournier C, Gourbeyre O,
Meynard D, Roth MP and Coppin H: Deletion of BMP6 worsens the
phenotype of HJV-deficient mice and attenuates hepcidin levels
reached after LPS challenge. Blood. 130:2339–2343. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pauk M, Grgurevic L, Brkljacic J, Kufner
V, Bordukalo-Niksic T, Grabusic K, Razdorov G, Rogic D, Zuvic M,
Oppermann H, et al: Exogenous BMP7 corrects plasma iron overload
and bone loss in Bmp6-/- mice. Int Orthop. 39:161–172. 2015.
View Article : Google Scholar
|
|
21
|
Meynard D, Vaja V, Sun CC, Corradini E,
Chen S, López-Otín C, Grgurevic L, Hong CC, Stirnberg M, Gütschow
M, et al: Regulation of TMPRSS6 by BMP6 and iron in human cells and
mice. Blood. 118:747–756. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Altamura S, Kessler R, Gröne HJ, Gretz N,
Hentze MW, Galy B and Muckenthaler MU: Resistance of ferroportin to
hepcidin binding causes exocrine pancreatic failure and fatal iron
overload. Cell Metab. 20:359–367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lunova M, Schwarz P, Nuraldeen R, Levada
K, Kuscuoglu D, Stützle M, Vujić Spasić M, Haybaeck J, Ruchala P,
Jirsa M, et al: Hepcidin knockout mice spontaneously develop
chronic pancreatitis owing to cytoplasmic iron overload in acinar
cells. J Pathol. 241:104–114. 2017. View Article : Google Scholar
|
|
24
|
Cooksey RC, Jouihan HA, Ajioka Rs, Hazel
MW, Jones DL, Kushner JP and McClain DA: Oxidative stress,
beta-cell apoptosis, and decreased insulin secretory capacity in
mouse models of hemochromatosis. Endocrinology. 145:5305–5312.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Council of Europe: European Convention for
the Protection of Vertebrate Animals used for Experimental and
other Scientific Purposes. ETS 123; Strasbourg: 1986
|
|
26
|
Solloway MJ, Dudley AT, Bikoff EK, Lyons
KM, Hogan BL and Robertson EJ: Mice lacking Bmp6 function. Dev
Genet. 22:321–339. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bhatia M, Saluja AK, Hofbauer B, Frossard
JL, Lee HS, Castagliuolo I, Wang CC, Gerard N, Pothoulakis C and
Steer ML: Role of substance P and the neurokinin 1 receptor in
acute pancreatitis and pancreatitis-associated lung injury. Proc
Natl Acad Sci uSA. 95:4760–4765. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rangan GK and Tesch GH: Quantification of
renal pathology by image analysis. Nephrology (Carlton).
12:553–558. 2007. View Article : Google Scholar
|
|
29
|
Roldan PS, Chereul E, Dietzel O, Magnier
L, Pautrot C, Rbah-Vidal L, Sappey-Marinier D, Wagner A, Zimmer L,
Janier MF, et al: Raytest ClearPET (TM), a new generation small
animal PET scanner. Nucl Instrum Methods Phys Res A Accel Spectrom
Detect Assoc Equip. 571:498–501. 2007. View Article : Google Scholar
|
|
30
|
Hansen JB, Moen IW and Mandrup-Poulsen T:
Iron: The hard player in diabetes pathophysiology. Acta Physiol
(Oxf). 210:717–732. 2014. View Article : Google Scholar
|
|
31
|
Buysschaert M, Paris I, Selvais P and
Hermans MP: Clinical aspects of diabetes secondary to idiopathic
haemochromatosis in French-speaking Belgium. Diabetes Metab.
23:308–313. 1997.PubMed/NCBI
|
|
32
|
Hatunic M, Finucane FM, Brennan AM, Norris
S, Pacini G and Nolan JJ: Effect of iron overload on glucose
metabolism in patients with hereditary hemochromatosis. Metabolism.
59:380–384. 2010. View Article : Google Scholar
|
|
33
|
Dichmann DS, Miller CP, Jensen J, Scott
Heller R and Serup P: Expression and misexpression of members of
the FGF and TGFbeta families of growth factors in the developing
mouse pancreas. Dev Dyn. 226:663–674. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pratt DS and Kaplan MM: Evaluation of
abnormal liver-enzyme results in asymptomatic patients. N Engl J
Med. 342:1266–1271. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ramos E, Kautz L, Rodriguez R, Hansen M,
Gabayan V, Ginzburg Y, Roth MP, Nemeth E and Ganz T: Evidence for
distinct pathways of hepcidin regulation by acute and chronic iron
loading in mice. Hepatology. 53:1333–1341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Canali S, Wang CY, Zumbrennen-Bullough KB,
Bayer A and Babitt JL: Bone morphogenetic protein 2 controls iron
homeostasis in mice independent of Bmp6. Am J Hematol.
92:1204–1213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xiao X, Dev S, Canali S, Bayer A, Xu Y,
Agarwal A, Wang CY and Babitt JL: Endothelial bone morphogenetic
protein 2 (Bmp2) knockout exacerbates hemochromatosis in
homeostatic iron regulator (Hfe) knockout mice but not bmp6
knockout mice. Hepatology. 72:642–655. 2020. View Article : Google Scholar
|
|
38
|
Huang FW, Pinkus JL, Pinkus GS, Fleming MD
and Andrews NC: A mouse model of juvenile hemochromatosis. J Clin
Invest. 115:2187–2191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wagner J, Fillebeen C, Haliotis T,
Charlebois E, Katsarou A, MUI J, Vali H and Pantopoulos K: mouse
models of hereditary hemochromatosis do not develop early liver
fibrosis in response to a high fat diet. PLoS one. 14:e02214552019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Padda RS, Gkouvatsos K, Guido M, Mui J,
Vali H and Pantopoulos K: A high-fat diet modulates iron metabolism
but does not promote liver fibrosis in hemochromatotic
Hjv−/− mice. Am J Physiol Gastrointest Liver Physiol.
308:G251–G261. 2015. View Article : Google Scholar
|
|
41
|
Mareninova OA, Sung KF, Hong P, Lugea A,
Pandol SJ, Gukovsky I and Gukovskaya AS: Cell death in
pancreatitis: Caspases protect from necrotizing pancreatitis. J
Biol Chem. 281:3370–3381. 2006. View Article : Google Scholar
|
|
42
|
Pauk M, Bordukalo-Niksic T, Brkljacic J,
Paralkar VM, Brault AL, Dumic-Cule I, Borovecki F, Grgurevic L and
Vukicevic S: A novel role of bone morphogenetic protein 6 (BMP6) in
glucose homeostasis. Acta Diabetol. 56:365–371. 2019. View Article : Google Scholar :
|
|
43
|
Guo Q, Wang W, Abboud R and Guo Z:
Impairment of maturation of BMP-6 (35 kDa) correlates with delayed
fracture healing in experimental diabetes. J Orthop Surg Res.
15:1862020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Westerweel PE, van Velthoven CT, Nguyen
TQ, den Ouden K, de Kleijn DP, Goumans MJ, Goldschmeding R and
Verhaar MC: Modulation of TGF-β/BMP-6 expression and increased
levels of circulating smooth muscle progenitor cells in a type I
diabetes mouse model. Cardiovasc Diabetol. 9:552010. View Article : Google Scholar
|
|
45
|
Nguyen TQ, Chon H, van Nieuwenhoven FA,
Braam B, Verhaar MC and Goldschmeding R: myofibroblast progenitor
cells are increased in number in patients with type 1 diabetes and
express less bone morphogenetic protein 6: A novel clue to adverse
tissue remodelling? Diabetologia. 49:1039–1048. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vinci MC, Gambini E, Bassetti B, Genovese
S and Pompilio G: When good guys turn bad: Bone marrow's and
hematopoietic stem cells' role in the pathobiology of diabetic
complications. Int J mol Sci. 21:38642020. View Article : Google Scholar
|
|
47
|
Jenkitkasemwong S, Wang CY, Coffey R,
Zhang W, Chan A, Biel T, Kim JS, Hojyo S, Fukada T and Knutson MD:
SLC39A14 is required for the development of hepatocellular iron
overload in murine models of hereditary hemochromatosis. Cell
Metab. 22:138–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kautz L, Meynard D, Monnier A, Darnaud V,
Bouvet R, Wang RH, Deng C, Vaulont S, Mosser J, Coppin H and Roth
MP: Iron regulates phosphorylation of Smad1/5/8 and gene expression
of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood.
112:1503–1509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vujić Spasić M, Sparla R, Mleczko-Sanecka
K, Migas MC, Breitkopf-Heinlein K, Dooley S, Vaulont S, Fleming RE
and Muckenthaler MU: Smad6 and Smad7 are co-regulated with hepcidin
in mouse models of iron overload. Biochim Biophys Acta. 1832:76–84.
2013. View Article : Google Scholar
|