Introduction
The liver is the metabolic organ of the majority of
substances in the human organism, particularly various drugs
(1,2). Drug-induced liver disease (DLD) is a
kind of disease that causes liver damage when patients receive
conventional drugs, Chinese herbal medicine, and health care
products (3,4). With the advent of various new drugs,
the occurrence of DLD is also showing an increasing trend (3-5).
The proportion of DLD in acute hepatitis and liver failure, is
gradually increasing, which has aroused widespread concern of
people and clinicians (3,4). The main clinical manifestations of
DLD include fever, fatigue, anorexia and jaundice (3,4,6,7).
Laboratory examinations mainly focus on abnormal elevation of
alanine aminotransferase (ALT) and aspartate aminotransferase (AST)
(8). In severe cases, cirrhosis,
hypoproteinemia, coagulation dysfunction and even liver failure may
occur, threatening life (6,7).
Pirarubicin (THP) is a common anthracycline antineoplastic drug,
but its clinical application is often affected by its toxic and
side effects, mainly including hepatotoxicity, cardiotoxicity and
myelotoxicity (9-11). A recent study reported that THP
can induce accumulation of reactive oxygen species in hepatocytes,
induce apoptosis and ferroptosis of hepatocytes, and finally cause
liver damage (9). On the other
hand, in the process of liver injury, inflammatory reaction also
plays an important role (12,13). How to prevent and treat DLD and
broaden the clinical application of corresponding drugs has become
a thorny problem.
With the rapid development of the modernization of
traditional Chinese medicine, finding effective ingredients from
natural plants and developing new drugs from natural sources have
become an effective means to protect the liver (14,15). Flavonoids are one of the important
categories of secondary metabolites of plants, which are not only
widely found in numerous medicinal plants, but also in almost all
vegetables and fruits (16,17). They have excellent biological
activities including anti-oxidation, anti-inflammatory, anticancer
and organ protection, which are of great benefit to human health
(16,18-20). Scutellarein (Sc), (chemical name
4′, 5,6,7-tetrahydroxyflavone) is the aglycone and active
metabolite of scutellarin, which is widely distributed in
Scutellaria genus of Labiatae family and
Erigeron genus of Compositae family, particularly in
the stems and leaves of Erigeron breviscapus (Vaniot) and
Scutellaria baicalensis Georgi (21). It is a natural flavonoid compound
with stronger biological activity, particularly anti-inflammatory
effect (21,22). Previous studies have found that Sc
has strong anti-inflammatory, antioxidant, anti-insulin resistance
and regulatory effects on nonalcoholic fatty liver disease caused
by obesity (23,24). However, it is unknown whether Sc
has potential protective effect on DLDs, particularly those induced
by THP.
PTEN is a key molecule in the development of certain
inflammatory diseases, which is widely expressed in the liver and
mediates its physiological activities (25-27). For example, PTEN can promote the
transformation of PIP3 to PIP2, weaken the phosphorylation process
of AKT promoted by PIP3, and then indirectly activate the
expression of NFκB, affect the secretion of inflammatory factors in
hepatocytes, and finally regulate liver inflammation (28-30). However, it is unknown whether the
anti-inflammatory effect of Sc can be achieved by regulating
PTEN.
The purpose of the present study was to investigate
the effect of Sc on the expression of PTEN and inflammatory
response in THP-induced rat liver and its possible mechanism, so as
to provide experimental basis for exploring the specific mechanism
of Sc in improving THP induced hepatotoxicity and screening
therapeutic targets. It provides a theoretical basis for the
development and application of Sc as a potential PTEN natural
agonist to treat liver diseases.
Materials and methods
Reagents
THP (cat. no. HY-13725) and Sc (cat. no. HY-N0752)
were purchased from MedChemExpress. Hematoxylin-eosin staining kit
(cat. no. C0105M) and Cell Counting Kit-8 (CCK-8; cat. no. C0039)
were purchased from Shanghai Biyuntian Biotechnology Co., Ltd. ALT
(cat. no. C009-2-1), AST (cat. no. C010-2-1), CRP (cat. no.
H126-1-2), monocyte chemoattractant protein-1 (MCP-1; cat. no.
H115), IL-1β (cat. no. H002-1-2) and IL-6 (cat. no. H007-1-2) assay
kits were purchased from Nanjing Jiancheng Bioengineering Research
Institute. Lentiviral particles carrying PTEN (LvPTEN) and empty
vector (LvControl) were constructed by Biotechnology Co., Ltd. The
primary antibodies of PTEN (cat. no. 22034-1-AP), AKT (cat. no.
10176-2-AP), phosphorylated (p)-AKT (cat. no. 80455-1-RR) and GAPDH
(cat. no. 10494-1-AP) were purchased from Wuhan Protein Technology
Biotechnology Co., Ltd. The primary anti-bodies of IκBα (cat. no.
4812S), p-p65 (cat. no. 3033T) and t-p65 (cat. no. 8242T) were
purchased from Cell Signaling Technology, Inc. Secondary antibody
[HRP-conjugated goat anti rabbit IgG H + L (cat. no. 31460)] was
purchased from Thermo Fisher Scientific, Inc. All reagents were of
analytical grade.
Animals
The present study was approved by the Ethics
Committee for Experimental Animals of The Affiliated Hospital of
Chengdu University (Chengdu, China). The IACUC number of animal
experiment is CDFS12020220056. A total of 20 male SD rats (8
weeks-old, 180-200 g) were purchased and raised in the Experimental
Animal Center of Chengdu University. Rat grouping was as follows:
i) Control group, normal saline; ii) Sc group, 100 mg/kg Sc +
normal saline; iii) THP group, normal saline + 3 mg/kg THP; and iv)
Sc + THP group, 100 mg/kg Sc + 3 mg/kg THP. Sc was administered by
gavage and THP was administered by intravenous injection. Sc was
dissolved in a very small amount of DMSO before being dissolved in
normal saline (DMSO: normal saline ≈1:1,000). The animal model was
established for 6 weeks, venous blood was received once a week, and
body weight and food intake were measured once a week. The rats
were housed under normal laboratory conditions (21±2°C, 12/12-h
light/dark cycle, humidity 50-60%) with free access to standard
pellet diet and water.
Sample collection and stain
The rats were anesthetized with pentobarbital sodium
(40 mg/kg intraperitoneally) and then sacrificed by cervical
dislocation. Blood and liver tissue were collected from aorta
rapidly, and the bleeding serum was centrifuged (22°C, 1,000 × g,
15 min). The venous blood obtained every week was also separated by
this method. The liver tissue was cleaned in normal saline,
partially fixed in paraformaldehyde (4%) solution (22°C, 48 h),
followed by dehydration, transparency, waxing, and embedding. The
tissue was then sliced into sections of ~5 µm using a
slicing machine. The other parts of the liver were frozen at −80°C
for subsequent molecular research. TUNEL staining was performed
according to the kit instructions. The paraffin sections of the
liver were dewaxed, rinsed and soaked with protease K dropwise
(37°C, 30 min), followed by DNase I reaction solution dropwise
(37°C, 30 min). Subsequently, after cleaning, TdT enzyme reaction
solution was added dropwise to the sample (37°C, 60 min, away from
light). Then, after cleaning, streptavidin TRITC working solution
was added (37°C, 30 min, away from light) dropwise to the sample.
Finally, DAPI staining solution was used to stain the nucleus
(37°C, 10 min, away from light). The sample was then cleaned, an
appropriate amount of sealing agent (glycerol: PBS=6:4) was added
dropwise, sealed and observed under an optical microscope.
Serum biomarkers of liver function and
inflammatory factor
The levels of ALT, AST, CRP, MCP-1, IL-1β and IL-6
in serum were determined according to the corresponding kit
protocol.
Histological analysis
The liver tissue was paraffin-embedded through
fixation, dehydration, transparency, wax penetration and embedding,
and finally sectioned to become 4-5-µm thick paraffin
sections. Then, paraffin sections were used for hematoxylin and
eosin (H&E) staining for histopathology. Staining images were
viewed using a Nikon eclipse 80i microscope (Nikon Corporation) at
a magnification of ×200.
Cell extraction of primary rat
hepatocytes
A male SD rat (4 week) was purchased from the Animal
Experiment Center of Chengdu University and fixed after
disinfection. The abdominal cavity was opened to expose the hepatic
portal vein and inferior vena cava. The hepatic portal vein was
perfused (5 ml/min), and the inferior vena cava was opened to clear
the blood. Then collagenase IV was replaced and perfusion was
continued until the liver became soft. After removing the blood
vessels and capsule in the liver tissue, the liver tissue was
separated, filtered with 100 µm sieve, and then washed.
After centrifugation at 1,800 × g for 5 min (22°C), it was
resuspended in DMEM medium (containing 10% FBS). Cells were counted
(primary hepatocytes were magnified at ×100 under a light
microscope for observation), and the mixed cell suspension was
transferred into a cell culture dish for 48 h. Later, it was found
that the morphology of primary hepatocytes was consistent, and
island like connections were formed between the cells. The
extraction scheme of primary hepatocytes in the present study was
derived from a previous study (31).
CCK-8 method to explore the optimal
concentration of THP and Sc and the viability of hepatocytes
Hepatocytes were treated with increasing
concentrations of THP (0, 1, 5, 10 and 20 µmol/l) for the
following time intervals: 0, 6, 12, 24 and 48 h. Under the optimal
THP treatment concentration and time, the Sc treatment
concentration was set to 0, 20, 40, 80, 160 and 320 µmol/l.
According to the instructions of CCK-8 test kit, the cell viability
of primary hepatocytes in each group was detected. Briefly,
hepatocytes suspension was inoculated into a 96-well plate (~5,000
cells per well), and after drug stimulation, 10 µl of CCK-8
solution was added to each well. After further incubation for 1 h
in the cell culture chamber (pH, 7.2-7.4; temperature, 37°C;
humidity, 95%; CO2, 5%), the absorbance value at 450 nm
was measured using an enzyme-linked immunosorbent assay.
Lentiviral particles processing
In the present study, PTEN gene was overexpressed by
lentiviral particles (Lv). PTEN specific lentivirus (shRNA
sequence: 5′GCT AGA ACT TAT CAA ACC CTT-3′) and non-targeted
control lentivirus (shRNA sequence: 5′CAA CAA GAT GAA GAG CAC
CAA-3′) were purchased from Qingke Biotechnology Co., Ltd. The
packaging and transfection of lentivirus and the screening of cells
were all completed by Qingke Biotechnology Co., Ltd. Before
transfection, 293T cells (Qingke Biotechnology Co., Ltd.) were
cultured to 80-90% degree of polymerization, PEI (1
µg/µl) and plasmid were dissolved in Optimem
respectively, and then mixed. After 48 h, centrifugation was
performed (4°C, 18,00 × g, 10 min), and the supernatant was
received and mixed with 5X PEG8000. Then centrifugation (4°C, 2,500
× g, 20 min) was performed to obtain the virus. According to the
manufacturer's instructions, lentiviral vector was transferred into
primary hepatocytes under the 15 µg/ml Polybrene with a
complex multiplicity of infection (MOI) of 10. The DMEM was changed
24 h after infection. After 72 h, primary hepatocytes were screened
using 2.0 µg/ml puromycin and cultured at 37°C in an
incubator containing 95% air and 5% carbon dioxide. The generation
system used was 3rd. All the aforementioned reagents were obtained
from Qingke Biotechnology Co., Ltd. The corresponding operation
scheme can be observed at the following link: https://tsingke.com.cn/equipment/Modified_synthesis.
Primary hepatocytes grouping and
treatment
Briefly, primary hepatocytes were divided into 7
groups and treated for 24 h: i) Control group, ii) THP group (5
µmol/l THP), iii) Sc + THP group (80 µmol/l Sc + 5
µmol/l THP), iv) LvControl group (non-targeted Control
lentivirus vector), v) LvControl + THP group (non-targeted Control
lentivirus vector + 5 µmol/l THP), vi) LvPTEN group
(lentiviral vector of PTEN), and vii) LvPTEN + Sc group (lentivirus
vector of PTEN + 80 µmol/l Sc).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from frozen pulverized rat
liver and primary hepatocytes using TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc.), then was transcribed by two-step method
using Super script First-Strand Synthesis System. The RT-qPCR
thermocycling conditions were as follows: Initial denaturation at
95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 64°C
for 30 sec. The 2−ΔΔCq method was used to calculate the
relative number of tested genes (32). The PCR products were quantified
with the SYBR Green PCR Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.), and the results were normalized to
β-actin gene expression. The primer sequences were as follows: PTEN
forward, 5′-CAA TGA CAG CCA TCA TCA AAG AG-3′ and reverse, 5′-GCT
CAG ACT TTT GTA ATT TGT G-3′; NFκB forward, 5′-AGA GGA TTT CGA TTC
CGC TA-3′ and reverse, 5′-CGT GAA GTA TTC CCA GGT TTG-3′; IL-1β
forward, 5′-GAC CTG TTC TTT GAG GCT GAC-3′ and reverse, 5′-TTC ATC
TCG AAG CCT GCA GTG-3′; IL-6 forward, 5′-AAC CAC GGC CTT CCC TAC
TTC-3′ and reverse, 5′-GAT GAA TTG GAT GGT CTT GGT C-3′; TNF-α
forward, 5′-GCC TCT TCT CAT TCC TGC TT-3′ and reverse, 5′-TGG GAA
CTT CTC ATC CCT TTG-3′; VCAM-1 forward, 5′-AAG TGG AGG TCT ACT CAT
TCC-3′ and reverse, 5′-GGT CAA AGG GGT ACA CAT TAG-3′; and β-actin
forward, 5′-AGC TGA GAG GGA AAT CGT GC-3′ and reverse 5′-ACC AGA
CAG CAC TGT GTT GG-3′.
Western blotting
Firstly, radioimmunoprecipitation assay buffer (cat.
no. P0013B; Shanghai Biyuntian Biotechnology Co., Ltd.) was added
to the tissue or cells to extract proteins, and the protein
concentration was measured using a BCA protein concentration
detection kit (cat. no. P0010; Shanghai Biyuntian Biotechnology
Co., Ltd.). Subsequently, protein loading buffer was added (cat.
no. P0015; Shanghai Biyuntian Biotechnology Co., Ltd.) and lysates
were heated at 95°C for 10 min to denature the protein. In turn,
liver tissue lysates or cell lysates (~20 µg) were subjected
to SDS-PAGE (10%). Subsequently, the protein was transferred to the
PVDF membrane at 4°C and PVDF membrane was soaked in QuickBlock™
Blocking Buffer (cat. no. P0220; Shanghai Biyuntian Biotechnology
Co., Ltd.) at room temperature for 15 min. The membrane was
incubated at 4°C for 14 h with the following primary antibodies
against: PTEN (1:1,000), AKT (1:1,000), p-AKT (1:1,000), IκBα
(1:1,000), p-p65 (1:1,000), t-p65 (1:1,000) and GAPDH (1:5,000).
Following the primary incubation, the membrane was incubated with
secondary antibody (1:10,000) at room temperature for 1 h.
Subsequently, protein visualization was performed using BeyoECL
Plus (Beyotime Institute of Biotechnology) and Image Lab 2.5.2
software (Bio Rad Laboratories, Inc.). GAPDH was used as an
internal reference protein.
Statistical analysis
The SPSS software (version 18.0; SPSS, Inc.) was
used for statistical analysis. Data are expressed as the mean ±
SEM. The normal distribution and homogeneity of variance of the
data was detected using one-way or two-way ANOVA. Tukey's multiple
comparison post hoc test was used to analyze the significant
differences between the groups. P≤0.05 was considered to indicate a
statistically significant difference.
Results
Effects of Sc and THP on body weight and
feed intake of rats
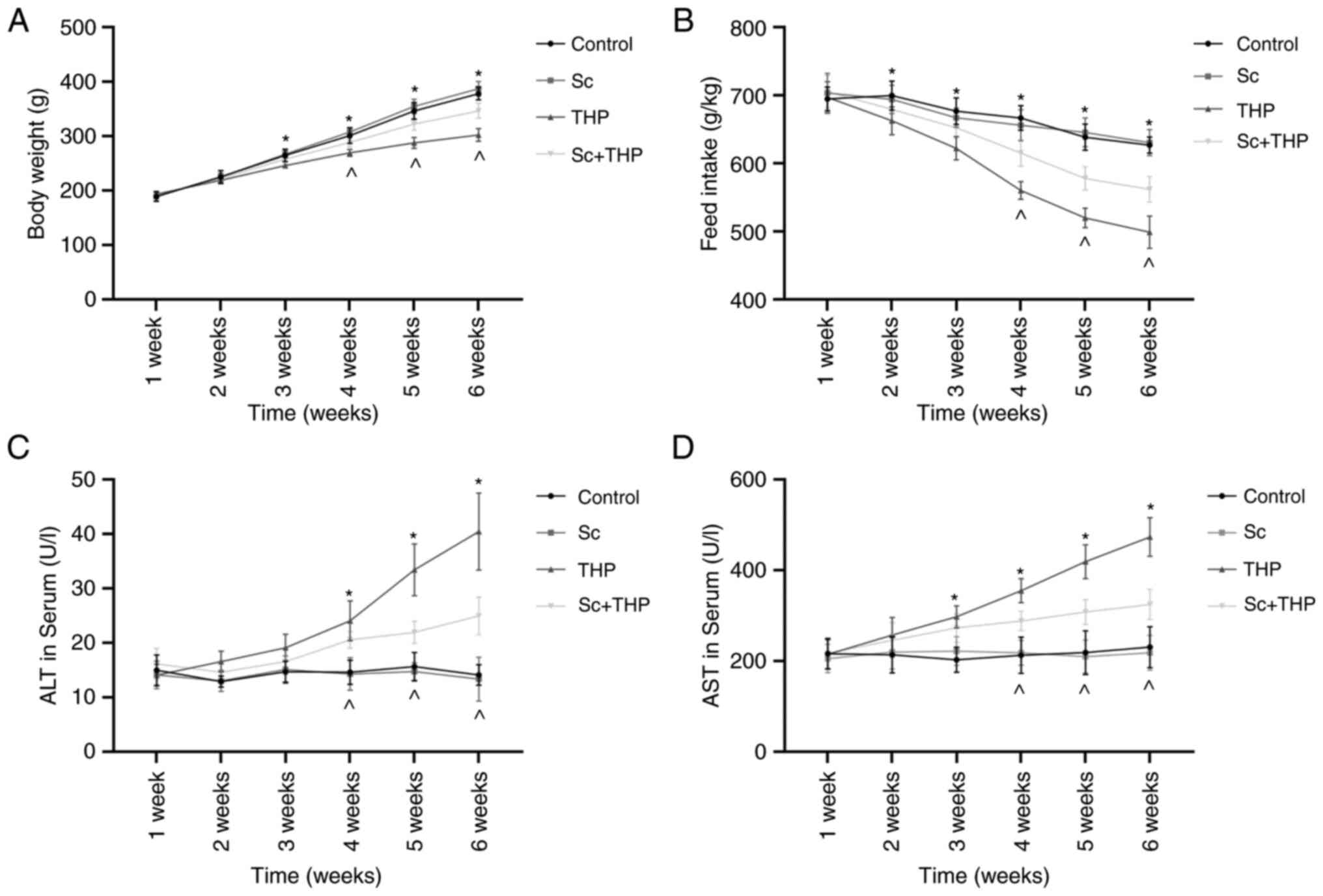
During the 6-week period, in THP group rats, the
body weight decreased significantly from the third week (vs.
Control, Fig. 1A), and the feed
intake decreased significantly from the second week (vs. Control,
Fig. 1B). The body weight and
food intake of rats in the Sc + THP group began to increase at week
4 (vs. THP, Fig. 1A and B). Sc
alone had no statistically significant effect on the weight and
feed intake of rats (vs. Control, Fig. 1A and B).
Effects of Sc and THP on ALT and AST in
serum and holism of rats
During the 6-week period, in THP group rats, the ALT
increased significantly from the fourth week (vs. Control, Fig. 1C), and the AST increased
significantly from the third week (vs. Control, Fig. 1D). The ALT and AST in serum of
rats in the Sc + THP group began to decrease at week 4 (vs. THP,
Fig. 1A and B). Sc alone had no
statistically significant effect on the ALT and AST in serum of
rats (vs. Control, Fig. 1A and
B).
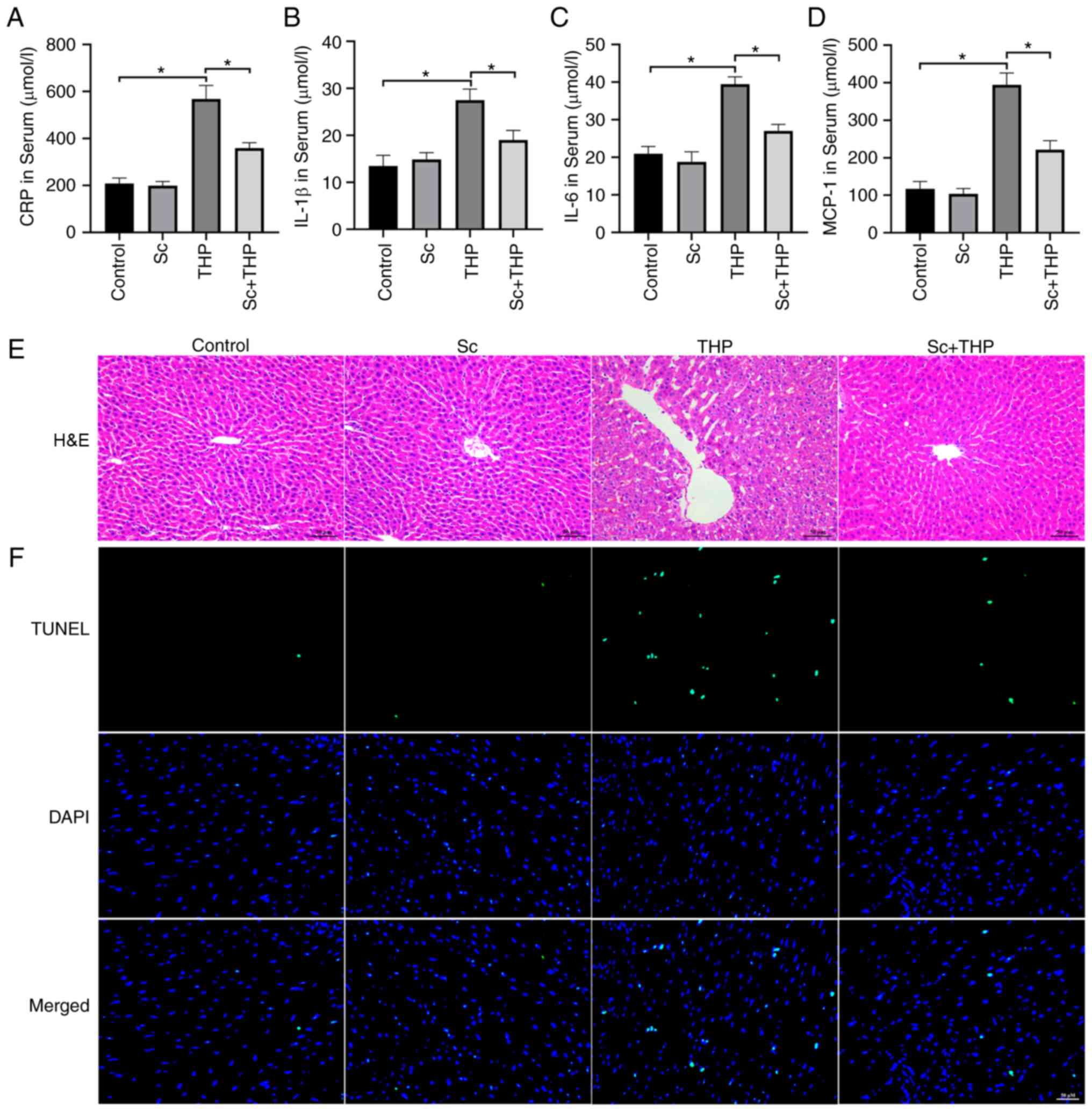
Effects of Sc and THP on serum
inflammatory factor and liver histomorphology of rats
After 6 weeks, the serum CRP (Fig. 2A), IL-1β (Fig. 2B), IL-6 (Fig. 2C) and MCP-1 (Fig. 2D) of THP group rats were
significantly increased (vs. Control). Serum CRP (Fig. 2A), IL-1β (Fig. 2B), IL-6 (Fig. 2C) and MCP-1 (Fig. 2D) of Sc + THP group rats were
significantly decreased compared with the THP group. Sc alone has
no statistically significant effect on inflammatory factors in rat
serum (vs. Control, Fig.
2A-D).
As revealed in Fig.
2E, the liver tissue morphology of rats in the control group
was normal, while that of rats in the THP group exhibited disorder
of hepatocyte fusion and arrangement, increased cell gap, abnormal
nucleus and blood cell infiltration, while that of rats in the Sc +
THP group was relatively light. Sc alone had no significant effect
on the liver histomorphology of rats.
The results demonstrated also that there were almost
no apoptotic cells in the liver tissues of rats in the control
group and Sc group, while some apoptotic cells appeared in the
liver tissues of rats in the THP group, while those in the Sc + THP
group were relatively light (Fig.
2F).
Effects of Sc and THP on liver
PTEN/AKT/NFκB signal pathway in rats
The expression of p-AKT/t-AKT and IκBα protein in
the liver of THP group rats decreased significantly, and the
expression of p-p65/t-p65 and PTEN protein increased significantly
(vs. Control, Fig. 3A-E). The
corresponding protein expression in Sc + THP group was
significantly reversed (vs. THP, Fig.
3A-E). The liver protein expression in Sc alone group was not
significantly abnormal (vs. Control). Semi-quantitative analysis of
PTEN (Fig. 3B), p-AKT/t-AKT
(Fig. 3C), IκBα (Fig. 3D) and p-p65/t-p65 (Fig. 3E) provided more evidence. Sc alone
had no significant effect on PTEN/AKT/NFκB signal pathway in rat
liver (vs. Control).
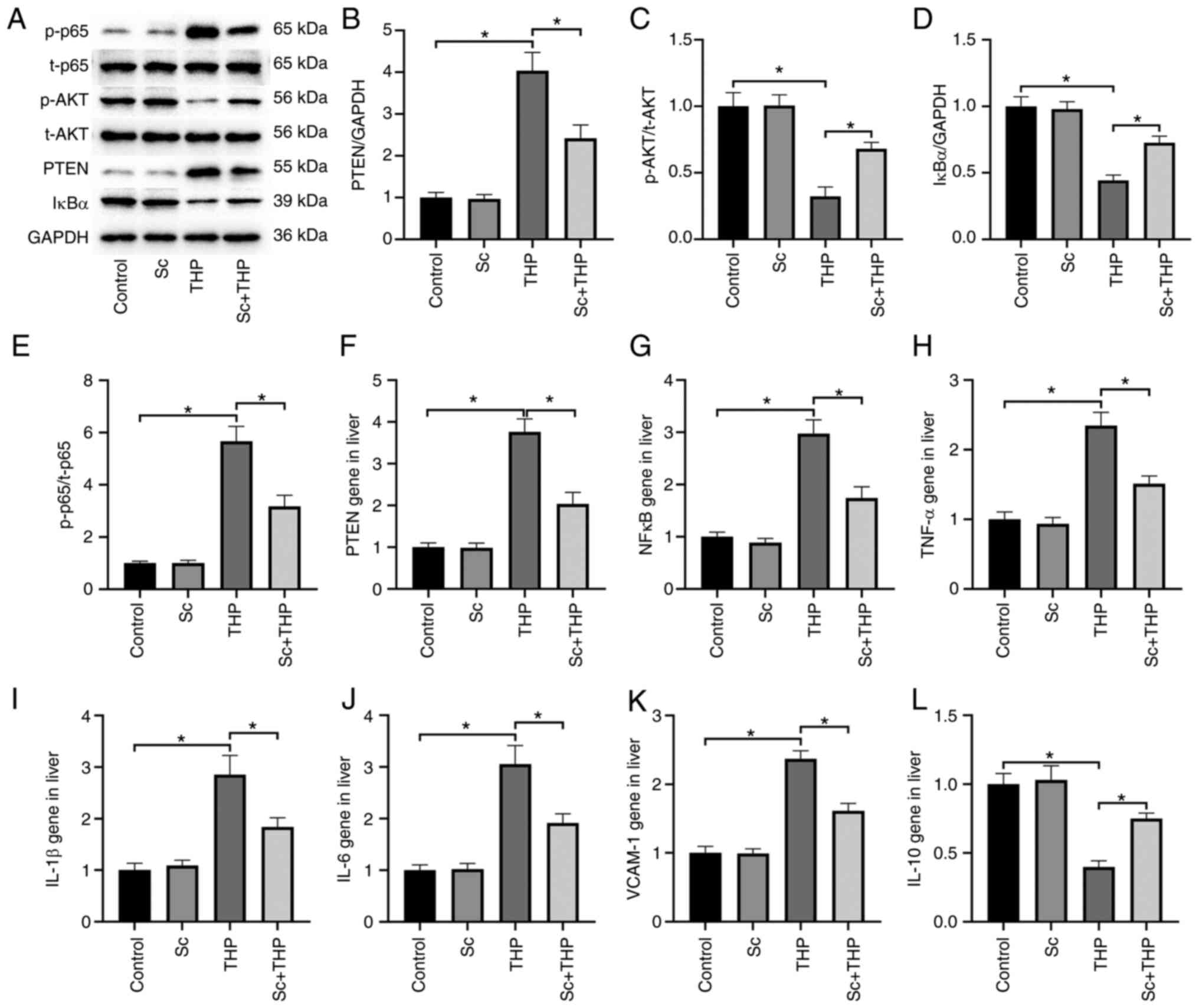 | Figure 3Changes of the hepatic PTEN/AKT/NFκB
signaling pathway and inflammation-related genes in rats. (A) The
effect of THP and Sc on the p65, AKT, PTEN and IκBα protein
expression in liver of rats. (B-E) Semi-quantitative analysis of
(B) PTEN, (C) AKT, (D) IκBα and (E) p65 protein expression. (F-L)
The effect of THP and Sc on (F) PTEN, (G) NFκB, (H) TNF-α, (I)
IL-1β, (J) IL-6, (K) VCAM-1 and (L) IL-10 gene expression in liver
of rats. Values are expressed as the mean ± SEM.
*P<0.05. THP, pirarubicin; Sc, scutellarein; p-,
phosphorylated. |
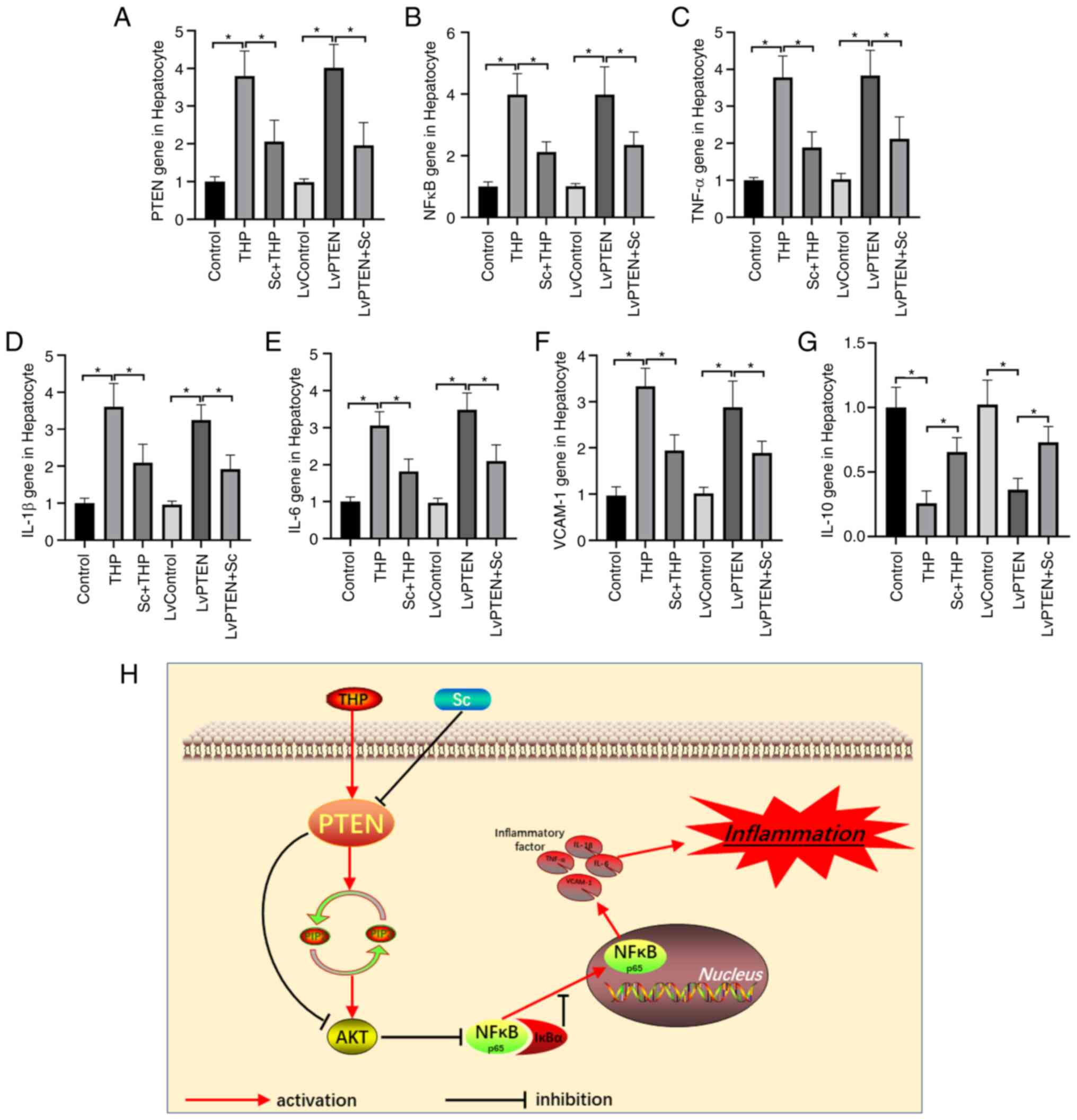
Effects of Sc and THP on liver PTEN gene
and inflammatory gene in rats
PCR results of rat liver demonstrated that PTEN gene
(Fig. 3F) and inflammatory gene
NFκB (Fig. 3G), TNF-α (Fig. 3H), IL-1β (Fig. 3I), IL-6 (Fig. 3J) and VCAM-1 (Fig. 3K) were significantly increased and
IL-10 (Fig. 3L) gene was
significantly decreased in the liver of rats in THP group (vs.
Control), while the level of Sc + THP group was significantly
reversed (vs. THP, Fig. 3F-L). Sc
alone had no significant effect on PTEN gene and inflammatory gene
in rat liver (vs. Control).
Optimum concentrations of THP and Sc in
primary hepatocytes
As revealed in Fig.
4A, in the time-concentration gradient relationship of THP
treatment of primary hepatocytes, 1 µmol/l THP had little
effect on the viability of hepatocytes within 48 h. However, 10 and
20 µmol/l THP had significant effect on the viability of
hepatocytes within 48 h; Therefore, 5 µmol/l THP was
selected as the treatment concentration. At 24 h, 5 µmol/l
THP inhibited the viability of hepatocytes to ~50%, which was
suitable for the experiment. Therefore, 5 µmol/l THP and 24
h were selected as the final treatment concentration and time.
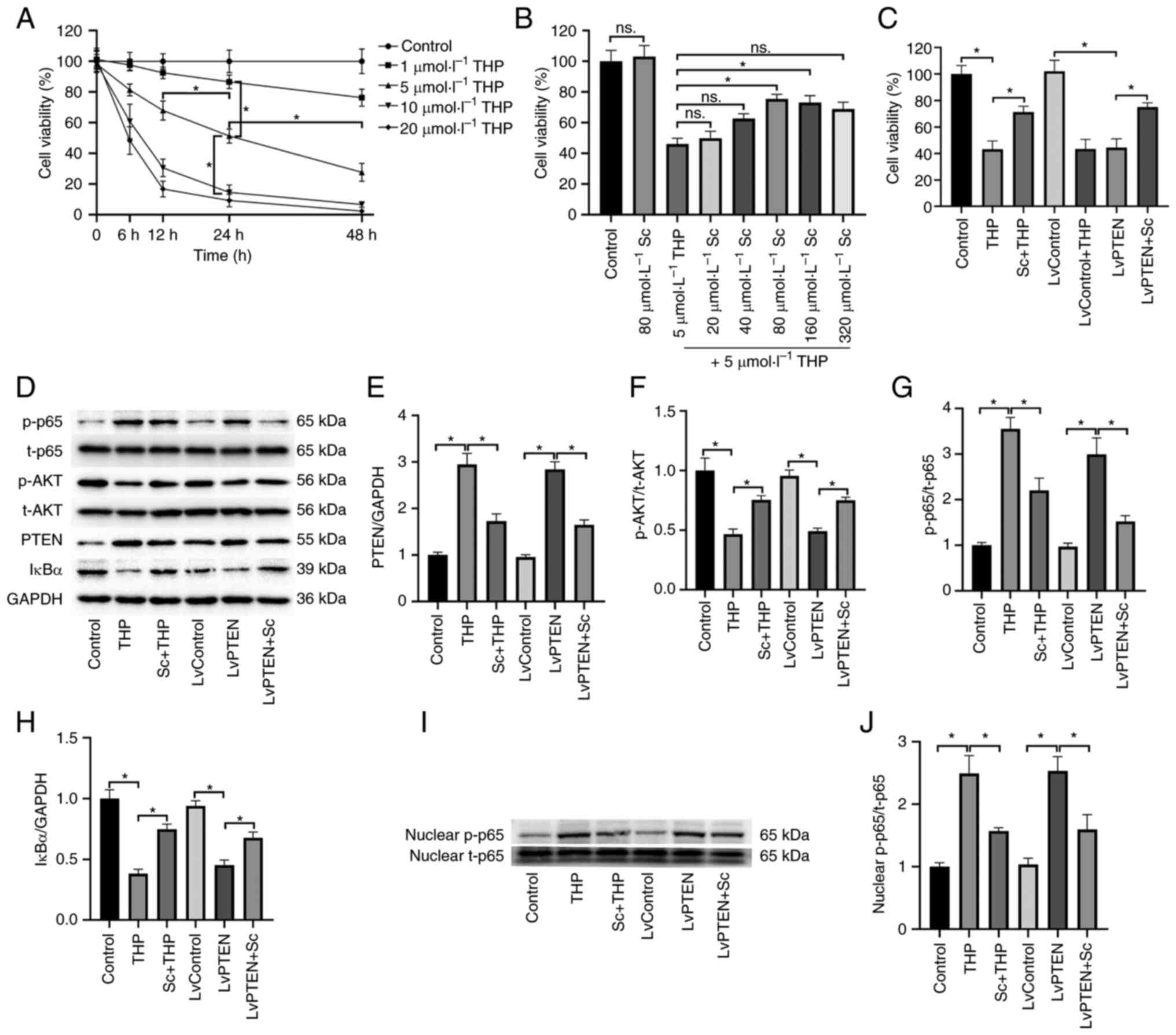 | Figure 4Changes of hepatocyte viability and
the PTEN/AKT/NFκB signaling pathway. (A) Selection of the optimal
concentration of THP for hepatocyte treatment. (B) Under the
condition of 5 µmol/l THP treatment, the optimal
concentration of Sc for hepatocyte treatment was selected. (C)
Effects of THP, Sc and Lentivirus on the viability of hepatocytes.
(D) The effect of THP, Sc and Lentivirus on the p65, AKT, PTEN and
IκBα protein expression in hepatocytes. (E-H) Semi-quantitative
analysis of (E) PTEN, (F) AKT, (G) p65 and (H) IκBα protein
expression. (I) The effect of THP, Sc and Lentivirus on the nucleus
p65 protein expression in hepatocytes. (J) Semi-quantitative
analysis of nucleus p65 protein expression. Values are expressed as
the mean ± SEM. *P<0.05. THP, pirarubicin; Sc,
scutellarein; p-, phosphorylated. |
It was identified that in the concentration gradient
relationship of primary hepatocytes treated with Sc, 20 and 40
µmol/l Sc have little effect on the viability of THP-treated
hepatocytes (vs. THP, Fig. 4B). A
total of 80 and 160 µmol/l Sc significantly increased the
viability of hepatocytes (vs. THP), thus 80 µmol/l Sc was
selected as the treatment concentration. Similarly, 80
µmol/l Sc alone did not significantly affect the viability
of hepatocytes (vs. Control).
Effects of Sc and THP on hepatocyte
viability
As shown in Fig.
4C, both THP (vs. Control) and LvPTEN (vs. LvControl)
significantly decreased the viability of hepatocytes, while the
viability of hepatocytes in the Sc + THP (vs. THP) and LvPTEN + Sc
groups (vs. LvControl + THP) increased significantly. LvControl
alone did not significantly affect the viability of hepatocytes
(vs. Control).
Effects of Sc and THP on PTEN/AKT/NFκB
signal pathway in hepatocytes
The expression of p-AKT/t-AKT and IκBα protein in
the THP (vs. Control) and LvPTEN groups (vs. LvControl) decreased
significantly, and the expression of p-p65/t-p65 and PTEN protein
in the THP (vs. Control) and LvPTEN groups (vs. LvControl)
increased significantly (Fig.
4D-H). The corresponding protein expression in the Sc + THP
(vs. THP) and LvPTEN + Sc (vs. LvControl + THP) groups was
significantly reversed (Fig.
4D-H). The liver protein expression in LvControl alone group
was not significantly aberrant (vs. Control). Semi-quantitative
analysis of PTEN (Fig. 4E),
p-AKT/t-AKT (Fig. 4F),
p-p65/t-p65 (Fig. 4G) and IκBα
(Fig. 4I) provided more
evidence.
The results also revealed that the expression of
nuclear p-p65/t-p65 protein was significantly increased in the THP
(vs. control group) and LvPTEN groups (vs. LvControl), while it was
significantly reversed in the Sc + THP group (vs. THP) and LvPTEN +
Sc group (vs. LvControl + THP, Fig.
4I). There was no significant abnormality in nuclear protein
expression in the single LvControl group (compared with the control
group). Semi-quantitative analysis provides additional evidence for
nuclear p-p65/t-p65 (Fig.
4J).
Effects of Sc and THP on PTEN gene and
inflammatory gene in hepatocytes
PCR results of hepatocytes showed that PTEN gene
(Fig. 5A) and inflammatory gene
NFκB (Fig. 5B), TNF-α (Fig. 5C), IL-1β (Fig. 5D), IL-6 (Fig. 5E) and VCAM-1 (Fig. 5F) increased significantly and
IL-10 (Fig. 5G) decreased
significantly in the THP (vs. Control) and LvPTEN groups (vs.
LvControl), and the corresponding gene expression in the Sc + THP
(vs. THP) and LvPTEN + Sc (vs. LvControl + THP) groups was
significantly reversed. The hepatocytes gene expression in
LvControl alone group was not significantly aberrant (vs.
Control).
Diagram of PTEN/AKT/NFκB signal pathway
and inflammation
As shown in the diagram of Fig. 5H, THP activates the expression of
PTEN in hepatocytes, leading to the decrease of PIP3, which leads
to the decrease of AKT phosphorylation, and finally activates the
expression of NFκB. NFκB binds IκBα and then transfers to the
nucleus, eventually causing the explosion of inflammatory factors
in the hepatocytes, leading to liver inflammation. Sc effectively
inhibits the expression of PTEN activated by THP, and finally
alleviates the liver inflammatory reaction.
Discussion
In the present study, it was found that the
hepatotoxicity caused by THP increased in a dose-dependent and
time-dependent manner. For example, during the 6-week THP
administration period, the body weight and food intake of rats
decreased continuously, and the serum ALT and AST increased
continuously. When hepatocyte necrosis is caused by various
reasons, ALT and AST are released into the blood in large
quantities; as a result, they are important indicators and
sensitive markers for the diagnosis of viral hepatitis and
drug-induced hepatitis (33,34). Drugs and compounds that are toxic
to the liver, including chlorpromazine, isoniazid, quinine,
salicylic acid preparations, ampicillin, carbon tetrachloride and
organic phosphorus, can lead to increased serum ALT and AST
activities (3,35).
In order to find out how THP causes liver damage,
the relevant inflammatory markers and genes were examined. The
results showed that inflammatory markers and inflammatory genes
were significantly increased in serum and hepatocytes. The
expression of p-AKT and IB decreased, while the expression of PTEN
and p-p65 increased. Similarly, the gene expression of PTEN also
increased. In further cell research, it was found that
overexpression of PTEN gene showed a similar phenomenon to THP
treatment, such as downregulation of p-AKT and upregulation of
p-p65, and the expression of corresponding inflammatory genes,
including NFκB, TNF-α, IL-1β, IL-6 and VCAM-1, were significantly
upregulated. PTEN gene exists in almost all tissues in the organism
and can modify other proteins and fats by removing phosphate groups
(36). Therefore, it is
characterized as a phosphatase gene. It is the first tumor
suppressor gene with double specific phosphatase activity found so
far and plays an important role in cell growth, apoptosis,
adhesion, migration and invasion (27,36,37). PIP3 is the main substrate of PTEN,
while AKT is the serine and threonine kinase downstream of
PI3K/PTEN (29,36). PTEN converts PIP3 into PIP2
through dephosphorylation, maintaining a low level of PIP3 in cells
(38). PIP3 is an important
second messenger, which can recruit AKT and PDK to the inner side
of the cell membrane and activate PDK to promote the
phosphorylation of AKT (30,36). PTEN attenuates this change,
thereby negatively regulating the AKT signal.
AKT is an important target to interfere with
inflammation (39). When AKT
signal is activated, it can inhibit the release of proinflammatory
factors including NFκB, IL-1β, IL-6 and TNF-α and promote the
expression of anti-inflammatory factors such as IL-10 and TGF
(39,40). As a key nuclear transcription
factor in inflammatory reaction, NFκB usually binds to the
inhibitor proteins of NFκB (IκB) in the form of heterodimer formed
by p50 and p65, and exists in the cytoplasm in an inactive form
(41,42). When subjected to upstream stimulus
signal, IκB is phosphorylated and degraded under IKK induction to
activate NFκB (42-44). The activated NFκB enters the
nucleus to initiate gene transcription, and produces and releases
inflammatory factors including IL-1β, IL-6 and TNF-α, and the
released inflammatory factors can in turn act again and activate
NFκB, forming positive feedback regulation, thus amplifying the
inflammatory reaction cascade (45,46).
Another outstanding finding of the present study was
that Sc appears to have the potential to prevent THP-induced liver
inflammation. Similar to its pathogenesis, Sc can inhibit the
activation of PTEN, in turn activate the phosphorylation of AKT,
inhibit the release of numerous proinflammatory factors, and
ultimately improve the liver inflammatory state. As one of the
representative drugs of natural flavonoids, Sc has great potential
in anti-inflammatory efficacy (21,22). In the study of non-alcoholic fatty
liver disease, it was found that Sc has significant anti-obesity,
anti-insulin resistance, anti-inflammatory and antioxidant effects
in the liver (23,24). The specific mechanism is closely
related to the inhibition of gene expression of inflammatory
cytokines and the fine regulation of genes responsible for energy
metabolism (23,24). However, it is worth noting that Sc
can inhibit the proliferation and metastasis of HepG2 cells by
upregulating PTEN and the PI3K/AKT/NFκB signaling pathway in the
study of liver cancer (20). A
previous study also found that Sc induced Fas-mediated exogenous
apoptosis and G2/M cell cycle arrest in Hep3B hepatoma cells,
indicating that Sc may be used as a potential natural drug for the
treatment of liver cancer (47).
Although this is in contrast to the results of the present study in
hepatocytes, it is considered that this may be due to the
heterogeneity between liver cancer cells and normal hepatocytes. In
short, Sc is beneficial in both anti inflammation and elimination
of liver cancer cells in the liver. The therapeutic effect and even
adjuvant anti-tumor effects of Sc have been demonstrated in studies
targeting other organs (48,49). For example, in the study of
bleomycin (BLM)-induced pulmonary fibrosis, it was found that Sc
not only alleviated BLM induced pulmonary fibrosis, but also
increased tumor cell apoptosis in combination with BLM treatment
(49). In another study, Sc was
confirmed to play a role in treating atherosclerosis by regulating
the Hippo-FOXO3A and PI3K/AKT signaling pathways (48). These studies have proven that Sc
is a powerful organ-protective compound, but there remains a long
way to go in terms of its specific application in clinical
treatment of human diseases.
In conclusion, increasing evidences have
demonstrated that inhibiting the activation of PTEN is considered
to be an effective therapeutic strategy to alleviate hepatitis,
whether viral or pharmaceutical. Chinese traditional herbal
extracts have attracted increasing researchers' interest due to
their competitive advantages of safety, cheapness and easy access.
Although most Chinese herbal extracts have not been fully
characterized, the research is also in the preliminary stage, and
the clinical efficacy remains to be determined. Nevertheless, a
large number of studies have identified that Sc has numerous
beneficial effects on various liver diseases, particularly
anti-inflammatory effects. It is worth noting that the current
study demonstrated that Sc, as a potential natural inhibitor of
PTEN, regulates the AKT/NFB signaling pathway, effectively
alleviates the upregulation of PTEN and the outbreak of
inflammatory factors in the liver caused by THP, and ultimately
protects liver function, providing a theoretical and experimental
basis for clarifying the pharmacological role of Sc in liver
protection.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZL was responsible for the conception and design of
the research, the key revision of the knowledge content and the
approval of the final version of the manuscript to be published,
and agrees to be responsible for all aspects of the work, YL made
substantial contributions to data acquisition, analysis and
interpretation, and participated in the drafting of the manuscript.
LC, JZ and HL made substantial contributions to data analysis and
interpretation. YL, LC, JZ, HL and ZL confirm the authenticity of
all the raw data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
CDFS12020220056) by and followed the guidelines of the Ethics
Committee for Experimental Animals of The Affiliated Hospital of
Chengdu University (Chengdu, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by Chengdu University Clinical
Medical College Affiliated Hospital Innovation Team Project (grant
no. CDFYCX202202).
References
|
1
|
Trefts E, Gannon M and Wasserman DH: The
liver. Curr Biol. 27:R1147–R1151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jetter A and Kullak-Ublick GA: Drugs and
hepatic transporters: A review. Pharmacol Res. 154:1042342020.
View Article : Google Scholar
|
|
3
|
Andrade RJ, Chalasani N, Björnsson ES,
Suzuki A, Kullak-Ublick GA, Watkins PB, Devarbhavi H, Merz M,
Lucena MI, Kaplowitz N and Aithal GP: Drug-induced liver injury.
Nat Rev Dis Primers. 5:582019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
European Association for the Study of the
Liver. Electronic address easloffice@easloffice.eu; Clinical
Practice Guideline Panel: Chair:; Panel members; EASL Governing
Board representative: EASL Clinical practice guidelines:
Drug-induced liver injury. J Hepatol. 70:1222–1261. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saithanyamurthi H and Faust AJ:
Drug-Induced liver disease: Clinical course. Clin Liver Dis.
21:21–34. 2017. View Article : Google Scholar
|
|
6
|
Luedde T, Kaplowitz N and Schwabe RF: Cell
death and cell death responses in liver disease: Mechanisms and
clinical relevance. Gastroenterology. 147:765–783.e4. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giordano CM and Zervos XB: Clinical
manifestations and treatment of drug-induced hepatotoxicity. Clin
Liver Dis. 17:565–573, viii. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Zhao P, Cheng M, Yu L, Cheng Z, Fan
L and Chen C: AST to ALT ratio and arterial stiffness in non-fatty
liver Japanese population:a secondary analysis based on a
cross-sectional study. Lipids Health Dis. 17:2752018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi H, Yan Y, Yang H, Pu P and Tang H:
Schisandrin B diet inhibits oxidative stress to reduce ferroptosis
and lipid peroxidation to prevent pirarubicin-induced
hepatotoxicity. Biomed Res Int. 2022:56235552022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Ma XY, Zhang T, Qin M, Sun B, Li
Q, Hu DW and Ren LQ: Protective effects of apocynum venetum against
pirarubicin-induced cardiotoxicity. Am J Chin Med. 47:1075–1097.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hori H, Kudoh T, Nishimura S, Oda M,
Yoshida M, Hara J, Tawa A, Usami I, Tanizawa A, Yumura-Yagi K, et
al: Acute and late toxicities of pirarubicin in the treatment of
childhood acute lymphoblastic leukemia: Results from a clinical
trial by the Japan Association of Childhood Leukemia Study. Int J
Clin Oncol. 22:387–396. 2017. View Article : Google Scholar
|
|
12
|
Jiménez-Castro MB, Cornide-Petronio ME,
Gracia-Sancho J and Peralta C: Inflammasome-mediated inflammation
in liver ischemia-reperfusion injury. Cells. 8:11312019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai C, Xiao X, Li D, Tun S, Wang Y, Velkov
T and Tang S: Chloroquine ameliorates carbon tetrachloride-induced
acute liver injury in mice via the concomitant inhibition of
inflammation and induction of apoptosis. Cell Death Dis.
9:11642018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu YT, Qi SL and Sun KW: Traditional
Chinese medicine, liver fibrosis, intestinal flora: Is there any
connection?-a narrative review. Ann Palliat Med. 10:4846–4857.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen M, Xie Y, Gong S, Wang Y, Yu H, Zhou
T, Huang F, Guo X, Zhang H, Huang R, et al: Traditional Chinese
medicine in the treatment of nonalcoholic steatohepatitis.
Pharmacol Res. 172:1058492021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Serafini M, Peluso I and Raguzzini A:
Flavonoids as anti-inflammatory agents. Proc Nutr Soc. 69:273–278.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koirala N, Thuan NH, Ghimire GP, Thang DV
and Sohng JK: Methylation of flavonoids: Chemical structures,
bioactivities, progress and perspectives for biotechnological
production. Enzyme Microb Technol. 86:103–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yi YS: Regulatory roles of flavonoids on
inflammasome activation during inflammatory responses. Mol Nutr
Food Res. 62:e18001472018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krych-Madej J, Stawowska K and Gebicka L:
Oxidation of flavonoids by hypochlorous acid: Reaction kinetics and
antioxidant activity studies. Free Radic Res. 50:898–908. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ha SE, Kim SM, Vetrivel P, Kim HH, Bhosale
PB, Heo JD, Lee HJ and Kim GS: Inhibition of cell proliferation and
metastasis by scutellarein regulating PI3K/Akt/NF-κB signaling
through PTEN activation in hepatocellular carcinoma. Int J Mol Sci.
22:88412021. View Article : Google Scholar
|
|
21
|
Russo M, Moccia S, Spagnuolo C, Tedesco I
and Russo GL: Roles of flavonoids against coronavirus infection.
Chem Biol Interact. 328:1092112020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chagas MDSS, Behrens MD, Moragas-Tellis
CJ, Penedo GXM, Silva AR and Gonçalves-de-Albuquerque CF: Flavonols
and flavones as potential anti-inflammatory, antioxidant, and
antibacterial compounds. Oxid Med Cell Longev. 2022:99667502022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin Y, Ren N, Li S, Chen M and Pu P: Novel
anti-obesity effect of scutellarein and potential underlying
mechanism of actions. Biomed Pharmacother. 117:1090422019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao L, Tang H, Zeng Q, Tang T, Chen M and
Pu P: The anti-insulin resistance effect of scutellarin may be
related to antioxidant stress and AMPKα activation in diabetic
mice. Obes Res Clin Pract. 14:368–374. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brenner C, Galluzzi L, Kepp O and Kroemer
G: Decoding cell death signals in liver inflammation. J Hepatol.
59:583–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cavalli G and Dinarello CA: Suppression of
inflammation and acquired immunity by IL-37. Immunol Rev.
281:179–190. 2018. View Article : Google Scholar
|
|
27
|
Peyrou M, Bourgoin L and Foti M: PTEN in
non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and
cancer. Dig Dis. 28:236–246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Álvarez-Garcia V, Tawil Y, Wise HM and
Leslie NR: Mechanisms of PTEN loss in cancer: It's all about
diversity. Semin Cancer Biol. 59:66–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Piao Y and Yin D: Mechanism underlying
treatment of diabetic kidney disease using Traditional Chinese
Medicine based on theory of Yin and Yang balance. J Tradit Chin
Med. 38:797–802. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu G, Yu H, Yang Q, Wang J, Fan H, Liu G,
Wang L, Bello BK, Zhao P, Zhang H and Dong J: Vibrio harveyi
infections induce production of proinflammatory cytokines in murine
peritoneal macrophages via activation of p38 MAPK and NF-κB
pathways, but reversed by PI3K/AKT pathways. Dev Comp Immunol.
127:1042922022. View Article : Google Scholar
|
|
31
|
Pu P, Wang XA, Salim M, Zhu LH, Wang L,
Chen KJ, Xiao JF, Deng W, Shi HW, Jiang H and Li HL: Baicalein, a
natural product, selectively activating AMPKα(2) and ameliorates
metabolic disorder in diet-induced mice. Mol Cell Endocrinol.
362:128–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Sookoian S and Pirola CJ: Liver enzymes,
metabolomics and genome-wide association studies: From systems
biology to the personalized medicine. World J Gastroenterol.
21:711–725. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Korver S, Bowen J, Pearson K, Gonzalez RJ,
French N, Park K, Jenkins R and Goldring C: The application of
cytokeratin-18 as a biomarker for drug-induced liver injury. Arch
Toxicol. 95:3435–3448. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stravitz RT and Lee WM: Acute liver
failure. Lancet. 394:869–881. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Worby CA and Dixon JE: PTEN. Annu Rev
Biochem. 83:641–669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ho J, Cruise ES, Dowling RJO and Stambolic
V: PTEN nuclear functions. Cold Spring Harb Perspect Med.
10:a0360792020. View Article : Google Scholar
|
|
38
|
Chen J, Zhang XD and Proud C: Dissecting
the signaling pathways that mediate cancer in PTEN and LKB1
double-knockout mice. Sci Signal. 8:pe12015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang F, Wang Y, Hemmings BA, Rüegg C and
Xue G: PKB/Akt-dependent regulation of inflammation in cancer.
Semin Cancer Biol. 48:62–69. 2018. View Article : Google Scholar
|
|
40
|
Sun X, Chen L and He Z: PI3K/Akt-Nrf2 and
anti-inflammation effect of macrolides in chronic obstructive
pulmonary disease. Curr Drug Metab. 20:301–304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mishra V, Banga J and Silveyra P:
Oxidative stress and cellular pathways of asthma and inflammation:
Therapeutic strategies and pharmacological targets. Pharmacol Ther.
181:169–182. 2018. View Article : Google Scholar
|
|
42
|
Mulero MC, Huxford T and Ghosh G: NF-κB,
IκB, and IKK: Integral components of immune system signaling. Adv
Exp Med Biol. 1172:207–226. 2019. View Article : Google Scholar
|
|
43
|
Lin JK: Cancer chemoprevention by tea
polyphenols through modulating signal transduction pathways. Arch
Pharm Res. 25:561–571. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kondylis V, Kumari S, Vlantis K and
Pasparakis M: The interplay of IKK, NF-κB and RIPK1 signaling in
the regulation of cell death, tissue homeostasis and inflammation.
Immunol Rev. 277:113–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gasparini C and Feldmann M: NF-κB as a
target for modulating inflammatory responses. Curr Pharm Des.
18:5735–5745. 2012. View Article : Google Scholar
|
|
46
|
Dumortier C, Danopoulos S, Velard F and Al
Alam D: Bone cells differentiation: How CFTR mutations may rule the
game of stem cells commitment? Front Cell Dev Biol. 9:6119212021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sang Eun H, Seong Min K, Ho Jeong L,
Vetrivel P, Venkatarame Gowda Saralamma V, Jeong Doo H, Eun Hee K,
Sang Joon L and Gon Sup K: Scutellarein induces Fas-mediated
extrinsic apoptosis and G2/M cell cycle arrest in Hep3B
hepatocellular carcinoma cells. Nutrients. 11:2632019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fu Y, Sun S, Sun H, Peng J, Ma X, Bao L,
Ji R, Luo C, Gao C, Zhang X and Jin Y: Scutellarin exerts
protective effects against atherosclerosis in rats by regulating
the Hippo-FOXO3A and PI3K/AKT signaling pathways. J Cell Physiol.
234:18131–18145. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nie J, Yang HM, Sun CY, Liu YL, Zhuo JY,
Zhang ZB, Lai XP, Su ZR and Li YC: Scutellarin enhances antitumor
effects and attenuates the toxicity of bleomycin in H22 ascites
tumor-bearing mice. Front Pharmacol. 9:6152018. View Article : Google Scholar : PubMed/NCBI
|