Introduction
Head and neck cancers are among the top seven most
prevalent cancer types worldwide, of which, the majority (90%) are
squamous cell carcinoma (1).
There are several risk factors associated with the development of
this cancer type, including premalignant conditions such as
consumption of tobacco, betel nut, and alcohol, poor oral hygiene,
UV radiation exposure, Epstein Barr virus infection, and human
papillomavirus (HPV) infection (2,3).
Oral cancer is a subtype of head and neck cancer that affects
various oral mucosal regions, including the anterior two-thirds of
the tongue, the gingival tissue, the mucosal lining of the lips and
cheeks, the sublingual floor, the hard palate, and the retromolar
region (4,5). Although significant progress has
been made in oral cancer treatment, further studies are required to
understand the mechanism of action of the therapeutic agents
commonly used and the heterogeneous nature of this disease.
Podophyllotoxin (PPT) is a naturally occurring
arylnaphthalene class of lignans found in the plants of the
Podophyllum genus, such as Podophyllum peltatum and
Podophyllum emodi (6). It
is a folk remedy that exhibits antiproliferative properties similar
to colchicine (7). PPT and its
derivatives exhibit significant anticancer activity by
destabilizing microtubules, which are structural elements of the
cytoskeleton (8-10). PPT also exerts a broad range of
highly effective antiviral and antibacterial properties (11). In 1990, the WHO recommended 0.5%
PPT as a first-line drug for the treatment of condyloma acuminatum,
which is usually caused by HPV (12). Two semi-synthesized
podophyllotoxin glycosyl derivatives, etoposide (VP-16) and
teniposide (VM-26), were approved by the FDA in 1983 and 1992 for
anticancer therapy (12).
Etoposide is used in frontline cancer therapy against various
cancer types, such as small-cell lung cancer and testicular cancer
(13-16), whereas teniposide is primarily
used for the treatment of acute lymphoblastic leukemia (17). With respect to the molecular
mechanisms, PPT exhibits potent anticancer effects by inhibiting
DNA topoisomerase II and tubulin, respectively, which cause cell
cycle arrest and DNA damage (11). The remarkable anticancer
properties of PPT have been extensively studied. Moreover, a
variety of novel compounds have been tested and demonstrated to be
effective at treating various neoplasms. PPT derivatives cause cell
cycle arrest and apoptosis associated with Bcl-2 in hepatocellular
carcinoma and lung cancer (18,19). In addition, the synthesis and
evaluation of a hybrid combining podophyllotoxin and coumarin was
studied and found to be associated with the AKT/mTOR pathway in
oral cancer (20). However,
studies determining the effectiveness of PPT in treating oral
squamous cell carcinoma (OSCC) (20) are less common compared with those
in other tumor types.
To identify the potent anticancer mechanism of
action of PPT in OSCC, apoptosis associated with the Bcl-2 family
of proteins, particularly myeloid cell leukemia-1 (Mcl-1) was
assessed. In OSCC, the Mcl-1 gene is commonly amplified and
upregulated, which affects the clinical course and survival of
patients with this disease (21-24). Mcl-1 is also upregulated and is
associated with poor outcomes in other malignant tumors, such as
hematologic malignancies (25),
breast cancer (26,27), lung (28), and gastric cancer (29). Mcl-1 blocks the oligomerization
of the pro-apoptotic Bcl-2 family members, Bax and Bak, which
sequesters them to produce protein-permeable holes in the
mitochondrial outer membrane (30-32). Finally, a reduction in Mcl-1
expression promotes cytochrome C release into the cytoplasm, which
activates the caspase cascade and induces apoptosis (21). Based on these findings, targeting
Mcl-1 may represent a promising approach for treating OSCC.
In the present study, the cytotoxic effects of PPT
against OSCC cell lines were assessed. It was found that PPT
induced apoptosis in OSCC cells by activating the mitochondrial
pathway through the downregulation of Mcl-1 at the
post-translational level.
Materials and methods
Cell culture and reagents
The OSCC cell lines (HSC3 and HSC4) were obtained
from Hokkaido University (Hokkaido, Japan) and cultured in DMEM/F12
medium (Welgene, Inc.) supplemented with 10% FBS and 1% antibiotics
[penicillin/streptomycin (P/S)] (Welgene, Inc.) at 37°C in a 5%
CO2 incubator. The Yonsei University kindly provided the
Immortalized human oral keratinocyte (IHOK) cells. KSF medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with BPE/EGFR
was used for maintaining IHOK cells. All experiments were performed
with cells cultured to 50-60% confluence. PPT and cycloheximide
(CHX) were purchased from MilliporeSigma (cat. no. P4405) and MG132
was obtained from Santa Cruz Biotechnology Inc. All chemicals were
dissolved in DMSO and stored at -20°C.
Trypan blue exclusion assay
The trypan blue exclusion assay was used to
determine the effect of PPT on cell viability. The OSCC cells
(1.8×105/ml HSC3 cells or 2.8×105/ml HSC4
cells) were seeded into 6-well plates and incubated with 100 nM PPT
for 24 h, followed by staining with 0.4% trypan blue (Gibco; Thermo
Fisher Scientific, Inc.). The viable cells were counted using a
Corning® Cell counter. All experiments were performed
independently three times with triplicate samples for each
experiment.
CCK-8 cell proliferation assay
To assess cell proliferation, the cells were seeded
into 96-well plates and incubated with 100 nM PPT for 24 h. Then,
10 µl CCK-8 solution (cat. no. CK04-500; Dojindo Molecular
Technologies, Inc.) was added to each well, thoroughly mixed with
the culture media, and incubated at 37°C for 2 h. The optical
density (OD) of each well was measured using a Chameleon microplate
reader (Hidex) at 450 nm and the OD values are reported as the mean
± SD.
Soft colony formation agar assay
Basal Medium Eagle (BME) with sodium carbonate was
dissolved in sterile distilled water to prepare a 2× BME solution,
which was then purified using a 0.2 µM syringe filter. The
bottom agar (0.5%) was prepared by mixing 2× BME, L-glutamine,
gentamicin, PBS, FBS, and 1.25% agar. The wells were pre-coated
with 3 ml per well along with 0.3% top agar. After curing at room
temperature (RT) for 1 h, 200 µl culture medium was added
every 3 days with DMSO and 100 nM PPT, and the cells were incubated
at 37°C for 10-14 days. Images of the colonies were randomly
captured (4 images per well) under a bright field microscope
(Olympus Corporation) at a 40× magnification. The number of
colonies with varying diameters was determined using ImageJ version
1.53t (National Institutes of Health).
Sphere formation assay
Cells were cultured in a serum-free medium
containing 25 ng/ml EGF (Gibco; Thermo Fisher Scientific, Inc.) and
bFGF (Invitrogen; Thermo Fisher Scientific, Inc.), 0.01× N-2, B27
supplement (Gibco; Thermo Fisher Scientific, Inc.), and 1% P/S and
were seeded into ultra-low attachment 6-well plates
(Corning®, Inc.) at a density of 1×104 of
cells/well. A total of 7-10 days after seeding, images of the
spheres were randomly captured using an inverted light microscope
(x20 magnification; Nikon Corporation) and counted in ImageJ.
Cell cycle distribution analysis
After treatment with PPT, HSC3 and HSC4 cells were
collected, resuspended, washed with PBS, and fixed in 70% ethanol
overnight at -20°C. The cells were incubated with 20 µg/ml
propidium iodide solution (PI; cat. no. P4170, MilliporeSigma) and
RNase A (20 µg/ml) for 15 min at 37°C. Cell cycle
distribution was determined using an LSRFortessa™ Cell Analyzer (BD
Biosciences). A minimum of 10,000 cells were analyzed for each
sample using BD FACSDIVA™ software 6.0 (BD Biosciences). The
percentage of the sub-G1 fraction was quantified using
FlowJo version 9/10 (FlowJo LLC).
Annexin V/PI double staining
Apoptosis was assessed using a FITC-Annexin V
Apoptosis Detection kit (BD Pharmingen) according to the
manufacturer's instructions. The floating and attached cells were
gathered, washed with ice-cold PBS twice, and spun down in a
centrifuge (180 × g, 5 min, 4°C). The cells were then resuspended
in Annexin V binding buffer and treated with 3 µl Annexin
V-FITC for 15 min at room temperature in the dark, followed by the
addition of 1 µl PI. The cells were then transferred to a
FACS tube and analyzed by flow cytometry. The Annexin V(+)/PI(-)
staining of cells indicated early-stage apoptosis, while Annexin
V(+)/PI(+) staining indicated late-stage apoptosis. The percentage
of cells displaying Annexin V/PI staining was determined by
analyzing a minimum of 10,000 cells. The flow cytometry data were
reanalyzed using FlowJo software.
Western blot analysis
Total protein was isolated from cells using RIPA
lysis buffer (MilliporeSigma) containing 1% phosphatase inhibitor
(Thermo Fisher Scientific Inc.) and protease inhibitor cocktails
(Roche Diagnostics, GmbH). The protein concentration of each sample
was measured using a DC Protein Assay kit (Bio-Rad Laboratories,
Inc.). The protein lysates, containing around 30-50 µg
protein, were normalized and then mixed with 5× protein sample
buffer. The mixture was heated at 95°C for 5 min, separated on 8,
12, or 15% SDS gels, resolved using SDS-PAGE, and then transferred
to Immuno-Blot PVDF membranes (MilliporeSigma). The membranes were
added to 5% skimmed milk in Tris-buffered saline with Tween20 at RT
for 2 h for blocking non-specific binding and incubated with
primary antibodies overnight at 4°C. The membranes were incubated
with horseradish peroxidase-conjugated secondary antibodies for 2 h
and developed using a WestGlow™ PICO PLUS Chemiluminescent
substrate (Biomax, Inc.). The immunoreactive bands were visualized
using an ImageQuant™ LAS 500 (GE Healthcare Life Sciences) or x-ray
film. Densitometry analysis of the western blots was performed
using Image J version 1.51k (National Institutes of Health). The
primary antibodies used were: Cleaved poly (ADP-ribose) polymerase
(PARP) (cat. no. 9541, 1:1,000), Mcl-1 (cat. no. 5453, 1:1,000),
Bcl-2 (cat. no. 2870, 1:1,000), and Bcl-xL (cat. no. 2764,
1:1,000), all from Cell Signaling Technology, Inc. β-actin (cat.
no. sc-47778, 1:3,000) and α-tubulin (cat. no. sc-5286, 1:3,000)
were obtained from Santa Cruz Biotechnology Inc. Cytochrome C (cat.
no. BD556433, 1:3,000) and CoxIV (cat. no. ab16056, 1:3,000)
antibodies were obtained from BD Biosciences and Abcam,
respectively.
Mitochondrial membrane potential (MMP)
assay
ΔΨm was measured by flow cytometry using the
lipophilic fluorescent dye JC-1 and the MitoScreen kit (BD
Pharmingen). After trypsinization, the cells were washed with PBS,
and collected by centrifugation at 1,120 × g at 4°C for 5 min. The
cell pellets were resuspended in 1× JC-1 working solution and
incubated at 37°C for 30 min in the dark. After staining, the cells
were rinsed with 1× assay buffer and spun down in a centrifuge at
3500 rpm for 5 min The supernatant was removed, and the cells were
resuspended in 1× assay buffer. Subsequently, the cells were
transferred to FACS tubes and analyzed with an LSRFortessa™ Cell
Analyzer. The proportion of stained cells was quantified using
10,000 cells per sample and analyzed using BD FACSDIVA™ software.
The flow cytometry data were reanalyzed using FlowJo software.
Cytosolic and mitochondrial
fractions
The isolation of the cytosolic and mitochondrial
fractions was performed using the Mitochondria/Cytosol
Fractionation Kit (Abcam). The procedure involved washing the cells
with ice-cold PBS, spinning them down (4°C for 5 min at 15,500 ×
g), and then resuspending the cell pellet in a mixture of 1×
cytosol extraction buffer, DTT, and protease inhibitor for 10 min
on ice. After centrifugation at 4°C for 15 min at 15,500 × g, the
supernatants, which contained cytosolic proteins, were collected
and the pellets were resuspended in the mitochondrial extraction
buffer. The supernatant containing the mitochondrial proteins was
then obtained by another round of centrifugation (4°C for 15 min at
15,500 × g).
Reverse transcription-quantitative PCR
(RT-qPCR)
The total RNA was extracted using TRIzol®
Reagent. Then, 1 µg of the extracted RNA was
reverse-transcribed using the AMPIGENE cDNA Synthesis kit (Enzo
Life Sciences, Inc.) according to the manufacturer's protocol, and
the resulting cDNA was subjected to qPCR using AMPIGENE qPCR Green
Mix Hi-Rox (Enzo Life Sciences, Inc.). qPCR was performed using the
Applied Biosystems StepOnePlus Real-Time PCR System with the
following thermocycling conditions: 95°C for 2 min; followed by 40
cycles of 95°C for 10 sec and 60°C for 30 sec. The relative
expression levels of each gene were normalized to GAPDH and
calculated using the 2−∆∆Cq method (33). The sequences of the primers used
are: Mcl-1 forward, 5′-GTA TCA CAG ACG TTC TCG-3′, and reverse,
5′-AGA GGA CCT AGA AGG TGG-3′, and GAPDH forward, 5′-GTG GTC TCC
TCT GAC TTC AAC-3′ and reverse, 5′-CCT GTT GCT GTA GCC AAA
TTC-3′.
Construction of the Mcl-1 overexpression
vector and transient transfection
The pcDNA3.1-Mcl-1 overexpression vector was
generated as described previously (32). HSC3 and HSC4 cells were treated
with either 1 µg blank pcDNA3.1 vector or the pcDNA3.1-Mcl-1
vector using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.) for 12 h as per the manufacturer's
guidelines.
Statistical analysis
Statistical analyses were performed using SPSS
version 25.0 (IBM Corp.). A two-tailed Student's t-test was used
for comparisons between two groups, and a one-way ANOVA followed by
a Tukey's post-hoc test was used for comparisons between multiple
groups. All graphs reflect the means and standard deviations of
three separate experiments. P<0.05 was used to indicate a
statistically significant difference.
Results
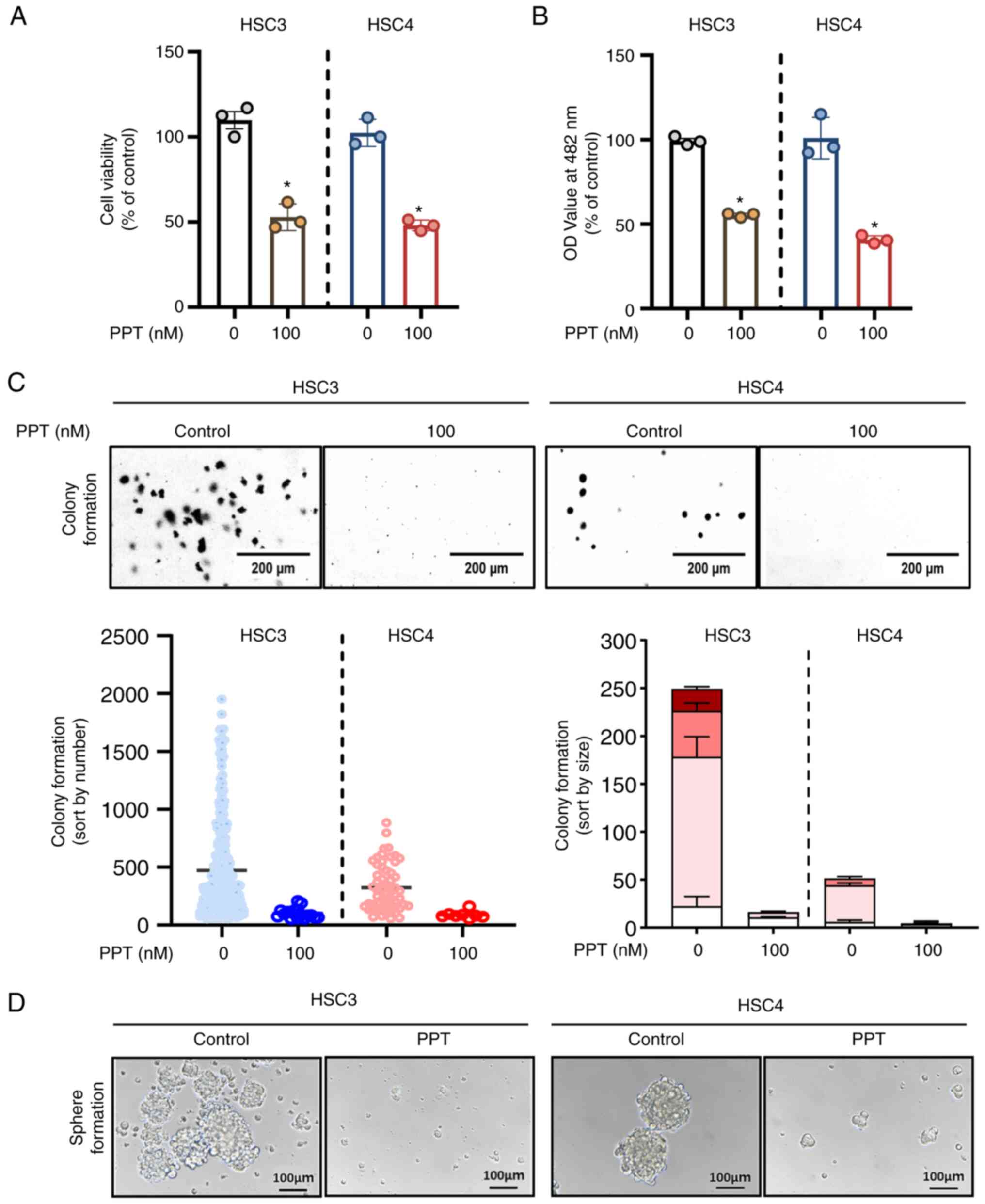
PPT inhibits the growth and colony
formation of OSCC cell lines
To measure the cytotoxic effects of PPT on HSC3 and
HSC4 cells, trypan blue exclusion and CCK-8 assays were performed
following treatment with 100 nM PPT for 24 h. PPT significantly
decreased the growth of both OSCC cell lines (Fig. 1A and B). To evaluate the effect
of the PPT, the treatment period was extended from 48 to 72 h. This
extended treatment revealed a sustained effect of PPT, confirming
its prolonged efficacy (Fig.
S1A). To ascertain the toxic effects on normal cell lines, IHOK
cells were treated with 100 nM PPT for 24 h, and it was found that
PPT did not affect the viability of the IHOK cells (Fig. S1B). To determine whether PPT
influenced anchorage-independent growth in the HSC3 and HSC4 cell
lines, soft agar assays were also performed by exposing the cell
lines to PPT for 7-28 days. As evidenced by the size and quantity
of the colonies, PPT significantly reduced the ability of cells to
form colonies compared with the control (Fig. 1C). To explore the various facets
of tumor behavior and drug response, spheroids were used as they
are more capable of simulating cell-microenvironment interactions
that closely mimic the 3D architecture of in vivo tumors.
Notably, the results revealed the inhibition of spheroid formation
upon PPT treatment (Fig. 1D).
These results suggest that PPT inhibited malignant cancer cell
growth in OSCC cell lines.
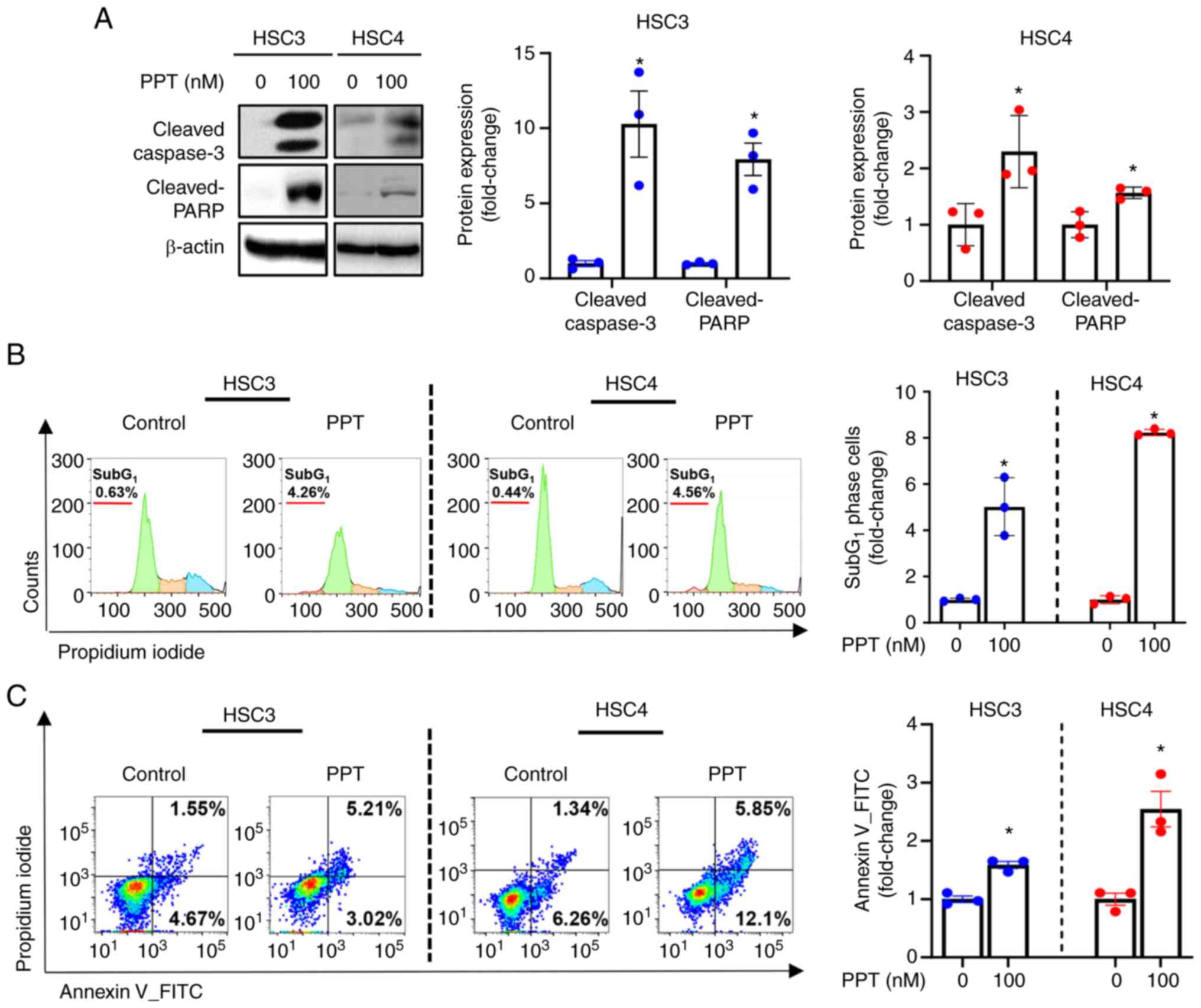
PPT increases the number of cells that
undergo apoptosis in human OSCC cell lines
PPT treatment of cells led to cleavage of caspase3
and PARP, as revealed by western blot analysis. This suggests that
PPT may induce apoptosis (Fig.
2A). Accordingly, a significant accumulation of apoptotic cells
in the sub-G1 phase of the cell cycle was observed in PPT-treated
cells (Fig. 2B). Flow cytometry
analysis was performed to further evaluate the effect of PPT on
apoptosis. This analysis provided information on the extent of cell
death in response to PPT treatment. Compared with the solvent
control, the percentage of Annexin V+ cells undergoing early-stage
(Annexin V+/PI−) or late-stage (Annexin
V+/PI+) apoptosis was increased following PPT
treatment (Fig. 2C).
Collectively, these results indicated that PPT induced apoptosis
via a caspase-mediated pathway in human OSCC cell lines.
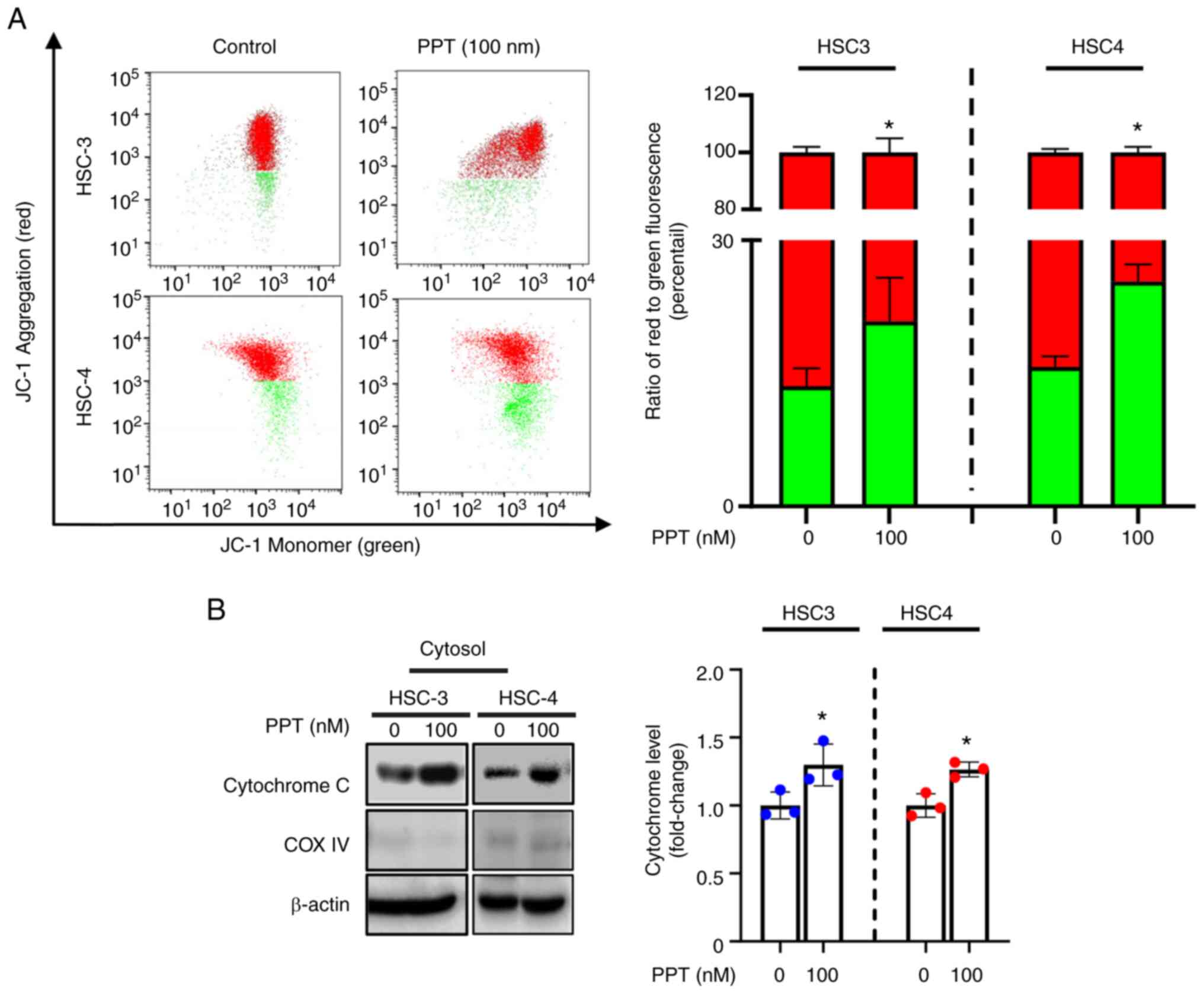
PPT influences the dysregulation of the
MMP and the release of cytochrome C
To determine whether PPT-induced apoptosis in HSC3
and HSC4 cell lines was accompanied by mitochondrial dysfunction,
the cationic dye JC-1 was used to detect changes in the MMP.
Compared with the solvent control group, PPT treatment resulted in
an increase in the monomeric form of JC-1, as indicated by an
increase in cytosolic green fluorescence. This suggests that PPT
may affect the MMP in cells (Fig.
3A). Furthermore, PPT induced the release of cytochrome C into
the cytosol (Fig. 3B). Taken
together, these results indicate that PPT promotes mitochondrial
membrane depolarization and translocation of cytochrome C into the
cytosol, which is considered an apoptotic event (34).
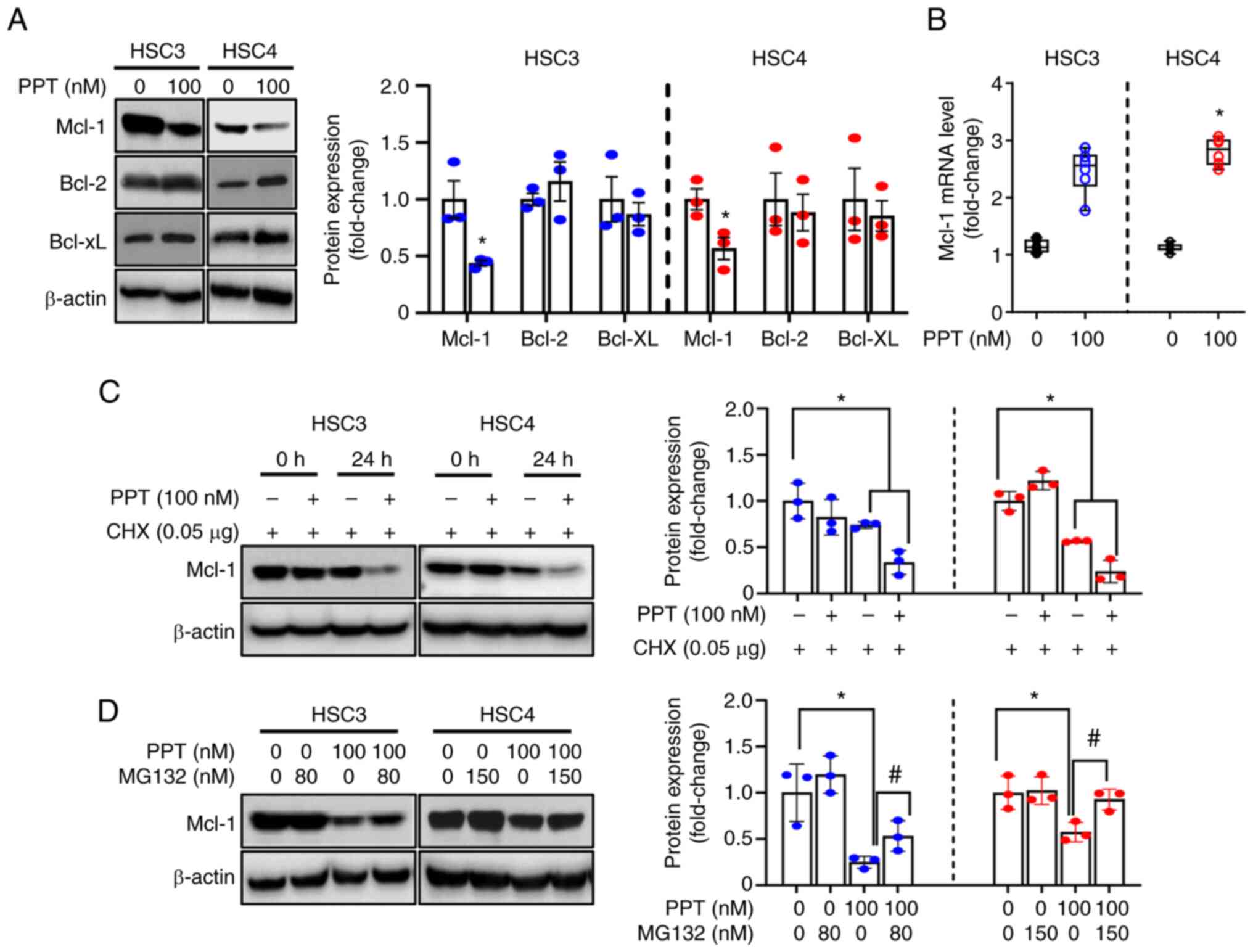
Downregulation of Mcl-1 protein by PPT is
associated with post-translational modifications
To understand the mechanisms of mitochondrial
membrane depolarization induced by PPT treatment, the expression of
Bcl-2 family proteins (anti-apoptotic: Mcl-1, Bcl-2, Bcl-xL and
pro-apoptotic: Bax, Bak) was evaluated. These proteins regulate the
integrity of the mitochondria prior to apoptosis (34). The study aimed to identify the
key molecules involved in the process. Significantly decreased
Mcl-1 expression was observed in both cell lines following PPT
treatment, whereas Bcl-2, Bcl-xL, Bax, and Bak were unchanged
(Figs. 4A and S2). qPCR was performed to determine
the effect of PPT on Mcl-1 mRNA levels. PPT had no obvious effects
on Mcl-1 mRNA levels in either cell line (Fig. 4B). This suggested that Mcl-1
protein is regulated at the translational or post-translational
level by PPT. To further assess whether PPT regulated the stability
of Mcl-1, cells were treated with CHX to block new protein
synthesis. Mcl-1 expression in the PPT and CHX-treated groups was
reduced compared with the groups treated with CHX alone (Fig. 4C). The study found that
pretreatment with MG132, a proteasome inhibitor, partially
prevented the decrease in Mcl-1 expression caused by PPT treatment.
This suggests that the proteasome pathway may play a role in the
effects of PPT on Mcl-1 expression (Fig. 4D). Based on these findings, PPT
contributed to a decrease in Mcl-1 protein levels through
proteasome-mediated degradation. This suggests that the
downregulation of Mcl-1 through proteasomal degradation is an
important step in the death of human OSCC cell lines following PPT
therapy.
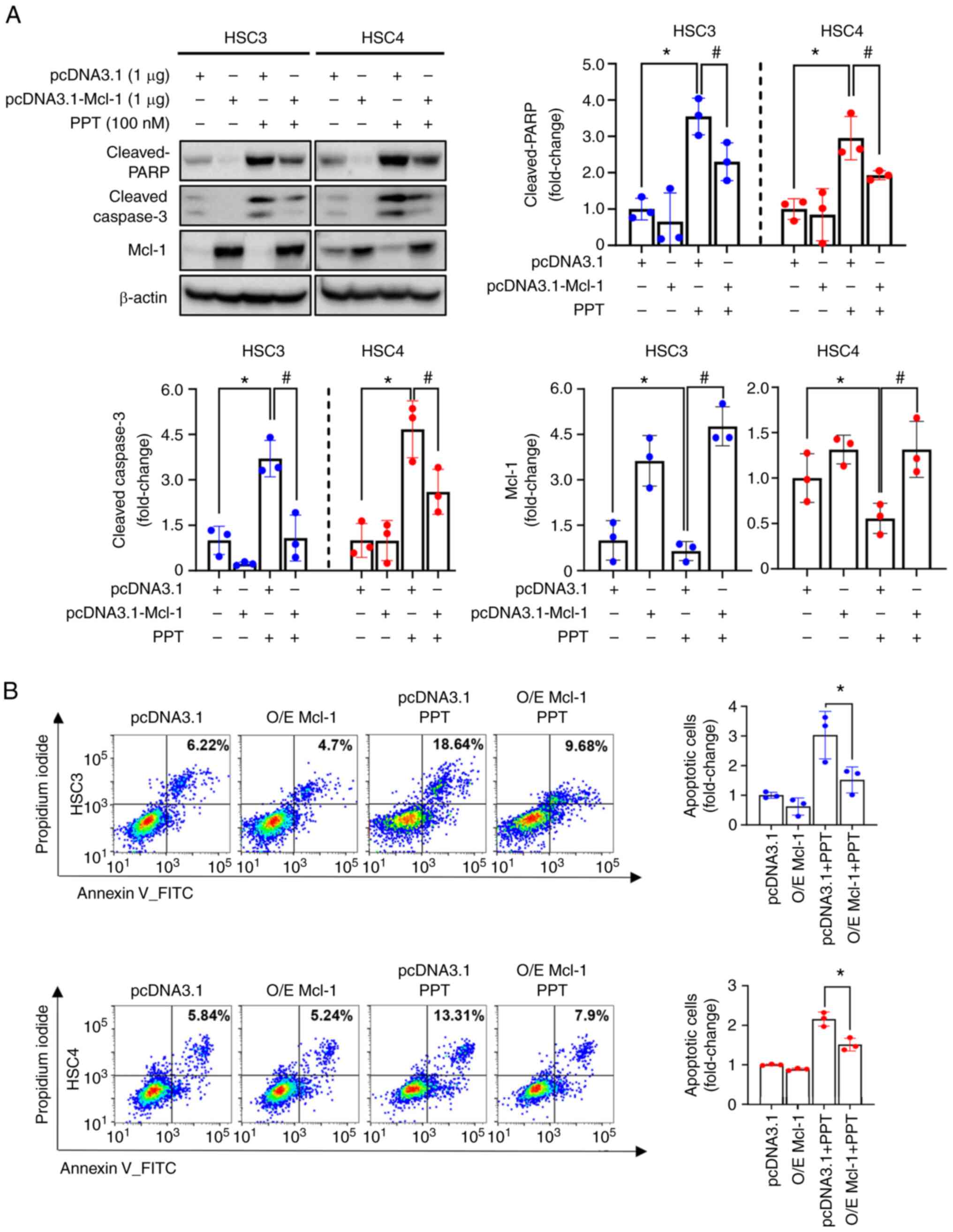
Suppression of Mcl-1 following PPT
treatment determines the susceptibility to apoptosis in human OSCC
cell lines
The role of Mcl-1 in PPT-induced apoptosis was
further investigated by overexpressing the Mcl-1 protein in human
OSCC cell lines. This allowed for the study of the effect of
increased Mcl-1 levels on the apoptotic response to PPT treatment.
Compared with the vector control (pcDNA3.1), Mcl-1 overexpression
(pcDNA3.1-Mcl-1) reduced the levels of cleaved caspase3 and cleaved
PARP induced by PPT treatment (Fig.
5A). These data indicate that Mcl-1 acts as a determinant of
PPT-induced apoptotic cell death in human OSCC cells. The
suppressive effect of Mcl-1 in PPT-induced apoptosis was further
established using flow cytometry analysis. The percentage of
Annexin V-positive cell lines following PPT treatment was decreased
following Mcl-1 overexpression (Fig.
5B). These findings indicated that Mcl-1 suppression may be
necessary for PPT-induced apoptosis in human OSCC cell lines.
Discussion
Currently, it is very difficult to predict the
disease progression of OSCC, given the anatomical complexities and
varied pathological subtypes (3). Thus, studies are ongoing to address
this issue by developing novel methods for the management of OSCC.
Numerous anticancer strategies are focused on inducing or restoring
apoptosis, which may inhibit cancer cell growth when apoptotic
signaling pathways are reactivated (35). Indeed, growth inhibition through
apoptosis induction using pharmacological approaches are
established effective anticancer strategies in OSCC (23,36-39). In the present study, the growth
of OSCC cells through induction of apoptosis using PPT was
assessed. PPT treatment significantly reduced the growth of OSCC
and inhibited anchorage-independent colony formation as determined
using soft agar assays. Spheroid formation assays were used to
better mimic the complex 3D structures of tumors in vivo
(40). The ability of PPT on
additional dimensions of tumor behavior and drug response in OSCC
was assessed and the results showed that spheroid formation was
inhibited when treated with PPT. In addition, PPT treatment
markedly increased the levels of apoptotic indicators, such as
cleaved-PARP, cleaved-caspase3, and Annexin V double staining.
Recently, Bai et al (20)
demonstrated that the novel combination of PPT and coumarin exerted
potent anti-cancer activity in OSCC via regulation of the AKT/mTOR
pathway, supporting the results of the present study. Together,
these suggest that PPT induces apoptosis, which in turn affects
OSCC cell survival.
An important mechanism of several chemotherapeutic
drugs is the disruption of normal tubulin function (41,42). PPT, a natural tubulin inhibitor,
inhibits the growth of cancer cells by disrupting tubulin
polymerization, which causes cell cycle arrest and inhibition of
microtubule synthesis during the formation of mitotic spindles
(43). Numerous studies have
demonstrated the anticancer properties of PPT and its derivatives
against various types of cancer by targeting different molecular
targets (11,20,43,44). For example, PPT led to the
production of reactive oxygen species and r-H2AX and activated the
ATM/p53/p21 pathway to trigger DNA damage in human breast cancer
(43). PPT also induced
apoptosis in human lung cancer cells by inhibiting c-MET kinase
activity (45). However,
additional studies are still required to identify the anticancer
mechanism of PPT in OSCC. OSCC cell lines exhibit significantly
high expression of Mcl-1, which occurs through genetic
amplification (21,46). As a commonly amplified and
upregulated protein in OSCC malignant lesions, Mcl-1 promotes the
development of cancer and reduces the survival of patients with
cancer (21,47). As targeting Mcl-1 may be an
important step in inducing programmed cell death, the use of
natural compounds to attenuate Mcl-1 expression is considered a
promising strategy for developing preventative and therapeutic
regimens in OSCC (22,48). Previously, it was found that
nitidine chloride, a naturally derived compound, decreased Mcl-1
protein expression by inhibiting the STAT3 pathway (23) and several natural products
including Sanguisorba officinalis were found to reduce Mcl-1
expression via specificity protein 1 and thus induce apoptosis in
OSCC cell lines (49,50). Fisetin also suppressed oral
cancer growth by modulating the SESN2/mTOR/Mcl-1 signaling axis
(39) suggesting that targeting
Mcl-1 by PPT may be a promising treatment strategy for the
management of OSCC. In the present study, whether PPT affected
Mcl-1 protein in OSCC cell lines was assessed. PPT treatment
specifically decreased Mcl-1 expression and Mcl-1 overexpression
(pcDNA3.1-Mcl-1) restored PPT-induced apoptosis. Mcl-1 mRNA levels
were unchanged following PPT treatment, which ruled out the
possibility that PPT affected the transcriptional regulation of
Mcl-1. Numerous studies have demonstrated that decreased Mcl-1
expression, which may be caused by various compounds in cancer
cells, is dependent on proteasomal processes (23,51). Thus, it is hypothesized that PPT
induces a rapid turnover of Mcl-1 protein in OSCC cell lines. In
the present study, PPT affected Mcl-1 turnover, as evidenced based
on the effects of the protein synthesis inhibitor and MG-132. These
findings indicated that proteasome-dependent degradation, rather
than transcriptional effects, was responsible for the observed
PPT-induced Mcl-1 decrease in OSCC cell lines. Other studies have
demonstrated that Mcl-1 is a key regulator for the apoptotic
effects of anti-tubulin drugs, which cause a reduction in Mcl-1
protein at the post-translational level, to potentiate cell death
(52,53), are consistent with the findings
of the present study. Overall, these data suggested that Mcl-1
contributes to PPT-induced apoptotic cell death in OSCC cell lines
such as other tubulin inhibitors or naturally derived
compounds.
In conclusion, it was found that PPT triggers
anti-tumor growth and colony formation in OSCC cell lines by
inducing mitochondrial-dependent apoptosis. These results were
associated with decreased expression of Mcl-1 at the protein level.
Taken together, these findings suggest that PPT may serve as a
potential novel therapeutic drug candidate for targeting Mcl-1 in
OSCC.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HJY and SJC conceived the study, acquired and
analyzed the data, and drafted the manuscript. SJC and JAS analyzed
and interpreted the data. SDC designed and interpreted the study.
SJC and SDC reviewed and edited the manuscript, and participated in
contributing to the contents of the revised manuscript. SJC and SDC
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This work was supported by the National Research Foundation of
Korea through a grant funded by the Korean government (MSIT) (grant
no. 2022R1A2C1091608) and the Ministry of Education (grant no.
2019R1A2C1085896).
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hernandez BY, Zhu X, Goodman MT, Gatewood
R, Mendiola P, Quinata K and Paulino YC: Betel nut chewing, oral
premalignant lesions, and the oral microbiome. PLoS One.
12:e01721962017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Usman S, Jamal A, The MT and Waseem A:
Major molecular signaling pathways in oral cancer associated with
therapeutic resistance. Front Oral Health. 1:6031602021. View Article : Google Scholar
|
|
4
|
Hanna GJ, Patel N, Tedla SG, Baugnon KL,
Aiken A and Agrawal N: Personalizing surveillance in head and neck
cancer. Am Soc Clin Oncol Educ Book. 43:e3897182023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gormley M, Creaney G, Schache A,
Ingarfield K and Conway DI: Reviewing the epidemiology of head and
neck cancer: Definitions, trends and risk factors. Br Dent J.
233:780–786. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu H, Lv M and Tian X: A review on
hemisynthesis, biosynthesis, biological activities, mode of action,
and structure-activity relationship of podophyllotoxins: 2003-2007.
Curr Med Chem. 16:327–349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
King ML and Sullivan MM: The similarity of
the effect of podophyllin and colchicine and their use in the
treatment of condylomata acuminata. Science. 104:244–245. 1946.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Desbène S and Giorgi-Renault S: Drugs that
inhibit tubulin polymerization: The particular case of
podophyllotoxin and analogues. Curr Med Chem Anticancer Agents.
2:71–90. 2002. View Article : Google Scholar
|
|
9
|
Chen SW, Wang YH, Jin Y, Tian X, Zheng YT,
Luo DQ and Tu YQ: Synthesis and anti-HIV-1 activities of novel
podophyllotoxin derivatives. Bioorg Med Chem Lett. 17:2091–2095.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu H and Xiao X: Natural products-based
insecticidal agents 4. Semisynthesis and insecticidal activity of
novel esters of 2-chloropodophyllotoxin against Mythimna separata
Walker in vivo. Bioorg Med Chem Lett. 19:5415–5418. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao W, Cong Y, Li HM, Li S, Shen Y, Qi Q,
Zhang Y, Li YZ and Tang YJ: Challenges and potential for improving
the druggability of podophyllotoxin-derived drugs in cancer
chemotherapy. Nat Prod Rep. 38:470–488. 2021. View Article : Google Scholar
|
|
12
|
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N,
Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH,
et al: Durvalumab plus platinum-etoposide versus platinum-etoposide
in first-line treatment of extensive-stage small-cell lung cancer
(CASPIAN): A randomised, controlled, open-label, phase 3 trial.
Lancet. 394:1929–1939. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Conlon KC, Sportes C, Brechbiel MW, Fowler
DH, Gress R, Miljkovic MD, Chen CC, Whatley MA, Bryant BR, Corcoran
EM, et al: 90Y-daclizumab (anti-CD25), high-dose
carmustine, etoposide, cytarabine, and melphalan chemotherapy and
autologous hematopoietic stem cell transplant yielded sustained
complete remissions in 4 patients with recurrent Hodgkin's
lymphoma. Cancer Biother Radiopharm. 35:249–261. 2020.PubMed/NCBI
|
|
14
|
Tsukamoto Y, Kiyasu J, Choi I, Kozuru M,
Uike N, Utsunomiya H, Hirata A, Fujioka E, Ohno H, Nakashima E, et
al: Efficacy and safety of the modified EPOCH regimen (etoposide,
vincristine, doxorubicin, carboplatin, and prednisolone) for adult
T-cell leukemia/lymphoma: A multicenter retrospective study. Clin
Lymphoma Myeloma Leuk. 20:e445–e453. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang J, Bi N, Wu S, Chen M, Lv C, Zhao L,
Shi A, Jiang W, Xu Y, Zhou Z, et al: Etoposide and cisplatin versus
paclitaxel and carboplatin with concurrent thoracic radiotherapy in
unresectable stage III non-small cell lung cancer: A multicenter
randomized phase III trial. Ann Oncol. 28:777–783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manapov F and Eze C: Survival advantage
for etoposide/cisplatin over paclitaxel/carboplatin concurrent
chemoradiation in patients with inoperable stage III NSCLC: A
subgroup analysis for ECOG 2 patients would be of great interest.
Ann Oncol. 28:2319–2320. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bugg BY, Danks MK, Beck WT and Suttle DP:
Expression of a mutant DNA topoisomerase II in CCRF-CEM human
leukemic cells selected for resistance to teniposide. Proc Natl
Acad Sci USA. 88:7654–7658. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamal A, Nayak VL, Bagul C, Vishnuvardhan
MV and Mallareddy A: Investigation of the mechanism and apoptotic
pathway induced by 4β cinnamido linked podophyllotoxins against
human lung cancer cells A549. Apoptosis. 20:1518–1529. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Sun H, Xiao Z, Zhang D, Bao X and
Wei N: XWL-1-48 exerts antitumor activity via targeting
topoisomerase II and enhancing degradation of Mdm2 in human
hepatocellular carcinoma. Sci Rep. 7:99892017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai G and Zhao D, Ran X, Zhang L and Zhao
D: Novel hybrids of podophyllotoxin and coumarin inhibit the growth
and migration of human oral squamous carcinoma cells. Front Chem.
8:6260752021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi SJ, Swarup N, Shin JA, Hong SD and
Cho SD: Myeloid cell leukemia-1 expression in cancers of the oral
cavity: A scoping review. Cancer Cell Int. 22:1822022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shin JA, Jung JY, Ryu MH, Safe S and Cho
SD: Mithramycin A inhibits myeloid cell leukemia-1 to induce
apoptosis in oral squamous cell carcinomas and tumor xenograft
through activation of Bax and oligomerization. Mol Pharmacol.
83:33–41. 2013. View Article : Google Scholar
|
|
23
|
Yang IH, Jung W, Kim LH, Shin JA, Cho NP,
Hong SD, Hong KO and Cho SD: Nitidine chloride represses Mcl-1
protein via lysosomal degradation in oral squamous cell carcinoma.
J Oral Pathol Med. 47:823–829. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung M, Han DJ, Ahn CH, Hong KO, Choi YS,
Kim JS, Yoon HJ, Hong SD, Shin JA and Cho SD: In vitro induction of
mitotic catastrophe as a therapeutic approach for oral cancer using
the ethanolic extract of Juniperus squamata. Oncol Rep. 45:1032021.
View Article : Google Scholar
|
|
25
|
Wei AH, Roberts AW, Spencer A, Rosenberg
AS, Siegel D, Walter RB, Caenepeel S, Hughes P, McIver Z, Mezzi K,
et al: Targeting MCL-1 in hematologic malignancies: Rationale and
progress. Blood Rev. 44:1006722020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Campbell KJ, Dhayade S, Ferrari N, Sims
AH, Johnson E, Mason SM, Dickson A, Ryan KM, Kalna G, Edwards J, et
al: MCL-1 is a prognostic indicator and drug target in breast
cancer. Cell Death Dis. 9:192018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wein L and Loi S: Mechanisms of resistance
of chemotherapy in early-stage triple negative breast cancer
(TNBC). Breast. 34(Suppl 1): S27–S30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakano T, Go T, Nakashima N, Liu D and
Yokomise H: Overexpression of antiapoptotic MCL-1 predicts worse
overall survival of patients with non-small cell lung cancer.
Anticancer Res. 40:1007–1014. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee WS, Park YL, Kim N, Oh HH, Son DJ, Kim
MY, Oak CY, Chung CY, Park HC, Kim JS, et al: Myeloid cell
leukemia-1 is associated with tumor progression by inhibiting
apoptosis and enhancing angiogenesis in colorectal cancer. Am J
Cancer Res. 5:101–113. 2014.
|
|
30
|
Perciavalle RM and Opferman JT: Delving
deeper: MCL-1's contributions to normal and cancer biology. Trends
Cell Biol. 23:22–29. 2013. View Article : Google Scholar
|
|
31
|
Levine B, Sinha S and Kroemer G: Bcl-2
family members: Dual regulators of apoptosis and autophagy.
Autophagy. 4:600–606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Willis SN, Chen L, Dewson G, Wei A, Naik
E, Fletcher JI, Adams JM and Huang DC: Proapoptotic Bak is
sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by
BH3-only proteins. Genes Dev. 19:1294–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
34
|
Wang C and Youle RJ: The role of
mitochondria in apoptosis*. Annu Rev Genet. 43:95–118. 2009.
View Article : Google Scholar
|
|
35
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hsu S, Singh B and Schuster G: Induction
of apoptosis in oral cancer cells: Agents and mechanisms for
potential therapy and prevention. Oral Oncol. 40:461–473. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang IH, Hong SH, Jung M, Ahn CH, Yoon HJ,
Hong SD, Cho SD and Shin JA: Cryptotanshinone chemosensitivity
potentiation by TW-37 in human oral cancer cell lines by targeting
STAT3-Mcl-1 signaling. Cancer Cell Int. 20:4052020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi SJ, Ahn CH, Yang IH, Jin B, Lee WW,
Kim JH, Ahn MH, Swarup N, Hong KO, Shin JA, et al: Pseudolaric acid
B induces growth inhibition and caspase-dependent apoptosis on head
and neck cancer cell lines through death receptor 5. Molecules.
24:37152019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Won DH, Chung SH, Shin JA, Hong KO, Yang
IH, Yun JW and Cho SD: Induction of sestrin 2 is associated with
fisetinmediated apoptosis in human head and neck cancer cell lines.
J Clin Biochem Nutr. 64:97–105. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kapałczyńska M, Kolenda T, Przybyła W,
Zajączkowska M, Teresiak A, Filas V, Ibbs M, Bliźniak R, Łuczewski
Ł and Lamperska K: 2D and 3D cell cultures-a comparison of
different types of cancer cell cultures. Arch Med Sci. 14:910–919.
2018.
|
|
41
|
Pang YQ, Lin H, Ou C, Cao Y, An B, Yan J
and Li X: Design, synthesis, and biological evaluation of novel
benzodiazepine derivatives as anticancer agents through inhibition
of tubulin polymerization in vitro and in vivo. Eur J Med Chem.
182:1116702019. View Article : Google Scholar
|
|
42
|
Mukhtar E, Adhami VM and Mukhtar H:
Targeting microtubules by natural agents for cancer therapy. Mol
Cancer Ther. 13:275–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang X, Rakesh KP, Shantharam CS,
Manukumar HM, Asiri AM, Marwani HM and Qin HL: Podophyllotoxin
derivatives as an excellent anticancer aspirant for future
chemotherapy: A key current imminent needs. Bioorg Med Chem.
26:340–355. 2018. View Article : Google Scholar
|
|
44
|
Xiao J, Gao M, Sun Z, Diao Q, Wang P and
Gao F: Recent advances of podophyllotoxin/epipodophyllotoxin
hybrids in anticancer activity, mode of action, and
structure-activity relationship: An update (2010-2020). Eur J Med
Chem. 208:1128302020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen JY, Tang YA, Li WS, Chiou YC, Shieh
JM and Wang YC: A synthetic podophyllotoxin derivative exerts
anti-cancer effects by inducing mitotic arrest and pro-apoptotic ER
stress in lung cancer preclinical models. PLoS One. 8:e620822013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ribeiro IP, Marques F, Barroso L,
Rodrigues J, Caramelo F, Melo JB and Carreira IM: Genomic profile
of oral squamous cell carcinomas with an adjacent leukoplakia or
with an erythroleukoplakia that evolved after the treatment of
primary tumor: A report of two cases. Mol Med Rep. 16:6780–6786.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shin JA, Seo JM, Oh S, Cho SD and Lee KE:
Myeloid cell leukemia-1 is a molecular indicator for malignant
transformation of oral lichen planus. Oncol Lett. 11:1603–1607.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nagata M, Wada K, Nakajima A, Nakajima N,
Kusayama M, Masuda T, Iida S, Okura M, Kogo M and Kamisaki Y: Role
of myeloid cell leukemia-1 in cell growth of squamous cell
carcinoma. J Pharmacol Sci. 110:344–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shin JA, Kim JS, Kwon KH, Nam JS, Jung JY,
Cho NP and Cho SD: Apoptotic effect of hot water extract of
Sanguisorba officinalis L. in human oral cancer cells. Oncol Lett.
4:489–494. 2012. View Article : Google Scholar
|
|
50
|
Shin JA, Kim JJ, Choi ES, Shim JH, Ryu MH,
Kwon KH, Park HM, Seo JY, Lee SY, Lim DW, et al: In vitro apoptotic
effects of methanol extracts of Dianthus chinensis and Acalypha
australis L. targeting specificity protein 1 in human oral cancer
cells. Head Neck. 35:992–998. 2013. View Article : Google Scholar
|
|
51
|
Choi SJ, Ahn CH, Hong KO, Kim JH, Hong SD,
Shin JA and Cho SD: Molecular mechanism underlying the apoptotic
modulation by ethanol extract of Pseudolarix kaempferi in
mucoepidermoid carcinoma of the salivary glands. Cancer Cell Int.
21:4272021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wertz IE, Kusam S, Lam C, Okamoto T,
Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, et al:
Sensitivity to antitubulin chemotherapeutics is regulated by MCL1
and FBW7. Nature. 471:110–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang W, Wang YQ, Meng T, Yi JM, Huan XJ,
Ma LP, Tong LJ, Chen Y, Ding J, Shen JK and Miao ZH: MCL-1
degradation mediated by JNK activation via MEKK1/TAK1-MKK4
contributes to anticancer activity of new tubulin inhibitor MT189.
Mol Cancer Ther. 13:1480–1491. 2014. View Article : Google Scholar : PubMed/NCBI
|