Introduction
Sepsis is a life-threatening organ dysfunction,
causing 11 million deaths yearly (1,2).
Sepsis-induced acute lung injury (ALI) is a common complication
causing 74,500 deaths/year in Europe and the United States
(3-5). Due to rapid onset of widespread
inflammation and acute epithelial cell injury in the lungs, severe
respiratory syndrome and symptoms occur, including shortness of
breath, rapid breathing and bluish skin coloration (6). Though some conventional treatments
including mechanical ventilation and fluid management based on the
underlying diseases and clinical care relieve symptoms, high
morbidity and mortality of ALI are a significant challenge
requiring novel therapeutic options.
Mesenchymal stem cells (MSCs) could modify
pathophysiology, regulate immunization and support tissue
regeneration (7,8). MSCs also have been researched in
inflammatory disease for their multifunctionality (9,10).
Specifically, studies have highlighted the therapeutic applications
of MSCs in Coronavirus disease 2019 (COVID-19) by inhibiting
inflammatory cytokine storm (11,12). However, the mechanism by which
MSCs play their functions is unknown. It is hypothesized that the
therapeutic effects are mediated by cell communications and the
replacement of MSCs (13,14). Therapy based on MSCs replacement
is hindered by the harsh microenvironment in the site.
Theoretically, the rationale for treatment may primarily relied on
MSCs-activated cell-to-cell communication by paracrine effects to
improve niche via delivering signals to endogenous cell (15-17). Immunomodulation in innate and
adaptive immune systems is mediated by the secretome (Sec) of MSCs
(18). The Sec, containing
cytokines, chemokines and extracellular vesicles (EVs) directly
regulates the microenvironment to achieve immunological balance
(19). Using Sec from MSCs is a
therapeutic strategy for inflammatory disease. Ectodermal (E)MSCs,
a type of MSC developed from neural crest (NC), are extensively
obtained from nasal mucosa without direct invasive injury and have
potential clinical applications. Simultaneously, potent tissue
regeneration potential makes EMSCs a candidate as seed cells
(20,21). Thus, EMSC-Sec deserves further
investigation in inflammation.
NF-κB(p50/p65) is a crucial molecular driver of the
inflammatory response (22).
Under physiological conditions, most NF-κB(p50/p65) dimers exist
with an inactive state in the cytoplasm because of binding of
inhibitors (IκB). However, pathological changes frequently trigger
pattern recognition receptor and antigen receptor-mediated
signaling cascades and activate the IκB kinase (IKK) complex.
Phosphorylation of IκB results in ubiquitination and degradation of
IκBα by proteases, allowing NF-κB(p50/p65) to be translocated to
the nucleus (23). NF-κB(p50/p65)
initiates gene transcription, increases expression of inflammatory
factors, including Nod-like receptor thermal protein domain
associated protein 3 (NLRP3) (24), and promotes inflammatory cytokine
cascade. This cascade caused by NLRP3-dependent pyroptosis, a type
of programmed death, triggers inflammation by extensively releasing
cell content (25,26). Hence, regulating the
NF-κB(p50/p65)/NLRP3 pathway is crucial to improve
inflammation.
Atomized drugs are a user-friendly means of
administering medication and could maintain efficacious
concentrations of drugs at different sites in the lung, allowing
the drug to penetrate readily through the lung mucosa to the blood
supply (27). Aerosolization of
immunotherapeutic agents has potential to manipulate the local
mucosal-specific microenvironment (28). Compared with intratracheal
administration, the delivery of drugs via a mucosal atomization
device could be more likely to achieve therapeutic concentrations
with pharmacokinetic effect (29). The present study used
aerosolization to ameliorate inflammation utilizing EMSC-Sec. It
was hypothesized that EMSC-Sec in the gaseous form restores the
inflammatory microenvironment. The present study aimed to explore
the anti-inflammation capabilities of EMSC-Sec in ALI and
elucidating the underlying mechanism.
Materials and methods
Extraction and identification of
EMSCs
EMSCs were collected using a tissue adhesion method
as previously described (30). A
total of five 3-week-old (30 g) male rats (Jiangsu University
Animal Center) were sacrificed by intraperitoneal sodium
pentobarbital overdose (200 mg/kg). Experiments were performed ~10
min after cessation of breathing. The nasal septum was removed
after disinfection with iodine. The nasal mucosa was cut into 1 mm
thick pieces on ice after washing with PBS. Tissue was suspended in
DMEM/F12 containing 10% fetal bovine serum (FBS, both HyClone;
Cytiva). The suspension was transferred to a plate at 37°C for
cultivation for 3 days. Cells at 80% confluence were subsequently
subcultured following trypsin digestion. EMSCs at three passages
were identified by detection of surface markers CD44, Connexin 43
(Cx43), SRY-related high-mobility group box-containing protein 9
(Sox9) and vimentin (all of antibodies purchased from Wuhan Boster
Biological Technology, Ltd.) with immunofluorescent staining. The
study protocol was approved by the Animal Care and Use Committee of
Jiangsu University (Zhenjiang, China).
Preparation and analysis of EMSC-Sec
The EMSCs (passage 3) were used. After the cell
confluence reached 80%, the complete medium containing 10% FBS was
replaced with DMEM/F12 with FBS (1%) at 37°C for 24 h. EMSCs were
cultured in complete medium at 37°C with 5% CO2 for
another 24 h. This process was repeated for at least 10 times to
accumulate EMSC-Sec. A total of 50 ml EMSC-Sec was centrifuged at
2,000 × g at 4°C for 10 min to remove non-adherent cells and
cellular debris. The supernatant was collected and concentrated by
lyophilized method (31). Then, 5
ml EMSC-Sec concentrate derived from 50 ml EMSC-Sec was evaluated
by BCA tests. Immunosuppressive agents (IL-10, TGF-β), neurotrophic
factors (Sonic hedgehog, SHH), and bioactive substances (EVs) have
emerged as pivotal factors in the regulation of the inflammatory
microenvironment (32-35). Proteins were analyzed using
western blot to determine whether EMSC-Sec possesses therapeutic
potential. ELISA test was conducted to assess the levels of IL-10
in EMSC-Sec.
Evaluation of EMSC-Sec in vitro
LPS is used to construct inflammatory cell models as
previously reported (36). MLE-12
cells (Feng Hui Biological Co., Ltd.) were cultivated in
high-glucose DMEM (HyClone, Cytiva) supplemented with 10% FBS.
MLE-12 (1×106 cells/ml) in the culture plate were
exposed to 10 μg/ml LPS (Macklin Biology Co., Ltd.) at 37°C
for 24 h to establish a cell model. To confirm whether EMSC-Sec
alleviates inflammatory injuries, cells were treated with EMSC-Sec
(0, 4, 6, 8 mg/ml) following LPS intervention at 37°C for 48 h.
Cell Counting Kit-8 (CCK-8) kit purchased from
Macklin company were adopted to evaluate viability. The LPS
challenged MLE-12 cells after EMSC-Sec treatment (0, 4, 6, 8 mg/ml)
were incubated with CCK-8 in an incubator containing 5%
CO2 at 37°C for 30 min. Then, data was detected and
analyzed at 450 nm by the microplate reader.
Animal model and experimental design
A total of 40 male C57BL/6 mice (25 g, 8 weeks) were
purchased from Henan Sikebeisi Biotechnology Co., Ltd. Following 3
days adaptive feeding with enough food and water in an appropriate
environment (temperature, 24-27°C; humidity, 40-50%; light-dark
cycle, 12-12 h), the mice were randomized into four groups:
Control, LPS, EMSC-Sec and LPS + EMSC-Sec (n=10/group). ALI model
was constructed by injecting 200 μl LPS dissolved in PBS (20
mg/kg) intraperitoneally for 24 h (37,38). Mice in the EMSC-Sec group without
LPS challenge and LPS + EMSC-Sec group received 4 mg/ml EMSC-Sec
once treatment before and after LPS injection. The first treatment
was performed using an atomizer (Omron Co., Ltd.) 6 h ago before
LPS injection. The second treatment was performed immediately
following LPS treatment using the same atomizer. Multiple mice were
placed in the atomizer for each administration. Subsequently, 20 ml
EMSC-Sec (4 mg/ml) was delivered six times at 6 h intervals between
each intervention until the mice were sacrificed in EMSC-Sec group
and LPS+EMSC-Sec group. The health of mice was monitored every 6 h
by observing the fur, behavior and hogback performance. In light of
the LPS-induced ALI causing multiple organ failure, we considered
humane endpoints, which included noticeable arching of the back and
reduced behavioral activity.
Mice in each group (n=3) were euthanized by
intraperitoneal injection of sodium pentobarbital (50 mg/kg). Then,
1 ml blood sample was immediately extracted from the left
ventricle. To enhance the assessment of the impact, the lung,
spleen, and liver from each group of mice were excised for
morphological observation. All procedures were in accordance with
the animal research institute guidance and ethical standards of
Jiangsu University (approval no. UJS-IACUC-2022051901).
Wet/dry (W/D) lung weight ratio
Lung samples were collected and weighed (wet
weight), then dried in an oven at 60°C for 24 h to measure dry
weight. Tissue edema was evaluated using the W/D ratio.
ELISA
IL-10 in EMSC-Sec was analyzed by ELISA (cat. no.
EK0417, Wuhan Boster Biological Technology, Ltd.) following the
manufacturer's instructions. The blood samples were centrifuged at
12,000 × g at 4°C for 5 min. The murine serum was prepared for the
detection of IL-10 and TNF-α. The optical density was measured at
the wavelength of 450 nm using a microplate reader.
Histopathology in pulmonary tissue
Lung tissues were collected and fixed with 4%
paraformaldehyde at 4°C overnight. Samples were embedded in
paraffin and then sectioned (thickness, 4 μm). Next, slices
were incubated with xylene solution for 20 min. Then, a sequential
cleaning was performed using anhydrous ethanol, followed by 95, 85,
75% ethanol, and Double Distilled Water (ddH2O) for a
duration of 5 min. The samples were subjected to 0.01 M citrate
buffer incubation in a microwave oven at 92 to 98°C for a duration
of 10 min to retrieve antigens. The slices were submerged in a 3%
hydrogen peroxide solution and left to incubate at room temperature
(RT) in a light-protected environment for 25 min, followed by three
rinses with PBS. The tissue was uniformly coated with a 3% BSA
solution and left to incubate at RT for 30 min. Following that, we
applied the primary antibody IL-17 (1:100, obtained from Boster,
catalog number A00421-2) to incubate the tissue slices at 4°C
overnight. Following PBS washing, the tissue was subsequently
treated with an HRP-labeled secondary antibody and incubated at RT
for 1 h. Ultimately, the color was induced using a DAB chromogenic
solution. The DAB Kit, which includes a secondary antibody and DAB
solution, was acquired from Boster (AR1027-3). The pictures were
captured using a light microscope at magnifications of ×100 and
×200.
Subsequently, the sections were stained with
hematoxylin and eosin (H&E) to determine pathological changes.
The sections were immersed in hematoxylin solution for 5 min at
room temperature (RT) to stain the cell nuclei, followed by water
washing. It is then briefly subjected to 1% hydrochloric acid
alcohol differentiation and rinsed in tap water. Next, the slides
were submerged in eosin solution for 2 min at RT to stain the
cytoplasm and other tissue components. The severity of the lung
injury was evaluated by infiltration of the inflammatory cells, the
degree of swelling and the thickness of the alveolar partition in
at least three randomly selected fields of view. Lung injury was
scored on a scale of 0 to 5 (39)
as follows: 0, no damage; 1, injury covering 1-10% of the area; 2,
injury covering 11-25% of the area; 3, injury covering 26-50% of
the area; 4, injury covering 51-75% of the area and 5, injury
covering >75% area. Qualitative hematoxylin and eosin (H&E)
staining was performed for observation under a light microscope at
a magnification of 100 and 200. Images were analyzed with GraphPad
Prism 8 (GraphPad Software, Inc.; Dotmatics).
Western blotting
Lung tissues were homogenized and lysed in RIPA
buffer containing cocktail inhibitors (Sigma-Aldrich; Merck KGaA)
and centrifuged at 4°C and 12,000 g for 10 min. The protein
concentration was detected with bicinchoninic acid kit (Sango
Biotech). A total of 10 microliters of protein samples were loaded
per lane, and electrophoresis was conducted under a constant
voltage of 80V. Polypeptides were separated by 10% SDS-PAGE. The
protein bands in gel were electrically transferred to
polyvinylidene fluoride membranes (MilliporeSigma) for 100 min
using a constant current of 350 mA. The membranes were blocked with
5% Bovine Serum Albumin (BSA, Macklin) at RT for 1 h and then
incubated with anti-SHH (1:500, Boster, A00058-1), anti-Toll-like
receptor 4 (TLR4) (1:500, Boster, A00017-3), anti-MPO (1:500,
Boster, BA0544), anti-IL-10 (1:2,000, Proteintech, 60269-1-Ig),
anti-IL-1 (1:500, Boster, A00101-1), anti-TNF-α (1:500, Boster,
cat. no. BA0131), anti-NF-κB (p50/p65; 1:500, Boster, A00284-1),
anti-IκB (1:500, Boster, PB9291), anti-phosphorylated (p-)IκB
(1:500, Boster, P01139-1), anti-NLRP3 (1:500, Boster, A00034-2) and
anti-β-actin (1:500, Boster, BA2305) antibodies at 4°C overnight.
Blots were washed three times with TBS containing 0.1% Tween-20 and
incubated with horseradish peroxidase-conjugated secondary antibody
(1:5,000, Boster, BM3894) for 1 h at room temperature. Finally,
protein expression levels were measured by enhanced
chemiluminescence kit (Millipore, Sigma). All of the bands were
analyzed by ImageJ software (National Institutes of Heath).
For cell samples, the cells were subjected to
western blot analyses after stimulation. Briefly, MLE-12 cells were
collected with a chemical lysis method (40). After loading the boiled protein,
the antibodies of IL-10 and TNF-α were applied to the detection and
eventually analyzed by ImageJ software.
Immunofluorescence
Cells or tissues were fixed in 4% paraformaldehyde.
The tissue samples were embedded in paraffin and cut into slices
with a thickness of 4 μm. After 30 min in a 60°C oven, the
slices were subjected to low-grade alcohol dewaxing and then
hydration, following the above-described method in the text. Then,
the samples were subjected to a 0.01M citrate buffer incubation in
a microwave oven at temperatures ranging from 92 to 98°C for a
duration of 10 min to retrieve antigens. Next, the cell membrane
was permeabilized by 0.1% Triton-100 at RT for 10 min. After three
washes with PBS, 5% BSA was adopted to block the protein at 37°C
for 30 min. Then, the sections were exposed to a mixture solution
comprising IL-10 (1;100, Proteintech, Inc.; 60269-1-Ig) and TNF-α
(1:100, Boster, BA0131) and were kept at 4°C overnight. Following
three PBS washes, the samples were also exposed to a mixture
solution including CY3-labeled goat-anti-rabbit (1:100, Boster,
BA1032) and CY3-labeled goat-anti-mouse (1:100, Boster, BA1031)
antibodies in a dark, humid environment at 37°C for 60 min. The
nucleus was stained by DAPI (Beijing Solarbio, C0065) at RT for 10
min.
For cell samples, procedure of immunofluorescence is
also same. Briefly, samples in 24-well plates were incubated with
primary antibodies for CD44 (1:100, Boster, A00052), Cx43 (1:100,
Boster, BA1727), Sox9 (1:100, Boster, PA1026-1), Vimentin (1:100,
Boster, PB9359) after being blocked with BSA. Following that,
CY3-labeled goat-anti-rabbit (1:100, Boster, BA1032) antibody and
DAPI was adopted. The fluorescence was determined at a
magnification of 100 and ×200 under a Nikon fluorescence microscope
(TE200-U). The data was analyzed using Image J software.
Statistical analysis
All data derived from three independent experimental
repeats are presented as the mean ± SEM. Statistical analysis was
performed with SPSS 19.0 software (SPSS Inc.). One-way ANOVA
followed by Tukey's post hoc test was adopted to determine the
statistical significance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characterization and identification of
EMSCs
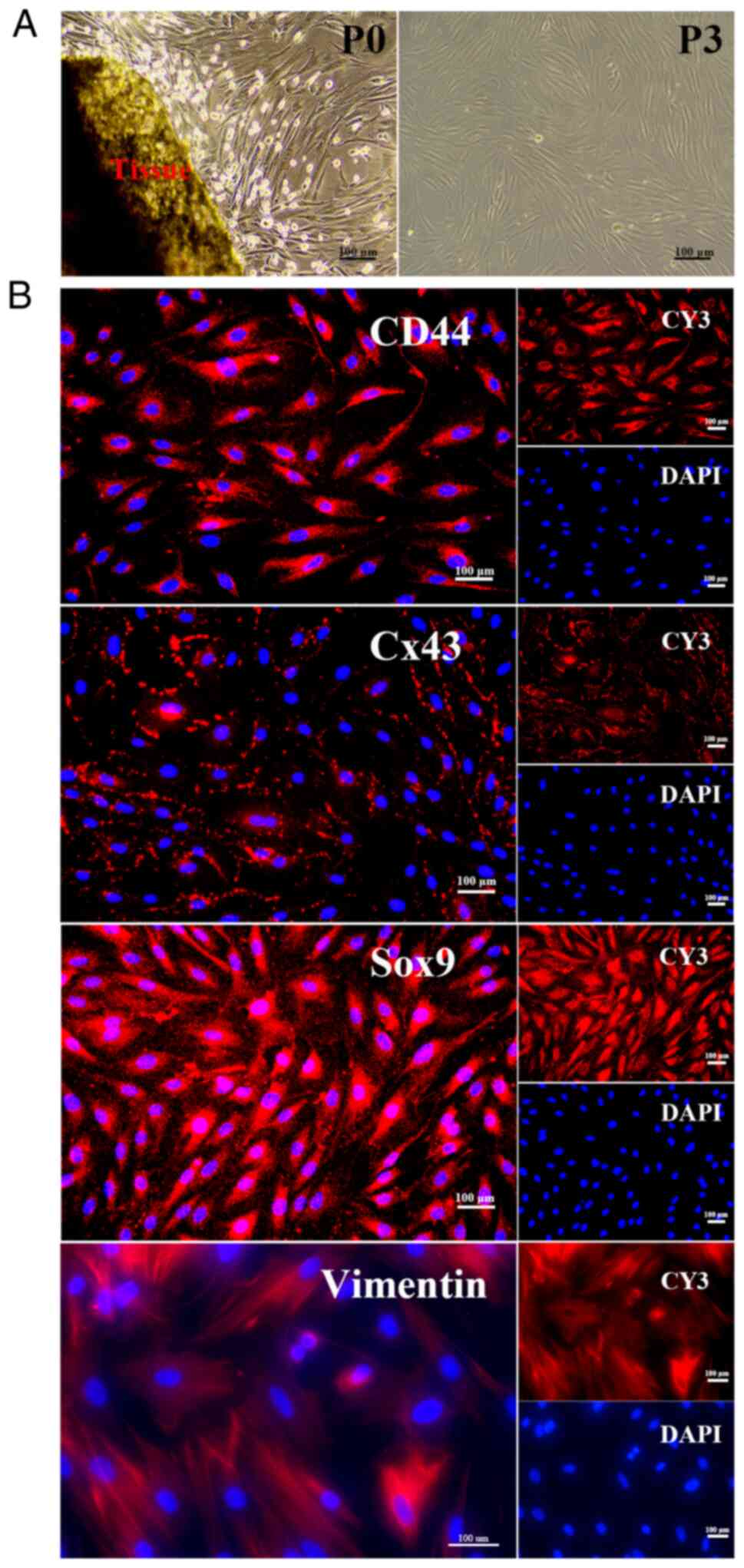
Primary EMSCs (P0) proliferated after 3 days of
culture in DMEM/F12 containing 20% FBS (Fig. 1A). Prolonged cultivation over
three generations of purified EMSCs is depicted on the right side
of Fig. 1A. The
immunofluorescence results revealed that EMSCs expressed MSC
markers (CD44, Cx43). Also, positive staining of the biomarker of
NC stem cells (Cx43) and NC-related marker (Sox9) in EMSCs revealed
that EMSCs originated from NC SCs (Fig. 1B).
Components of EMSC-Sec
To determine the potential role of EMSC-Sec in
inflammation and recovery, the present study evaluated the
expression of anti-inflammatory cytokines (IL-10, TGF-β), trophic
factor (SHH) and exosome markers (CD9, CD63) in concentrated
EMSC-Sec. The data in Fig. 2A
showed that EMSC-Sec may be therapeutic in inflammatory diseases.
In addition, BCA and ELISA were performed to quantify protein and
cytokines in EMSC-Sec. The concentration of EMSC-Sec lyophilized
powder in a 10-fold dilution was approximately 20 mg/ml, aligning
with the findings observed for EMSC-Sec at 2 mg/ml (as shown in
Fig. 2B). IL-10 holds a pivotal
position in regulating inflammation, influencing the course and
progression of inflammatory diseases (33). A total of 320 pg/ml IL-10 was
present in concentrated EMSC-Sec, indicating the potential of
EMSC-Sec to regulate inflammation.
Role of EMSC-Sec in MLE-12 following LPS
challenge
MLE-12 cells in good condition were cultured on
6-well plates. As shown in Fig.
3A, MLE-12 displayed a fibroblastic appearance and clustered
into formations resembling petals. LPS-exposed MLE-12 cells were
rescued using enriched EMSC-Sec (Fig.
3C). To verify the influence of EMSC-Sec, the present study
conducted western blotting (Fig.
3B). The LPS+EMSC-Sec group (4, 6, 8 mg/ml) expressed lower
levels of TNF-α than the LPS group, suggesting a significant
anti-inflammatory effect in the LPS+EMSC-Sec group (Fig. 3F). Likewise, elevated expression
of IL-10 showed that EMSC-Sec regulated inflammation (Fig. 3G). The ELISA outcomes depicted in
Fig. 3D and E also confirm that
EMSC-Sec elevate the expression of IL-10 and modulate the
regulation of TNF-α. Above data showed that 4 mg/ml EMSC-Sec
inducing immune equilibrium via up-expression of IL-10 (Fig. 3E and G). Additionally, EMSC-Sec
promoted proliferation (Fig. 3H).
After exposure to LPS (10 μg/ml), MLE-12 cells exhibited a
swift increase in activity before the 12-h mark. However, between
the 12 and 24th h, the secretion from EMSCs significantly promoted
the proliferation of MLE-12 cells, particularly at a concentration
of 4 μg/ml.
Aerosol inhalation of EMSC-Sec improves
ALI
The mice in the EMSC-Sec group received pretreatment
with an atomizer. After injection of LPS, the mice continued to
receive aerosol inhalation of EMSC-Sec for 24 h (Fig. 4A). All ALI mice exhibited obvious
arching of the back and less activity. Mice in the treatment group
exhibited more activity and sleek fur compared with LPS group.
Morphological observation of the lung, liver and spleen showed that
the lung was swollen and the surface of the liver was coarse and
dull under the LPS challenge (Fig.
4B). Notably, EMSC-Sec improved this phenomenon. Furthermore,
we observed a darker appearance in the spleens of both the LPS and
the EMSC-Sec group. Splenic melanosis in mice is a reflex of
melanogenesis in the skin, which is unrelated to the inflammatory
disease (41,42). W/D ratio demonstrated that
EMSC-Sec could reduce the degree of pulmonary edema significantly
(Fig. 4D). H&E staining
showed that the alveolar structure was destroyed and numerous
inflammatory cells infiltrated the LPS group (Fig. 4C). However, EMSC-Sec reversed this
phenomenon. EMSC-Sec significantly decreased lung damage score,
suggesting a positive effect of EMSC-Sec in ALI (Fig. 4E).
EMSC-Sec protects the lung via inhibiting
inflammation
Immunohistochemical staining demonstrated that the
EMSC-Sec group exhibited good morphological structure and low
expression of IL-17, indicating decreased inflammation (Fig. 5).
EMSC-Sec regulates the inflammatory
response at the protein level
ELISA showed that EMSC-Sec inhibited the
inflammatory cytokines (TNF-α) and upregulated anti-inflammatory
cytokines (IL-10; Fig. 6B-C).
Myeloperoxidase (MPO), which has proven to be a local mediator of
tissue injury, causes various inflammatory diseases (43). Dysregulation of TNF-α is a
hallmark of inflammatory disease (44). EMSC-Sec prevented the induction of
MPO and TNF-α in LPS-induced ALI, revealing the anti-inflammatory
effect (Fig. 6A). There was an
increase in IL-10 in the EMSC-Sec group. Compared with LPS group,
lung tissue in the EMSC-Sec group expressed lower levels of MPO and
TNF-α (Fig. 6D and E), which may
be due to the significant increase of IL-10 (Fig. 6G). Additionally, there was
significant upregulation of SHH following LPS stimulation, which
may be due to tissue injury. Compared with the EMSC-Sec group, the
LPS group had higher levels of SHH, indicating that the mice in the
LPS group had more lesions (Fig.
6F).
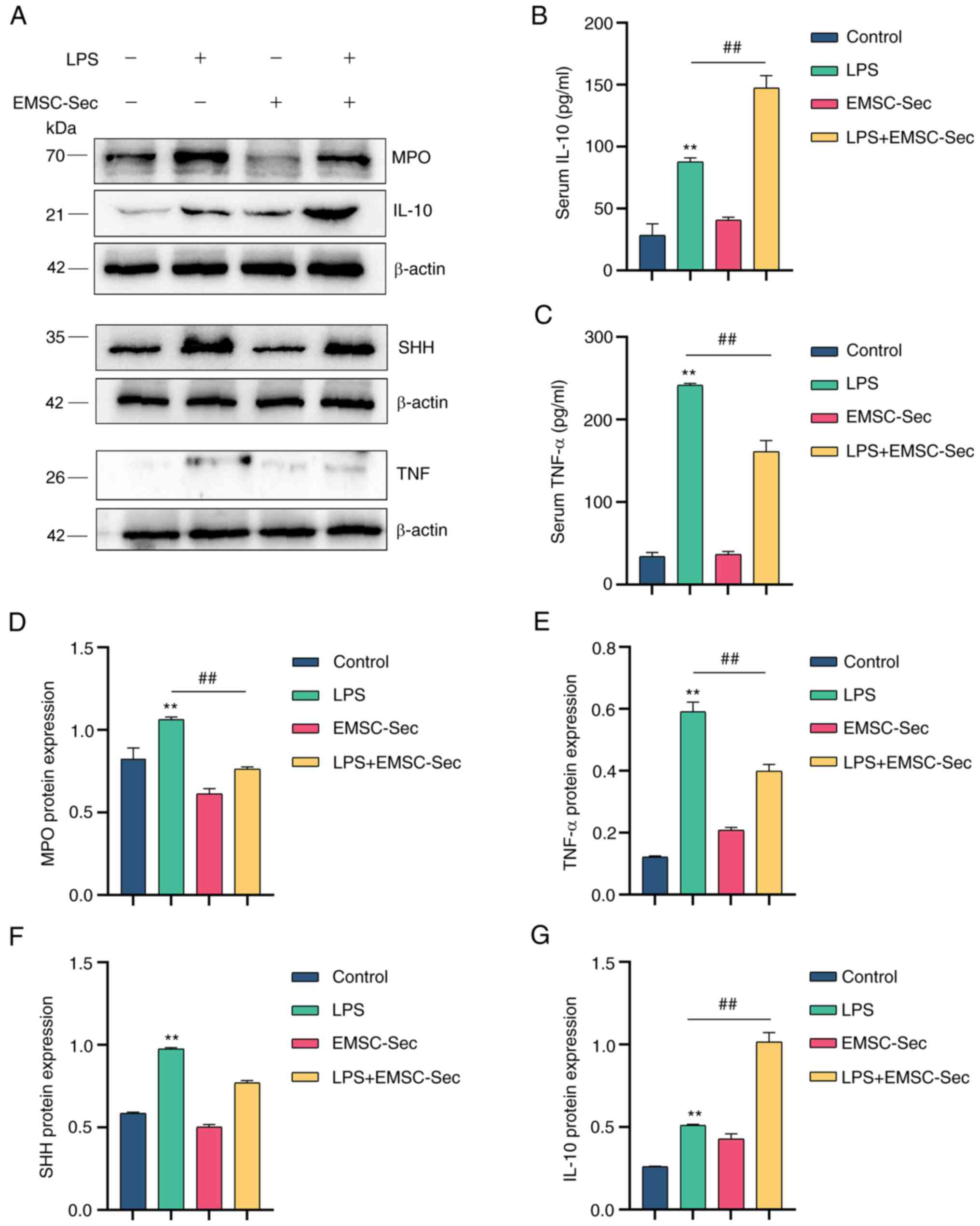 | Figure 6EMSC-Sec decreases pro-inflammatory
cytokines produced by LPS. (A) Expression of MPO, TNF-α, IL-10, and
SHH in mice using western blotting. Levels of (B) IL-10 and (C)
TNF-α in serum using ELISA (n=3 in each group). Protein levels of
(D) MPO, (E) TNF-α, (F) SHH and (G) IL-10, and SHH following LPS
treatment (n=3/group). **P<0.01 vs. control;
##P<0.01. EMSC-Sec, ectodermal mesenchymal stem
cell-Sectome; LPS, lipopolysaccharide; MPO, myeloperoxidase; SHH,
Sonic hedgehog. |
Upregulation of IL-10 suppresses
inflammation in ALI
To explore the association between IL-10 and TNF-α,
immunofluorescence was performed. LPS and EMSC-Sec groups expressed
increased IL-10 in the injury site (Fig. 7). Of note, EMSC-Sec group
expressed higher levels of IL-10 compared with the LPS group.
Moreover, the EMSC-Sec group provided better protection to lung
tissue structure and reduced TNF-α levels. IL-10 within
inflammatory lesions resulted in subdued TNF-α expression, implying
that the increased IL-10 levels induced by EMSC-Sec had an
inhibitory effect on inflammation.
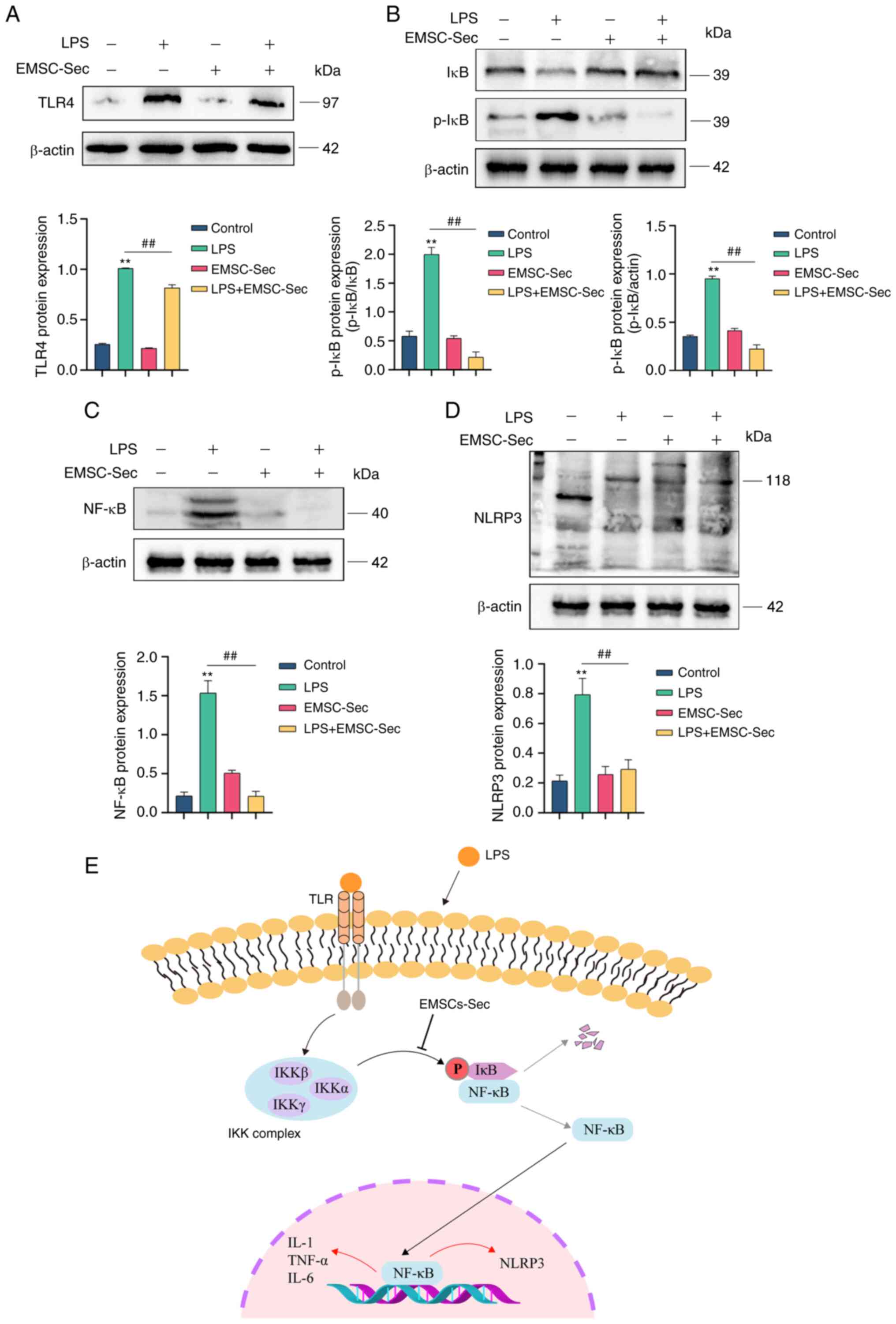
EMSC-Sec inhibits inflammation via the
NF-κB(p50/p65)/NLRP3 pathway
EMSC-Sec significantly inhibited the inflammatory
response in ALI. The anti-inflammatory effect may not be due to
changes in IL-10 alone. NF-κB(p50/p65) disorder typically results
in uncontrolled inflammation (22). LPS stimulation increased the
expression of TLR4 which is a component of Gram-negative bacteria,
to induce the production of pro-inflammatory mediators and kill
bacteria (Fig. 8A) (45). EMSC-Sec restored the balance of
NF-κB (p50/p65) and IκB following LPS treatment (Fig. 8B and C). EMSC-Sec also decreased
the expression of NF-κB (Fig. 8C)
and increased p-IκB (Fig. 8B).
Relative to the LPS group, NLRP3 was significantly inhibited by
EMSC-Sec (Fig. 8D), which
demonstrated the anti-inflammatory properties of EMSCs-Se. Fig. 8E illustrates the mechanism by
which EMSC-Sec operates. Briefly, Inhibiting IKB phosphorylation by
EMSC-Sec diminishes NF-κB (P50/P65) activation, resulting in a
lowered inflammation level.
Discussion
The present study investigated whether EMSC-Sec
possesses an anti-inflammatory influence in vitro and in
vivo. EMSC-Sec improved inhibited inflammation by increasing
IL-10 and proliferation of MLE-12 cells. Additionally, EMSC-Sec
performed biological functions to regulate LPS-induced ALI.
EMSC-Sec efficiently controlled the inflammatory conditions and
triggered an increase in IL-10 levels upon arrival at the site of
lung injury. EMSC-Sec was also found to inhibit the
NF-κB(p50/p65)/NLRP3 pathway, contributing to downregulation of
inflammatory cytokines in vivo. Collectively, the present
study demonstrated therapeutic effects of EMSC-Sec in ALI and the
underlying mechanism.
The pandemic caused by severe acute respiratory
distress syndrome coronavirus 2 (SARS-CoV-2) provided novel insight
into the host's reaction to infections and cytokine storms
(46). As a disease associated
with host immune response, severe sepsis leads to a cascade of
production of cytokines (47).
The uncontrolled production of cytokines seriously threatens human
health in sepsis (48,49). Acute respiratory distress syndrome
caused by sepsis directly results in high mortality (35-45%)
(50). Although drugs such as
antibiotics, antiviral medications, and vasodilators have been
developed, there is still a lack of effective treatment methods
(51).
Recently, mesenchymal stem cells (MSCs) have been
the subject of investigation due to their ability to modulate the
immune system and their trophic activity (19). Many researchers posit that the
bioactive molecules released by MSCs could potentially reshape the
local microenvironment where the lesion is located (52,53). Thus, MSCs-Sec may be a therapeutic
option for inflammatory diseases.
Inhaled aerosols are a promising candidate for
delivering drugs for lung disease due to rapid achievement of high
tissue concentrations after application (54). Aerosolized medication exhibits
encouraging results in lung diseases (55-57). For example, when adelmidrol is
administered via aerosol, it effectively diminishes oxidative
stress and mitigates inflammatory damage in cases of ALI (58). However, the effects of EMSC-Sec on
sepsis-induced ALI are unclear. The present study investigated the
role of EMSC-Sec in regulating inflammation in ALI. EMSC-Sec
limited inflammation and improved ALI. IL-10 serves an essential
role in combating damage caused by inflammation. Previous studies
have reported that IL-10 maintains tissue homeostasis by
restricting excessive inflammatory responses and promoting damaged
tissue regeneration (59,60). The potent immunosuppressive
capabilities of IL-10 are ascribed to its role as a target for both
the innate and adaptive immune responses during the infection
resolution phase (61,62). IL-10 may be an effective strategy
for autoimmune diseases such as rheumatoid arthritis (63), psoriasis (64), allergic asthma (65) and inflammatory bowel disease
(66). Therefore, the present
study evaluated levels of IL-10 in ALI. While EMSC-Sec may have
anti-inflammatory potential, the anti-inflammatory effects of
EMSC-Sec in ALI are unknown (67). IL-17 is a key proinflammatory
cytokine in the T helper 17 pathway (68). IL-17 levels are elevated in
various inflammatory conditions, including sepsis, pneumonia,
systemic lupus erythematosus, rheumatoid arthritis, allograft
rejection and cancer (69). Thus,
the detection of IL-17 could be meaningful. Increased IL-10
suggested the anti-inflammation potential of EMSC-Sec and may
explain the lower levels of inflammatory cytokines, especially
IL-17, in the LPS + EMSC-Sec group. Quiescence in the adult lung is
an actively maintained state and is regulated by hedgehog
signaling. Hedgehog controls epithelial quiescence and regeneration
in response to injury via a mesenchymal feedback mechanism
(70). The current investigation
identified exogenous SHH within EMSC-Sec, showcasing its
therapeutic relevance due to its capacity for regeneration. SHH is
a morphogen that regulates tissue development during embryogenesis
(71). Here, SHH was also
elevated obviously in the LPS + EMSC-Sec group. This may be
associated with active components (MicroRNA125b) and exosomes
(72,73). EMSC-Sec interfered with nuclear
localization of NF-κB(p50/p65) and ultimately inhibited the
NF-κB(p50/p65)/NLRP3 pathway. Aberrant activation of NLRP3
inflammation is associated with pathogenesis of various
inflammatory conditions. NLRP3 inflammasome can activate pyroptosis
and ultimately induce the inflammatory cytokines storm (74). Fragile signals from NLRP3 not only
directly reduce the release of the proinflammatory cytokines IL-1β
and IL-18 but also dampen Gasdermin D (GSDMD)-mediated pyroptosis,
thereby preventing widespread inflammation (75). EMSC-Sec-induced NLRP3 suppression
may also explain low levels of inflammation in the EMSC-Sec group.
The upregulation of IL-10 and NF-κB(p50/p65)/NLRP3 pathway
inhibition may explain how EMSC-Sec inhibits inflammatory cytokine
storm.
Non-invasive inhalation of EMSC-Sec aims to open up
new possibilities for clinical treatment. Here, EMSC-Sec could
alleviate LPS-induced ALI via decreasing inflammatory cytokines.
However, the mechanism needs to be confirmed due to the complex
components. Expression of p-IκB in the LPS + EMSC-Sec group was
less than that in the control group. Under physiological
conditions, the body is in equilibrium and protein expression is
stable. LPS and EMSC-Sec act as separate influencing factors
disrupting the equilibrium. LPS disrupts the inflammatory balance
of the body. This inflammatory stress response induces transient
overexpression of IκB. EMSC-Sec induces a second inflammatory
stress response because of its potent anti-inflammation effects
(76). This response may account
for low expression of IκB in the LPS + EMSC-Sec group.
Proinflammatory proptosis caused by inflammatory activation
frequently triggers cytokine storms (77,78). While EMSC-Sec achieved
anti-inflammatory effects in ALI, the relationship between EMSC-Sec
and pyroptosis remains to be studied.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JT and FL designed and conceived the study,
constructed figures and wrote the manuscript. JT, ZZ and XW
collected samples and performed experiments. YQ and YZ analyzed
data. All authors have read and approved the final manuscript. JT
and FL confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The animal experiments were approved by the animal
research institute of Jiangsu University (approval no.
UJS-IACUC-2022051901).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Technology Project of 'Ke
Jiao Xing Wei' in Suzhou City (grant no. KJXW2020066).
References
|
1
|
Zheng Q, Wang YC, Liu QX, Dong XJ, Xie ZX,
Liu XH, Gao W, Bai XJ and Li ZF: FK866 attenuates sepsis-induced
acute lung injury through c-jun-N-terminal kinase (JNK)-dependent
autophagy. Life Sci. 250:1175512020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang D, Wang H, Billiar TR, Kroemer G and
Kang R: Emerging mechanisms of immunocoagulation in sepsis and
septic shock. Trends Immunol. 42:508–522. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao B, Lu R, Chen J, Xie M, Zhao X and
Kong L: S100A9 blockade prevents lipopolysaccharide-induced lung
injury via suppressing the NLRP3 pathway. Respir Res. 22:452021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu C, Li H, Zhang P, Tian C, Luo J, Zhang
W, Bhandari S, Jin S and Hao Y: Lymphatic flow: A potential target
in sepsis-associated acute lung injury. J Inflamm Res. 13:961–968.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Yu W, Han D, Meng J, Wang H and
Cao G: L-lysine ameliorates sepsis-induced acute lung injury in a
lipopolysaccharide-induced mouse model. Biomed Pharmacother.
118:1093072019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou M, Fang H, Du M, Li C, Tang R, Liu H,
Gao Z, Ji Z, Ke B and Chen XL: The modulation of regulatory T cells
via HMGB1/PTEN/β-catenin axis in LPS induced acute lung injury.
Front Immunol. 10:16122019. View Article : Google Scholar
|
|
7
|
Weiss ARR and Dahlke MH: Immunomodulation
by mesenchymal stem cells (MSCs): Mechanisms of action of living,
apoptotic, and dead MSCs. Front Immunol. 10:11912019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loke XY, Imran SAM, Tye GJ, Wan Kamarul
Zaman WS and Nordin F: Immunomodulation and regenerative capacity
of MSCs for long-COVID. Int J Mol Sci. 22:124212021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu Y, Ni J and Chen J, Ma G, Zhao M, Zhu
S, Shi T, Zhu J, Huang Z, Zhang J and Chen J: Dual-functionalized
MSCs that express CX3CR1 and IL-25 exhibit enhanced therapeutic
effects on inflammatory bowel disease. Mol Ther. 28:1214–1228.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu W, Xiao ZX, Zeng D, Huang F, Wang J,
Liu Y, Bellanti JA, Olsen N and Zheng SG: B7-H1 promotes the
functional effect of human gingiva-derived mesenchymal stem cells
on collagen-induced arthritis murine model. Mol Ther. 28:2417–2429.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meng F, Xu R, Wang S, Xu Z, Zhang C, Li Y,
Yang T, Shi L, Fu J, Jiang T, et al: Human umbilical cord-derived
mesenchymal stem cell therapy in patients with COVID-19: A phase 1
clinical trial. Signal Transduct Target Ther. 5:1722020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu R, Yan T, Feng Y, Liu Y, Cao H, Peng
G, Yang Y, Xu Z, Liu J, Hou W, et al: Mesenchymal stem cell
treatment improves outcome of COVID-19 patients via multiple
immunomodulatory mechanisms. Cell Res. 31:1244–1262. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu S, Liu F, Zhou Y, Jin B, Sun Q and Guo
S: Immunosuppressive property of MSCs mediated by cell surface
receptors. Front Immunol. 11:10762020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu C, Wu Z and Li L: Mesenchymal stromal
cells promote liver regeneration through regulation of immune
cells. Int J Biol Sci. 16:893–903. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
L PK, Kandoi S, Misra R, S V, K R and
Verma RS: The mesenchymal stem cell secretome: A new paradigm
towards cell-free therapeutic mode in regenerative medicine.
Cytokine Growth Factor Rev. 46:1–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Phan J, Kumar P, Hao D, Gao K, Farmer D
and Wang A: Engineering mesenchymal stem cells to improve their
exosome efficacy and yield for cell-free therapy. J Extracell
Vesicles. 7:15222362018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wechsler ME, Rao VV, Borelli AN and Anseth
KS: Engineering the MSC secretome: A hydrogel focused approach. Adv
Healthc Mater. 10:e20019482021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang C, Yan J, Yao Z, Zhang C, Li X and
Mao HQ: Effects of mesenchymal stem cell-derived paracrine signals
and their delivery strategies. Adv Healthc Mater. 10:e20016892021.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song N, Scholtemeijer M and Shah K:
Mesenchymal stem cell immunomodulation: Mechanisms and therapeutic
potential. Trends Pharmacol Sci. 41:653–664. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jakob M, Hemeda H, Janeschik S, Bootz F,
Rotter N, Lang S and Brandau S: Human nasal mucosa contains
tissue-resident immunologically responsive mesenchymal stromal
cells. Stem Cells Dev. 19:635–644. 2010. View Article : Google Scholar
|
|
21
|
Hong CG, Chen ML, Duan R, Wang X, Pang ZL,
Ge LT, Lu M, Xie H and Liu ZZ: Transplantation of nasal olfactory
mucosa mesenchymal stem cells benefits Alzheimer's disease. Mol
Neurobiol. 59:7323–7336. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Afonina IS, Zhong Z, Karin M and Beyaert
R: Limiting inflammation-the negative regulation of NF-κB and the
NLRP3 inflammasome. Nat Immunol. 18:861–869. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Halova I, Rönnberg E, Draberova L,
Vliagoftis H, Nilsson GP and Draber P: Changing the
threshold-Signals and mechanisms of mast cell priming. Immunol Rev.
282:73–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo B, Huang F, Liu Y, Liang Y, Wei Z, Ke
H, Zeng Z, Huang W and He Y: NLRP3 inflammasome as a molecular
marker in diabetic cardiomyopathy. Front Physiol. 8:5192017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang Y, Xu W and Zhou R: NLRP3
inflammasome activation and cell death. Cell Mol Immunol.
18:2114–2127. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He X, Yang W, Zeng Z, Wei Y, Gao J, Zhang
B, Li L, Liu L, Wan Y, Zeng Q, et al: NLRP3-dependent pyroptosis is
required for HIV-1 gp120-induced neuropathology. Cell Mol Immunol.
17:283–299. 2020. View Article : Google Scholar :
|
|
27
|
Hickey AJ: Emerging trends in inhaled drug
delivery. Adv Drug Deliv Rev. 157:63–70. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sudduth ER, Trautmann-Rodriguez M, Gill N,
Bomb K and Fromen CA: Aerosol pulmonary immune engineering. Adv
Drug Deliv Rev. 199:1148312023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takaenoki Y, Masui K, Oda Y and Kazama T:
The pharmacokinetics of atomized lidocaine administered via the
Trachea: A randomized trial. Anesth Analg. 123:74–81. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi W, Wang Z, Bian L, Wu Y, HuiYa M, Zhou
Y, Zhang Z, Wang Q, Zhao P and Lu X: Periodic heat stress licenses
EMSC differentiation into osteoblasts via YAP signaling pathway
activation. Stem Cells Int. 2022:37154712022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peng W, Chang M, Wu Y, Zhu W, Tong L,
Zhang G, Wang Q, Liu J, Zhu X, Cheng T, et al: Lyophilized powder
of mesenchymal stem cell supernatant attenuates acute lung injury
through the IL-6-p-STAT3-p63-JAG2 pathway. Stem Cell Res Ther.
12:2162021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lainé A, Labiad O, Hernandez-Vargas H,
This S, Sanlaville A, Léon S, Dalle S, Sheppard D, Travis MA,
Paidassi H and Marie JC: Regulatory T cells promote cancer
immune-escape through integrin αvβ8-mediated TGF-β activation. Nat
Commun. 12:62282021. View Article : Google Scholar
|
|
33
|
Ouyang W, Rutz S, Crellin NK, Valdez PA
and Hymowitz SG: Regulation and functions of the IL-10 family of
cytokines in inflammation and disease. Annu Rev Immunol. 29:71–109.
2011. View Article : Google Scholar
|
|
34
|
Garg C, Khan H, Kaur A, Singh TG, Sharma
VK and Singh SK: Therapeutic implications of sonic hedgehog pathway
in metabolic disorders: Novel target for effective treatment.
Pharmacol Res. 179:1061942022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao X, Zhao Y, Sun X, Xing Y, Wang X and
Yang Q: Immunomodulation of MSCs and MSC-derived extracellular
vesicles in osteoarthritis. Front Bioeng Biotechnol. 8:5750572020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Huang J, Foley NM, Xu Y, Li YP, Pan
J, Redmond HP, Wang JH and Wang J: B7H3 ameliorates LPS-induced
acute lung injury via attenuation of neutrophil migration and
infiltration. Sci Rep. 6:312842016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suzuki K, Okada H, Takemura G, Takada C,
Tomita H, Yano H, Muraki I, Zaikokuji R, Kuroda A, Fukuda H, et al:
Recombinant thrombomodulin protects against LPS-induced acute
respiratory distress syndrome via preservation of pulmonary
endothelial glycocalyx. Br J Pharmacol. 177:4021–4033. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baumgarten G, Knuefermann P, Wrigge H,
Putensen C, Stapel H, Fink K, Meyer R, Hoeft A and Grohé C: Role of
Toll-like receptor 4 for the pathogenesis of acute lung injury in
Gram-negative sepsis. Eur J Anaesthesiol. 23:1041–1048. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shaaban AA, El-Kashef DH, Hamed MF and
El-Agamy DS: Protective effect of pristimerin against LPS-induced
acute lung injury in mice. Int Immunopharmacol. 59:31–39. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang X, Zhang P, An L, Sun N, Peng L,
Tang W, Ma D and Chen J: Miltirone induces cell death in
hepatocellular carcinoma cell through GSDME-dependent pyroptosis.
Acta Pharm Sin B. 10:1397–1413. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Plonka PM, Michalczyk D, Popik M,
Handjiski B, Slominski A and Paus R: Splenic eumelanin differs from
hair eumelanin in C57BL/6 mice. Acta Biochim Pol. 52:433–441. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Michalczyk D, Popik M, Salwinski A and
Plonka PM: Extradermal melanin transfer? Lack of macroscopic spleen
melanization in old C57BL/6 mice with de-synchronized hair cycle.
Acta Biochim Pol. 56:343–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Davies MJ: Myeloperoxidase: Mechanisms,
reactions and inhibition as a therapeutic strategy in inflammatory
diseases. Pharmacol Ther. 218:1076852021. View Article : Google Scholar
|
|
44
|
van Loo G and Bertrand MJM: Death by TNF:
A road to inflammation. Nat Rev Immunol. 23:289–303. 2023.
View Article : Google Scholar
|
|
45
|
Płóciennikowska A, Hromada-Judycka A,
Borzęcka K and Kwiatkowska K: Co-operation of TLR4 and raft
proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life
Sci. 72:557–581. 2015. View Article : Google Scholar
|
|
46
|
Fajgenbaum DC and June CH: Cytokine storm.
N Engl J Med. 383:2255–2273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chousterman BG, Swirski FK and Weber GF:
Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol.
39:517–528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kumar V: Toll-like receptors in
sepsis-associated cytokine storm and their endogenous negative
regulators as future immunomodulatory targets. Int Immunopharmacol.
89:1070872020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim JS, Lee JY, Yang JW, Lee KH,
Effenberger M, Szpirt W, Kronbichler A and Shin JI:
Immunopathogenesis and treatment of cytokine storm in COVID-19.
Theranostics. 11:316–329. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Matthay MA, Zemans RL, Zimmerman GA, Arabi
YM, Beitler JR, Mercat A, Herridge M, Randolph AG and Calfee CS:
Acute respiratory distress syndrome. Nat Rev Dis Primers. 5:182019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Huang M, Cai S and Su J: The pathogenesis
of sepsis and potential therapeutic targets. Int J Mol Sci.
20:53762019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Asgari Taei A, Khodabakhsh P, Nasoohi S,
Farahmandfar M and Dargahi L: Paracrine effects of mesenchymal stem
cells in ischemic stroke: Opportunities and challenges. Mol
Neurobiol. 59:6281–6306. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tran C and Damaser MS: Stem cells as drug
delivery methods: Application of stem cell secretome for
regeneration. Adv Drug Deliv Rev. 82-83:1–11. 2015. View Article : Google Scholar
|
|
54
|
Fröhlich E and Salar-Behzadi S: Oral
inhalation for delivery of proteins and peptides to the lungs. Eur
J Pharm Biopharm. 163:198–211. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gelfand CA, Sakurai R, Wang Y, Liu Y,
Segal R and Rehan VK: Inhaled vitamin A is more effective than
intramuscular dosing in mitigating hyperoxia-induced lung injury in
a neonatal rat model of bronchopulmonary dysplasia. Am J Physiol
Lung Cell Mol Physiol. 319:L576–L584. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lee WH, Loo CY, Traini D and Young PM:
Development and evaluation of paclitaxel and curcumin dry powder
for inhalation lung cancer treatment. Pharmaceutics. 13:92020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sakurai R, Lee C, Shen H, Waring AJ,
Walther FJ and Rehan VK: A combination of the aerosolized PPAR-γ
agonist pioglitazone and a synthetic surfactant protein B peptide
mimic prevents hyperoxia-induced neonatal lung injury in rats.
Neonatology. 113:296–304. 2018. View Article : Google Scholar
|
|
58
|
Interdonato L, D'amico R, Cordaro M,
Siracusa R, Fusco R, Peritore AF, Gugliandolo E, Crupi R, Coaccioli
S, Genovese T, et al: Aerosol-administered adelmidrol attenuates
lung inflammation in a murine model of acute lung injury.
Biomolecules. 12:13082022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ouyang W and O'Garra A: IL-10 family
cytokines IL-10 and IL-22: From basic science to clinical
translation. Immunity. 50:871–891. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Saraiva M, Vieira P and O'Garra A: Biology
and therapeutic potential of interleukin-10. J Exp Med.
217:e201904182020. View Article : Google Scholar :
|
|
61
|
Engelhardt KR and Grimbacher B: IL-10 in
humans: Lessons from the gut, IL-10/IL-10 receptor deficiencies,
and IL-10 polymorphisms. Curr Top Microbiol Immunol. 380:1–18.
2014.PubMed/NCBI
|
|
62
|
Mollazadeh H, Cicero AFG, Blesso CN, Pirro
M, Majeed M and Sahebkar A: Immune modulation by curcumin: The role
of interleukin-10. Crit Rev Food Sci Nutr. 59:89–101. 2019.
View Article : Google Scholar
|
|
63
|
Chen Z, Bozec A, Ramming A and Schett G:
Anti-inflammatory and immune-regulatory cytokines in rheumatoid
arthritis. Nat Rev Rheumatol. 15:9–17. 2019. View Article : Google Scholar
|
|
64
|
Asadullah K, Döcke WD, Sabat RV, Volk HD
and Sterry W: The treatment of psoriasis with IL-10: Rationale and
review of the first clinical trials. Expert Opin Investig Drugs.
9:95–102. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tumes DJ, Papadopoulos M, Endo Y, Onodera
A, Hirahara K and Nakayama T: Epigenetic regulation of T-helper
cell differentiation, memory, and plasticity in allergic asthma.
Immunol Rev. 278:8–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Koelink PJ, Bloemendaal FM, Li B, Westera
L, Vogels EWM, van Roest M, Gloudemans AK, van't Wout AB, Korf H,
Vermeire S, et al: Anti-TNF therapy in IBD exerts its therapeutic
effect through macrophage IL-10 signalling. Gut. 69:1053–1063.
2020. View Article : Google Scholar
|
|
67
|
Wang Z, Zhang X, Qi L, Feng W, Gu Y and
Ding Y: Olfactory mucosa tissue-derived mesenchymal stem cells
lysate ameliorates LPS-induced acute liver injury in mice. BMC Pulm
Med. 22:4142022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tan HL and Rosenthal M: IL-17 in lung
disease: Friend or foe? Thorax. 68:788–790. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ge Y, Huang M and Yao YM: Biology of
interleukin-17 and its pathophysiological significance in sepsis.
Front Immunol. 11:15582020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Peng T, Frank DB, Kadzik RS, Morley MP,
Rathi KS, Wang T, Zhou S, Cheng L, Lu MM and Morrisey EE: Hedgehog
actively maintains adult lung quiescence and regulates repair and
regeneration. Nature. 526:578–582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pepicelli CV, Lewis PM and McMahon AP:
Sonic hedgehog regulates branching morphogenesis in the mammalian
lung. Curr Biol. 8:1083–1086. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hyun J, Wang S, Kim J, Kim GJ and Jung Y:
MicroRNA125b-mediated Hedgehog signaling influences liver
regeneration by chorionic plate-derived mesenchymal stem cells. Sci
Rep. 5:141352015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Qi J, Zhou Y, Jiao Z, Wang X, Zhao Y and
Li Y, Chen H, Yang L, Zhu H and Li Y: Exosomes derived from human
bone marrow mesenchymal stem cells promote tumor growth through
hedgehog signaling pathway. Cell Physiol Biochem. 42:2242–2254.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhao N, Di B and Xu LL: The NLRP3
inflammasome and COVID-19: Activation, pathogenesis and therapeutic
strategies. Cytokine Growth Factor Rev. 61:2–15. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Coll RC, Schroder K and Pelegrín P: NLRP3
and pyroptosis blockers for treating inflammatory diseases. Trends
Pharmacol Sci. 43:653–668. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Latour A, Gu Y, Kassis N, Daubigney F,
Colin C, Gausserès B, Middendorp S, Paul JL, Hindié V, Rain JC, et
al: LPS-induced inflammation abolishes the effect of DYRK1A on IkB
stability in the brain of mice. Mol Neurobiol. 56:963–975. 2019.
View Article : Google Scholar
|
|
77
|
Wei Y, Yang L, Pandeya A, Cui J, Zhang Y
and Li Z: Pyroptosis-induced inflammation and tissue damage. J Mol
Biol. 434:1673012021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
de Vasconcelos NM and Lamkanfi M: Recent
insights on inflammasomes, gasdermin pores, and pyroptosis. Cold
Spring Harb Perspect Biol. 12:a0363922020. View Article : Google Scholar
|