Introduction
Alzheimer's disease (AD) is a progressive
neurodegenerative disease characterized by gradual cognitive loss,
which is caused by the accumulation of amyloid-β (Aβ) plaques and
hyper-phosphorylated τ tangles across the brain (1-3).
Despite advances in AD research (4-6),
the precise pathogenesis of remains obscure.
Recent studies have indicated that AD is not
confined solely to the brain but extends to the gut (7,8).
Patients with AD often exhibit symptoms of gastrointestinal
dysfunction, inflammation and immune dysregulation (9-11).
The gut/brain axis is considered to play a key role in the
pathogenesis of AD (7). As the
'second brain' of the body, the gut exhibits intricate interactions
with other organs, tissues and systems within the body (12). It communicates with the brain via
multiple neural, endocrine and immune system pathways and can
modulate behavior, mood and cognitive function by influencing
neurons and synapses in the brain (13-15). Consequently, the gut is
increasingly acknowledged as a vital topic of research for
understanding the pathogenesis of AD (7,16).
The gut barrier comprises the epithelial layer and
underlying lamina propria and serves as the first line of defense
against external threats such as pathogenic bacteria and toxins
(17). The epithelial layer is
composed of a monolayer of polarized epithelial cells held together
by tight junctions (TJs) (18).
The maintenance of gut barrier integrity is regulated by various
factors, including TJs, mucin (MUC) synthesis, antimicrobial
peptides and immune cells (19).
Recent findings suggest that disruption of the gut barrier,
commonly known as 'leaky gut', is associated with various central
nervous system diseases, including AD (20). A leaky gut allows gut bacteria and
their products to enter the bloodstream, which activates the immune
system and triggers inflammation (21); chronic inflammation is associated
with the development and progression of AD (22).
Previous studies have shown that gut tissue in
patients with AD undergoes changes similar to those observed in the
brain tissue, such as Aβ deposition and neuronal loss (23,24). However, understanding of gut
tissue alterations in patients with AD remains limited. The present
study aimed to investigate pathological changes in the gut tissue
of an AD mouse model and to explore the potential involvement of
the gut barrier in AD pathology. The results may offer novel
insight into the pathogenesis of AD and provide promising prospects
for the treatment of this condition.
Materials and methods
Animals
The double transgene (dTg) Aβ precursor protein
(APP)/presenilin 1 (PS1) mice rely on an ectopic promoter to
express Swedish (swe) mutations in the APP695cDNA and dE9 mutations
in the PS1 gene, leading to supraphysiological levels of APP and
secretion of human Aβ (25).
APPswe/PS1dE9 (APP/PS1) dTg mice and non-transgenic [C57BL/6,
wild-type (WT)] homologues were purchased from Institute of
Biomedical Sciences, Nanjing University, Nanjing, China. The mice
were housed in a room with a 12/12-h light/dark cycle, temperature
controlled at 22-24°C and humidity maintained at 50-60%. The mice
had ad libitum access to water and food until they were 6 or
12 months old, respectively. A total of 32 male APP/PS1 mice (6-
and 12-month-old; 30-38 g) and WT mice (age- and sex-matched) were
used (n=8/group; Fig. 1). All
experimental procedures were approved by the Ethics Committee of
Chongqing Medical University (approval no. 116/2021) and followed
the National Institutes of Health Guidelines for the Care and Use
of Experimental Animals (26).
Intestinal permeability analysis
As previously described (27), intestinal permeability was
assessed using fluorescein isothiocyanate (FITC)-dextran (4 kDa)
test (Sigma-Aldrich; Merck KGaA). Briefly, mice were fasted for 4 h
before receiving a 60 mg/100 g body weight oral gavage of
FITC-Dextran (28). After 4 h,
200 µl of whole blood was collected from the retro-orbital
venous plexus of mice deeply anesthetized with 5% isoflurane,
centrifuged (4°C) at 5,000 × g for 5 min and plasma was transferred
into fresh tubes. The fluorescence intensity of FITC-dextran in
separated plasma was determined using a multimode plate reader
(EnSpire Plate Reader; PerkinElmer, Inc.; excitation, 485 nm;
emission, 525 nm). FITC-dextran dilutions in PBS were used to
obtain standard curves to calculate FITC concentration. The mice
were returned to their cage after awakening from anesthesia and
raised until they were sacrificed for harvesting tissue 1 week
later.
Preparation of tissue
As described by Stacchiotti et al (29), mice were sacrificed by cervical
dislocation under 5% isoflurane anesthesia. Sterile physiological
saline solution (0.9% sodium chloride) was injected in the left
ventricle of the heart until liver blanching. Perfused organs,
including brain, ileum and proximal colon, were carefully
removed.
The brain and intestinal tissue samples were rinsed
with sterile saline for 1 min. The brain was divided into two
halves: One half was stored at -80°C for western blotting, while
the other half was post-fixed with freshly prepared 4%
paraformaldehyde (PFA) for 24 h. The ileum and proximal colon were
divided equally into three segments: The first segment was fixed
with 4% PFA or Carnoy's fixative (60% methanol, 30% chloroform and
10% acetic acid) for 24 h before paraffin embedding, the second
segment was fixed in 2.5% glutaraldehyde for 4 h for
ultrastructural investigation and the third segment was stored at
-80°C for western blotting and reverse transcription-quantitative
PCR (RT-qPCR). All procedures were performed on ice.
Hematoxylin and eosin (H&E)
staining
Serial sections of paraffin-embedded ileum samples
(4-µm thickness) were cut using a microtome. The paraffin
sections were then deparaffinized by heating at 60°C, washed with
xylene as the deparaffinization reagent, and rehydrated through a
graded series of ethanol before being stained with H&E (Beijing
Solarbio Science & Technology Co., Ltd.) at room temperature
for 10 min. The stained sections were observed under a light
microscope (Olympus Corporation) at a magnification of ×30 and
images of the intestinal mucosa, villi and small intestinal
epithelial cells were captured.
Alcian blue-periodic acid Schiff (AB-PAS)
staining
Specimens from the ileum and proximal colon were
collected and stained with AB-PAS for histochemical analysis of GCs
as previously described (30).
Firstly, 3-4 sections were prepared from each specimen. The
sections (4-µm thick) were deparaffinized in xylene at 60°C
for 10 min and dehydrated in ethanol solution for 5 min. Oxidation
of tissues was performed in 0.5% PA solution at room temperature
for 5 min, followed by rinsing in distilled water. Next, sections
were stained with Schiff reagent (Beijing Solarbio Science &
Technology Co., Ltd.) at room temperature for 10 min and rinsed in
tap water for 5 min. Subsequently, the sections were stained with
1% AB in an aqueous solution of 3% acetic acid (Ph 2.5) at room
temperature for 15 min. Finally, they were counterstained with
hematoxylin at room temperature for 2 min. All samples were
observed under a light microscope at a magnification of ×20.
Immunohistochemistry (IHC) staining
Paraffin-embedded specimens were sliced (4-µm
thickness), deparaffinized in xylene at 60°C for 10 min and
hydrated in graded alcohol for 5 min each. Antigen retrieval was
performed in boiling water with citric acid (pH 6.0) at 95°C for 30
min. Endogenous peroxidase was blocked for 10 min with 3% hydrogen
peroxide at 25°C. PBS (pH 7.4) containing 5% fetal bovine serum
(HyClone; Cytiva) was used to block the specimens in 0.3% Triton
X-100 for 30 min at 37°C. Samples were incubated overnight with
primary antibodies at 4°C and then with peroxidase-conjugated
anti-rabbit IgG (1:200; cat. no. MP-7451 Vector Laboratories, Inc.)
at 25°C for 30 min. Following incubation at room temperature for 30
sec with diaminobenzidine (Vector Laboratories, Inc.), the sections
were rinsed with distilled water and counterstained with Mayer's
hematoxylin (Vector Laboratories, Inc.) at room temperature for 2
min. The primary antibodies were mouse anti-Aβ (1: 200; cat. no.
NBP2-13075; Novus Biologicals, LLC) and rabbit anti-MUC2 (1: 50;
cat. no. #DF8390; Affinity Biosciences).
Immunopositivity was quantitatively evaluated using
a light microscope (Olympus Corporation) at a magnification of ×20.
Images were analyzed using ImageJ software (version 2.1.0; National
Institutes of Health) by researchers blinded to the health status
of the mice and were calculated as the number of positive cells and
percentage of positive area.
Immunofluorescence (IF) staining
Intestinal tissue was fixed in methanol-Carnoy and
immunostained, as aforementioned. Sections were immunostained with
a rabbit anti-MUC2 antibody (1:100; red; cat. no. #DF8390; Affinity
Biosciences) and incubated at 4°C overnight. Goat anti-rabbit IgG
conjugated to Alexa Fluor 488 (1:1,000; cat. no. #A-11094; Thermo
Fisher Scientific, Inc.) was used as the secondary antibody and
applied for 1 h at room temperature. N-acetylglucosamine residues
were stained with Alexa Fluor 633-labeled wheat germ agglutinin
(WGA; 1:50; Novus Biologicals, LLC; green) at 37 °C for 30 min.
Finally, sections were counterstained with DAPI (1:1,000;
Sigma-Aldrich; Merck KGaA) at room temperature for 10 min.
Immunopositivity was evaluated quantitatively using
a fluorescence microscope (Olympus Corporation) at ×20
magnification. Images were evaluated using ImageJ software (version
2.1.0; National Institutes of Health) by researchers who were
blinded to the health status of the mice and were measured as the
percentage of positive area.
Transmission electron microscopy
(TEM)
As previously described (31), fresh intestinal tissue was removed
and cut into 1.5 mm3 pieces, which were fixed in a 2.5%
glutaraldehyde solution at 4°C for 2 h, followed by fixation in 1%
osmium tetroxide at room temperature for 1 h, before being embedded
in epoxy resin. Thin sections (~80 nm) were transferred onto 100
mesh copper grids and then stained with uranyl acetate at room
temperature for 10 min and lead citrate for 5 min. The sections
were visualized by TEM using an HT7700 device, as previously
described (32).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was conducted for specific differentially
expressed genes (DEGs). The primer sequences (Table I) were designed using the online
Primer3 tool (primer3.ut.ee/) for the complete coding sequences of
Notch1, Hairy and enhancer of split-1 (Hes1), Mouse atonal homolog
1 (Math1), Growth factor independence 1 (Gfi1), SAM pointed
domain-containing ETS transcription factor (spdef), Kinesin family
member 4 (Kif4), and β-actin. These sequences were obtained from
the NCBI Genbank (ncbi.nlm.nih.gov) database. Primer specificity was
confirmed using the Primer-BLAST tool (blast.ncbi.nlm.nih.gov/Blast.cgi), and the primers
were synthesized by Beijing Tsingke Biotech Co., Ltd. For RT-qPCR,
total RNA was extracted from proximal colonic tissue using
TRIzol® (cat. no. #9108; Takara Biotechnology Co.,
Ltd.). RT was carried out using the PrimeScript™ RT Master Mix
according to the manufacturer's protocol (cat. no. #RR037A; Takara
Biotechnology Co., Ltd.) to synthesize cDNA. Subsequently, SYBR
Premix Ex Taq™ (cat. no. #RR820A; Takara Biotechnology Co., Ltd.)
was used to perform qPCR to assess changes in gene expression. The
qPCR thermal cycling conditions were as follows: an initial
denaturation step at 95°C for 30 sec, followed by 40 cycles of
denaturation at 95°C for 5 sec and annealing/extension at 60°C for
30 sec. Each sample was subjected to PCR amplification in
triplicate. The 2−∆∆Cq method (33) was employed to normalize the RNA
expression levels of genes against β-actin.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Forward,
(5′-3′) | Reverse
(5′-3′) | Accession no. |
|---|
| β-actin |
GGCTCCTAGCACCATGAAGA |
AAAACGCAGCTCAGTAACAGT | NM_007393.5 |
| Notch1 |
CCCGCTGTGAGATTGATGTTAAT |
ACACCTTCATAACCTGGCATACA | NM_008714.3 |
| Hes1 |
GGCAGACATTCTGGAAATGA |
TTGATCTGGGTCATGCAGTT | NM_001416728.1 |
| Math1 |
GAGTGGGCTGAGGTAAAAGAGT |
GGTCGGTGCTATCCAGGAG | NM_061763.5 |
| Gfi1 |
ATCAAATGCAGCAAGGTGTTCTC |
GTGTCCGAGTGAATGAGCAGATG | NM_010278.2 |
| Spdef |
CTGGGAGCACGTTGGATGAG |
CGGTACTGGTGTTCTGTCCA | NM_001414277.1 |
| Kif4 |
CTCGAAGCCAAATGTGCCATAAA |
TCCACTTTGACCAGCTCTTCTTT | NM_008446.3 |
Western blot analysis
Proteins were extracted using RIPA lysis buffer and
protease inhibitor (both Beijing Solarbio Science & Technology
Co., Ltd.). Protein concentration was measured with a BCA Protein
Detection kit (Beijing Solarbio Science & Technology Co.,
Ltd.). Equal quantities of protein (20 µg/lane) were
separated by 10% SDS-PAGE and transferred to PVDF membranes. The
membranes were blocked with 5% skimmed milk at room temperature for
2 h and incubated with primary antibodies (Table II) at 4°C overnight, followed by
incubation with horseradish peroxidase-conjugated secondary
antibodies (goat anti-mouse, cat. no. S0002, or anti-rabbit IgG,
cat. no. S0001, both Affinity Biosciences) for 1 h at 25°C. The
membranes were finally visualized using immobilon forte western HRP
substrate (MilliporeSigma). The intensity of the signal was
evaluated by densitometry using ImageJ (version 2.1.0; National
Institutes of Health), and the value was normalized to the loading
control (α-tubulin).
 | Table IIMonoclonal primary antibodies in
western blotting. |
Table II
Monoclonal primary antibodies in
western blotting.
| Antibody | Source | Cat. no. | Manufacturer | Dilution |
|---|
| APP | Rabbit | #29765 C | ST | 1:1,000 |
| BACE1 | Rabbit | #5606 | CST | 1:1,000 |
| MUC2 | Rabbit | #DF8390 | Affinity
Biosciences | 1:1,000 |
| ZO-1 | Rabbit | ab276131 | Abcam | 1:1,000 |
| Occludin | Rabbit | ab216327 | Abcam | 1:1,000 |
| Claudin1 | Rabbit | ab180158 | Abcam | 1:2,000 |
| LBP | Rabbit | ab254559 | Abcam | 1:1,000 |
| IL1β | Rabbit | ab254360 | Abcam | 1:1,000 |
| TNFα | Rabbit | ab183218 | Abcam | 1:1,000 |
| α-tubulin | Mouse | ab7291 | Abcam | 1:10,000 |
Statistical analysis
Data are presented as the mean ± SEM from three or
more independent experiments. GraphPad Prism 6 software (GraphPad
Software, Inc.; Dotmatics) was used for all analyses. Data were
compared using unpaired Student's t test or two-way ANOVA followed
by Bonferroni's post hoc test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Increased Aβ immunoreactivity in brain
and intestine of dTg mice
The present study first examined Aβ immunoreactivity
and expression of its substrate APP and limiting enzyme β-secretase
1 (BACE1) in the brain of dTg mice and their WT counterparts.
Senile plaques (SP) in the cortex and hippocampus were detected by
immunohistochemical staining with anti-Aβ antibodies. IHC showed
that Aβ-positive plaques were only observed in dTg mice. More
plaques were observed in the cortex and hippocampus in 12-month
than in 6-month dTg mice. No plaques were observed in the brain of
WT mice (Fig. 2A and B). Western
blotting showed that APP protein was overexpressed in the brain of
both 6 and 12-month dTg compared with WT mice. Accordingly, the
expression of BACE1 protein was significantly increased in the
brain of both groups of dTg mice (Fig. 2C-E).
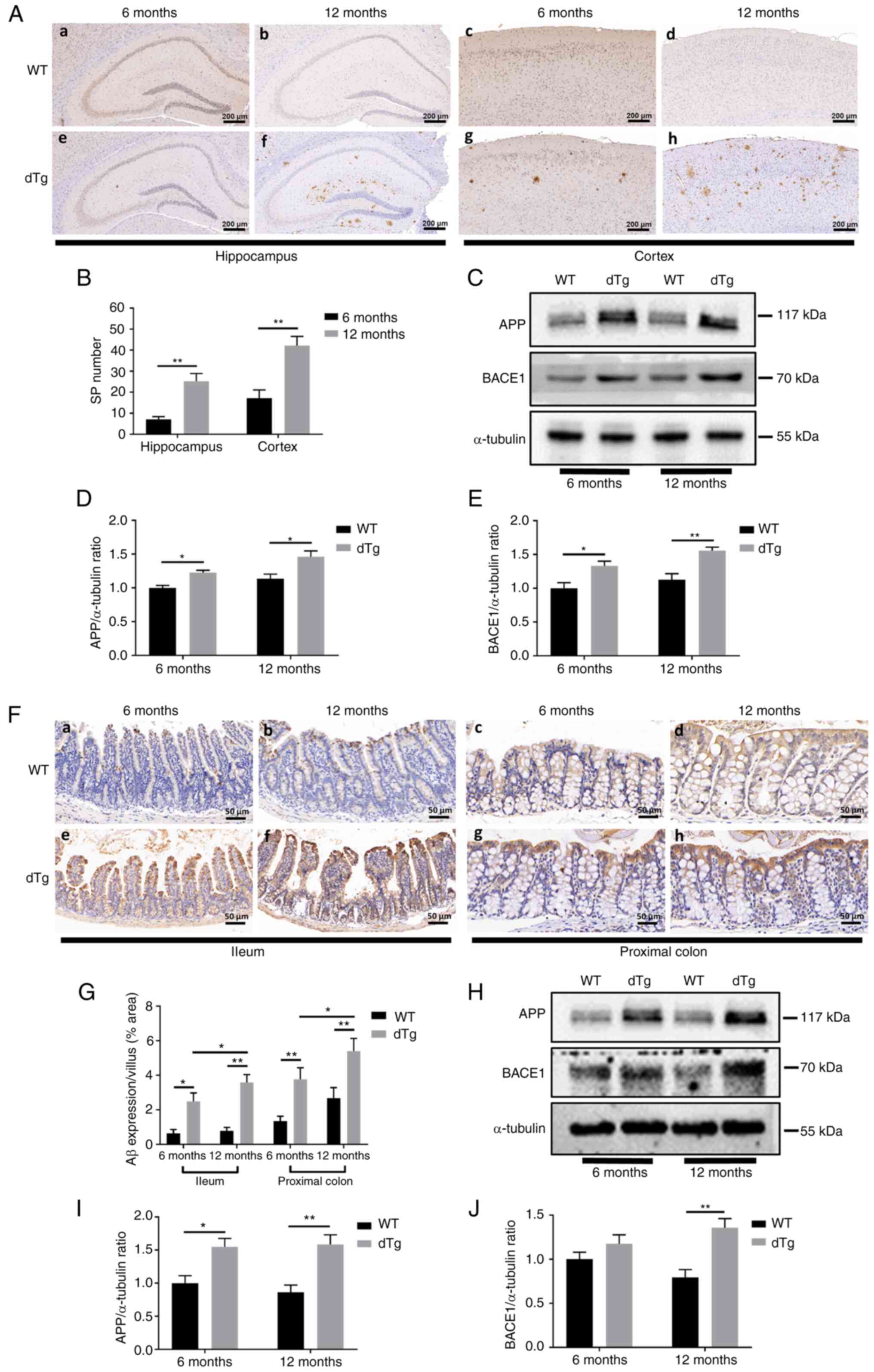 | Figure 2SP deposition in cortex and
hippocampus and Aβ aggregation in intestinal mucosa.
Immunohistochemistry showed SP in (Aa-h) cortex and hippocampus of
WT and dTg mice at 6 and 12 months, respectively. (B) The number of
SP were determined by immunohistochemistry in the cortex and
hippocampus (scale bar, 200 µm; n=6/group). (C) Protein
levels of APP and BACE1 in extracts of cortex and hippocampus
tissue were detected by western blotting. Western blot
quantification of (D) APP and (E) BACE1 (n=4/group).
Immunohistochemistry showed Aβ aggregation in (Fa-h) intestinal
mucosa of WT and dTg mice at 6 and 12 months, respectively. (G) Aβ
expression area were determined by immunohistochemistry in ileum
and proximal colon (scale bar, 50 µm; n=6/group). (H)
Protein levels of APP and BACE1 in extracts of intestinal tissue
were detected by western blotting. Western blot quantification of
(I) APP and (J) BACE1 (n=4/group). *P<0.05,
**P<0.01. SP, senile plaque; Aβ, amyloid-β; dTg,
double transgene; WT, wild-type; APP, amyloid precursor protein;
BACE1, β site APP cleaving enzyme 1. |
Aβ immunoreactivity was examined in the ileum and
proximal colon. The results showed higher Aβ levels in dTg compared
with WT mice, and Aβ-positive expression was more pronounced in the
12- than in the 6-month group, which was consistent with
observations in the cortex and hippocampus. Aβ expression was
significantly increased in dTg compared with WT mice of the same
age (Fig. 2F and G). Western
blotting revealed overexpression of APP in the 6- and 12-month dTg
groups compared with WT mice, while BACE1 expression was
significantly higher only in the 12-month dTg group (Fig. 2H-J). These results indicated
pathological changes in the gut of AD model mice, similar to those
in AD brain.
Increased intestinal permeability in dTg
mice
To investigate whether increased intestinal Aβ
exerted an effect on the intestinal barrier, an intestinal
permeability assay was performed. The variation in epithelial
permeability was measured by FITC-dextran assay. Compared with WT,
dTg mice showed a significant increase in intestinal mucosal
permeability in 6- and 12-month groups, respectively. Furthermore,
intestinal permeability of 12-month dTg mice was significantly
higher than that of 6-month dTg mice (Fig. 3).
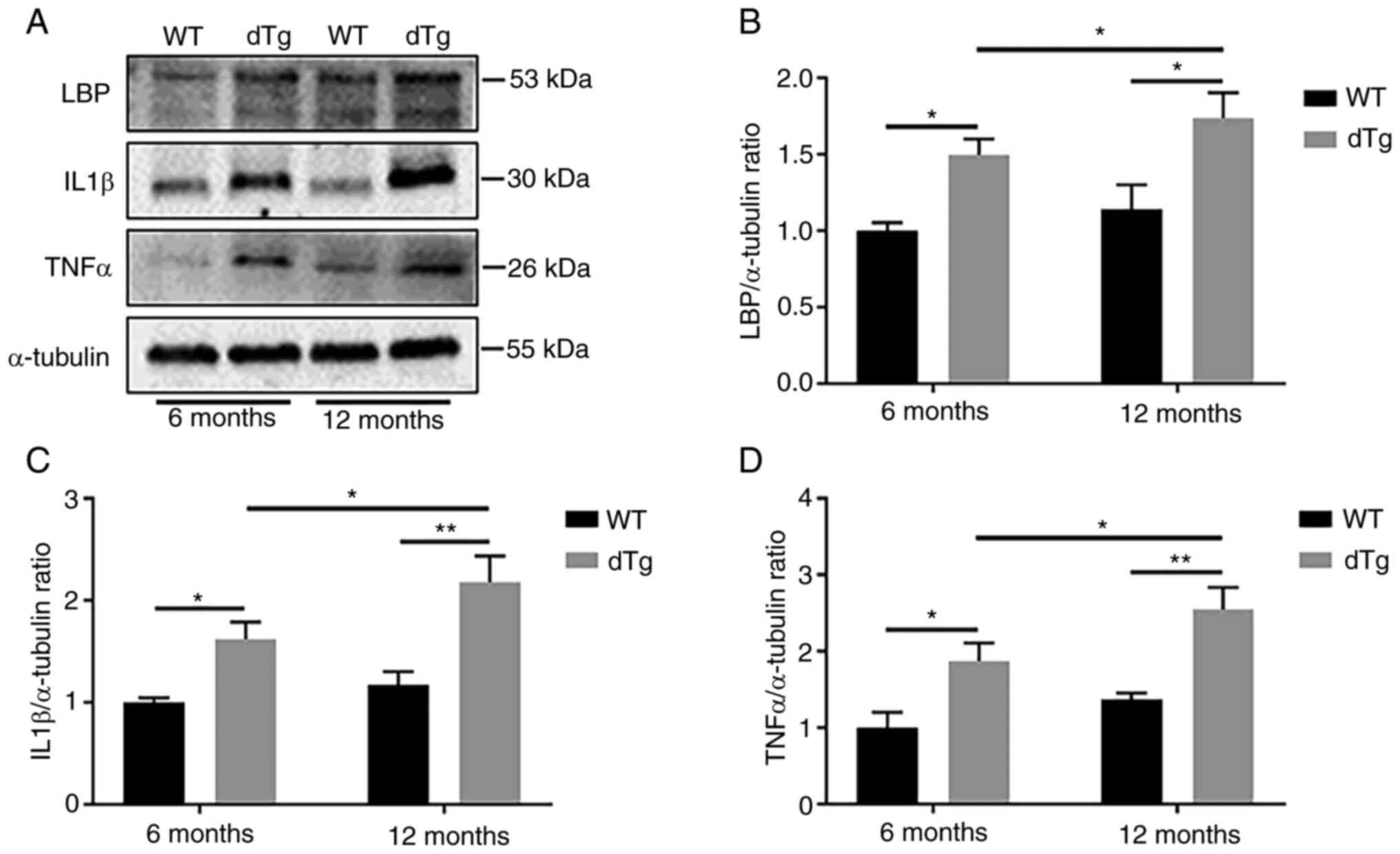
Elevated lipopolysaccharide-binding
protein (LBP) and cytokine protein expression in dTg mice
Increased intestinal permeability can lead to
susceptibility to inflammation (34). To evaluate intestinal
inflammation, the present study measured LBP, IL1β and TNFα protein
expression in intestinal tissue, which play crucial roles in
mediating inflammatory and anti-inflammatory responses (35-37). Notably, dTg mice exhibited
significantly higher LBP protein expression at 6 and 12 months
compared with their WT counterparts. Additionally, dTg mice
displayed increased production of proinflammatory cytokines (IL-1β
and TNFα) in the 6- and 12-month groups compared with WT mice.
Furthermore, expression levels of LBP, IL1β and TNFα were
significantly elevated in the 12-month dTg mice compared with the
6-month group (Fig. 4A-D).
Damaged intestinal epithelial TJ in dTg
mice
The disruption of TJs in intestinal epithelium,
which is associated with intestinal inflammation (38), was examined using TEM images to
show the ultrastructure of the ileum and proximal colon mucosa
(Fig. 5A). WT mice had normal
intestinal epithelial morphology with uniform distribution of
microvilli, tight cell junctions and normal organelle morphology
(Fig. 5Aa-d). However, intestinal
epithelial integrity was disrupted in all dTg mice, which was
evidenced by microvilli breakage or absence, TJ damage and
bacterial invasion (Fig.
5Ae-h).
The protein expression of zonula occludens-1 (ZO-1),
occludin, and claudin 1 in proximal colon was analyzed using
western blotting. The expression of these proteins was
significantly decreased in both 6- and 12-month dTg mice compared
with their WT counterparts. Additionally, expression of ZO-1,
occludin and claudin 1 was lower in the 12-month group of dTg mice
compared with the 6-month group (Fig.
5B-E).
Increased number of goblet cells (GCs)
and elevated expression and glycosylation of MUC2 in dTg mouse
intestine
Mucus, which is synthesized by GCs, is an essential
component of the intestinal barrier (39). To investigate the impact of Aβ on
GCs, AB-PAS staining was conducted to evaluate the quantity and
morphology of GCs. The results showed a significant increase in
number of GCs in the ileum and proximal colon of dTg mice at 6 and
12 months compared with WT mice. Notably, GCs in the 12-month group
of dTg mice were significantly decreased compared with the 6-month
group of dTg mice in the proximal colon (Fig. 6A and C). Similar results were
observed in the analysis of Paneth cells (PCs; Fig. S1A and B). Compared with WT mice,
dTg mice had significantly increased PCs in the ileum at 6 and 12
months. PCs in the ileum of 12-month group of dTg mice were
significantly reduced compared with 6-month group of dTg mice.
The present study assessed whether changes in GCs
affected MUC synthesis by immunostaining GCs with specific markers
such as MUC2. In both the ileum and proximal colon, the area of
MUC2-positive cells in the crypts of dTg mice were significantly
increased compared with those in WT mice at 6 and 12 months.
Notably, expression of MUC2 was significantly reduced in 12-
compared with 6-month dTg mice (Fig.
6B and D). Consistent with the IHC staining, compared with WT
mice, protein expression of MUC2 was elevated in the 6- and
12-month groups of dTg mice, while the 12-month dTg mice had
significantly lower expression than the 6-month group in the
proximal colon (Fig. 6E and
F).
To evaluate the function of mucus as a barrier, the
present study assessed MUC glycosylation using IF staining with
anti-MUC2 antibody and WGA lectin in the ileum and proximal colon.
MUC2 was glycosylated and confined to GCs in intestinal mucosa. IF
staining revealed a significant increase in MUC2 in dTg compared
with WT mice in both the ileum and proximal colon at 6 and 12
months, while 12-month dTg mice had lower expression than 6-month
dTg mice. WGA staining also showed similar results. WGA expression
in ileum and proximal colon of 6- and 12-month dTg mice was
significantly increased compared with WT mice, while WGA expression
in 12-month dTg mice was significantly lower than in 6-month dTg
mice. In addition, O-glycosylation was most abundant in GCs at the
bottom of intestinal crypts and on the mucosal surface of the
intestine (Fig. 7A-F).
Disrupted Notch signaling and GC
differentiation markers in intestine of dTg mice
GCs were increased in dTg mice. Notch signaling is
known to regulate stem cell fate determination in crypts via
Notch1. Upon activation, Notch1 promotes the expression of Hes-1,
which in turn suppresses Math1 downstream, leading to increased
enterocytes and decreased differentiation into GCs and expression
of MUC2 (40). Therefore, the
present study examined the status of Notch signaling. RT-qPCR
analysis (Fig. 8) revealed that
the mRNA levels of Notch1 and Hes-1 were significantly decreased in
proximal colon of 6- and 12-month dTg mice compared with WT mice,
while levels of downstream Math1, Gfi1, Spdef and Klf4 were
increased in 6- and 12-month dTg compared with WT mice.
Discussion
Transgenic mouse models of AD provide a useful tool
for studying pathogenesis of AD, as well as novel therapeutic
interventions. The first mouse models of AD were based on
transgenic expression of human APP and exhibited robust amyloid
pathology and memory deficits, but most of these models need at
least 10 months to develop SP or exhibit learning and memory
deficits. Singly transgenic human presenilin 1 (PS1) or PS2 mutant
mice do not develop AD pathology or cognitive deficit, although
they increase Aβ42 levels with no effect on Aβ40. The most widely
used APP/PS1 mouse model was developed through co-injecting the APP
and PS1 transgenes. APP/PS1 created by David Borchelt combines
human APP containing the swe mutation and PS1 containing e δE9
mutation (41). Amyloid plaques
begin to appear in the mouse cortex ~4 months and in the
hippocampus ~6 months, and they increase in size and number with
age. Deficits in learning and memory arise between 6 and 10 months
and worsen with age (42,43).
The intestinal barrier consists of cellular and
extracellular elements (44).
Intestinal epithelial cells constitute the primary cellular element
of the intestinal barrier and are interconnected by junctional
complexes. The barrier permits uptake of nutrients, electrolytes
and water, while constituting a defense against intraluminal
toxins, antigens and enteric flora (45,46). TJs are a fundamental junctional
complex consisting of TJ proteins, including transmembrane proteins
such as claudin-1 and occludin, and the peripheral membrane adaptor
protein ZO-1, which serves a vital role in preserving the integrity
of the intestinal barrier and regulating intestinal permeability
(47). The loss of TJ proteins
increases intestinal permeability (48). In the present study, intestinal
concentrations of ZO-1, occludin, and claudin-1 were significantly
decreased in dTg mice. Ultrastructural alterations, including
microvilli breakage and absence, TJ damage and bacterial invasion,
were demonstrated by TEM. Overall, these observations suggested
disruption of barrier integrity in dTg mice, which exhibited
greater intestinal permeability. This increase in intestinal
permeability was confirmed by FITC-dextran assay in both the ileum
and proximal colon mucosa of dTg mice.
Inflammatory-induced intestinal barrier dysfunction
is a key factor in regulating intestinal permeability. Chronic
low-grade enteric inflammation causes alterations and/or
disassembly of intercellular junctions, which contribute to severe
pathological alterations of the intestinal wall and intestinal
barrier dysfunction (49).
Significantly elevated LBP, IL-1β and TNFα in intestinal tissue of
6-month-old dTg mice suggested the presence of an inflammatory
response in the early stages of AD and the inflammatory response
increased with the progression of AD. The inflammatory response
interferes with TJs and increases intestinal permeability due to
dysregulation of TJ protein expression (50). Furthermore, the present data
showed positive immunoreactivity for Aβ in the intestinal
epithelium, suggesting immune and inflammatory responses. However,
Aβ is an inflammatory stimulator as well as a peptide at the center
of AD that triggers inflammatory alterations and releases strong
neurotoxic products such as oxygen-free radicals, chemokines,
cytokines and other inflammatory proteins (51-53). Additionally, a significant
increase in PC count was also observed in dTg mice. PCs are
critical for regulating immune responses and resisting microbial
invasion by releasing antimicrobial peptides (54).
The extracellular environment of the intestine
comprises mucus serving as a protective barrier, enzymes involved
in digestion, immune cells and cytokines participating in immune
responses, as well as nutrients, waste products, and
microorganisms, among others. (55-57) These extracellular components
contribute to the complex and dynamic environment of the intestinal
tract. Among the extracellular components, MUC2, which is a typical
secretory gel-forming MUC produced by GCs, is highly expressed in
the colon with lower levels in the small intestine (58). Before secretion, glycosylated MUC2
is densely packed and stored in secretory GC vesicles. MUC2
contains several O-linked saccharidic chains and MUC-type O-glycans
are key factors in protecting the intestinal mucus barrier from
damage (59). Lectins linked to
fluorescein were used as carbohydrate probes to detect the primary
residuals in the oligosaccharidic chains of the GC MUC (60). FITC-WGA recognizes the residues
both of N-Acetylglucosamine and sialic acid (61). The present results showed
increased amounts of glycosylated residues in the dTg mice, which
was consistent with the upregulation of MUC2 synthesis observed in
the dTg mice. However, the amount and glycosylation pattern of MUC2
reduced in 12-compared with 6-month dTg mice, potentially
indicating mucosal barrier impairment after prolonged stimulation.
This may be due to prolonged mucosal glycosylation burden, leading
to gradual fatigue of the glycosylation system and decline in MUC2
glycosylation. Furthermore, there were differences in morphology of
MUC2 and WGA between GCs of ileum and those of proximal colon.
However, further investigations are needed. The present study
showed mucous metaplasia in the proximal colonic epithelium of dTg
mice, with an increased number of GCs filled with acid and
heterochromatic MUC. MUC and mucinous metaplasia were increased
presence in response to the rapid turnover of epithelial cells
within proximal colonic crypts. The Notch signaling pathway is
associated with GC proliferation (40). Notch1 and Hes-1 are expressed in
intestinal crypts, and further analysis of Hes-1 and Math1 [which
are Notch targets and downstream effectors (62,63)], indicated a role of Notch
signaling in regulating the development and homeostasis of
intestinal epithelia. In secretory lineages, such as GCs, Hes-1
directly binds to the Math1 transcription factor, leading to
decreased Math1 expression and downstream Gfi1, Spdef and Klf4
(64-67). The present data suggested that GCs
were upregulated in dTg mice by suppressing Notch signaling and
increasing differentiation of Intestinal Stem Cells to form
goblets, enhancing mucus and MUC formation. Here, protein levels of
MUC2 were significantly increased in proximal colonic tissue. In
light of the single effect of Notch signaling on mucus and GCs,
Notch signaling was aberrantly inhibited in dTg mice. The present
results suggested that dTg mouse mucosa may compensate for MUC2 and
GC proliferation by inhibiting the Notch signaling pathway, thereby
strengthening the mucus barrier. In addition, claudin-1 is a key
component of the TJ complex; however, research has suggested other
potential functions of claudin-1 (68). Claudin-1 modifies intestinal
epithelial homeostasis via Notch-signaling regulation. Upregulated
expression of claudin-1 induces MMP-9 and phosphorylated-ERK
signaling to activate Notch signaling, which inhibits the
differentiation of GCs, thus decreasing the number of GCs and MUC2
expression (69). However,
downregulation of claudin-1 causes an increase in GC number
(70), which remains to be
confirmed in dTg mouse intestines. Mucus secretion increased with
GC number in the present study, which may result from the
inflammatory response in the intestine. This suggested that the
increased proliferative activity of GCs may be a protective
mechanism to strengthen the intestinal barrier. However, over time,
this protective mechanism weakened. The present study further
confirmed that in AD model mice, there was an overabundance of Aβ
accumulation within the intestinal epithelium. This was accompanied
by heightened permeability, inflammation-related alterations, and a
reduction in tight junction (TJ) proteins. These changes led to
heightened reactive proliferation of goblet cells (GCs), along with
an increase in the production of mucus (Fig. 9).
 | Figure 9Schematic representation of brain and
intestinal variation between wild-type and dTg mice. Alzheimer's
disease model dTg mice exhibited aggregation of amyloid plaques in
both the brain and intestinal epithelium, increased intestinal
permeability, elevated levels of lipopolysaccharide-binding
protein, IL-1β and TNFα, decreased tight junction proteins,
bacterial infiltration into the intestinal epithelium and increased
goblet cell count and mucus synthesis. dTg, double transgene; LBP,
lipopolysaccharide-binding-protein; Math1, Mouse atonal homolog 1;
Gfi1, Growth factor independence 1; Spdef, SAM pointed
domain-containing ETS transcription factor; Kif4, Kinesin family
member 4. |
In summary, the present study demonstrated that
intestinal changes in permeability, TJ and mucin synthesis in a
mouse model of AD. The present findings may provide novel insight
into the pathogenesis of AD and potential options for the treatment
of this disease. Furthermore, the present findings warrant further
investigation to elucidate the mechanisms responsible for the
interaction between intestinal Aβ deposition and gut barrier
disruption in AD.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GH and SL conceived and designed the study. JH and
YL performed the experiments and drafted the manuscript, YZ, HJ and
JL conducted the statistical analysis. GH, SL, YL and JH confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures involving animals were performed
under institutional guidelines. The present study was approved by
The Ethics Committee of Chongqing Medical University (approval no.
116/2021).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
AD
|
Alzheimer's disease
|
|
Aβ
|
amyloid-β
|
|
WT
|
wild-type
|
|
APP
|
amyloid precursor protein
|
|
PS1
|
presenilin 1
|
|
dTg
|
double transgene
|
|
LBP
|
lipopolysaccharide binding-protein
|
|
ZO-1
|
zonula occludens-1
|
|
GC
|
goblet cell
|
|
MUC2
|
mucin 2
|
|
WGA
|
wheat germ agglutin
|
|
PC
|
Paneth cell
|
|
TJ
|
tight junction
|
Acknowledgments
Not applicable.
Funding
The present study was supported by Scientific and Technological
Research Program of Chongqing Municipal Education Commission (grant
nos. KJCXZD2020021 and KJZD-K201900403), Chongqing Medical
University Program for Youth Innovation in Future Medicine (grant
no. W0044) and General Project of Chongqing Natural Science
Foundation (grant no. cstc2021jcyj-msxmX0442).
References
|
1
|
Lane CA, Hardy J and Schott JM:
Alzheimer's disease. Eur J Neurol. 25:59–70. 2018. View Article : Google Scholar
|
|
2
|
Soria Lopez JA, Gonzalez HM and Leger GC:
Alzheimer's disease. Handb Clin Neurol. 167:231–55. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun BL, Li WW, Zhu C, Jin WS, Zeng F, Liu
YH, Bu XL, Zhu J, Yao XQ and Wang YJ: Clinical Research on
Alzheimer's Disease: Progress and Perspectives. Neurosci Bull.
34:1111–1118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Monteiro AR, Barbosa DJ, Remiao F and
Silva R: Alzheimer's disease: Insights and new prospects in disease
pathophysiology, biomarkers and disease-modifying drugs. Biochem
Pharmacol. 211:1155222023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cervellati C and Zuliani G: Frontier on
Alzheimer's Disease. Int J Mol Sci. 24:77482023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wood PL: Failure of current Alzheimer's
disease hypotheses. Aging (Albany NY). 15:5959–560. 2023.PubMed/NCBI
|
|
7
|
Jin J, Xu Z, Zhang L, Zhang C, Zhao X, Mao
Y, Zhang H, Liang X, Wu J, Yang Y and Zhang J: Gut-derived
β-amyloid: Likely a centerpiece of the gut-brain axis contributing
to Alzheimer's pathogenesis. Gut Microbes. 15:21671722023.
View Article : Google Scholar
|
|
8
|
Chen C, Zhou Y, Wang H, Alam A, Kang SS,
Ahn EH, Liu X, Jia J and Ye K: Gut inflammation triggers
C/EBPβ/δ-secretase-dependent gut-to-brain propagation of Aβ and Tau
fibrils in Alzheimer's disease. EMBO J. 40:e1063202021. View Article : Google Scholar
|
|
9
|
Wang D, Zhang X and Du H: Inflammatory
bowel disease: A potential pathogenic factor of Alzheimer's
disease. Prog Neuropsychopharmacol Biol Psychiatry. 119:1106102022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eiser AR and Fulop T: Alzheimer's Disease
Is a Multi-Organ Disorder: It may already be preventable. J
Alzheimers Dis. 91:1277–1281. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
MahmoudianDehkordi S, Arnold M, Nho K,
Ahmad S, Jia W, Xie G, Louie G, Kueider-Paisley A, Moseley MA,
Thompson JW, et al: Altered bile acid profile associates with
cognitive impairment in Alzheimer's disease-An emerging role for
gut microbiome. Alzheimers Dement. 15:76–92. 2019. View Article : Google Scholar
|
|
12
|
Dupont HL, Jiang ZD, Dupont AW and Utay
NS: The intestinal microbiome in human health and disease. Trans Am
Clin Climatol Assoc. 131:178–197. 2020.PubMed/NCBI
|
|
13
|
De la Fuente M: The Role of the
Microbiota-Gut-Brain axis in the health and illness condition: A
focus on Alzheimer's disease. J Alzheimers Dis. 81:1345–160. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kesika P, Suganthy N, Sivamaruthi BS and
Chaiyasut CL: Role of gut-brain axis, gut microbial composition,
and probiotic intervention in Alzheimer's disease. Life Sci.
264:1186272021. View Article : Google Scholar
|
|
15
|
Glinert A, Turjeman S, Elliott E and Koren
O: Microbes, metabolites and (synaptic) malleability, oh my! The
effect of the microbiome on synaptic plasticity. Biol Rev Camb
Philos Soc. 97:582–599. 2022. View Article : Google Scholar :
|
|
16
|
Nafady MH, Sayed ZS, Abdelkawy DA, Shebl
ME, Elsayed RA, Ashraf GM, Perveen A, Attia MS and Bahbah EI: The
effect of gut microbe dysbiosis on the pathogenesis of Alzheimer's
Disease (AD) and related conditions. Curr Alzheimer Res.
19:274–284. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu L, Yin M, Gao J, Yu C, Lin J, Wu A,
Zhu J, Xu C and Liu X: Intestinal barrier function in the
pathogenesis of nonalcoholic fatty liver disease. J Clin Transl
Hepatol. 11:452–458. 2023.PubMed/NCBI
|
|
18
|
Jia J, Zheng W, Zhang C, Zhang P, Guo X,
Song S and Ai C: Fucoidan from Scytosiphon lomentaria protects
against destruction of intestinal barrier, inflammation and lipid
abnormality by modulating the gut microbiota in dietary
fibers-deficient mice. Int J Biol Macromol. 224:556–567. 2023.
View Article : Google Scholar
|
|
19
|
Srugo SA, Bloise E, Nguyen TTN and Connor
KL: Impact of maternal malnutrition on gut barrier defense:
Implications for pregnancy health and fetal development. Nutrients.
11:13752019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neri I, Boschetti E, Follo MY, De Giorgio
R, Cocco LI, Manzoli L and Ratti S: Microbiota-Gut-Brain axis in
neurological disorders: From leaky barriers microanatomical changes
to biochemical processes. Mini Rev Med Chem. 23:307–319. 2023.
View Article : Google Scholar
|
|
21
|
El-Hakim Y, Bake S, Mani KK and Sohrabji
F: Impact of intestinal disorders on central and peripheral nervous
system diseases. Neurobiol Dis. 165:1056272022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oh E, Kang JH, Jo KW, Shin WS, Jeong YH,
Kang B, Rho TY, Jeon SY, Lee J, Song IS and Kim KT: Synthetic PPAR
Agonist DTMB Alleviates Alzheimer's disease pathology by inhibition
of chronic microglial inflammation in 5xFAD Mice.
Neurotherapeutics. 19:1546–1565. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Manocha GD, Floden AM, Miller NM, Smith
AJ, Nagamoto-Combs K, Saito T, Saido TC and Combs CK: Temporal
progression of Alzheimer's disease in brains and intestines of
transgenic mice. Neurobiol Aging. 81:166–176. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Puig KL, Lutz BM, Urquhart SA, Rebel AA,
Zhou X, Manocha GD, Sens M, Tuteja AK, Foster NL and Combs CK:
Overexpression of mutant amyloid-beta protein precursor and
presenilin 1 modulates enteric nervous system. J Alzheimers Dis.
44:1263–1278. 2015. View Article : Google Scholar
|
|
25
|
Niidome T, Taniuchi N, Akaike A, Kihara T
and Sugimoto H: Differential regulation of neurogenesis in two
neurogenic regions of APPswe/PS1dE9 transgenic mice. Neuroreport.
19:1361–1364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
27
|
Nakanishi T, Fukui H, Wang X, Nishiumi S,
Yokota H, Makizaki Y, Tanaka Y, Ohno H, Tomita T, Oshima T and Miwa
H: Effect of a High-Fat Diet on the Small-Intestinal environment
and mucosal integrity in the Gut-Liver axis. Cells. 10:31682021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schroeder JA, Kuether EA, Fang J, Jing W,
Weiler H, Wilcox DA, Montgomery RR and Shi Q: Thromboelastometry
assessment of hemostatic properties in various murine models with
coagulopathy and the effect of factor VIII therapeutics. J Thromb
Haemost. 19:2417–2427. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stacchiotti A, Ricci F, Rezzani R, Li
Volti G, Borsani E, Lavazza A, Bianchi R and Rodella LF: Tubular
stress proteins and nitric oxide synthase expression in rat kidney
exposed to mercuric chloride and melatonin. J Histochem Cytochem.
54:1149–1157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Phillippi DT, Daniel S, Nguyen KN,
Penaredondo BA and Lund AK: Probiotics function as immunomodulators
in the intestine in C57Bl/6 male mice exposed to inhaled diesel
exhaust particles on a High-Fat diet. Cells. 11:14452022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tizro P, Choi C and Khanlou N: Sample
preparation for transmission electron microscopy. Methods Mol Biol.
1897:417–424. 2019. View Article : Google Scholar
|
|
32
|
Yan JT, Liu XY, Liu JH, Li GW, Zheng LF,
Zhang XL, Zhang Y, Feng XY and Zhu JX: Reduced acetylcholine and
elevated muscarinic receptor 2 in duodenal mucosa contribute to the
impairment of mucus secretion in 6-hydroxydopamine-induced
Parkinson's disease rats. Cell Tissue Res. 386:249–260. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
34
|
Szymanska E, Szymanska S, Dadalski M and
Kierkus J: Biological markers of disease activity in inflammatory
bowel diseases. Prz Gastroenterol. 18:141–147. 2023.PubMed/NCBI
|
|
35
|
Gurram PC, Manandhar S, Satarker S, Mudgal
J, Arora D and Nampoothiri M: Dopaminergic signaling as a plausible
modulator of astrocytic toll-like receptor 4: A crosstalk between
neuroinflammation and cognition. CNS Neurol Disord Drug Targets.
22:539–557. 2023. View Article : Google Scholar
|
|
36
|
Dinarello CA: Interleukin-1 in the
pathogenesis and treatment of inflammatory diseases. Blood.
117:3720–3732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brenner D, Blaser H and Mak TW: Regulation
of tumour necrosis factor signalling: Live or let die. Nat Rev
Immunol. 15:362–374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grosheva I, Zheng D, Levy M, Polansky O,
Lichtenstein A, Golani O, Dori-Bachash M, Moresi C, Shapiro H, Del
Mare-Roumani S, et al: High-Throughput screen identifies host and
microbiota regulators of intestinal barrier function.
Gastroenterology. 159:1807–1823. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Melhem H, Regan-Komito D and Niess JH:
Mucins dynamics in physiological and pathological conditions. Int J
Mol Sci. 22:136422021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Okamoto R, Tsuchiya K, Nemoto Y, Akiyama
J, Nakamura T, Kanai T and Watanabe M: Requirement of Notch
activation during regeneration of the intestinal epithelia. Am J
Physiol Gastrointest Liver Physiol. 296:G23–G35. 2009. View Article : Google Scholar
|
|
41
|
Jankowsky JL, Slunt HH, Ratovitski T,
Jenkins NA, Copeland NG and Borchelt DR: Co-expression of multiple
transgenes in mouse CNS: A comparison of strategies. Biomol Eng.
17:157–165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Garcia-Alloza M, Robbins EM, Zhang-Nunes
SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai
BJ and Frosch MP: Characterization of amyloid deposition in the
APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis.
24:516–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Duyckaerts C, Potier MC and Delatour B:
Alzheimer disease models and human neuropathology: Similarities and
differences. Acta Neuropathol. 115:5–38. 2008. View Article : Google Scholar
|
|
44
|
Turner JR: Intestinal mucosal barrier
function in health and disease. Nat Rev Immunol. 9:799–809. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Choonara BF, Choonara YE, Kumar P,
Bijukumar D, du Toit LC and Pillay V: A review of advanced oral
drug delivery technologies facilitating the protection and
absorption of protein and peptide molecules. Biotechnol Adv.
32:1269–1282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Groschwitz KR and Hogan SP: Intestinal
barrier function: Molecular regulation and disease pathogenesis. J
Allergy Clin Immunol. 124:3–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Odenwald MA and Turner JR: The intestinal
epithelial barrier: A therapeutic target? Nat Rev Gastroenterol
Hepatol. 14:9–21. 2017. View Article : Google Scholar :
|
|
48
|
Camilleri M: Leaky gut: Mechanisms,
measurement and clinical implications in humans. Gut. 68:1516–1526.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pellegrini C, Ippolito C, Segnani C, Dolfi
A, Errede M, Virgintino D, Fornai M, Antonioli L, Garelli F,
Nericcio A, et al: Pathological remodelling of colonic wall
following dopaminergic nigrostriatal neurodegeneration. Neurobiol
Dis. 139:1048212020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nighot P and Ma T: Endocytosis of
intestinal tight junction proteins: In Time and Space. Inflamm
Bowel Dis. 27:283–290. 2021. View Article : Google Scholar :
|
|
51
|
Szczepanik AM, Rampe D and Ringheim GE:
Amyloid-beta peptide fragments p3 and p4 induce pro-inflammatory
cytokine and chemokine production in vitro and in vivo. J
Neurochem. 77:304–317. 2001.PubMed/NCBI
|
|
52
|
Zhou WW, Lu S, Su YJ, Xue D, Yu XL, Wang
SW, Zhang H, Xu PX, Xie XX and Liu RT: Decreasing oxidative stress
and neuroinflammation with a multifunctional peptide rescues memory
deficits in mice with Alzheimer disease. Free Radic Biol Med.
74:50–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fan Q, Liu Y, Wang X, Zhang Z, Fu Y, Liu
L, Wang P, Ma H, Ma H, Seeram NP, et al: Ginnalin a inhibits
aggregation, reverses fibrillogenesis, and alleviates cytotoxicity
of amyloid β(1-42). ACS Chem Neurosci. 11:638–647. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Conway KL, Kuballa P, Song JH, Patel KK,
Castoreno AB, Yilmaz OH, Jijon HB, Zhang M, Aldrich LN, Villablanca
EJ, et al: Atg16l1 is required for autophagy in intestinal
epithelial cells and protection of mice from Salmonella infection.
Gastroenterology. 145:1347–1357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Weiss GA, Grabinger T, Glaus Garzon J,
Hasler T, Greppi A, Lacroix C, Khanzhin N and Hennet T: Intestinal
inflammation alters mucosal carbohydrate foraging and
monosaccharide incorporation into microbial glycans. Cell
Microbiol. 23:e132692021. View Article : Google Scholar
|
|
56
|
De Gregorio V, Imparato G, Urciuolo F and
Netti PA: Micro-patterned endogenous stroma equivalent induces
polarized crypt-villus architecture of human small intestinal
epithelium. Acta Biomater. 81:43–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
McCaffrey C, Corrigan A, Moynagh P and
Murphy R: Effect of yeast cell wall supplementation on intestinal
integrity, digestive enzyme activity and immune traits of broilers.
Br Poult Sci. 62:771–782. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Escande F, Porchet N, Bernigaud A,
Petitprez D, Aubert JP and Buisine MP: The mouse secreted
gel-forming mucin gene cluster. Biochim Biophys Acta. 1676:240–250.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Luis AS and Hansson GC: Intestinal mucus
and their glycans: A habitat for thriving microbiota. Cell Host
Microbe. 31:1087–100. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Muthupalani S, Ge Z, Joy J, Feng Y, Dobey
C, Cho HY, Langenbach R, Wang TC, Hagen SJ and Fox JG: Muc5ac null
mice are predisposed to spontaneous gastric antro-pyloric
hyperplasia and adenomas coupled with attenuated H. pylori-induced
corpus mucous metaplasia. Lab Invest. 99:1887–1905. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Higuchi R, Song C, Hoshina R and Suzaki T:
Endosymbiosis-related changes in ultrastructure and chemical
composition of Chlorella variabilis (Archaeplastida, Chlorophyta)
cell wall in Paramecium bursaria (Ciliophora, Oligohymenophorea).
Eur J Protistol. 66:149–155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Papaspyropoulos A, Angelopoulou A,
Mourkioti I, Polyzou A, Pankova D, Toskas K, Lanfredini S,
Pantazaki AA, Lagopati N, Kotsinas A, et al: RASSF1A disrupts the
NOTCH signaling axis via SNURF/RNF4-mediated ubiquitination of
HES1. EMBO Rep. 23:e512872022. View Article : Google Scholar
|
|
63
|
Yagishita Y, Joshi T, Kensler TW and
Wakabayashi N: Transcriptional Regulation of Math1 by Aryl
Hydrocarbon Receptor: Effect on Math1+ Progenitor Cells in Mouse
Small Intestine. Mol Cell Biol. 43:43–63. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kim YS and Ho SB: Intestinal goblet cells
and mucins in health and disease: Recent insights and progress.
Curr Gastroenterol Rep. 12:319–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shinoda M, Shin-Ya M, Naito Y, Kishida T,
Ito R, Suzuki N, Yasuda H, Sakagami J, Imanishi J, Kataoka K, et
al: Early-stage blocking of Notch signaling inhibits the depletion
of goblet cells in dextran sodium sulfate-induced colitis in mice.
J Gastroenterol. 45:608–617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
van der Flier LG and Clevers H: Stem
cells, self-renewal, and differentiation in the intestinal
epithelium. Annu Rev Physiol. 71:241–260. 2009. View Article : Google Scholar
|
|
67
|
Wu H, Chen QY, Wang WZ, Chu S, Liu XX, Liu
YJ, Tan C, Zhu F, Deng SJ, Dong YL, et al: Compound sophorae
decoction enhances intestinal barrier function of dextran sodium
sulfate induced colitis via regulating notch signaling pathway in
mice. Biomed Pharmacother. 133:1109372021. View Article : Google Scholar
|
|
68
|
Pope JL, Ahmad R, Bhat AA, Washington MK,
Singh AB and Dhawan P: Claudin-1 overexpression in intestinal
epithelial cells enhances susceptibility to adenamatous polyposis
coli-mediated colon tumorigenesis. Mol Cancer. 13:1672014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Pope JL, Bhat AA, Sharma A, Ahmad R,
Krishnan M, Washington MK, Beauchamp RD, Singh AB and Dhawan P:
Claudin-1 regulates intestinal epithelial homeostasis through the
modulation of Notch-signalling. Gut. 63:622–634. 2014. View Article : Google Scholar
|
|
70
|
Battagin AS, Bertuzzo CS, Carvalho PO,
Ortega MM and Marson FAL: Single nucleotide variants c.-13G → C
(rs17429833) and c.108C → T (rs72466472) in the CLDN1 gene and
increased risk for familial colorectal cancer. Gene.
768:1453042021. View Article : Google Scholar
|