Introduction
Lung cancer is one of the most common malignant
tumors with the highest incidence and mortality worldwide in 2020,
of which non-small cell lung cancer (NSCLC) accounts for ~85% of
all lung cancer cases (1,2).
The main types of NSCLC are adenocarcinoma and squamous and large
cell carcinoma (3). Improved
diagnosis, the availability of novel effective chemotherapeutic
agents, advanced surgical techniques and declining smoking rates
are contributing factors to gradually improving outcomes for NSCLC
(4). However, the 5-year
survival rate of patients with NSCLC is ~26% due to chemotherapy
resistance (5). Cisplatin (DDP)-based therapy is the most widely
used regimen for advanced NSCLC (6). Therefore, the reversal of DDP
resistance should be addressed to improve the treatment efficacy of
NSCLC.
Disulfiram (DSF), a drug used for the treatment of
alcohol dependence, inhibits activity of aldehyde dehydrogenase 1
(ALDH1) (7). DSF promotes tumor
cell apoptosis and inhibits tumor growth, tumor cell invasion and
metastasis (8,9). In a clinical trial, DSF was shown
to prolong survival in patients with metastatic NSCLC when added to
a combination regimen of DDP and vinorelbine (10). An additional study indicated that
DSF reverses DDP resistance in NSCLC by inhibiting ALDH1 expression
(11). In view of the antitumor
effect of DSF in NSCLC, it is necessary to explore its antitumor
molecular mechanism of action.
DSF combined with copper (DSF/Cu) exhibits an
antitumor effect, which could inhibit proliferation of a variety of
tumor cells including melanoma, colorectal and lung cancer, glioma
and breast cancer (12-14). The antitumor effect of DSF
requires the participation of Cu2+; its metabolite,
diethyldithiocarbamate (DTC), in vivo forms chelate
complexes with Cu2+ (CuET). CuET promotes coagulation of
nuclear protein localization protein 4 and binds p97 protein, which
affects its function in tumor cells and leads to CuET degradation,
resulting in excessive accumulation of a large amount of waste
protein in the cell, leading to the death of tumor cells (15). Cuproptosis is caused by direct
binding of copper to the fatty acylated components of the
tricarboxylic acid cycle, resulting in aggregation of fatty
acylated proteins, loss of iron and sulfur cluster proteins and
changes in mitochondrial respiration levels, which eventually leads
to proteotoxic stress and cell death (16). Solute carrier family 31 member 1
(SLC31A1) and ATPase copper-transporting β (ATP7B), the key
proteins of the cell membrane that control copper metabolism and
homeostasis, trigger changes in intracellular copper concentration
and are key factors that lead to cuproptosis (16). Zhou et al (17) demonstrated that DSF significantly
upregulates expression of programmed death-ligand 1 (PD-L1) in
hepatocellular cancer cells, which may enhance immunosuppression
and immune escape of tumors. According to GEO database (ncbinlm.nih.gov/geo/), DSF significantly increased the
level of ATP7B in hepatocellular carcinoma, which inhibits
cuproptosis to a certain extent. Mi et al (18) indicated that downregulation of
ATP7B expression downregulates the hypoxia inducible factor (HIF)-1
pathway, which downregulates PD-L1 expression. PX478, an
experimental HIF-1 inhibitor, causes significant tumor regression
and delays tumor growth in mice (19,20). PX478 inhibits HIF-1α protein
levels and transactivation in a number of cancer cell lines
(21). Cui et al
(22) reported that a
combination of PX-478 and anti-PD-L1 exerts a tumor inhibition
effect compared with single treatment in lung carcinoma. Also, in
renal cancer, subtype of succinate dehydrogenase B (SDHB), a ferric
sulfur subunit protein of mitochondrial complex II that serves a
key role in cuproptosis, is expressed at low levels and PD-L1
exhibits nearly undetectable expression. Low expression of the SDHB
is one of the primary characteristics of cuproptosis, indicating
that there may be an association between cuproptosis and PD-L1
expression (23).
Therefore, the present study aimed to investigate
whether DSF upregulates the expression of ATP7B to activate the
HIF-1 signaling pathway, thereby inducing the upregulation of PD-L1
expression and enhancing the effect of immunosuppression and immune
escape of NSCLC. The present study aimed to assess whether the
combined treatment of DSF with anti-PD-L1 could enhance the
anticancer role of cuproptosis induction caused by single treatment
of DSF in inhibiting NSCLC resistance.
Materials and methods
Cell culture
The human NSCLC cell line A549 was obtained from
American Type Culture Collection (cat. no. CRM-CCL-185). The cells
were cultured in RPMI-1640 supplemented with 10% fetal bovine serum
(both Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2.
Cell treatment
A549 cells were treated with DSF (0.1, 0.2, 0.5, 1.0
and 2.0 μM) in the presence or absence 0.2 μM
CuCl2 at 37°C for 24 h. One experiment involved
treatment of A549 cells with 2 μg/ml DDP in the presence or
absence of DSF treatment at 0.1 and 1.0 μM for 48 h at 37°C.
An additional experiment involved treatment of A549 cells with 2
μg/ml DDP in the presence or absence of 1 μM DSF and
5 μM JQ-1 (PD-L1 inhibitor) for 48 h at 37°C. Moreover, A549
cells were treated in the presence or absence of 1 μM DSF
(0.2 μM Cu2+) and 5 μM JQ-1 for 48 h at
37°C. Finally, A549 cells were treated in the presence or absence
of 1 μM DSF (0.2 μM Cu2+) and 10 μM
PX478 (HIF-1A inhibitor) for 48 h at 37°C.
Cell Counting Kit-8 (CCK-8) assay
A549 cells were seeded in 96-well plates
(2×103 cells/well) and continuously cultured at 37°C for
24 h. Subsequently, cells were incubated with CCK-8 solution (10
μl; Beyotime Institute of Biotechnology) for 2 h at 37°C
with 5% CO2. The absorbance of each well was detected by
a spectrophotometer at 450 nm.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was isolated from A549 cells using
TRIzol® (Thermo Fisher Scientific, Inc.) according to
the manufacturer's specification. cDNA was synthesized from RNA
using the TransScript All-in-One First-Strand cDNA Synthesis kit
(TransGen Biotech Co. Ltd.) according to the manufacturer's
instructions. The relative expression levels of each gene were
detected by qPCR using SYBR® Premix EX Taq™ kit (Takara
Bio, Inc.) in an ABI 9600 (Applied Biosystems; Thermo Fisher
Scientific, Inc.) thermal cycler according to the manufacturer's
instructions and quantified by 2−ΔΔCq (24). The following were the
thermocycling conditions: Initial denaturation at 95°C for 3 min;
followed by 40 cycles of denaturation at 95°C for 30 sec, annealing
at 60°C for 30 sec and extension at 72°C for 30 sec. The primer
sequences were as follows: ATP7B forward, 5′-CCT CCT CTC CCG GGA
CTT TA-3′ and reverse, 5′-TGG CAA GTC ATG CCC AAG AT-3′; PD-L1
forward, 5′-TTT GCT GAA CGC CCC AT A CA-3′ and reverse, 5′-GGA ATT
GGT GGT GGT GGT CT' and GAPDH forward 5′-GGG AAA CTG TGG CGT GAT-3′
and reverse, 5′-GAG TGG GTG TCG CTG TTG A-3′. GAPDH was used as an
internal control.
Western blot analysis
A549 cells were collected, lysed in RIPA buffer
(Beyotime Institute of Biotechnology, Shanghai, China) and
centrifuged at 12,000 × g at 4°C for 10 min to obtain supernatant.
The levels of the proteins in the supernatant were analyzed by
bicinchoninic acid protein assay kit. The protein (30
μg/lane) were separated by 10% SDS-PAGE, transferred onto a
polyvinylidene fluoride membrane and blocked with 5% skimmed milk
in 0.1% TBS-Tween-20 for 1 h at room temperature. The membranes
were incubated with the following primary antibodies: ATP7B (cat.
no. ab124973; 1:1,000; Abcam), PD-L1 (cat. 0no. ab213524; 1:1,000;
Abcam), ferredoxin-1 (FDX1; cat. no. 12592-1-AP; 1:1,000;
Proteintech), SLC31A1 (cat. no. ab129067; 1:1,000; Abcam), SDHB
(cat. no. ab175225; 1:50,000; Abcam), HIF-1A (cat. no. #36169;
1:1,000; Cell Signaling Technology) and GAPDH (cat. no. ab9485;
1:2,500; Abcam) overnight at 4°C. Subsequently, the membranes were
washed with TBST and incubated with horseradish
peroxidase-conjugated secondary antibody (cat. no. ab6721; 1:2,000;
Abcam) for 1 h at room temperature. The protein bands were
visualized by an enhanced chemiluminescence kit (Thermo Fisher
Scientific, Inc.) and quantified by Image J 1.8.0 software
(National Institutes of Health).
Flow cytometry
The apoptosis of A549 cells was detected by flow
cytometry using an Annexin V/propidium iodide (PI) kit (Guangzhou
RiboBio Co., Ltd.). Briefly, A549 cells were collected and washed
with ice-cold PBS twice. Subsequently, A549 cells were resuspended
in 100 μl binding buffer (2×105 cells) and
stained with 5 μl Annexin V and 5 μl PI at 4°C for 15
min in the dark. Finally, a flow cytometer (FACSCalibur; BD
Biosciences) and Flowjo vX.0.7 software (FlowJo LLC) were used to
detect the apoptosis of A549 cells. The apoptotic rate was
calculated using the formula: Apoptotic rate=Q2+Q3.
Detection of oxidative stress
A549 cells were lysed in cell lysis solution
(Beyotime Institute of Biotechnology), then centrifuged at 10,000 ×
g at 4°C for 10 min to obtain the cell supernatant. The levels of
reactive oxygen species (ROS), malondialdehyde (MDA) and superoxide
dismutase (SOD) were determined in the supernatant of A549 cells
with ELISA kits (cat. no. E004-1-1; Nanjing Jiancheng
Bioengineering Institute), MDA assay kits (cat. no. A003-1-2;
Nanjing Jiancheng Bioengineering Institute) and SOD assay kits
(cat. no. S0086; Beyotime Institute of Biotechnology) (Beyotime
Institute of Biotechnology) according to the manufacturer's
instructions.
Statistical analysis
The data are presented as mean ± standard deviation
of three independent experimental repeats. Statistical analysis was
performed using GraphPad Prism 8.0.1 (GraphPad Software, Inc.;
Dotmatics). Statistical differences were evaluated by one-way
analysis of variance followed by Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
DSF decreases cell viability and
upregulated expression levels of ATP7B and PD-L1 of A549 cells
DSF suppressed the viability of A549 cells; the
inhibitory effect of DSF on the viability of A549 cells was
enhanced by CuCl2 (Fig.
1A). In the presence or absence of CuCl2, the
expression levels of ATP7B and PD-L1 were upregulated in A549 cells
by DSF (Fig. 1B-D). Not only did
CuCl2 significantly inhibit the cell viability, but also
significantly up-regulate the expression levels of ATP7B and
PD-L1.
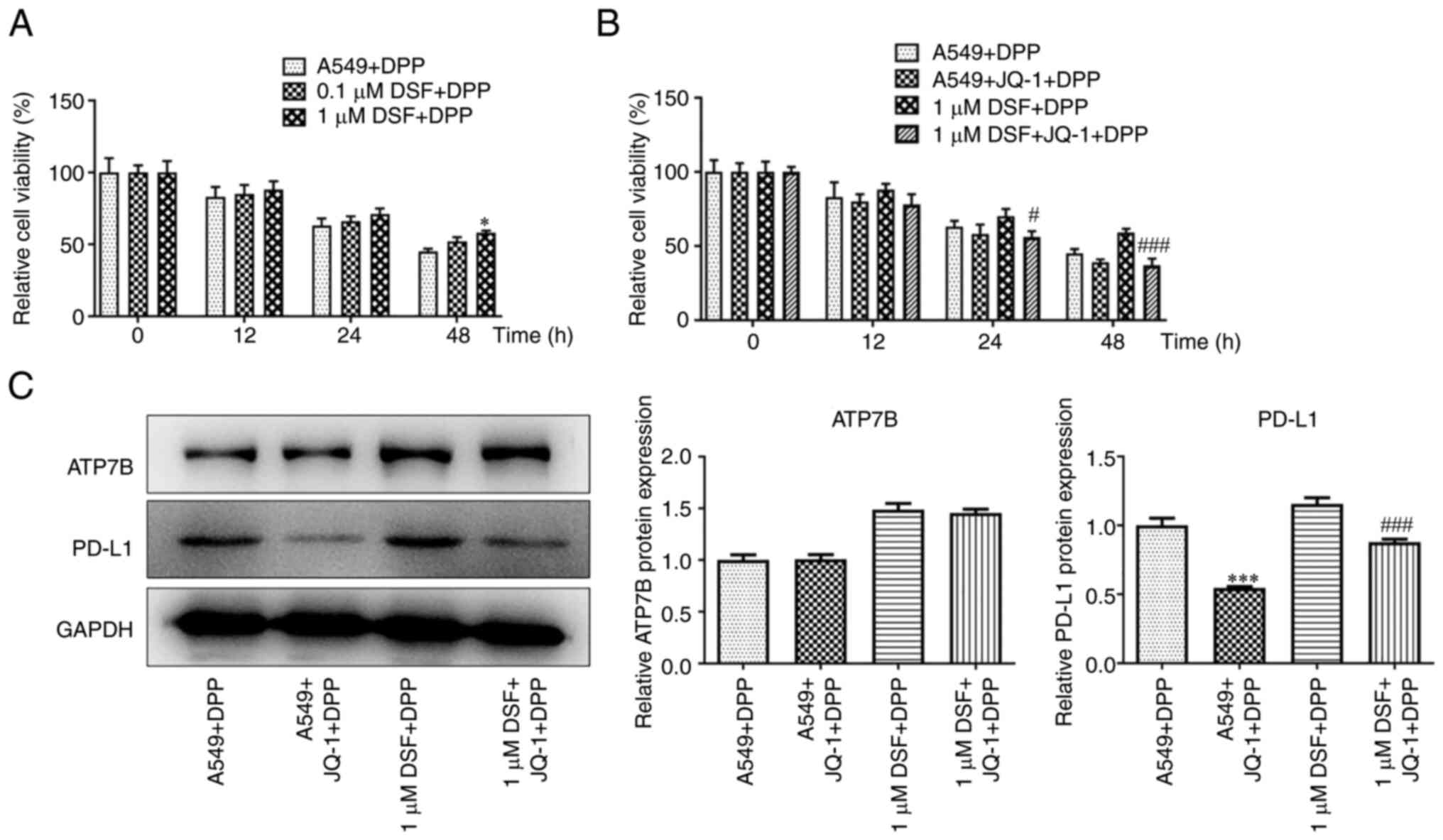
The combination treatment of DSF and JQ-1
decreased the viability and the expression of PD-L1 in A549
cells
DSF improved the viability of DPP-treated A549 cells
at 48 h (Fig. 2A). JQ-1
suppressed the viability of DPP-treated A549 cells treated with DSF
at 24 and 48 h (Fig. 2B). DSF
increased expression levels of ATP7B and PD-L1. JQ-1 exhibited no
significant effect on ATP7B expression but inhibited expression
levels of PD-L1 in DPP-treated A549 cells (Fig. 2C).
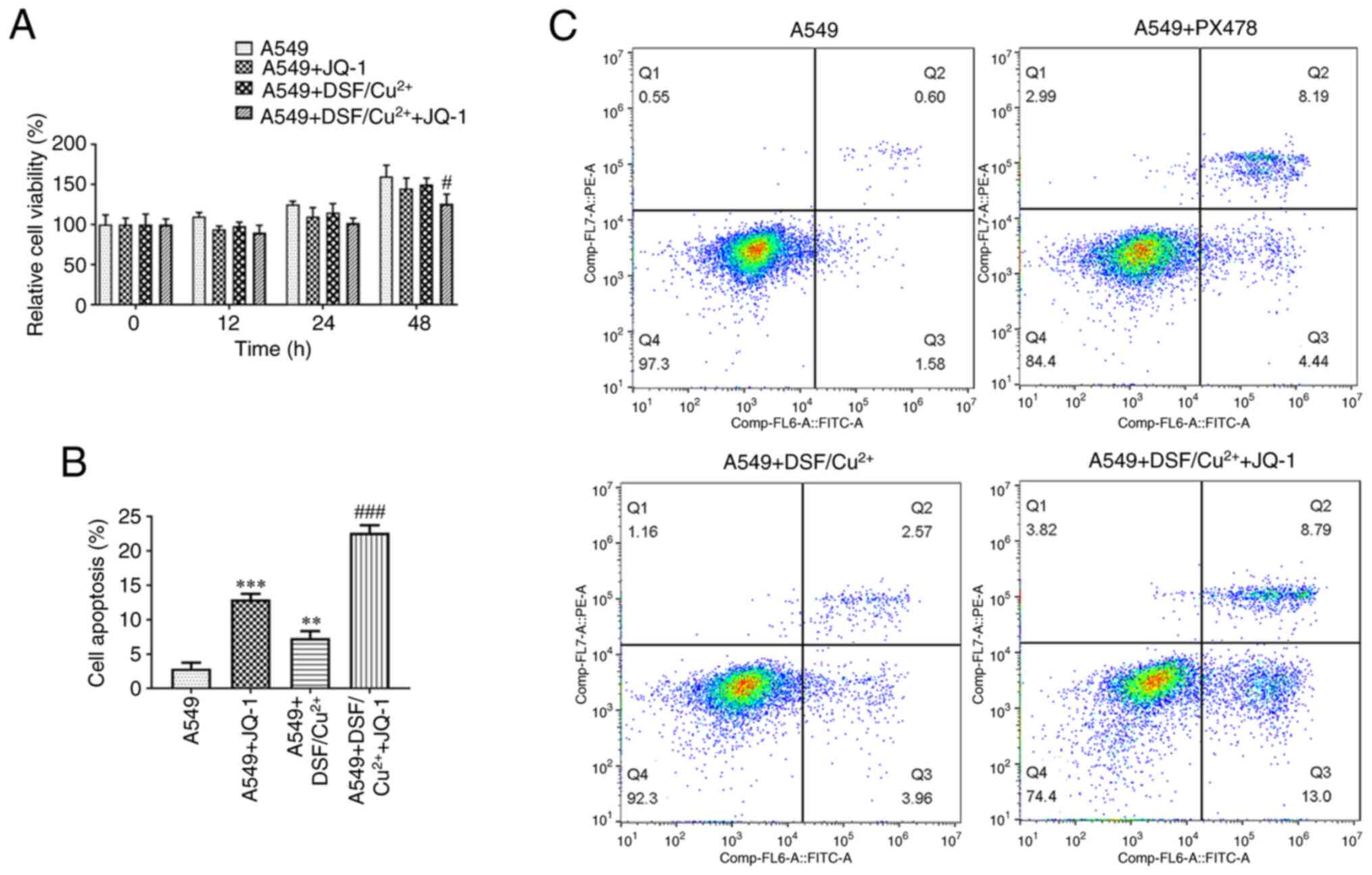
The combination treatment of DSF and JQ-1
decreased the viability and induced the apoptosis in A549
cells
Combination of DSF and JQ-1 suppressed the viability
of A549 cells (Fig. 3A). JQ-1
promoted the apoptosis of A549 cells and DSF suppressed apoptosis.
The combined effect on apoptosis of A549 cells caused by DSF and
JQ-1 was more potent than that caused by single treatment of JQ-1
(Fig. 3B and C).
Combination treatment of DSF and JQ-1
promoted the oxidative stress and cuproptosis of A549 cells
DSF and JQ-1 independently increased levels of ROS
and MDA, while decreased levels of SOD in A549 cells. The combined
treatment of DSF and JQ-1 further increased levels of ROS and MDA,
while decreasing levels of SOD in A549 cells (Fig. 4A-C). JQ-1 upregulated expression
of FDX1 and SLC31A1, while it downregulated the expression of
ATP7B, SDHB, PD-L1 and HIF-1A in A549 cells. DSF caused an
upregulation in expression levels of FDX1, ATP7B, SLC31A1, PD-L1
and HIF-1A, while it downregulated expression levels of SDHB in
A549 cells. The combination of DSF and JQ-1 increased expression
levels of FDX1 and SLC31A1, while suppressing expression of ATP7B,
SDHB, PD-L1 and HIF-1A in A549 cells (Fig. 4D).
 | Figure 4Effect of DSF and/or JQ-1 on oxidative
stress and cuproptosis of A549 cells. Levels of (A) ROS, (B) MDA
and (C) SOD in A549 cells treated with DSF/Cu2+ and/or
JQ-1 were detected by commercial assay kits. (D) Expression of
cuproptosis-associated proteins in A549 cells treated with
DSF/Cu2+ and/or JQ-1 was detected by western blot.
***P<0.001 vs. A549. #P<0.05 and
###P<0.001 vs. A549 + DSF/Cu2+. DSF,
disulfiram; ROS, reactive oxygen species; MDA, malondialdehyde;
SOD, superoxide dismutase; FDX1, ferredoxin-1; ATP7B, ATPase
copper-transporting β; SLC31A1, solute carrier family 31 member 1;
SDHB, succinate dehydrogenase B; PD-L1, programmed death-ligand 1;
HIF-1A, hypoxia inducible factor-1A. |
PX478 decreases viability and promoted
the apoptosis in DSF-treated A549 cells
PX478 increased the effect of DSF and enhanced the
inhibitory effect on the viability of A549 cells (Fig. 5A). DSF promoted apoptosis and
PX478 enhanced the effect of DSF on the induction of apoptosis in
A549 cells (Fig. 5B and C).
PX478 promotes oxidative stress and
induced cuproptosis in DSF-treated A549 cells
DSF increased levels of ROS and MDA, while
decreasing the levels of SOD in A549 cells. PX478 upregulated the
levels of ROS and MDA, while downregulating SOD in DSF-treated A549
cells (Fig. 6A-C). The
expression levels of FDX1, ATP7B, SLC31A1, PD-L1, and HIF-1A were
increased, while those of SDHB were decreased in A549 cells treated
with DSF. PX478 promoted the expression levels of FDX1 and SLC31A1,
while suppressing expression levels of ATP7B, PD-L1 and HIF-1A in
DSF-treated A549 cells (Fig.
6D).
 | Figure 6Effect of PX478 on oxidative stress
and cuproptosis of DSF-treated A549 cells. Levels of (A) ROS, (B)
MDA and (C) SOD in A549 cells treated with DSF/Cu2+
and/or PX478 were detected by commercial assay kits. (D) Expression
of cuproptosis-associated proteins in A549 cells treated with
DSF/Cu2+ and/or PX478 was detected by western blot.
**P<0.01 and ***P<0.001 vs. A549.
###P<0.001 vs. A549 + DSF/Cu2+. DSF; ROS,
reactive oxygen species; MDA, malondialdehyde; SOD, superoxide
dismutase; FDX1, ferredoxin-1; ATP7B, ATPase copper-transporting β;
SLC31A1, solute carrier family 31 member 1; SDHB, succinate
dehydrogenase B; PD-L1, programmed death-ligand 1; HIF-1A, hypoxia
inducible factor-1A. |
Discussion
A number of studies have confirmed that DSF exerts
anticancer activity via the induction of cell apoptosis by
Cu2+ (25,26). The present study indicated that
DSF/Cu2+ inhibited the viability of A549 cells to a
greater extent than single treatment with DSF. In the presence or
absence of Cu2+, the effects of DSF on the expression
levels of ATP7B and PD-L1 were not significant. Hassani et
al (27) used DSF/Cu to
treat acute myeloid leukemia cell lines, confirming that DSF/Cu
inhibited the proliferation of these cells in a
concentration-dependent manner. Papaioannou et al (28) used DSF combined with 1
μmol/l Cu to treat six ovarian cancer cell lines, which
promoted cell death.
DSF causes a significant upregulation in expression
of PD-L1 in hepatocellular cancer cells, which may enhance
immunosuppression and immune escape of tumors (17). The present study indicated that
following pretreatment with DSF, the viability of DPP-treated A549
cells was improved by upregulating expression levels of ATP7B and
PD-L1, which was consistent with previous studies (29,30). It was hypothesized that although
DSF independently demonstrated tumor inhibitory activity, it
upregulated the expression levels of PD-L1 to enhance
immunosuppression and immune escape of tumors.
A previous study indicated that combination of
anti-PD-L1 anticancer therapy with DSF promotes the anticancer
effect of anti-PD-L1 on breast cancer (29). However, it is possible that the
overall anticancer effect may be weakened by upregulation of PD-L1
expression by DSF treatment. Following combined treatment of cells
with anti-PD-L1, DSF alone was more efficient in inducing
cuproptosis and anticancer effects. Moreover, anti-PD-L1 promoted
and improved the anticancer effect of DSF. The present study
indicated that addition of JQ-1 (PD-L1 inhibitor) further inhibited
viability of A549 cells; however, when A549 cells were pretreated
with DSF, JQ-1 reversed the increased DPP resistance of A549 cells
caused by upregulation of PD-L1 expression.
A previous study demonstrated that overexpression of
ATP7B decreases accumulation and accelerates efflux of Cu, which
decreases the effects of DSF on cancer cells. The HIF-1 signaling
pathway is suppressed in ATP7B-knockout HepG2 cells (18). HIF-1 is a DNA-binding protein
involved in cell signal transduction under hypoxic conditions in
vivo. The A subunit of HIF-1 is induced and activated by
hypoxia to control activity of HIF-1 and regulate its expression
(31). PD-L1 is an important
factor mediating the immune response of the body. Following
combination with PD-1, the expression of tyrosine phosphatase
non-receptor type 11 protein is increased via tyrosine-based switch
motif to promote phosphorylation of several key molecules of the T
cell antigen receptor signaling pathway and inhibit proliferation
and activity of T cells. The expression of PD-L1 on the surface of
tumor cells is an important factor leading to immune escape of
tumor cells (32,33). Zhao et al (34) demonstrated that inhibition of
enhancer of zeste homolog 2 expression inhibits the expression of
PD-L1 by decreasing levels of HIF-1A, indicating that both PD-L1
and HIF-1A are associated with occurrence and development of NSCLC.
Previous studies have shown that HIF-1A induces immune tolerance of
tumor cells and promotes malignant development of cancer by
regulating PD-L1 expression under hypoxic conditions (35-37). In the present study, HIF-1
inhibitor PX478 was used to investigate the protective effect
caused by treatment with DSF on NSCLC. PX478 acted with DSF to
suppress cell viability and oxidative stress and promote apoptosis
and cuproptosis of A549 cells. However, there are several
limitations in the present study. A single cell line, which was
established in cell culture, was used; our future experiments will
verify the present findings and potential mechanism in other NSCLC
cell lines. In addition, the present results require confirmation
using animals, as well as clinical studies.
In conclusion, DSF inhibited viability and promoted
expression of ATP7B and PD-L1 in A549 cells. The viability of
DPP-treated A549 cells was increased following DSF treatment. JQ-1
and PX478 promoted the ability of DSF to suppress cell viability
and enhanced the induction of oxidative stress, as well as
induction of apoptosis and cuproptosis of A549 cells. Overall,
combination of DSF with anti-PD-L1 treatment increased the
induction of cuproptosis caused by DSF in NSCLC, which reveals a
novel mechanism underlying the combined antitumor effect of DSF
with anti-PD-L1 treatment and may facilitate discovery of a lead
compound or drug candidate for the development of novel antitumor
drugs.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL and LZ designed the study and drafted and revised
the manuscript. QS, SB and HW analyzed the data and performed the
literature review. PL and QS confirmed the authenticity of all the
raw data. All authors performed the experiments. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.
|
|
2
|
Freeman B, Mamallapalli J, Bian T, Ballas
K, Lynch A, Scala A, Huo Z, Fredenburg KM, Bruijnzeel AW, Baglole
CJ, et al: Opportunities and challenges of kava in lung cancer
prevention. Int J Mol Sci. 24:95392023.
|
|
3
|
Akamine T, Toyokawa G, Tagawa T and Seto
T: Spotlight on lorlatinib and its potential in the treatment of
NSCLC: The evidence to date. Onco Targets Ther. 11:5093–5101.
2018.
|
|
4
|
Chang A: Chemotherapy, chemoresistance and
the changing treatment landscape for NSCLC. Lung cancer. 71:3–10.
2011.
|
|
5
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016.
|
|
6
|
Rossi A and Di Maio M: Platinum-based
chemotherapy in advanced non-small-cell lung cancer: Optimal number
of treatment cycles. Expert Rev Anticancer Ther. 16:653–660.
2016.
|
|
7
|
Lopez J, Ramchandani D and Vahdat L:
Copper depletion as a therapeutic strategy in cancer. Met Ions Life
Sci. 19:2019.
|
|
8
|
Bu W, Wang Z, Meng L, Li X, Liu X, Chen Y,
Xin Y, Li B and Sun H: Disulfiram inhibits epithelial-mesenchymal
transition through TGFβ-ERK-Snail pathway independently of Smad4 to
decrease oral squamous cell carcinoma metastasis. Cancer Manag Res.
11:3887–3898. 2019.
|
|
9
|
Li Y, Wang LH, Zhang HT, Wang YT, Liu S,
Zhou WL, Yuan XZ, Li TY, Wu CF and Yang JY: Disulfiram combined
with copper inhibits metastasis and epithelial-mesenchymal
transition in hepatocellular carcinoma through the NF-κB and TGF-β
pathways. J Cell Mol Med. 22:439–451. 2018.
|
|
10
|
Bucci M: Cancer therapy: A path of DSF
destruction. Nat Chem Biol. 14:1072018.
|
|
11
|
Liu X, Wang L, Cui W, Yuan X, Lin L, Cao
Q, Wang N, Li Y, Guo W, Zhang X, et al: Targeting ALDH1A1 by
disulfiram/copper complex inhibits non-small cell lung cancer
recurrence driven by ALDH-positive cancer stem cells. Oncotarget.
7:58516–58530. 2016.
|
|
12
|
Morrison BW, Doudican NA, Patel KR and
Orlow SJ: Disulfiram induces copper-dependent stimulation of
reactive oxygen species and activation of the extrinsic apoptotic
pathway in melanoma. Melanoma Res. 20:11–20. 2010.
|
|
13
|
Liu P, Brown S, Goktug T, Channathodiyil
P, Kannappan V, Hugnot JP, Guichet PO, Bian X, Armesilla AL,
Darling JL and Wang W: Cytotoxic effect of disulfiram/copper on
human glioblastoma cell lines and ALDH-positive cancer-stem-like
cells. Br J Cancer. 107:1488–1497. 2012.
|
|
14
|
Cheriyan VT, Wang Y, Muthu M, Jamal S,
Chen D, Yang H, Polin LA, Tarca AL, Pass HI, Dou QP, et al:
Disulfiram suppresses growth of the malignant pleural mesothelioma
cells in part by inducing apoptosis. PLoS One. 9:e937112014.
|
|
15
|
Skrott Z, Mistrik M, Andersen KK, Friis S,
Majera D, Gursky J, Ozdian T, Bartkova J, Turi Z, Moudry P, et al:
Alcohol-abuse drug disulfiram targets cancer via p97 segregase
adaptor NPL4. Nature. 552:194–199. 2017.
|
|
16
|
Tsvetkov P, Coy S, Petrova B, Dreishpoon
M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R,
Spangler RD, et al: Copper induces cell death by targeting
lipoylated TCA cycle proteins. Science. 375:1254–1261. 2022.
|
|
17
|
Zhou B, Guo L, Zhang B, Liu S, Zhang K,
Yan J, Zhang W, Yu M, Chen Z, Xu Y, et al: Disulfiram combined with
copper induces immunosuppression via PD-L1 stabilization in
hepatocellular carcinoma. Am J Cancer Res. 9:2442–2455. 2019.
|
|
18
|
Mi X, Li Z, Yan J, Li Y, Zheng J, Zhuang
Z, Yang W, Gong L and Shi J: Activation of HIF-1 signaling
ameliorates liver steatosis in zebrafish atp7b deficiency (Wilson's
disease) models. Biochim Biophys Acta Mol Basis Dis.
1866:1658422020.
|
|
19
|
Luo F, Lu FT, Cao JX, Ma WJ, Xia ZF, Zhan
JH, Zeng KM, Huang Y, Zhao HY and Zhang L: HIF-1α inhibition
promotes the efficacy of immune checkpoint blockade in the
treatment of non-small cell lung cancer. Cancer Lett. 531:39–56.
2022.
|
|
20
|
Sun S, Guo C, Gao T, Ma D, Su X, Pang Q
and Zhang R: Hypoxia enhances glioma resistance to
sulfasalazine-induced ferroptosis by upregulating SLC7A11 via
PI3K/AKT/HIF-1α Axis. Oxid Med Cell Longev. 2022:78624302022.
|
|
21
|
Koh MY, Spivak-Kroizman T, Venturini S,
Welsh S, Williams RR, Kirkpatrick DL and Powis G: Molecular
mechanisms for the activity of PX-478, an antitumor inhibitor of
the hypoxia-inducible factor-1alpha. Mol Cancer Ther. 7:90–100.
2008.
|
|
22
|
Cui Z, Ruan Z, Li M, Ren R, Ma Y, Zeng J,
Sun J, Ye W, Xu W, Guo X, et al: Intermittent hypoxia inhibits
anti-tumor immune response via regulating PD-L1 expression in lung
cancer cells and tumor-associated macrophages. Int Immunopharmacol.
122:1106522023.
|
|
23
|
Walter B, Gil S, Naizhen X, Kruhlak MJ,
Linehan WM, Srinivasan R and Merino MJ: Determination of the
expression of PD-L1 in the morphologic spectrum of renal cell
carcinoma. J Cancer. 11:3596–3603. 2020.
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
25
|
Xu R, Zhang K, Liang J, Gao F, Li J and
Guan F: Hyaluronic acid/polyethyleneimine nanoparticles loaded with
copper ion and disulfiram for esophageal cancer. Carbohydr Polym.
261:1178462021.
|
|
26
|
Chen SY, Chang YL, Liu ST, Chen GS, Lee SP
and Huang SM: Differential cytotoxicity mechanisms of copper
complexed with disulfiram in oral cancer cells. Int J Mol Sci.
22:37112021.
|
|
27
|
Hassani S, Ghaffari P, Chahardouli B,
Alimoghaddam K, Ghavamzadeh A, Alizadeh S and Ghaffari SH:
Disulfiram/copper causes ROS levels alteration, cell cycle
inhibition, and apoptosis in acute myeloid leukaemia cell lines
with modulation in the expression of related genes. Biomed
Pharmacother. 99:561–569. 2018.
|
|
28
|
Papaioannou M, Mylonas I, Kast RE and
Brüning A: Disulfiram/copper causes redox-related proteotoxicity
and concomitant heat shock response in ovarian cancer cells that is
augmented by auranofin-mediated thioredoxin inhibition.
Oncoscience. 1:21–29. 2013.
|
|
29
|
Zheng X, Liu Z, Mi M, Wen Q, Wu G and
Zhang L: Disulfiram Improves the Anti-PD-1 Therapy Efficacy by
Regulating PD-L1 expression via epigenetically reactivation of IRF7
in triple negative breast cancer. Front Oncol. 11:7348532021.
|
|
30
|
Falls-Hubert KC, Butler AL, Gui K,
Anderson M, Li M, Stolwijk JM, Rodman SN III, Solst SR,
Tomanek-Chalkley A, Searby CC, et al: Disulfiram causes selective
hypoxic cancer cell toxicity and radio-chemo-sensitization via
redox cycling of copper. Free Radic Biol Med. 150:1–11. 2020.
|
|
31
|
Du H, Chen Y, Hou X, Huang Y, Wei X, Yu X,
Feng S, Wu Y, Zhan M, Shi X, et al: PLOD2 regulated by
transcription factor FOXA1 promotes metastasis in NSCLC. Cell Death
Dis. 8:e31432017.
|
|
32
|
Patsoukis N, Brown J, Petkova V, Liu F, Li
L and Boussiotis VA: Selective effects of PD-1 on Akt and Ras
pathways regulate molecular components of the cell cycle and
inhibit T cell proliferation. Sci Signal. 5:ra462012.
|
|
33
|
Munari E, Zamboni G, Marconi M, Sommaggio
M, Brunelli M, Martignoni G, Netto GJ, Moretta F, Mingari MC,
Salgarello M, et al: PD-L1 expression heterogeneity in non-small
cell lung cancer: Evaluation of small biopsies reliability.
Oncotarget. 8:90123–90131. 2017.
|
|
34
|
Zhao Y, Wang XX, Wu W, Long H, Huang J,
Wang Z, Li T, Tang S, Zhu B and Chen D: EZH2 regulates PD-L1
expression via HIF-1α in non-small cell lung cancer cells. Biochem
Biophys Res Commun. 517:201–209. 2019.
|
|
35
|
Noman MZ, Desantis G, Janji B, Hasmim M,
Karray S, Dessen P, Bronte V and Chouaib S: PD-L1 is a novel direct
target of HIF-1α, and its blockade under hypoxia enhanced
MDSC-mediated T cell activation. J Exp Med. 211:781–790. 2014.
|
|
36
|
Daniel SK, Sullivan KM, Labadie KP and
Pillarisetty VG: Hypoxia as a barrier to immunotherapy in
pancreatic adenocarcinoma. Clin Transl Med. 8:102019.
|
|
37
|
Barsoum IB, Smallwood CA, Siemens DR and
Graham CH: A mechanism of hypoxia-mediated escape from adaptive
immunity in cancer cells. Cancer Res. 74:665–674. 2014.
|