Introduction
Lung cancer is among the most lethal diseases
worldwide. For non-small cell lung cancer (NSCLC) in particular,
the 5-year survival rate is very low (1). Three main therapeutic modalities are
used to treat NSCLC: surgery, radio- and chemotherapy. Both radio-
and chemotherapy often evoke therapeutic resistance, which is a
major obstacle encountered during the treatment of all types of
cancer, including NSCLC. One approach that is being used to enhance
therapeutic efficacy and improve cancer patient survival in a
variety of cancer settings is combination treatment with anticancer
drugs and radio-therapy. This approach is based on the premise that
anticancer drugs act via a different mechanism than radiotherapy
and, importantly, may enhance the sensitivity of the cancer to the
effects of ionizing radiation (IR). Among existing anticancer drugs
that have been used as radiotherapy-enhancing agents, or
‘radiosensitizers’, are non-steroidal anti-inflammatory drugs,
5-fluorouracil, paclitaxel (and related taxane derivatives), and
gemcitabine (2–7). Although many chemo-radiotherapy
trials have been conducted using well-known anticancer drugs,
developing new radiosensitizers that are more effective and less
toxic remains an important priority (8–10).
With the advent of molecular biology has come the
identification of new molecular markers of cancer with the
potential to serve as targets of therapeutic drug candidates
(11). There is considerable
research interest in these markers, with both academic and industry
researchers seeking to develop novel, targeted drugs or therapeutic
antibodies for personalized therapy. Likewise, research on
radiosensitizer development has also focused on developing targeted
agents (8). But the development of
novel therapeutic reagents is a costly and time-consuming process;
therefore, conventional agents with confirmed biological safety or
that have shown anticancer effects are also receiving renewed
attention (12,13).
3′,4′,5′,7′-Tetrahydroxyflavone (luteolin) is a
flavonoid isolated from various plants, including edible and
traditional medicinal plants. Flavonoids are secondary metabolites
of plants characterized by their diphenylpropane structure
(C6-C3-C6). Numerous investigations have sought to identify links
between the consumption of food containing high concentrations of
luteolin and beneficial effects on several chronic diseases, but
have been unable to confirm a correlation. Although physiological
effects of luteolin in foods have not been established, purified
luteolin and several of its derivatives have been shown to exhibit
various, significant biological effects, including antioxidant,
anti-inflammatory, antimicrobial, and cancer
chemotherapeutic/chemoprevention activity, among others (14). The anticancer effects of luteolin,
in particular, have been actively investigated. These studies have
reported that luteolin modulates various aspects of the cancer cell
machinery, inhibiting CDK2 and causing cell cycle arrest in G0/G1,
disrupting cellular homeostasis by depleting ATP and inhibiting
glucose uptake, and inducing apoptosis through activation of
caspases or promotion of mitochondrial dysfunction by proapoptotic
B-cell lymphoma 2 (Bcl-2) proteins, among other actions. Moreover,
numerous studies have shown that luteolin suppresses the growth of
cancer cell lines in vitro as well as cancer xenografts
in vivo (15).
In this study, we examined the anticancer effects of
luteolin and determined its 50% inhibitory concentration
(IC50) values against NSCLC cell lines. We also assessed
the effects of combined treatment with luteolin and IR on
IC50 values and demonstrated that combination treatment
enhanced apoptotic cell death in vitro and in vivo
through activation of a p38/ROS/caspase cascade.
Materials and methods
Cell culture and chemicals
The human NSCLC cell lines, NCI-H1299 and -H460,
were purchased from American Type Culture Collection (Rockville,
MD, USA). SB203580, N-acetyl-L-cysteine (NAC),
2′,7′-dichlorofluorescin diacetate (DCF-DA) and
carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone
(z-VAD-fmk) were obtained from Calbiochem (La Jolla, CA, USA).
Luteolin was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Immunoblot analysis
NCI-H1299 and -H460 cells were seeded in 60-mm cell
culture dishes and treated under various experimental conditions.
Treated cells were trypsinized, washed with ice-cold
phosphate-buffered saline (PBS), and collected by centrifugation.
Whole-cell lysates were prepared from harvested cells by incubating
cell pellets in RIPA buffer [50 mmol/l Tris pH 8.0, 150 mmol/l
NaCl, 1% NP-40, 0.5% deoxycholic acid, and 0.1% sodium dodecyl
sulfate (SDS)] containing a protease and phosphatase inhibitor
cocktail (Sigma-Aldrich). Proteins in cell lysates were separated
SDS-polyacrylamide gel electrophoresis (PAGE) on 12% gels and
transferred to nitrocellulose membranes (Invitrogen Life
Technologies, Carlsbad, CA, USA). Membranes were incubated with
primary antibodies against caspase-3, -8 and -9, Bcl-2,
phospho-p38, and p38 (Cell Signaling Technology, Inc., Beverly, MA,
USA). An anti-β-actin antibody (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) was used as a control for equal loading. After
washing with PBS/Tween-20 (PBST), membranes were incubated with the
appropriate secondary antibody. Immunoreactive proteins were
detected using a chemiluminescence kit (Thermo Fisher Scientific,
Inc., Rockford, IL, USA). Relative band densities of target
proteins, determined densitometrically and normalized to those of
β-actin in each experiment, were analyzed using ImageJ software
(NIH, Bethesda, MD, USA).
MTT assay and IC50
determination
NCI-H460 and -H1299 cells were seeded onto 96-well
plates (4×103 cells/well) and treated with different
concentrations (10, 20, 30, 40, 50 and 100 μM) of luteolin. After
incubating for 72 h, 50 μl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
solution (2 mg/ml) were added to each well and the plates were
incubated for additional 2 h at 37°C. Dark-blue formazan crystals
generated by the activity of live cells were dissolved in 150 μl of
dimethyl sulfoxide (DMSO), and the absorbance of individual wells
at 545 nm was determined using a microplate reader (Original
Multiskan; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
IC50 values were calculated from a
concentration-response analysis performed using SoftMax Pro
(Molecular Devices, Sunnyvale, CA, USA).
Clonogenic assay
NCI-H460 and -H1299 cells were seeded in triplicate
60-mm dishes at cell concentrations estimated to yield 20–100
colonies/dish (100, 200, 400, 600 and 1,000 cells/dish). After 24 h
of incubation, NCI-H460 and -H1299 cells were pre-treated with 20
or 30 μM luteolin for 6 h and then exposed to different doses of IR
(1, 3, 5, or 7 Gy) using 137Cs as a radiation source
[Atomic Energy of Canada Limited (AECL), Mississauga, ON, Canada].
Cells were cultured for 10–14 days, and colonies >200 μm in
diameter were counted using a colony counter (Imaging Products
International, Inc., Chantilly, VA, USA). Dose enhancement ratios
(DERs) were calculated as described previously (16).
Cell counting assay
Cells were seeded at a density of 1×105
cells in 60-mm dishes, and treated with luteolin (20 or 30 μM) or
left untreated. After 6 h, cells were exposed to IR (2 or 3 Gy) and
incubated for 72 h. Thereafter, the number of cells in each group
was determined by counting under a microscope using a
hemocytometer.
Propidium iodide uptake assay
Propidium iodide (PI) (Sigma-Aldrich) was used to
detect apoptotic cell death. Cells were seeded at a density of
1×105 cells/well in 6-well plates and then pre-incubated
with or without luteolin for 6 h. Cells were then exposed to IR (2
or 3 Gy) and incubated for 72 h. Cells were then washed twice with
cold PBS and resuspended in 200 μl of 5 μg/ml PI solution.
Apoptosis was detected and analyzed using a FACSort flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA).
Xenograft size determination and TUNEL
assay
A xenograft model for evaluating the in vivo
effect of luteolin was created by injecting 6-week-old
BALB/cAnNCrj-nu/nu strain mice (Charles River Laboratories Japan,
Inc., Kanagawa, Japan) with NCI-H460 cells (1×107). Mice
were divided into four groups (5 mice/group): control (mock
treated), IR only, luteolin only, and luteolin and IR (combination
treatment). When xenografts reached ~100–120 mm3, mice
in luteolin only and combination treatment groups were
subcutaneously injected with 10 mg/kg of luteolin; for IR-only and
control groups, mice were injected with an equal volume of vehicle
solution (DMSO). After 6 h, IR-only and combination treatment
groups were irradiated with 5 Gy. This protocol was repeated three
times at 5-day intervals for 35 days. Tumor dimensions (long and
short axis) were detected over 35 days and tumor volumes were
calculated as (short axis2 × long axis)/2. For
irradiation, mice were anesthetized by intraperitoneal injection of
100 μl of Zoletil (Virbac Laboratories, Carros, France), then fixed
to an acrylic plate and locally irradiated with a 60Co
γ-ray source (Theratrom 780; AECL). Body parts other than tumor
xenografts were protected with lead blocks. For terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays,
xenografts were extracted, fixed with formaldehyde, and then
embedded in a paraffin block. Sliced tissues were stained and
analyzed with an ApopTag TUNEL assay kit (Merck KGaA, Darmstadt,
Germany) as described by the manufacturer. Tumor growth delay
values were calculated as described in Table I.
 | Table IAnalysis of tumor growth delay. |
Table I
Analysis of tumor growth delay.
| Treatment | Daysa | Growth
delayb |
|---|
| Control | 7.8 | |
| Luteolin only | 12 | 4.2 |
| IR only | 17.4 | 9.6 |
| Luteolin + IR | 29.6 | 21.8 |
| Enhancement
factorc | 1.83 | |
ROS detection assay
Reactive oxygen species (ROS) detection assays were
performed as described previously (17). Cells were seeded at a density of
1×105 cells/well in 6-well plates and pre-incubated with
or without luteolin for 6 h. Cells in IR-only and combination
treatment groups were then exposed to 2 Gy of IR, with or without
pharmacological inhibitors. After treating for 24 h, cells were
trypsinized and incubated with 20 μM DCF-DA for 5 min. ROS were
detected and analyzed using a FACSort flow cytometer
(Becton-Dickinson).
Statistical analysis
Data were analyzed using GraphPad Prism software
(GraphPad Software, Inc., La Jolla, CA, USA), and the significance
of differences between experimental groups was determined using
Student’s t-test. P<0.05 was considered significant; individual
p-values are denoted by asterisks in figures (p<0.05, 0.01 and
0.001). The number above each point or bar in every graph indicates
the mean percentage of three independent experiments, and error
bars signify standard deviation (SD).
Results
Combined treatment with luteolin and IR
enhances cell death
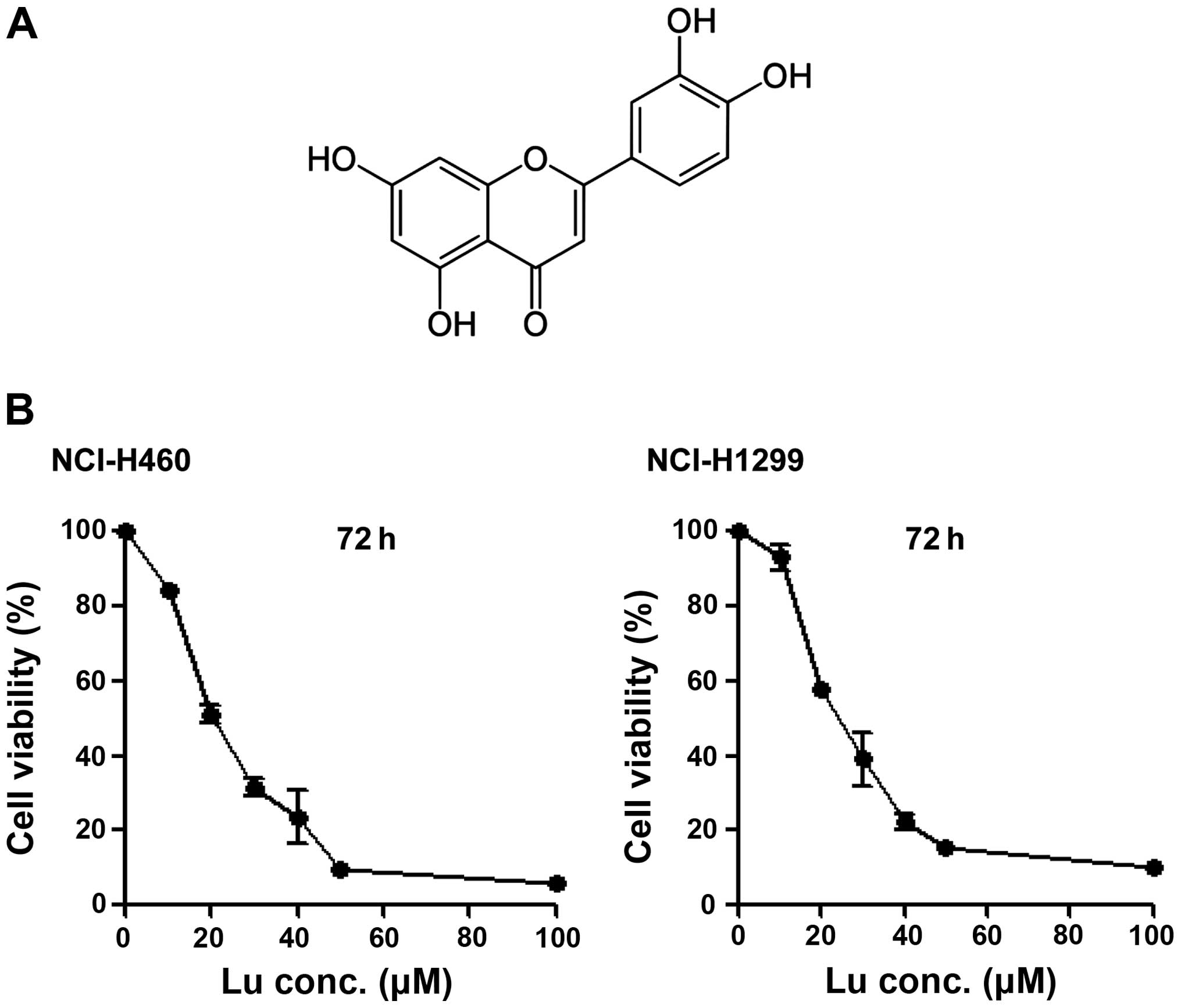
Luteolin (Fig. 1A)
is considered an anticancer drug candidate. Accordingly, we
determined IC50 values of luteolin against NSCLC cells
using MTT assays. The IC50 values were determined to be
20.706 μM in NCI-H460 cells and 25.291 μM in NCI-H1299 cells
(Fig. 1B), showing that luteolin
alone is capable of killing NSCLC cells, as previously reported
(18). To test the
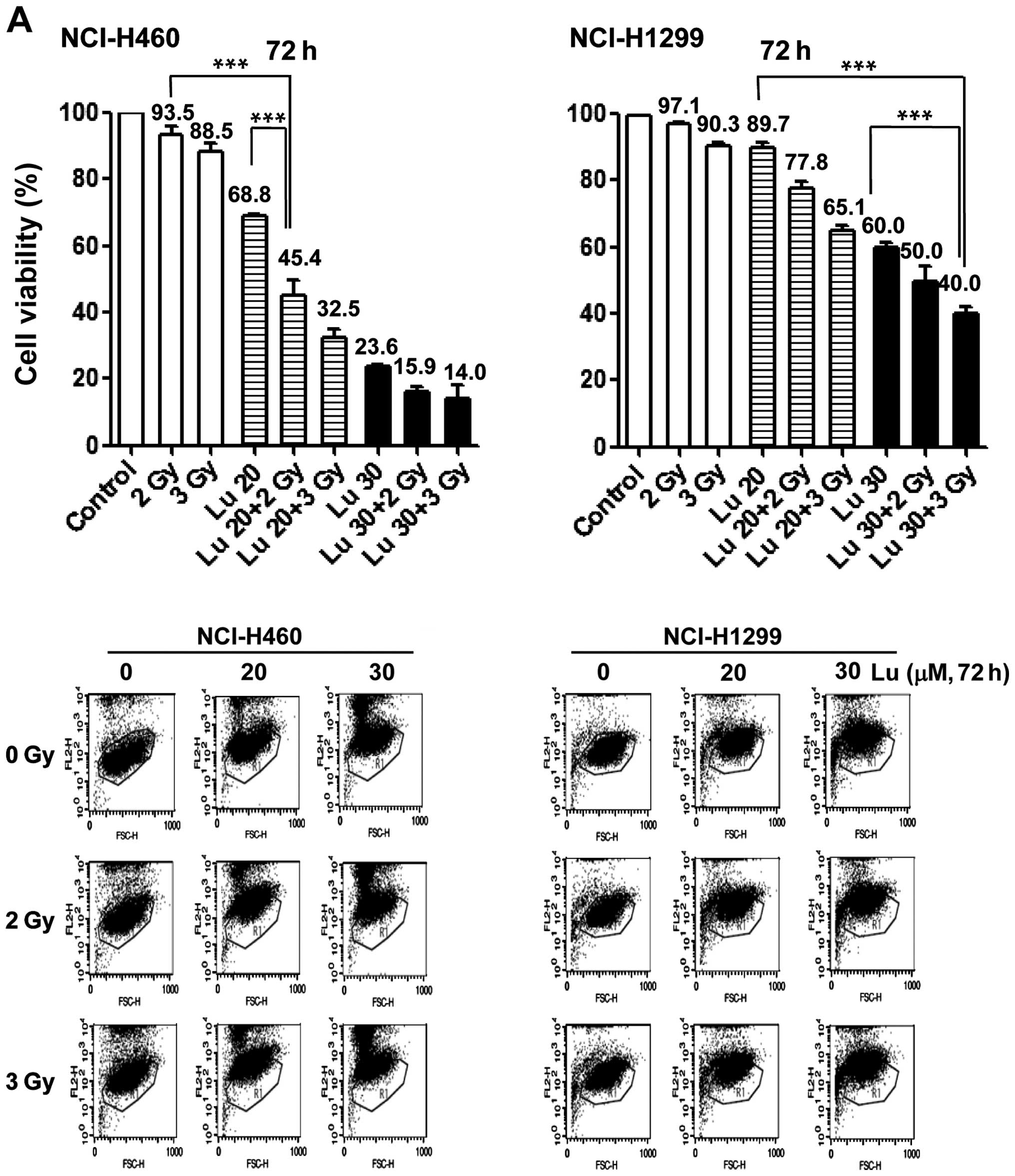
radiosensitizing effect of luteolin, we pre-treated NCI-H460 and
-H1299 cells with 20 or 30 μM luteolin for 6 h, and then exposed
cells to different doses of IR (1, 3, 5 or 7 Gy). Clonogenic
(Fig. 2A) and cell counting
(Fig. 2B) assays confirmed the
radiosensitizing effect of luteolin. Clonogenic assays showed that
the survival fraction in the combination treatment group decreased
compared with that in the IR-only treatment group. At a survival
fraction of 0.25, the DER value was calculated to be 1.22 and 1.35
for NCI-H460 and -H1299 cells, respectively (Fig. 2A). Cell counting assays also showed
that the combination of luteolin (20 or 30 μM) and IR (2 or 3 Gy)
enhanced cell death (Fig. 2B).
Differences in the mean survival rates of NCI-H460 cells between
the luteolin-only and combination treatment group (2 Gy IR) were
~36% at 20 μM luteolin and ~15% at 30 μM luteolin (Fig. 2B, upper panel), indicating that the
combination of 20 μM luteolin and 2 Gy IR was most effective in
these cells. For NCI-H1299 cells (Fig.
2B, lower panel), differences in the mean survival rates
between the luteolin-only and combination group (3 Gy IR) were ~12%
at 20 μM luteolin and ~17% at 30 μM luteolin, indicating that the
combination of 30 μM luteolin and 3 Gy IR was most effective in
this cell line. Collectively, these results indicate that luteolin
acts as a radiosensitizer against NSCLCs and exerts a much stronger
radiosensitizing effect in NCI-H460 cells.
Combined treatment with luteolin and IR
enhances apoptotic cell death
Next, we tested which cell death pathway mediated
the radiosensitizing effect of luteolin. NCI-H460 and -H1299 cells
were pre-treated with 20 or 30 μM luteolin for 6 h, and then
irradiated with 2 or 3 Gy IR. After 72 h, cells were harvested and
PI uptake was analyzed (Fig. 3A).
In NCI-H460 cells, the combination of 20 μM luteolin and 2 Gy IR
increased apoptotic cell death by ~48% compared with 2 Gy IR only
and by ~23% vs. 20 μM luteolin only (Fig. 3A, left panel). In NCI-H1299 cells,
the combination of 30 μM luteolin and 3 Gy IR increased apoptotic
cell death by ~50% compared with 3 Gy IR only, and by ~20% vs. 30
μM luteolin only (Fig. 3A, right
panel). Immunoblot analyses also showed that these
combination-treatment conditions (20 μM luteolin + 2 Gy IR in
NCI-H460 and 30 μM luteolin + 3 Gy IR in NCI-H1299) promoted
activation of caspase-3, -8 and -9, and decreased Bcl-2 levels
(Fig. 3B). Pre-treatment with
z-VAD-fmk, a chemical pan-caspase inhibitor, suppressed apoptotic
death of NCI-H460 cells induced by combination treatment with
luteolin and IR (Fig. 3C).
 | Figure 3Combined treatment with
3′,4′,5′,7′-tetrahydroxyflavone (luteolin) and ionizing radiation
(IR) enhances apoptotic cell death in vitro. (A) Propidium
iodide (PI) uptake assay for NCI-H460 and -H1299 cells receiving
mock treatment (control) or treated with luteolin only, IR only, or
a combination of luteolin and IR. Control, mock-treated control; Lu
20 and 30, groups treated with 20 and 30 μM luteolin only,
respectively; 2 and 3 Gy, groups treated with IR only; Lu 20/30 + 2
Gy/3 Gy, combinations of 20 or 30 μM of luteolin and 2 or 3 Gy of
IR. (B) Immunoblot detection of caspase-3, -8 and -9, and B-cell
lymphoma 2 (Bcl-2). NCI-H460 cells were mock-treated (control) or
were treated with 20 μM luteolin only, 2 Gy IR only, or the
combination of 20 μM luteolin and 2 Gy IR. NCI-H1299 cells were
mock-treated (control) or were treated with 30 μM luteolin only, 3
Gy IR only, or the combination of 30 μM luteolin and 3 Gy IR. (C)
PI uptake assay for NCI-H460 cells treated with a combination of
luteolin and IR with or without pre-treatment with 20 μM
carbobenzoxy-valyl-alanyl-aspartyl- [O-methyl]-fluoromethylketone
(z-VAD-fmk). Samples were harvested after treating for 72 h.
**P<0.01 and ***p<0.001. |
Phosphorylation of p38 increases ROS
production and apoptotic cell death under conditions of combined
treatment with luteolin and IR
We next examined modulation of apoptosis-related
mitogen-activated protein kinase (MAPK) proteins in NCI-H460 cells
by immunoblot analysis, and found that the combination of 20 μM
luteolin and 2 Gy IR induced p38 phosphorylation (Fig. 4A). Pre-treatment with SB250358, a
specific chemical inhibitor of p38, attenuated apoptotic cell death
induced by combination treatment, decreasing the percentage of
apoptotic cells by ~18% (Fig. 4B).
Blockade of p38 also suppressed activation of caspases (Fig. 4C). Moreover, combined treatment
induced ROS production, increasing ROS levels by ~55% compared with
controls. Interestingly, inhibition of p38 with SB250358 also
blocked ROS production (Fig. 4D).
Collectively, these results suggest that p38 might be a major
signaling mediator of the radiosensitizing effects of luteolin
through its effects on ROS production and apoptotic cell death.
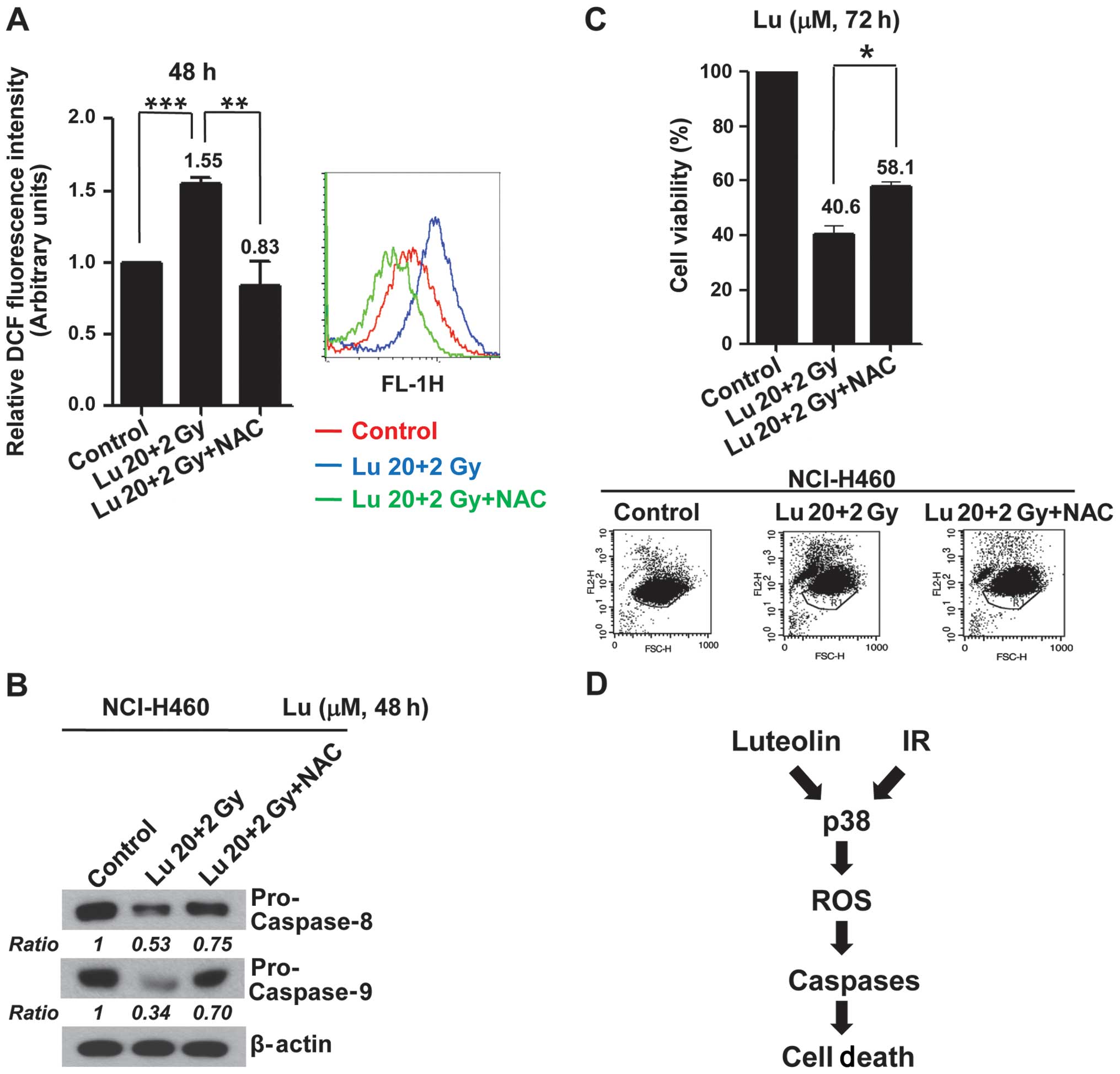
Increased ROS production induced by
combined treatment with luteolin and IR mediates apoptotic cell
death in vitro
To examine the functional linkage between the
p38-dependent generation of ROS and apoptosis, we tested the
effects of the ROS scavenger NAC on the caspase activation and
apoptosis induced by combined treatment with luteolin and IR.
Pre-treatment of NCI-H460 cells with NAC caused a decrease in ROS
production induced by combination treatment (Fig. 5A) in association with inhibition of
caspase activations (Fig. 5B) and
a decrease (~17%) in apoptotic cell death (Fig. 5C). These results indicate that
activation of a p38/ROS/caspase cascade might enhance apoptotic
cell death and constitute a major intracellular signaling pathway
for the radiosensitizing effects of luteolin (Fig. 5D).
Combined treatment with luteolin and IR
enhances apoptotic cell death in vivo
On the basis of the above in vitro results,
we tested the radiosensitizing effects of luteolin in vivo
using an NCI-H460 cell tumor xenograft model, measuring the time
for tumors in each group to reach a volume of 1,500 mm3.
Compared with controls, combination treatment resulted in a tumor
growth delay of 21.8 days, yielding an enhancement factor of 1.83
(Fig. 6A and Table I). These results suggest that
luteolin enhances radiation-induced cell death both in vitro
and in vivo. Tumor tissue was excised from mice in each
treatment group, as described in Materials and methods, and TUNEL
assays were performed (Fig. 6B).
Apoptotic cells were counted and plotted as a percentage of the
total cell population. The number of apoptotic cells in the
combination treatment group was ~3-fold higher than that in the
IR-only group, and ~5-fold higher than that in the luteolin-only
group. These results clearly suggest that the combination of
luteolin and IR enhances cell death by enhancing apoptosis in
vivo as well as in vitro.
Discussion
In the present study, we demonstrate that luteolin
exerts radio-sensitizing effects that enhance apoptotic cell death
in NSCLC cells both in vitro and in vivo, and further
show that these effects are mediated by activation of a
p38/ROS/caspase cascade. First, we observed that the combination of
luteolin and IR enhanced death of both NCI-H460 and -H1299 cells
(Fig. 1). Several previous reports
have shown that both p53 and phosphatase and tensin homolog
(PTEN) are major tumor suppressors that regulate drug and
radiation responses (19,20). However, because the NCI-H460 cell
line contains wild-type p53 and PTEN but the
NCI-H1299 cell line is p53- and PTEN-null, our
results indicate that enhancement of cell death by the combination
of luteolin and IR is independent of intracellular p53 and
PTEN status (21). Second,
we found based on PI uptake that the combination of luteolin and IR
enhances apoptotic cell death (Fig.
3A). Consistent with this, Bcl-2 protein levels were decreased
and activation of caspase-3, -8, and -9 was increased by
combination treatment (Fig. 3B).
Numerous apoptosis-related proteins, including p53,
Bcl-2-associated X protein (Bax), p21, Bcl-2 and caspases, are
involved in the radiation-induced apoptotic death of cancer cells
(22). These proteins might also
be responsible for radiosensitizing effects and could be targets
for the development of radio-enhancing reagents. Therefore, we
could conclude that radiosensitizing effect of luteolin is
dependent on apoptosis.
There are two major pathways of apoptosis: intrinsic
and extrinsic (or death receptor-mediated). Activation of the
intrinsic pathway is induced by external stress and is followed by
changes in mitochondrial permeability transition (MPT) and
activation of caspase-9. The extrinsic pathway begins with death
receptor/ligand binding and proceeds through caspase-8 activation.
Caspase activation is a common event in both apoptotic pathways.
Caspase-8 and -9 are ‘initiator’ caspases of the extrinsic and
intrinsic pathway, respectively, whereas caspase-3 is a common
‘executioner’ caspase in all apoptotic pathways. Bcl-2 inhibits
apoptosis by regulating MPT and thereby blocking cytochrome
c release from mitochondria. Because the combination of
luteolin and IR increased activation of both the intrinsic and
extrinsic apoptotic caspases and decreased Bcl-2 protein levels, we
postulate that the radiosensitizer action of luteolin results from
regulation of caspase activation and MPT (23). In addition to these apoptotic
proteins, various intracellular proteins, including
phosphatidylinositol 3-kinase, Akt, cell cycle-related molecules
and elements of the DNA repair system, serve as targets of
radiosensitizers. Radiosensitizing reagents that target these
proteins can enhance radiation-induced cell death by perturbing
various physiological phenomena-inhibiting angiogenesis, arresting
or disrupting the cell cycle, inducing apoptosis, or blocking cell
survival signaling pathways (24).
Experiments were performed to identify the
intracellular signaling pathway involved in mediating the cell
death enhancement induced by combined treatment with luteolin and
IR implicated activation of a p38/ROS/caspase cascade (Figs. 4 and 5). Phosphorylation of p38 MAPK, one of
three kinases that form the core of a MAPK cascade, was increased
by the combination of luteolin and IR. MAP kinase kinase kinase
(MAPKKK, also known as MEKK), the first of the three kinases, is
located downstream of the original signaling protein, such as small
GTPase, and phosphorylates the second kinase, MAP kinase kinase
(MAPKK, also known as MEK or MKK) in this kinase cascade. Activated
MAPKK isoforms, including MKK3, 4 and 6, then phosphorylate the
last kinase of the cascade including p38. Several previous studies
have shown that p38 MAPK is a stress-response molecule and involved
in cell death by radiation and radiosensitizers. It has been
reported that activation of c-Abl-PKCδ-Rac1-p38 MAPK signaling by
IR induces conformational changes in Bak and Bax, resulting in
mitochondrial activation-mediated apoptotic cell death in human
NSCLC cells (25). We also
previously reported that the combination of a small chemical
molecule and IR enhances cancer cell death through p38-mediated
Bcl-2 degradation (26). In
addition, we previously showed that IR activates c-Jun N-terminal
kinase (JNK), another MAPK, and that activated JNK induces ROS
production by disrupting mitochondrial membrane potential (17). Here, we found that p38 MAPK
activation induced ROS production, which enhanced apoptotic cell
death. ROS generated by IR damages cells through oxidation of
lipids, DNA, and proteins. ROS production is mediated primarily by
membrane-associated enzymes, such as NADPH oxidase, or is triggered
intracellularly in the mitochondria through the electron transport
chain (27). It is also known that
oxidative damage caused by IR-induced ROS promotes apoptosis
through activation of caspases. Park et al (28) reported that the combination of
phytosphingosine and IR enhanced apoptosis via ROS-induced
mitochondrial relocalization of Bax and nuclear translocation of
apoptosis-inducing factor (AIF). Lee et al (29) also reported that IR can act through
protein kinase C-δ (PKCδ)-mediated ROS production to induce
apoptosis. Therefore, the enhancement of apoptosis by the
combination of luteolin and IR through activation of
p38/ROS/caspases reported here is in accord with the previous
reports (28,29). However, inhibition of
p38/ROS/caspase did not complete abrogate the cell death induced by
combined treatment with luteolin and IR. This implies that other
cell death or growth-retardation mechanisms might modulate the
radiosensitizing effect of luteolin, raising interesting questions
for further research.
Taken together, our results suggest a novel role for
luteolin as a radiosensitizer in NSCLC cells, where it acts by
increasing apoptotic cell death by the activation of the
p38/ROS/caspase cascade, and it is independent of the expression of
p53 and PTEN. Development of an ideal radiosensitizer
must consider two aspects, increased efficiency and protection of
normal tissues (10). We did not
determine if luteolin protected normal tissue from damage by IR,
but the combination of luteolin and IR enhanced cancer cell death
in vivo as well as in vitro. The radiosensitization
of cells by the combination of luteolin and IR shares a common
stress-response signaling pathway containing p38 MAPK and ROS
(30,31). Elucidation of the exact mechanism
of radiosensitization is important in order to develop new drugs
that are synergistic with IR. Our findings on the radiosensitizing
effects of luteolin may be useful to develop therapeutic techniques
for the treatment of NSCLC patients regardless of p53 and PTEN
expression status.
Acknowledgements
This study was supported by the Nuclear Research and
Development Program of the National Research Foundation of Korea
(NRF) (Seoul, Korea) grant funded by the Korean government (MEST)
(2012M2A2A7010459) and, in part, by the Basic Science Research
Program through the NRF (2008-0062611).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer Statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim JS, Amorino GP, Pyo H, Cao Q, Price JO
and Choy H: The novel taxane analogs, BMS-184476 and BMS-188797,
potentiate the effects of radiation therapy in vitro and in vivo
against human lung cancer cells. Int J Radiat Oncol Biol Phys.
51:525–534. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Palayoor ST, Bump EA, Calderwood SK,
Bartol S and Coleman CN: Combined antitumor effect of radiation and
ibuprofen in human prostate carcinoma cells. Clin Cancer Res.
4:763–771. 1998.PubMed/NCBI
|
|
4
|
Dicker AP, Williams TL and Grant DS:
Targeting angiogenic processes by combination rofecoxib and
ionizing radiation. Am J Clin Oncol. 24:438–442. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim KY, Seol JY, Jeon GA and Nam MJ: The
combined treatment of aspirin and radiation induces apoptosis by
the regulation of bcl-2 and caspase-3 in human cervical cancer
cells. Cancer Lett. 189:157–166. 2003. View Article : Google Scholar
|
|
6
|
Zhu AX and Willett CG: Chemotherapeutic
and biologic agents as radiosensitizers in rectal cancer. Semin
Radiat Oncol. 13:454–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones PD, de Lorimier LP, Kitchell BE and
Losonsky JM: Gemcitabine as a radiosensitizer for nonresectable
feline oral squamous cell carcinoma. J Am Anim Hosp Assoc.
39:463–467. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Begg AC, Stewart FA and Vens C: Strategies
to improve radiotherapy with targeted drugs. Nat Rev Cancer.
11:239–253. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitsudomi T, Suda K and Yatabe Y: Surgery
for NSCLC in the era of personalized medicine. Nat Rev Clin Oncol.
10:235–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moding EJ, Kastan MB and Kirsch DG:
Strategies for optimizing the response of cancer and normal tissues
to radiation. Nat Rev Drug Discov. 12:526–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Travis WD, Brambilla E, Noguchi M, et al:
International Association for the Study of Lung Cancer/American
Thoracic Society/European Respiratory Society: international
multi-disciplinary classification of lung adenocarcinoma: executive
summary. Proc Am Thorac Soc. 8:381–385. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Swinney DC and Anthony J: How were new
medicines discovered? Nat Rev Drug Discov. 10:507–519. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rask-Andersen M, Almén MS and Schiöth HB:
Trends in the exploitation of novel drug targets. Nat Rev Drug
Discov. 10:579–590. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seelinger G, Merfort I, Wölfle U and
Schempp CM: Anti-carcinogenic effects of the flavonoid luteolin.
Molecules. 13:2628–2651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin Y, Shi R, Wang X and Shen HM:
Luteolin, a flavonoid with potential for cancer prevention and
therapy. Curr Cancer Drug Targets. 8:634–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Albert JM, Cao C, Kim KW, et al:
Inhibition of poly(ADP-ribose) polymerase enhances cell death and
improves tumor growth delay in irradiated lung cancer models. Clin
Cancer Res. 13:3033–3042. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim EM, Yang HS, Kang SW, Ho JN, Lee SB
and Um HD: Amplification of the gamma-irradiation-induced cell
death pathway by reactive oxygen species in human U937 cells. Cell
Signal. 20:916–924. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leung HW, Kuo CL, Yang WH, Lin CH and Lee
HZ: Antioxidant enzymes activity involvement in luteolin-induced
human lung squamous carcinoma CH27 cell apoptosis. Eur J Pharmacol.
534:12–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rebucci M and Michiels C: Molecular
aspects of cancer cell resistance to chemotherapy. Biochem
Pharmacol. 85:1219–1226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JK, Jung HY, Park SH, Kang SY, Yi MR,
Um HD and Hong SH: Combination of PTEN and gamma-ionizing radiation
enhances cell death and G(2)/M arrest through regulation of AKT
activity and p21 induction in non-small-cell lung cancer cells. Int
J Radiat Oncol Biol Phys. 70:1552–1560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prise KM, Schettino G, Folkard M and Held
KD: New insights on cell death from radiation exposure. Lancet
Oncol. 6:520–528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Katz D, Ito E and Liu FF: On the path to
seeking novel radiosensitizers. Int J Radiat Oncol Biol Phys.
73:988–996. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Munshi A and Ramesh R: Mitogen-activated
protein kinases and their role in radiation response. Genes Cancer.
4:401–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park JK, Chung YM, Kim BG, Yoo YA, Yang
BS, Kim JS and Yoo YD: N′-(phenyl-pyridin-2-yl-methylene)-hydrazine
carbodithioic acid methyl ester enhances radiation-induced cell
death by targeting Bcl-2 against human lung carcinoma cells. Mol
Cancer Ther. 3:403–407. 2004.PubMed/NCBI
|
|
27
|
Caputo F, Vegliante R and Ghibelli L:
Redox modulation of the DNA damage response. Biochem Pharmacol.
84:1292–1306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park MT, Kim MJ, Kang YH, et al:
Phytosphingosine in combination with ionizing radiation enhances
apoptotic cell death in radiation-resistant cancer cells through
ROS-dependent and -independent AIF release. Blood. 105:1724–1733.
2005. View Article : Google Scholar
|
|
29
|
Lee YJ, Lee DH, Cho CK, et al: HSP25
inhibits radiation-induced apoptosis through reduction of
PKCdelta-mediated ROS production. Oncogene. 24:3715–3725. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ju W, Wang X, Shi H, Chen W, Belinsky SA
and Lin Y: A critical role of luteolin-induced reactive oxygen
species in blockage of tumor necrosis factor-activated nuclear
factor-kappaB pathway and sensitization of apoptosis in lung cancer
cells. Mol Pharmacol. 71:1381–1388. 2007. View Article : Google Scholar : PubMed/NCBI
|