Introduction
Human mesenchymal stem cells (hMSC) are
undifferentiated cells capable of self-renewal and proliferation
(1). Under certain conditions hMSC
are able to differentiate into diverse mesenchymal cell types,
e.g., osteocytes, adipocytes and chondrocytes (2). Due to their ability to migrate as
well as the capability to differentiate, hMSC play an important
role in the preservation and re-creation of tissue integrity.
Inflammation and tissue damage are strong chemoattractants for
hMSC, similar to the recruitment of leucocytes into inflamed tissue
sites (3–5). Tissue-specific homing of leukocytes
depends on cytokines, tissue-specific adhesion molecules, and
homing receptors (6). Von
Lüttichau and colleagues demonstrated functional expression of
several chemokine receptors on primary isolated hMSC (7).
Cancer tissue exhibits similar cytokine patterns as
inflamed tissue (8). Hence, there
is a strong tropism of hMSC towards tumors (9). Furthermore, hMSC integrate into the
tumor directly and build parts of the cancer microenvironment
(9,10). Thus, several studies have suggested
that hMSC would be an appropriate biological carrier, for example,
for drug delivery therapy (11,12).
However, interactions between hMSC and cancer are
ambiguous. Some studies show tumor progression and enhancement of
the tumor metastatic potential by hMSC. This progression is induced
by cell-cell contact as well as by the secretion of several
cytokines and growth factors by hMSC such as interleukin-6 (IL-6),
epidermal growth factor (EGF), vascular epidermal growth factor
(VEGF), insulin-like growth factor (IGF)-1 or transforming growth
factor (TGF)-β in a paracrine manner (13–18).
Some of these, especially EGF and IL-6, may play an important role
in tumor progression and metastasis. The activation of
mitogen-activated protein kinase (MAPK) pathways was shown to play
an important role in cytokine-induced tumor progression (19,20).
MAPK pathways link extracellular signals to the machinery that
controls fundamental cellular processes (21). Especially the extracellular
signal-regulated kinase (ERK)1/2 is activated by growth factors,
which in turn is responsible for cell proliferation,
differentiation, apoptosis and migration (21).
In a previous study, we were able to show an
increase in tumor proliferation as well as motility in the presence
of hMSC. Cancer cell lines showed resistance towards paclitaxel
after co-cultivation with hMSC (18,22).
The aim of the present study was to evaluate whether hMSC induce an
activation of the MAPK pathway in vitro. Furthermore, the
effect of IL-6 on cell proliferation was also investigated.
Materials and methods
hMSC isolation and culture
The cells were harvested and prepared from 5
voluntary patients with written informed consent from the
Department of Orthopedics, Koenig-Ludwig-Haus. The study was
approved by the Ethics Committee of the Medical Faculty of the
University of Wuerzburg (12/06). Bone marrow was collected under
aseptic conditions and cells were isolated according to the
protocol described by Lee et al (23). Ficoll density-gradient
centrifugation was used in order to isolate hMSC (30 min, 1,300
rpm, density=1,077 g/ml, Biochrom AG, Berlin, Germany). After
collection of the cells from the interphase, a washing step with
phosphate buffered saline (PBS) (Roche Diagnostics GmbH, Mannheim,
Germany) containing 2% FCS followed. After centrifugation the cell
pellet was resuspended in expansion medium (DMEM-EM), which was
made of Dulbecco’s modified Eagle’s medium (DMEM) (Gibco
Invitrogen, Karlsruhe, Germany) containing 10% FCS (Linaris,
Wertheim-Bettingen, Germany), 1% penicillin and streptomycin
(Sigma-Aldrich, Schnelldorf, Germany). Cells were incubated for 24
h at 37°C and 5% CO2. The medium was changed every other
day. The morphology of hMSC was analyzed by inverted microscopy
(Leica DMI 4000B Inverted Microscope, Leica Microsystems, Wetzlar,
Germany).
Multidifferentiation capacity
Osteogenic differentiation was carried out in a
24-well plate (BD Falcon, Heidelberg, Germany) with
1×104 cells/well until 70% confluence was reached. The
expansion medium DMEM-EM additionally contained 10−7 M
dexamethasone, 10−3 M β-glycerophosphate and
2−4 M ascorbate-2-phosphate (all Sigma-Aldrich). The von
Kossa method was used to show the presence of calcium mineral
components. Adipogenic differentiation medium was made of DMEM-EM
containing 10−7 M dexamethasone and 10−9 g/ml
recombinant human insulin (both Sigma-Aldrich). Staining with Oil
Red O confirmed the presence of intra-cellular lipid droplets.
For chondrogenic differentiation the pellet culture
system was used. The cell pellets were cultured in a defined
chondrogenic differentiation medium (Lonza, Basel, Switzerland)
supplemented with 10−9 g/ml transforming growth
factor-β3 (Sigma-Aldrich). Cells were cultivated for 3 weeks.
Thereafter, the pellets were embedded in Optimal Cutting
Temperature Paraffin (Tissue-Tek® O.C.TTM;
Sakura Finetek, Zoeterwoude, The Netherlands). Cryosections were
stained with Alcian blue to show the presence of
glycosamineglycane.
Expression of cell surface markers
Flow cytometry was used in order to confirm surface
antigen markers. hMSC (1×106) were incubated with
anti-CD105, anti-CD90, anti-CD44 and anti-CD34 (all antibodies were
purchased from BD Bioscience, Heidelberg, Germany). Cell surface
analyses were performed by flow cytometry (FACSCanto™; BD
Bioscience).
HNSCC cell line FaDu and HLaC78
The head and neck squamous carcinoma cell lines FaDu
and HLaC78 were used (24,25). Cells were grown in DMEM-EM and
cultured at 37°C with 5% CO2 in culture flasks. Every
other day the medium was replaced. After reaching 70–80% confluence
cells were trypsinized with 0.25% trypsin (Gibco Invitrogen),
washed with PBS and seeded in new flasks or treatment wells.
Experiments were performed using cells in the exponential growth
phase. FaDu and HLaC78 were incubated with the supernatants of hMSC
(hMSC-sup). The following experiments were carried out in order to
evaluate the effects of cytokines released by hMSC on FaDu and
HLaC78.
Cytokine analysis of hMSC-sup with the
dot blot assay
The dot blot assay (RayBiotech Inc., Norcross, GA,
USA) was used as a semi-quantitative method for determining hMSC
cytokine secretion. After an incubation period of 48 h in DMEM
without supplements, the supernatants were collected and
investigated for the presence of cytokines. The assay was performed
according to the manufacturer’s protocol. The labeled proteins were
observed by enhanced chemiluminescence using detection buffer and
exposure to X-ray film. The cytokines were represented as dots with
different intensity and growth size.
Quantitative analysis of IL-6 by
ELISA
Supernatants of hMSC were collected. IL-6
concentration was measured using the ELISA kit human IL-6 (Disclose
SAS, Besancon Codex, France). All experiments were investigated in
duplicate. The plate was read at 450 nm (Titertek Multiskan PLUS;
Labsystems, Helsinki, Finland). IL-6 concentration (pg/ml) was
ascertained by creating a standard curve using recombinant IL-6.
DMEM without supplements served as the control.
Cell proliferation analysis
Cells (2×104) (FaDu and HLaC78) were
incubated with hMSC-sup at 37°C with 5% CO2 for 4 days,
while electronically counting the cell number each day
(Casy® Technologies, Innovatis AG, Reutlingen, Germany).
DMEM-EM served as the control (n=5).
Simultaneously, the effect of IL-6 on cell
proliferation was evaluated by using the MTT assay. Cells were
seeded at a density of 1×104 in a 96-well rounded bottom
plate. After an incubation period of 24 h in DMEM-EM, hMSC-sup +/−
anti-IL-6 500 ng/ml (R&D Systems, Wiesbaden-Nordenstadt,
Germany) cells were washed with PBS. Then all plates were incubated
with 100 μl of MTT solution (1 mg/ml) followed by 5 h of incubation
at 37°C with 5% CO2. After removal of MTT, 100 μl of
isopropanol was added for 1 h at 37°C with 5% CO2. The
plate was read at a wavelength of 570 nm (Titertek Multiskan)
(n=5).
Total RNA extraction, cDNA synthesis and
real-time PCR
The expression of EGFR in FaDu and HLaC78 were
investigated as follows: According to the manufacturer’s protocol,
the TRIzol method was used in order to extract total RNA from
cells. Next, the extracted total RNA was reverse transcribed to
cDNA. For the quantification of gene expression, the SYBR Green PCR
master mix kit (Applied Biosystems, Foster City, CA, USA) was used.
The EGFR primer was purchased from Applied Biosystems (forward
primer 5′-GCGTTCGGCACGGTGTATAA-3′ and reverse primer
5′-GGCTTTCGGAGATGTTGCTTC-3′). The conditions for gene amplification
were as follows: 50°C for 2 min; 95°C for 10 min, and 40 cycles at
95°C for 15 sec and 60°C for 1 min. As an endogenous control, the
GAPDH gene was used.
Western blotting
Cells (FaDu and HLaC78) were harvested by
trypsinization and dissolved in RIPA buffer (PBS, containing 1%
NP40, 0.5% sodium deoxycholate and 0.1% SDS), supplemented with 10
μg/ml phenylmethanesulfonyl fluoride (PMSF). Protein concentration
was then determined. Equal amounts of total protein lysates were
loaded on a 10% SDS-polyacrylamide gel and transferred by
electroblotting to a polyvinylidene difluoride membrane. Blots were
blocked for 1 h at room temperature with TBST (10 mM Tris, 150 mM
NaCl, 0.05% Tween-20, pH 8.0), containing 5% nonfat dry milk.
Afterwards, the membrane was incubated with primary antibody ERK1/2
(Cell Signaling Technology, Beverly, MA, USA) overnight at 4°C.
Subsequently, the membrane was washed and incubated with a
species-specific IgG secondary antibody for 1 h to visualize the
specific bindings. The protein expression was detected with a
chemiluminescence system (ECL, Amersham Biosciences, Freiburg,
Germany), according to the manufacturer’s protocol.
Statistical analysis
All data were transferred to standard spreadsheets.
Differences between groups were examined for significance by the
Kruskal-Wallis test using GraphPad Prism 6.0 statistics software
(GraphPad Software, Inc., San Diego, CA, USA). Differences were
considered statistically significant when the P-value was
<0.05.
Results
hMSC morphology and differentiation
capability
hMSC exhibited a fibroblast-shaped morphology. They
were able to differentiate into chondrocytes, adipocytes and
osteocytes, which were confirmed by Alcian blue, Oil Red O and van
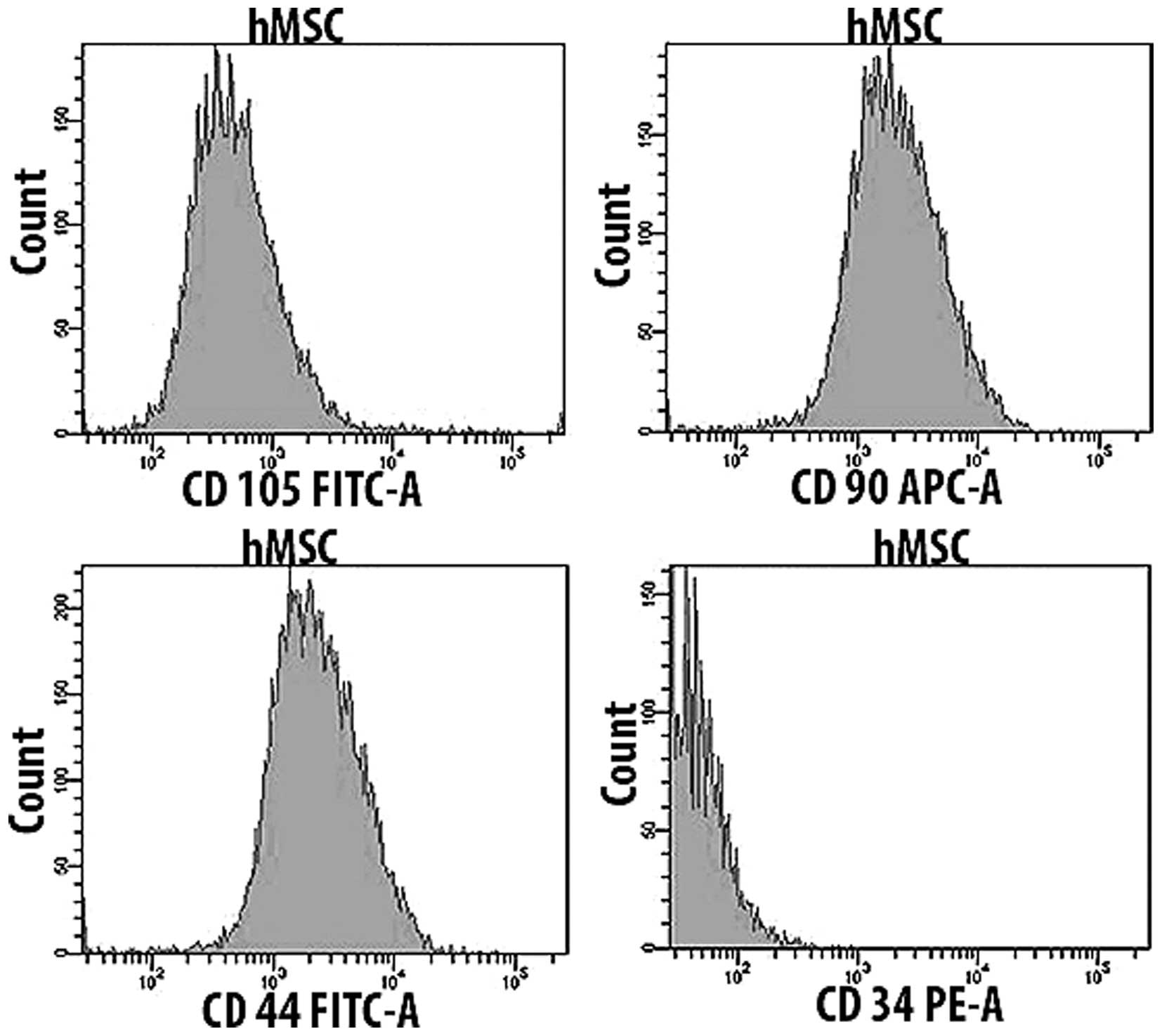
Kossa staining. The flow cytometric analysis revealed the presence
of typical surface markers. hMSC were positive for CD105, CD90 and
CD44, and negative for CD34 (Figs.
1 and 2).
HNSCC cell line morphology before and
after cultivation with hMSC-sup
Morphology of FaDu and HLaC78 was evaluated before
and after cultivation with hMSC-sup for 48 h. Microscopy revealed
no differences in cell morphology between each group for both cell
lines.
Cytokine secretion by hMSC
The dot blot assay was used to evaluate different
cytokines in the hMSC supernatants. hMSC supernatants contained a
variety of cytokines responsible for pro- and anti-inflammation,
chemotaxis, angiogenesis and growth factors. The dots of the
following cytokines had a strong intensity: interleukin (IL)-3;
IL-6, IL-8, IL-10, Growth regulated oncogene (GRO), GRO-α, Monocyte
chemotactic protein (MCP)-1, Macrophage colony-stimulating factor,
Stem cell factor, Tumor necrosis factor (TNF)-α and OncostatinM
(Fig. 3 and Table I).
 | Table ICytokines map. |
Table I
Cytokines map.
| + | + | − | − | ENA-78 | GCSF | GM-CSF | GRO | GRO-α | I-309 | IL-1α | IL-1β |
| + | + | − | − | ENA-78 | GCSF | GM-CSF | GRO | GRO-α | I-309 | IL-1α | IL-1β |
| IL-2 | IL-3 | IL-4 | IL-5 | IL-6 | IL-7 | IL-8 | IL-10 | IL-12 | IL-13 | IL-15 | IFN-γ |
| IL-2 | IL-3 | IL-4 | IL-5 | IL-6 | IL-7 | IL-8 | IL-10 | IL-12 | IL-13 | IL-15 | IFN-γ |
| MCP-1 | MCP-2 | MCP-3 | MCSF | MCD | MIG | MIP-1d | RANTES | SCF | SDF-1TA | RC | TGF-β1 |
| MCP-1 | MCP-2 | MCP-3 | MCSF | MCD | MIG | MIP-1d | RANTES | SCF | SDF-1TA | RC | TGF-β1 |
| TNF-α | TNF-β | EGF | IGF-1 | Angiogenin | OncostatinM | Thrombopoietin | VEGF | PDGF-BB | Leptin | − | + |
| TNF-α | TNF-β | EGF | IGF-1 | Angiogenin | OncostatinM | Thrombopoietin | VEGF | PDGF-BB | Leptin | − | + |
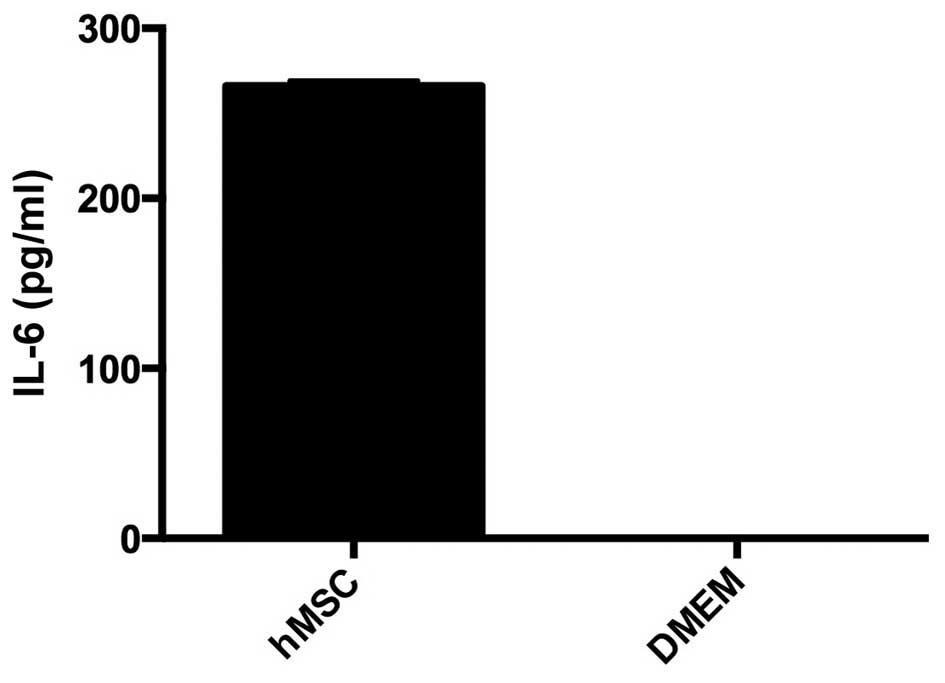
Quantitative analysis of IL-6
The secretion of IL-6 was quantified with the ELISA
method. hMSC in monolayer culture released 260 pg/ml IL-6. DMEM
without supplements as control did not contain IL-6 (Fig. 4).
Effects of hMSC on HNSCC proliferation
+/− anti-IL-6
The cultivation of FaDu and HLaC78 with hMSC-sup
induced a significant enhancement of cell proliferation compared to
cultivation in DMEM-EM. The MTT assay confirmed the results of the
cell counting. FaDu and HLaC78 showed a significant enhancement of
cell proliferation. The impact of IL-6 on cell proliferation was
investigated by the addition of anti-IL-6 into hMSC-sup. Anti-IL-6
induced the inhibition of cell proliferation. FaDu and HLaC78
showed a reduction in cell proliferation compared to cultivation
with hMSC-sup without anti-IL-6 (Figs.
5 and 6).
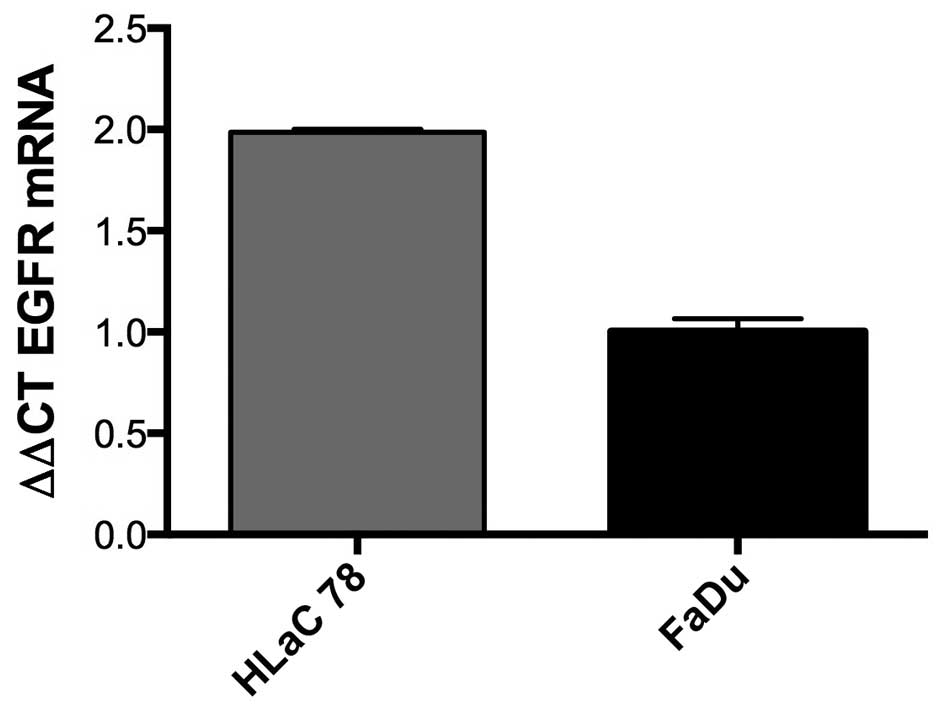
Expression of EGFR in HNSCC
fGFR gene expression was assessed by real-time PCR
in FaDu and HLaC78. Values were expressed using the ΔΔCt method to
derive relative fold change. Both cell lines were positive for EGFR
expression. The EGFR expression was almost 2-fold more in HLaC78
compared to FaDu (Fig. 7).
Activation of ERK1/2
The expression of ERK1/2 and the corresponding
phosphorylated protein in FaDu and HLaC78 was investigated with the
western blotting. Western blot analysis revealed expression of
ERK1/2 as well as its phosphorylated protein after cultivation in
hMSC-sup. The cultivation in DMEM-EM revealed total absence of the
phosphorylated protein (Fig.
8).
Discussion
Solid tumors are embedded into a complex
microenvironment, which includes several types of non-malignant
cells, malignant cells as well as extracellular matrix. On one
hand, cytokines produced by tumor cells alter the gene expression
of stromal cells and their phenotypical properties. On the other
hand, the cancer cells release growth factors, cytokines and
proteases which enhance tumor growth (26,27).
Cancer and stroma interact in an autocrine as well as paracrine
manner. This implies the symbiotic relationship between cancer and
cancer surrounding stroma. Several cytokines responsible for
inflammation and angiogenesis are elevated in the serum of patients
with HNSCC compared to the control group (28). IL-6, a multi-functional regulator
of immune responses and hematopoiesis, influences the proliferation
and invasion potential of head and neck cancer directly (29). IL-6 regulates a complex network of
cytokines responsible for inflammation, growth factors as well as
angiogenic proteins which lead to malignant and invasive tumor
growth (30). In the current
study, the cultivation of the HNSCC cell lines FaDu and HLaC78 with
hMSC-sup resulted in proliferation enhancement of tumor cells. The
dot blot of hMSC-sup revealed the secretion of various cytokines
and growth factors by hMSC. The dots of several cytokines,
especially IL-6, had a strong intensity.
The addition of anti-IL-6 counteracted these
pro-mitotic effects of hMSC-sup. In the current study, a
significant attenuation of HNSCC cell proliferation in the culture
system treated with anti-IL-6 compared to cells treated with
hMSC-sup was detected. This is an indirect marker for the impact of
IL-6 on cancer progression. Furthermore, IL-6 can be responsible
for the development of resistance towards anti-cancer drugs such as
cisplatin (31). This was achieved
by an increased expression of the cellular inhibitor of apoptosis 2
(cIAP-2) via IL-6. The effect was reversible by the addition of
anti-IL-6 and anti-cIAP-2.
In the current study, the proliferation enhancement
of FaDu and HLaC78 was induced via activation of ERK1/2. The Erk1/2
pathway is a critical signal transduction pathway in the
mitogen-activated protein kinase family, and is closely associated
with tumorigenesis and tumor progression (32). Erk1/2 targets different molecules
that are responsible for cell growth, survival, adhesion, motility
and differentiation (32,33). The activation of ERK1/2 via hMSC
seems to be crucial for cell proliferation. IL-6 was shown by Shi
et al to stimulate tumor growth by activation of Ras, Raf,
MEK, and Erk1 and 2 (34). IL-6
induces different intracellular signaling cascades, e.g. JAK-STAT,
MAPK and PI3K (35,36).
In malignant mammary carcinoma the activation of
Erk1/2 has been associated with a poor prognosis (20). Due to their ability to be recruited
by damaged tissues, inflammation and cancer, hMSC have been
discussed as being a very promising vehicle for cancer treatment.
However, many studies in this area do not consider the interactions
between stem cells and cancer. There are studies showing a
tumorigenic effect of hMSC (13,15,18),
as well as reports on cancer inhibition by hMSC (37,38).
Karnoub et al were able to show that hMSC
enhanced the metastatic potential of breast carcinoma cells by
de novo secretion of RANTES, which represents a potent
cytokine responsible for cancer cell motility, invasion and
migration (13). The dot blot
assay revealed the secretion of RANTES by hMSC. In a previous study
by our group, the enhancement of cancer cell motility was
demonstrated (22). Co-injection
of hMSC and breast cancer cells resulted in cancer progression
(15).
One important issue in cancer progression is the
ability of hMSC to integrate into tumor stroma and differentiate
into cancer-associated fibroblasts (CAF) (10,39,40).
Long-term co-culture of hMSC with cancer supernatants resulted in a
differentiation of hMSC into myofibroblasts (41). These CAF (differentiated hMSC) are
able to release cytokines responsible for cancer growth and matrix
remodeling (42). Furthermore, CAF
are able to express proteins involved in lactate absorption,
lactate oxidation and reduced glucose absorption (43). CAF sustain cancer cell survival by
buffering and recycling products of anaerobic metabolism (43). Cancer-associated fibroblasts are
involved in the initiation of cancer invasion (44).
In contrast to studies showing the tumor-supporting
potential of hMSC, there are also studies indicating anti-cancer
effects: As shown by Khakoo, the inhibition of Kaposi sarcoma was
induced via suppression of Akt protein kinase by hMSC.
Qiao et al demonstrated inhibition of human
hepatoma cell lines via the Wnt signaling pathway (37). Zhu et al presented the DKK-1
(dickkopf-1) protein as a negative regulator of the Wnt signaling
pathway (38). In a study
conducted by Qiao et al hMSC inhibited cancer cell growth by
secretion of DKK-1 (45). The
reason for these contradictory findings may be explained by the use
of different stem cell sources as well as different donor ages.
Thus, the use of stem cells as a carrier for cancer therapy must be
critically discussed. A possible solution may be the use of
genetically engineered stem cells with, for example, the absence of
IL-6 secretion. Therefore, a challenging future mission will be to
identify an optimal stem cell with enhanced tumor tropism and
highly anti-tumorigenic potential.
References
|
1
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bianco P, Riminucci M, Gronthos S and
Robey PG: Bone marrow stromal stem cells: Nature, biology, and
potential applications. Stem Cells. 19:180–192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coffelt SB, Marini FC, Watson K, Zwezdaryk
KJ, Dembinski JL, LaMarca HL, Tomchuck SL, Honer zu Bentrup K,
Danka ES, Henkle SL, et al: The pro-inflammatory peptide LL-37
promotes ovarian tumor progression through recruitment of
multipotent mesenchymal stromal cells. Proc Natl Acad Sci USA.
106:3806–3811. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Asahara T, Masuda H, Takahashi T, Kalka C,
Pastore C, Silver M, Kearne M, Magner M and Isner JM: Bone marrow
origin of endothelial progenitor cells responsible for postnatal
vasculogenesis in physiological and pathological
neovascularization. Circ Res. 85:221–228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malek S, Kaplan E, Wang JF, Ke Q, Rana JS,
Chen Y, Rahim BG, Li M, Huang Q, Xiao YF, et al: Successful
implantation of intravenously administered stem cells correlates
with severity of inflammation in murine myocarditis. Pflugers Arch.
452:268–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Campbell JJ and Butcher EC: Chemokines in
tissue-specific and microenvironment-specific lymphocyte homing.
Curr Opin Immunol. 12:336–341. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Von Lüttichau I, Notohamiprodjo M,
Wechselberger A, Peters C, Henger A, Seliger C, Djafarzadeh R, Huss
R and Nelson PJ: Human adult CD34− progenitor cells
functionally express the chemokine receptors CCR1, CCR4, CCR7,
CXCR5, and CCR10 but not CXCR4. Stem Cells Dev. 14:329–336. 2005.
View Article : Google Scholar
|
|
8
|
Dvorak HF: Tumors: Wounds that do not
heal. Similarities between tumor stroma generation and wound
healing. N Engl J Med. 315:1650–1659. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kidd S, Caldwell L, Dietrich M, Samudio I,
Spaeth EL, Watson K, Shi Y, Abbruzzese J, Konopleva M, Andreeff M,
et al: Mesenchymal stromal cells alone or expressing
interferon-beta suppress pancreatic tumors in vivo, an effect
countered by anti-inflammatory treatment. Cytotherapy. 12:615–625.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spaeth EL, Dembinski JL, Sasser AK, Watson
K, Klopp A, Hall B, Andreeff M and Marini F: Mesenchymal stem cell
transition to tumor-associated fibroblasts contributes to
fibrovascular network expansion and tumor progression. PLoS One.
4:e49922009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang Q, Liu XZ, Kang CS, Wang GX, Zhong Y
and Pu PY: The anti-glioma effect of suicide gene therapy using
BMSC expressing HSV/TK combined with overexpression of Cx43 in
glioma cells. Cancer Gene Ther. 17:192–202. 2010. View Article : Google Scholar
|
|
12
|
Cavarretta IT, Altanerova V, Matuskova M,
Kucerova L, Culig Z and Altaner C: Adipose tissue-derived
mesenchymal stem cells expressing prodrug-converting enzyme inhibit
human prostate tumor growth. Mol Ther. 18:223–231. 2010. View Article : Google Scholar :
|
|
13
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin WR, Brittan M and Alison MR: The role
of bone marrow-derived cells in fibrosis. Cells Tissues Organs.
188:178–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rhodes LV, Muir SE, Elliott S, Guillot LM,
Antoon JW, Penfornis P, Tilghman SL, Salvo VA, Fonseca JP, Lacey
MR, et al: Adult human mesenchymal stem cells enhance breast
tumorigenesis and promote hormone independence. Breast Cancer Res
Treat. 121:293–300. 2010. View Article : Google Scholar
|
|
16
|
Kemp KC, Hows J and Donaldson C: Bone
marrow-derived mesenchymal stem cells. Leuk Lymphoma. 46:1531–1544.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Wang M, Abarbanell AM, Weil BR,
Herrmann JL, Tan J, Novotny NM, Coffey AC and Meldrum DR: MEK
mediates the novel cross talk between TNFR2 and TGF-EGFR in
enhancing vascular endothelial growth factor (VEGF) secretion from
human mesenchymal stem cells. Surgery. 146:198–205. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scherzed A, Hackenberg S, Froelich K,
Kessler M, Koehler C, Hagen R, Radeloff A, Friehs G and Kleinsasser
N: BMSC enhance the survival of paclitaxel treated squamous cell
carcinoma cells in vitro. Cancer Biol Ther. 11:349–357. 2011.
View Article : Google Scholar
|
|
19
|
Park JI: Growth arrest signaling of the
Raf/MEK/ERK pathway in cancer. Front Biol (Beijing). 9:95–103.
2014. View Article : Google Scholar
|
|
20
|
Whyte J, Bergin O, Bianchi A, McNally S
and Martin F: Key signalling nodes in mammary gland development and
cancer. Mitogen-activated protein kinase signalling in experimental
models of breast cancer progression and in mammary gland
development. Breast Cancer Res. 11:2092009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scherzed A, Hackenberg S, Radeloff A,
Froelich K, Rak K, Hagen R and Kleinsasser N: Human mesenchymal
stem cells promote cancer motility and cytokine secretion in vitro.
Cells Tissues Organs. 198:327–337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh
K, Bae YC and Jung JS: Characterization and expression analysis of
mesenchymal stem cells from human bone marrow and adipose tissue.
Cell Physiol Biochem. 14:311–324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rangan SR: A new human cell line (FaDu)
from a hypopharyngeal carcinoma. Cancer. 29:117–121. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zenner HP, Lehner W and Herrmann IF:
Establishment of carcinoma cell lines from larynx and submandibular
gland. Arch Otorhinolaryngol. 225:269–277. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Witz IP: Tumor-microenvironment
interactions: Dangerous liaisons. Adv Cancer Res. 100:203–229.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zigrino P, Löffek S and Mauch C:
Tumor-stroma interactions: Their role in the control of tumor cell
invasion. Biochimie. 87:321–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Z, Malhotra PS, Thomas GR, Ondrey FG,
Duffey DC, Smith CW, Enamorado I, Yeh NT, Kroog GS, Rudy S, et al:
Expression of proinflammatory and proangiogenic cytokines in
patients with head and neck cancer. Clin Cancer Res. 5:1369–1379.
1999.PubMed/NCBI
|
|
29
|
Kanazawa T, Nishino H, Hasegawa M, Ohta Y,
Iino Y, Ichimura K and Noda Y: Interleukin-6 directly influences
proliferation and invasion potential of head and neck cancer cells.
Eur Arch Otorhinolaryngol. 264:815–821. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lederle W, Depner S, Schnur S, Obermueller
E, Catone N, Just A, Fusenig NE and Mueller MM: IL-6 promotes
malignant growth of skin SCCs by regulating a network of autocrine
and paracrine cytokines. Int J Cancer. 128:2803–2814. 2011.
View Article : Google Scholar
|
|
31
|
Cohen S, Bruchim I, Graiver D, Evron Z,
Oron-Karni V, Pasmanik-Chor M, Eitan R, Bernheim J, Levavi H,
Fishman A, et al: Platinum-resistance in ovarian cancer cells is
mediated by IL-6 secretion via the increased expression of its
target cIAP-2. J Mol Med Berl. 91:357–368. 2013. View Article : Google Scholar
|
|
32
|
Chang H, Shi Y, Tuokan T, Chen R and Wang
X: Expression of aquaporin 8 and phosphorylation of Erk1/2 in
cervical epithelial carcinogenesis: Correlation with
clinicopathological parameters. Int J Clin Exp Pathol. 7:3928–3937.
2014.PubMed/NCBI
|
|
33
|
Hsu YL, Hou MF, Kuo PL, Huang YF and Tsai
EM: Breast tumor-associated osteoblast-derived CXCL5 increases
cancer progression by ERK/MSK1/Elk-1/snail signaling pathway.
Oncogene. 32:4436–4447. 2013. View Article : Google Scholar
|
|
34
|
Shi Y, Hsu JH, Hu L, Gera J and
Lichtenstein A: Signal pathways involved in activation of p70S6K
and phosphorylation of 4E-BP1 following exposure of multiple
myeloma tumor cells to interleukin-6. J Biol Chem. 277:15712–15720.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Levy DE and Darnell JE Jr: Stats:
Transcriptional control and biological impact. Nat Rev Mol Cell
Biol. 3:651–662. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rose-John S, Scheller J, Elson G and Jones
SA: Interleukin-6 biology is coordinated by membrane-bound and
soluble receptors: Role in inflammation and cancer. J Leukoc Biol.
80:227–236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao
RC, Ye L and Zhang X: Suppression of tumorigenesis by human
mesenchymal stem cells in a hepatoma model. Cell Res. 18:500–507.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian
C, Li J, Yan X, Liu Y, Shao C, et al: Human mesenchymal stem cells
inhibit cancer cell proliferation by secreting DKK-1. Leukemia.
23:925–933. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu R, Wei S, Chen J and Xu S: Mesenchymal
stem cells in lung cancer tumor microenvironment: Their biological
properties, influence on tumor growth and therapeutic implications.
Cancer Lett. 353:145–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Direkze NC, Hodivala-Dilke K, Jeffery R,
Hunt T, Poulsom R, Oukrif D, Alison MR and Wright NA: Bone marrow
contribution to tumor-associated myofibroblasts and fibroblasts.
Cancer Res. 64:8492–8495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mishra PJ, Mishra PJ, Humeniuk R, Medina
DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW and Banerjee D:
Carcinoma-associated fibroblast-like differentiation of human
mesenchymal stem cells. Cancer Res. 68:4331–4339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Silzle T, Kreutz M, Dobler MA, Brockhoff
G, Knuechel R and Kunz-Schughart LA: Tumor-associated fibroblasts
recruit blood monocytes into tumor tissue. Eur J Immunol.
33:1311–1320. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Koukourakis MI, Giatromanolaki A, Harris
AL and Sivridis E: Comparison of metabolic pathways between cancer
cells and stromal cells in colorectal carcinomas: A metabolic
survival role for tumor-associated stroma. Cancer Res. 66:632–637.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
De Wever O and Mareel M: Role of tissue
stroma in cancer cell invasion. J Pathol. 200:429–447. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qiao L, Xu ZL, Zhao TJ, Ye LH and Zhang
XD: Dkk-1 secreted by mesenchymal stem cells inhibits growth of
breast cancer cells via depression of Wnt signalling. Cancer Lett.
269:67–77. 2008. View Article : Google Scholar : PubMed/NCBI
|