Introduction
Pancreatic cancer is known to be the fifth most
frequent cause of cancer-related death in Europe and the United
States (1,2). It is a lethal disease and the 5-year
actual survival rate after potentially curative resection ranges
between 8 and 19% (3–6). Multidisciplinary approach has been
actively pursued to improve the outcome of this disease, but
effective treatment strategy remains to be established.
Plasma is referred to as ‘the fourth state of
matter’ that is subsequent to solid, liquid and gas, and resides in
a high-energy state composed of negative electrons, positive ions,
free radicals, excited molecules and energetic photons (7). Conventionally, plasma has been
generated under high temperature and low pressure; however, owing
to technical developments, non-equilibrium atmospheric pressure
plasma (NEAPP), also known as cold plasma or non-thermal
atmospheric pressure plasma, has actually entered into the realm of
practical use (8).
Recently, NEAPP therapy has attracted attention as
‘the fourth cancer therapy’, which is subsequent to surgery,
chemotherapy and radiotherapy. Previously, the antitumor effects of
plasma have been reported in various cancer cell lines (7,9–12),
and were thought to be associated with generation of reactive
oxygen species (ROS), leading to DNA damage, cell cycle arrest and
finally induction of apoptosis (13,14).
Because selective targeting of tumor cells is one of the most
important aspects of anticancer therapy, some previous studies have
reported on the direct effects of plasma treatment on cancer cells,
although it might result in adverse effects on adjacent
non-cancerous tissues (12,15).
In recent years, however, it has been reported that glioblastoma
brain tumor cells and ovarian and gastric cancer cells could be
selectively induced to undergo apoptosis when treated indirectly
with plasma-activated medium (PAM) (16–18).
In the present study, selective antitumor effects of
the PAM exposure on cell viability of pancreatic cancer cells were
explored. Furthermore, the underlying mechanism whereby indirect
plasma treatment could induce apoptosis was investigated. To the
best of our knowledge, this is the first report to study the
effectiveness of indirect plasma-activated medium exposure on
pancreatic cancer cells.
Materials and methods
Cell lines and culture condition
Pancreatic cancer cell lines (PANC-1, Capan-2,
BxPC-3 and MIA PaCa-2) were obtained from the American Type Culture
Collection (Manassas, VA, USA) and maintained in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS), 100 U/ml of
penicillin and 100 μg/ml of streptomycin (Life Technologies Corp.,
Grand Island, NY, USA). Normal human pancreatic duct epithelial
cells (HPDE6/C7) were kindly provided by Dr Sarah Thayer
(Massachusetts General Hospital, Boston, MA, USA). Cells were grown
in keratinocyte serum-free medium (KSFM) (Life Technologies Corp.)
containing 30 μg/ml bovine pituitary extract (BPE) and 0.2 ng/ml
epidermal growth factor (EGF). All cells were cultured at 37°C in a
humidified atmosphere with 5% CO2.
Experimental system for production of
PAM
The experimental system for production of PAM was
described in a previous report (17). NEAPP with an ultra-high electron
density (~2×1016 cm−3) was provided with an
estimated O density of approximately 4×1015
cm−3 (19, 20). While argon gas was flowing, plasma
in the discharge region was excited by applying 10 kV from a 60-Hz
commercial power supply to two electrodes 8 mm apart (12). The flow rate of the argon gas was
set at two standard liters/min (slm), and the separate distance
between the plasma source and the medium (L) was fixed at L = 15
mm. Six ml of RPMI-1640 medium without 10% FBS was placed in a
60-mm dish and was treated with plasma at several exposure times
(30 sec, 1 min, 2 min, 3 min and 5 min).
Cell proliferation assay
To evaluate the antitumor effects of PAM treatment,
a WST-1 (Takara-Bio, Tokyo, Japan) cell proliferation assay was
performed according to the manufacturer's instructions. Cells were
seeded in a 96-well plate at a density of 1×103,
5×103 and 1×104 cells per 100 μl of culture
medium. On the following day, the culture medium was replaced with
100 μl of PAM. After PAM treatment for 24 h, 10 μl of the WST-1
solution was added to each well and plates were incubated at 37°C
for 90 min. Absorbance at 440 and 630 nm was measured in a
microplate reader. Each experiment was performed using six wells
and repeated independently at least three times.
Cell apoptosis assay
To assess the effects of PAM treatment on cells,
induction of apoptosis, morphological changes and caspase-3/7
activation were examined. Cells were seeded at a density of
5×104 cells/well in a 12-well plate. The following day,
the culture medium was replaced with PAM. Morphological changes
were evaluated every few hours using light microscopy. Cells were
also seeded in an 8-well imaging chamber at a density of
1×104 cells/well in 200 μl of culture medium. The next
day, the culture medium was replaced with 200 μl of PAM or 200 μl
of RPMI-1640 as a control. After 2 h, CellEvent™ caspase-3/7 Green
Detection reagent (Life Technologies Corp.) was added to the wells.
Three hours after PAM treatment, cells were observed under a
Keyence BZ9000 microscope (Osaka, Japan).
Detection of intracellular ROS
generation
Cells were seeded in an 8-well imaging chamber at a
density of 1×104 cells/well in 200 μl of culture medium.
The following day, the culture medium was replaced with 200 μl of
PAM or 200 μl of RPMI-1640 as a control. After 2 h, the medium was
replaced with 10 μM CM-H2DCFDA (Life Technologies
Corp.), and cells were incubated at 37°C in a humidified atmosphere
with 5% CO2. One hour later, cells were observed under a
Keyence BZ9000 microscope (Osaka, Japan).
Cell proliferation assay with ROS
inhibition
To assess the role of ROS generation in cells, the
antitumor effects of PAM on pancreatic cancer cells treated with
N-acetyl cysteine (NAC, Sigma-Aldrich, St. Louis, MO, USA), an
intracellular ROS scavenger, were examined. Cells were seeded in a
96-well plate at a density of 1×103, 5×103
and 1×104 cells/well in 100 μl of culture medium. The
next day, the culture medium was replaced with 100 μl of PAM and 4
mM NAC. Twenty-four hours later, 10 μl of WST-1 solution was added
to each well and the cell proliferation assay was performed.
Animal studies
Six-week-old male nude mice (BALB/C) (N=10) were
obtained from Chubu Kagaku Shizai (Nagoya, Japan). A total of
5×103 Capan2 cells were suspended in 50 μl of RPMI-1640
and 150 μl of Matrigel (BD Biosciences, San Jose, CA, USA), and
subcutaneously injected into the bilateral flank of mice. Then,
mice were divided into a control group and a PAM-treated group.
Mice in the control group received 200 μl of RPMI-1640, whereas the
PAM-treated group received 200 μl of PAM by subcutaneous injection.
In this animal study, PAM was prepared as follows: 4 ml of
RPMI-1640 medium was placed in a 21-mm dish and treated with plasma
for 10 min. The subcutaneous injection was performed thrice-weekly
starting 24 h after cell injection. To evaluate antitumor effects,
the tumor volume was calculated using the formula: π/6 × (largest
diameter) × (smallest diameter)2. At 29 days after cell
injection, the mice were sacrificed and tumors were harvested and
weighed. To assess pathological differences, hematoxylin-eosin
(H&E) staining of sections from paraffin-embedded tumors was
examined. Animal studies were performed in accordance with the
guidelines issued by the Animal Experimental Committee of Nagoya
University, Graduate School of Medicine.
Statistical analysis
All data are presented as means ± SD. Statistical
analysis of the data was performed using a Student's t-test.
p-values <0.05 were considered statistically significant.
Results
Effects of PAM on pancreatic cancer
cells
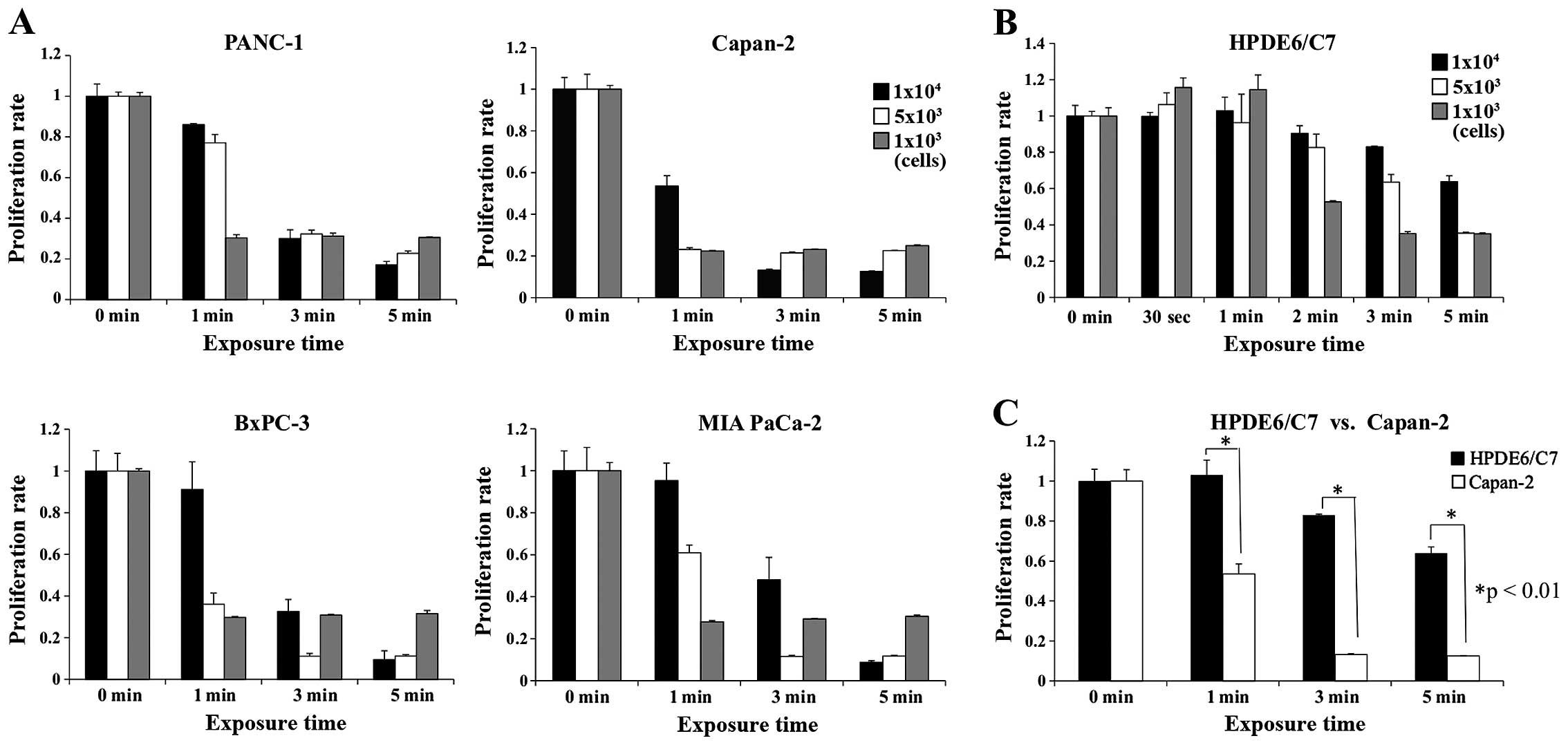
Antitumor effects of PAM treatment on four
pancreatic cancer cell lines, PANC-1, Capan-2, BxPC-3 and MIA
PaCa-2, were examined using a cell proliferation assay. The results
are shown stratified by the length of time culture medium was
exposed to the NEAPP (Fig. 1A).
When the culture medium was treated with NEAPP for 1 min,
1×103 cells of each cell line were effectively killed.
However, when 5×103 or 1×104 cells were
treated similarly, the cell proliferation assay showed differential
sensitivity depending on the cell line. That is, 1×104
Capan-2 cells were decreased by 47%, whereas cells from other cell
lines (PANC-1, BxPC-3 and MIA PaCa-2) were decreased by 4, 9 and
5%, respectively. When the culture medium was treated with plasma
for 3 or 5 min, 5×103 or 1×104 cells from
every cell line were effectively killed by PAM.
Effects of PAM on human pancreatic duct
epithelial cells
Next, to evaluate the effect of PAM on human
pancreatic duct epithelial cells (HPDE6/C7), the cell proliferation
assay was conducted (Fig. 1B).
When the culture medium was treated with NEAPP for ≤1 min, there
was a 15% increase in cell proliferation. When the culture medium
was treated with NEAPP for 5 min, cell proliferation decreased by
65% in wells with 1×103 and 5×103 cells,
whereas it decreased by 37% in wells with 1×104 cells.
When the cell proliferation rate was compared between HPDE6/C7 and
Capan-2 cells, it was significantly higher for HPDE6/C7 than for
Capan-2 cells, indicating low sensitivity of non-cancer cells to
PAM (Fig. 1C).
Apoptosis induced by PAM treatment on
pancreatic cancer cells
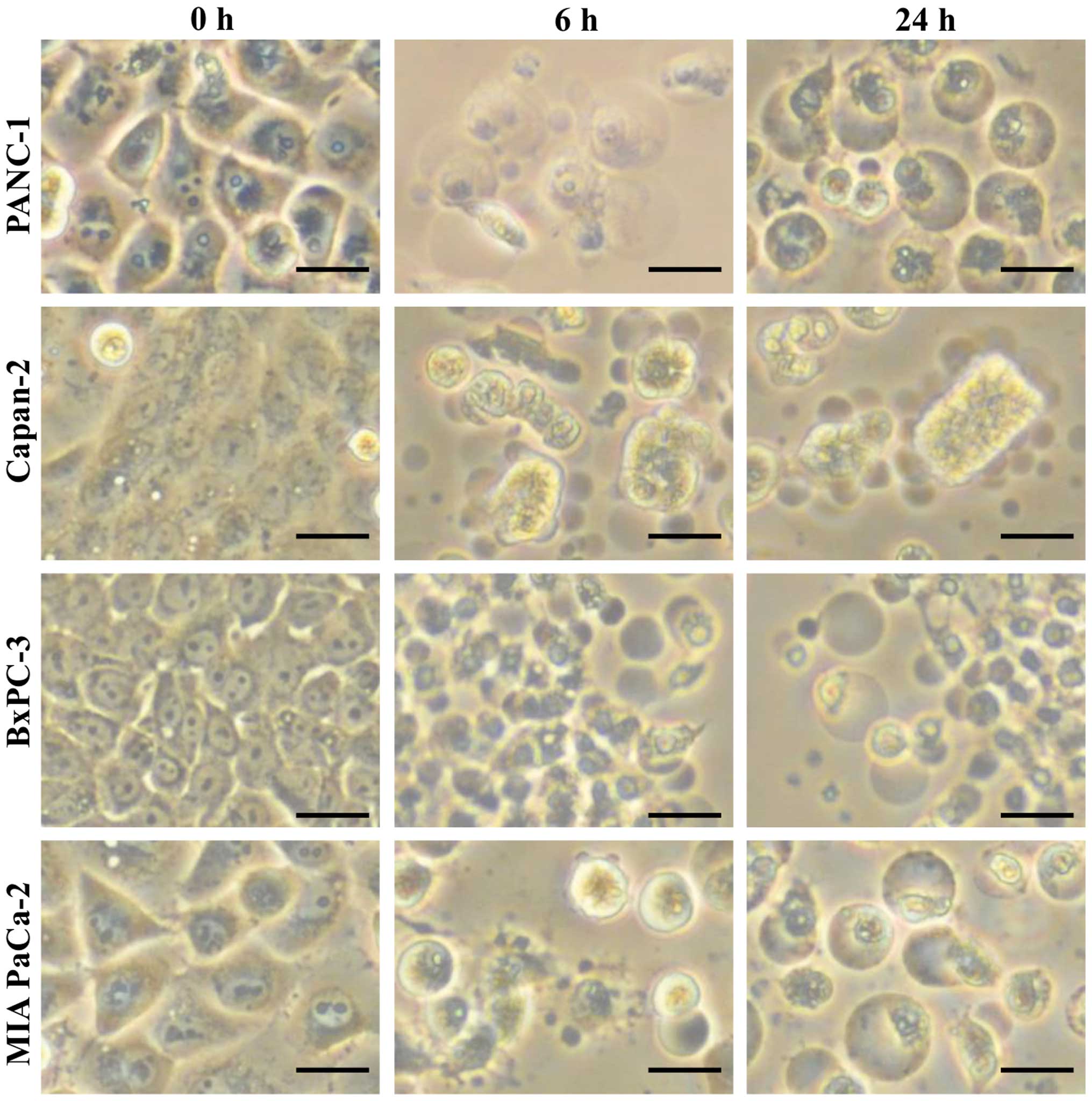
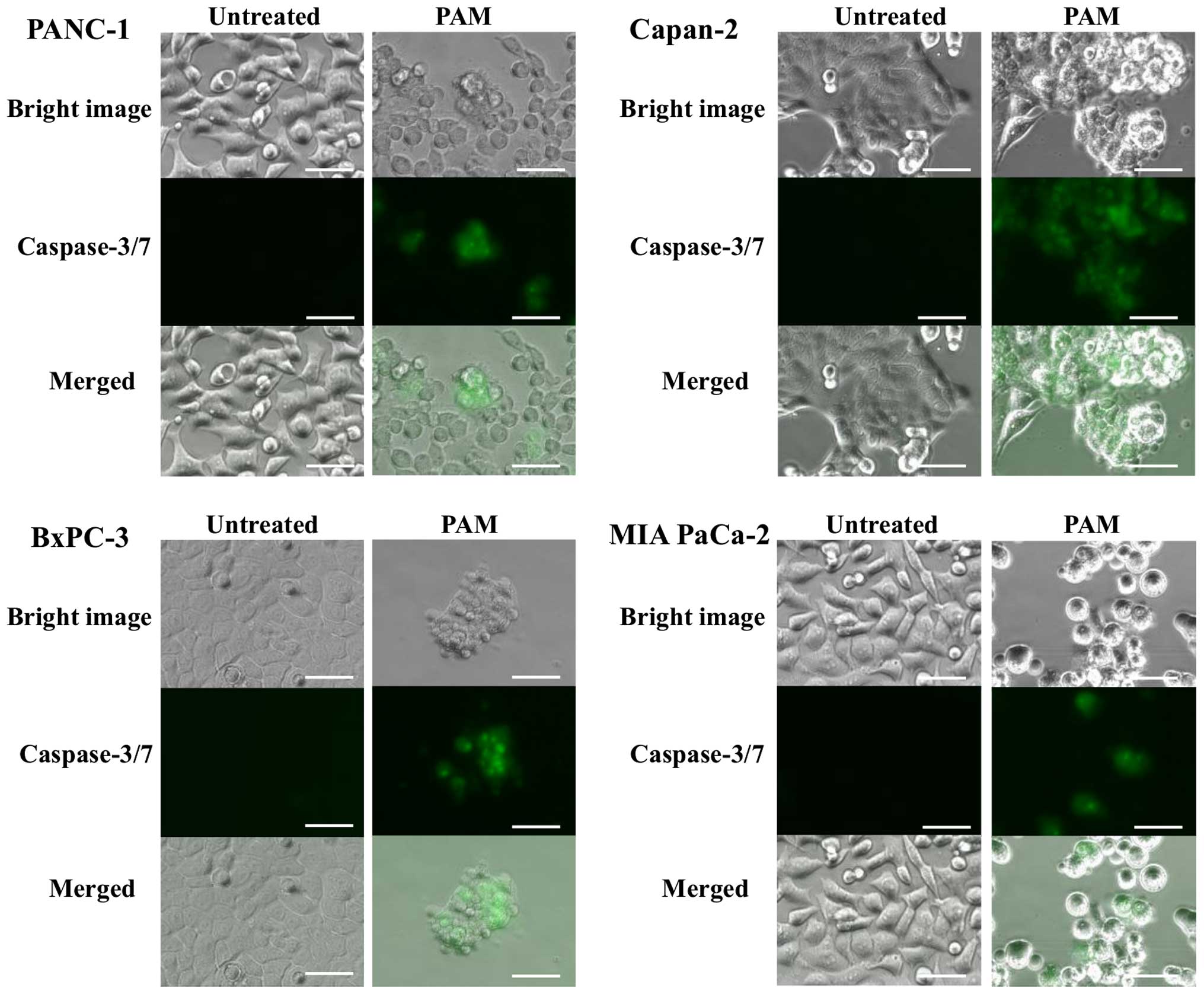
Morphological changes and caspase-3/7 activation in
pancreatic cancer cells by PAM treatment were explored to assess
the induction of apoptosis. Vacuolization of cell membranes and
aggregation of cell nuclei as well as transformation into small and
round cells were observed after 4 h of PAM treatment. Ultimately,
most of the treated cells were deformed and shrunk, and some cells
were detached from the dish after 24 h, indicating typical
morphological changes of apoptosis (Fig. 2). On the other hand, caspase-3/7
activation was detected in accordance with these morphological
changes, as shown in Fig. 3. These
results suggested that the antitumor effects of PAM could be
attributed to cell apoptosis, rather than necrosis.
A mechanism of apoptosis induced by PAM
treatment in pancreatic cancer cells
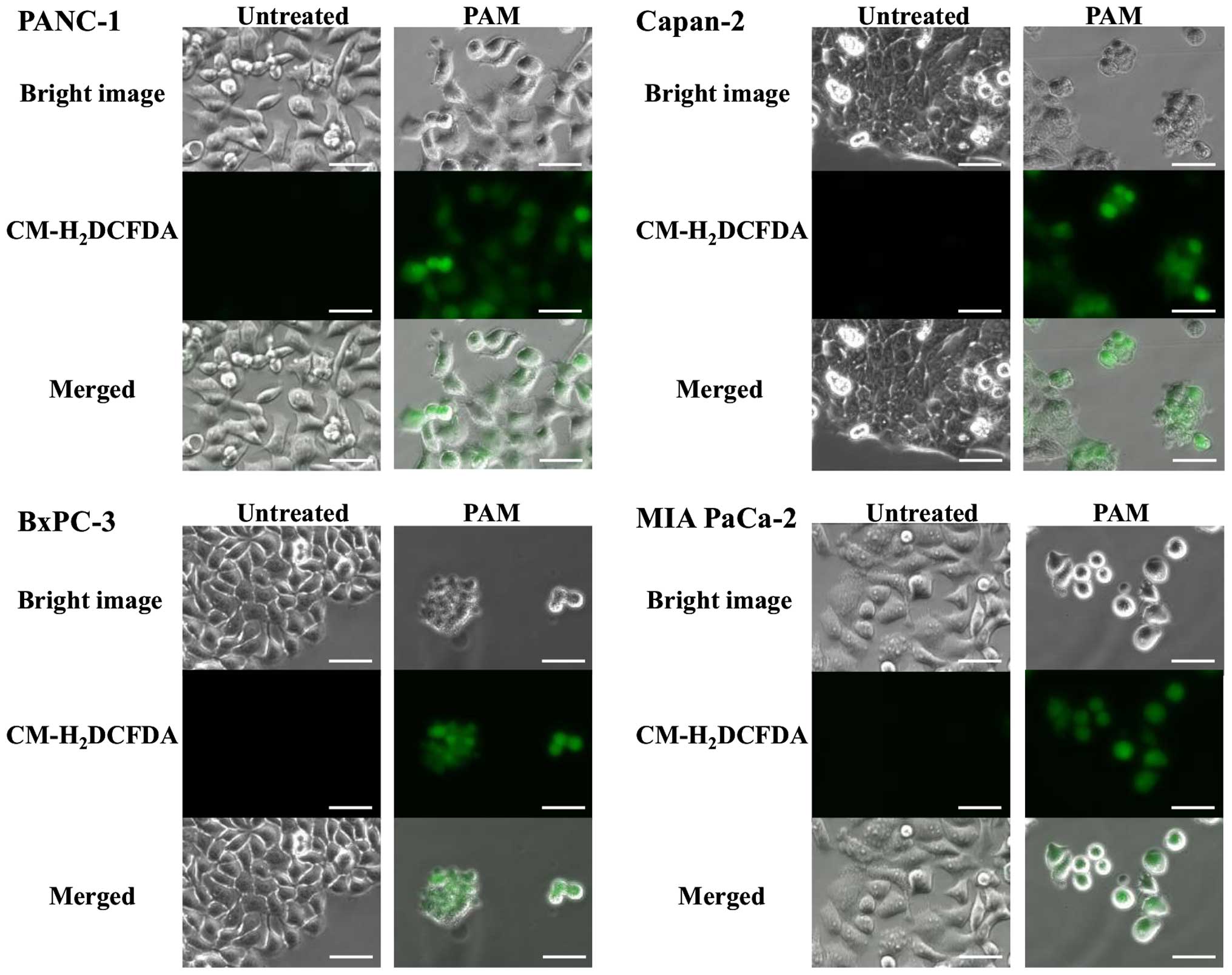
To better understand the underlying mechanism of
apoptosis which occurred after PAM treatment, intracellular ROS
generation in pancreatic cancer cell lines was investigated. After
3 h of PAM treatment, ROS uptake into every cell line was observed
using fluorescence microscopy (Fig.
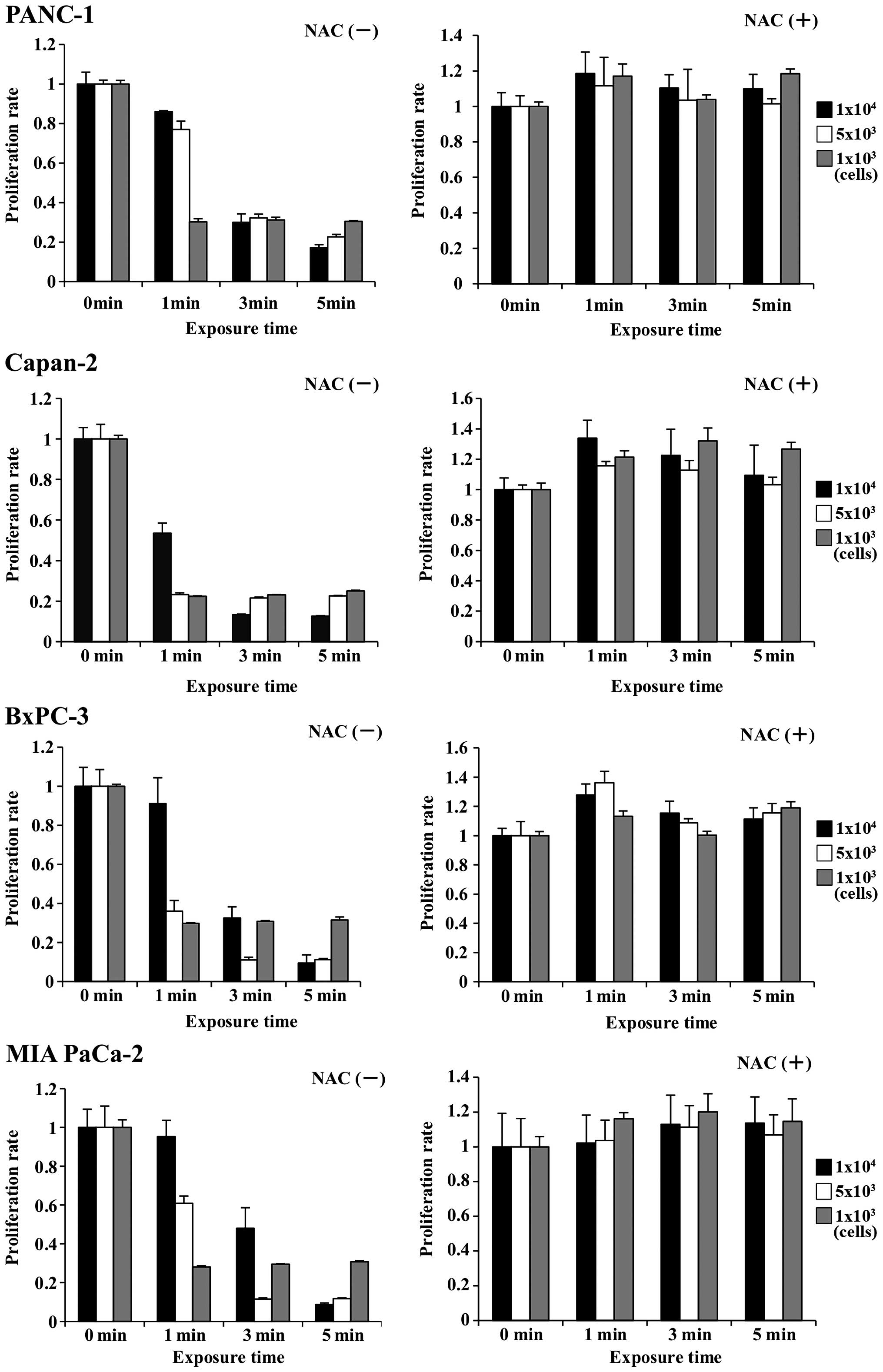
4). To assess the role of ROS generation in cancer cells, the
effect of NAC, an intracellular ROS scavenger, over the antitumor
activity of PAM were examined with the pancreatic cancer cell
lines. The antitumor activity was completely inhibited in every
cell line (Fig. 5), indicating
that apoptosis caused by PAM treatment could be induced by
intracellular ROS generation.
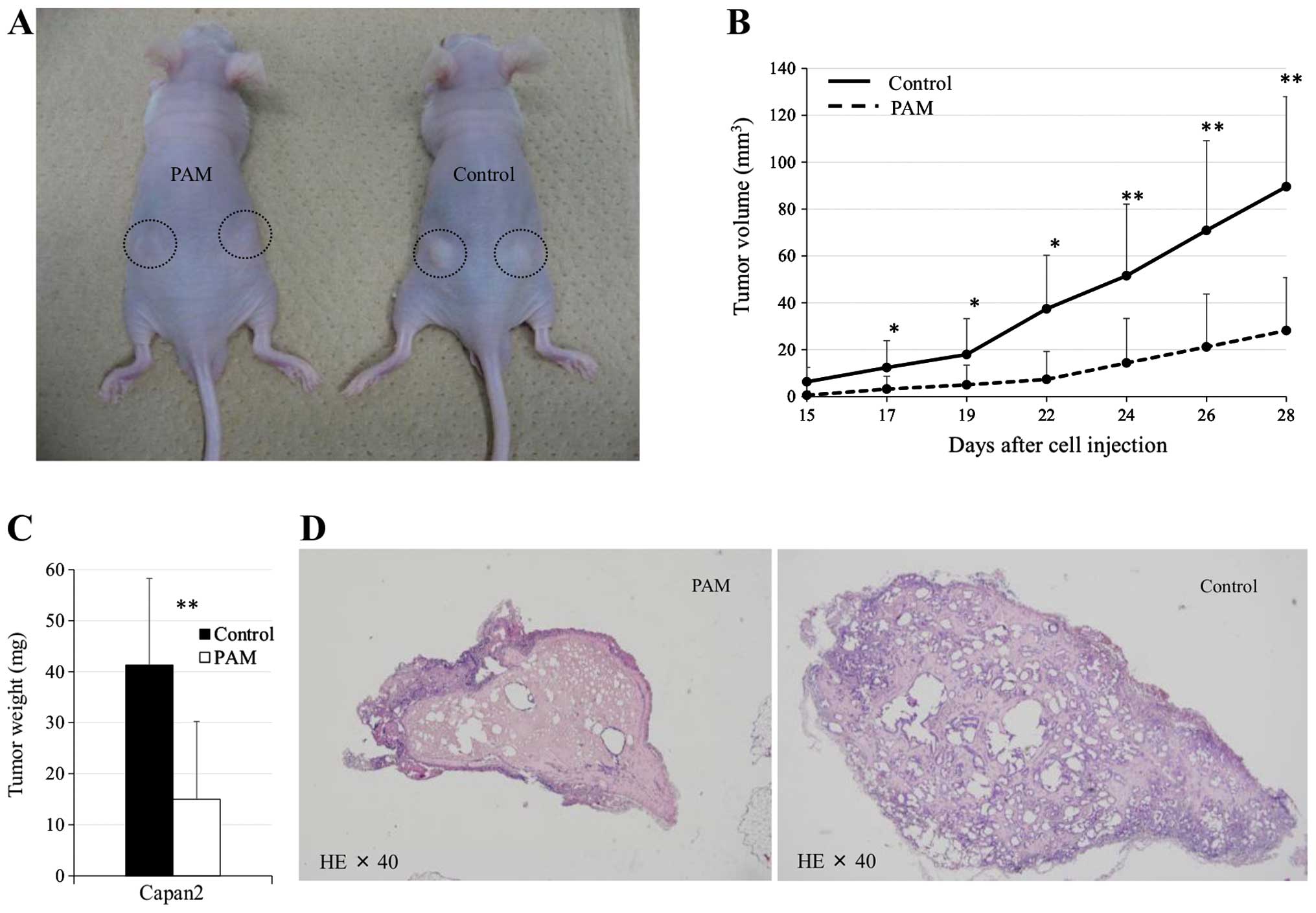
Antitumor effect of PAM on Capan2 tumor
xenografts in mice
The antitumor effect of PAM treatment was
investigated in a mouse xenograft model in which Capan2 cells were
injected subcutaneously. Tumor formation was observed as early as
day 10 post-cell injection in the control group, whereas it was not
observed before day 14 in the PAM-treated group. The calculated
tumor volume on day 28 in the PAM-treated group was significantly
reduced compared with the control group [28±22 vs. 89±38
(mm3 ± SD), p=0.0031]. The tumor weight on day 29 was
significantly less in the PAM-treated group and amounted to 64% of
the control group (p=0.0018). Histological analysis of tumors from
each group showed that ~60% of tumor cells were degenerated in the
PAM-treated group. No apparent adverse effects were observed in
this animal study (Fig. 6).
Discussion
Pancreatic cancer has the worst prognosis of all
gastrointestinal malignancies, and the difficulty of diagnosing the
disease in its early stage results in 70–80% of patients being
deemed unresectable, either because the disease is locally advanced
or accompanied with distant metastasis (21,22).
Peritoneal dissemination is one of common pathway for metastasis in
advanced pancreatic cancer. Although various effective anticancer
drugs have been introduced into the clinical management of
pancreatic cancer, the efficacy of these systemic chemotherapies
remains elusive in the treatment of peritoneal metastasis.
Recently, intraperitoneal administration of cytotoxic agents has
been attempted in the treatment of peritoneal dissemination
(23,24), but its clinical impact remains to
be evaluated.
Some previous reports have demonstrated that plasma
could exert anti-proliferative effects on various cancer cells by
inducing apoptosis (10,25,26).
Apoptosis is well known as programmed cell death that removes
damaged cells; therefore, it serves as a crucial mechanism to
defend tissues and organs from various types of stress and cell
damage (27). Induction of
apoptosis in cancer cells is beneficial compared with that of
necrosis, because apoptosis does not cause inflammatory response
that could influence adjacent normal cells as in the case of
necrosis. In this study, PAM treatment was found to have
anti-proliferative effects on pancreatic cancer cell lines through
induction of apoptosis, as has been proved by typical morphological
changes and caspase-3/7 activation.
Recent studies indicate that NEAPP can generate ROS,
such as superoxide radicals (O2−), hydrogen
peroxide (H2O2), hydroxyl radicals (OH) and
nitric oxide (NO), inducing apoptosis to the target cells (14,28,29).
In the present study, we found that ROS uptake was observed in all
cell lines treated with PAM while the anti-proliferative effect of
PAM was completely inhibited with NAC. NAC has been widely used as
an antioxidant and directly scavenges hydroxyl radicals (OH),
hydrogen peroxide (H2O2) and hypochlorous
acid (HClO) but not superoxide radicals (O2−)
(30). Hence, the antitumor
effects on pancreatic cancer cells might be caused by at least one
of these ROS. More recently, Ninomiya et al demonstrated
that NEAPP jets cause OH radical generation both in the liquid
phase (extracellular culture medium) and within cancer cells, and
consequently induce apoptotic cell death in breast cancer cell
lines (31). Therefore, direct
effect of NEAPP irradiation will also have to be explored in future
through measurements of extracellular and intracellular ROS.
Some previous reports have demonstrated that there
is a relationship between the level of ROS generation and the cell
proliferation rate is complex (32,33).
Quite paradoxically, lower levels of ROS have been shown by some
researchers to enhance rather than inhibit mitosis and cell
proliferation (34,35). In the present study, cell
proliferation was actually increased by 15% in normal cells when
exposed to plasma for a shorter period of time. The mechanism that
induces the contradictory responses to the medium with short
exposure to the NEAPP, cytotoxicity in pancreatic cancer cells and
proliferation in normal cells, remains unclear.
The most problematic aspect of conventional cancer
therapies, such as chemotherapy and radiotherapy, is unwanted
adverse events caused by damage to the normal cells. However,
previous reports looking at various plasma treatments documented
their selective cytotoxicity to the tumor cells while leaving
normal cells intact in ovarian cancer, glioblastoma and lung cancer
(12,14,16).
Our results also showed that HPDE6/C7 cells were more tolerant to
the PAM treatment than pancreatic cancer cells, although even
HPDE6/C7 cells were killed when exposed to the PAM generated by
long exposure to the NEAPP. In this regard, Barrera et al
showed that normal cells were more tolerant to exogenous ROS
stress, owing to their antioxidant reserve compared with cancer
cells (36). The selective
cytotoxicity of PAM treatment on cancer cells might offer a
promising alternative approach in addition to the conventional
anticancer therapies.
On the basis of our in vitro study, we also
performed an in vivo animal study. We started PAM treatment
24 h post-cell injection, and confirmed the effect of PAM on
pancreatic cancer cells while they remain relatively small in
number, mimicking micrometastasis. In a previous report, PAM
inhibited the tumor growth of ovarian cancer cells in vivo
(17); however, tumor growth was not completely inhibited by
PAM. One reason for this result may have been that various ROS
scavengers in the living organism neutralized some ROS generated by
the plasma. In future experiments, we hope to use the PAM, both
alone and in combination with cytotoxic agents or ROS scavengers,
to treat peritoneal metastasis of in vivo model.
In conclusion, we demonstrated that PAM treatment,
which induced apoptosis through intracellular ROS generation, had
antitumor effects on pancreatic cancer cell lines. Furthermore, the
cytotoxic effects were selective to cancer cells at optimal
experimental conditions, showing potential of the PAM treatment as
a novel mode of treatment for pancreatic cancer.
References
|
1
|
Malvezzi M, Bertuccio P, Levi F, La
Vecchia C and Negri E: European cancer mortality predictions for
the year 2012. Ann Oncol. 23:1044–1052. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Conlon KC, Klimstra DS and Brennan MF:
Long-term survival after curative resection for pancreatic ductal
adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann
Surg. 223:273–279. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schnelldorfer T, Ware AL, Sarr MG, Smyrk
TC, Zhang L, Qin R, Gullerud RE, Donohue JH, Nagorney DM and
Farnell MB: Long-term survival after pancreatoduodenectomy for
pancreatic adenocarcinoma: Is cure possible? Ann Surg. 247:456–462.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferrone CR, Pieretti-Vanmarcke R, Bloom
JP, Zheng H, Szymonifka J, Wargo JA, Thayer SP, Lauwers GY,
Deshpande V, Mino-Kenudson M, et al: Pancreatic ductal
adenocarcinoma: Long-term survival does not equal cure. Surgery.
152(Suppl 1): S43–S49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Winter JM, Brennan MF, Tang LH, D'Angelica
MI, Dematteo RP, Fong Y, Klimstra DS, Jarnagin WR and Allen PJ:
Survival after resection of pancreatic adenocarcinoma: Results from
a single institution over three decades. Ann Surg Oncol.
19:169–175. 2012. View Article : Google Scholar
|
|
7
|
Vandamme M, Robert E, Pesnel S, Barbosa E,
Dozias S, Sobilo J, Lerondel S, Le Pape A and Pouvesle J-M:
Antitumor effect of plasma treatment on U87 glioma xnografts:
Preliminary results. Plasma Process Polym. 7:264–273. 2010.
View Article : Google Scholar
|
|
8
|
Yamazaki H, Ohshima T, Tsubota Y,
Yamaguchi H, Jayawardena JA and Nishimura Y: Microbicidal
activities of low frequency atmospheric pressure plasma jets on
oral pathogens. Dent Mater J. 30:384–391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stoffels E, Kieft IE and Sladek REJ:
Superficial treatment of mammalian cells using plasma needle. J
Phys D Appl Phys. 36:29082003. View Article : Google Scholar
|
|
10
|
Fridman G, Shereshevsky A, Jost MM, Brooks
AD, Fridman A, Gutsol A, Vasilets V and Friedman G: Floating
electrode dielectric barrier discharge plasma in air promoting
apoptotic behavior in melanoma skin cancer cell lines. Plasma Chem
Plasma Process. 27:163–176. 2007. View Article : Google Scholar
|
|
11
|
Kim CH, Bahn JH, Lee SH, Kim GY, Jun SI,
Lee K and Baek SJ: Induction of cell growth arrest by atmospheric
non-thermal plasma in colorectal cancer cells. J Biotechnol.
150:530–538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iseki S, Nakamura K, Hayashi M, Tanaka H,
Kondo H, Kajiyama H, Kano H, Kikkawa F and Hori M: Selective
killing of ovarian cancer cells through induction of apoptosis by
nonequilibrium atmospheric pressure plasma. Appl Phys Lett.
100:1137022012. View Article : Google Scholar
|
|
13
|
Georgescu N and Lupu AR: Tumoral and
normal cells treatment with high-voltage pulsed cold atmospheric
plasma jets. Plasma Science IEEE Trans. 38:1949–1955. 2010.
View Article : Google Scholar
|
|
14
|
Keidar M, Walk R, Shashurin A, Srinivasan
P, Sandler A, Dasgupta S, Ravi R, Guerrero-Preston R and Trink B:
Cold plasma selectivity and the possibility of a paradigm shift in
cancer therapy. Br J Cancer. 105:1295–1301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brullé L, Vandamme M, Riès D, Martel E,
Robert E, Lerondel S, Trichet V, Richard S, Pouvesle JM and Le Pape
A: Effects of a non thermal plasma treatment alone or in
combination with gemcitabine in a MIA PaCa2-luc orthotopic
pancreatic carcinoma model. PLoS One. 7:e526532012. View Article : Google Scholar
|
|
16
|
Tanaka H, Mizuno M, Ishikawa K, Nakamura
K, Kajiyama H, Kano H, Kikkawa F and Hori M: Plasma-activated
medium selectively kills glioblastoma brain tumor cells by
down-regulating a survival signaling molecule, AKT kinase. Plasma
Med. 1:265–277. 2011. View Article : Google Scholar
|
|
17
|
Utsumi F, Kajiyama H, Nakamura K, Tanaka
H, Mizuno M, Ishikawa K, Kondo H, Kano H, Hori M and Kikkawa F:
Effect of indirect nonequilibrium atmospheric pressure plasma on
anti-proliferative activity against chronic chemo-resistant ovarian
cancer cells in vitro and in vivo. PLoS One. 8:e815762013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Torii K, Yamada S, Nakamura K, Tanaka H,
Kajiyama H, Tanahashi K, Iwata N, Kanda M, Kobayashi D, Tanaka C,
et al: Effectiveness of plasma treatment on gastric cancer cells.
Gastric Cancer. 18:635–643. 2015. View Article : Google Scholar
|
|
19
|
Iwasaki M, Inui H, Matsudaira Y, Kano H,
Yoshida N, Ito M and Hori M: Nonequilibrium atmospheric pressure
plasma with ultrahigh electron density and high performance for
glass surface cleaning. Appl Phys Lett. 92:p0815032008. View Article : Google Scholar
|
|
20
|
Jia F, Sumi N, Ishikawa K, Kano H, Inui H,
Kularatne J, Takeda K, Kondo H, Sekine M, Kono A, et al: Laser
scattering diagnosis of a 60-Hz non-equilibrium atmospheric
pressure plasma jet. Appl Phys Express. 4:0261012011. View Article : Google Scholar
|
|
21
|
Wray CJ, Ahmad SA, Matthews JB and Lowy
AM: Surgery for pancreatic cancer: Recent controversies and current
practice. Gastroenterology. 128:1626–1641. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furuse J, Ishii H and Okusaka T: The
Hepatobiliary and Pancreatic Oncology (HBPO) Group of the Japan
Clinical Oncology Group (JCOG): History and future direction. Jpn J
Clin Oncol. 43:2–7. 2013. View Article : Google Scholar
|
|
23
|
Ishigami H, Kitayama J, Kaisaki S,
Hidemura A, Kato M, Otani K, Kamei T, Soma D, Miyato H, Yamashita
H, et al: Phase II study of weekly intravenous and intraperitoneal
paclitaxel combined with S-1 for advanced gastric cancer with
peritoneal metastasis. Ann Oncol. 21:67–70. 2010. View Article : Google Scholar
|
|
24
|
Takahara N, Isayama H, Nakai Y, Sasaki T,
Ishigami H, Yamashita H, Yamaguchi H, Hamada T, Uchino R, Mizuno S,
et al: Intravenous and intraperitoneal paclitaxel with S-1 for
refractory pancreatic cancer with malignant ascites: An interim
analysis. J Gastrointest Cancer. 45:307–311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim G, Kim W, Kim K and Lee J: DNA damage
and mitochondria dysfunction in cell apoptosis induced by
nonthermal air plasma. Appl Phys Lett. 96:0215022010. View Article : Google Scholar
|
|
26
|
Ma Y, Ha CS, Hwang SW, Lee HJ, Kim GC, Lee
KW and Song K: Non-thermal atmospheric pressure plasma
preferentially induces apoptosis in p53-mutated cancer cells by
activating ROS stress-response pathways. PLoS One. 9:e919472014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kalghatgi S, Kelly CM, Cerchar E, Torabi
B, Alekseev O, Fridman A, Friedman G and Azizkhan-Clifford J:
Effects of non-thermal plasma on mammalian cells. PLoS One.
6:e162702011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sensenig R, Kalghatgi S, Cerchar E,
Fridman G, Shereshevsky A, Torabi B, Arjunan KP, Podolsky E,
Fridman A, Friedman G, et al: Non-thermal plasma induces apoptosis
in melanoma cells via production of intracellular reactive oxygen
species. Ann Biomed Eng. 39:674–687. 2011. View Article : Google Scholar
|
|
30
|
Aruoma O: Free radicals, oxidative stress,
and antioxidants in human health and disease. J Am Oil Chem Soc.
75:199–212. 1998. View Article : Google Scholar
|
|
31
|
Ninomiya K, Ishijima T, Imamura M,
Yamahara T, Enomoto H, Takahashi K, Tanaka Y, Uesugi Y and Shimizu
N: Evaluation of extra- and intracellular OH radical generation,
cancer cell injury, and apoptosis induced by a non-thermal
atmospheric-pressure plasma jet. J Phys D Appl Phys. 46:4254012013.
View Article : Google Scholar
|
|
32
|
Nicco C, Laurent A, Chereau C, Weill B and
Batteux F: Differential modulation of normal and tumor cell
proliferation by reactive oxygen species. Biomed Pharmacother.
59:169–174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishikawa M: Reactive oxygen species in
tumor metastasis. Cancer Lett. 266:53–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arjunan KP, Friedman G, Fridman A and
Clyne AM: Non-thermal dielectric barrier discharge plasma induces
angiogenesis through reactive oxygen species. J R Soc Interface.
9:147–157. 2012. View Article : Google Scholar
|
|
35
|
Kalghatgi S, Friedman G, Fridman A and
Clyne AM: Endothelial cell proliferation is enhanced by low dose
non-thermal plasma through fibroblast growth factor-2 release. Ann
Biomed Eng. 38:748–757. 2010. View Article : Google Scholar
|
|
36
|
Barrera G: Oxidative stress and lipid
peroxidation products in cancer progression and therapy. ISRN
Oncol. 2012:1372892012.PubMed/NCBI
|