Introduction
Ovarian cancer is the second most common malignant
tumor in gynecology and is the fifth leading cause of
cancer-related mortality in women (1). Since early ovarian cancer is often
asymptomatic, more than half of patients are initially diagnosed at
advanced disease stages with peritoneal dissemination and ascites
(2). The standard therapy for
advanced ovarian cancer is a combination of cytoreductive surgery
and platinum/taxane-based chemotherapy. Ovarian cancer is
relatively sensitive to chemotherapy and many patients achieve
remission with this combinatorial therapy (3,4).
However, the benefit is temporary, and more than half of these
patients develop recurrence and die of the disease. Thus, there are
limitations to current treatment options, necessitating the
development of novel treatment strategies.
Angiogenesis is a phenomenon in which new blood
vessels develop from the existing vasculature. Normal angiogenesis
is a vital reaction observed only in strictly controlled
situations, such as organogenesis during fetal development, the
endometrial proliferative stage, and the wound healing process.
However, pathological angiogenesis is closely associated with the
progression of proliferative diseases (such as diabetic retinopathy
and rheumatoid arthritis) and solid tumors. In 1971, Folkman
proposed the theory that the growth of tumor cells depends on
angiogenesis and that inhibition of angiogenesis is a useful
antitumor strategy (5).
Subsequently, many studies have been published describing the
regulatory mechanisms of tumor angiogenesis and related
processes.
Angiogenesis is regulated by a balance between
angiogenic growth factors, such as VEGF (6) and FGF (7), and angiogenesis inhibitors, such as
angiostatin (8), endostatin
(9), pigment epithelium-derived
factor (10), 16 kDa prolactin
(11), and thrombospondin-1
(12). In 2004, the novel
angiogenesis inhibitor vasohibin-1 (VASH1) produced by human
umbilical vein endothelial cells (HUVEC) stimulated by VEGF and FGF
was identified (13). VASH1 is
expressed in VEGF-stimulated vascular endothelial cells and acts on
vascular endothelial cells themselves via a negative feedback
mechanism that regulates angiogenesis (13,14).
Mouse corneal assays have also confirmed that VASH1 has inhibitory
effects on pathological angiogenesis caused by several angiogenic
factors, including VEGF, FGF, and PDGF (13,15),
suggesting that the targets of the anti-angiogenic action are
wide-ranging. VASH1 lacks a classical secretory signal and its
secretion mechanism was previously unknown. However, it was
recently discovered that VASH1 binds small vasohibin-binding
protein (SVBP), thereby enabling its secretion (16). VASH1 has been associated with
diseases involving abnormal angiogenesis, including malignant
tumors (17–21), atherosclerosis (22), diabetic retinopathy (23), diabetic nephropathy (24,25),
and age-related macular degeneration (26,27).
It has also been shown that VASH1 inhibits lymphangiogenesis and
lymph node metastasis as well as angiogenesis (15). VASH1 could be effective for the
treatment of various diseases, including malignant tumors. Animal
experiments using murine lung cancer cell lines have demonstrated
the anti-angiogenic and antitumor activity of VASH1 (13). In this study, we investigated the
previously unreported antitumor effect of VASH1 on ovarian cancer
in an experimental animal model of ovarian cancer.
Materials and methods
Cell lines and culture
The human ovarian cancer cell line SKOV-3 (28) was purchased from the American Type
Culture Collection (Manassas, VA, USA). The SKOV-3 cell line, which
has previously been reported to produce VEGF (29,30),
was cultured in Dulbecco's modified Eagle's medium/F12 (DMEM/F12)
medium (Life Technologies, Carlsbad, CA, USA) containing 10% fetal
calf serum (Sigma-Aldrich, St. Louis, MO, USA) and 1%
penicillin/streptomycin (Life Technologies). HUVECs were obtained
from Kurabo Industries, Ltd. (Osaka, Japan) and cultured in a
10-cm, type 1 collagen-coated culture dish (Asahi Glass Co., Ltd.,
Tokyo, Japan) containing EBM-2 medium (Lonza, Walkersville, MD,
USA) supplemented with EGM-2-MV-SingleQuots (Lonza), VEGF, FGF,
insulin-like growth factor-1, epidermal growth factor, and 2% fetal
calf serum. All cells were cultured at 37°C in a 5% carbon dioxide
atmosphere.
Establishment of a VASH1-expressing cell
line
Human VASH1 cDNA (NCBI database; Gene ID 22846) was
inserted into the cloning site (SmaI, XbaI) of
pCMV-IRES-bsr (31) to generate a
VASH1 expression vector. The VASH1 expression vector or the control
plasmid vector expressing luciferase [pCMV-LUC-IRES-bsr (31)] was transfected into SKOV-3 cells
using Lipofectamine LTX Plus reagent (Invitrogen, Carlsbad, CA,
USA) according to the manufacturer's instructions. Subsequently,
the transfected cells were selectively cultured in medium
containing blasticidin S HCl (Funakoshi Co., Ltd., Tokyo, Japan)
and blasticidin-resistant cells were obtained. These cells were
cloned by limiting dilution and VASH1-expressing and control clones
were established.
Quantitative reverse
transcriptase-polymerase chain reaction (RT-PCR)
Extraction of mRNA from cultured cells was performed
using an RNeasy Mini kit (Qiagen, Valencia, CA, USA) according to
the manufacturer's instructions. The mRNA was subjected to
quantitative RT-PCR using a One Step SYBR PrimeScript PLUS RT-PCR
kit (Perfect Real-Time, Takara, Otsu, Japan) and Thermal Cycler
Dice Real Time System II (Takara) according to the manufacturer's
protocol. The amount of each mRNA was expressed as a ratio of the
glyceraldehyde 3-phosphate dehydrogenase (GAPDH)-corrected
fluorescent signal intensity. The primer sequences were as follows:
GAPDH forward, ACCACAGTCCATGCCATCAC; reverse, GGGCCTCTTTGGTCATTTCC;
VASH1 forward, AGATCCCCATACCGAGTGTG; reverse,
GGGCCTCTTTGGTCATTTCC.
In vitro cell growth
A total of 1×103 cancer cells were seeded
in each well of a 96-well plate and an XTT assay kit (Roche
Diagnostics, Mannheim, Germany) was used every 24 h. The absorbance
was measured 24 h later at 490 nm, and cell growth curves were
generated.
Endothelial cell growth in vitro
A total of 1×106 cancer cells were seeded
in each well of a 6-well plate and cultured in EBM-2 medium
containing no growth factors. The culture supernatant was collected
24 h later. A total of 2×103 HUVEC were seeded in each
well of a 96-well plate and cultured using the culture supernatant.
An XTT assay kit was used after 48 h and the absorbance was
measured 24 h later at 490 nm.
Western blotting
Cells were lysed using a lysis buffer (1% NP-40, 150
mM NaCl, and 50 mM Tris-HCl, pH 8.0), and proteins were extracted.
These samples were mixed with an SDS sample buffer [10 mM Tris-HCl
(pH 7.5), 150 mM NaCl, 1% SDS, and EDTA-free proteinase inhibitor
cocktail (Roche)]; the mixtures were electrophoresed on 10%
polyacrylamide gels, followed by transfer to polyvinylidene
fluoride membranes (Merck Millipore, Ltd., Billerica, MA, USA). The
membranes were incubated at room temperature for 1 h in Tris buffer
(pH 7.6) containing 5% skimmed milk (Wako Pure Chemical Industries,
Ltd., Tokyo, Japan), and were incubated at 4°C overnight with 1
μg/ml of mouse monoclonal anti-VASH1 (13) or rabbit anti-actin antibody
(Sigma-Aldrich). After washing three times with phosphate-buffered
saline-Tween-20 (PBS-T), the membranes were incubated with
peroxidase-conjugated anti-mouse or anti-rabbit IgG antibody (GE
Healthcare, Buckinghamshire, UK) at room temperature for 1 h. After
washing with PBS-T three times, the membranes were subjected to
chemiluminescence using an ECL kit (American Biosciences,
Piscataway, NJ, USA) and chemiluminescence was detected using a
cooled CCD camera system (LAS-4000 mini, GE Healthcare).
Animal experiments
Four- to six-week-old BALB/c nude mice (Clea Japan,
Tokyo, Japan) were kept in a specific pathogen-free environment.
All animal experiments followed the Guidelines for Animal
Experiments of Tohoku University and Jichi Medical University.
Subcutaneous tumor transplant model
Nude mice were subcutaneously transplanted with
5×106 tumor cells. The tumors formed were measured with
a caliper and tumor volumes [long diameter x (short
diameter)2 x 1/2] were calculated.
Peritoneal dissemination model and
survival time
A total of 5×106 cancer cells were used
to inoculate the peritoneal cavity of nude mice, and the amount of
ascitic fluid and number of tumor nodules on the surface of the
intestine and mesentery were measured. Mouse survival was
determined twice per day and survival curves were constructed using
the Kaplan-Meier method.
Immunohistochemical staining
Subcutaneous tumor transplant model mice were
sacrificed using isoflurane inhalation. The tumor was removed,
embedded in OCT compound (Sakura Finetek Japan Co., Ltd., Tokyo,
Japan) and frozen. Sections 7-mm thick were cut, fixed in methanol
at −20°C for 20 min, blocked with 1% BSA at room temperature for 30
min, and incubated with a 1:500 dilution of anti-mouse CD31
antibody (Research Diagnosis, Flanders, NJ, USA) overnight at 4°C.
After washing three times with PBS, sections were incubated with a
1:500 dilution of Alexa 488-conjugated anti-rat IgG antibody
(Molecular Probes, Eugene, OR, USA) at room temperature for 1 h,
washed three times with PBS and embedded in fluorescent mounting
medium. Specimens were analyzed using a BZ-9000 fluorescence
microscope (Keyence, Osaka, Japan). The vascular area was
calculated as the average blood vessel area in five microscopic
fields. Quantitative analysis was performed using BZ-HIC software
(Keyence).
Statistics
Student's t-test was used for comparison between two
groups. The log-rank test was used to compare Kaplan-Meier survival
curves. p-values <0.05 were considered significant.
Results
Establishment of a VASH1-expressing cell
line
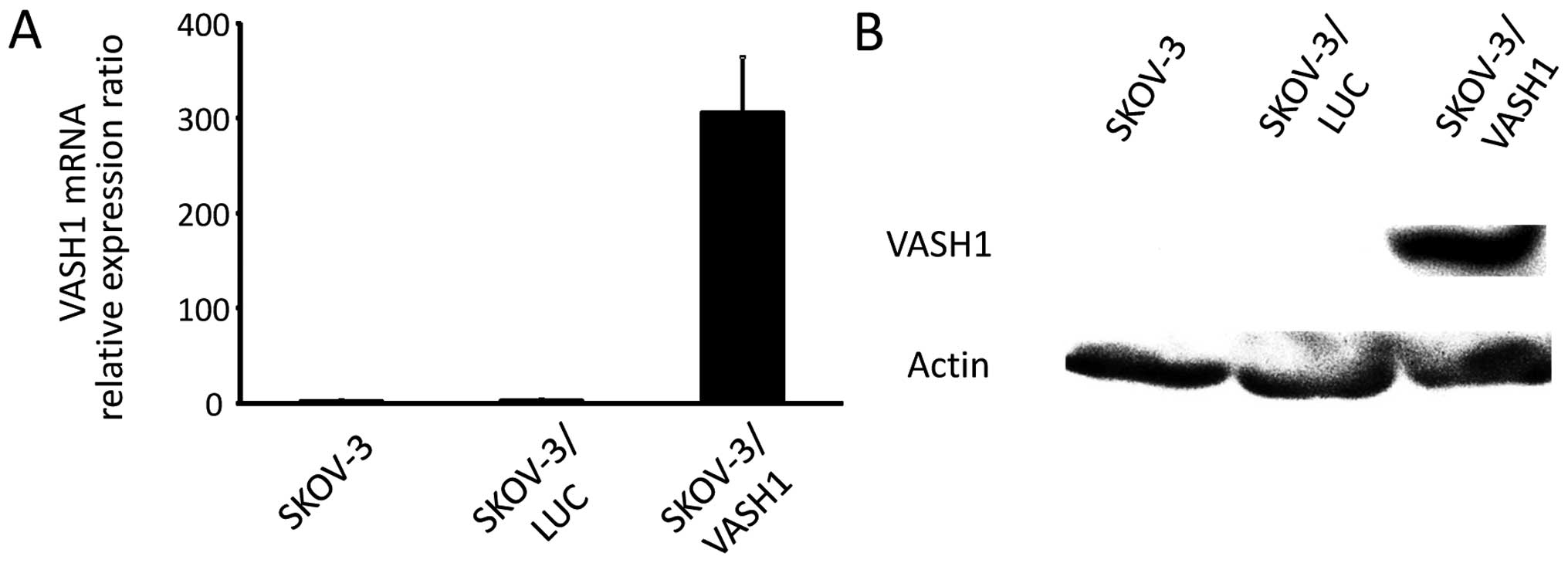
Fig. 1 shows the
results for VASH1 expression analyzed using quantitative RT-PCR and
western blotting, respectively, for SKOV-3, SKOV-3/LUC, and
SKOV-3/VASH1. Expression of VASH1 mRNA and protein was only
observed in SKOV-3/VASH1, indicating the establishment of the
VASH1-expressing ovarian cancer cell line SKOV-3.
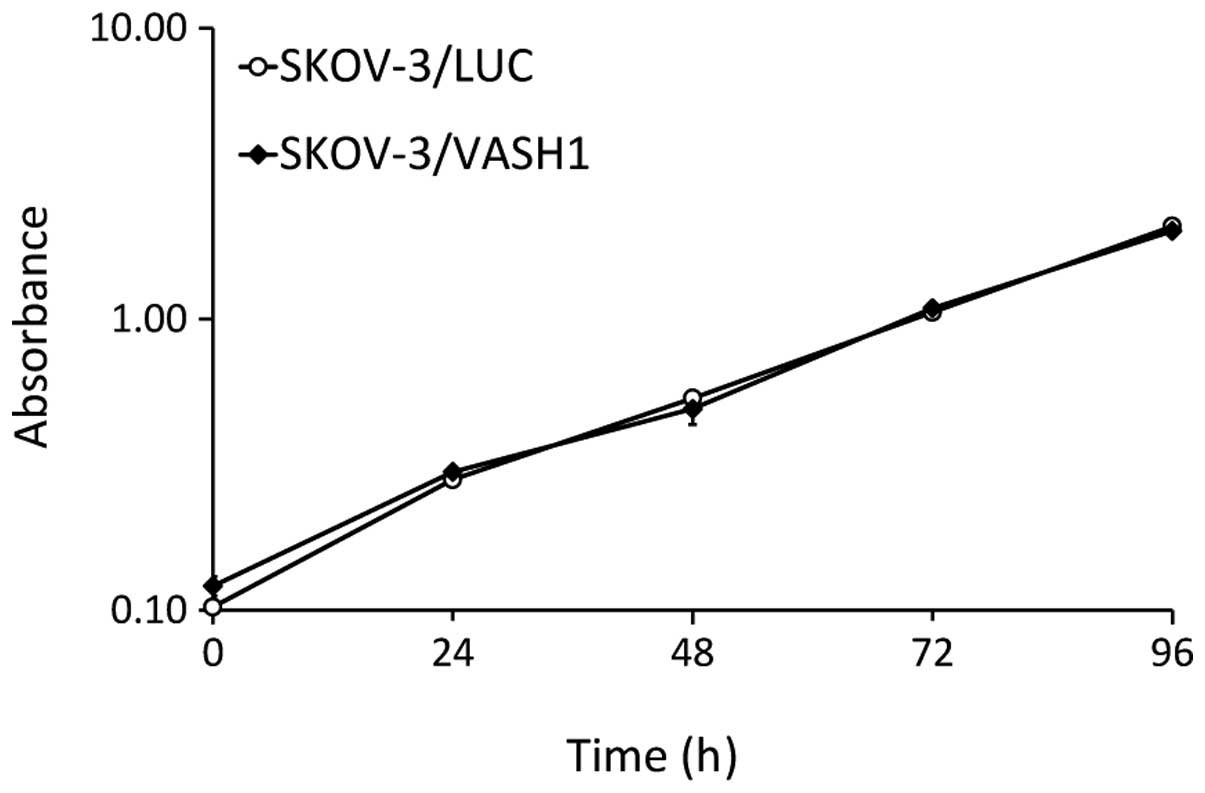
Effect of VASH1 on cell growth in
vitro
The effects of SKOV-3/LUC and SKOV-3/VASH1 on cell
growth in vitro were compared. As shown in Fig. 2, there was no significant
difference in the cell growth curve, indicating that VASH1
expression did not influence the growth of SKOV-3 cells in
vitro.
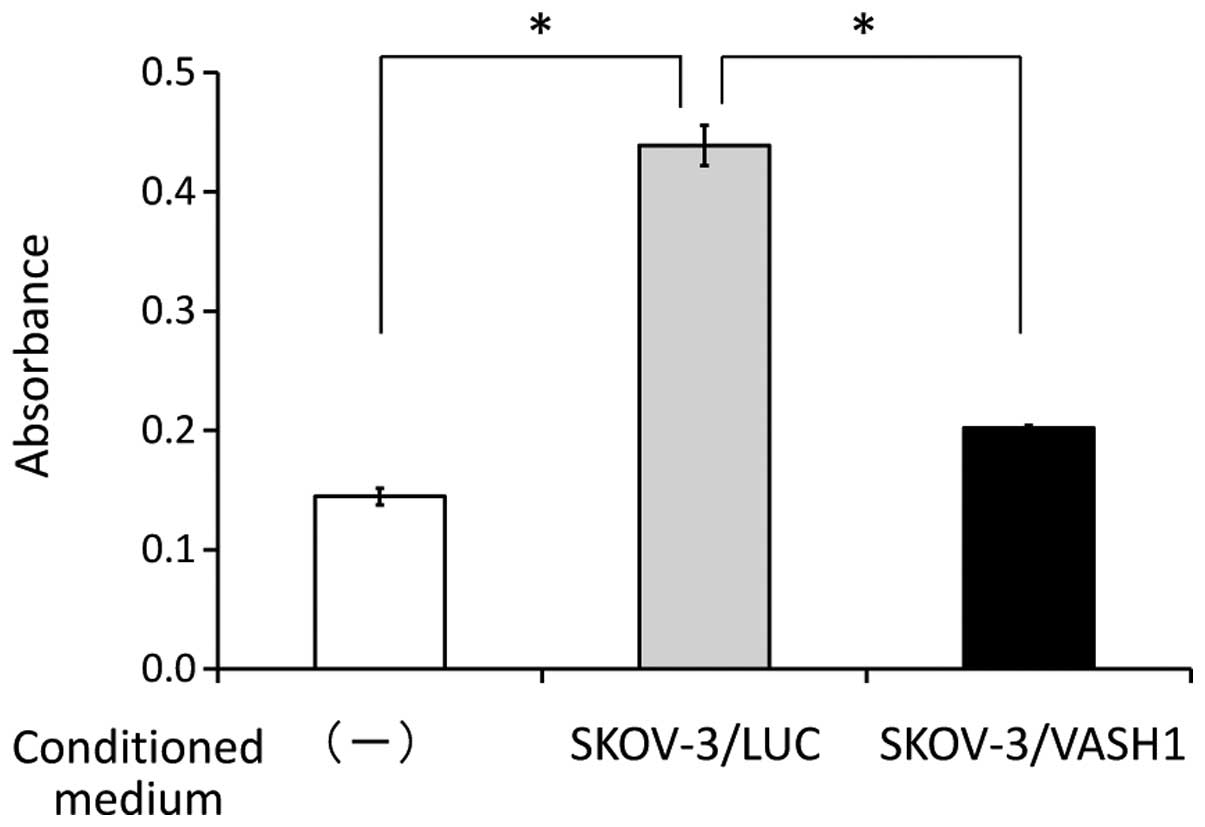
The effect of VASH1 secretion by
SKOV-3/VASH1 on HUVEC
The effect of VASH1 secretion by SKOV-3/VASH1 on
HUVEC was examined. As shown in Fig.
3, the absorbance indicating the number of HUVEC 48 h later was
significantly higher in the group supplemented with the culture
supernatant of SKOV-3/LUC (n=3) than in the non-supplemented group
(n=3) (0.44±0.02 vs. 0.15±0.01, respectively; p<0.01), but was
significantly lower in the group supplemented with the culture
supernatant of SKOV-3/VASH1 (n=3) than in the group supplemented
with the supernatant of SKOV-3/LUC (n=3) (0.20±0.002 vs. 0.44±0.02,
respectively; p<0.01). This indicates that VASH1 secreted by
SKOV-3/VASH1 inhibited the growth of HUVEC in vitro.
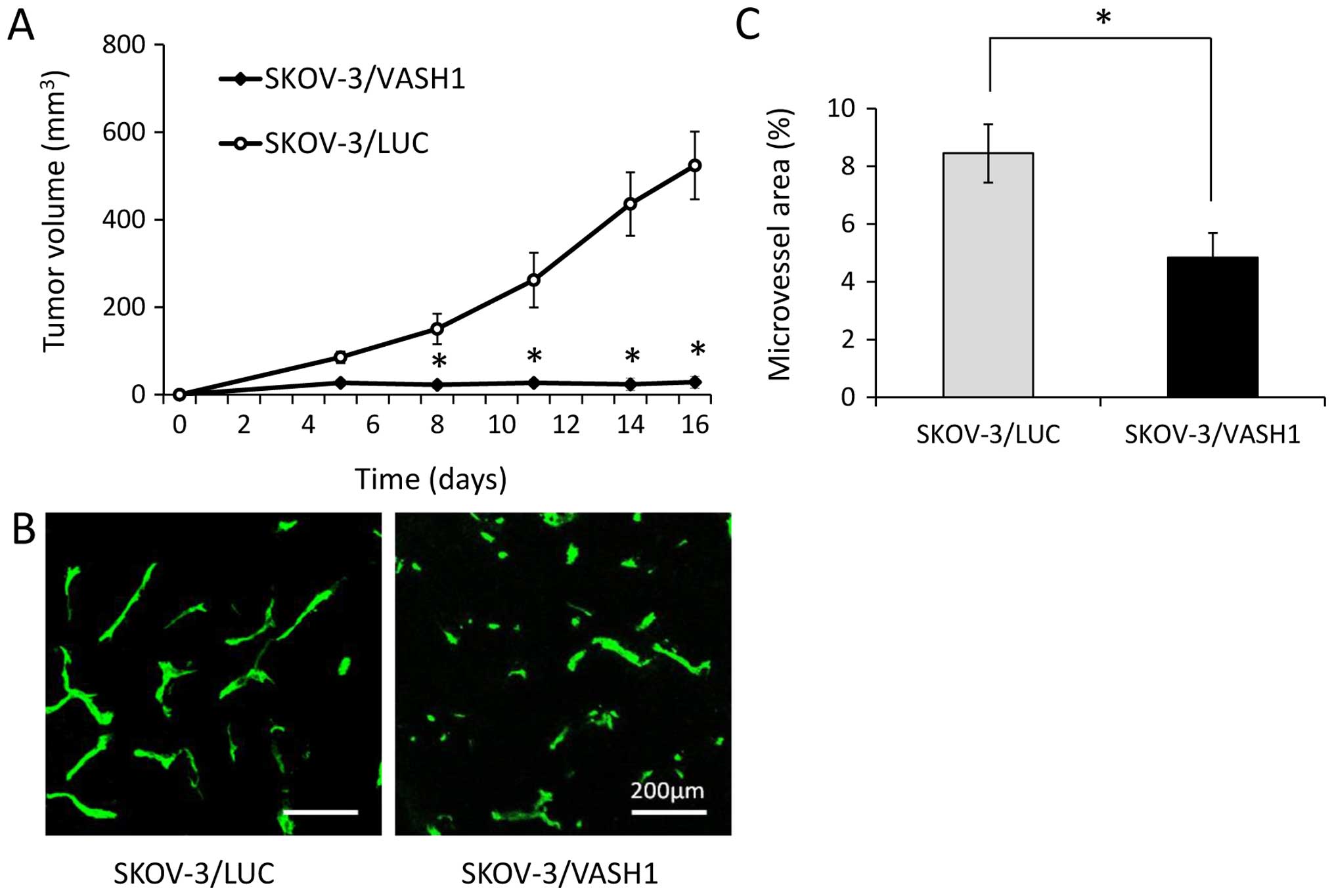
Antitumor and anti-angiogenic effects of
VASH1
Fig. 4A shows the
growth curves of SKOV-3/LUC and SKOV-3/VASH1 subcutaneous tumors.
Both SKOV-3/LUC and SKOV-3/VASH1 groups formed tumor nodules 1 week
after inoculation. Subsequently, tumor enlargement was observed in
the SKOV-3/LUC group but not in the SKOV/VASH1 group. Thus, VASH1
expression inhibited the subcutaneous growth of SKOV-3 tumors in
vivo. Next, new blood vessel formation in resected SKOV-3/LUC
and SKOV-3/VASH1 tumors was evaluated using immunohistochemical
staining (Fig. 4B). The
microvessel density in SKOV-3/VASH1 tumors (n=3) per high-power
field (4.9±0.9%) was significantly lower than that in SKOV-3/LUC
tumors (n=3) (8.5±1.0%, p<0.01) (Fig. 4C). Thus, VASH1 expression inhibited
angiogenesis and growth in SKOV-3 tumors.
Peritoneal dissemination-inhibiting
effect of VASH1
The effect of VASH1 expression on ascites and
peritoneal dissemination was studied in murine models of
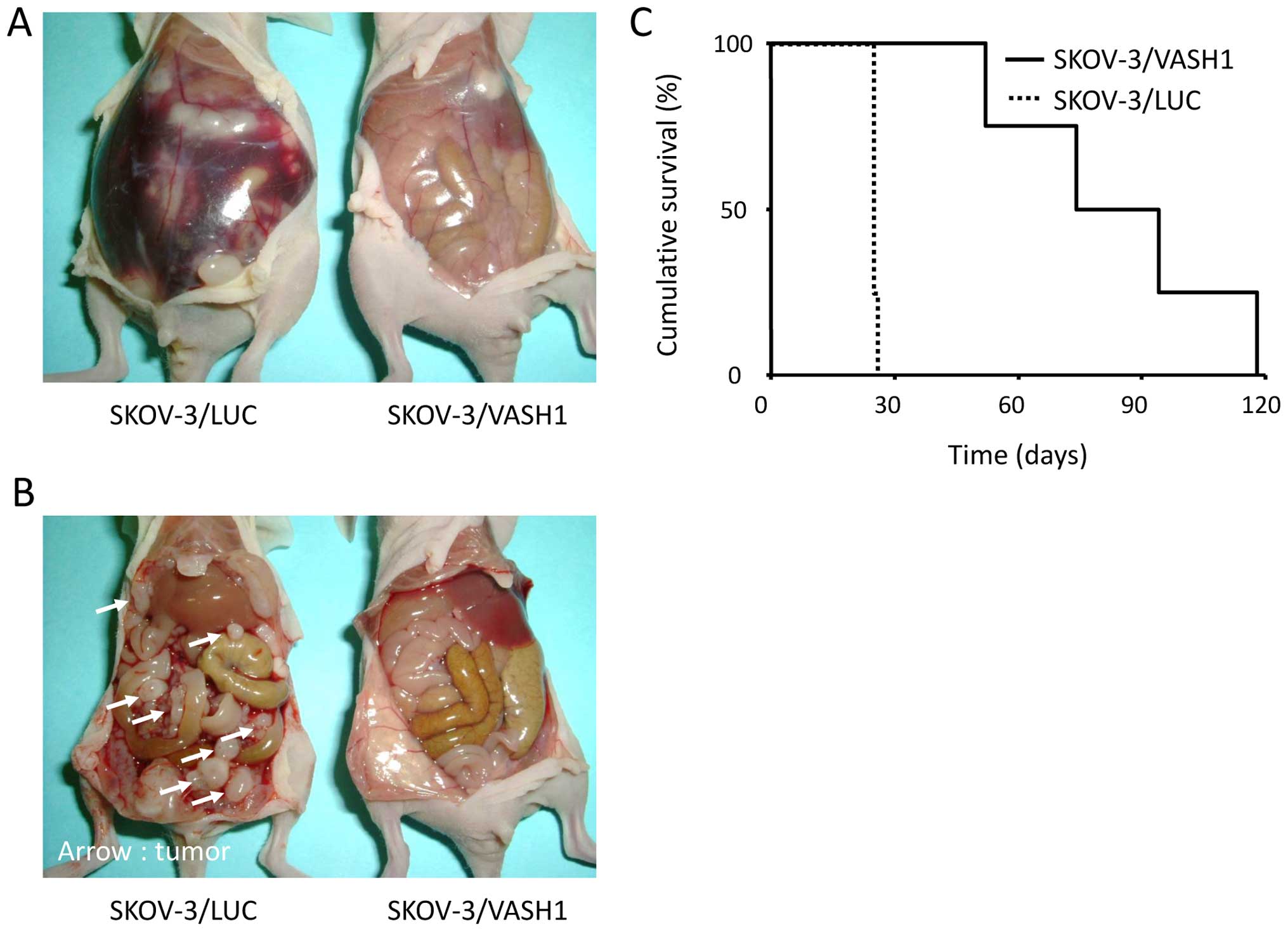
peritoneally disseminated ovarian cancer. Fig. 5A and B show findings at laparotomy
21 days after the inoculation of the peritoneal cavity of nude mice
with SKOV-3/LUC or SKOV-3/VASH1 cells. Marked ascites (Fig. 5A, left) and many tumor nodules
disseminated in the peritoneum (Fig.
5B, left) were observed in SKOV-3/LUC-inoculated mice but
little or no ascites or peritoneal dissemination was observed in
SKOV-3/VASH1-inoculated mice (Fig.
5A, right and B, right). The number of disseminated tumor
nodules on the surface of the intestine and mesentery was
significantly lower in SKOV-3/VASH1-inoculated (n=4) than in
SKOV-3/LUC-inoculated (n=4) mice (3±4 vs. 87±48 nodules,
respectively; p<0.01). Thus, VASH1 expression inhibited
peritoneal dissemination of SKOV-3 cells.
Next, the survival of SKOV-3/LUC- and
SKOV-3/VASH1-inoculated mice was examined. Survival curves are
shown in Fig. 5C. All mice in the
SKOV-3/LUC group (n=4) died by day 28 post-inoculation, whereas
survival of SKOV-3/VASH1 mice (n=4) was significantly increased
(p<0.01). Thus, VASH1 expression inhibited the peritoneal
dissemination of SKOV-3 cells, significantly prolonging mouse
survival.
Discussion
Animal studies using lung or breast cancer cell
lines have reported the anti-angiogenic and antitumor activity of
VASH1 (13,15,17).
However, no studies have reported its effects on ovarian cancer,
which spreads by peritoneal dissemination, a unique pattern of
tumor progression. Therefore, we investigated the antitumor effect
of VASH1 on ovarian cancer in murine models of peritoneally
disseminated ovarian cancer.
In this study, the growth ability of ovarian cancer
cells engineered to express the VASH1 gene remained unchanged. An
experiment using HUVECs showed that VASH1 secreted by tumor cells
inhibited the growth of vascular endothelial cells. Next, animal
experiments showed that VASH1 expression inhibited angiogenesis in
tumors. In addition, in a murine model of peritoneal dissemination
of cancer cells, VASH1 inhibited peritoneal dissemination and
ascites, thus significantly prolonging survival. This indicates
that VASH1 exerts an antitumor effect on ovarian cancer by
inhibiting angiogenesis in the tumor environment.
In recent years, molecular-targeted therapy against
VEGF has been clinically applied and has demonstrated some
effectiveness. Bevacizumab, an anti-VEGF monoclonal antibody, has
been widely used in the clinical setting in the treatment of
colorectal cancer, non-small cell lung cancer, and breast cancer.
In addition, a phase III clinical trial of bevacizumab for ovarian
cancer showed that the progression-free survival time was
significantly higher in a group of patients treated with
bevacizumab and chemotherapy followed by continued bevacizumab
monotherapy than in a group of patients treated with chemotherapy
alone (32). Furthermore,
large-scale clinical studies reported similar midterm results
(33,34), supporting the efficacy of
bevacizumab therapy for ovarian cancer. However, a phenomenon
called ‘evasive resistance’, which is observed in tumors after
anti-VEGF therapy, has attracted attention (35). Proposed mechanisms for the
acquisition of this resistance include the induction of angiogenic
factors other than VEGF, mobilization of bone marrow-derived
endothelial progenitor cells, covering of tumor vascular
endothelial cells by pericytes, and an increase in the invasive
capacity of tumor cells. An animal experiment using an antibody
targeting the VEGF receptor (VEGFR) 2 showed that vascular
regression and tumor reduction occurred first, followed by the
induction of angiogenesis, leading to tumor regrowth (36). Analysis of the mRNA of regrown
tumors revealed increased expression of angiogenic factors other
than VEGF, such as FGF, ephrin, and angiopoietin (36). In addition, a clinical study
reported that the treatment of glioblastoma patients with VEGFR
inhibitors resulted in increased serum FGF levels (37). Since various angiogenic factors are
involved in tumor angiogenesis, there are limitations to treatment
targeting a single angiogenic factor. Therefore, to achieve
sufficient therapeutic benefit, it may be necessary to
simultaneously target multiple angiogenic factors. In the
above-cited study, the combined use of anti-VEGFR2 antibodies and
soluble FGF receptor (an FGF antagonist) successfully delayed tumor
regrowth (36). In our study,
VASH1 inhibited angiogenesis mediated by various angiogenic factors
other than VEGF (13,15). These observations suggest that
VASH1, which acts alone to inhibit multiple angiogenic factors, is
a more effective therapeutic agent compared with VEGF inhibitors in
terms of overcoming evasive resistance.
Physiologically, VASH1 is induced in vascular
endothelial cells to prevent excessive angiogenesis associated with
VEGF stimulation. The application of VASH1 to the treatment of
diseases caused by abnormal angiogenesis requires doses that far
exceed the physiological VASH1 expression (38). To date, the local administration of
purified VASH1 protein preparations (27,38)
and local (38,39) or systemic (15,17,22,24,25)
administration of an adenovirus vector encoding VASH1 have been
attempted. We previously reported the efficacy of therapeutic
strategies using adeno-associated virus (AAV) vectors carrying
various therapeutic factors (40,41).
AAV vectors have advantages, in that they are derived from
non-pathogenic viruses with a strong safety profile; also,
non-dividing cells, such as neurons and muscle cells, can also be
efficiently transfected, with transfected genes expressed over a
prolonged period of time (42). If
these advantages were exploited, it would be possible to transfect
skeletal muscle cells with therapeutic genes using AAV vectors
carrying therapeutic factors to induce muscle cells expression and
secretion of these factors into the blood and to deliver them to
target lesions. Using an animal model, we demonstrated the
feasibility of inhibiting ovarian cancer progression by
constructing an AAV vector containing soluble VEGFR-1 (sFlt-1), a
VEGF antagonist, and injecting this vector into skeletal muscle
(40). Using a similar strategy,
we showed that an AAV vector carrying soluble VEGFR-3 (sFlt-4), an
antagonist of VEGF-C that promotes tumor lymphangiogenesis and
lymphatic metastasis, successfully inhibited lymph node metastasis
of endometrial carcinoma (41). A
gene therapy strategy using vectors such as AAV is a promising
application of VASH1 therapy. In this case, since SVBP is required
for the secretion of VASH1, co-transfection of VASH1 and SVBP
expression vectors could be effective in increasing VASH1 secretion
into the blood.
Unlike sFlt-1 and sFlt-4, the underlying inhibitory
mechanism of VASH1 is still unknown. We are currently trying to
elucidate this mechanism. If this mechanism is understood in
future, it might be possible to identify patients who will benefit
from VASH1.
Anti-angiogenic therapy is now approved for several
types of cancers and drugs targeting the VEGF signals are in
clinical use. However, such drugs can have side effects that
include hypertension and proteinuria due to the impairment of
normal quiescent vessels. It was recently reported that VASH1
protects endothelial cells from premature senescence and
stress-induced cell death via the induction of superoxide dismutase
2 and sirtuin 1 (43). Animal
experiments demonstrated that VASH1 did not increase mean blood
pressure and urinary albumin excretion (44). It has also been reported that VASH1
does not affect any morphological changes in normal blood vessels
(15), wound healing, body weight,
and peripheral blood flow (45) in
adenoviral VASH1 gene-treated mice. These findings suggest that
VASH1 can be a potential candidate for anti-angiogenic
treatment.
In conclusion, this study showed that VASH1, a
negative feedback regulator of angiogenesis, inhibited tumor
angiogenesis and peritoneal dissemination in ovarian tumors,
thereby prolonging survival in mouse models of ovarian cancer.
These results suggest that a novel therapy based on VASH1 could be
a very useful therapeutic strategy for ovarian cancer.
Acknowledgements
This study was supported by The Reserch Award to
Jichi Medical University Graduate Student (Y.T.).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heintz AP: Surgery in advanced ovarian
carcinoma: Is there proof to show the benefit? Eur J Surg Oncol.
14:91–99. 1988.PubMed/NCBI
|
|
3
|
McGuire WP, Hoskins WJ, Brady MF, Kucera
PR, Partridge EE, Look KY, Clarke-Pearson DL and Davidson M:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takei Y, Suzuki M, Ohwada M, Saga Y, Kohno
T, Machida S and Sato I: A feasibility study of paclitaxel and
carboplatin therapy in Japanese patients with epithelial ovarian
cancer. Oncol Rep. 10:951–955. 2003.PubMed/NCBI
|
|
5
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leung DW, Cachianes G, Kuang WJ, Goeddel
DV and Ferrara N: Vascular endothelial growth factor is a secreted
angiogenic mitogen. Science. 246:1306–1309. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fahmy RG, Dass CR, Sun LQ, Chesterman CN
and Khachigian LM: Transcription factor Egr-1 supports
FGF-dependent angiogenesis during neovascularization and tumor
growth. Nat Med. 9:1026–1032. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Reilly MS, Holmgren L, Shing Y, Chen C,
Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH and Folkman J:
Angiostatin: A novel angiogenesis inhibitor that mediates the
suppression of metastases by a Lewis lung carcinoma. Cell.
79:315–328. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Reilly MS, Boehm T, Shing Y, Fukai N,
Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR and Folkman J:
Endostatin: An endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dawson DW, Volpert OV, Gillis P, Crawford
SE, Xu H, Benedict W and Bouck NP: Pigment epithelium-derived
factor: A potent inhibitor of angiogenesis. Science. 285:245–248.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clapp C, Martial JA, Guzman RC,
Rentier-Delure F and Weiner RI: The 16-kilodalton N-terminal
fragment of human prolactin is a potent inhibitor of angiogenesis.
Endocrinology. 133:1292–1299. 1993.PubMed/NCBI
|
|
12
|
Tolsma SS, Volpert OV, Good DJ, Frazier
WA, Polverini PJ and Bouck N: Peptides derived from two separate
domains of the matrix protein thrombospondin-1 have anti-angiogenic
activity. J Cell Biol. 122:497–511. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe K, Hasegawa Y, Yamashita H,
Shimizu K, Ding Y, Abe M, Ohta H, Imagawa K, Hojo K, Maki H, et al:
Vasohibin as an endothelium-derived negative feedback regulator of
angiogenesis. J Clin Invest. 114:898–907. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimizu K, Watanabe K, Yamashita H, Abe M,
Yoshimatsu H, Ohta H, Sonoda H and Sato Y: Gene regulation of a
novel angiogenesis inhibitor, vasohibin, in endothelial cells.
Biochem Biophys Res Commun. 327:700–706. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heishi T, Hosaka T, Suzuki Y, Miyashita H,
Oike Y, Takahashi T, Nakamura T, Arioka S, Mitsuda Y, Takakura T,
et al: Endogenous angiogenesis inhibitor vasohibin1 exhibits
broad-spectrum anti-lymphangiogenic activity and suppresses lymph
node metastasis. Am J Pathol. 176:1950–1958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki Y, Kobayashi M, Miyashita H, Ohta
H, Sonoda H and Sato Y: Isolation of a small vasohibin-binding
protein (SVBP) and its role in vasohibin secretion. J Cell Sci.
123:3094–3101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hosaka T, Kimura H, Heishi T, Suzuki Y,
Miyashita H, Ohta H, Sonoda H, Moriya T, Suzuki S, Kondo T, et al:
Vasohibin-1 expression in endothelium of tumor blood vessels
regulates angiogenesis. Am J Pathol. 175:430–439. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshinaga K, Ito K, Moriya T, Nagase S,
Takano T, Niikura H, Yaegashi N and Sato Y: Expression of vasohibin
as a novel endothelium-derived angiogenesis inhibitor in
endometrial cancer. Cancer Sci. 99:914–919. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshinaga K, Ito K, Moriya T, Nagase S,
Takano T, Niikura H, Sasano H, Yaegashi N and Sato Y: Roles of
intrinsic angiogenesis inhibitor, vasohibin, in cervical
carcinomas. Cancer Sci. 102:446–451. 2011. View Article : Google Scholar
|
|
20
|
Tamaki K, Moriya T, Sato Y, Ishida T,
Maruo Y, Yoshinaga K, Ohuchi N and Sasano H: Vasohibin-1 in human
breast carcinoma: A potential negative feedback regulator of
angiogenesis. Cancer Sci. 100:88–94. 2009. View Article : Google Scholar
|
|
21
|
Tamaki K, Sasano H, Maruo Y, Takahashi Y,
Miyashita M, Moriya T, Sato Y, Hirakawa H, Tamaki N, Watanabe M, et
al: Vasohibin-1 as a potential predictor of aggressive behavior of
ductal carcinoma in situ of the breast. Cancer Sci. 101:1051–1058.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamashita H, Abe M, Watanabe K, Shimizu K,
Moriya T, Sato A, Satomi S, Ohta H, Sonoda H and Sato Y: Vasohibin
prevents arterial neointimal formation through angiogenesis
inhibition. Biochem Biophys Res Commun. 345:919–925. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sato H, Abe T, Wakusawa R, Asai N,
Kunikata H, Ohta H, Sonoda H, Sato Y and Nishida K: Vitreous levels
of vasohibin-1 and vascular endothelial growth factor in patients
with proliferative diabetic retinopathy. Diabetologia. 52:359–361.
2009. View Article : Google Scholar
|
|
24
|
Nasu T, Maeshima Y, Kinomura M,
Hirokoshi-Kawahara K, Tanabe K, Sugiyama H, Sonoda H, Sato Y and
Makino H: Vasohibin-1, a negative feedback regulator of
angiogenesis, ameliorates renal alterations in a mouse model of
diabetic nephropathy. Diabetes. 58:2365–2375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saito D, Maeshima Y, Nasu T, Yamasaki H,
Tanabe K, Sugiyama H, Sonoda H, Sato Y and Makino H: Amelioration
of renal alterations in obese type 2 diabetic mice by vasohibin-1,
a negative feedback regulator of angiogenesis. Am J Physiol Renal
Physiol. 300:F873–F886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wakusawa R, Abe T, Sato H, Yoshida M,
Kunikata H, Sato Y and Nishida K: Expression of vasohibin, an
antiangiogenic factor, in human choroidal neovascular membranes. Am
J Ophthalmol. 146:235–243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wakusawa R, Abe T, Sato H, Sonoda H, Sato
M, Mitsuda Y, Takakura T, Fukushima T, Onami H, Nagai N, et al:
Suppression of choroidal neovascularization by vasohibin-1, a
vascular endothelium-derived angiogenic inhibitor. Invest
Ophthalmol Vis Sci. 52:3272–3280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fogh J, Wright WC and Loveless JD: Absence
of HeLa cell contamination in 169 cell lines derived from human
tumors. J Natl Cancer Inst. 58:209–214. 1977.PubMed/NCBI
|
|
29
|
Mesiano S, Ferrara N and Jaffe RB: Role of
vascular endothelial growth factor in ovarian cancer: Inhibition of
ascites formation by immunoneutralization. Am J Pathol.
153:1249–1256. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wiener JR, Nakano K, Kruzelock RP, Bucana
CD, Bast RC Jr and Gallick GE: Decreased Src tyrosine kinase
activity inhibits malignant human ovarian cancer tumor growth in a
nude mouse model. Clin Cancer Res. 5:2164–2170. 1999.PubMed/NCBI
|
|
31
|
Urabe M, Hasumi Y, Ogasawara Y, Matsushita
T, Kamoshita N, Nomoto A, Colosi P, Kurtzman GJ, Tobita K and Ozawa
K: A novel dicistronic AAV vector using a short IRES segment
derived from hepatitis C virus genome. Gene. 200:157–162. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burger RA, Brady MF, Bookman MA, Fleming
GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE,
et al; Gynecologic Oncology Group. Incorporation of bevacizumab in
the primary treatment of ovarian cancer. N Engl J Med.
365:2473–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Perren TJ, Swart AM, Pfisterer J,
Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P,
Cervantes A, Kurzeder C, et al; ICON7 Investigators. A phase 3
trial of bevacizumab in ovarian cancer. N Engl J Med.
365:2484–2496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aghajanian C, Blank SV, Goff BA, Judson
PL, Teneriello MG, Husain A, Sovak MA, Yi J and Nycum LR: OCEANS: A
randomized, double-blind, placebo-controlled phase III trial of
chemotherapy with or without bevacizumab in patients with
platinum-sensitive recurrent epithelial ovarian, primary
peritoneal, or fallopian tube cancer. J Clin Oncol. 30:2039–2045.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bergers G and Hanahan D: Modes of
resistance to anti-angiogenic therapy. Nat Rev Cancer. 8:592–603.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Casanovas O, Hicklin DJ, Bergers G and
Hanahan D: Drug resistance by evasion of antiangiogenic targeting
of VEGF signaling in late-stage pancreatic islet tumors. Cancer
Cell. 8:299–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Batchelor TT, Sorensen AG, di Tomaso E,
Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M,
et al: AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor,
normalizes tumor vasculature and alleviates edema in glioblastoma
patients. Cancer Cell. 11:83–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shen J, Yang X, Xiao WH, Hackett SF, Sato
Y and Campochiaro PA: Vasohibin is up-regulated by VEGF in the
retina and suppresses VEGF receptor 2 and retinal
neovascularization. FASEB J. 20:723–725. 2006.PubMed/NCBI
|
|
39
|
Zhou SY, Xie ZL, Xiao O, Yang XR, Heng BC
and Sato Y: Inhibition of mouse alkali burn induced-corneal
neovascularization by recombinant adenovirus encoding human
vasohibin-1. Mol Vis. 16:1389–1398. 2010.PubMed/NCBI
|
|
40
|
Takei Y, Mizukami H, Saga Y, Yoshimura I,
Hasumi Y, Takayama T, Kohno T, Matsushita T, Okada T, Kume A, et
al: Suppression of ovarian cancer by muscle-mediated expression of
soluble VEGFR-1/Flt-1 using adeno-associated virus serotype
1-derived vector. Int J Cancer. 120:278–284. 2007. View Article : Google Scholar
|
|
41
|
Takahashi K, Mizukami H, Saga Y, Takei Y,
Urabe M, Kume A, Machida S, Fujiwara H, Suzuki M and Ozawa K:
Suppression of lymph node and lung metastases of endometrial cancer
by muscle-mediated expression of soluble vascular endothelial
growth factor receptor-3. Cancer Sci. 104:1107–1111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mueller C and Flotte TR: Clinical gene
therapy using recombinant adeno-associated virus vectors. Gene
Ther. 15:858–863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Miyashita H, Watanabe T, Hayashi H, Suzuki
Y, Nakamura T, Ito S, Ono M, Hoshikawa Y, Okada Y, Kondo T, et al:
Angiogenesis inhibitor vasohibin-1 enhances stress resistance of
endothelial cells via induction of SOD2 and SIRT1. PLoS One.
7:e464592012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miyashita H, Suzuki H, Ohkuchi A and Sato
Y: Mutual balance between vasohibin-1 and soluble VEGFR-1 in
endothelial cells. Pharmaceuticals. 4:782–793. 2011. View Article : Google Scholar
|
|
45
|
Li D, Zhou K, Wang S, Shi Z and Yang Z:
Recombinant adeno-virus encoding vasohibin prevents tumor
angiogenesis and inhibits tumor growth. Cancer Sci. 101:448–452.
2010. View Article : Google Scholar
|