Introduction
Gene therapy holds great promise for treating
intractable tumors, and suicide gene therapy is one of the most
promising strategies (1). The
herpes simplex virus (HSV) thymidine kinase (TK) gene is the most
widely applied suicide gene in clinical trials (2–5). TK
catalyzes the phosphorylation of the prodrug ganciclovir (GCV) to
generate the mono-phosphate form, which is converted by cellular
kinases into di- and triphosphate forms that inhibit DNA synthesis
(6) and induce apoptosis (7). Furthermore, by exerting the so-called
‘bystander effect’, toxic metabolites of GCV can kill neighboring
non-transfected tumor cells, enhancing cytotoxicity (8). However, the effects of the TK-GCV
system are suboptimal, and combination with another strategy is
usually recommended (4,5).
Mammalian target of rapamycin (mTOR) is an
evolutionally conserved serine/threonine protein kinase and is a
target of antitumor therapy because it regulates cell growth and
metabolism and also because its aberrant activation occurs in many
types of tumors (9–11). In addition to rapamycin, the
prototypical inhibitor of mTOR, several rapamycin analogs
(rapalogs) show improved pharmacokinetic bioavailability and
reduced toxicity (12–14). Moreover, rapalogs such as
temsirolimus and everolimus have been approved by the United States
Food and Drug Administration (FDA) for treatment of renal cell
cancer and lymphoma (14,15). However, the mechanism of mTOR
inhibition deserves further scrutiny because many tumors are
extremely heterogeneous regarding their sensitivity to rapalogs.
This can be explained in part by the complexity of mTOR signaling.
mTOR comprises the mTOR complex 1 (mTORC1) and mTOR complex 2
(mTORC2), which interact in a negative feedback loop (11). Thus, inactivation of mTORC1 after
the administration of traditional rapalogs leads to paradoxical
activation of Akt, the upstream stimulator of mTORC1, by
reactivating either the insulin receptor substrate-1 pathway or the
mTORC2 pathway, diminishing the antitumor effects of a single
rapalog (14–16). Further, simultaneous inhibition of
mTORC1 and mTORC2 pathways or suppression of all mTORCs and
PI3K-Akt pathways using chemical inhibitors is often unacceptably
toxic to normal cells despite the improved antitumor effects
(11,14). Therefore, a highly sophisticated
approach is required if mTOR is to be considered as a therapeutic
target for gene therapy.
Here, we developed a novel dual gene therapy
strategy and confirmed its potential as a combination gene therapy
in a uterine cervical carcinoma model. We employed a suicide gene
(HSV-TK) and its prodrug (GCV) to inhibit DNA synthesis and a small
hairpin RNA (shRNA) that targeted mTOR expression. Recombinant
adeno-associated virus (rAAV) vectors were used for each strategy
(17,18). Herein, we show that the combination
of the TK-GCV system and mTOR inhibition was efficacious in our
model system.
Materials and methods
Cell culture and reagents
Human cervical carcinoma HeLa cells were obtained
from the American Type Culture Collection (Manassas. VA, USA). The
cells were maintained in Dulbecco's modified Eagle's medium
(Gibco-Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (Gibco-Invitrogen), GlutaMAX-1 (2 mM) and 1%
penicillin (100 IU/ml)/streptomycin (50 μg/ml) in a humidified
atmosphere containing 5% carbon dioxide at 37°C.
Construction of a recombinant
adeno-associated virus
The rAAV vectors were constructed as previously
described (19,20). The rAAV2 plasmid expressing
splice-corrected 39TK (sc39TK) under the control of the CMV
promoter was constructed by replacing the BamHI-SalI
site. The PCR product of the sc39TK gene from pcDNA3.1(+)-sc39TK
was inserted into the BamHI-SalI site of the
pSp72-self complementary AAV-GFP vector (20). The rAAV2 vectors that expressed
mTOR-shRNA or control shRNA driven by an H1 promoter were
constructed as described previously (19). The sequences of the mTOR-shRNA were
as follows: 5′-GAT CCG
AATGTT GAC CAATGC TAT TTC AAG AGA ATA GCA TTG GTC AACATT CTT TTT
TGG AAA AGC T-3′ (sense) with a BamHI linker and 5′-AGC TTT
TCC AAA AAAGAATGT TGA CCA
ATG CTA TTC TCT TGA AAT AGCATT GGT CAA CAT TCG-3′
(antisense) with a HindIII linker. Nucleotides specific for
mTOR are underlined. The generation, preparation, and titration of
rAAV2 stocks were performed according to a published method
(21).
Cytotoxicity assay
Exponentially growing HeLa cells were seeded in
6-well plates. After overnight incubation, cells were infected with
the rAAV2-sc39TK virus at a multiplicity of infection (MOI) of
1,000. At day 1 post-infection, the cells were re-seeded in 96-well
plates (10,000 cells per well), and different concentrations of GCV
(Cymevene, Roche Diagnostics, Indianapolis, IN, USA) were added to
the media, followed by incubation for 4 days. Each treatment
condition was tested in triplicate. The CCK-8 assay was performed
according to the manufacturer's instructions (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan). Cell viability was calculated
relative to that of control cells.
Western blot analysis and
immunocytochemical analysis
For western blot analysis, proteins were separated
using gel electrophoresis through SDS-polyacrylamide gels and then
transferred to a PVDF membrane. The membranes were blocked with
Tris-buffered saline (TBS) containing 0.1% Tween-20 and 5% (w/v)
skim milk. After washing with TBST, the membranes were incubated
overnight at 4°C with antibodies against mTOR (Cell Signaling
Technology #2983, Boston, MA, USA), GFP (Millipore, #AB16901,
Temecula, CA, USA) or TK (from William Summers, Yale University,
USA), diluted with TBST containing 1% skim milk. After washing with
TBST, the membranes were incubated for 1 h at room temperature with
the secondary antibodies (Jackson ImmunoResearch Laboratories,
Inc., West Grove, PA, USA). Bands were detected using an ECL system
(Thermo Scientific, Rockford, IL, USA). For immunocytochemistry,
cells were plated in 6-well plates and infected with rAAV2-sc39TK
at various MOIs. Cells were blocked with TBS containing 5% bovine
serum albumin (BSA). After washing, the anti-TK antibody (1:200)
and Cy3-labeled secondary antibody (1:500) were sequentially added
to the fixed cells. Fluorescence signals were analyzed using a
fluorescence microscope.
Cell cycle analysis
HeLa cells were infected with either rAAV2-shCont or
rAAV2-shmTOR. After 48 h, the cells were trypsinized and fixed with
ice-cold ethanol. The cells were then incubated with 0.05%
propidium iodide and analyzed using a flow cytometer (FACSCalibur,
Becton-Dickinson, San Jose, CA, USA) and dedicated software
(CellQuest, Becton-Dickinson).
Mouse tumor xenograft models
Six-week-old BALB/c nu/nu male mice (Orient Bio
Inc., Seongnam, Korea) were used to establish a tumor xenograft
model. The institutional animal care and use committee (IACUC) of
the Biomedical Research Center at Asan Medical Center approved all
procedures involving mice. HeLa cells were infected with
rAAV2-shCont, rAAV2-shmTOR, or rAAV2-sc39TK at MOI 1,000. After 24
h of incubation, 3×106 cells were injected
subcutaneously into the forelimbs of nude mice. Two weeks later,
when the tumors formed by the transplanted cells grew to a volume
of 100 mm3, the mice were divided into groups of 4–6,
and each mouse was administered one intra-peritoneal injection of
GCV (10 mg/kg) daily for 16 days. Tumor diameter and body weight
were measured every 3 days. Tumor volume was calculated as follows:
V = (L×W2) × 0.5, where V = volume, L = length, and W =
width. The mice were sacrificed at designated times, and the tumors
were harvested and subjected to further analysis.
Data presentation and statistical
analysis
Data were reported as the mean ± standard deviation
(SD). Differences between experimental groups and controls were
determined using the Mann-Whitney test and were considered
significant at two-tailed p<0.05.
Results
Recombinant adeno-associated virus
vectors
The expression of the GFP or mutant TK (sc39TK)
genes was controlled by the constitutive promoter (pCMV) in the
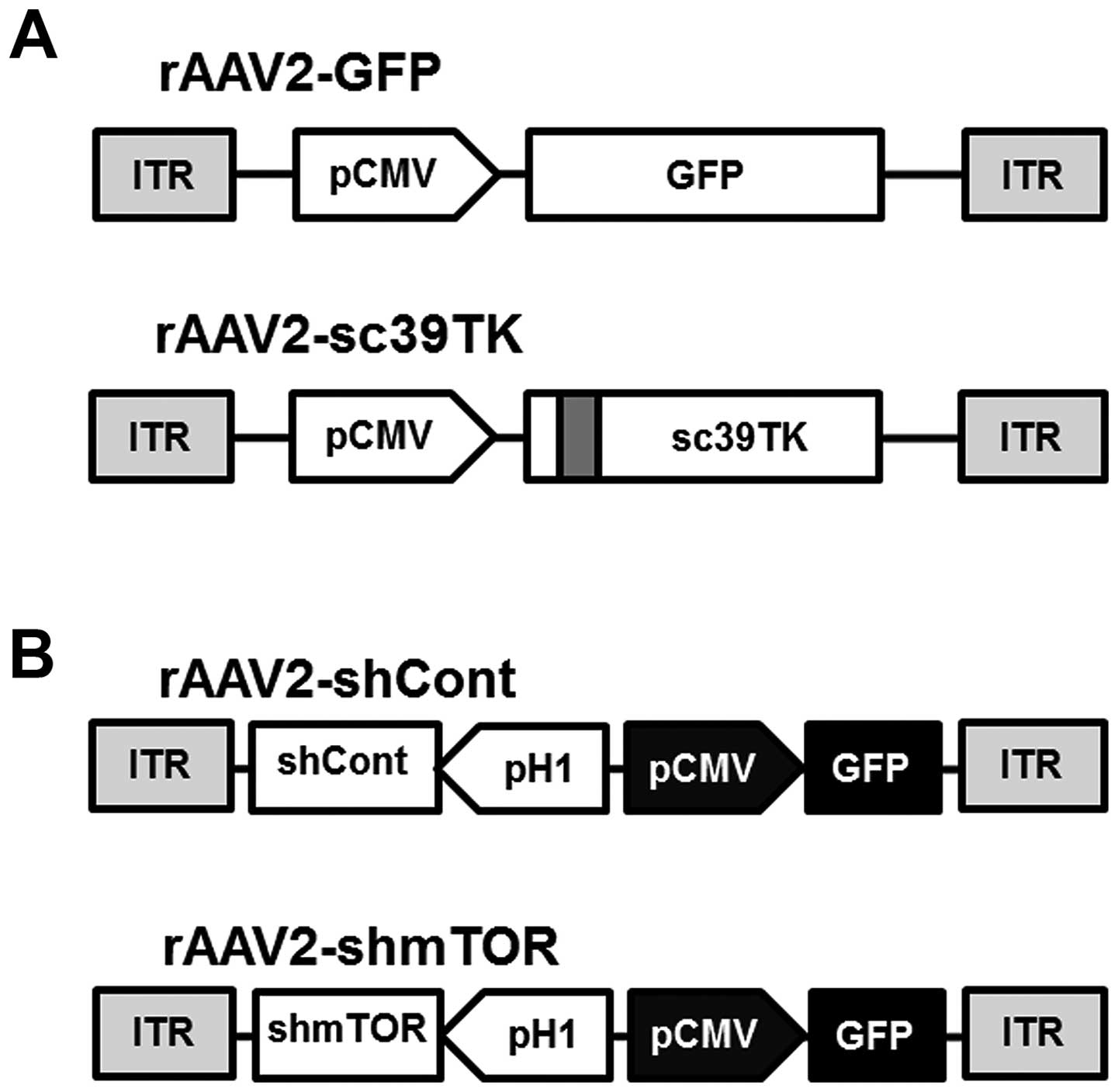
rAAV2-GFP or rAAV2-sc39TK vectors, respectively (Fig. 1A). The sc39TK gene was generated by
introducing a five-codon substitution and a silent mutation in the
GCV-resistant splice acceptor and donor sequences (22). These mutations enhance the drug
sensitivity of tumor cells (20,22).
In contrast, the expression of the control shRNA (shCont) and the
mTOR-shRNA (shmTOR) was regulated by the H1 promoter in the
rAAV2-shCont and the rAAV2-shmTOR vectors, respectively (Fig. 1B). The GFP gene controlled by the
CMV promoter was inserted as a reporter to detect the expression of
the corresponding shRNAs. The mTOR-shRNA was designed using our
custom siRNA screening algorithm (Convenient Application Program
for siRNA Design, CAPSID), which identifies highly specific siRNAs
with minimal off-target effects (19,23).
Suicide gene therapy using the sc39TK-GCV
system
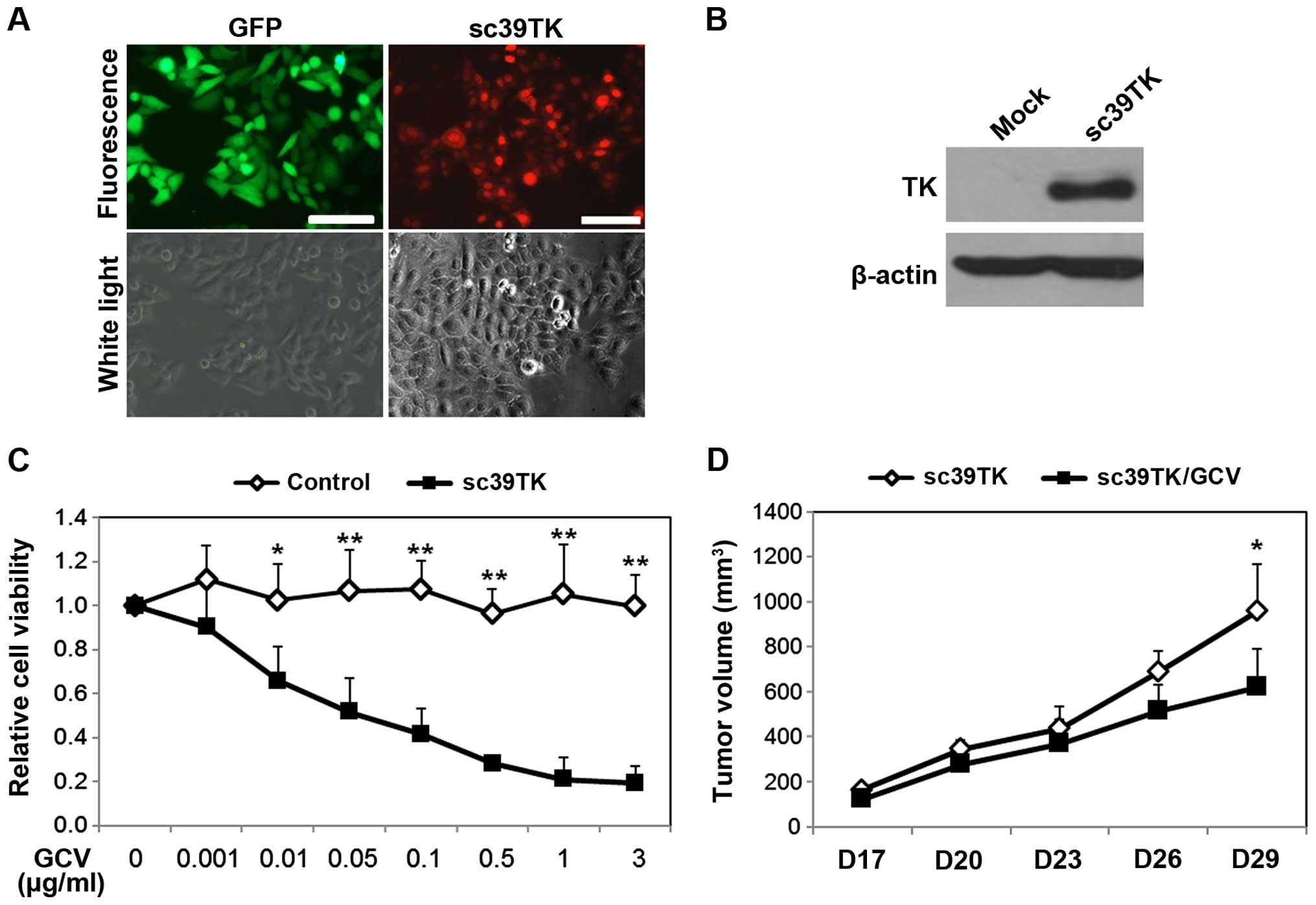
We first determined the transduction efficiency of
rAAV2-sc39TK for HeLa cells at various MOIs. The control vector
(rAAV2-GFP) transduced ~90% of the HeLa cells at a MOI 1,000, and
this rate was recapitulated in the cells infected with
rAAV2-sc39TK. More than 90% of the cells expressed sc39TK after
infection with rAAV2-sc39TK at MOI 1,000, which was used in
subsequent experiments (Fig. 2A).
Western blot analysis demonstrated that induction of sc39TK
expression (Fig. 2B) continued up
to 8 days after infection, which was the last time-point for the
in vitro experiments. To determine the cytotoxicity of the
sc39TK-GCV system, HeLa cells infected with rAAV2-sc39TK were
treated with various concentrations of GCV. There was a
concentration-dependent reduction in the cell viability of the
rAAV2-sc39TK-infected cells (Fig.
2C). The relative viability of the rAAV2-sc39TK-infected cells
decreased to 0.52±0.15 at 0.05 μg/ml GCV and was completely
abolished at 1 μg/ml GCV (Fig.
2C). In xenografted mice, cells infected with rAAV2-sc39TK
without GCV treatment continued to grow as tumors until day 29
after engraftment, whereas tumor formation was impaired when the
cells were treated with GCV. This effect was apparent at day 26,
and the differences in tumor volume on day 29 were statistically
significant (p<0.05) (Fig. 2D).
Therefore, the results indicate efficient transduction of HeLa
cells with rAAV2-sc39TK and effective tumor inhibition after GCV
administration.
mTOR inhibition using shRNA
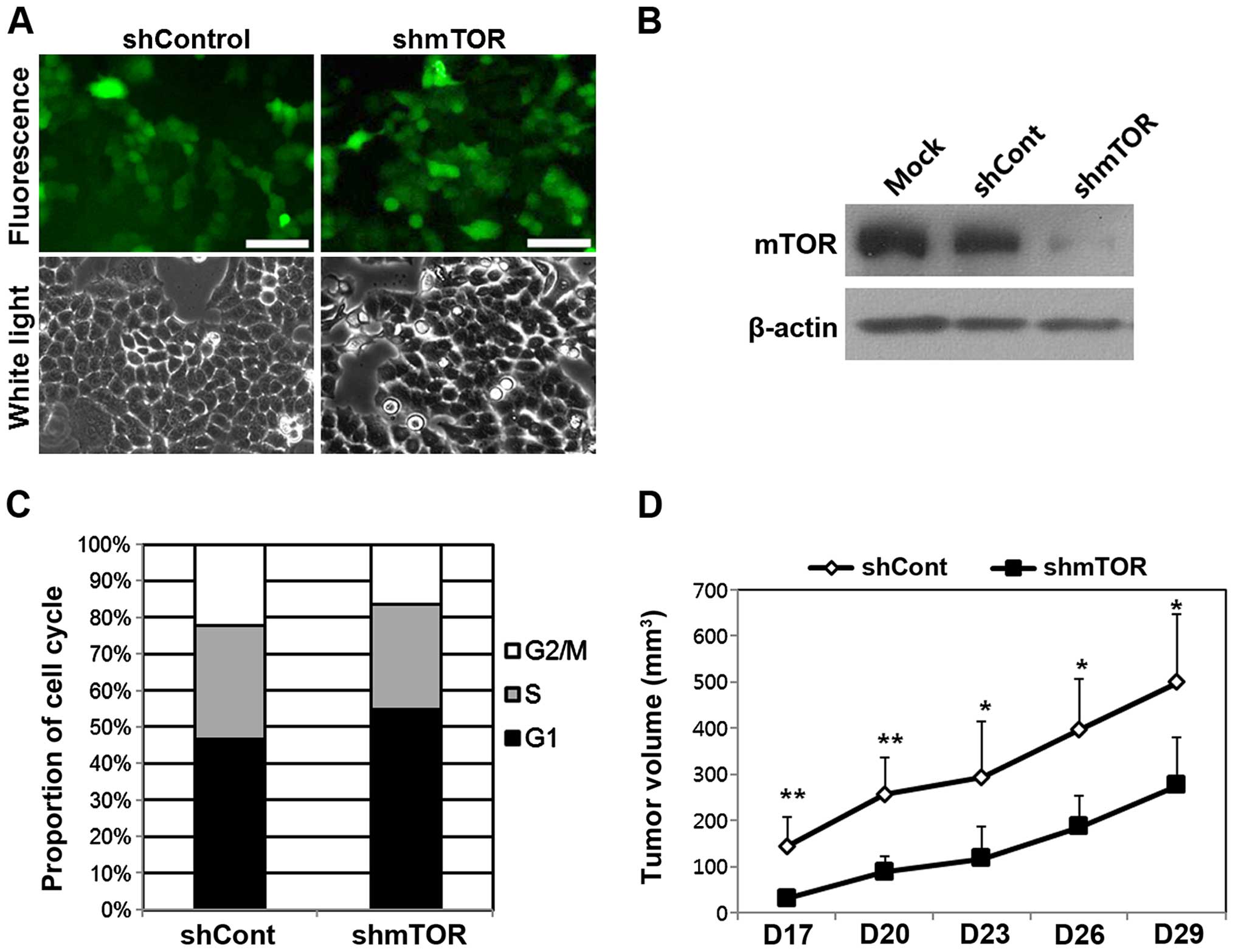
The transduction efficiency of the rAAV2-shRNA
vectors was assessed by determining the levels of GFP expression at
various MOIs. At MOI 1,000, ~90% of HeLa cells expressed GFP, and
the expression levels of rAAV2-shmTOR-infected cells were
equivalent to those of rAAV2-shCont-infected cells (Fig. 3A). The expression of mTOR was
specifically inhibited in cells infected with rAAV2-shmTOR
(Fig. 3B). The suppression of mTOR
expression in vitro continued up to 8 days after infection
with the rAAV2-shmTOR vectors. The in vitro cellular
response to mTOR inhibition was determined by analyzing the cell
cycle. Infection with rAAV2-shmTOR notably increased the proportion
of cells in the G1 phase and decreased the proportion of cells in
the S and G2/M phases. The proportion of rAAV2-shmTOR-infected
cells in the G1 phase increased by 8.4% relative to the proportion
in cells infected with rAAV2-shCont (Fig. 3C). In xenografted mice, the volume
of tumors induced by engrafted cells infected with rAAV2-shmTOR was
significantly reduced compared with those induced by cells infected
with rAAV2-shCont (Fig. 3D).
Combination gene therapy using the
sc39TK-GCV system and mTOR inhibition
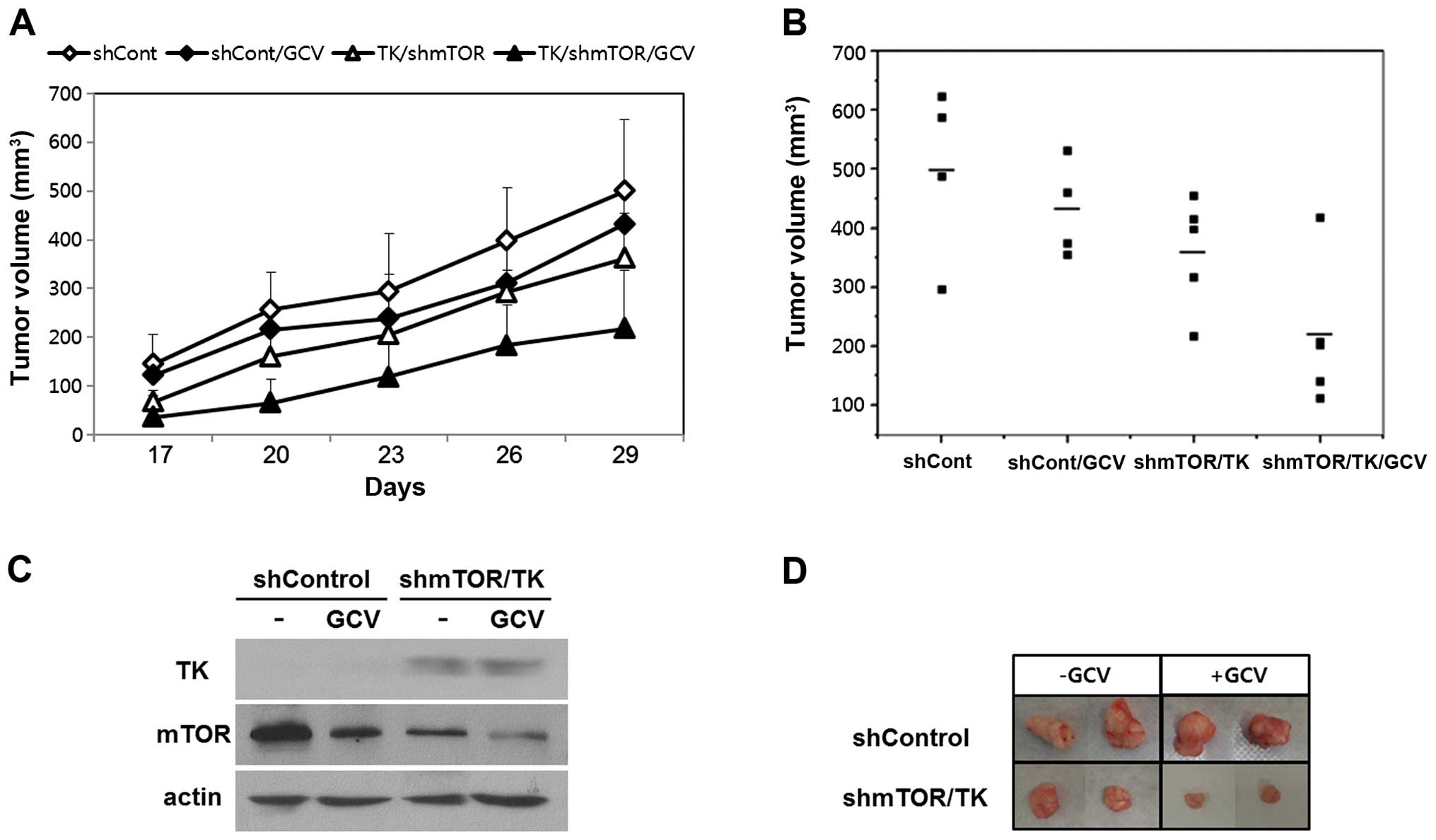
To investigate the effects of combined gene therapy,
the mice were subjected to the treatments as follows: i)
rAAV2-shCont, ii) rAAV2-shCont with GCV, iii) rAAV2-sc39TK and
rAAV2-shmTOR without GCV, and iv) rAAV2-sc39TK and rAAV2-shmTOR
with GCV. On day 17, tumors generated by cells infected with
rAAV2-shmTOR and rAAV2-sc39TK were smaller than those generated by
cells that were infected with rAAV2-shCont (Fig. 4A). Administration of GCV further
reduced the growth of tumors generated by cells co-infected with
rAAV2-sc39TK and rAAV-shmTOR, and the tumor volumes were smallest
in this group (Fig. 4A). On day 29
after implantation, compared with the group of mice engrafted with
cells infected with rAAV2-shCont, there was a decrease in tumor
volume of 27.9±6.3% in the group not treated with GCV but implanted
with cells co-infected with rAAV2-shmTOR/rAAV2-sc39TK. Furthermore,
there was a 56.8±8.2% decrease in tumor volume in the group treated
with GCV and implanted with cells co-infected with
rAAV2-shmTOR/rAAV2-sc39TK (Fig.
4B). On day 29, western blot analysis revealed maintained TK
expression and suppressed mTOR expression in tumors formed by cells
co-infected with rAAV2-shmTOR/rAAV2-sc39TK (Fig. 4C). The gross size of the dissected
tumors formed by cells co-infected with rAAV2-shmTOR/rAAV2-sc39TK
was smaller than that of tumors formed from cells infected with
rAAV2-shCont. Addition of GCV further reduced the size of the
tumors of the former group (Fig.
4D).
Effects of combination gene therapy on
transgene expression
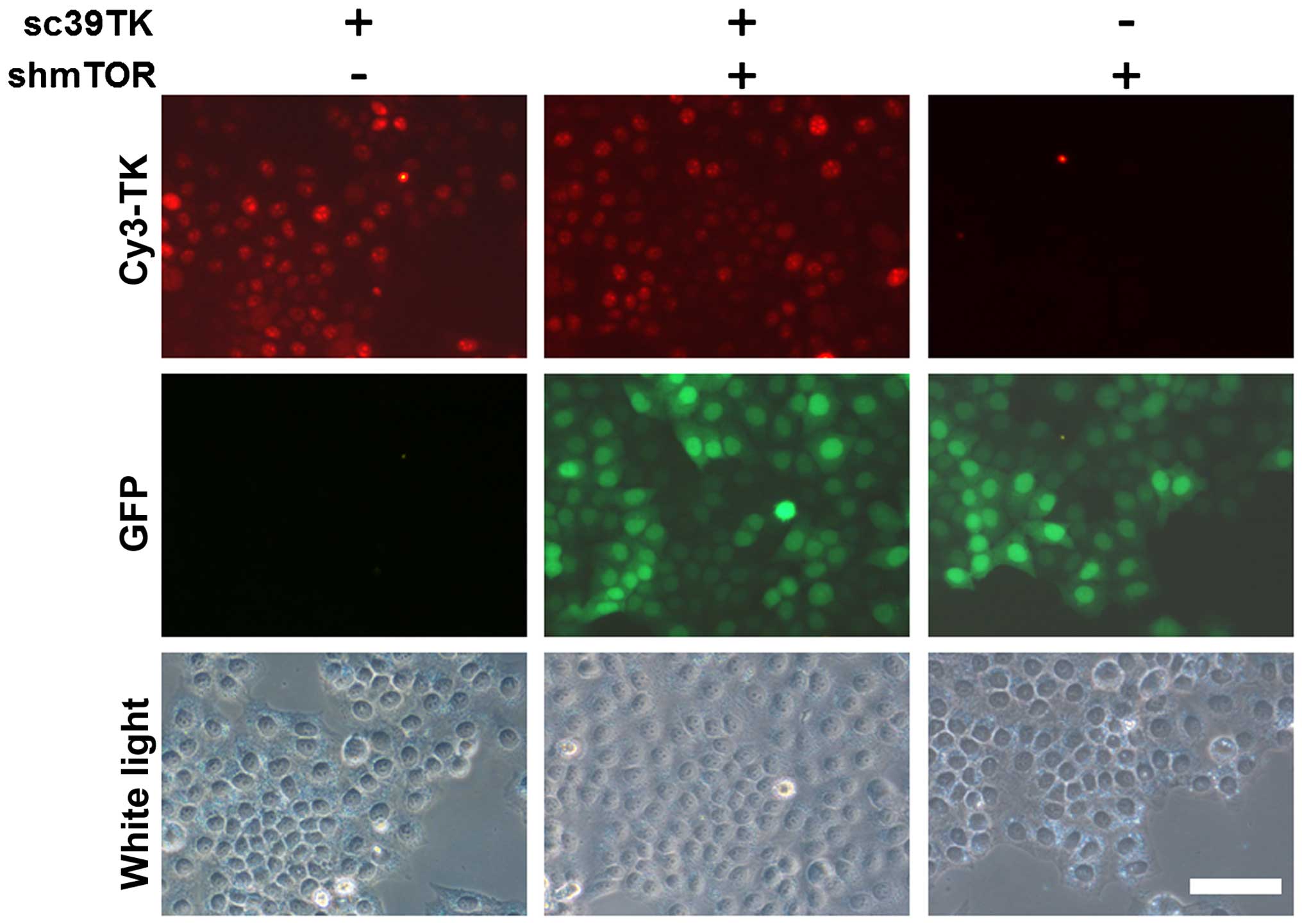
We further investigated whether combined
transduction of cells with rAAV2-sc39TK and rAAV2-shmTOR mutually
inhibited the expression of the transgenes. It is of note that
there is no GFP signal from rAAV2-sc39TK (Fig. 1A). In the HeLa cells infected with
both rAAV2-sc39TK and rAAV2-shmTOR, the GFP signals were comparable
to those from rAAV2-shmTOR alone (Fig.
5). Furthermore, TK expression by cells infected with
rAAV2-sc39TK was not inhibited by co-infection with rAAV2-shmTOR.
Moreover, when the cells were infected at MOI ≤5,000, neither the
GFP signal nor TK expression level was reduced (data not
shown).
Discussion
In the present study, we demonstrate for the first
time to our knowledge that the combination of suicide gene therapy
(sc39TK-GCV system) and mTOR inhibition using an mTOR-specific
shRNA enhanced antitumor effects without mutual interference of
transgene expression. Taking into consideration that the tumors
increase in size because of proliferation (increase of cell number)
and growth (increase of cell size) of the individual tumor cells,
the cytocidal effects of the sc39TK-GCV system (Fig. 2) combined with the cytostatic
effect of mTOR inhibition (Fig. 3)
serve as an ideal antitumor mechanism (24). The sc39TK-GCV system effectively
inhibits the proliferation of tumor cells by inhibiting DNA
synthesis (1,6,20),
whereas the mTOR-specific shRNA inhibits the growth of tumor cells
by downregulating tumorigenic pathways (19).
The TK-GCV system has been evolved in many aspects.
The original version of HSV-TK was modified to sc39TK to enhance
its antitumor effects upon GCV treatment (20,22,25).
A theranostic (simultaneous therapy and diagnosis) approach for
individualized therapy was successfully achieved using TK activity
that incorporates therapeutics such as GCV and diagnostic imaging
agents such as 18F-FHBG (a radiolabeled penciclovir
analog for positron emission tomography) (4,20).
However, because the therapeutic efficacy of the TK-GCV system
alone is usually insufficient (4,5), the
TK-GCV system has been tested in combination with other antitumor
strategies such as another suicide gene, conventional chemotherapy,
radiotherapy, and immunotherapy (1). In fact, the combination here of
TK-GCV with RNAi is a relatively new approach to cancer therapy
(26).
mTOR is an emerging target of antitumor gene therapy
because it functions in the PI3K/Akt/mTOR signaling pathway that
mediates tumorigenesis (13,27).
mTOR regulates the growth, senescence, survival, and metabolic
homeostasis of cells (11).
Elevated mTOR activity is present in many tumors, and suppression
of mTOR induces tumor regression (9,10).
Moreover, gene therapy targeting mTOR is a promising anti-tumor
approach, because, as an evolutionally conserved target, relatively
few mutant forms are known (13,28).
However, mTOR inhibitors have been found to be successful for
treating only a few types of tumors such as renal cell carcinoma or
lymphoma, and the responses of many other tumors to such drugs are
highly heterogeneous (13). In
this regard, the RNAi approach used in the present and previous
studies shows promise because mTOR-shRNA inhibits both mTORC1 and
mTORC2 without serious off-target effects (19,23).
Using rAAV as a vector for gene therapy may have
contributed substantially to the success of the combination
approach. Wide applicability to a variety of tumors and long-term
expression of transgenes without serious toxicity are advantages of
using rAAV vectors (29,30). Furthermore, rAAV has been
FDA-approved for gene therapy (29,31).
We have used rAAV vectors for transgene packaging, delivery, and
expression and for in vitro and in vivo monitoring in
our previous studies (20,32–34).
In the present study, the transduction efficiencies were well
balanced between the HSV-TK gene and the mTOR-shRNA (Figs. 2 and 3). Transgene expression was well
maintained until the end of the experiments (Fig. 4) without mutual interference
(Fig. 5). The demonstration of the
combined effects of the dual gene therapy strategy might have been
difficult without this expertise of rAAV vectors.
However, some questions remain unanswered regarding
the utility of the dual gene therapy presented here. In the TK-GCV
system, actively dividing tumor cells are killed directly, and the
non-dividing quiescent tumor cells may be indirectly affected by
toxic GCV metabolites through the bystander effect. Whether or not
the bystander effect contributes to tumor inhibition in conjunction
with mTOR inhibition is unknown (8). Additionally, the present study does
not address techniques for delivery to patients or the effects of
treatment for >1 month (3).
These questions require further study.
In the present proof-of-concept study, we
demonstrate that the TK-GCV system combined with an mTOR-specific
shRNA enhances the suppression of tumor growth compared to the use
of either technique alone. This promising result may lead to the
development of a novel gene therapy strategy against intractable
tumors.
Acknowledgements
This study was supported by grants from Medical
Research Center Program (2008-0062286 to H. Lee), Basic Science
Research Program (NRF-2011-0014821 to H.N. Woo), and Nuclear
Research and Development Program (NRF-2014M2B2A9030104 and
NRF-2012M2A2A7035589 to W.W. Lee), Republic of Korea.
Abbreviations:
|
HSV
|
herpes simplex virus
|
|
TK
|
thymidine kinase
|
|
GCV
|
ganciclovir
|
|
shRNA
|
small hairpin RNA
|
|
mTOR
|
mammalian target of rapamycin
|
|
rAAV
|
recombinant adeno-associated virus
|
References
|
1
|
Duarte S, Carle G, Faneca H, de Lima MC
and Pierrefite-Carle V: Suicide gene therapy in cancer: Where do we
stand now? Cancer Lett. 324:160–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Immonen A, Vapalahti M, Tyynelä K,
Hurskainen H, Sandmair A, Vanninen R, Langford G, Murray N and
Ylä-Herttuala S: AdvHSV-tk gene therapy with intravenous
ganciclovir improves survival in human malignant glioma: A
randomised, controlled study. Mol Ther. 10:967–972. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rainov NG: A phase III clinical evaluation
of herpes simplex virus type 1 thymidine kinase and ganciclovir
gene therapy as an adjuvant to surgical resection and radiation in
adults with previously untreated glioblastoma multiforme. Hum Gene
Ther. 11:2389–2401. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sangro B, Mazzolini G, Ruiz M, Ruiz J,
Quiroga J, Herrero I, Qian C, Benito A, Larrache J, Olagüe C, et
al: A phase I clinical trial of thymidine kinase-based gene therapy
in advanced hepatocellular carcinoma. Cancer Gene Ther. 17:837–843.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Voges J, Reszka R, Gossmann A, Dittmar C,
Richter R, Garlip G, Kracht L, Coenen HH, Sturm V, Wienhard K, et
al: Imaging-guided convection-enhanced delivery and gene therapy of
glioblastoma. Ann Neurol. 54:479–487. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moolten FL: Tumor chemosensitivity
conferred by inserted herpes thymidine kinase genes: Paradigm for a
prospective cancer control strategy. Cancer Res. 46:5276–5281.
1986.PubMed/NCBI
|
|
7
|
Wei SJ, Chao Y, Hung YM, Lin WC, Yang DM,
Shih YL, Ch'ang LY, Whang-Peng J and Yang WK: S- and G2-phase cell
cycle arrests and apoptosis induced by ganciclovir in murine
melanoma cells transduced with herpes simplex virus thymidine
kinase. Exp Cell Res. 241:66–75. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mesnil M and Yamasaki H: Bystander effect
in herpes simplex virus-thymidine kinase/ganciclovir cancer gene
therapy: Role of gap-junctional intercellular communication. Cancer
Res. 60:3989–3999. 2000.PubMed/NCBI
|
|
9
|
Bjornsti MA and Houghton PJ: The TOR
pathway: A target for cancer therapy. Nat Rev Cancer. 4:335–348.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Q, Thoreen C, Wang J, Sabatini D and
Gray NS: mTOR Mediated Anti-Cancer Drug Discovery. Drug Discov
Today Ther Strateg. 6:47–55. 2009. View Article : Google Scholar
|
|
11
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
From growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View
Article : Google Scholar
|
|
12
|
Cornu M, Albert V and Hall MN: mTOR in
aging, metabolism, and cancer. Curr Opin Genet Dev. 23:53–62. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Faivre S, Kroemer G and Raymond E: Current
development of mTOR inhibitors as anticancer agents. Nat Rev Drug
Discov. 5:671–688. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fasolo A and Sessa C: Current and future
directions in mammalian target of rapamycin inhibitors development.
Expert Opin Investig Drugs. 20:381–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pópulo H, Lopes JM and Soares P: The mTOR
signalling pathway in human cancer. Int J Mol Sci. 13:1886–1918.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue
P, Fu H and Khuri FR: Activation of Akt and eIF4E survival pathways
by rapamycin-mediated mammalian target of rapamycin inhibition.
Cancer Res. 65:7052–7058. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hermonat PL and Muzyczka N: Use of
adeno-associated virus as a mammalian DNA cloning vector:
Transduction of neomycin resistance into mammalian tissue culture
cells. Proc Natl Acad Sci USA. 81:6466–6470. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park K, Kim WJ, Cho YH, Lee YI, Lee H,
Jeong S, Cho ES, Chang SI, Moon SK, Kang BS, et al: Cancer gene
therapy using adeno-associated virus vectors. Front Biosci.
13:2653–2659. 2008. View
Article : Google Scholar
|
|
19
|
Ahn J, Woo HN, Ko A, Khim M, Kim C, Park
NH, Song HY, Kim SW and Lee H: Multispecies-compatible antitumor
effects of a cross-species small-interfering RNA against mammalian
target of rapamycin. Cell Mol Life Sci. 69:3147–3158. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JY, Kim JH, Khim M, Lee HS, Jung JH,
Moon DH, Jeong S and Lee H: Persistent anti-tumor effects via
recombinant adeno-associated virus encoding herpes thymidine kinase
gene monitored by PET-imaging. Oncol Rep. 25:1263–1269.
2011.PubMed/NCBI
|
|
21
|
Shin O, Kim SJ, Lee WI, Kim JY and Lee H:
Effective transduction by self-complementary adeno-associated
viruses of human dendritic cells with no alteration of their
natural characteristics. J Gene Med. 10:762–769. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Black ME, Kokoris MS and Sabo P: Herpes
simplex virus-1 thymidine kinase mutants created by semi-random
sequence mutagenesis improve prodrug-mediated tumor cell killing.
Cancer Res. 61:3022–3026. 2001.PubMed/NCBI
|
|
23
|
Lee HS, Ahn J, Jun EJ, Yang S, Joo CH, Kim
YK and Lee H: A novel program to design siRNAs simultaneously
effective to highly variable virus genomes. Biochem Biophys Res
Commun. 384:431–435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmelzle T and Hall MN: TOR, a central
controller of cell growth. Cell. 103:253–262. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Black ME, Newcomb TG, Wilson HM and Loeb
LA: Creation of drug-specific herpes simplex virus type 1 thymidine
kinase mutants for gene therapy. Proc Natl Acad Sci USA.
93:3525–3529. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park SY, Lee W, Lee J and Kim IS:
Combination gene therapy using multidrug resistance (MDR1) gene
shRNA and herpes simplex virus-thymidine kinase. Cancer Lett.
261:205–214. 2008. View Article : Google Scholar
|
|
27
|
Bartholomeusz C and Gonzalez-Angulo AM:
Targeting the PI3K signaling pathway in cancer therapy. Expert Opin
Ther Targets. 16:121–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grabiner BC, Nardi V, Birsoy K, Possemato
R, Shen K, Sinha S, Jordan A, Beck AH and Sabatini DM: A diverse
array of cancer-associated MTOR mutations are hyperactivating and
can predict rapamycin sensitivity. Cancer Discov. 4:554–563. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mingozzi F and High KA: Therapeutic in
vivo gene transfer for genetic disease using AAV: Progress and
challenges. Nat Rev Genet. 12:341–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mueller C and Flotte TR: Clinical gene
therapy using recombinant adeno-associated virus vectors. Gene
Ther. 15:858–863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ylä-Herttuala S: Endgame: Glybera finally
recommended for approval as the first gene therapy drug in the
European union. Mol Ther. 20:1831–1832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim SJ, Lee WI, Heo H, Shin O, Kwon YK and
Lee H: Stable gene expression by self-complementary
adeno-associated viruses in human MSCs. Biochem Biophys Res Commun.
360:573–579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim SJ, Lee WI, Lee YS, Kim DH, Chang JW,
Kim SW and Lee H: Effective relief of neuropathic pain by
adeno-associated virus-mediated expression of a small hairpin RNA
against GTP cyclohydrolase 1. Mol Pain. 5:672009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee HS, Shin OK, Kim SJ, Lee WI, Jeong S,
Park K, Choe H and Lee H: Efficient gene expression by
self-complementary adeno-associated virus serotype 2 and 5 in
various human cancer cells. Oncol Rep. 18:611–616. 2007.PubMed/NCBI
|