Introduction
Cutaneous malignant melanoma is a highly aggressive
tumour originating from melanocytes situated in the stratum basale
of the epidermis (1). Malignant
transformation of melanocytes can be induced endogenously via
genetic predisposition or by exogenous factors such as UV
irradiation. In either case, alterations in the composition of
pericellular matrix and/or cell surface receptor pattern could
occur. It is widely accepted, that any modulation of the
cell-matrix interaction may trigger changes in the activity of
various signalling pathways leading to uncontrolled cellular
proliferation or motility (2–4).
Hyaluronic acid (HA) is a non-sulphated high-molecular-mass
glycos-aminoglycan (GAG) built by disaccharide units composed of
N-acetyl-glucosamine and glucuronic acid. HA is the most abundant
component of the extracellular matrix either in normal or
malignantly transformed tissues. Polyanionic character of HA may
provide a highly hydrated, anti-adhesive pericellular matrix to
malignantly transformed cells, including melanoma cells, playing a
critical role in the invasion of surrounding tissues (5). Three isotypes of HA synthases (HAS)
HAS1, HAS2, and HAS3 are responsible for the production of HA
(6,7). HAS are transmembrane molecules
producing HA extruded into the extracellular space and they also
connect it to the cells in the meantime. Major differences of the
isoforms are in the length of the HA produced and the ease of the
release (7–10). HAS1 and HAS2 produce higher
molecular mass (106 Da) HA chains forming a pericellular
HA coat, while HA produced by HAS3 is shorter (105 Da)
(11,12). Besides the synthesising enzymes,
pericellular HA also binds to cell surface receptors, of which CD44
is the best known, but an atypical variant of hyaladherins, RHAMM
(receptor for hyaluronan mediated motility), also plays an
important role in cell-HA interactions (13,14).
RHAMM fulfils primarily intracellular functions but may also appear
in the plasma membrane, where it acts as a co-receptor for HA
together with CD44. It is worth to mention, that presence of
extracellular RHAMM is very frequent on highly malignant, invasive,
metastatic tumour cells (15).
Engagement of CD44 and RHAMM with HA can modulate motility and
invasion of tumour cells (14),
and may activate a great variety of signalling cascades (16). It is also known, that cells can
incorporate small HA oligosaccharide particles by endocytosis
(17) and intracellular HA may
influence activity of various signal transduction pathways
regulating proliferation, adhesion and motility either in normal or
malignantly transformed cells (18–20).
It has been published that a direct intracellular interaction of
RHAMM and HA can activate ERK1/2 and can result in a consequent
enhancement of cell proliferation (21).
Signalling elements connected to the HA homeostasis
of tumour cells can be activated by reversible phosphorylation on
Ser/Thr amino acid residues by several protein kinases such as PKA,
classical PKCs (22) or Ras
(23). It has also been reported
that enzymatic activity of HAS2 and HAS3 isotypes are regulated by
Ser/Thr phosphorylation (21). In
contrast, little is known about the role of phosphoprotein
phosphatases (PP) which can dephosphorylate the target proteins in
the regulation of HA synthesis or binding of HA by cells.
Calcineurin or PP2B is a Ca2+-calmodulin-dependent
Ser/Thr specific PP, which is present in the majority of mammalian
cells and tissues (24). The
presence of PP2B in epidermis-related cells, such as keratinocytes
and melanocytes was reported only few years ago (25,26).
Importance of PP2B in skin homeostasis is proved by the fact, that
pharmacological inhibitors of this PP are applied in the
dermatological practice for topic treatment of various inflammatory
diseases of skin accompanied with activation of T-lymphocytes,
e.g., atopic dermatitis (27). Our
group has proven that calcineurin influenced migration of melanoma
cells (28) but the connection of
this PP and HA homeostasis has not been investigated yet.
In this study we provide evidence that unlike the
melanocytes, HA is abundantly produced by melanoma cells and serves
as a chemoattractant for their migration in Boyden chamber. HAS2
and HAS3 enzymes were detectable in melanoma metastases and in
various human melanoma cell lines, while we failed to detect any
presence of HAS1. The amount of the secreted HA can be reduced by
inhibition of calcineurin with CsA, while the inhibition of ERK1/2
exerted an opposite effect. HAS3 activity was likely regulated by
reversible phosphorylation controlled by PP2B and ERK1/2. We also
found that inhibition of ERK1/2 activity increased the HA-guided
migration of melanoma cells favouring lower molecular weight HA as
a chemoattractant.
Materials and methods
Culturing melanoma and melanocyte cell
lines
Human melanoma HT168 cell line (kind gift of
professor József Tímár, Semmelweis University, Hungary) was
established from A2058 cell line according to its metastasis
formation in immunosuppressed mice (29), while WM35 obtained from ATCC
(ATCC® CRL-1661™, Manassas, VA, USA) originally was
isolated from a primary cutaneous melanoma of radial growth phase
(30). Cells were nourished with
RPMI-1640 culture medium (Sigma-Aldrich, St. Louis, MO, USA)
supplemented with 10% fetal bovine serum (PAA, Piscataway, NJ,
USA), 4.1 g/l glucose, 2 mmol/l L-glutamine (Gibco, Gaithersburg,
MD, USA), penicillin (100 U/ml) and streptomycin (100 μg/ml). NHEM
cell line (PromoCell GmbH, Heidelberg, Germany) was cultured in
Melanocyte Medium (PromoCell GmbH) according to the instructions of
the manufacturer. Cells were incubated at 37°C in the presence of
95% air and 5% CO2 atmosphere and 80% humidity in
25-cm2 flasks (PAA Laboratories) until −70%
confluence.
Inhibition of calcineurin and ERK
1/2
Activity of calcineurin and ERK1/2 was inhibited
with the continuous application of 2 μM cyclosporine A (CsA)
(Sigma-Aldrich) (dissolved in sterile DMSO) and 5 μM PD098059 (PD)
(Sigma-Aldrich) (dissolved in sterile DMSO), respectively, started
2 days before confluence. DMSO was administered as vehicle-control
but no alterations were shown in any of the experiments comparing
with the untreated controls (data not shown).
bHABC-histochemistry and
immunocytochemistry
Histological samples with melanoma metastases
(mesenterial lymph node and lung lesions) as well as control
tissues from healthy human bodies were collected from cadavers with
the assistance of the Pathology and the Forensic Medicine
Departments (n=3). The study was approved by the Ethics Committee
of University of Debrecen, under licence number 3244-7/2011. For
morphological analysis, cell lines were cultured on rectangular
cover glasses (Menzel-Gläser, Menzel GmbH, Braunschweig, Germany).
Cadaver tissue samples and cell cultures were fixed in
Saint-Marie's fixative (99% ethanol and 1% anhydrous acetic acid)
for 24 or 1 h, respectively. Tissues were embedded into paraffin
and 7-μm thick sections were cut. After removal of paraffin and
rehydration in ethanol, PBS supplemented with 1% bovine serum
albumin (BSA, Amresco LLC, Solon, OH, USA) at 37°C for 1 h was
applied to block nonspecific antibody binding. HA was detected by
using a biotinylated HA-binding complex in 5 μg/ml concentration
(bHABC was kindly provided by R. Tammi and M. Tammi, Department of
Anatomy, University of Kuopio, Kuopio, Finland) at 4°C overnight.
The reaction was visualized with Streptavidin-Alexa 555 (2 μg/ml,
Invitrogen Corp., Carlsbad, CA, USA) for fluorescence microscopy.
Cultures and tissues were mounted in Vectashield Hard Set mounting
medium (Vector Laboratories Ltd., Peterborough, UK) containing DAPI
to visualise the nuclei of cells. In cadaver tissue samples,
monoclonal MelanA antibody (Novocastra Laboratories Ltd.,
Newcastle, UK) was used to demonstrate melanin-positive cells
labelling with an Alexa 488 conjugated anti-mouse antibody
(Invitrogen Corporation) at a dilution 1:1,000.
For HAS2, HAS3, RHAMM and CD44 immunocytochemistry
cell tissues were incubated with primary antibodies at a dilution
described in Table I, at 4°C
overnight. For visualisation of the primary antibodies Alexa Flour
488-conjugated goat anti-rabbit (Invitrogen Corp.) and Alexa Fluor
488-conjugated goat anti-mouse (Invitrogen Corp.) secondary
antibodies were used at a dilution of 1:1,000. Samples were mounted
in Vectashield Hard Set mounting medium (Vector Laboratories, Ltd.)
containing DAPI to visualise the nuclei of cells. Photomicrographs
of the samples were taken using an Olympus DP72 camera on a Nikon
Eclipse E800 microscope (Nikon Corp., Tokyo, Japan). Images were
acquired using cellSense Entry 1.5 software (Olympus, Shinjuku,
Tokyo, Japan) using constant camera settings to allow comparison of
fluorescent signal intensities.
 | Table IThe antibodies used in the
experiments. |
Table I
The antibodies used in the
experiments.
| Antibody | Host animal | Dilution for
western blotting | Dilution for
immunocytochemistry | Distributor; cat
no. |
|---|
| Anti-HAS1 | Goat,
polyclonal | 1:200 | 1:50 | Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA
sc-23145 |
| Anti-HAS2 | Rabbit,
polyclonal | 1:200 | 1:50 | Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA
sc-66916 |
| Anti-HAS3 | Rabbit,
polyclonal | 1:400 | 1:400 | Abcam, Camridge,
UK
ab154104 |
| Anti-HAS3 | Rabbit,
polyclonal | 1:400 | 1:1,000 | Sigma-Aldrich, St.
Louis, MO, USA
SAB1101156 |
| Anti-CD44 | Mouse,
monoclonal | 1:400 | 1:100 | Gift from: Tammi
& Tammi University of Kuopio, Finland |
| Anti-CD44 | Mouse, monoclonal
Clone: 2C5 | 1:400 | 1:100 | R&D Systems,
Minneapolis, MN, USA
BBA10 |
| Anti-RHAMM | Mouse, monoclonal,
clone: 2D6 | 1:400 | 1:100 | Novocastra,
Newcastle, UK
NCL-CD168 |
| Anti-β-actin | Mouse, monoclonal,
clone: AC-15 | 1:10,000 | - | Sigma-Aldrich, St.
Louis, MO, USA
A5441 |
Migration assays in Boyden chemotaxis
chamber
Cells were washed twice in CMF-PBS, harvested with
0.25% trypsin (Sigma-Aldrich) and resuspended in RPMI-1640 medium
in a density of 2×105 cells/ml. Lower wells of 48-well
Boyden chemotaxis chamber (Neuro Probe Inc., Gaithersburg, MD, USA)
were filled with human umbilical cord HA (1,600 kDa) or
Streptomyces HA (300–800 kDa) (Sigma-Aldrich) dissolved in CMF-PBS
at a concentration of 400 and 800 μg/ml, respectively and covered
with a polycarbonate filter (Neuro Probe Inc.) containing pores
with a diameter of 3 μm. Cell suspension (50 μl) was inoculated
into the wells on the top of the membrane and the chamber was
incubated for 3 h at 37°C in a humidified atmosphere (5%
CO2-95% air). Non-migrated cells were removed from the
surface of the membrane and after fixation in methanol, migrated
cells were stained with 1% toluidine blue (Sigma-Aldrich) dissolved
in water. Membranes were air-dried and mounted with Pertex
(Sigma-Aldrich). Absolute cell numbers were counted using a light
microscope. Six-wells were counted in each experimental group and
three independent assays were performed.
Measurement of cell proliferation with
[3H]-thymidine labelling
Medium containing 1 μCi/ml [3H]-thymidine
(185 GBq/mM) [3H]-thymidine (American Radiolabeled
Chemicals, Inc. St. Louis, MO, USA) was added to the cells cultured
in wells of 24-well plates for 16 h after 2 days of PD treatment.
After washing with PBS, proteins were precipitated with ice-cold 5%
trichloroacetic acid for 20 min. After washing with PBS again,
cells were harvested using 0.25% trypsin for 10 min. Cells were
collected with centrifugation at 2,000 rpm, the pellet was
resuspended in 10 μl CMF-PBS and placed into wells of special,
opaque 96-well plates (Wallac, Perkin-Elmer Life and Analytical
Sciences, Shelton, CT, USA). The plates were placed in an
exsiccator containing phosphorous pentoxide in order to absorb
moisture. Prior to the measurements, 50 μl scintillation solution
(MaxiLight; Hidex, Turku, Finland) was added to each well, and
radioactivity was counted by a liquid scintillation counter
(Chameleon Microplate Reader, Hidex). Measurements were carried out
in 6 samples of each experimental group in 3 independent
experiments.
RNA isolation and reverse transcriptase
PCR analysis
Cell cultures were dissolved in TRIzol (Applied
Biosystems, Foster City, CA, USA) and after the addition of 20%
RNase free chloroform, samples were centrifuged at 4°C at 10,000 x
g for 20 min. Samples were incubated in 500 μl of RNase-free
isopropanol at −20°C for 1 h. After washing the centrifuged pellet
by 70% ethanol the total RNA was resuspended in RNase-free water
and stored at −20°C. The assay mixture for reverse transcriptase
reaction containing 2 μg RNA was performed by High Capacity RT kit
(Applied Biosystems) according to the manufacturer's instructions.
Amplifications were performed in a thermal cycler (Labnet
MultiGene™ 96-well Gradient Thermal Cycler; Labnet International,
Edison, NJ, USA) in a final volume of 25 μl [containing 1 μl
forward and reverse primers (0.4 μM), 0.5 μl dNTP (200 μM), and 5 U
of Promega GoTaq® DNA polymerase in 1X reaction buffer]
as follows: 95°C, 2 min, followed by 35 cycles (denaturation, 94°C,
1 min; annealing at optimised temperatures as given in Table II for 1 min; extension, 72°C, 90
sec) and then 72°C, 10 min. The sequences of primer pairs, and
further details of polymerase chain reactions, are given in
Table II. PCR products were
analysed by electrophoresis in 1.2% agarose gel containing ethidium
bromide. GAPDH was used as internal control. Optical density of
signals was measured by ImageJ 1.40 g freeware and results were
normalised to the optical density of untreated control
cultures.
 | Table IINucleotide sequences, amplification
sites, GenBank accession numbers, amplimer sizes and PCR reaction
conditions for each primer pair are shown. |
Table II
Nucleotide sequences, amplification
sites, GenBank accession numbers, amplimer sizes and PCR reaction
conditions for each primer pair are shown.
| Gene | Primer | Nucleotide sequence
(5′-3′) | GenBank ID | Annealing
temperature | Amplimer size
(bp) |
|---|
| HAS1 | Sense | CCT ACG AGG CGG TGG
TCT (1261–1278) | NM_001297436 | 57°C | 306 |
| Antisense | GCA GAG GGA CGT AGT
TAG CG (1566–1547) |
| HAS2 | Sense | ACA GGC ATC TCA CGA
ACC (1479–1496) | NM_005328 | 49°C | 415 |
| Antisense | ATC TTG GCG GGA AGT
AAA (1893–1876) |
| HAS3 | Sense | TCG GCG ATT CGG TGG
ACT (835–852) | NM_001199280 | 57°C | 280 |
| Antisense | TGC TGG AGG AGG CTG
TTG C (1114–1096) |
| RHAMM | Sense | AAA GTT AAG TCT TCG
GAA TC (384–403) | NM_001142556 | 46°C | 371 |
| Antisense | CCT TCT TGC TTA GCC
ATC (754–737) |
| CD44 | Sense | TTG TGG CAT TTA TTC
ATC AG (4076–4095) | NM_000610.3 | 46°C | 321 |
| Antisense | GGT AGA CAG GGA GGA
GCA (4396–4379) |
| GAPDH | Sense | CCA GAA GAC TGT GGA
TGG CC (740–759) | NM_002046 | 54°C | 411 |
| Antisense | CTG TAG CCA AAT TCG
TTG TC (1150–1131) |
Preparation of total cell lysates and
western blot analysis
Cell cultures were washed in physiological NaCl
solution and then harvested. After centrifugation (2,000 rpm, 10
min), cell pellets were suspended in 100 μl of homogenization RIPA
(radioimmunoprecipitation assay)-buffer (150 mM sodium chloride;
1.0% NP40, 0.5% sodium deoxycholate; 50 mM Tris, pH 8.0) containing
protease inhibitors [aprotinin (10 μg/ml), 5 mM benzamidine,
leupeptin (10 μg/ml), trypsine inhibitor (10 μg/ml), 1 mM PMSF, 5
mM EDTA, 1 mM EGTA, 8 mM Na-Fluoride, 1 mM Na-orthovanadate].
Samples were stored at −70°C. Suspensions were sonicated by pulsing
burst for 30 sec at 40 A (Cole-Parmer, Illinois, USA). For western
blotting, total cell lysates were used.
Samples for SDS-PAGE were prepared by the addition
of Laemmli electrophoresis sample buffer (4% SDS, 10%
2-mercaptoethanol, 20% glycerol, 0.004% bromophenol blue, 0.125 M
Tris-HCl pH 6.8) to cell lysates to set equal protein concentration
of samples, and boiled for 10 min. Approximately 40 μg of protein
was separated by 7.5% SDS-PAGE gel for detection of HAS1, HAS2,
HAS3, RHAMM, CD44, and actin. Proteins were transferred
electrophoretically to nitrocellulose membranes. After blocking
with 5% non-fat dry milk in PBS, membranes were washed and exposed
to the primary antibodies (Table
I) overnight at 4°C. PhosphoSer/Thr detection kit (Millipore,
Billerica, MA, USA) was used to detect phosphorylation level of
Ser/Thr amino acid side chains. After washing for 3×10 min in PBST,
membranes were incubated with HRP conjugated anti-rabbit IgG
(Bio-Rad Laboratories, CA, USA) in 1:1,500 or anti-mouse IgG
(Bio-Rad Laboratories) in 1:1,500 dilution. Signals were detected
by enhanced chemiluminescence (Millipore) according to the
instructions of the manufacturer. Signals were manually developed
on X-ray film (Agfa-Gevaert Group, Mortsel, Belgium). Optical
density of western blot signals was measured by using ImageJ 1.40 g
freeware and the results were normalised to the values of untreated
control cultures.
Statistical analysis
Optical density (OD) of RT-PCR and western blot
results were normalised to the inner controls, then the ODs of the
control samples of every experimental group and CsA or PD treated
cultures of each melanoma cell line were used for statistical
analysis. Statistical comparisons between control and test samples
were performed by using Student's paired t-test where statistical
method reported significant differences among the groups
(P<0.05). The data are representative of at least three
different experiments. Where applicable, data are expressed as mean
± SEM.
Results
HA homeostasis of normal skin and
melanoma metastasis
Although presence of HA around epidermal
keratinocytes (31) and expression
of HAS enzymes by these cells have been described long ago
(32,33). Involvement of melanocytes in
epidermal HA homeostasis is less evident. Therefore, first we
investigated the HA secretion of normal melanocytes locating in the
stratum basale of human epidermis. We were able to demonstrate only
a weak signal for HA in the basal cell layer of the epithelium
(Fig. 1A-a.1). Expression of all
the three HAS enzymes was also monitored. We failed to demonstrate
either HAS1 (data not shown) or HAS2 in the epidermis of human skin
tissue samples (Fig. 1B-b.1), but
a well visible HAS3 immunopositivity was detected in the stratum
basale where melanocytes reside accompanied with undifferentiated
keratinocytes (Fig. 1C-c.1). As
tumour invasion and motility are highly dependent on the ECM
surrounding the malignant cells and HA has been proven to be one of
the extracellular macromolecules which can be responsible for the
regulation of infiltration of tissues in the vicinity of primary
tumours (34), next we
investigated HA and HAS content of melanoma samples. Two
differently evolved metastases of melanoma were screened
(mesenterial lymph node and lung lesions) and compared with normal
tissues from healthy human cadavers. HA was modestly detectable in
the normal lung and mesenteric lymph node (Fig. 1A-a.2 and a.4) but a strongly
enhanced HA signal was observed either in lymph node (Fig. 1.A-a.3) or lung with melanoma
metastases (Fig. 1A-a.5). HAS1 was
detectable neither in normal skin, nor in malignant lesions (data
not shown). The expression of HAS2 and HAS3 was not detectable with
immunohistochemistry in normal lymph nodes (Fig. 1B-b.2 and c.2) or the lung (Fig. 1B-b.4 and c.4), but a strong signal
of HAS2 immunopositivity was observed in lymph node melanoma
metastases (Fig. 1B-b.3). In spite
of the strong autofluorescence signal of elastic fibres in the
lung, a well visible colocalization of HAS2 and MelanA was detected
(Fig. 1B-b.5). Similarly to HAS2,
HAS3 was not detected in the normal healthy tissues with this
method (Fig. 1C-c.2 and c.4), but
a pronounced expression was demonstrated in metastatic lymph nodes
and lung metastases (Fig. 1C-c.3 and
c.5). Strong HAS3 immunopositivity was seen close to the nuclei
of MelanA expressing cells, as well as in the surrounding stromal
cell population.
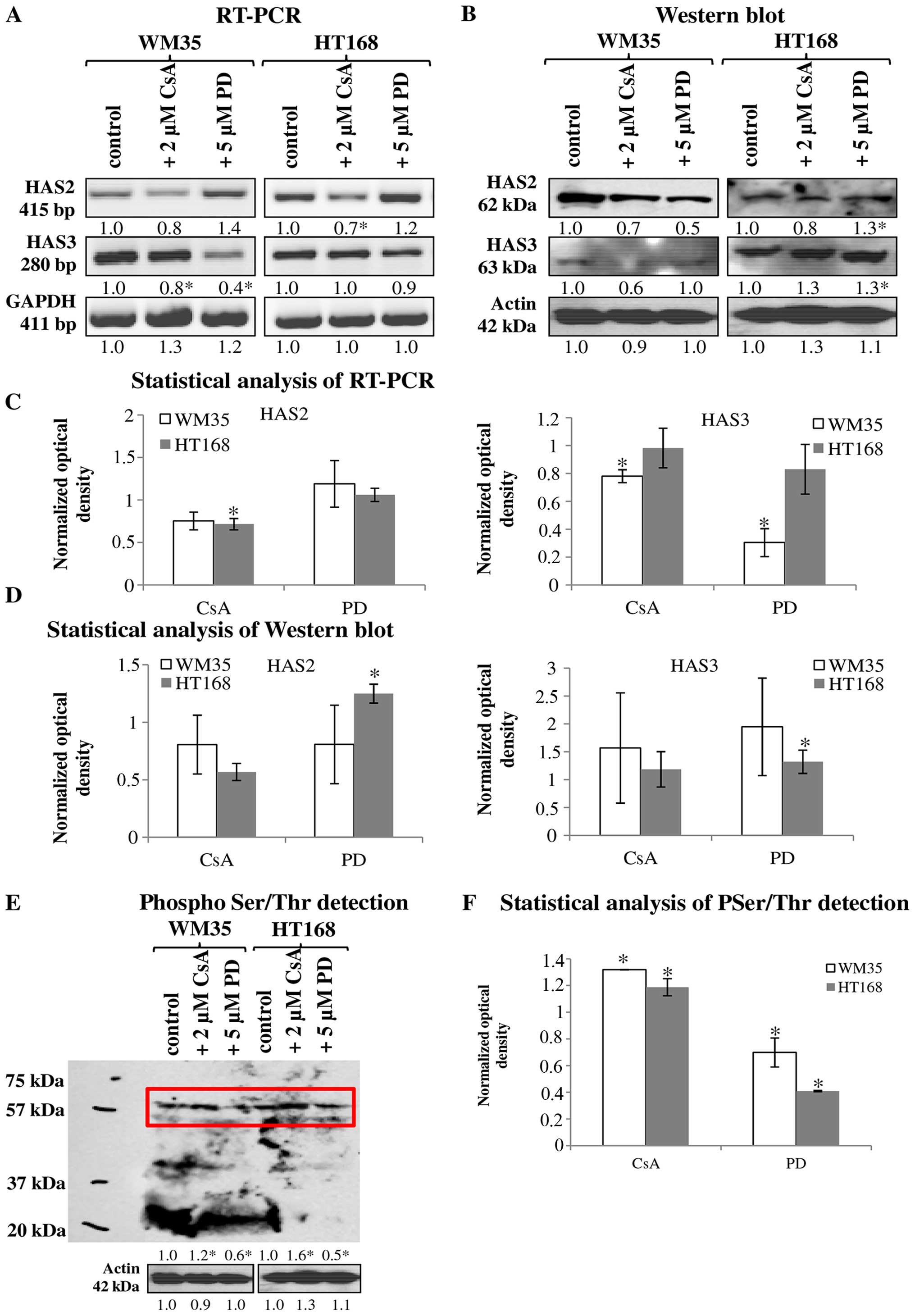
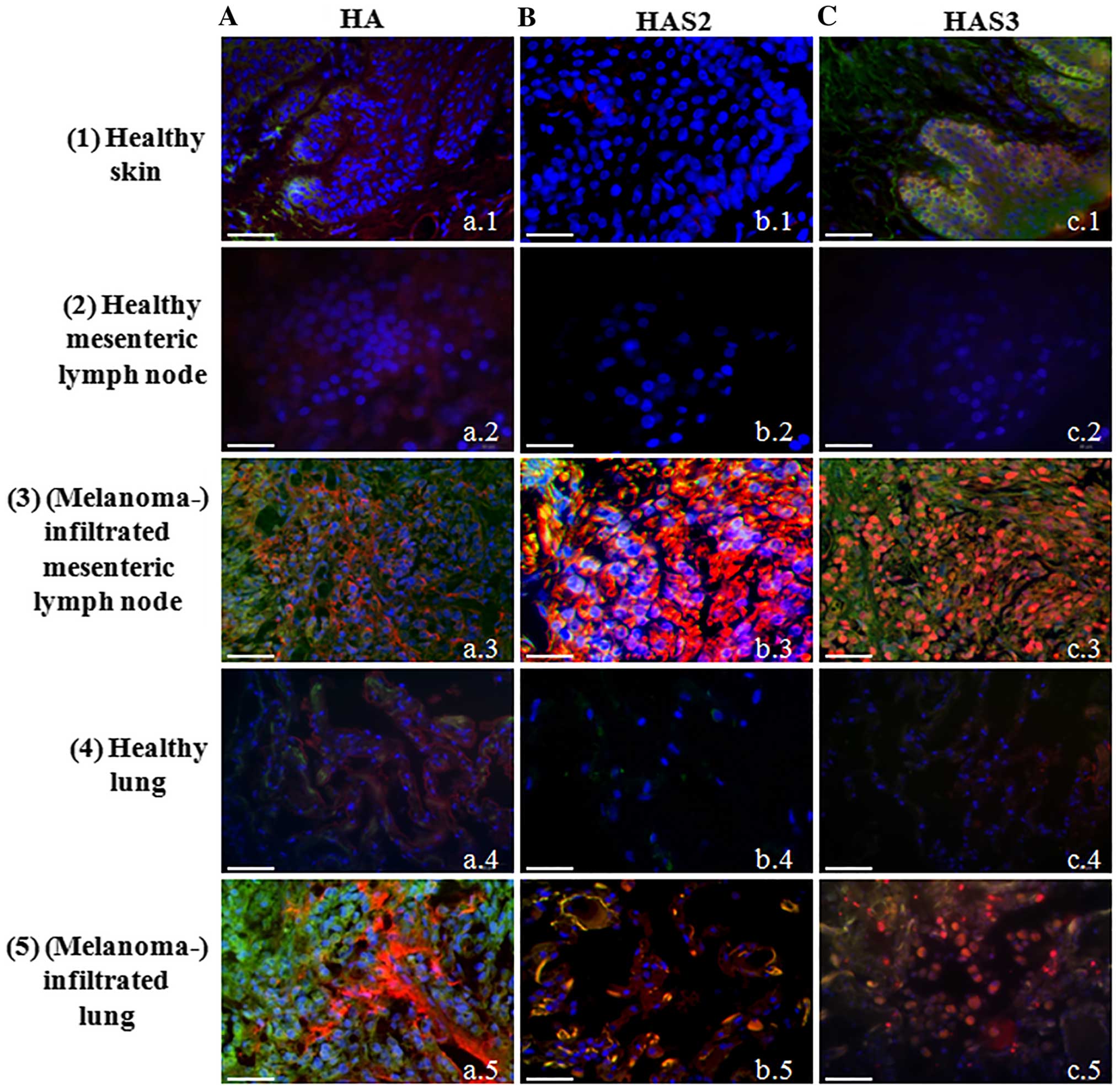 | Figure 1Detection of hyaluronic acid, HAS2
and HAS3 in normal healthy tissues and malignant metastases. Row 1,
normal skin, 2, mesenteric lymph node, 3, melanoma metastasis from
mesenteric lymph node, 4, normal lung, 5, melanoma metastasis from
lung. (A) HA affinity histochemistry. (B) HAS2. (C) HAS3
immunohistochemistry. HA is labelled with Streptavidin-Alexa 555
(red), HAS2 and HAS3 anti-rabbit-Alexa 555 (red). Melanocytes and
melanoma cells are shown with MelanA-Alexa 488 (green). Nuclei are
demonstrated with DAPI (blue). Scale bar, 50 μm. Representative
photomicrographs of 3 independent experiments are shown. |
Regulation of HA secretion of in vitro
melanocyte cell culture
Since the HA secretion and HAS expression were
markedly altered in tissue samples with melanoma metastases we
investigated the HA homeostasis of normal human melanocyte (NHEM)
in a cell culture system. Similarly to the melanocytes locating in
the stratum basale of epidermis, cultured melanocytes secreted only
a low amount of HA, hardly detectable with affinity cytochemistry
(Fig. 2A). As secretion of HA can
be regulated by reversible phosphorylation, we investigated the
involvement of ERK1/2 in the HA production and we also aimed to
identify the involvement of a possible PP, calcineurin in this
process. PD098059 was administered as a MAPK and CsA as a
calcineurin inhibitor. Inhibition of PP2B activity resulted in an
undetectably low HA production of cultured melanocytes (Fig. 2A). In contrast, the inhibition of
ERK1/2 elevated the HA secretion of normal human melanocytes
(Fig. 2A). These data suggest that
the two enzymes exert the opposite effect on HA production of NHEM
cells. In terms of the synthesising enzymes, mRNA of HAS1 was not
present (data not shown), while the mRNAs of HAS2 and HAS3 were
present in the NHEM cells (Fig.
2B), although only weak signals of protein expression were seen
with western blot method (Fig.
2C). HAS2 was hardly detectable in NHEM (Fig. 2E) while HAS3 gave evident, strong
signals with immunocytochemistry (Fig.
2F). Inhibition of calcineurin did not alter the mRNA
expression of HAS3 and HAS2 (Fig. 2B
and D), but reduction in the protein expression of the
synthases was observed (Fig.
2C–F). Inhibition of MAPK pathway exerted different effect on
HAS expression of NHEM cells. It did not alter the expression of
HAS2 (Fig. 2B–E), but resulted in
a modest elevation of the mRNA expression (Fig. 2B and D) and a pronounced increase
of the protein expression of HAS3 (Fig. 2C, D and F). Interestingly, a strong
accumulation of HAS3 was visible around the nuclear area in NHEM
cells after ERK1/2 inhibition (Fig.
2F).
Cells of both malignant melanoma cell
lines secrete HA, but show various HAS expression patterns
It is already known that HA regulates the migration,
proliferation and other cell biological features of malignant cells
(5), so we aimed to detect the
presence of this extracellular matrix component and to identify the
HAS enzymes responsible for the production of HA in melanoma cell
lines established from various stages of cutaneous melanoma.
HA was detected both intracellularly and
pericellularly in the examined cell lines (Fig. 3A-a and d). The detected HA is the
product of HAS2 and/or HAS3 enzymes because their mRNA (Fig. 4A and C) and protein expressions
(Fig. 4B and D) were proven by
reverse transcriptase PCR and western blot analyses, respectively.
Neither mRNA nor protein expression of HAS1 was detected (data not
shown). Similarly to NHEM, both melanoma cell lines expressed the
mRNAs of both HAS enzymes (Fig.
4A) but the protein expression of the synthases showed slight
differences. HAS2 protein expression was stronger in WM35 cells
(Figs. 3B-a, 4B and D), while it remained weaker in
HT168 cells (Figs. 3B-d, 4B and D). HAS3 protein expression showed
the opposite pattern, it was stronger in HT168 cells and was almost
undetectable in WM35 cells (Figs.
3C-d, 4B and D). In both cell
types, HAS3 formed filamentous arrangement in the cytoplasm and
positive signals associated to the nuclear region were also
detected (Fig. 3C-a and d).
 | Figure 3Detection of HA, HAS enzymes in WM35
and HT168 melanoma cell lines. (A) HA affinity cytochemistry
demonstrating secreted HA in red (Streptavidin-Alexa 555).
Untreated cells of WM35 (a), WM35 cells treated with 2 μM of CsA
(b), and with 5 μM of PD098059 (c), untreated HT168 cells (d),
HT168 cells treated with 2 μM of CsA (e), and with 5 μM of PD098059
(f). DAPI was used for visualisation of the nuclei. Scale bar, 20
μm. Representative photomicrographs of 3 independent experiments.
(B and C) Immunocytochemistry of HAS2 and HAS3, positive signals
appear in green (Alexa 488). Untreated cells of WM35 (a), WM35
cells treated with 2 μM of CsA (b), with 5 μM of PD098059 (c),
untreated HT168 cells (d), HT168 cells treated with 2 μM of CsA
(e), and with 5 μM of PD098059 (f). DAPI was used for visualisation
of the nuclei. Scale bar, 20 μm. Representative photomicrographs of
three independent experiments are shown. |
Inhibition of ERK1/2 and PP2B altered the
HA secretion of melanoma cells
Treatment of melanoma cells with 2 μM CsA notably
decreased the HA secretion (Fig. 3A-b
and e), while the inhibition of ERK 1/2 pathway with
administration of 5 μM of PD098059 increased the HA production of
both cell lines (Fig. 3A-c and f).
Parallel with the HA production, application of CsA also decreased
HAS2 protein expression (Figs. 3B-b
and e, 4B and D). Presence of
the dominantly expressed HAS3 isoform of aggressive metastatic
HT168 cells was significantly elevated by PD098059 treatment but no
alteration was detected after the administration of CsA (Figs. 3C-c and f, 4B and D). Expression of HAS3 isoform
showed only a modest decrease in WM35 cells after PP2B inhibition
(Figs. 3C-b, 4B and D). At the level of ~60 kDa
molecular weight we detected increased Ser/Thr phosphorylation at
the presence of CsA. Administration of PD098059 exerted an opposite
effect it resulted in weaker signal for phosphorylated proteins at
the same molecular weight. These observations suggest altered
phosphorylation and the likelihood of a consequent change in the
enzymatic activity of HAS2 and/or HAS3 under the effect of the
applied enzyme inhibitors (Fig. 4E and
F).
Involvement of receptors of hyaluronan
binding in reversible dephosphorylation
It has been published that both CD44 and RHAMM have
important roles in the signal transduction of melanoma cells
(35). In vitro, both of
these receptors can play a role in the regulation of the migration
of melanoma cell lines. Expression of mRNA and protein of RHAMM and
CD44 were demonstrated in both cell lines (Fig. 5A–D). RHAMM gave strong
intracellular signals and the observed filamentous organization of
the positivity suggests association to cytoskeletal elements. CD44
also showed cytoplasmic positivity but membrane associated signals
were also detected (Fig. 5E-a and
F-a). Protein expression of RHAMM was higher in HT168 cell line
than in WM35, while CD44 exhibited similar expression pattern both
in HT168 and WM35 cell lines (Fig. 5B
and D). Not every cell of the WM35 cells showed RHAMM
positivity with immunocytochemistry (Fig. 5E-a). Administration of 2 μM CsA did
not significantly alter the protein expression of RHAMM in HT168
and in WM35 melanoma cells (Fig. 5B, D
and E-b and e). In contrast, the application of 5 μM PD098059
intensely elevated the protein expression of RHAMM (Fig. 5B, D and E-c and f). Furthermore,
none of the inhibitors had prominent effect on the CD44 protein
expression (Fig. 5B, D and F).
Intra- and/or extracellular function of
secreted HA
Since incorporated HA can be partly responsible for
the regulation of proliferation of tumour cells (5) we investigated the effects of
inhibitors on cellular division of WM35 and HT168 melanoma cells.
We have previously published results that CsA reduces the
proliferation of both cell lines (28). In contrast, PD098059 elevated the
proliferation rate of HT168 cells (Fig. 6A).
As strong HA signals localized in the pericellular
matrix of melanoma cells either in the melanoma cell cultures or in
the tissue samples containing melanoma metastases, a supportive
effect of HA on cell motility seemed very likely (5). Hence, we investigated the migratory
properties and invasiveness of HT168 and WM35 melanoma cell lines.
An in vitro migration assay was performed in Boyden chamber
in the presence of hyaluronic acid (a higher, 1,600 kDa and a
lower, 300–800 kDa molecular weight HA solution) as a
chemoattractant. We did not find significant differences between
the migrations of these cell lines towards different size HA
chemoattractants (Fig. 6B). As a
result of the 2 μM CsA or 5 μM PD098059 treatments, the average
number of the migrated HT168 cells toward lower molecular weight of
HA was elevated but no significant alteration was shown in the
presence of 1,600 kDa HA (Fig.
6D). While the administration of CsA markedly diminished the
migration of WM35 cells, the presence of PD098059 significantly
facilitated the migration toward 300–800 kDa HA (Fig. 6C). In contrast, cell motility in
the presence of the 1600 kDa HA was not significantly altered by
PD098059 administration (Fig.
6C).
Discussion
Melanoma is one of the most aggressive and rapidly
invading tumours with the worst prognosis in clinical dermatology.
Formation of metastasis of malignantly transformed melanocytes is
highly dependent on the cell surface receptor composition and any
alterations in the composition and/or organization of the
pericellular matrix (2–4). Presence of HA at the vicinity of
keratinocytes has been proved in human skin (36) and its function in the metastasis
formation during melanoma progression has also been demonstrated
(5,37). Accumulation of HA and the
activation of HA synthases during skin injury (31) or by keratinocyte growth factor
(38) play a crucial role in the
reconstruction of the integrity of epidermis and the subsequent
tissues. Different molecular sized HA was produced by each of the
HAS1, -2 and -3, proven to exert diverse effects on the normal life
cycle of cells and can influence invasiveness of malignant cells
(39). The altered expression of
each HAS has been published in different stages of melanoma and HA
accumulation surrounding primer tumours was also detected (37). In the present study, we proved the
presence of HA and HAS3 in the MelanA positive melanocytes along
with a weak expression of HAS2 in the stratum basale of the normal
epidermis. In contrast to the data published (37), we found elevated HA, HAS2 and also
HAS3 expression but did not detect any HAS1 in malignant lesions
such as lung and mesenteric lymph node metastases. The lack of HAS1
enzyme can be a result of metabolic differences of the three HAS
enzymes, as HAS1 requires higher concentration of HA precursors
(40). Nonetheless, abundant
expression and prognostic correlation with the presence of HAS1 was
found in case of breast cancer (41). Some studies indicated that
inhibition of HA synthesis and accumulation of HA in the cell coat
with 4-methyl-umbelliferion can also diminish migration of some
type of tumour cells such as BF16 melanoma cell lines while it has
no effect on other malignant cells such as breast cancer cells
(42). These observations may
reflect on differences in the enzymatic source of the HA-rich
pericellular matrix in various malignancies. There are data
demonstrating, that overexpression of HAS3 results in increase of
cell surface HA and enhances cell locomotion (43) and mutations and aberrant splicing
of HAS may alter the migration of tumour cells (18). Clearly, the regulation of the HA
production by various HAS enzymes in different malignant tissues is
an important factor which should have a deep impact on the
behaviour of malignant cells. Therefore, we investigated the
molecular regulation of HA synthesis focusing on Ser/Thr
phosphorylation of HAS enzymes.
HA stimulates migration of melanoma cell lines in
vitro via the interaction with CD44 and RHAMM (44–46).
A variety of signalling pathways have been identified which
associate with RHAMM (47–51) and CD44 (52–54).
CD44 phosphorylation is one of the most important
posttranscriptional modifications in the activation of cell-cell or
cell-matrix interactions during migration (14). Another HA receptor, RHAMM regulates
signalling cascades which are linked to the MAP kinase pathway,
whose Ser/Thr specific protein kinase elements can be mutated in
melanoma cells (55). ERK1/2
regulates proliferation of cancer cells (56) via a direct contact formed with the
RHAMM-HA complex (15,16). Thus, the HA induced signal
transduction and ERK have a well-defined role in tumour
progression, but only sporadic data exist in connection with HA
synthases. It has been proven that HAS2 can be Ser/Thr
phosphorylated which may modify the HA synthesis of cells (57). As ERK1/2 has an indirect link to HA
homeostasis via RHAMM binding, and it could be a question of
interest whether this RHAMM-HA-ERK1/2 complex has any effect on HA
synthesis (58). We found that the
inhibition of ERK1/2 increased the secreted HA and elevated the
expression of HAS3 in melanoma cell cultures. A similar phenomenon
was described in a synoviocyte cell culture system (59). We also demonstrated, that ERK1/2
inhibition reduced the Ser/Thr phosphorylated proteins at the
molecular weight of 60 kDa, which suggests that HAS2 and/or HAS3
can be targets of ERK signalling. It was an interesting phenomenon
that HAS3 immunopositivity showed a filamentous organization and
signals also appeared close to the nuclear area of the cells in
melanoma cell lines. NHEM cells showed a strong accumulation of
HAS3 around the nuclear area after the inhibition of ERK1/2. These
findings strengthen the idea that ERK signalling pathways play role
in the regulation of HA synthases (58) and suggest that MAPK-signalling may
influence the intracellular localization of HAS enzymes,
particularly HAS3. Another interesting finding was that the ERK
inhibition enhanced migration towards smaller HA. As HAS3 produces
lower molecular weight HA, this observation further strengthens the
probability of the functional link of ERK1/2 and this isoform of
HAS (60). Smaller HA (40–70 kDa)
can be incorporated to cells by endocytosis (17) and enhances proliferation, thus the
increased level of HA after ERK inhibition can be one of the
factors that have positive effect on cellular division as well
(5). Indeed, we found that upon
inhibition of MAPK pathway with PD098059, proliferation of the
highly aggressive HT168 cells significantly increased.
We have published that inhibition of the
Cacalmodulin dependent cellular PP, PP2B also known as calcineurin,
with cyclosporine A attenuated proliferation and enhanced
expression and phosphorylation of ERK1/2 in HT168 and WM35 cell
lines (28). In the present study
we aimed to explore if the inhibition of this PP exerts any effect
on the HA homeostasis of melanoma cells. To the best of our
knowledge, there are no published experimental data on the
involvement of PPs in the regulation of HA synthesis so far. Here
we demonstrated that the inhibition of PP2B (calcineurin), reduced
the amount of HA both in NHEM and melanoma cell lines and we
detected a significant increase of Ser/Thr phoshoproteins at 60
kDa, the molecular weight of HAS2 and HAS3, during administration
of CsA. These observations together with our above mentioned
previous findings suggest, that PP2B may influence phosphorylation
of the HAS enzymes indirectly, via modulation of the activity of
MAPK pathway. Moreover, the inverse correlation between the amount
of the phosphoproteins at 60 kDa to the detected amount of HA raise
the probability that phosphorylation of HAS enzymes at the Ser/Thr
residues, which are targeted either directly or indirectly by an
ERK1/2-PP2B axis, have negative effect on their biosynthetic
activity in the investigated melanoma cell lines. Although this
hypothesis requires further experimental support, Vigetti et
al (61) observed a similar
phenomenon in aortic smooth muscle cells, when activation of MAPK
caused phosphorylation on threonine 110 in HAS2 and enzymatic
activity became markedly lowered as the consequence of this
posttranslational modification (61). In contrast, we found that
pharmacological inhibition of MAPK pathway enhanced HA synthesis,
while Bourguignon et al reported, that ERK activation
mediated serine phosphorylation increased the HA secretion by all
the three HAS isoforms (62).
Clearly, the site of the phosphorylation has deep impact on the
activity of HAS enzymes, it can either increase or decrease it.
Moreover, certain cell specific aspects of the final outcome of HAS
phosphorylation should also be taken into consideration.
Expression of the HA receptors RHAMM and CD44 has
been proven both in malignant melanoma and in normal melanocytes
(5,63). RHAMM can be in a direct interaction
with intracellular HA through which it can bind to ERK1/2.
Therefore, the elevation of the expression of this HA binding
receptor together with the strong HA signals upon ERK inhibition in
both melanoma cell lines can be factors which promote migration
and/or proliferation (5). Either
the HA receptor RHAMM or ERK1/2 can bind to microtubules in the
cells. Both of RHAMM and HAS3 immunocytochemistry gave a sort of
filamentous arrangement in the cells in our experiments, supporting
the probability of RHAMM-ERK-HAS3 crosstalk in both investigated
melanoma cell lines (64). The
expression of HAS3 protein was significantly lower in WM35 cells
which may correlate with the milder phenotype of this cell line as
it was isolated from early stage of melanoma progression (65).
In conclusion, our results reflect on differences in
the chemoattractant behaviour of HA with various molecular weights.
Moreover, a complex regulatory mechanism via protein
phosphorylation of HAS2 and HAS3 is suggested in which PP2B plays a
promoting role, while MAPK pathway attenuates HA production and
RHAMM protein expression in WM35 and HT168 melanoma cell lines.
This latter observation may suggest that application of MAPK-ERK
pathway inhibitors requires careful, personalized therapeutic
design in case of melanoma patients.
Acknowledgements
The authors thank Mrs. Krisztina Bíró of the
Department of Anatomy, Medical and Health Science Centre,
University of Debrecen, Hungary for her skilful and excellent
technical assistance and Ms. Krisztina Körmendi and Ms. Renáta Sütő
medical students for their skilful help during the study. Our
grateful thanks are extended to Margit Balázs, and József Tímár for
providing melanoma cell lines during the initial phase of our
studies. This study was supported by grants from the Hungarian
Science Research Fund (OTKA-CNK80709) and by the
TÁMOP-4.2.2/B-10/1-2010-0024; and
TÁMOP-4.2.2.A-11/1/KONV-2012-0025. Éva Katona was supported by
TÁMOP 4.2.4. A/2-11-1-2012-0001 ‘National Excellence Program -
Elaborating and operating an inland student and researcher personal
support system’ project. The project is co-financed by the European
Union and the European Social Fund. Tamás Juhász was supported by
Bolyai Janos Research Scholarship, Szodoray Lajos and Magyary
Zoltán Funds by Hungarian Academy of Science and the European Union
and the State of Hungary, co-financed by the European Social Fund
in the framework of TÁMOP 4.2.4. A/2-11-1-2012-0001 ‘National
Excellence Program’. Tamás Juhász and Róza Zákány were supported by
GOP-1.1.1-11-2012-0197 financed by the Hungarian government and the
EU.
Abbreviations:
|
CsA
|
cyclosporin A
|
|
dNTP
|
deoxynucleotide triphosphate
|
|
ECM
|
extracellular matrix
|
|
EDTA
|
ethylene diamine tetra-acetic acid
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
FBS
|
fetal bovine serum
|
|
FGF
|
fibroblast growth factor
|
|
GAG
|
glycos-aminoglycan
|
|
HA
|
hyaluronic acid
|
|
HAS
|
hyaluronan synthase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
PBS
|
phosphate-buffered saline
|
|
PBST
|
phosphate-buffered saline supplemented
with 1% Tween-20
|
|
PKA
|
protein kinase A
|
|
PKC
|
protein kinase C
|
|
PP2B
|
protein phosphatase 2B
|
|
RHAMM
|
receptor for hyaluronan-mediated
motility
|
|
RT-PCR
|
reverse transcription followed by
polymerase chain reaction
|
|
TBE
|
Tris-boric acid-EDTA
|
References
|
1
|
Tsao H, Chin L, Garraway LA and Fisher DE:
Melanoma: From mutations to medicine. Genes Dev. 26:1131–1155.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mandalà M, Merelli B and Massi D: Nras in
melanoma: Targeting the undruggable target. Crit Rev Oncol Hematol.
92:107–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marshall JF, Nesbitt SA, Helfrich MH,
Horton MA, Polakova K and Hart IR: Integrin expression in human
melanoma cell lines: Heterogeneity of vitronectin receptor
composition and function. Int J Cancer. 49:924–931. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pocheć E, Janik M, Hoja-Łukowicz D,
Link-Lenczowski P, Przybyło M and Lityńska A: Expression of
integrins α3β1 and α5β1 and GlcNAc β1,6 glycan branching influences
metastatic melanoma cell migration on fibronectin. Eur J Cell Biol.
92:355–362. 2013. View Article : Google Scholar
|
|
5
|
Ahrens T, Assmann V, Fieber C, Termeer C,
Herrlich P, Hofmann M and Simon JC: CD44 is the principal mediator
of hyaluronic-acid-induced melanoma cell proliferation. J Invest
Dermatol. 116:93–101. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Itano N, Atsumi F, Sawai T, Yamada Y,
Miyaishi O, Senga T, Hamaguchi M and Kimata K: Abnormal
accumulation of hyaluronan matrix diminishes contact inhibition of
cell growth and promotes cell migration. Proc Natl Acad Sci USA.
99:3609–3614. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prehm P: Hyaluronate is synthesized at
plasma membranes. Biochem J. 220:597–600. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brinck J and Heldin P: Expression of
recombinant hyaluronan synthase (HAS) isoforms in CHO cells reduces
cell migration and cell surface CD44. Exp Cell Res. 252:342–351.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Itano N, Sawai T, Yoshida M, Lenas P,
Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y,
et al: Three isoforms of mammalian hyaluronan synthases have
distinct enzymatic properties. J Biol Chem. 274:25085–25092. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weigel PH, Hascall VC and Tammi M:
Hyaluronan synthases. J Biol Chem. 272:13997–14000. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goentzel BJ, Weigel PH and Steinberg RA:
Recombinant human hyaluronan synthase 3 is phosphorylated in
mammalian cells. Biochem J. 396:347–354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuroda Y, Kasai K, Nanashima N, Nozaka H,
Nakano M, Chiba M, Yoneda M and Nakamura T: 4-Methylumbelliferone
inhibits the phosphorylation of hyaluronan synthase 2 induced by
12-O-tetradecanoyl-phorbol-13-acetate. Biomed Res. 34:97–103. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sironen RK, Tammi M, Tammi R, Auvinen PK,
Anttila M and Kosma VM: Hyaluronan in human malignancies. Exp Cell
Res. 317:383–391. 2011. View Article : Google Scholar
|
|
14
|
Turley EA, Noble PW and Bourguignon LY:
Signaling properties of hyaluronan receptors. J Biol Chem.
277:4589–4592. 2002. View Article : Google Scholar
|
|
15
|
Maxwell CA, McCarthy J and Turley E:
Cell-surface and mitotic-spindle RHAMM: Moonlighting or dual
oncogenic functions? J Cell Sci. 121:925–932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Telmer PG, Tolg C, McCarthy JB and Turley
EA: How does a protein with dual mitotic spindle and extracellular
matrix receptor functions affect tumor susceptibility and
progression? Commun Integr Biol. 4:182–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiu L, Li Z, Qiao M, Long M, Wang M, Zhang
X, Tian C and Chen D: Self-assembled pH-responsive hyaluronic
acid-g-poly((L)-histidine) copolymer micelles for targeted
intracellular delivery of doxorubicin. Acta Biomater. 10:2024–2035.
2014. View Article : Google Scholar
|
|
18
|
Adamia S, Pilarski PM, Belch AR and
Pilarski LM: Aberrant splicing, hyaluronan synthases and
intracellular hyaluronan as drivers of oncogenesis and potential
drug targets. Curr Cancer Drug Targets. 13:347–361. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JY and Spicer AP: Hyaluronan: A
multifunctional, mega-Dalton, stealth molecule. Curr Opin Cell
Biol. 12:581–586. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Voelcker V, Gebhardt C, Averbeck M,
Saalbach A, Wolf V, Weih F, Sleeman J, Anderegg U and Simon J:
Hyaluronan fragments induce cytokine and metalloprotease
upregulation in human melanoma cells in part by signalling via
TLR4. Exp Dermatol. 17:100–107. 2008. View Article : Google Scholar
|
|
21
|
Hatano H, Shigeishi H, Kudo Y, Higashikawa
K, Tobiume K, Takata T and Kamata N: RHAMM/ERK interaction induces
proliferative activities of cementifying fibroma cells through a
mechanism based on the CD44-EGFR. Lab Invest. 91:379–391. 2011.
View Article : Google Scholar
|
|
22
|
Soares AS, Costa VM, Diniz C and Fresco P:
Inosine strongly enhances proliferation of human C32 melanoma cells
through PLC-PKC-MEK1/2-ERK1/2 and PI3K pathways. Basic Clin
Pharmacol Toxicol. 116:25–36. 2015. View Article : Google Scholar
|
|
23
|
Haydn JM, Hufnagel A, Grimm J, Maurus K,
Schartl M and Meierjohann S: The MAPK pathway as an apoptosis
enhancer in melanoma. Oncotarget. 5:5040–5053. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rusnak F and Mertz P: Calcineurin: Form
and function. Physiol Rev. 80:1483–1521. 2000.PubMed/NCBI
|
|
25
|
Dotto GP: Calcineurin signaling as a
negative determinant of keratinocyte cancer stem cell potential and
carcinogenesis. Cancer Res. 71:2029–2033. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smit NP, Van Rossum HH, Romijn FP, Sellar
KJ, Breetveld M, Gibbs S and Van Pelt J: Calcineurin activity and
inhibition in skin and (epi)dermal cell cultures. J Invest
Dermatol. 128:1686–1690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Breuer K, Werfel T and Kapp A: Allergic
manifestations of skin diseases--atopic dermatitis. Chem Immunol
Allergy. 91:76–86. 2006. View Article : Google Scholar
|
|
28
|
Juhász T, Matta C, Veress G, Nagy G,
Szíjgyártó Z, Molnár Z, Fodor J, Zákány R and Gergely P: Inhibition
of calcineurin by cyclosporine A exerts multiple effects on human
melanoma cell lines HT168 and WM35. Int J Oncol. 34:995–1003.
2009.PubMed/NCBI
|
|
29
|
Ladányi A, Tímár J, Paku S, Molnár G and
Lapis K: Selection and characterization of human melanoma lines
with different liver-colonizing capacity. Int J Cancer. 46:456–461.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Herlyn M: Human melanoma: Development and
progression. Cancer Metastasis Rev. 9:101–112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tammi R, Pasonen-Seppänen S, Kolehmainen E
and Tammi M: Hyaluronan synthase induction and hyaluronan
accumulation in mouse epidermis following skin injury. J Invest
Dermatol. 124:898–905. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sugiyama Y, Shimada A, Sayo T, Sakai S and
Inoue S: Putative hyaluronan synthase mRNA are expressed in mouse
skin and TGF-beta upregulates their expression in cultured human
skin cells. J Invest Dermatol. 110:116–121. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tammi R, Ripellino JA, Margolis RU and
Tammi M: Localization of epidermal hyaluronic acid using the
hyaluronate binding region of cartilage proteoglycan as a specific
probe. J Invest Dermatol. 90:412–414. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schmaus A, Klusmeier S, Rothley M, Dimmler
A, Sipos B, Faller G, Thiele W, Allgayer H, Hohenberger P, Post S,
et al: Accumulation of small hyaluronan oligosaccharides in tumour
interstitial fluid correlates with lymphatic invasion and lymph
node metastasis. Br J Cancer. 111:559–567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Raso-Barnett L, Banky B, Barbai T, Becsagh
P, Timar J and Raso E: Demonstration of a melanoma-specific CD44
alternative splicing pattern that remains qualitatively stable, but
shows quantitative changes during tumour progression. PLoS One.
8:e538832013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Malaisse J, Bourguignon V, De Vuyst E,
Lambert de Rouvroit C, Nikkels AF, Flamion B and Poumay Y:
Hyaluronan metabolism in human keratinocytes and atopic dermatitis
skin is driven by a balance of hyaluronan synthases 1 and 3. J
Invest Dermatol. 134:2174–2182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Siiskonen H, Poukka M, Tyynelä-Korhonen K,
Sironen R and Pasonen-Seppänen S: Inverse expression of
hyaluronidase 2 and hyaluronan synthases 1-3 is associated with
reduced hyaluronan content in malignant cutaneous melanoma. BMC
Cancer. 13:1812013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Karvinen S, Pasonen-Seppänen S, Hyttinen
JM, Pienimäki JP, Törrönen K, Jokela TA, Tammi MI and Tammi R:
Keratinocyte growth factor stimulates migration and hyaluronan
synthesis in the epidermis by activation of keratinocyte hyaluronan
synthases 2 and 3. J Biol Chem. 278:49495–49504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ichikawa T, Itano N, Sawai T, Kimata K,
Koganehira Y, Saida T and Taniguchi S: Increased synthesis of
hyaluronate enhances motility of human melanoma cells. J Invest
Dermatol. 113:935–939. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rilla K, Oikari S, Jokela TA, Hyttinen JM,
Kärnä R, Tammi RH and Tammi MI: Hyaluronan synthase 1 (HAS1)
requires higher cellular UDP-GlcNAc concentration than HAS2 and
HAS3. J Biol Chem. 288:5973–5983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Auvinen P, Rilla K, Tumelius R, Tammi M,
Sironen R, Soini Y, Kosma VM, Mannermaa A, Viikari J and Tammi R:
Hyaluronan synthases (HAS1-3) in stromal and malignant cells
correlate with breast cancer grade and predict patient survival.
Breast Cancer Res Treat. 143:277–286. 2014. View Article : Google Scholar
|
|
42
|
Edward M, Quinn JA, Pasonen-Seppänen SM,
McCann BA and Tammi RH: 4-Methylumbelliferone inhibits tumour cell
growth and the activation of stromal hyaluronan synthesis by
melanoma cell-derived factors. Br J Dermatol. 162:1224–1232. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu N, Gao F, Han Z, Xu X, Underhill CB
and Zhang L: Hyaluronan synthase 3 overexpression promotes the
growth of TSU prostate cancer cells. Cancer Res. 61:5207–5214.
2001.PubMed/NCBI
|
|
44
|
Savani RC, Cao G, Pooler PM, Zaman A, Zhou
Z and DeLisser HM: Differential involvement of the hyaluronan (HA)
receptors CD44 and receptor for HA-mediated motility in endothelial
cell function and angiogenesis. J Biol Chem. 276:36770–36778. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thomas L, Byers HR, Vink J and Stamenkovic
I: CD44H regulates tumor cell migration on hyaluronate-coated
substrate. J Cell Biol. 118:971–977. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Turley EA, Austen L, Vandeligt K and Clary
C: Hyaluronan and a cell-associated hyaluronan binding protein
regulate the locomotion of ras-transformed cells. J Cell Biol.
112:1041–1047. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Du YC, Chou CK, Klimstra DS and Varmus H:
Receptor for hyaluronan-mediated motility isoform B promotes liver
metastasis in a mouse model of multistep tumorigenesis and a tail
vein assay for metastasis. Proc Natl Acad Sci USA. 108:16753–16758.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hall CL, Lange LA, Prober DA, Zhang S and
Turley EA: pp60(c-src) is required for cell locomotion regulated by
the hyaluronanreceptor RHAMM. Oncogene. 13:2213–2224.
1996.PubMed/NCBI
|
|
49
|
Kouvidi K, Berdiaki A, Nikitovic D,
Katonis P, Afratis N, Hascall VC, Karamanos NK and Tzanakakis GN:
Role of receptor for hyaluronic acid-mediated motility (RHAMM) in
low molecular weight hyaluronan (LMWHA)-mediated fibrosarcoma cell
adhesion. J Biol Chem. 286:38509–38520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Park D, Kim Y, Kim H, Kim K, Lee YS, Choe
J, Hahn JH, Lee H, Jeon J, Choi C, et al: Hyaluronic acid promotes
angiogenesis by inducing RHAMM-TGFβ receptor interaction via
CD44-PKCδ. Mol Cells. 33:563–574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang S, Chang MC, Zylka D, Turley S,
Harrison R and Turley EA: The hyaluronan receptor RHAMM regulates
extra-cellular-regulated kinase. J Biol Chem. 273:11342–11348.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hanagiri T, Shinohara S, Takenaka M,
Shigematsu Y, Yasuda M, Shimokawa H, Nagata Y, Nakagawa M, Uramoto
H, So T, et al: Effects of hyaluronic acid and CD44 interaction on
the proliferation and invasiveness of malignant pleural
mesothelioma. Tumour Biol. 33:2135–2141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ohta S, Yoshida J, Iwata H and Hamaguchi
M: Hyaluronate activates tyrosine phosphorylation of cellular
proteins including focal adhesion kinase via CD44 in human glioma
cells. Int J Oncol. 10:561–564. 1997.PubMed/NCBI
|
|
54
|
Okamoto I, Kawano Y, Murakami D, Sasayama
T, Araki N, Miki T, Wong AJ and Saya H: Proteolytic release of CD44
intracellular domain and its role in the CD44 signaling pathway. J
Cell Biol. 155:755–762. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fecher LA, Amaravadi RK and Flaherty KT:
The MAPK pathway in melanoma. Curr Opin Oncol. 20:183–189. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kohno M, Tanimura S and Ozaki K: Targeting
the extracellular signal-regulated kinase pathway in cancer
therapy. Biol Pharm Bull. 34:1781–1784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vigetti D, Viola M, Karousou E, De Luca G
and Passi A: Metabolic control of hyaluronan synthases. Matrix
Biol. 35:8–13. 2014. View Article : Google Scholar
|
|
58
|
Li L, Asteriou T, Bernert B, Heldin CH and
Heldin P: Growth factor regulation of hyaluronan synthesis and
degradation in human dermal fibroblasts: Importance of hyaluronan
for the mitogenic response of PDGF-BB. Biochem J. 404:327–336.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
David-Raoudi M, Deschrevel B, Leclercq S,
Galéra P, Boumediene K and Pujol JP: Chondroitin sulfate increases
hyaluronan production by human synoviocytes through differential
regulation of hyaluronan synthases: Role of p38 and Akt. Arthritis
Rheum. 60:760–770. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Törrönen K, Nikunen K, Kärnä R, Tammi M,
Tammi R and Rilla K: Tissue distribution and subcellular
localization of hyaluronan synthase isoenzymes. Histochem Cell
Biol. 141:17–31. 2014. View Article : Google Scholar
|
|
61
|
Vigetti D, Clerici M, Deleonibus S,
Karousou E, Viola M, Moretto P, Heldin P, Hascall VC, De Luca G and
Passi A: Hyaluronan synthesis is inhibited by adenosine
monophosphate-activated protein kinase through the regulation of
HAS2 activity in human aortic smooth muscle cells. J Biol Chem.
286:7917–7924. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bourguignon LY, Gilad E and Peyrollier K:
Heregulin-mediated ErbB2-ERK signaling activates hyaluronan
synthases leading to CD44-dependent ovarian tumor cell growth and
migration. J Biol Chem. 282:19426–19441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Herbold KW, Zhou J, Haggerty JG and
Milstone LM: CD44 expression on epidermal melanocytes. J Invest
Dermatol. 106:1230–1235. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tolg C, Hamilton SR, Morningstar L, Zhang
J, Zhang S, Esguerra KV, Telmer PG, Luyt LG, Harrison R, McCarthy
JB, et al: RHAMM promotes interphase microtubule instability and
mitotic spindle integrity through MEK1/ERK1/2 activity. J Biol
Chem. 285:26461–26474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tofuku K, Yokouchi M, Murayama T, Minami S
and Komiya S: HAS3-related hyaluronan enhances biological
activities necessary for metastasis of osteosarcoma cells. Int J
Oncol. 29:175–183. 2006.PubMed/NCBI
|