Introduction
Malignant melanoma (MM) is one of the most
aggressive forms of cutaneous neoplasm, the incidence of which has
increased markedly in recent decades (1). An estimated 87,110 newly diagnosed MM
cases and 9,730 MM-associated mortalities were predicted for 2017
in the United States (1). Despite
the notable improvements in diagnosis and treatment made in recent
years, the prognosis remains poor for patients diagnosed at the
metastatic stage, with a median survival time of 6-9 months and a
5-year survival rate of only 10% (2-4).
Thus, it is necessary to establish effective biomarkers for the
early diagnosis and efficient evaluation of prognosis following
surgery.
Long non-coding RNAs (lncRNAs) are a class of
transcripts >200 nucleotides in length with limited protein
coding potential (5-7). Recently, studies have demonstrated
that lncRNAs have multiple functions in a wide range of biological
process, including proliferation (8), apoptosis (9), cell migration (10) and cell invasion (11). A novel regulatory mechanism whereby
lncRNAs function as competing endogenous (ce)RNAs to sponge
microRNAs (miRNAs) and regulate their downstream signaling pathways
has been proposed and confirmed preliminarily (12-14).
Although it has been reported that a number of lncRNA transcripts
are involved in the carcinogenesis and development of MM (15-17),
the miRNA sponge roles of lncRNAs in the modulation of cell
proliferation and migration in MM remain unclear.
The present study screened for candidate lncRNAs
responsible for the malignant phenotype of MM from the Gene
Expression Omnibus (GEO) datasets. It was identified that long
non-coding RNA activated by transforming growth factor (TGF)-β
(lncRNA-ATB) was the most markedly upregulated lncRNA in MM tissues
and A375 cells compared with benign nevus cells and human
melanocytes. The roles and underlying mechanism of lncRNA-ATB in
the proliferation, apoptosis and invasion-metastasis cascade of MM
cells were investigated and identified in vivo and in
vitro.
Materials and methods
Cell culture
Highly invasive human MM A2058 cells, moderately
invasive human MM A375 cells, normal epidermal melanocyte HEMa-LP
cells, 293 and B16/F10 were obtained from the Cell Bank of the
Chinese Academy of Sciences (Shanghai, China), where they were
characterized by mycoplasma detection, DNA-fingerprinting, isozyme
detection and cell vitality detection. A2058, A375, 293 and B16/F10
cells were cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA) at 37°C in a humidified atmosphere
of 95% air and 5% CO2. HEMa-LP cells were cultured in
Medium 254 (Invitrogen; Thermo Fisher Scientific, Inc.) suplemented
with human melanocyte growth supplement (Hyclone; GE Healthcare
Life Sciences) at 37°C in a humidified atmosphere of 95% air and 5%
CO2.
Oligonucleotide transfection
pGMLV-lncRNA-ATB negative control (NC),
pGMLV-lncRNA-ATB short hairpin (sh)RNA, miRNA (miR)-590-5p NC,
miR-590-5p mimics and miR-590-5p inhibitors were purchased from
GenePharma Co. Ltd. (Shanghai, China). The sequence for miR-590-5p
was GAGCUUAUUCAUAAAAUGCAG; miR-NC was GUCCAGUGAAUUCCCAG; and
miR-590-5p inhibitors was GACGUAAAAUACUUAUUCGAG. The lncRNA-ATB
overexpressing plasmid was constructed by Shanghai GeneChem Co.,
Ltd. (Shanghai, China) using pCDNA3.1. An empty vector was used as
the control (Shanghai GeneChem Co., Ltd.). When the cell confluence
of A375 and A2058 cells reached 50-60%, oligonucleotide
transfections were performed using Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Oligonucleotides (50 nmol) were mixed with 5 µl
Lipofectamine® 2000 in 500 µl medium. The
transfection solutions were added to each well containing 500
µl medium. Following transfection, cell samples were
collected at 48 h for further analyses.
RNA/miRNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
For lncRNA-ATB quantification, total RNA was
extracted from the cells or tissues using TRI reagent
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), according to the
manufacturer's protocol. RNA samples were reverse transcribed into
cDNA using RT regeants (Takara Bio, Inc., Otsu, Japan) with lncRNA
specific primers, and β-actin was used as internal loading
controls. RT was performed at 45°C for 60 min and 70°C for 10 min.
SYBR Mix (Takara Bio, Inc.) was used to detect and quantify
lncRNA-ATB and β-actin expression. For miR-590-5p quantification,
total miRNA was extracted from the cells or tissues using the
miRNeasy RNA isolation kit (Qiagen, Inc., Valencia, CA, USA),
according to the manufacturer's protocol. Total miRNA samples (1
µl; 1 µg/µl) were reverse transcribed into
cDNA using the miScript RT kit (Qiagen GmbH, Hilden, Germany) with
miR-590-5p specific primers, and universal small nuclear U6 RNA was
used as an internal loading control. An Applied Biosystems™ SYBR™
Green dye miRNA RT-qPCR kit (Thermo Fisher Scientific, Inc.) was
used to detect and quantify miR-590-5p and U6 expression. Data were
analyzed with 7500 software v.2.0.1 (Applied Biosystems; Thermo
Fisher Scientific, Inc.), with the automatic Cq setting for
adapting baseline and threshold for Cq determination (18). Each sample was examined in
triplicate. The primer sequences used in the present study are
listed in Table I. RT-qPCR assays
were performed under the following thermocycling conditions: 95°C
for 5 min, followed by 45 cycles of 95°C for 15 sec, 60°C for 30
sec and 72°C for 30 sec.
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene name | Forward primer
(5′→3′) | Reverse primer
(5′→3′) |
|---|
| lncRNA-ATB |
CTTCACCAGCACCCAGAGA |
AAGACAGAAAAACAGTTCCGAGTC |
| β-actin |
CGTCTTCCCCTCCATCGT |
GAAGGTGTGGTGCCAGATTT |
| miR-590-5p |
GGAATTCTTCAGTTGTAACCCAG |
CGGGATCCTTGAGATGTCACCAA |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
Western blotting
Cells were washed in PBS three times prior to the
proteins being extracted. Cells were lysed using
radioimmunoprecipitation assay buffer (JingCai Technologies, Inc.,
Xi'an, China) and the protein concentration was determined using
the bicinchoninic acid method. Each protein sample (30 µg)
was denatured in SDS sample buffer and separated via 10% SDS-PAGE.
Separated proteins were transferred to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA), blocked with 5%
bovine serum albumin (BSA; Beyotime Institute of Biotechnology,
Haimen, China) for 2 h at room temperature, and incubated overnight
with primary antibodies at 4°C. Blotting was performed with primary
antibodies against Yes associated protein 1 (YAP1; 1:300; cat. no.
14074; Cell Signaling Technology, Inc., Danvers, MA, USA). Goat
anti-rabbit immunoglobulin horseradish peroxidase-linked F(ab)2
fragments (1:5,000; cat. no. TA130071; OriGene Technologies, Inc.,
Beijing, China) were used as secondary antibodies for 2 h at room
temperature. β-actin (1:4,000; cat. no. ab8226; Abcam, Cambridge,
UK) was used as a loading control. Blots were then washed three
times (10 min/wash) in TBS-Tween-20 and developed using an enhanced
chemiluminescence system (JingCai Technologies, Inc.). ImageJ
(National Institutes of Health, Bethesda, MD, USA) was used to
analyze the gray values of each blot.
Cell counting kit-8 (CCK-8) assay
A2058 cells transfected with lncRNA-ATB NC or
lncRNA-ATB shRNA 24 h previously, and A375 cells transfected with
empty vector or lncRNA-ATB overexpressing vector 24 h previously,
were seeded in a 96-well culture plate at 2,000 cells/well. Each
group was established in nine wells. CCK-8 reagents (cat. no.
40203ES60; Yeasen Biological Technology, Inc., Shanghai, China)
were added into each well at 24, 48, 72, 96 and 120 h post-seeding,
and each group was cultured for a further 50 min at 37°C in a
humidified atmosphere of 95% air and 5% CO2. The optical
density values was measured at 490 nm using a microplate
reader.
Flow cytometry
MM cells in a 6-well culture plate were harvested by
trypsinization, and washed three times with PBS. For cellular
apoptosis analysis, cells were suspended in 500 µl binding
buffer at a density of 2×106 cells/ml, and incubated
with Annexin V-fluoroscein isothiocyanate and propidium iodide (PI;
BD Biosciences, San Jose, CA, USA) for 15 min in the dark at room
temperature. For cell cycle analysis, PI was added into A2058 cells
tranfected with lncRNA-ATB NC or lncRNA-ATB, and A375 cells
transfected with empty vector or lncRNA-ATB overexpressing vector,
and incubated for 20 min in the dark at room temperature. Cellular
apoptosis and the cell cycle were analyzed using a flow cytometer
and Kaluza analysis software version 2.0 (both Beckman Coulter,
Inc., Brea, CA, USA).
Cell migration and invasion
Cell migration and invasion capacity were measured
in vitro using Transwell migration assays (EMD Millipore).
A2058 cells transfected with lncRNA-ATB NC or lncRNA-ATB, and A375
cells transfected with empty vector or lncRNA-ATB overexpressing
vector were suspended in DMEM with 10 g/l BSA at a density of
5×105 cells/ml. Cell suspensions (200 µl) were
seeded in the upper chamber with membrane coated with (for the
Transwell invasion assay) or without (for the migration assay)
Matrigel (BD Biosciences). To attract the cells, 600 µl DMEM
with 10% serum was added to the bottom chamber. When the cells had
been allowed to migrate for 24 h or to invade for 48 h, the
penetrated cells on the filters were fixed in dried methanol for 1
min and stained with 4 g/l crystal violet for 10 min at room
temperature. The numbers of migrated or invasive cells were
determined from five random fields using a light microscope
(Olympus Corporation, Tokyo, Japan) at ×10 magnification.
Ethics statement
All animals were treated in accordance with the
Guide for the Care and Use of Laboratory Animals, 1996, by the
National Research Council (US) Institute for Laboratory Animal
Research (19). Animal experiments
were approved by and carried out according to the guidelines of the
Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China).
RNA pull-down assay
The full length lncRNA-ATB sequence or NC sequence
was amplified by PCR kit (Takara Bio, Inc.) using a T7-containing
primer. The following primer sequences were used: lncRNA-ATB
forward, 5′-TCCCTGACTCCTCTATGGCATCTGTGG-3′; lncRNA-ATB reverse,
5′-CCTTTGCTTCCTCTTTTCTCATCTACTC-3′. The thermocycling conditions
were as stated above. The DNA fragments were cloned into pcDNA3.1.
The restriction enzyme XhoI was used to linearize the
plasmids. T7 RNA polymerase (Takara Bio, Inc.) and Biotin RNA
Labeling Mix (Roche Diagnostics, Indianapolis, IN, USA) was used to
make biotin-labeled RNAs that were reverse transcribed. The
products were treated with RNase-free DNase I (Roche Diagnostics)
and purified with an RNeasy Mini kit (Qiagen, Inc.), and
subsequently mixed with RNA extract from A2058 cells and incubated
at room temperature for 1 h. The pull-down complexes were analyzed
by RT-qPCR as previously stated following subsequent washes.
Tumor xenograft assays
All mice were housed and maintained under specific
pathogen-free conditions at 18-22°C, with 20% humidity, a 12-h
light and 12-h dark cycle, with feeding ad libitum. All
experiments were approved by the Animal Care and Use Committee of
Xi'an Jiaotong University and performed in accordance with
institutional guidelines. For the tumorigenesis assays, xenograft
tumors were generated via subcutaneous injection of A2058 cells
(5×106), including A2058 lncRNA-ATB NC and A2058
lncRNA-ATB shRNA, into the hind limbs of 18-20-g 4-6-week-old
Balb/C female athymic nude mice (nu/nu; Animal Center of Xi'an
Jiaotong University, Xi'an, China; total number, 10 mice; n=5
mice/group). Tumor size was measured using a vernier caliper. Tumor
volume was determined by the following formula: 0.5× A×
B2, where A represents the diameter of the base of the
tumor and B represents the corresponding perpendicular value. After
35 days, the mice were sacrificed and the tumors were collected and
weighed. For the lung colonization assay, B16/F10 cells
(5×106), which included lncRNA-ATB NC-luciferase and
lncRNA-ATB shRNA-luciferase were intravenously injected into
18-20-g 6-week-old female C57/B6 mice (total number, 10 mice; n=5
mice/group). Lung colonization was measured using Xenogen IVIS
Kinetic imaging systems (PerkinElmer, Inc., Waltham, MA, USA) on
days 0, 7 and 14 post-injection.
Luciferase assays
The putative binding sites of miR-590-5p for
lncRNA-ATB were predicted using RNA hybrid (20) and miRDB (http://www.mirdb.org/). Two hundreds and ninety-three
cells were seeded in a 96-well plate at 70% confluence. The
lncRNA-ATB was cloned into pMir-Report (Ambion; Thermo Fisher
Scientific, Inc.), yielding pMir-Report-lncRNA-ATB. Mutations were
introduced in potential miR-590-5p binding sites using the
QuikChange site-directed mutagenesis kit (Stratagene; Agilent
Technologies, Inc., Santa Clara, CA, USA). miR-590-5p NC or mimics
were transfected into 293 cells with 30 ng wild-type (WT) or mutant
(Mut) 3′-untranslated region (UTR) of lncRNA-ATB using
Lipofectamine® 2000, as previously stated. Cells were
collected 48 h post-transfection, and luciferase assays were
performed using a Photinus pyralis-Renilla reniformis
dual luciferase reporter assay system, according to the
manufacturer's protocol (Promega Corporation, Madison, WI, USA).
The luciferase activity of each lysate was normalized to
Renilla luciferase activity.
Statistical analysis
Differentially expressed lncRNAs were identified
from the GEO database GSE3189 with false discovery rate (FDR)
<0.01 and |logFC| >1 using the R package (21). The raw P-value was corrected using
the Benjamini and Hochberg method (22) to circumvent the multi-test bias. A
fold-change value >2 or <0.25 and FDR <0.01 were selected
as cutoff criteria for differentially expressed lncRNAs.
Statistical analysis was performed using SPSS statistical software
(version 21.0; IBM Corp., Armonk, NY, USA). The differences in
characteristics between two groups were examined by the Student's
t-test. The differences in characteristics between three groups
were examined by analysis of variance, and the least significant
difference test was applied to detect the differences between each
pair of groups. The correlation between miR-590-5p expression and
YAP1 expression was analyzed by Pearson correlation analysis. All
P-values were determined from two-sided tests, and P<0.05 was
considered to indicate a statistically significant difference. All
experiments were repeated three times and the data are presented as
the mean ± standard deviation from three independent
experiments.
Results
lncRNA-ATB is upregulated in MM cell
lines
To elucidate the critical lncRNAs involved in the
carcinogenesis and progression of MM, comparative lncRNA profiling
was performed in 45 MM, 18 benign skin nevus cell (BN), and seven
normal skin tissue specimens from the GEO dataset GSE3189 (23). Vectorgram analysis further
identified that lncRNA-ATB was upregulated in MM specimens compared
with BN (Fig. 1A and Table II). The volcano plot illustrates
that the lncRNA-ATB expression level was >4-fold increased
between cases and controls (P<0.001; Fig. 1B). The significant upregulation of
lncRNA-ATB was further confirmed using RT-qPCR in the human
epidermal melanocyte cell line HEMa-LP and in MM cell lines
(Fig. 1C).
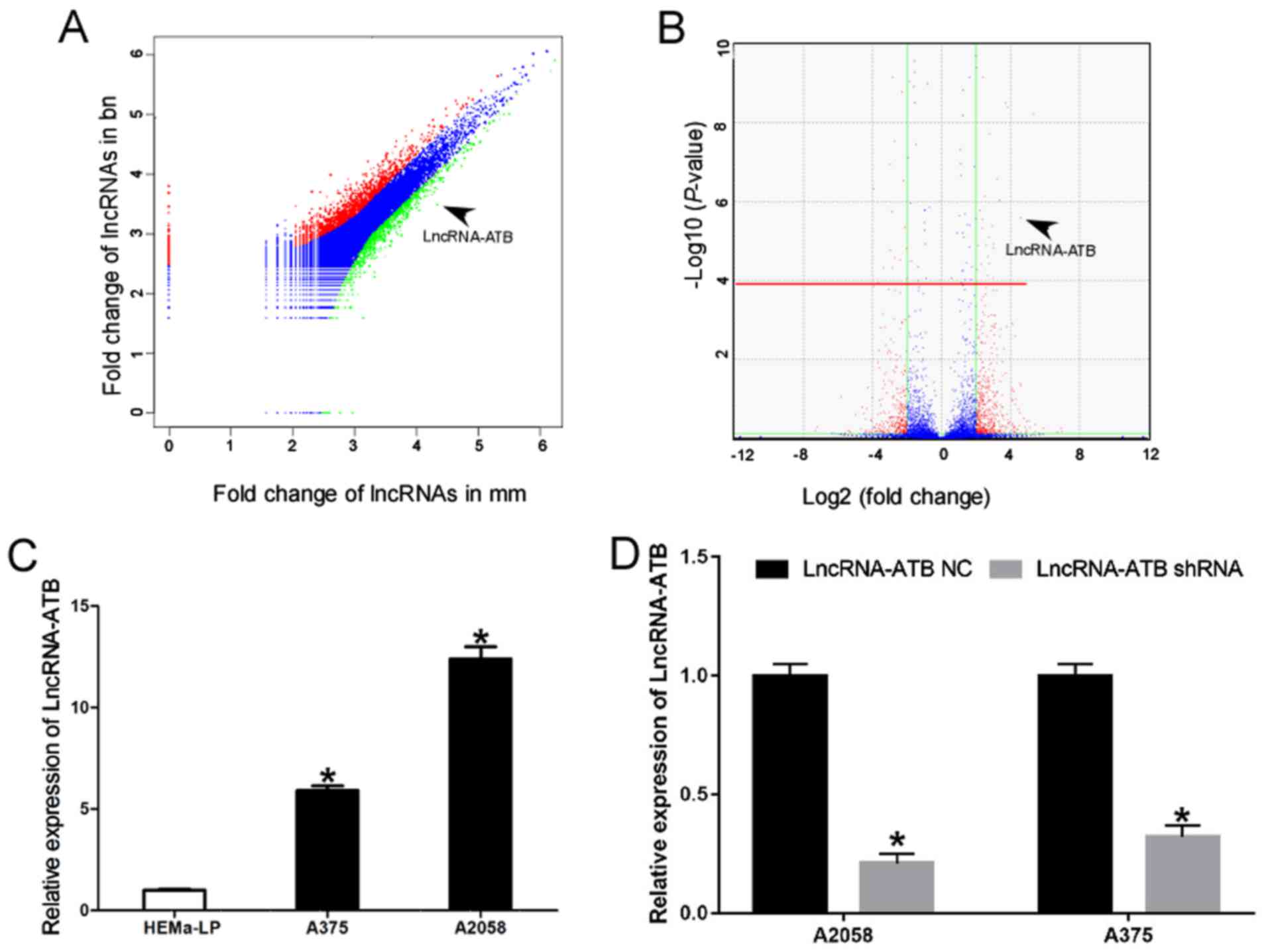 | Figure 1lncRNA-ATB is upregulated in MM cell
lines of malignant melanoma. (A) Vectorgram analysis of lncRNAs in
MM and BN specimens. The x-axis indicates the Log2
fold-change in lncRNA expression in MM tissues, and the y-axis
indicates the Log2 fold-change of lncRNA expression in
BN tissues. Red, lncRNAs upregulated by ≥2-fold in MM compared with
BN. Green, lncRNAs upregulated by ≥2-fold in BN compared with MM.
Blue, lncRNAs upregulated by <2-fold in MM compared with BN or
lncRNAs upregulated by <2-fold in BN compared with MM. Data from
the GEO datasets (GSE3189): Differentially expressed lncRNAs from
the GEO dataset GSE3189 with FDR <0.01 and |logFC| >2 were
identified using the R package. The raw P-value was corrected using
the Benjamini and Hochberg method to circumvent the multi-test
bias. A fold-change value >2 or <0.25 and FDR <0.01 were
selected as cutoff criteria for differentially expressed lncRNAs.
(B) Volcano plot of lncRNAs in MM and BN. The x-axis indicates the
Log2 fold-change in lncRNA expression between MM and BN
tissues, while the y-axis indicates the Log10 of the
adjusted P-value for each lncRNA. Values above the red line were
identified to be statistically significant. (P<0.01) following
application of the Benjamini and Hochberg method. lncRNA-ATB
expression level was >4-fold increased between cases and
controls (P<0.001 vs. BN). (C) Relative expression of lncRNA-ATB
in human epidermal melanocytes and MM cells. *P<0.05
vs. HEMa-LP cells. (D) Knockdown efficiency of lncRNA-shRNA in MM
cells. Data are from three experiments and are presented as the
mean ± standard deviation. *P<0.05 vs. respective
lncRNA-ATB NC group (Student's t-test). MM, malignant melanoma; BN,
benign nevi; lncRNA, long noncoding RNA; ATB, activated by
transforming growth factor-β; GEO, Gene Expression Omnibus; FDR,
false discovery rate; shRNA, short hairpin RNA; NC, negative
control. |
 | Table IITop 30 lncRNAs upregulated in
malignant melanoma. |
Table II
Top 30 lncRNAs upregulated in
malignant melanoma.
| lncRNA name | Log2
fold-change |
|---|
| LncRNA-ATB | 4.585437915 |
| Linc00662 | 3.029954694 |
| AC144652.1 | 3.057751736 |
| RP11-654D12.2 | 3.081628575 |
| PVT1 | 3.139985752 |
| PGM5-AS1 | 3.151644278 |
| MIR24-2 | 3.1683935 |
| SNHG19 | 3.233861413 |
| AC112721.2 | 3.301811151 |
| CTD-2066L21.3 | 3.34462291 |
| MIR378D2 | 3.356163777 |
| NUP50-AS1 | 3.420937034 |
| SNHG9 | 3.427925853 |
| AP006621.5 | 3.441188157 |
| LINC00973 | 3.515399942 |
| LUCAT | 3.567557456 |
| LINC01588 | 3.582647048 |
| YTHDF3-AS1 | 3.589774049 |
| BANCR | 3.678548044 |
| TUG1 | 3.78577717 |
| SPRY4-IT1 | 3.826319081 |
| H19 | 3.855857029 |
| HOXD-AS1 | 3.879582756 |
| FALEC | 3.881343407 |
| FTH1P3 | 3.915488817 |
| ILF3-AS1 | 3.980602573 |
| HOXA11-AS | 4.004958111 |
| HEIH | 4.061621096 |
| CCAT1 | 4.155603912 |
| MHENCR | 10.18103013 |
Effects of lncRNA-ATB on the cell
proliferation and apoptosis of MM cells
To investigate the functional role of lncRNA-ATB in
MM cells, gain- and loss-of function experiments were performed by
transfecting an shRNA, shLncRNA-ATB, into the highly invasive MM
cell line A2058 to knock down lncRNA-ATB expression (Fig. 1D; P<0.05). An lncRNA-ATB
overexpressing plasmid was also transfected into the moderately
invasive MM cell line A375 to stably overexpress lncRNA-ATB. CCK-8
assays indicated that the proliferation of A2058 cells transfected
with shLncRNA-ATB was inhibited compared with that in the control
(Fig. 2A). However, the
proliferation of A375 cells transfected with the lncRNA-ATB
overexpressing plasmid was enhanced compared with that in the
control (Fig. 2B). Cellular
apoptosis assays demonstrated that the percentage of early
apoptotic cells increased in A2058 cells transfected with
shLncRNA-ATB. Whereas, the percentage of early apoptotic cells
decreased in A375 cells transfected with the lncRNA-ATB
overexpressing plasmid (Fig. 2C and
D).
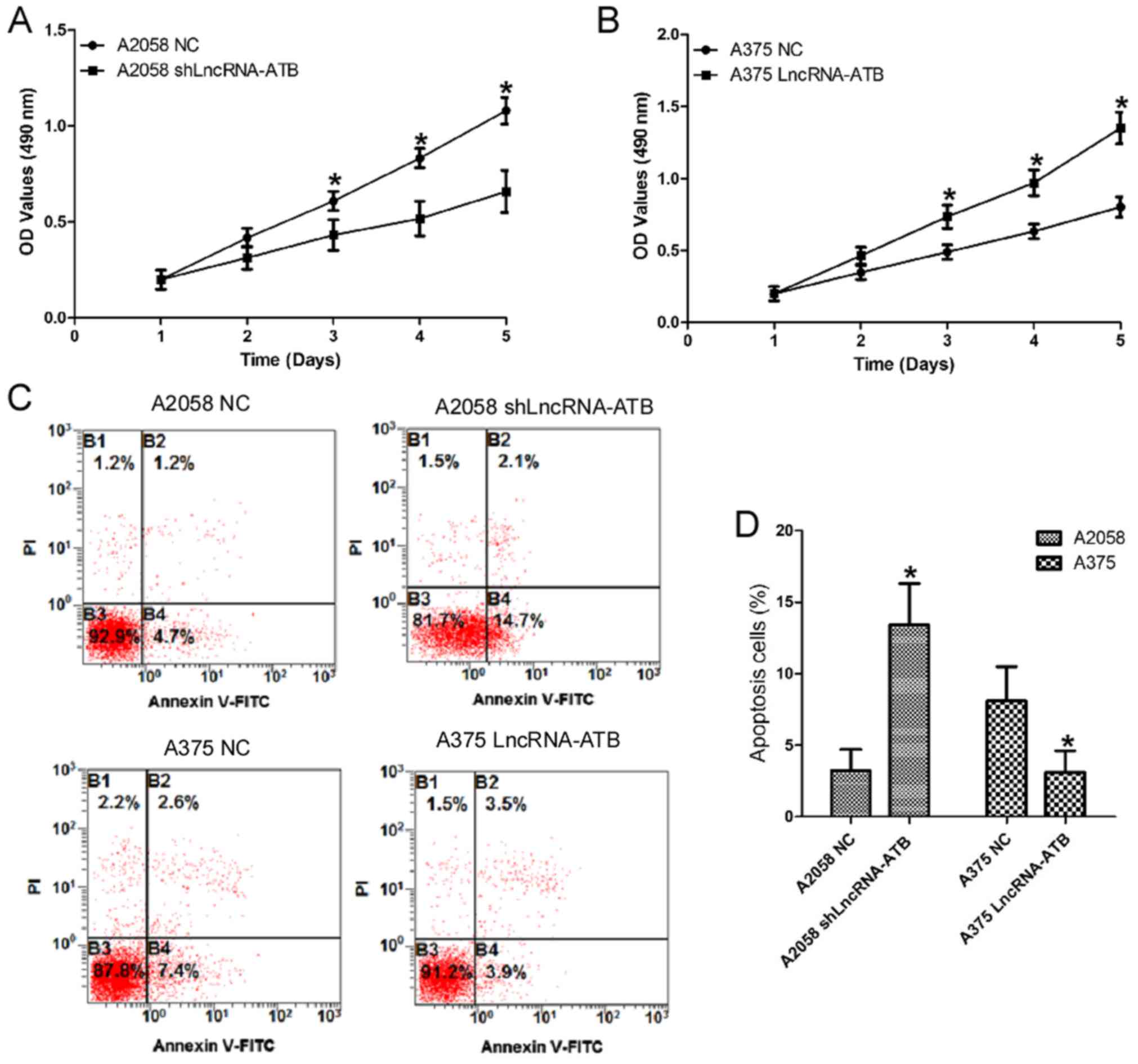 | Figure 2Effects of lncRNA-ATB on the cell
proliferation and apoptosis of MM cells. (A) The effects of
lncRNA-ATB shRNA on the proliferation of A2058 cells were detected
by CCK-8 assays. (B) Effects of lncRNA-ATB on the proliferation of
A375 cells were detected by CCK-8 assays. (C) Effects of lncRNA-ATB
on the cellular apoptosis of MM cells were detected by flow
cytometry using an Annexin V/PI kit, and (D) the results were
quantified. Data are from three experiments and are presented as
the mean ± standard deviation. *P<0.05 vs. respective
NC group (Student's t-test). MM, malignant melanoma; lncRNA, long
noncoding RNA; shRNA, short hairpin RNA; NC, negative control; PI,
propidium iodide; FITC, fluorescein isothiocyanate; OD, optical
density; CCK-8, cell counting kit-8; ATB, activated by transforming
growth factor-β. |
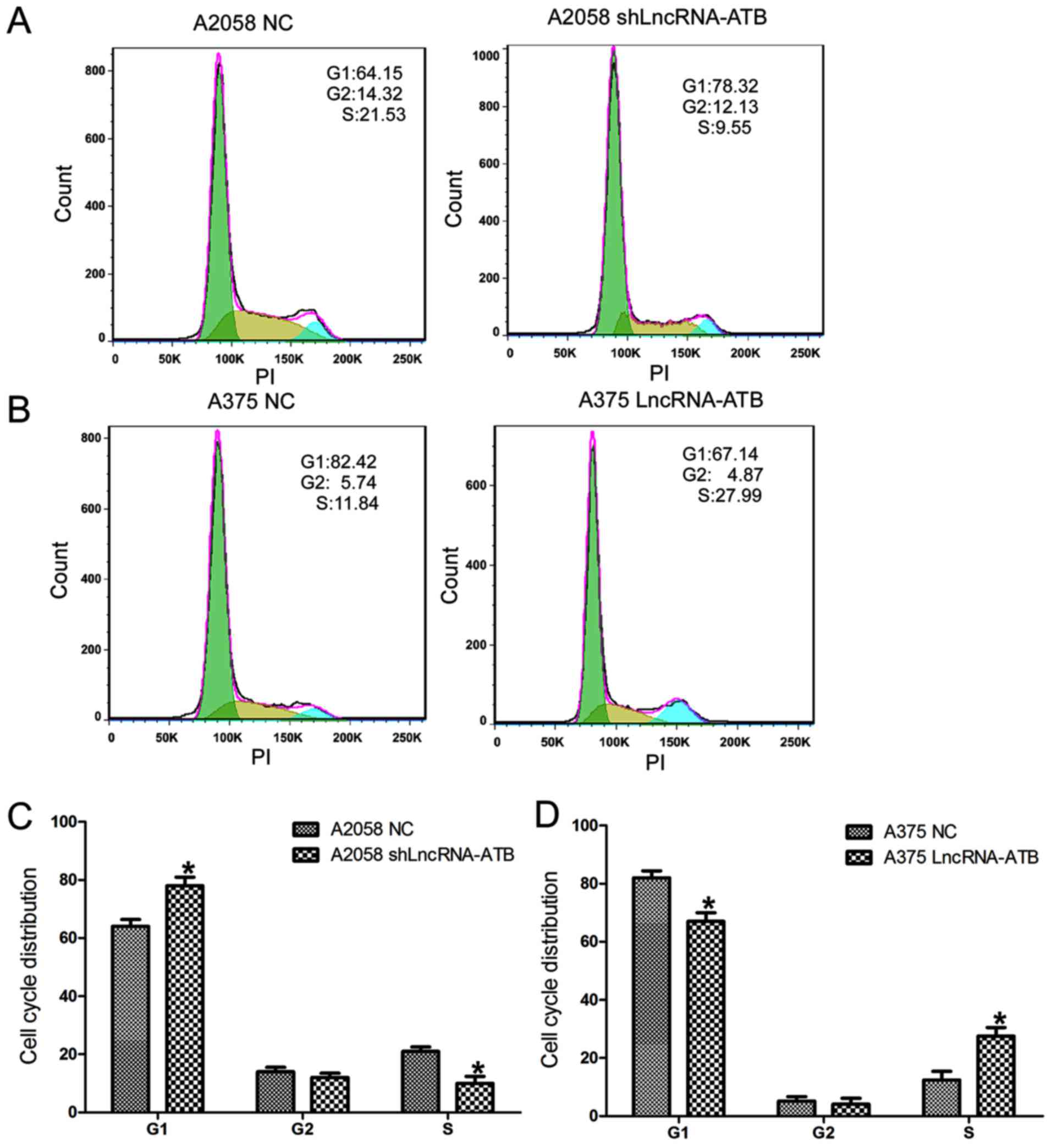
Effects of lncRNA-ATB on the cell cycle
distribution of MM cells
Cell cycle assays (Fig.
3) demonstrated that A2058 cells transfected with shLncRNA-ATB
displayed a significant increase in the percentage of cells in the
G1 phase and a decrease in the percentage of cells in the S phase
(Fig. 3A and C). By contrast, the
percentage of cells in the G1 phase decreased and the percentage of
cells in the S phase increased in A375 cells transfected with the
lncRNA-ATB overexpressing plasmid compared with that in the control
(Fig. 3B and D).
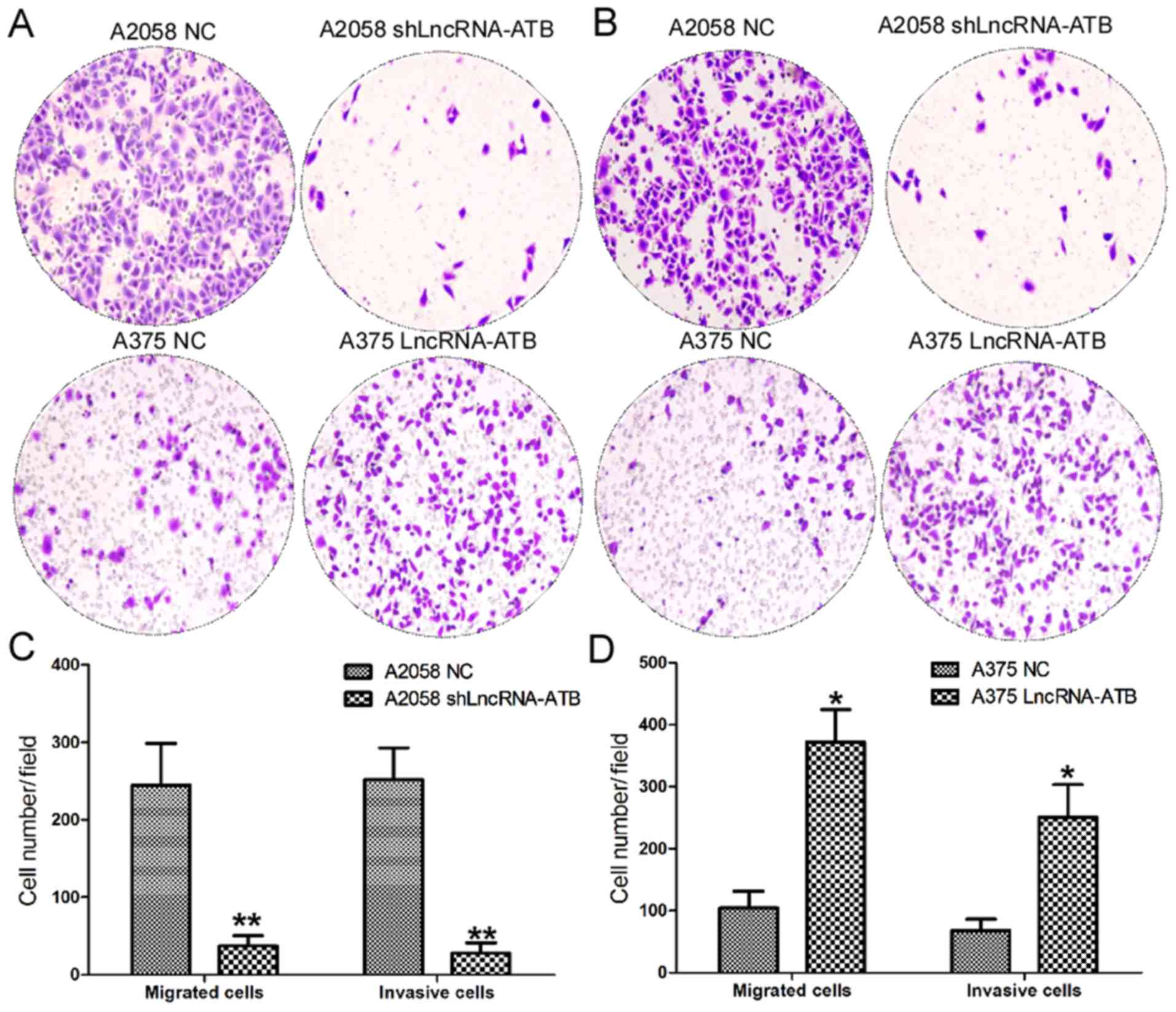
Effects of lncRNA-ATB on the cell
migration and invasion of MM cells
The present study further investigated the effects
of lncRNA-ATB on the cell migration and invasion of MM cells using
Transwell assays (Fig. 4). The
cell migration assays indicated a significant decrease in the
number of migratory cells among A2058 cells transfected with
shLncRNA-ATB compared with the control (Fig. 4A and C). By contrast, ectopic
expression of lncRNA-ATB significantly increased the number of
migratory A375 cells compared with the normal control (Fig. 4A and D). As expected, cell invasion
assays also confirmed a decrease in the number of invasive cells
among A2058 cells transfected with shLncRNA-ATB compared with the
control (Fig. 4B and C). Ectopic
expression of lncRNA-ATB significantly enhanced the invasive
ability of A375 cells compared with the control (Fig. 4B and D).
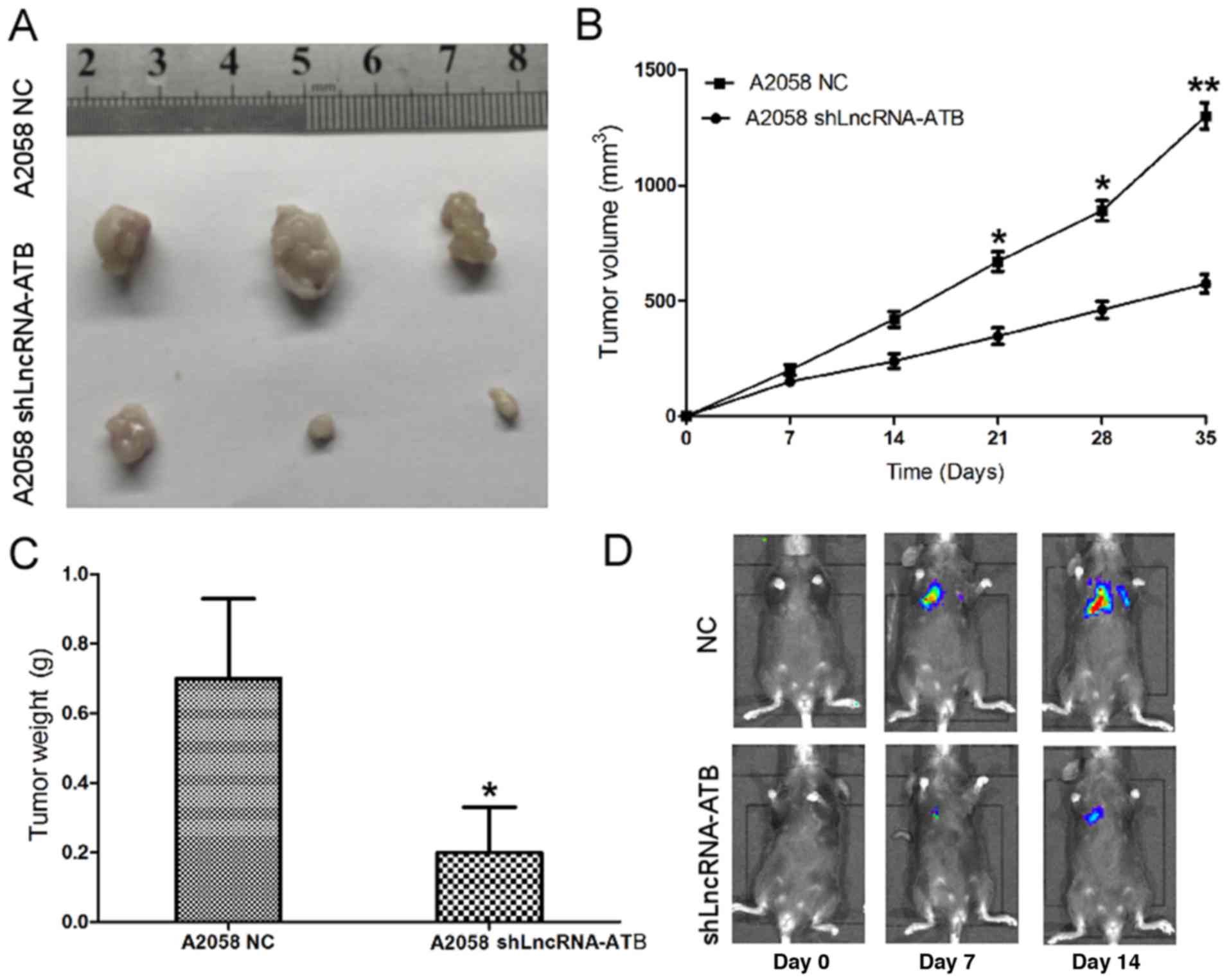
Effects of lncRNA-ATB on the tumorigenic
ability of MM cells
The effect of lncRNA-ATB on the tumorigenic ability
of MM cells was detected in vivo. shLncRNA-ATB and
luciferase were transfected into A2058 cells and tumorigenesis
assays were performed in nude mice. Cells (5×106) were
injected into the flanks of the nude mice. Tumor sizes were
measured using the Xenogen IVIS Kinetic imaging system and Vernier
calipers every 7 days. After 35 days, the mice were sacrificed, and
the tumors were collected and weighed. It was identified that
shLncRNA-ATB had a significant tumor growth-inhibitory effect,
causing significant reductions in tumor size (Fig. 5A and B) and weight (Fig. 5C) in the shLncRNA-ATB group
compared with the control group. B16/F10 melanoma cells transfected
with shLncRNA-ATB or controls were injected intravenously into
C57/B6 mice, and lung colonization was assessed using the Xenogen
IVIS200 System. It was identified that lung metastasis was
inhibited in mice injected with B16/F10 cells transfected with
shLn-cRNA-ATB, in comparison with the A2058 cells transfected with
a control shRNA (Fig. 5D). Taken
together, these results indicated that lncRNA-ATB was upregulated
in MM cells, and that knockdown of lncRNA-ATB was able to repress
cell proliferation, cell migration, cell invasion and tumor growth
in vitro and in vivo.
lncRNA-ATB directly sponges miR-590-5p to
upregulate YAP1 expression
To examine the potential interaction between YAP1
and its targeting miRNA, miR-590-5p was identified as a potential
targeting miRNA that has putative binding sites for lncRNA-ATB
(Fig. 6A; data from RNA hybrid and
miRDB), by searching in online bioinformatics databases. RT-qPCR
assays revealed that miR-590-5p mimics and inhibitors were able to
effectively upregulate and downregulate miR-590-5p expression,
respectively, in MM cells (Fig.
6B; P<0.01). The biotin-labeled pulldown assay indicated
that lncRNA-ATB was able to directly pull down miR-590-5p, which
suggested that lncRNA-ATB may directly and specifically sponge
miR-590-5p (Fig. 6C). Accordingly,
miR-590-5p expression was significantly increased in A2058 cells
transfected with shLncRNA-ATB, and significantly downregulated in
A375 cells transfected with the lncRNA-ATB overexpressing plasmid
(Fig. 6D; P<0.05). In addition,
a dual-luciferase reporter assay was performed to verify whether
lncRNA-ATB was the functional target of miR-590-5p. It was observed
that miR-590-5p was able to reduce the relative luciferase activity
of WT-LncRNA-ATB. Conversely, co-transfection of 293 cells with
Mut-LncRNA-ATB and miR-590-5p resulted in a non-significant
alteration in the relative luciferase activity (Fig. 6E). The RT-qPCR results showed that
ectopic expression of miR-590-5p in A2058 cells inhibited
lncRNA-ATB expression, while lncRNA-ATB expression was upregulated
in A375 cells transfected with miR-590-5p inhibitors (Fig. 6F). These results demonstrated that
miR-590-5p was also able to directly bind to lncRNA-ATB at specific
recognition sites. A previous study reported miR-590-5p may
function as a tumor suppressing miRNA in A375 cells (24). In addition, it may inhibit the cell
migration and invasion of A375 by directly targeting YAP1. Thus,
the present study investigated the effects of lncRNA-ATB on the
YAP1 protein expression level. The western blotting results
demonstrated that the expression of YAP1 protein was significantly
downregulated in A2058 cells transfected with shLncRNA-ATB. By
contrast, ectopic expression of lncRNA-ATB in A375 cells resulted
in increased YAP1 protein expression levels (Fig. 6G and H).
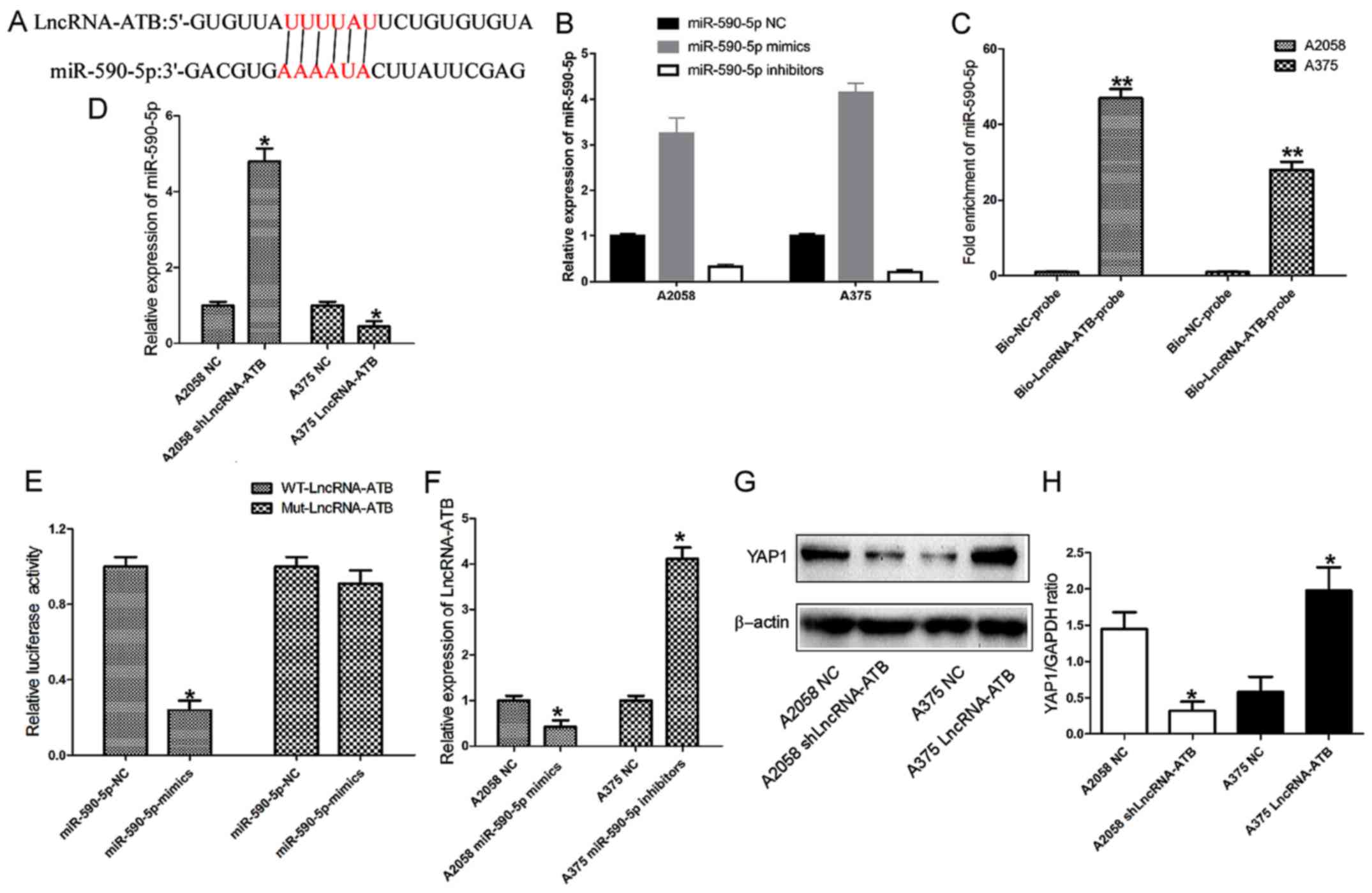 | Figure 6lncRNA-ATB sponges miR-590-5p to
promote YAP1 expression in MM cells. (A) lncRNA-ATB and its
putative binding sequence in miR-50-5p. (B) Efficacy of miR-590-5p
mimics or inhibitors in MM cells. (C) Relative expression of
miR-590-5p in the same sample pulled down by biotinylated
lncRNA-ATB and NC probe. (D) Relative expression of miR-590-5p in
MM cells transfected with lncRNA-ATB shRNA and lncRNA-ATB
overexpressing plasmid. (E) Relative luciferase activity in 293
cells transfected with WT or Mut lncRNA-ATB plasmid. (F) Relative
expression of lncRNA-ATB in MM cells transfected with miR-590-5p
mimics or inhibitors. (G) Immunoblotting analysis and (H) relative
quantification of YAP1 expression levels in MM cells transfected
with lncRNA-ATB shRNA or overexpressing plasmid. Data are from
three experiments and are presented as the mean ± standard
deviation. *P<0.05 and **P<0.01 vs.
respective NC group (Student's t-test). MM, malignant melanoma; NC,
negative control; shRNA, short hairpin RNA; lncRNA, long noncoding
RNA; ATB, activated by transforming growth factor-β; WT, wild-type;
Mut, mutant; YAP1, Yes associated protein 1; miR, microRNA; Bio,
biotinylated. |
Discussion
In the present study, it was demonstrated that
lncRNA-ATB, which had been reported to be activated by TGF-β
(25), was upregulated in A375
cells compared with human melanocytes. In addition, it was observed
that lncRNA-ATB was able to promote MM cell proliferation,
migration and invasion in vitro, and MM tumor growth in
vivo. Furthermore, it was demonstrated that lncRNA-ATB
attenuated cell cycle arrest and inhibited cellular apoptosis in MM
cells. Finally, the present study provided the first evidence, to
the best of our knowledge, that lncRNA-ATB functions as a ceRNA to
enhance YAP1 expression by competitively sponging miR-590-5p in MM
cells. Taken together, these results demonstrated that lncRNA-ATB
may function as a ceRNA to upregulate YAP1 expression, and promote
cell proliferation and invasion, in MM cells by sponging miR-590-5p
to reduce the binding of miR-590-5p to YAP1.
lncRNA-ATB has been reported to be upregulated in a
number of types of cancer, including papillary thyroid cancer
(26), renal cell carcinoma
(27) and gastric cancer (28). lncRNA-ATB was identified as a
TGF-β-activated lncRNA (25);
thus, the function of lncRNA-ATB may be consistent with TGF-β
pathways in different tumors, although certain lncRNAs, including
HOX transcript antisense RNA, serve entirely opposite function in
different types of cancer cells (29,30).
It is possible that lncRNA-ATB is an example of this type of
non-coding RNA. However, according to previous studies (25,31),
it was noted that lncRNA-ATB serves an oncogenic role in the
majority of types of cancers. In line with these previous studies,
the present study identified that lncRNA-ATB was significantly
upregulated in MM cell lines compared with normal human epidermal
melanocyte cell lines. In non-small cell lung cancer, lncRNA-ATB
promotes cellular apoptosis, and inhibits cell viability, cell
migration and invasion (31).
Furthermore, lncRNA-ATB was identified as a potential oncogene in
osteosarcoma (32), glioma
(33), prostate cancer (34) and colon cancer (35); the upregulation of lncRNA-ATB was
able to promote carcinogenesis and progression in these cancer
types. Notably, through searching electronic databases, a
meta-analysis reported that lncRNA-ATB was associated with adverse
clinical features, overall survival, progression-free survival and
prognosis in human cancer (36).
Studies have also confirmed that increased lncRNA-ATB expression is
an independent prognostic predictor in a number of types of cancer,
including non-small cell lung cancer (37), gastric cancer (38) and colorectal cancer (39).
To further determine the biological role of lncRNAs
in MM, gain or loss-of- function experiments were performed. The
results revealed that lncRNA-ATB promoted cell proliferation,
migration and invasion, and attenuated cell cycle arrest in
vitro. It was additionally demonstrated that knockdown of
lncRNA-ATB was able to inhibit tumor growth in vivo. In
addition to lncRNA-ATB, a number of other lncRNAs have been
demonstrated to be potential oncogenes or tumor suppressors in MM,
including homeobox A11-antisense (40), hepatocellular carcinoma upregulated
EZH2-associated lncRNA (41) and
colon cancer associated transcript 1 (42). In agreement with these studies,
lncRNA-ATB was identified in the present study to be a potential
oncogene in MM, and the critical roles of lncRNAs in the
carcinogenesis and progression of MM were further confirmed. As it
is difficult to perform gain-or-loss of function experiments using
normal epidermal melanocytes, the present study did not investigate
the role of lncRNA-ATB in HEMa-LP cells. Although lncRNA-ATB serves
an important role in carcinogenesis, it was considered likely that
the proliferation of HEMa-LP may not exhibit a marked alteration,
due to the number of tumor suppressor genes in HEMa-LP cells to
suppress the function of lncRNA-ATB. It was also observed that the
basal measurements of A2058 cells and A375 cells with respect to
the cell cycle, cell migration and cell invasion were different.
There may be a number of reasons to account for this discrepancy.
Firstly, the source of A2058 and A375 is different. Compared with
A375, A2058 is a more invasive cell line. Thus, the number of
migrated and invasive A2058 cells transfected with lncRNA-ATB NC
was greater compared with the A375 cells transfected with
lncRNA-ATB NC. Secondly, the different levels of lncRNA-ATB may be
one of the factors accounting for the increased invasive ability of
A2058 cells.
Recently, studies identified lncRNAs that were able
to act as ceRNAs to interfere with miRNAs and their downstream
pathways, which affect post-transcriptional regulation (43,44).
ceRNA regulatory networks are implicated in numerous biological
processes in cancer, including tumorigenesis (45), epithelialmesenchymal transition
(46) and the invasionmetastasis
cascade (47). For example, lncRNA
metastasis associated lung adenocarcinoma transcript 1 is
overexpressed in MM and upregulates the expression levels of matrix
metal-lopeptidase 14 and snail family transcriptional repressor 1
by competitively sponging miR-22, thus promoting tumor growth and
metastasis (48). Similarly, the
lncRNA melanoma highly expressed competing endogenous lncRNA for
miR-425 and miR-489 also functions as a ceRNA to upregulate insulin
like growth factor 1 and spindling 1 expression by sponging miR-425
and miR-489, thereby promoting tumor growth and metastasis in MM
(49). lncRNA-ATB was also
demonstrated to be a ceRNA in hepatocellular carcinoma,
upregulating zinc finger E-box binding homeobox 1 and 2 expression,
and promoting the invasion-metastasis cascade by competitively
binding miR-200 (50). The present
study further increased understanding of the ceRNA function of
lncRNA-ATB. The potential miR-590-5p binding sites were identified
in lncRNA-ATB transcripts, and it was demonstrated that lncRNA-ATB
expression was negatively regulated by miR-590-5p. Notably, it was
confirmed that lncRNA-ATB may function as a ceRNA by sponging
miR-590-5p to upregulate YAP1 expression in MM cells.
In conclusion, the present study demonstrated that
lncRNA-ATB is a potential oncogene in MM. lncRNA-ATB was able to
promote the tumor growth and metastasis of MM cells in vivo
and in vitro by sponging miR-590-5p, functionally releasing
YAP1 mRNA transcripts that are normally targeted by miR-590-5p. The
present study helped to reveal the regulatory mechanism of
lncRNA-ATB in MM and may lead to novel therapeutic strategies for
MM.
Abbreviations:
|
MM
|
malignant melanoma
|
|
lncRNA
|
long noncoding RNA
|
|
lncRNA-ATB
|
long noncoding RNA activated by
transforming growth factor-β
|
|
YAP1
|
Yes associated protein 1
|
Acknowledgments
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics. 2017.CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eggermont AM, Spatz A and Robert C:
Cutaneous melanoma. Lancet. 383:816–827. 2014. View Article : Google Scholar
|
|
3
|
Roukos DH: PLX4032 and melanoma:
Resistance, expectations and uncertainty. Expert Rev Anticancer
Ther. 11:325–328. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kondo Y, Shinjo K and Katsushima K: Long
non-coding RNAs as an epigenetic regulator in human cancers. Cancer
Sci. 108:1927–1933. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bolha L, Ravnik-Glavač M and Glavač D:
Long noncoding RNAs as biomarkers in cancer. Dis Markers.
2017.7243968:2017.
|
|
8
|
Zhao L, Sun H, Kong H, Chen Z, Chen B and
Zhou M: The Lncrna-TUG1/EZH2 axis promotes pancreatic cancer cell
proliferation, migration and EMT phenotype formation through
sponging Mir-382. Cell Physiol Biochem. 42:2145–2158. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang BY, Jin Z and Zhao Z: Long
intergenic noncoding RNA 00305 sponges miR-136 to regulate the
hypoxia induced apoptosis of vascular endothelial cells. Biomed
Pharmacother. 94:238–243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Peng Y, Xu Z, Ge B, Xiang X, Zhang
T, Gao L, Shi H, Wang C and Huang J: LncROR promotes bladder cancer
cell proliferation, migration, and epithelial-mesenchymal
transition. Cell Physiol Biochem. 41:2399–2410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Mao ZP, Wang L, Wu GH, Zhang FH,
Wang DY and Shi JL: Long non-coding RNA MALAT1 promotes
cholangiocarcinoma cell proliferation and invasion by activating
PI3K/Akt pathway. Neoplasma. 64:725–731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang C, Wu D, Gao L, Liu X, Jin Y, Wang D,
Wang T and Li X: Competing endogenous RNA networks in human cancer:
Hypothesis, validation, and perspectives. Oncotarget.
7:13479–13490. 2016.PubMed/NCBI
|
|
13
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo LL, Song CH, Wang P, Dai LP, Zhang JY
and Wang KJ: Competing endogenous RNA networks and gastric cancer.
World J Gastroenterol. 21:11680–11687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Richtig G, Ehall B, Richtig E,
Aigelsreiter A, Gutschner T and Pichler M: Function and clinical
implications of long non-coding RNAs in melanoma. Int J Mol Sci.
18:E7152017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Fan W, Wan B, Tu M, Jin F, Liu F,
Xu H and Han P: Characterization of long noncoding RNA and
messenger RNA signatures in melanoma tumorigenesis and metastasis.
PLoS One. 12:e01724982017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bian D, Gao C, Bao K and Song G: The long
non-coding RNA NKILA inhibits the invasion-metastasis cascade of
malignant melanoma via the regulation of NF-ĸB. Am J Cancer Res.
7:28–40. 2017.
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Guide for the Care and Use of Laboratory
Animals. National Academy Press; Washington (DC): 1996
|
|
20
|
Rehmsmeier M, Steffen P, Hochsmann M and
Giegerich R: Fast and effective prediction of microRNA/target
duplexes. RNA. 10:1507–1517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
RDevelopment CORE TEAM: R: A Language and
Environment for Statistical Computing. R foundation for statistical
computing; 2014, http://www.R-project.org/.
|
|
22
|
Tan YD and Xu H: A general method for
accurate estimation of false discovery rates in identification of
differentially expressed genes. Bioinformatics. 30:2018–2025. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Talantov D, Mazumder A, Yu JX, Briggs T,
Jiang Y, Backus J, Atkins D and Wang Y: Novel genes associated with
malignant melanoma but not benign melanocytic lesions. Clin Cancer
Res. 11:7234–7242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang L, Shi S, Zhou Y, Mu X, Han D, Ge R
and Mu K: miR-590-5p inhibits A375 cell invasion and migration in
malignant melanoma by directly inhibiting YAP1 expression. Xi Bao
Yu Fen Zi Mian Yi Xue Za Zhi. 33:326–330. 2017.In Chinese.
PubMed/NCBI
|
|
25
|
Shi SJ, Wang LJ, Yu B, Li YH, Jin Y and
Bai XZ: LncRNA-ATB promotes trastuzumab resistance and
invasion-metastasis cascade in breast cancer. Oncotarget.
6:11652–11663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu XM, Guo W, Li N, Liu HZ, Liu J, Qiu SQ,
Zhang Q, Wang LC, Li F and Li CL: The expression and function of
long noncoding RNA lncRNA-ATB in papillary thyroid cancer. Eur Rev
Med Pharmacol Sci. 21:3239–3246. 2017.PubMed/NCBI
|
|
27
|
Qi JJ, Liu YX and Lin L: High expression
of long non-coding RNA ATB is associated with poor prognosis in
patients with renal cell carcinoma. Eur Rev Med Pharmacol Sci.
21:2835–2839. 2017.PubMed/NCBI
|
|
28
|
Lei K, Liang X, Gao Y, Xu B, Xu Y, Li Y,
Tao Y, Shi W and Liu J: Lnc-ATB contributes to gastric cancer
growth through a MiR-141-3p/TGFβ2 feedback loop. Biochem Biophys
Res Commun. 484:514–521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HJ, Lee DW, Yim GW, Nam EJ, Kim S, Kim
SW and Kim YT: Long non-coding RNA HOTAIR is associated with human
cervical cancer progression. Int J Oncol. 46:521–530. 2015.
View Article : Google Scholar :
|
|
30
|
Chen Y, Bian Y, Zhao S, Kong F and Li X:
Suppression of PDCD4 mediated by the long non-coding RNA HOTAIR
inhibits the proliferation and invasion of glioma cells. Oncol
Lett. 12:5170–5176. 2016. View Article : Google Scholar
|
|
31
|
Cao Y, Luo X, Ding X, Cui S and Guo C:
LncRNA ATB promotes proliferation and metastasis in A549 cells by
down-regulation of microRNA-494. J Cell Biochem. Apr 25–2018.Epub
ahead of print. View Article : Google Scholar
|
|
32
|
Han F, Wang C, Wang Y and Zhang L: Long
noncoding RNA ATB promotes osteosarcoma cell proliferation,
migration and invasion by suppressing miR-200s. Am J Cancer Res.
7:770–783. 2017.PubMed/NCBI
|
|
33
|
Ma CC, Xiong Z, Zhu GN, Wang C, Zong G,
Wang HL, Bian EB and Zhao B: Long non-coding RNA ATB promotes
glioma malignancy by negatively regulating miR-200a. J Exp Clin
Cancer Res. 35:902016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu S, Yi XM, Tang CP, Ge JP, Zhang ZY and
Zhou WQ: Long non-coding RNA ATB promotes growth and
epithelial-mesenchymal transition and predicts poor prognosis in
human prostate carcinoma. Oncol Rep. 36:10–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yue B, Qiu S, Zhao S, Liu C, Zhang D, Yu
F, Peng Z and Yan D: LncRNA-ATB mediated E-cadherin repression
promotes the progression of colon cancer and predicts poor
prognosis. J Gastroenterol Hepatol. 31:595–603. 2016. View Article : Google Scholar
|
|
36
|
Fan YH, Ji CX, Xu B, Fan HY, Cheng ZJ and
Zhu XG: Long noncoding RNA activated by TGF-beta in human cancers:
A meta-analysis. Clin Chim Acta. 468:10–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ke L, Xu SB, Wang J, Jiang XL and Xu MQ:
High expression of long non-coding RNA ATB indicates a poor
prognosis and regulates cell proliferation and metastasis in
non-small cell lung cancer. Clin Transl Oncol. 19:599–605. 2017.
View Article : Google Scholar
|
|
38
|
Saito T, Kurashige J, Nambara S, Komatsu
H, Hirata H, Ueda M, Sakimura S, Uchi R, Takano Y, Shinden Y, et
al: A long non-coding RNA activated by transforming growth factor-β
is an independent prognostic marker of gastric cancer. Ann Surg
Oncol. 22(Suppl 3): S915–S922. 2015. View Article : Google Scholar
|
|
39
|
Iguchi T, Uchi R, Nambara S, Saito T,
Komatsu H, Hirata H, Ueda M, Sakimura S, Takano Y, Kurashige J, et
al: A long noncoding RNA, lncRNA-ATB, is involved in the
progression and prognosis of colorectal cancer. Anticancer Res.
35:1385–1388. 2015.PubMed/NCBI
|
|
40
|
Lu Q, Zhao N, Zha G, Wang H, Tong Q and
Xin S: LncRNA HOXA11-AS exerts oncogenic functions by repressing
p21 and miR-124 in uveal melanoma. DNA Cell Biol. 36:837–844. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao H, Xing G, Wang Y, Luo Z, Liu G and
Meng H: Long noncoding RNA HEIH promotes melanoma cell
proliferation, migration and invasion via inhibition of
miR-200b/a/429. Biosci Rep. 37:BSR201706822017. View Article : Google Scholar :
|
|
42
|
Lv L, Jia JQ and Chen J: LncRNA CCAT1
upregulates proliferation and invasion in melanoma cells via
suppressing miR-33a. Oncol Res. 26:201–208. 2018. View Article : Google Scholar
|
|
43
|
Qu J, Li M, Zhong W and Hu C: Competing
endogenous RNA in cancer: A new pattern of gene expression
regulation. Int J Clin Exp Med. 8:17110–17116. 2015.
|
|
44
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cui B, Li B, Liu Q and Cui Y: lncRNA CCAT1
promotes glioma tumorigenesis by sponging miR-181b. J Cell Biochem.
118:4548–4557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lv M, Zhong Z, Huang M, Tian Q, Jiang R
and Chen J: lncRNA H19 regulates epithelial-mesenchymal transition
and metastasis of bladder cancer by miR-29b-3p as competing
endogenous RNA. Biochim Biophys Acta. 1864.1887–1899. 2017.
|
|
47
|
Wang H, Huo X, Yang XR, He J, Cheng L,
Wang N, Deng X, Jin H, Wang N, Wang C, et al: STAT3-mediated
upregulation of lncRNA HOXD-AS1 as a ceRNA facilitates liver cancer
metastasis by regulating SOX4. Mol Cancer. 16:1362017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Luan W, Li L, Shi Y, Bu X, Xia Y, Wang J,
Djangmah HS, Liu X, You Y and Xu B: Long non-coding RNA MALAT1 acts
as a competing endogenous RNA to promote malignant melanoma growth
and metastasis by sponging miR-22. Oncotarget. 7:63901–63912. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen X, Dong H, Liu S, Yu L, Yan D, Yao X,
Sun W, Han D and Gao G: Long noncoding RNA MHENCR promotes melanoma
progression via regulating miR-425/489-mediated PI3K-Akt pathway.
Am J Transl Res. 9:90–102. 2017.PubMed/NCBI
|
|
50
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|