Introduction
Lung cancer is the primary cause of
cancer-associated mortality worldwide, and in 2017 it was also the
primary cause of cancer-associated death in the United States of
America (1). Furthermore, in 2015,
lung cancer in China was the leading cause of cancer-associated
mortality in men >75 years old (2). Therefore, effective lung cancer
therapy is urgently required for the improvement of public health.
Although progress has been made in lung cancer therapy, treatment
failure and decreases in the survival of patients with lung cancer
is attributed to the metastasis and recurrence of lung cancer
(3,4). One of the crucial causes of
metastasis and recurrence of lung cancer are lung cancer-initiating
cells (CICs) (3–5); therefore, the elimination of lung
CICs may contribute to the cure of lung cancer. Cluster of
differentiation (CD)133 is a marker of CICs in various types of
cancer, including lung cancer (5,6).
Bertolini et al demonstrated that CD133+ lung
cancer cells display stronger self-renewal capabilities and induce
more severe in vivo tumorigenicity compared with
CD133− lung cancer cells (6).
Numerous studies have proposed that differentiated
cancer cells have the ability to be converted into CICs (7–9). To
obtain improved cancer therapeutic efficacy, several combination
strategies that target both CICs and non-CICs have recently been
developed (10,11). Gong et al developed
salinomycin sodium (SS) and doxorubicin-loaded liposomes to
eliminate both liver CICs and cancer cells, and demonstrated that
this combination therapy is superior to single therapy with SS or
doxorubicin (10). Therefore, the
eradication of lung CICs, together with cancer cells, is expected
to obtain superior therapeutic efficacy, compared with targeting
only CD133+ lung CICs.
SS is an antibacterial therapeutic agent that exerts
potent activity against CICs in various types of cancer, including
lung cancer (10–12). The underlying mechanisms be which
SS targets CICs include inhibition of the Wnt pathway and induction
of apoptosis (13). Notably, SS
exerts marked cytotoxic effects against common lung and prostate
cancer cells (14,15). Therefore, SS represents a promising
candidate drug that may combat lung CICs together with cancer
cells. Nevertheless, the aqueous solubility of SS is poor;
therefore, researchers have developed SS nanoparticles (NPs) to
facilitate the preclinical application of SS in cancer therapy
(10–14). Lipid-polymer hybrid NPs of
biodegradable polymers and lipids represent superior candidate drug
delivery systems, since they combine the advantages of liposomes
and polymer NPs (16,17). Liposomes are characterized by
superior biocompatibility and easy modification of hydrophilic
polymers [for example, poly(ethylene glycol) (PEG)] and targeting
molecules (for example, antibodies, peptides and aptamers)
(18,19). The advantages of polymer NPs [for
example, poly(lactide-co-glycolide) acid (PLGA), which is the most
used polymer] include controlled and sustained release, high drug
loading and superior stability (16,17).
Therefore, the strengths of lipid-polymer hybrid NPs include their
superior biocompatibility, modification, controlled and sustained
release, stability and drug loading (16).
To promote the delivery of chemotherapy drugs to
cancer cells, considerable interest has been paid to
antibody-targeted NPs, which could obtain targeted cancer therapy
(18–20). It is well known that
antibody-targeted NPs have improved the therapeutic effect of
chemotherapy in various types of cancer (19). It has also been reported that
epidermal growth factor receptor (EGFR) is abundantly expressed in
various cancer types, including lung cancer (21,22).
Furthermore, amplification of EGFR is a typical genetic aberration
associated with lung cancer, thus suggesting that EGFR may be a
promising therapeutic target for the treatment of lung cancer
(21,22). Several studies have developed NPs
with EGFR antibodies to target cancer (23,24).
The present study hypothesized that, due to overexpression of EGFR
in lung cancer, a significant portion of CD133− lung
cancer cells may abundantly express EGFR, making it possible to
target CD133− lung cancer cells by targeting EGFR. Since
targeting CD133+ lung CICs could be realized by the
conjugation of a CD133 antibody to NPs, it was proposed that NPs
coupled with CD133 and EGFR antibodies may target lung CICs as well
as cancer cells. Furthermore, the targeting of two antigens could
enhance antigen density, and this increase in antigen density may
result in increased cellular delivery of drugs (25,26).
To target lung CICs together with cancer cells, the
present study generated SS lipid-PLGA hybrid NPs with CD133 and
EGFR antibodies (CD133/EGFR SS NPs). The characteristics, and
targeting and therapeutic effects of CD133/EGFR SS NPs towards lung
cancer were investigated, and the in vivo anti-tumor
activity of CD133/EGFR SS NPs was examined in lung cancer-bearing
mice.
Materials and methods
Materials
Lipids, including
1,2-distearoyl-sn-glycero-3-phos-phoethanolamine-N-[maleimide(PEG)-2000]
(DSPE-PEG-Mal), phosphatidylcholine and cholesterol were provided
by Avanti Polar Lipids (Alabaster, AL, USA). PLGA (50:50, 40–75
kDa), polyvinyl alcohol (PVA, 30–70 kDa), coumarin 6,
2-iminothiolane (Traut's reagent), SS and organic reagents were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). EGFR
(cat. no. MAB1095-500) and CD133 antibodies (cat. no. MAB11331)
were obtained from R&D Systems, Inc. (Minneapolis, MN, USA),
and EGFR and CD133 Fab' were obtained according to our previous
protocol (16). The Pierce
Bicinchoninic Acid (BCA) Protein Assay kit, Roswell Park Memorial
Institute 1640 (RPMI-1640) medium and fetal bovine serum (FBS) were
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
PerCP-Cy5.5 anti-EGFR antibodies (cat. no. sc-120 PCPC5) were
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Phycoerythrin (PE)-CD133 antibodies (cat. no. 130080801) and the
CD133 MicroBead kit (cat. no. 130-050-801) were provided by
Miltenyi Biotec Technology & Trading (Shanghai) Co., Ltd.
(Shanghai, China).
Lung cancer cell lines culture
The human lung cancer cell lines, A549 and H460,
were purchased from American Type Culture Collection (Manassas, VA,
USA), and were cultured in RPMI-1640 medium supplemented with 2 mM
L-glutamine and 10% FBS. Lung cancer cells were cultured in a
humidified atmosphere containing 5% CO2 at 37°C.
Antigen (EGFR and CD133) expression in
lung cancer cell lines
Double-staining flow cytometry was conducted to
analyze the expression of EGFR and CD133 in the lung cancer cell
lines. Briefly, the lung cancer cells were dissociated into single
cells, and the dissociated cells were incubated with PE-CD133 and
PerCP-Cy5.5 anti-EGFR antibodies (1 µg/ml) diluted in 1% FBS
for 0.5 h at 4°C. Subsequently, the cells were washed to remove
unconjugated antibodies. Finally, the washed cells were suspended
in PBS and a FACSCalibur flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA) was used to analyze the proportion of positively
stained cells. The data were analyzed using FlowJo (version 10;
FlowJo LLC, Ashland, OR, USA).
Magnetic cell sorting-based separation of
CD133+ cells
The separation of CD133+ cells from the
lung cancer cells was conducted according to the protocol provided
by the CD133 MicroBead kit [Miltenyi Biotec Technology &
Trading (Shanghai) Co., Ltd.]. A FACSCalibur flow cytometer was
used to analyze the proportion of positively stained cells, as
aforementioned.
Preparation of lipid-PLGA hybrid NPs
PLGA NPs were prepared according to the
emulsion-solvent evaporation procedure. Briefly, 0.5 mg SS and 5 mg
PLGA were completely dissolved in acetone to form the oil phase.
The oil solution was injected into 2% PVA solution, followed by
homogenization. Subsequently, the mini-emulsion was poured into
0.2% PVA solution, and quickly mixed for 6 h to remove any
remaining acetone by evaporation; the NPs were recovered using
ultra-centrifugation (80,000 × g) for 0.5 h at 25°C. Concurrently,
a lipid film composed of phosphatidylcholine, DSPE-PEG-Mal and
cholesterol (57:3:40 molar ratio) was formed in a round bottom
flask using a vacuum rotary evaporator. Once the lipid film was
formed, the prepared NPs were added to hydrate the film. A
hand-held extruder (Avanti Polar Lipids) with 200 nm membranes was
adopted to extrude the obtained lipid-polymer suspension to create
small and homogeneous NPs. The resultant lipid-polymer NPs were
washed by centrifugation (2,000 × g) with Amicon centrifugal
filters [molecular weight cut-off (MWCO) 100 kDa; EMD Millipore,
Billerica, MA, USA] using distilled water for 0.5 h at 25°C.
Furthermore, EGFR and CD133 Fab′ were thiolated using
2-iminothiolane (molar ratio of Fab′ to 2-iminothiolane, 1:80)
(16). Thiolated EGFR and CD133
Fab′ (1:1 molar ratio) were incubated with the NPs (molar ratio of
Fab′ to DSPE-PEG-Mal, 1:10) for 6 h at room temperature to
facilitate antibody conjugation to the NPs. Subsequently, Amicon
centrifugal filters (MWCO 100 kDa) were used to remove unconjugated
Fab′ for 0.5 h at 25°C by centrifugation (2,000 × g). Non-targeted
NPs were developed using a similar method as aforementioned,
without the addition of Fab′. Blank NPs were developed using a
similar method, without the initial addition of SS. The fluorescent
coumarin 6-loaded NPs were constructed using a similar method;
however, coumarin 6 was initially added.
The following abbreviations were used to designate
the NPs used in the present study: CD133/EGFR NPs, blank lipid-PLGA
NPs conjugated with CD133 and EGFR antibodies. The SS NPs were
designated as follows: SS NPs, SS lipid-PLGA NPs; CD133 SS NPs, SS
lipid-PLGA NPs with CD133 antibodies; EGFR SS NPs, SS lipid-PLGA
NPs with EGFR antibodies; CD133/EGFR SS NPs, SS lipid-PLGA NPs with
CD133 and EGFR antibodies. The fluorescent NPs were designated as
follows: Coumarin 6 NPs, coumarin 6 lipid-PLGA NPs; CD133 coumarin
6 NPs, coumarin 6 lipid-PLGA NPs with CD133 antibodies; EGFR
coumarin 6 NPs, coumarin 6 lipid-PLGA NPs with EGFR antibodies;
CD133/EGFR coumarin 6 NPs, coumarin 6 lipid-PLGA NPs with CD133 and
EGFR antibodies.
Conjugation efficacy of antibodies to
NPs
Ultrafiltration of the NPs was used to evaluate the
conjugation efficacy of antibodies to NPs. Briefly, the antibodies
were incubated with the NPs, and the antibody/NP mixtures were
centrifuged (2,000 × g) using Amicon centrifugal filters (MWCO 100
kDa) to remove unconjugated antibodies for 0.5 h at 25°C. The
concentration of unconjugated antibodies was measured using the
Pierce BCA Protein Assay Reagent kit. After measuring the antibody
concentration, the conjugation efficacy of antibodies to NPs was
evaluated using the following equation: (Mt –
Mu)/Mt; where Mt refers to the
mass of total antibodies and MU refers to the mass of unconjugated
antibodies.
Size, polydispersity index (PDI), zeta
potential, morphology and drug loading of lipid-PLGA NPs
A Zetasizer Nano ZS90 (Malvern Panalytical Ltd.,
Malvern, UK) was used to evaluate the particle size, PDI and zeta
potential of the lipid-PLGA NPs, after diluting 200 µl NPs
in 1.8 ml distilled water. To analyze the NP ultrastructure, the
samples were stained with phosphotungstic acid, air-dried and
images were captured by transmission electron microscopy (High
Resolution TEM; JEM2100F; JEOL, Ltd., Tokyo, Japan). SS
encapsulation efficiency (EE) and loading of the lipid-PLGA NPs was
determined using reversed-phase high performance liquid
chromatography (HPLC) with the universal reverse phase
Diamonsil® C-18 column (5 µm, 250×4.5 mm; Dikma
Technologies, Inc., Foothill Ranch, CA, USA). Briefly, 1 ml
dichloromethane was added to 2 mg lyophilized NPs to dissolve them.
Subsequently, dichloromethane was completely removed by evaporation
in a vacuum, and methanol was added to dissolve the residue after
thorough vortexing. Analysis (sample volume, 20 µl) was
carried out using the L-2000 HPLC system (Hitachi, Ltd., Tokyo,
Japan). The mobile phase was
water/tetrahydrofuran/acetonitrile/phosphoric acid (v/v/v/v:
10/4/86/0.01), and the flow rate of the mobile phase was set at 1.0
ml/min. The EE of SS was calculated according to the following
formula: QE/QT × 100%. QE and
QT were defined as the quantity of encapsulated SS and
the total quantity of added SS, respectively. Drug loading of SS
was calculated using the following formula:
QE/QN × 100%. QE and QN
were defined as the quantity of encapsulated SS and the quantity of
SS-loaded NPs, respectively. The detection wavelength of SS was set
at 210 nm. Furthermore, a coumarin 6 calibration curve was used to
examine the drug loading of coumarin 6-loaded NPs.
SS release of lipid-PLGA NPs
The lipid-PLGA NPs (0.5 mg/ml) were suspended in PBS
or PBS containing 10% FBS in a centrifuge tube. Subsequently, the
NPs were placed onto an orbital shaker and were gently agitated
(100 rpm) at 37°C. At various time points during the 120-h drug
release period, during which the sample was continuously agitated,
the centrifuge tubes were centrifuged (10,000 × g for 30 min) at
25°C to obtain the supernatant, which was further measured using
reverse-HPLC, as aforementioned. The accumulated SS release rate of
the NPs was measured using the following formula:
(Mi/Mt) × 100%; where Mi refers to
the mass of accumulated released SS, and Mt to the total
amount of SS.
In vitro targeting of fluorescent NPs to
lung cancer cells
The in vitro targeting of fluorescent NPs was
evaluated as described in our previous study (10). Briefly, lung cancer cells
(5×105 cells/well) were seeded into a 12-well cell
culture plate overnight at 37°C. Subsequently, the medium in each
well was replaced with fresh medium containing coumarin 6-loaded
NPs (equal to 20 ng/ml coumarin 6). Cells were incubated with the
fluorescent NPs for 2 h at 37°C. After incubation, the lung cancer
cells were washed with PBS to remove unbound NPs and were
trypsinized to obtain dissociated single cells. Finally, the cells
were directly suspended in PBS and analyzed using a FACSCalibur
flow cytometer. The data were analyzed using FlowJo (version 10;
FlowJo LLC).
Cytotoxic effects of NPs towards lung
cancer cell lines
The cytotoxic effects of NPs were evaluated using
the Cell Counting kit (CCK)-8 assay (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan), according to the
manufacturer's protocol. Briefly, lung cancer cells were
trypsinized into single cells, washed and inoculated into 96-well
cell culture plates at a density of 3×103 cells/well overnight at
37°C. Subsequently, the medium was replaced with fresh medium
containing free SS or NPs at a series of concentrations. After 72-h
treatment, the medium was discarded and was replaced with fresh
medium. Cell viability was determined using the CCK-8 assay and a
microplate reader (Multiskan MK3; Thermo Fisher Scientific, Inc.).
Finally, the data were processed by GraphPad Prism 5 (GraphPad
Software, Inc., La Jolla, CA, USA) to calculate the half maximal
inhibitory concentration (IC50) values.
Similarly, the cytotoxic effects of NPs
were also evaluated using the MTT assay
Briefly, lung cancer cells (3×103
cell/well) were seeded into 96-well plates and incubated overnight
at 37°C. The medium was then replaced with fresh medium containing
a series of concentrations of free SS or NPs. After 72-h treatment,
the cells were washed three times with PBS to remove the
formulations, and fresh medium was added to the cells.
Subsequently, 20 µl MTT solution (5 mg/ml in PBS) was added
to each well and incubated for an additional 4 h, after which, the
medium was replaced with 150 µl dimethyl sulfoxide per well.
Absorbance was measured at 490 nm using a microplate reader
(Multiskan MK3). Finally, the data were processed by GraphPad Prism
5 to calculate the IC50 values.
Therapeutic effects of NPs on mice
bearing lung cancer xenografts
Male nude mice (age, 5 weeks; weight, ~20 g) were
purchased from the Shanghai Chinese Academy of Sciences (Shanghai,
China). The present study was approved by the Animal Administrative
Committee of the Naval Medical University (Shanghai, China). The
mice were housed in separate cages (n=4 mice/cage) in a controlled
atmosphere (humidity, 50±5%; temperature, 21±1°C) under a 12-h
light/dark cycle. The mice were allowed free access to food and
water. A mouse model bearing a lung xenograft was established by
injecting 5×106 H460 cells suspended in PBS into the
flank of the mice. The volume of the tumors reached ~50
mm3 on day 5. The mice were randomly divided into the
following seven groups (n=8 mice/group): Saline, CD133/EGFR NPs,
SS, SS NPs, CD133 SS NPs, EGFR SS NPs and CD133/EGFR SS NPs. On
days 5, 7, 9, 11, 13, 15, 17, 19 and 21, the mice were treated with
NPs (suspended in PBS, 5 mg SS/kg, injected intravenously into the
tail vein) or free SS (dissolved in ethanol, 5 mg SS/kg, injected
intraperitoneally). Since the solubility of SS is very low in
water, SS could only be dissolved in ethanol. However, intravenous
injection of SS dissolved in ethanol causes toxicity to mice;
therefore, free SS dissolved in ethanol could only be injected
intraperitoneally. Previous studies have administered SS dissolved
in ethanol intraperitoneally (13,14).
The tumor volume was calculated using the following formula: Volume
= width2 × length/2), using digital calipers, and was
monitored once every 5 days. The weight of the mice was also
measured once every 5 days. At the end of the antitumor assay (day
35), the mice were sacrificed using carbon dioxide. Subsequently,
tumors were excised from the mice and weighed.
Tumorigenicity of lung cancer cells in
vivo
The tumorigenicity of lung cancer cells in
vivo was determined in 5-week old male nude mice (weight, ~20
g). Briefly, various densities of CD133+ or
CD133− lung cancer cells (between 5×102 and
1×106 cells) were isolated according to the magnetic
bead-based approach. Subsequently, the collected cells were mixed
with BD Matrigel™ (BD Biosciences), and implanted subcutaneously
into the mice. Tumor formation was recorded during the observation
period of 7 weeks.
Statistical analysis
Data were analyzed using SPSS (version 13; SPSS,
Inc., Chicago, IL, USA). All of the experiments were repeated three
times. Unless otherwise stated, all data are expressed as the means
± sstandard deviation. The differences between two groups were
measured using Student's non-paired t-test, whereas the differences
among three or more groups were measured using a one-way analysis
of variance with Newman-Keuls post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Generation of lipid-PLGA NPs
Two steps are used to prepare lipid-PLGA NPs: The
first step is to prepare the PLGA NP core by the emulsion-solvent
evaporation procedure; the second step refers to coating of the
PLGA NP core with the lipid shell using the lipid-film based
hydration approach (Fig. 1).
Subsequently, the thiolated antibodies are linked to the lipid-PLGA
NPS via reaction of the sulfhydryl and maleimide groups. The size,
zeta potential and drug loading of NPs are displayed in Fig. 2A. The size of SS NPs, which were
not conjugated with antibodies, was small (96.3 nm). However,
following conjugation of antibodies, the NP size increased to
>100 nm; CD133 SS NPs, EGFR SS NPs and CD133/EGFR SS NPs were
112.1, 111.7 and 107.8 nm, respectively. The zeta potential of all
NPs was ~−15 mV. The drug loading of all NPs ranged between 7 and
10%, and all of the NPs exhibited an EE of >75%. To determine
the conjugation efficiency of antibodies on the NPs, CD133 SS NPs,
EGFR SS NPs and CD133/EGFR SS NPs were analyzed; the conjugation
efficacies ranged between 15 and 18% (data not shown). TEM analysis
observed that all NPs exhibited a spherical shape and a
monodisperse pattern (Fig. 2B). As
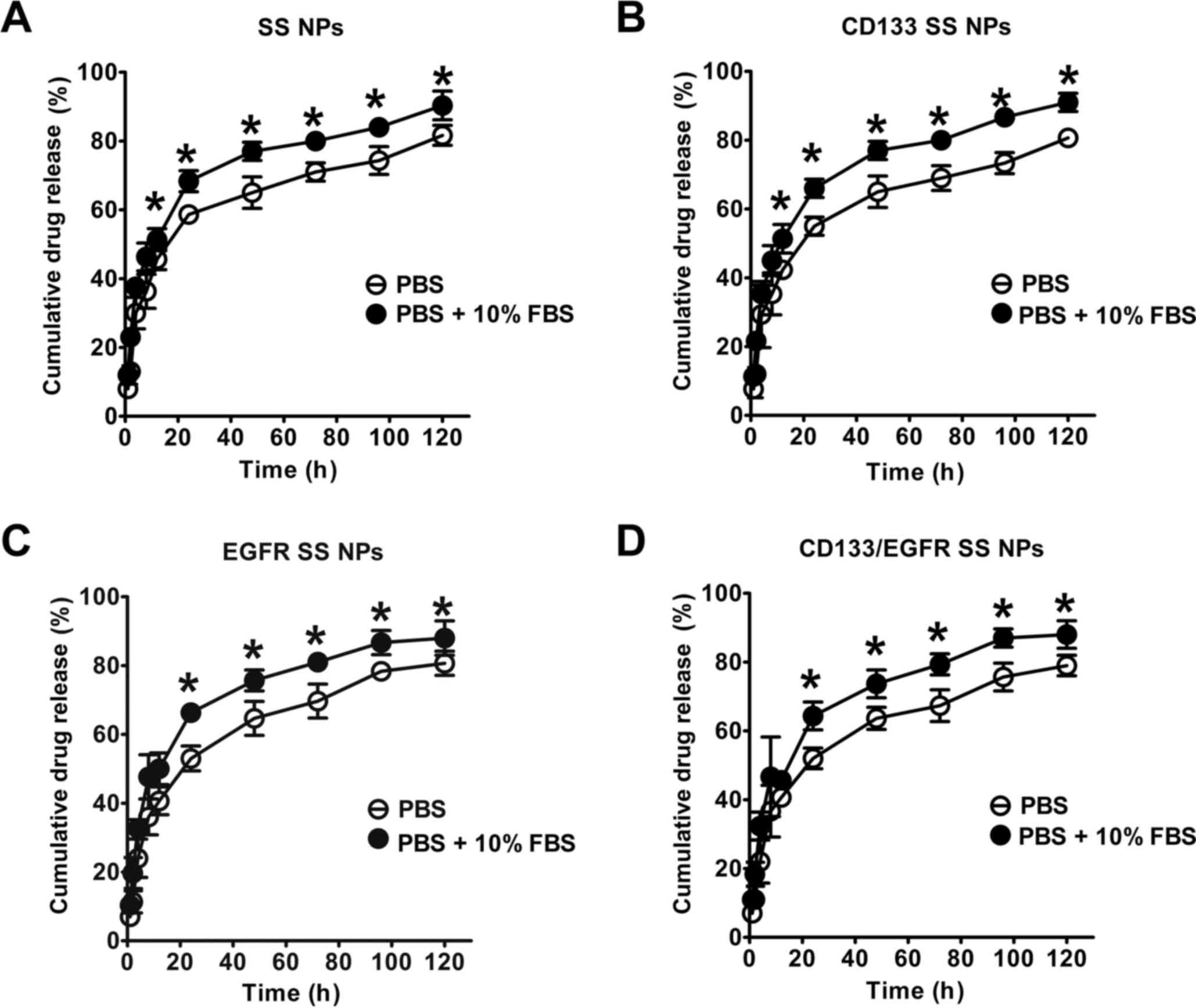
shown in Fig. 3, the SS release
assay indicated that all NPs displayed a burst release (~60% of SS
was released in the first 24 h). During the next 96 h, the
accumulated SS release arrived at 80%, thus suggesting that all NPs
displayed a sustained drug release in 120 h. Furthermore, the SS
release of the NPs was markedly higher in PBS supplemented with 10%
FBS (P<0.05) compared with in FBS-free PBS, thus suggesting that
serum may facilitate the drug release of NPs.
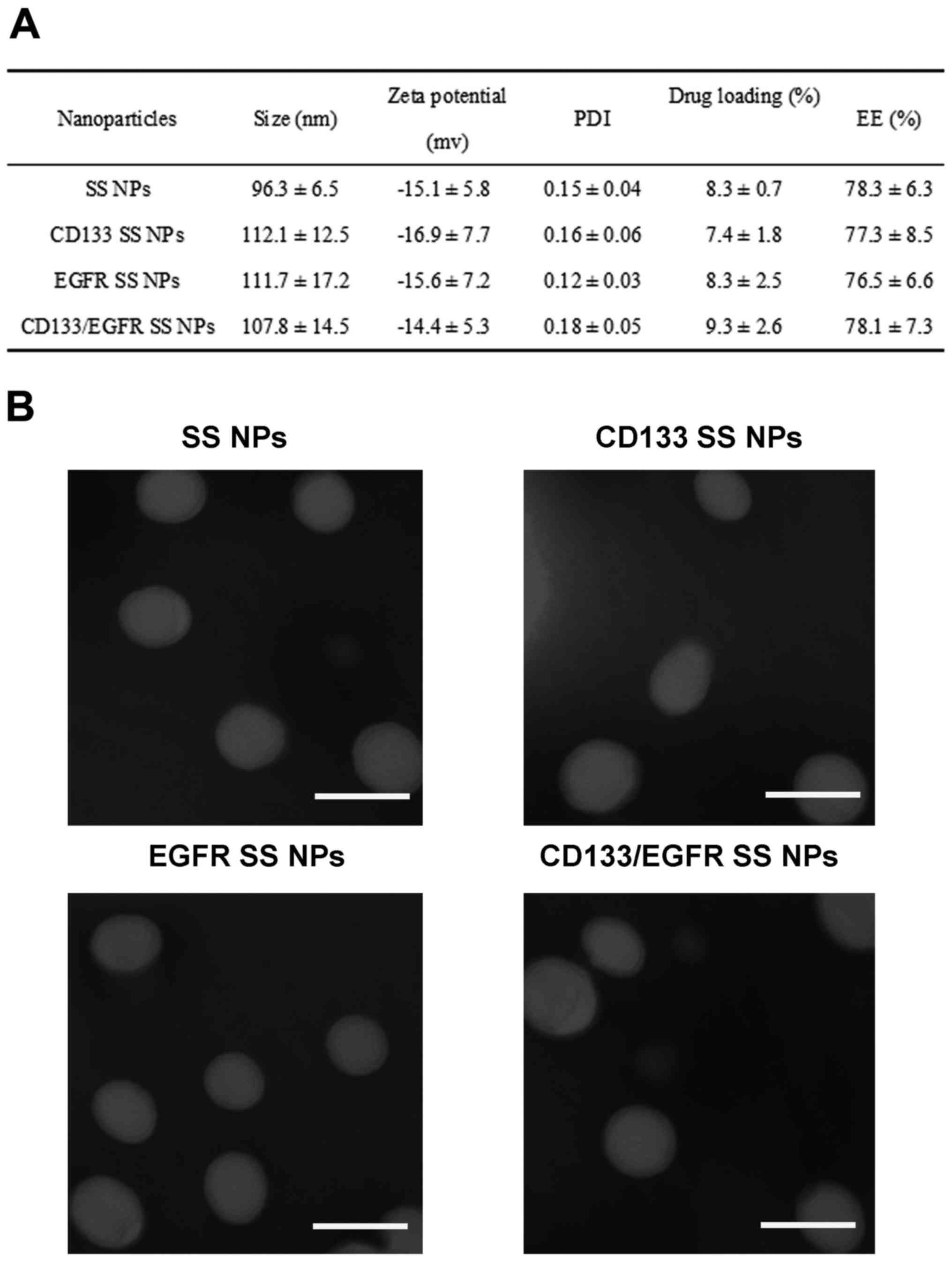 | Figure 2Characteristics of NPs. (A) Size,
zeta potential, PDI, EE and drug loading of
lipid-poly(lactide-co-glycolide) acid NPs. Data are presented as
the means ± standard deviation (n=3). (B) Analysis by TEM. Samples
were stained with phosphotungstic acid, air-dried and images were
captured by TEM. Scale bars represent 100 nm. CD133, cluster of
differentiation 133; EE, encapsulation efficiency; EGFR, epidermal
growth factor receptor; NPs, nanoparticles; PDI, polydispersity
index; SS, salinomycin sodium. |
Antigen expression and in vivo
tumorigenicity of lung cancer cells
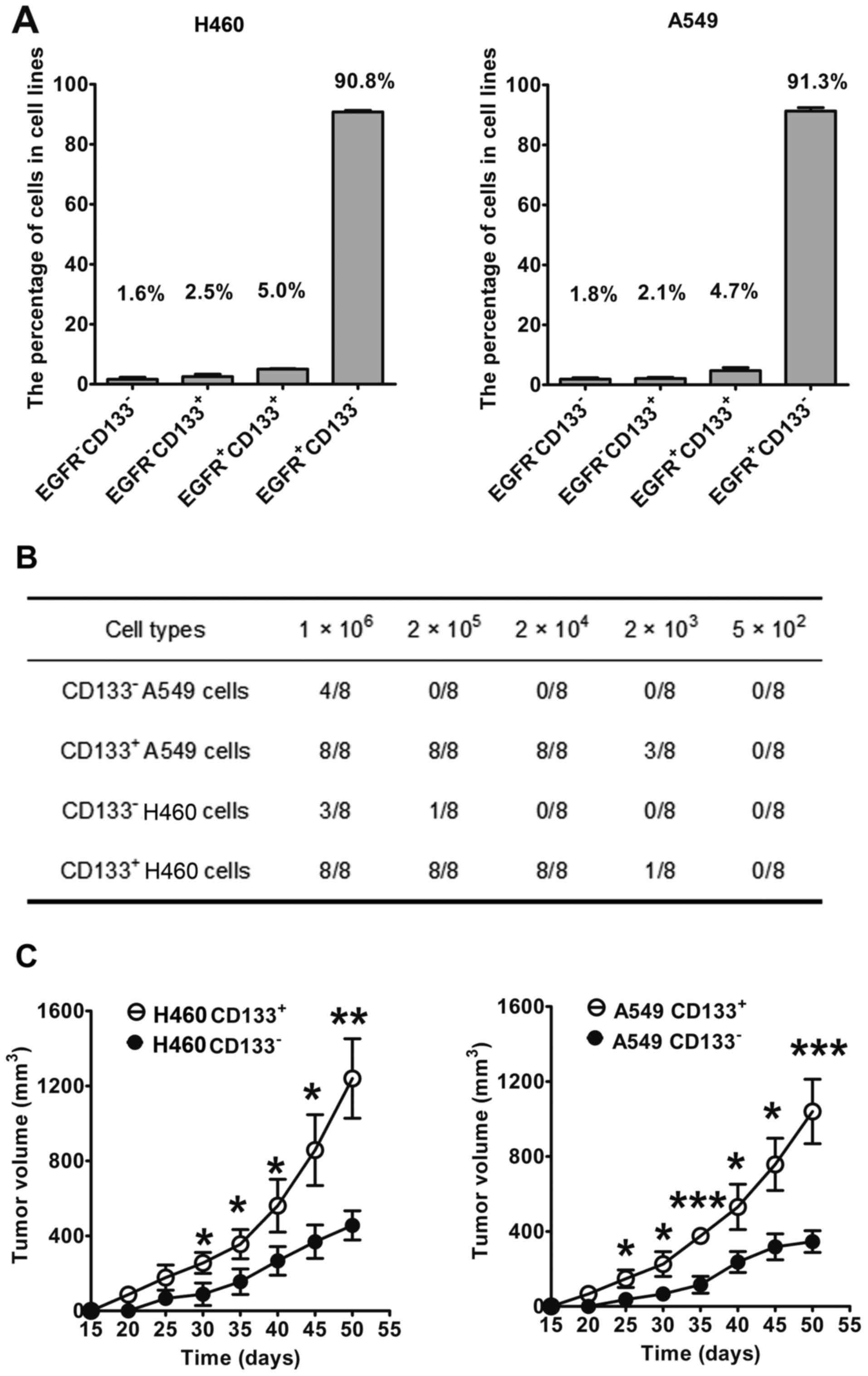
CD133 and EGFR expression was evaluated using
double-staining flow cytometry in lung cancer cells (Fig. 4A). The percentage of
EGFR−/CD133+,
EGFR−/CD133−,
EGFR+/CD133+ and
EGFR+/CD133− in H460 cells was 2.5, 1.6, 5.0
and 90.8%, respectively. In A549 cells, the percentage of
EGFR−/CD133+,
EGFR−/CD133−,
EGFR+/CD133+ and
EGFR+/CD133− was 2.1, 1.8, 4.7 and 91.3%,
respectively. These results indicated that the expression levels of
EGFR and CD133 in lung cancer cells are heterogeneous, as reflected
by the different subpopulations in the lung cancer cell lines.
Prior to magnetic sorting-based isolation, the percentage of
CD133+ cells was 7.5 and 6.8% in H460 and A549 cells,
respectively, whereas the percentage of CD133+ cells in
isolated CD133+ cells was >98% following magnetic
sorting-based isolation. Conversely, in sorted CD133−
cells, the percentage of CD133+ cells was <2%.
The present study also evaluated the tumorigenicity
of CD133+ and CD133− lung cancer cells in
mice (Fig. 4B and C). Notably,
100% tumor incidence (8/8) was detected in mice treated with
≥2×104 CD133+ A549 cells (Fig. 4B). Conversely, only 50% tumor
incidence (4/8) was detected in mice treated with CD133−
A549 cells, even when they were treated with the maximum cell
number (1×106), thus indicating that CD133+
A549 cells significantly increased tumorigenic potential compared
with CD133− A549 cells. Similarly, CD133+
H460 cells had significantly increased tumorigenic potential
compared with CD133− H460 cells. CD133+ H460
cells resulted in 100% tumor incidence in mice when the cell count
was ≥2×104 cells, whereas 1×106
CD133− H460 cells only produced 37.5% tumor incidence
(3/8). Furthermore, the growth curves of tumors in mice formed by
1×106 lung cancer cells were evaluated (Fig. 4C). After 30 days, the volume of
tumors induced by CD133+ H460 cells was significantly
larger compared with CD133− H460 cells (P<0.05). On
day 50, the volume of the tumors induced by CD133+ H460
cells was ~1,200 mm3, which was markedly larger than the
volume of tumors induced by CD133− H460 cells (~400
mm3; P<0.01; Fig.
4C). In A549 cells, similar results were obtained. These
findings indicated that the tumorigenicity of CD133+
lung cancer cells was markedly higher than CD133− lung
cancer cells, thus suggesting that CD133+ lung cancer
cells possessed the characteristics of lung CICs.
Targeting of fluorescent NPs to lung
cancer cells in vitro
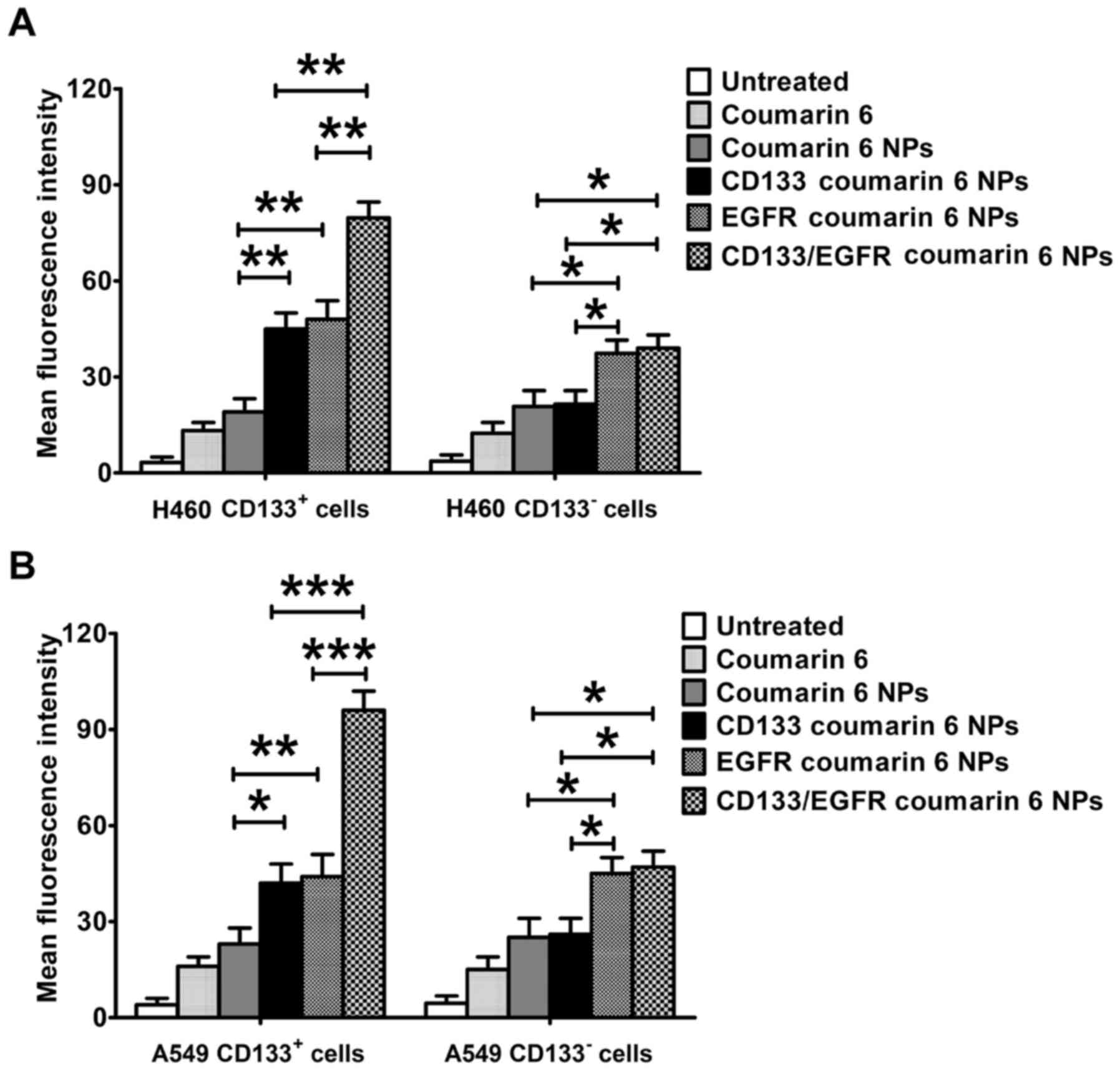
As a common green fluorescent tracer, coumarin 6 was
used to evaluate the in vitro targeting of fluorescent NPs
to lung cancer cells (Fig. 5). In
CD133+ H460 cells, the uptake of CD133/EGFR coumarin 6
NPs was significantly higher compared with EGFR coumarin 6 NPs and
CD133 coumarin 6 NPs (P<0.01; Fig.
5A). Both EGFR coumarin 6 NPs and CD133 coumarin 6 NPs
exhibited increased uptake compared with coumarin 6 NPs
(P<0.01). However, CD133/EGFR coumarin 6 NPs exhibited similar
uptake to EGFR coumarin 6 NPs in CD133− H460 cells, but
exhibited increased uptake compared with CD133 coumarin 6 NPs and
coumarin 6 NPs (P<0.05). In A549 cells, similar results were
achieved (Fig. 5B). CD133/EGFR
coumarin 6 NPs exhibited increased uptake compared with EGFR
coumarin 6 NPs and CD133 coumarin 6 NPs in CD133+ A549
cells (P<0.001). In addition, CD133/EGFR coumarin 6 NPs
exhibited increased uptake compared with CD133 coumarin 6 NPs and
coumarin 6 NPs in CD133− A549 cells (P<0.05). EGFR
coumarin 6 NPs also showed increased uptake compared with CD133
coumarin 6 NPs and coumarin 6 NPs in CD133− A549 cells
(P<0.05).
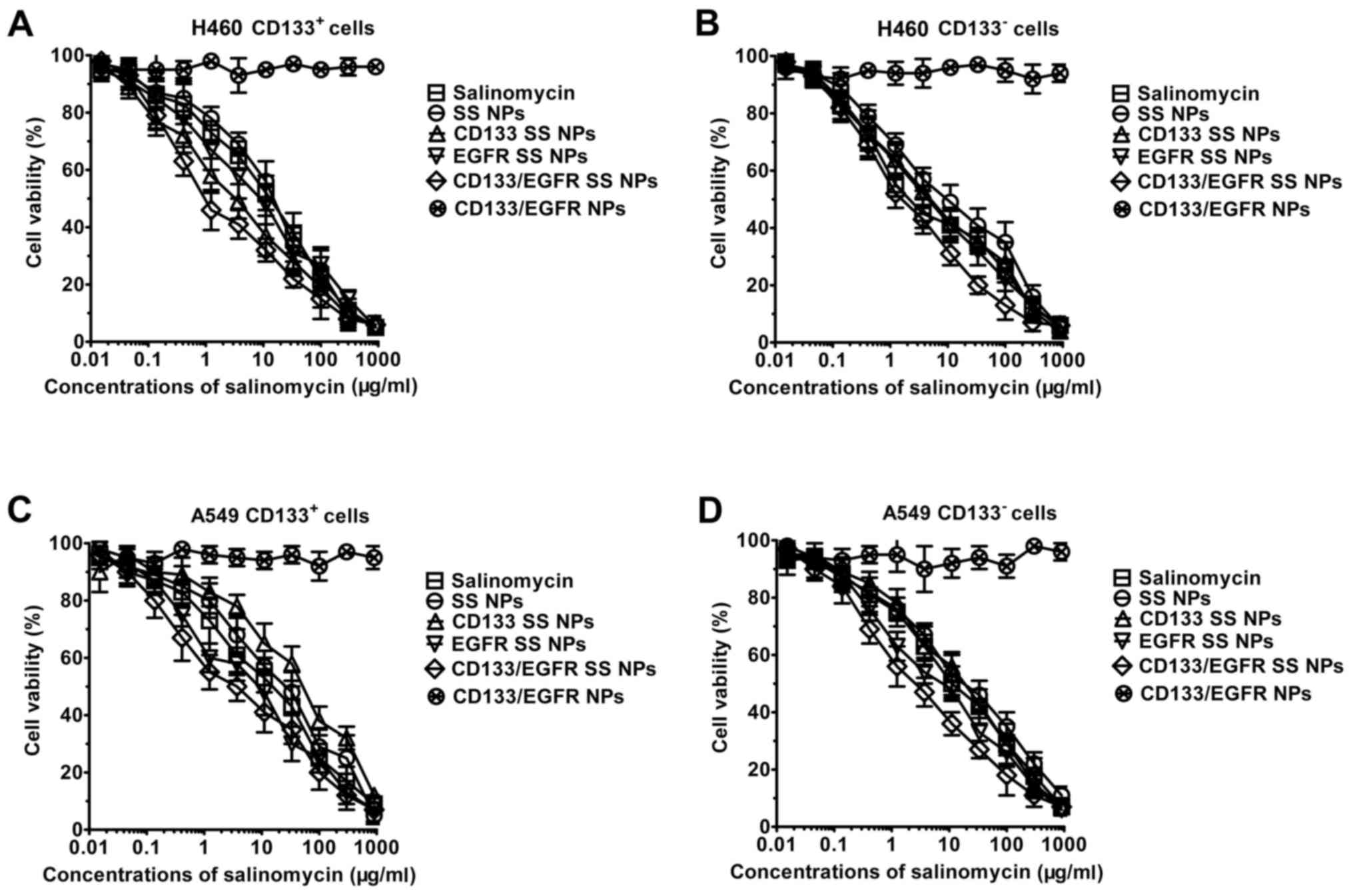
Cytotoxic effects of SS and lipid-PLGA
NPs on lung cancer cells
As shown in Fig. 6,
CD133/EGFR NPs, blank lipid-PLGA NPs with CD133 and EGFR
antibodies, displayed no marked cytotoxic effects towards lung
cancer cells, and the curve induced by CD133/EGFR NPs was almost
horizontal. Conversely, dose-dependent cytotoxicity was observed
for SS and SS NPs, as indicated by the inverse sigmoid curves. The
IC50 values of the drugs are presented in Table I. In CD133+ H460 cells,
SS NPs exhibited similar cytotoxic effects to SS (7.8 vs. 6.3
µg/ml). Compared with SS NPs, CD133 SS NPs and EGFR SS NPs
possessed significantly increased cytotoxic effects (3.2
µg/ml for CD133 SS NPs, 3.8 µg/ml for EGFR SS NPs;
P<0.05). Notably, the cytotoxic effects of CD133/EGFR SS NPs
were significantly higher than the other groups, including EGFR SS
NPs, CD133 SS NPs and SS NPs (P<0.05). Nevertheless, the
IC50 values of CD133 SS NPs (17.3 µg/ml) and SS
NPs (15.1 µg/ml) did not differ significantly in
CD133− H460 cells. In CD133− H460 cells,
compared with SS NPs (15.1 µg/ml) and CD133 SS NPs (17.3
µg/ml), the IC50 values of CD133/EGFR SS NPs (6.7
µg/ml) and EGFR SS NPs (7.3 µg/ml) were significantly
lower (P<0.05). In A549 cells, similar results were achieved. In
CD133+ A549 cells, the cytotoxic effects of CD133/EGFR
SS NPs were markedly higher compared with the other groups,
including SS NPs, CD133 SS NPs and EGFR SS NPs (P<0.05). In
addition, the cytotoxic effects of CD133/EGFR SS NPs were
significantly higher than the other groups, including SS NPs and
CD133 SS NPs in CD133− A549 cells (P<0.05).
Consistent with the CCK-8 assay, similar results were obtained
using the MTT assay. As shown in Table II, in CD133+ H460 and
A549 cells, the cytotoxic effects of CD133/EGFR SS NPs were
significantly higher than the other groups, including EGFR SS NPs,
CD133 SS NPs and SS NPs (P<0.05). In CD133− H460
cells and A549 cells, the cytotoxic effects of CD133/EGFR SS NPs
were significantly higher than the other groups, including SS NPs
and CD133 SS NPs (P<0.05).
 | Table ICytotoxicity of SS and NPs, as
reflected by IC50, on lung cancer cells. |
Table I
Cytotoxicity of SS and NPs, as
reflected by IC50, on lung cancer cells.
| Treatment | IC50
value (µg/ml)
|
|---|
H460
| A549
|
|---|
|
CD133+ |
CD133− |
CD133+ |
CD133− |
|---|
| SS | 6.3±2.5 | 13.6±3.5 | 6.6±2.4 | 17.5±5.8 |
| SS NPs | 7.8±1.6 | 15.1±3.8 | 8.7±2.9 | 21.2±4.8 |
| CD133 SS NPs | 3.2±1.1a | 17.3±4.9 | 5.3±2.7a | 25.9±6.9 |
| EGFR SS NPs | 3.8±1.3a | 7.3±2.8a | 5.2±2.3a | 7.8±1.8a |
| CD133/EGFR SS
NPs | 1.1±0.4a–c | 6.7±2.7a,b | 1.4±0.7a–c | 4.3±1.5a,b |
| CD133/EGFR NPs | >270.0 | >270.0 | >270.0 | >270.0 |
 | Table IICytotoxicity of SS and NPs, as
reflected by IC50, on lung cancer cells measured by the
MTT assay. |
Table II
Cytotoxicity of SS and NPs, as
reflected by IC50, on lung cancer cells measured by the
MTT assay.
| Treatment | IC50
value (µg/ml)
|
|---|
H460
| A549
|
|---|
|
CD133+ |
CD133− |
CD133+ |
CD133− |
|---|
| SS | 8.9±2.8 | 17.8±2.9 | 9.3±3.3 | 19.2±6.3 |
| SS NPs | 9.3±2.1 | 18.3±4.9 | 9.5±1.5 | 23.5±7.2 |
| CD133 SS NPs | 4.1±1.5 | 20.5±6.3 | 7.1±3.7 | 28.2±7.3 |
| EGFR SS NPs | 4.2±1.1 | 9.8±3.1 | 8.1±1.5 | 6.6±2.4 |
| CD133/EGFR SS
NPs | 1.5±0.3 | 7.8±3.6 | 3.2±1.3 | 5.8±2.6 |
| CD133/EGFR NPs | >270.0 | >270.0 | >270.0 | >270.0 |
Taken together, CD133/EGFR SS NPs exhibited enhanced
cytotoxic effects compared with CD133 SS NPs, EGFR SS NPs, SS NPs
and SS in CD133+ lung cancer cells. Furthermore,
CD133/EGFR SS NPs exhibited enhanced cytotoxic effects compared
with CD133 SS NPs, SS NPs and SS in CD133− lung cancer
cells.
Therapeutic effects of lipid-PLGA NPs on
lung cancer-bearing mice
The therapeutic effects of lipid-PLGA NPs were
examined in lung cancer-bearing mice (Fig. 7). The tumors of mice treated with
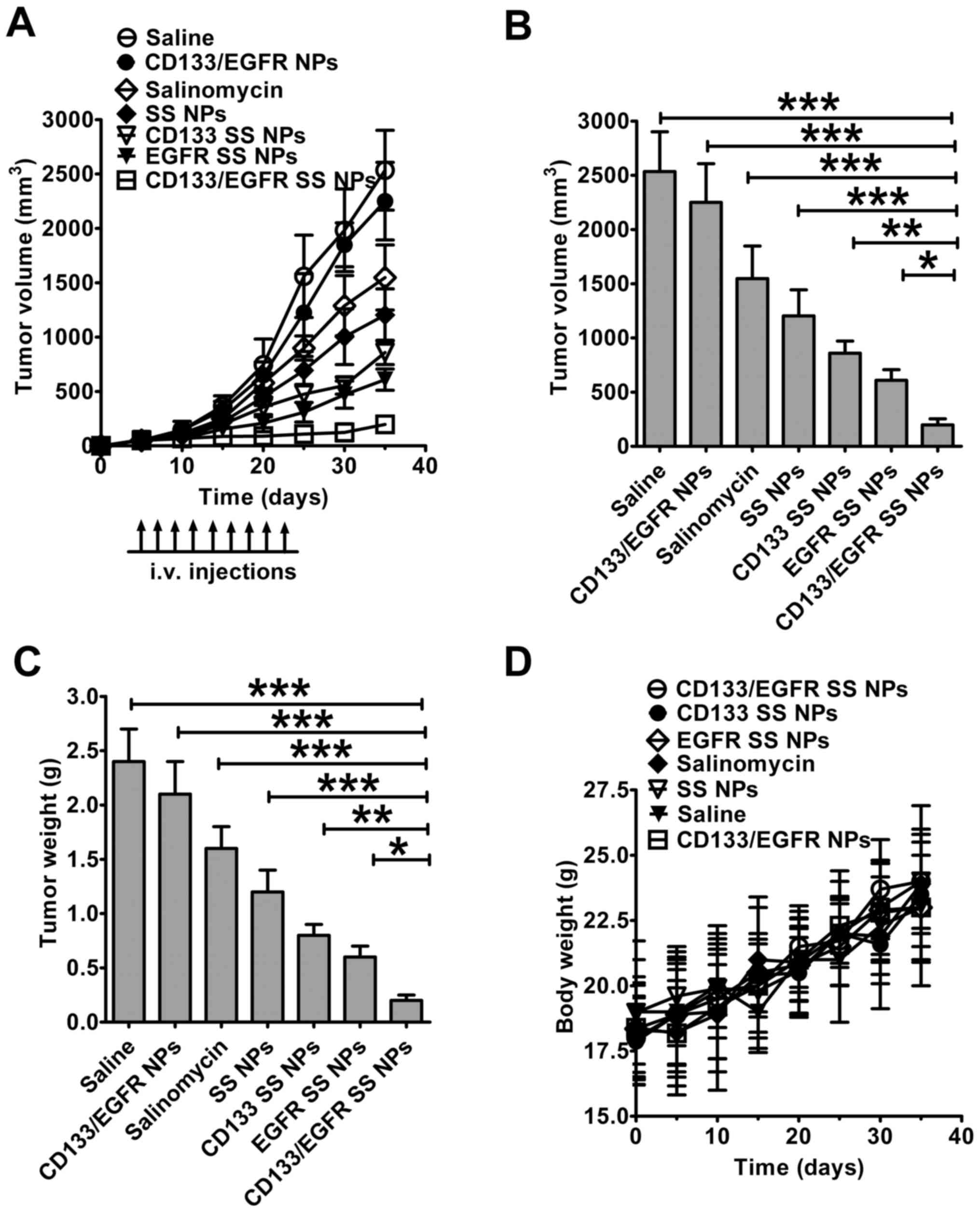
CD133/EGFR NPs progressed as rapidly as in saline-treated mice, due
to the absence of SS in CD133/EGFR NPs (Fig. 7A). SS and SS NPs achieved mild
therapeutic efficacy (39 and 52% decrease in tumor volume compared
with saline, respectively). Notably, CD133/EGFR SS NPs obtained a
92% decrease in tumor volume, whereas EGFR SS NPs and CD133 SS NPs
only obtained moderate (75 and 66% decrease in tumor volume)
therapeutic efficacy, respectively. Notably, tumor volume in the
CD133/EGFR SS NPs-treated group was the smallest among all of the
groups (CD133/EGFR SS NPs vs. SS NPs and SS, P<0.001; CD133/EGFR
SS NPs vs. CD133 SS NPs, P<0.01; CD133/EGFR SS NPs vs. EGFR SS
NPs, P<0.05; Fig. 7B). The
CD133/EGFR SS NPs-treated group exhibited the smallest tumor weight
compared with the other groups (CD133/EGFR SS NPs vs. SS NPs and
SS: P<0.001; CD133/EGFR SS NPs vs. CD133 SS NPs, P<0.01;
CD133/EGFR SS NPs vs. EGFR SS NPs, P<0.05; Fig. 7C). All of the mice showed a steady
weight increase during the treatment, and did not exhibit
significant weight loss (Fig.
7D).
Discussion
Recent evidence has indicated that CICs should be
targeted together with cancer cells to achieve a better therapeutic
effect (11,14). Since EGFR and CD133 are markers of
lung cancer cells and CICs, respectively, the present study
constructed dual-targeting lipid-PLGA hybrid NPs, named CD133/EGFR
SS NPs, to target lung CICs and cancer cells. In the present study,
CD133/EGFR SS NPs exhibited significantly improved therapeutic
effects in lung cancer compared with free SS, and non-targeted or
single-targeting NPs.
Poor safety hampers the potential use of inorganic
NPs in the clinic (25,27). Conversely, due to their superior
safety, biodegradable organic NPs are considered to be more
promising in clinical applications (25,27).
CD133/EGFR SS NPs are composed of PLGA, phosphatidylcholine and
cholesterol; all of these materials have been approved by the Food
and Drug Administration. In a pilot clinical trial containing
patients with cancer, the therapeutic effects of the polyether
antibiotic SS have been examined; the results indicated that SS may
be administered to patients with no severe side effects (13). In the present study, blank
lipid-PLGA NPs with EGFR and CD133 antibodies had good
biocompatibility as determined by the CCK-8 assay. Furthermore, no
significant weight loss was detected in mice following treatment
with the prepared SS NPs. Therefore, the NPs generated in the
present study have been demonstrated to possess good safety
profiles.
The selection of antibodies contributes
significantly to the specific targeting of developed NPs to lung
cancer cells. Since non-CICs can be transformed to CICs, it is
essential to increase the cytotoxicity of NPs towards
CD133− lung cancer cells. The present study developed
CD133/EGFR SS NPs, which are dual-targeting lipid-PLGA hybrid NPs,
to target and eliminate CD133+ and CD133−
lung cancer cells. The results demonstrated that, in
CD133+ lung cancer cells, CD133/EGFR SS NPs exhibited
significantly increased cytotoxic effects compared with CD133 SS
NPs, EGFR SS NPs and SS NPs. In CD133− lung cancer
cells, CD133/EGFR SS NPs also exhibited increased cytotoxic effects
compared with CD133 SS NPs and SS NPs. These data suggested that
CD133/EGFR SS NPs may increase targeting and therapeutic effects
towards both lung CICs and lung cancer cells.
The targeting of CD133/EGFR SS NPs to CD133, which
is a marker of stem cells, may have potential risk to normal
hematopoietic stem cells. In our further studies, the following
strategy may be adopted to reduce the potential risk to normal
hematopoietic stem cells; intratumoral administration of CD133/EGFR
SS NPs may increase the accumulation of NPs in tumors, thus
resulting in reduced distribution of NPs in bone marrow.
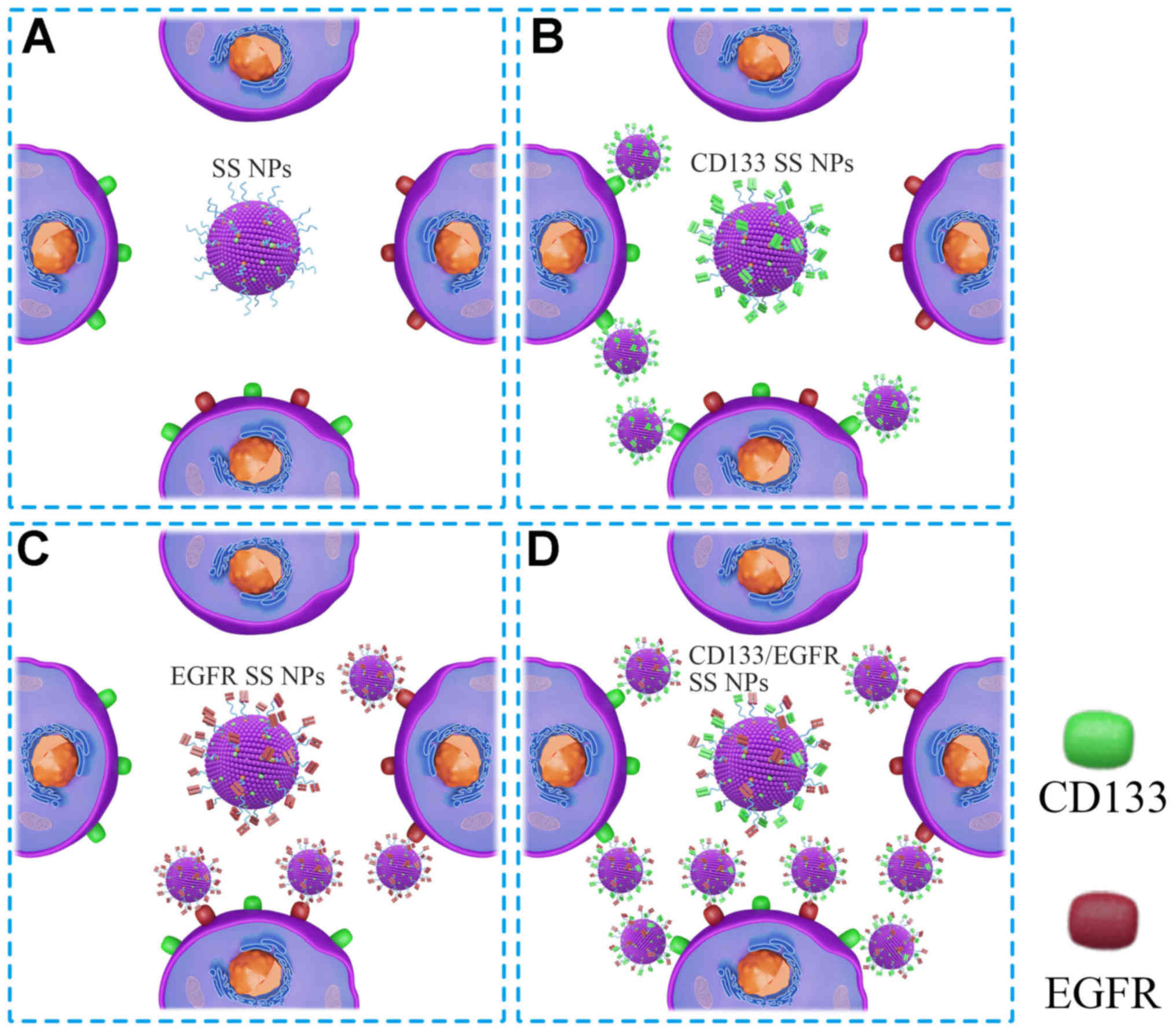
The present results helped to clarify the anticancer
mechanism underlying CD133/EGFR SS NPs (Fig. 8). As demonstrated by flow
cytometry, lung cancer cells did not exhibit homogeneous antigen
expression, and were composed of four cell populations
(EGFR+/CD133+,
EGFR−/CD133−,
EGFR+/CD133− and
EGFR−/CD133+). Due to the absence of EGFR or
CD133 antibodies, SS NPs did not target the four cell populations.
CD133 SS NPs exhibited enhanced targeting to
EGFR+/CD133+ and
EGFR−/CD133+ cells, whereas EGFR SS NPs
exhibited enhanced targeting to EGFR+/CD133+
and EGFR+/CD133− cells. Notably, the
targeting of CD133/EGFR SS NPs to
EGFR+/CD133+,
EGFR+/CD133− and
EGFR−/CD133+ cells was enhanced due to the
conjugation of double antibodies. Furthermore, CD133/EGFR SS NPs
exhibited enhanced targeting to EGFR+/CD133+
cells compared with CD133 SS NPs and EGFR SS NPs, since the antigen
density on target cells is closely associated with the targeting of
antibody-conjugated NPs, and the antigen density can be
artificially increased according to the targeting of two antigens
(EGFR and CD133) on target cells (27). Taken together, these findings may
explain why CD133/EGFR SS NPs exerted significantly increased
therapeutic efficacy towards CD133+ lung CICs compared
with CD133 SS NPs, EGFR SS NPs and SS NPs, and markedly enhanced
therapeutic efficacy to CD133− lung cancer cells
compared with CD133 SS NPs and SS NPs.
The effects of NP treatment on the immune response
against tumor growth may require evaluation, in order to prove the
efficacy of NPs. The main observations used to determine the
anticancer effects in the present study focused on tumor weight and
volume; the immune response was not analyzed in this study. The
in vivo anticancer effects of SS-loaded NPs or liposomes
have been investigated in previous studies (10,11),
whereas the effects of SS-based treatment on the immune response
against tumor growth have not been investigated. Therefore, the
present study did not test the effects of SS-based treatment on the
immune response against tumor growth. However, the importance of
testing the immune effects of this treatment have been recognized,
and will potentially be analyzed in future studies.
In conclusion, recent evidence has indicated that
CICs, together with cancer cells, should be targeted to achieve
superior therapeutic effects against cancer. The present study
developed CD133/EGFR SS NPs, which efficiently increased SS
delivery to lung CICs and lung cancer cells, and may therefore
represent an effective treatment for lung cancer.
Acknowledgments
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar
|
|
4
|
Kim JJ and Tannock IF: Repopulation of
cancer cells during therapy: An important cause of treatment
failure. Nat Rev Cancer. 5:516–525. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zakaria N, Satar NA, Abu Halim NH, Ngalim
SH, Yusoff NM, Lin J and Yahaya BH: Targeting lung cancer stem
cells: Research and clinical impacts. Front Oncol. 7:802017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bertolini G, Roz L, Perego P, Tortoreto M,
Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S,
et al: Highly tumorigenic lung cancer CD133+ cells
display stem-like features and are spared by cisplatin treatment.
Proc Natl Acad Sci USA. 106:16281–16286. 2009. View Article : Google Scholar
|
|
7
|
Chaffer CL, Brueckmann I, Scheel C,
Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y,
Polyak K, et al: Normal and neoplastic nonstem cells can
spontaneously convert to a stem-like state. Proc Natl Acad Sci USA.
108:7950–7955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gupta PB, Fillmore CM, Jiang G, Shapira
SD, Tao K, Kuperwasser C and Lander ES: Stochastic state
transitions give rise to phenotypic equilibrium in populations of
cancer cells. Cell. 146:633–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iliopoulos D, Hirsch HA, Wang G and Struhl
K: Inducible formation of breast cancer stem cells and their
dynamic equilibrium with non-stem cancer cells via IL6 secretion.
Proc Natl Acad Sci USA. 108:1397–1402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gong Z, Chen D, Xie F, Liu J, Zhang H, Zou
H, Yu Y, Chen Y, Sun Z, Wang X, et al: Codelivery of salinomycin
and doxorubicin using nanoliposomes for targeting both liver cancer
cells and cancer stem cells. Nanomedicine (Lond). 11:2565–2579.
2016. View Article : Google Scholar
|
|
11
|
Xie F, Zhang S, Liu J, Gong Z, Yang K,
Zhang H, Lu Y, Zou H, Yu Y, Chen Y, et al: Codelivery of
salinomycin and chloroquine by liposomes enables synergistic
antitumor activity in vitro. Nanomedicine (Lond). 11:1831–1846.
2016. View Article : Google Scholar
|
|
12
|
Wang Y: Effects of salinomycin on cancer
stem cell in human lung adenocarcinoma A549 cells. Med Chem.
7:106–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naujokat C and Steinhart R: Salinomycin as
a drug for targeting human cancer initiating cells. J Biomed
Biotechnol. 2012:9506582012. View Article : Google Scholar
|
|
14
|
Zhang Y, Zhang Q, Sun J, Liu H and Li Q:
The combination therapy of salinomycin and gefitinib using
poly(d,l-lactic-co-glycolic acid)-poly(ethylene glycol)
nanoparticles for targeting both lung cancer stem cells and cancer
cells. OncoTargets Ther. 10:5653–5666. 2017. View Article : Google Scholar
|
|
15
|
Ketola K, Hilvo M, Hyötyläinen T, Vuoristo
A, Ruskeepää AL, Orešič M, Kallioniemi O and Iljin K: Salinomycin
inhibits prostate cancer growth and migration via induction of
oxidative stress. Br J Cancer. 106:99–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao J, Xia Y, Chen H, Yu Y, Song J, Li W,
Qian W, Wang H, Dai J and Guo Y: Polymer-lipid hybrid nanoparticles
conjugated with anti-EGF receptor antibody for targeted drug
delivery to hepatocellular carcinoma. Nanomedicine (Lond).
9:279–293. 2014. View Article : Google Scholar
|
|
17
|
Kapoor DN, Bhatia A, Kaur R, Sharma R,
Kaur G and Dhawan S: PLGA: A unique polymer for drug delivery. Ther
Deliv. 6:41–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao J, Chen H, Song H, Su X, Niu F, Li W,
Li B, Dai J, Wang H and Guo Y: Antibody-targeted immunoliposomes
for cancer treatment. Mini Rev Med Chem. 13:2026–2035. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao J, Feng SS and Guo Y: Antibody
engineering promotes nanomedicine for cancer treatment.
Nanomedicine (Lond). 5:1141–1145. 2010. View Article : Google Scholar
|
|
20
|
Wang J, Wu Z, Pan G, Ni J, Xie F, Jiang B,
Wei L, Gao J and Zhou W: Enhanced doxorubicin delivery to
hepatocellular carcinoma cells via CD147 antibody-conjugated
immunoliposomes. Nanomedicine. Oct 16–2017.(Epub ahead of print).
pii: S1549-9634(17)30179-X.
|
|
21
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sos ML, Koker M, Weir BA, Heynck S,
Rabinovsky R, Zander T, Seeger JM, Weiss J, Fischer F, Frommolt P,
et al: PTEN loss contributes to erlotinib resistance in EGFR-mutant
lung cancer by activation of Akt and EGFR. Cancer Res.
69:3256–3261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
El-Sayed IH, Huang X and El-Sayed MA:
Surface plasmon resonance scattering and absorption of anti-EGFR
antibody conjugated gold nanoparticles in cancer diagnostics:
Applications in oral cancer. Nano Lett. 5:829–834. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang L, Mao H, Wang YA, Cao Z, Peng X,
Wang X, Duan H, Ni C, Yuan Q, Adams G, et al: Single chain
epidermal growth factor receptor antibody conjugated nanoparticles
for in vivo tumor targeting and imaging. Small. 5:235–243. 2009.
View Article : Google Scholar
|
|
25
|
Cushing BL, Kolesnichenko VL and O'Connor
CJ: Recent advances in the liquid-phase syntheses of inorganic
nanoparticles. Chem Rev. 104:3893–3946. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Allen TM: Ligand-targeted therapeutics in
anticancer therapy. Nat Rev Cancer. 2:750–763. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Auffan M, Rose J, Bottero JY, Lowry GV,
Jolivet JP and Wiesner MR: Towards a definition of inorganic
nanoparticles from an environmental, health and safety perspective.
Nat Nanotechnol. 4:634–641. 2009. View Article : Google Scholar : PubMed/NCBI
|