Introduction
Oral squamous cell carcinoma (OSCC) is the sixth
most common type of cancer worldwide (1). In the United States alone, >50,000
new cases are diagnosed annually, resulting in ~10,000 cases of
mortality (2,3). Surgery remains the main treatment
option for patients with OSCC, since the currently available
chemotherapeutic agents have proved to be of limited success.
Therefore, the 5-year survival rate for patients with OSCC over the
past four decades remains at 50-55%, despite novel treatment
modalities (4,5). A better understanding of the
biological nature of OSCC is required, in order to improve the
effectiveness of chemotherapeutic intervention and, consequently,
the survival of patients with OSCC.
IFNγ is an immune response-stimulating cytokine,
which orchestrates several distinct cellular functions. Its role
focuses on enhanced immune cell surveillance (6,7) and
induction of the major histocompatibility complex response in
numerous types of normal and neoplastic cells (8,9).
IFNγ, which is secreted by activated T cells and natural killer
cells, enhances macrophage activation, T helper cell (Th)1/Th2
balance, and regulates cellular proliferation and apoptosis
(10). Furthermore, IFNγ has been
reported to directly attack tumor cells by initiating a cascade of
signaling mechanisms that modulate cell viability (11,12).
Although the molecular basis of this action remains unclear,
previous studies have proposed potential effects of IFNγ on
endoplasmic reticulum (ER) stress proteins (13-15)
and ER calcium (Ca) homeostasis channel pumps (16).
The ER is responsible for protein folding and
targeting during protein synthesis, and represents the principal
intracellular Ca storage, which is essential for Ca signaling
pathways and the regulation of cellular Ca homeostasis (17,18).
Sarco/endoplasmic reticulum Ca-ATPase (SERCA)-type pumps are the
major carriers of Ca into the ER lumen, whereas inositol
1,4,5-trisphosphate receptors (IP3r) are family proteins that drive
Ca release channels (19). These
Ca influx or efflux channels are regulated by Ca-dependent
chaperones, including 78-kDa glucose-regulated protein (GRP78),
which bind or buffer intraluminal Ca (17,20).
ER homeostasis is a multifactorial process that is
affected by numerous environmental factors, including the redox
state, ischemia, nutrient and Ca level alterations, high protein
synthesis rate, and inflammation (21). Persistent stimuli may disrupt
proper ER function, thus leading to ER stress. This, in turn, may
result in activation of a cascade of signaling molecules and
pathways that constitute the unfolded protein response (UPR)
(21,22). The main aim of the UPR is to arrest
intraluminal accumulation and/or secretion of unfolded proteins and
enhance degradation of misfolded proteins (23,24).
Protein kinase R-like ER kinase (PERK), activating transcription
factor (ATF)6 (α and β), and the kinase endoribonuclease
inositol-requiring enzyme 1 (IRE1), constitute three ER
transmembrane protein-sensors that detect alterations in ER
homeostasis. Conversely, GRP78 is considered to be the master UPR
chaperone that negatively regulates PERK, ATF6 and IRE1 functions
(17,25). A nexus between ER stress and cancer
has previously been highlighted; UPR alterations may prevent ER
stress-induced apoptosis and help cancer cells survive in a
otherwise demanding microenvironment (23). Other studies have also revealed
that GRP78 cysteine oxidation favors cancer cell survival during
stress (22), and SERCA activity
has been reported as a potential target for cancer treatment
(17).
Dentin sialophosphoprotein (DSPP) is a member of the
small integrin-binding ligand N-linked glycoproteins (SIBLINGs)
family (26). Within the past
decade, the expression of some members of the SIBLINGs family,
along with their cognate matrix metalloproteinases (MMPs), have
been detected in various types of cancer, including OSCC (27-31).
Specifically, our previous studies have indicated that DSPP
expression is correlated with the transition of dysplastic oral
premalignant lesions to OSCC, with tumor aggressiveness, and with
the recurrence of OSCC at histologically negative ('tumor-free')
surgical margins of primary OSCC (28-31).
DSPP is expressed in the cytoplasm and perinuclear perimeter of
OSCC cells, with significantly elevated immunoreactivity in the
cytoplasm of poorly differentiated OSCC cells (28).
Our recent study reported a novel finding, that
matrix MMP20 is expressed and directly interacts with DSPP in human
OSCC tissues and cell lines (32).
This finding established MMP20 as the cognate MMP partner of DSPP
(32). Furthermore, dentin
sialoprotein (DSP), which is the cleaved N-terminal product of
DSPP, interacts with MMP20 promoter proximal elements (32). An earlier report by Joshi et
al demonstrated that DSPP silencing in OSCC cells results in
MMP2, MMP3, MMP9, vascular endothelial growth factor, p53, Ki-67
and epidermal growth factor receptor downregulation, as well as
altered cell morphology, cell proliferation, colony-formation and
invasion of OSCC cells (33). In
addition, DSPP silencing increases cisplatin sensitivity and
enhances apoptosis of OSCC cells, whereas subcutaneous injection of
OSCC xenografted Balb/c nude mice with DSPP-silenced OSCC cells
results in attenuated tumor growth (33). Our recent report proposed a
tumorigenic role for DSPP in OSCC cells, and presented a
relationship between DSPP and the ER chaperone GRP78 (34). Furthermore, our report suggested a
DSPP-associated modulatory effect on ER stress, Ca homeostasis and
UPR proteins, including sarco/endoplasmic reticulum
Ca2+-ATPase (SERCA2b), IRE1, PERK and ATF6 (34).
The present study aimed to investigate the role of
IFNγ signaling in DSPP expression. The study aimed to elucidate a
potential connection between this interaction and ER homeostasis,
and suggested an alternative mechanism responsible for IFNγ-induced
effects on OSCC cells. Therefore, the effects of IFNγ treatment on
specific ER stress-associated proteins, including SERCA2b, IP3r,
GRP78, IRE1 and PERK, were investigated in the OSC2 OSCC cell line,
and its effects on tumor cell proliferation, migration and
apoptosis were analyzed.
Materials and methods
Human cell lines and culture
conditions
The previously characterized human OSCC cell line,
OSC2, which was originally obtained from the American Type Culture
Collection (Manassas, VA, USA) and routinely authenticated in our
laboratory, was used for this study. Cells were cultured as a
monolayer in Dulbecco's modified Eagle's medium (DMEM)/F12
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 1% penicillin/streptomycin and
500 ng/ml hydrocortisone (Sigma Aldrich; Merck KGaA, Darmstadt,
Germany), and were maintained at 37°C in a humidified atmosphere
containing 5% CO2. Recombinant human IFNγ was purchased
from Abcam (Cambridge, MA, USA). For all experiments, OSC2 cells
were plated and cultured for 48 h prior to the addition of IFNγ at
a concentration of 500 U/ml for 24 or 48 h at 37°C. Time-points
were chosen with regards to time-response experiments on
interferon-regulated factor 1 (IRF1) mRNA expression following
treatment with 500 U/ml IFNγ for 6, 12, 24 or 48 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from cells using TRIzol®
reagent (cat. no. 15596-026; Invitrogen; Thermo Fisher Scientific,
Inc.), according to a standardized protocol, and the concentration
of each sample was determined. The qSTAR qPCR primer pairs against
human genes had the following sequences (5′-3′): IRF1, forward
CGAATCGCTCCTGCAGCAGA, reverse GCCCAGCTCCGGAACAAACA; DSPP, forward
CAACCATAGAGAAAGCAAACGCG, reverse TTTCTGTTGCCACTGCTGGGAC; MMP20,
forward GACCAGACCACAATGAACGT, reverse GTCCACTTCTCAGG ATTGTC; PERK,
forward ATCCCCCAT GGAACGACCTG, reverse ACCCGCCAGGGACAAAAATG;
SERCA2b, forward TCATCTTCCAGATCACACCGC, reverse
GTCAAGACCAGAACATATC; IP3r, forward GGTTTCATTTGCAAGTTAATAAAG,
reverse AATGCTTTCATGGAACACTCGGTC; IRE1, forward
CGGGAATTCGGCCGAGTCCTCGCCATG, reverse
CAAGCGGCCGCCTTTCCCAACTATCACCACGCT; GRP78, forward
TGTTCAACCAATTATCAGCAAACTC, reverse TTCTGCTGTATCCTCTTCACCAGT; and
β-actin, forward GTCTCCTCTGACTTCAACAGCG and reverse
ACCACCCTGTTGCTGTAGCCAA.
Total RNA (1 μg) was reverse transcribed
using iScript RT Supermix (cat. no. 1708841; Bio-Rad Laboratories,
Inc., Hercules, CA, USA), according to the manufacturer's protocol.
qPCR was performed using synthesized cDNA on a qPCR machine using
iTaq™ UniverSYBR® Green PCR Master Mix (cat. no. 1725124; Bio-Rad
Laboratories, Inc.). PCR thermocycling was conducted as follows:
94°C for 5 min, followed by 40 cycles at 94°C for 30 sec, 60°C for
20 sec and 72°C for 40 sec, and a final extension step at 72°C for
5 min. A standard curve was generated from three serial dilutions
of cDNA. Samples, including negative controls, were analyzed in
triplicate, and PCR products were verified using dissociation curve
analysis. mRNA expression levels were normalized to actin and were
analyzed using Bio-Rad CFX manager software (Version 3.0; Bio-Rad
Laboratories, Inc.).
Western blot analysis
Western blot analysis was performed as previously
described (34). Briefly, cells
were lysed and sonicated (20 kHz, 2×10 sec) in
radioimmunoprecipitation assay lysis buffer (Thermo Fisher
Scientific, Inc.). Subsequently, equal amounts of protein (30-50
μg, depending on the particular protein), as determined by
the Bradford protein assay method, were separated by 10% SDS-PAGE,
and electrophoretically separated proteins were transferred to
polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.).
Blotted membranes were then placed in blocking solution (PBS-0.5%
Tween-20, Sigma-Aldrich; Merck KGaA) for 1 h at room temperature
prior to probing with the following primary antibodies, which were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA):
Mouse monoclonal B-cell lymphoma 2 (Bcl-2; cat. no. sc-7382,
1:250); mouse monoclonal Bcl-2-associated X protein (Bax; cat. no.
sc-7480, 1:200); rabbit polyclonal cytochrome c (cat. no.
sc-7159, 1:200) and rabbit polyclonal proliferating cell nuclear
antigen (PCNA; cat. no. sc-7907, 1:200) overnight at 4°C. The
membranes were washed thoroughly with PBS (Sigma Aldrich; Merck
KGaA), and then incubated with goat polyclonal anti-rabbit
immunoglobulin G (IgG) horseradish peroxidase-conjugated secondary
antibody (cat. no. sc-2301, 1:3,000; Santa Cruz Biotechnology,
Inc.) or anti-mouse IgG horseradish peroxidase-conjugated secondary
antibody (cat. no. sc-2031, 1:3,000; Santa Cruz Biotechnology,
Inc.) with agitation at room temperature for 1 h. β-actin (1:2,000)
was used as a loading control (cat. no. sc-47778; Santa Cruz
Biotechnology, Inc.). Proteins were visualized using an enhanced
chemiluminescence (ECL) system (Pierce™ ECL; Thermo Fisher
Scientific, Inc.) and band intensity was semi-quantified using
ImageJ software 1.48 (National Institutes of Health, Bethesda, MD,
USA).
MTT assay
Cell viability was assessed by detecting the
conversion of MTT to formazan via mitochondrial oxidation.
IFNγ-treated and untreated OSC2 cells, at a density of
5×103 cells/well, were incubated with 0.5 mg/ml MTT for
3 h at room temperature in 96-well plates after 24 and 48 h in
culture. The formation of insoluble formazan purple crystals
indicated the presence of viable cells. Crystals were dissolved in
dimethyl sulfoxide and the optical density (OD) of the solutions
was measured using a spectrophotometer at a wavelength of 570 nm.
Assays were performed in triplicate and data are expressed as the
means of OD values ± standard deviation.
Apoptosis analysis by flow cytometry
For apoptosis analyses, Annexin V/propidium iodide
(PI; Sigma Aldrich; Merck KGaA) staining of IFNγ-treated and
untreated OSC2 cells was conducted after 24 and 48 h. Briefly,
cells were washed with 1X PBS and resuspended at 106
cells/ml in Annexin V-binding buffer, before aliquoting the
suspension into 100 μl/tube fractions. Subsequently, 5
μl Annexin V-fluorescein isothiocyanate (FITC) and 10
μl PI buffer were added to each tube and cells were
incubated in the dark for 15 min at room temperature. Finally, 400
μl 1X Annexin V-binding buffer was added to each tube and
flow cytometric analysis was conducted within 1 h. Samples were
analyzed on a FACSCalibur flow cytometer (BD Biosciences, San Jose,
CA, USA). Gates in the right angle scatter versus forward scatter
diagrams were used to exclude debris. At least 100,000 events were
collected prior to analysis. All flow cytometric data were analyzed
using BD CellQuest Pro software (Version 5.0; BD Biosciences).
Scratch wound-healing assay
OSC2 cells were cultured until they reached 90%
confluence in 35-mm dishes. Subsequently, scratches were generated
using a sterile 200-μl pipette tip prior to cells being
treated with IFNγ for 24 or 48 h. The border of the denuded area
was immediately marked with a fine line, and cells were incubated
in DMEM/F12 supplemented with 10% FBS. Images of the cell cultures
were captured at 24 and 48 h using an inverted phase contrast
microscope (Olympus Corporation, Tokyo, Japan). Assays were
performed in duplicate.
Statistical analysis
Results from IFNγ-treated cells were compared with
results from untreated (control) cells. Statistical analyses were
performed using SPSS version 21 (IBM Corp., Armonk, NY, USA).
Paired groups were compared using Student's t-test, whereas one-way
analysis of variance was applied for the comparison of multiple
groups, followed by post hoc pairwise comparisons with the
application of Dunn's test. All experiments were performed in
triplicate. P<0.05 was considered to indicate a statistically
significant difference.
Results
IFNγ (500 U/ml) activates intracellular
molecular signaling networks at specific time-points
The synthesis of IRF1 is induced by IFNγ (35). Therefore, to determine whether the
selected dose of 500 U/ml IFNγ effectively activated intracellular
molecular signaling pathways, alterations in the mRNA expression
levels of IRF1 were monitored by RT-qPCR analyses in a
time-dependent assay. As shown in Fig.
1, the mRNA expression levels of IRF1 exhibited a statistically
significant time-dependent increase in cells treated for 24 and 48
h compared with the control cells (0 h). Since 24 and 48 h IFNγ
treatment resulted in an increased IFNγ response, these time-points
(24 and 48 h) and dose (500 U/ml) were selected for subsequent
experiments.
IFNγ treatment downregulates DSPP, MMP20,
GRP78, SERCA2b and IRE1, but upregulates IP3r and PERK in OSC2
cells
To evaluate the effects of IFNγ treatment on DSPP
and MMP20, as well as on the ER stress response, UPR and Ca
homeostasis, alterations in the mRNA expression levels of DSPP,
MMP20, GRP78, SERCA2b, IP3r, PERK and IRE1 were determined by
RT-qPCR analysis at 24 and 48 h intervals. Table I summarizes the
upregulated/downregulated genes detected following treatment of
OSC2 cells with IFNγ. As shown in Fig.
2, DSPP, MMP20, GRP78, SERCA2b and, to a lesser extent, IRE1,
exhibited statistically significant differences (P<0.05). These
results suggested that IFNγ treatment may affect ER Ca homeostasis
by suppressing SERCA2b and inducing IP3r. Furthermore, the results
suggested that the IFNγ-induced downregulation of GRP78, and the
modest effects on UPR-associated proteins IRE1 and PERK, may induce
ER stress.
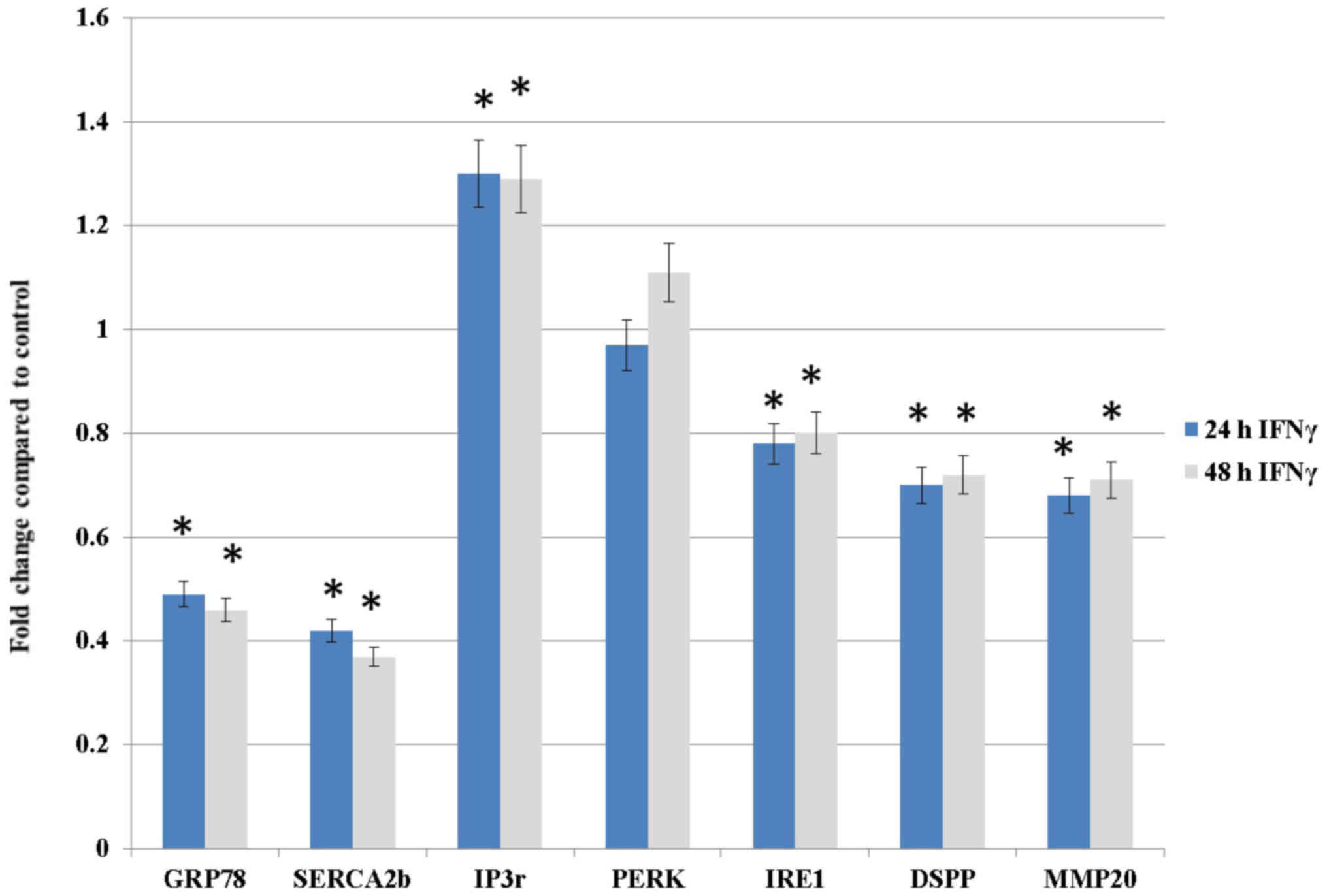 | Figure 2Effects of IFNγ treatment (500 U/ml
for 24 or 48 h) on the mRNA expression levels of proteins
associated with ER stress, the unfolded protein response and Ca
homeostasis, as well as DSPP and MMP20, as assessed by reverse
transcription-quantitative-polymerase chain reaction analysis.
DSPP, MMP20, GRP78, SERCA2b and, to a lesser extent, IRE1 exhibited
statistically significant decreases at both time-points compared
with the control group. Conversely, IP3r mRNA expression was
significantly increased at both time-points compared with the
control group, whereas PERK mRNA expression was not significantly
increased in response to IFNγ. Results are expressed as fold
changes relative to the expression levels in the control
(untreated) group. Data are presented as the means ± standard error
of the mean, and each experiment was performed in triplicate.
*P<0.05 vs. the control group. DSPP, dentin
sialophosphoprotein; ER, endoplasmic reticulum; GRP78, 78-kDa
glucose-regulated protein; IFNγ, interferon γ; IP3r, inositol
1,4,5-trisphosphate receptor; IRE1, inositol-requiring enzyme 1;
MMP20, matrix metalloproteinase 20; PERK, protein kinase R-like ER
kinase; SERCA2b, sarco/endoplasmic reticulum
Ca2+-ATPase. |
 | Table ISummary of upregulated/downregulated
mRNAs following treatment of OSC2 cells with interferon γ. |
Table I
Summary of upregulated/downregulated
mRNAs following treatment of OSC2 cells with interferon γ.
| Gene type | Upregulated | Downregulated |
|---|
| SIBLING | - | DSPP |
| DSPP cognate
partner | - | MMP20 |
| Ca homeostasis | IP3r | SERCA2b |
| ER chaperone | - | GRP78 |
| UPR-associated
proteins | - | IRE1 |
|
Apoptosis-associated proteins | Bax; cytochrome
c | Bcl-2 |
|
Proliferation-associated | - | PCNA |
| proteins | | |
IFNγ treatment decreases OSC2 cell
migration
To assess the effects of IFNγ treatment on the
migratory capacity of OSC2 cells, the rate of scratch wound closure
on cell culture plates was determined. As shown in Fig. 3, IFNγ treatment of cells
significantly delayed wound closure compared with the control cells
at 24 and 48 h (P<0.05). This finding may be associated with the
significantly reduced MMP20 mRNA expression detected in
IFNγ-treated cells compared with in the control cells (P<0.05;
Fig. 2), and suggested that IFNγ
may regulate the migratory capacity of OSC2 cells, possibly by
suppressing MMP20 along with its cognate partner DSPP.
IFNγ treatment inhibits proliferation and
increases apoptosis of OSC2 cells
To analyze mitochondrial activity following
treatment of OSC2 cells with IFNγ for various time-points, the MTT
colorimetric assay was conducted. As shown in Fig. 4, IFNγ-treated cells exhibited
significantly lower OD values at 24 and 48 h compared with the
control cells (P<0.05), thus indicating that OSC2 cell
proliferation was reduced following IFNγ treatment. This
observation is consistent with the results of western blotting;
IFNγ treatment induced a reduction in the expression levels of the
cell proliferation-associated marker PCNA (Fig. 5). In order to assess the rate of
apoptosis, IFNγ-treated OSC2 cells were analyzed by Annexin V-FITC
flow cytometry and apoptotic rates were compared with the control
(untreated) group. Cell sorting indicated that the apoptotic cell
fraction was significantly increased from 3.51% in the untreated
control group to 16.8 and 27.6% in IFNγ-treated cells at 24 and 48
h, respectively (Fig. 6). This
finding is consistent with the upregulation in the protein
expression levels of pro-apoptotic molecules, Bax and cytochrome
c, and the downregulation of the anti-apoptotic molecule,
Bcl-2, as shown in Fig. 5. Taken
together, these results suggested that IFNγ may exert an antitumor
effect on OSC2 cells by reducing cell proliferation and enhancing
apoptosis.
Discussion
To the best of our knowledge, the present study is
the first to determine the various effects of IFNγ treatment on the
mRNA expression levels of DSPP and MMP20, ER Ca homeostasis, ER
stress- and UPR-associated proteins, and on notable hallmarks of
oral carcinogenesis in OSCC cells. The downregulation of DSPP and
MMP20 mRNA expression following IFNγ treatment may account for the
reduced migratory potential of OSC2 cells. Our previous report
revealed that the strong binding of DSP to MMP20, and its
interaction with the promoter proximal element of MMP20, may
account for increased migration, invasion and metastasis in OSCC
(32). Recently, we reported that
DSPP silencing results in significantly reduced MMP20 mRNA
expression and in reduced migration of OSCC cells (34). Other investigators have reported
that IFNγ and IFNβ suppress MMP9 expression through a signal
transducer and activator of transcription (STAT)1α pathway in
primary astrocytes and human fibrosarcoma cells (36), and that IFNγ treatment reduces
migration of A172 human glioblastoma cells (37). IFNγ also inhibits MMP3-induced
invasiveness of T98G glioma cells (38). Therefore, the effects of DSPP and
MMP20 downregulation on the notable hallmarks of oral
carcinogenesis, including decreased cell viability and migration,
and increased apoptosis, noted in the present study following
treatment of OSC2 cells with IFNγ are consistent with our previous
findings (33,34).
It has previously been suggested that IFNs enhance
apoptosis in acute promyelocytic leukemia, chronic myelog-enous
leukemia, multiple myeloma, melanoma and ovarian cancer (39). Specifically, several reports have
highlighted the effects of IFNγ on head and neck squamous cell
carcinoma (HNSCC) cells. For example, a recent report revealed that
IFNγ induces apoptosis in two HNSCC cell lines, and leads to
overexpression and activation of indoleamine 2,3 protein (13). Furthermore, IFNγ activates Janus
kinase/STAT1, apoptosis signal-regulating kinase 1, p38,
c-jun-N-terminal kinase, the nuclear factor-κB pathway and IRF1
(13). IFNγ treatment of OSCC
cells has also been revealed to result in down-regulation of heat
shock protein 27, which is a proposed anti-apoptotic molecule, and
enhancement of cell death (40).
Gadkaree et al reported that the antitumor effects of
synthetic cyclic dinucleotides are associated with the upregulation
of IFN-γ+cluster of differentiation 8+
infiltrating T cells and programmed death-L1 protein in a HNSCC
xenograft-mouse model (41). Xu
et al revealed that IFNγ sensitizes HNSCC cells to
chemotherapy-induced apoptosis and necroptosis by upregulating
early growth response protein 1 (42). Conversely, administration of IFNα
or tumor necrosis factor α does not induce considerable alterations
in OSCC apoptosis (40).
The present results suggested that IFNγ treatment of
OSC2 cells suppressed PCNA and Bcl-2 expression, and upregulated
Bax and cytochrome c expression; these findings are
consistent with previous report of similar effects in human breast,
prostate and lung cancer cells. Ning et al reported that
IFNγ, but not IFNα or IFNβ, enhances IRF1 expression in
anti-estrogen-resistant human breast cancer cells, and IRF1
induction downregulates the expression of pro-survival proteins,
Bcl-2 and Bcl-2-like protein 2, and enhances pro-apoptotic Bcl-2
antagonist/killer (Bak) and Bax activity (43). Furthermore, IFNγ enhances the
apoptotic effects of polyinosinic:polycytidylic acid in human
prostate cancer cells by enhancing Bak expression (44). With regards to human lung cancer
cells, IFNγ induces phosphorylated-STAT1 activity in cells
expressing STAT1-CC, which are hyper-responsive to IFN, thus
resulting in downregulation of PCNA and c-fos (45). In OSCC cells of the tongue, Liu
et al reported that overexpression of interleukin-18
activates caspase-3, -7 and -9 pathways, and enhances IFNγ and
cytochrome c mRNA expression (46).
With regards to ER Ca homeostasis-, ER stress- and
UPR-associated proteins, the present data revealed that IFNγ
treatment decreased GRP78 and SERCA2b mRNA expression, and induced
IP3r expression. These findings supported the hypothesis that a
combination of increased ER Ca leakage (through IP3 channels) and a
blockage in Ca influx (via suppression of SERCA activity) may
perturb ER Ca homeostasis and enhance ER stress. Furthermore, the
high apoptotic rates observed in response to IFNγ treatment of OSC2
cells may be associated with GRP78 downregulation. This speculation
is based on the results of previous studies, which provide evidence
indicating that GRP78 promotes tumor progression (47,48),
and increased GRP78 expression is correlated with shorter
recurrence time and poor survival of patients with prostate and
breast cancers (49-51). Notably, in response to GRP78
inhibition, the response of patients with prostate cancer to
photodynamic therapy is improved (52). In in vitro and animal
experiments, GRP78 silencing suppresses tumor cell invasion, cell
growth and metastasis in xenograft models of gastric cancer
(53).
The present data also indicated that IFNγ treatment
resulted in moderate downregulation of IRE1 mRNA expression, and a
very modest induction of PERK activity after 48 h. These findings
are consistent with earlier reports indicating that treatment of
pancreatic rat cells with IFNγ decreases the basal levels of
spliced X-binding protein 1 (XBP1) mRNA; XBP1 is downstream of IRE1
(14). Similarly, Son et al
reported that IRE1 silencing results in accelerated Ca efflux
through IP3r and increased apoptotic rates in human neuroblastoma
cells (54), whereas Kanekura
et al indicated that IRE1 inhibition enhances ER stress and
apoptosis through oligomerization of Bax and Bak proteins (55). With regards to HNSCC cells, an
in vitro study by El Jamal et al suggested that IFNγ
treatment of HNSCC cells triggers ER stress and induces apoptosis
by upregulating PERK and IRE1 pathways (13). Furthermore, Fribley et al
demonstrated that treatment with Celastrol (a triterpenoid compound
isolated from the Celastraceae plant family) mediates the
pro-apoptotic UPR effects and apoptosis in OSCC cells via
PERK-eukaryotic translation initiation factor 2 (eIF2)-ATF4-C/EBP
homologous protein signaling (56). Similarly, Afatinib, which is an
inhibitor that targets ErbB family members, triggers the
PERK-eIF2α-ATF4 pathway and suppresses protein kinase B-mammalian
target of rapamycin activity leading to apoptosis of HNSCC cells
(57).
Notably, some studies appear to conflict with the
aforementioned findings, suggesting a tumorigenic role for PERK in
modulation of ER stress. For example, Fujimoto et al
reported that PERK inhibition induces apoptosis of cancer stem
cells (58), whereas Koumenis
reported that the expression of PERK, and its target molecule eIF2,
are correlated with increased tumor growth and survival under
hypoxic conditions (59).
Furthermore, it has been reported that PERK inhibition decreases
tumor growth in vitro and in vivo (60), and hampers metastasis in breast
cancer mice xenografts (61).
Our previous study suggested the effects of DSPP on
ER stress, the UPR and Ca homeostasis (34), and the present data indicated that
treatment of OSC2 cells with IFNγ resulted in downregulation of
DSPP and MMP20; therefore, it may be hypothesized that DSPP serves
an oncogenic role during the ER stress adaptive response in the
OSCC microenvironment. Therefore, IFNγ treatment-induced DSPP
downregulation may be directly associated with the observed
alterations in ER homeostasis, at least partially by mediating
alterations in major ER stress-associated proteins, including
GRP78, SERCA2b and UPR sensor proteins, thus contributing to UPR
collapse. Although the mechanisms by which IFNγ interacts with DSPP
are yet to be fully understood, these mechanisms may include
pathways that are yet to be characterized. Overall, the finding
that IFNγ modified OSCC properties through UPR modifications
warrants further investigation.
It has been suggested that the UPR serves a dual
role in cancer biology: Firstly, to ameliorate ER stress-associated
damage; and secondly, to activate apoptotic pathways in severe
conditions (22). For this reason,
it is often difficult to predict whether, or for how long, UPR
proteins inhibit tumor growth, or protect cancer cells within the
tumor microenvironment (62). It
would appear that the duration and severity of ER stress determines
the survival or apoptotic death of cancer cells (62). Conflicting reports regarding the
effects of UPR protein expression on cancer cell fate may reflect
the fact that individual UPR protein modifications result in
opposing signals between induction and attenuation under ER stress
(62,63).
In conclusion, the present data strongly supported
an anti-tumor role for IFNγ in OSCC cells through mechanisms that
downregulate DSPP and MMP20, leading to disturbances in ER
homeostasis, and alterations in proteins associated with ER stress
and Ca regulation. Notably, treatment of OSCC cells with IFNγ also
decreased cell viability and migration, and increased apoptosis.
Therefore, it may be speculated that either IFNγ interacts with
DSPP, which, in turn, at least partially mediates the observed
effects on ER stress molecules, or, alternatively, DSPP
modifications follow IFNγ treatment-induced alterations in ER
homeostasis. A recognized limitation of the present study is the
focus on the alterations in the mRNA expression levels of genes
encoding ER stress- or UPR-associated proteins. Nevertheless, the
data obtained presents background information for the design of
additional studies investigating alterations in protein expression
and related functional mechanisms. Therefore, further studies
beyond the scope of the present report may help to elucidate the
sequential mechanisms underlying IFNγ-DSPP interaction in OSCC, and
the consequential effects on ER stress response. These studies aim
to provide insight into potential targeted therapeutic methods and
interventional strategies for the treatment of patients with
OSCC.
Acknowledgments
Not applicable.
Funding
This study was supported by faculty startup research
funding (to KUEO) from the University of Texas Health Science
Center at Houston (Houston, TX, USA)
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
NGN and KUEO made substantial contributions to the
conception and design of the study, reviewed data, reviewed/edited
draft manuscripts, and reviewed/edited the final draft of the
manuscript. IG and JA carried out experiments related to the study,
acquired, analyzed and interptreted data, and provided the initial
draft of the manuscript. All authors gave their approval of the
final draft of the manuscript, and agree to be accountable for all
aspects of the study related to accuracy or integrity of all parts
of the study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Irani S: Distant metastasis from oral
cancer: A review and molecular biologic aspects. J Int Soc Prev
Community Dent. 6:265–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simard EP, Torre LA and Jemal A:
International trends in head and neck cancer incidence rates:
Differences by country, sex and anatomic site. Oral Oncol.
50:387–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Russo D, Merolla F, Mascolo M, Ilardi G,
Romano S, Varricchio S, Napolitano V, Celetti A, Postiglione L, Di
Lorenzo PP, et al: FKBP51 immunohistochemical expression: A new
prognostic biomarker for OSCC? Int J Mol Sci. 18:4432017.
View Article : Google Scholar :
|
|
4
|
Silverman S Jr: Demographics and
occurrence of oral and pharyngeal cancers. The outcomes, the
trends, the challenge. J Am Dent Assoc. 132(Suppl): S7–S11. 2001.
View Article : Google Scholar
|
|
5
|
Kujan O, Glenny AM, Duxbury J, Thakker N
and Sloan P: Evaluation of screening strategies for improving oral
cancer mortality: A Cochrane systematic review. J Dent Educ.
69:255–265. 2005.PubMed/NCBI
|
|
6
|
Wakita D, Chamoto K, Ohkuri T, Narita Y,
Ashino S, Sumida K, Nishikawa H, Shiku H, Togashi Y, Kitamura H, et
al: IFN-gamma-dependent type 1 immunity is crucial for
immunosurveillance against squamous cell carcinoma in a novel mouse
carcinogenesis model. Carcinogenesis. 30:1408–1415. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hayakawa Y, Sato-Matsushita M, Takeda K,
Iwakura Y, Tahara H and Irimura T: Early activation and
interferon-γ production of tumor-infiltrating mature CD27 high
natural killer cells. Cancer Sci. 102:1967–1971. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong M, Jiang Z and Zhou YF: Effects of
thermotherapy on Th1/Th2 cells in esophageal cancer patients
treated with radiotherapy. Asian Pac J Cancer Prev. 15:2359–2362.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Hou J, Zhang J, An Y, Zhang X, Yue
L, Liu J and Li X: Atorvastatin synergizes with IFN-γ in treating
human non-small cell lung carcinomas via potent inhibition of RhoA
activity. Eur J Pharmacol. 682:161–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gattoni A, Parlato A, Vangieri B,
Bresciani M and Derna R: Interferon-gamma: Biologic functions and
HCV therapy (type I/II) (1 of 2 parts). Clin Ter. 157:377–386.
2006.PubMed/NCBI
|
|
11
|
Chung TW, Tan KT, Chan HL, Lai MD, Yen MC,
Li YR, Lin SH and Lin CC: Induction of indoleamine 2,3-dioxygenase
(IDO) enzymatic activity contributes to interferon-gamma induced
apoptosis and death receptor 5 expression in human non-small cell
lung cancer cells. Asian Pac J Cancer Prev. 15:7995–8001. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hastie C: Interferon gamma, a possible
therapeutic approach for late-stage prostate cancer? Anticancer
Res. 28B:2843–2849. 2008.
|
|
13
|
El Jamal SM, Taylor EB, Abd Elmageed ZY,
Alamodi AA, Selimovic D, Alkhateeb A, Hannig M, Hassan SY,
Santourlidis S, Friedlander PL, et al: Interferon gamma-induced
apoptosis of head and neck squamous cell carcinoma is connected to
indoleamine-2,3-dioxygenase via mitochondrial and ER
stress-associated pathways. Cell Div. 11:112016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pirot P, Eizirik DL and Cardozo AK:
Interferon-gamma potentiates endoplasmic reticulum stress-induced
death by reducing pancreatic beta cell defence mechanisms.
Diabetologia. 49:1229–1236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watanabe Y, Suzuki O, Haruyama T and
Akaike T: Interferon-gamma induces reactive oxygen species and
endoplasmic reticulum stress at the hepatic apoptosis. J Cell
Biochem. 89:244–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cardozo AK, Ortis F, Storling J, Feng YM,
Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz
A and Eizirik DL: Cytokines downregulate the sarcoendoplasmic
reticulum pump Ca2+ ATPase 2b and deplete endoplasmic
reticulum Ca2+, leading to induction of endoplasmic
reticulum stress in pancreatic beta-cells. Diabetes. 54:452–461.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Denmeade SR and Isaacs JT: The SERCA pump
as a therapeutic target: Making a 'smart bomb' for prostate cancer.
Cancer Biol Ther. 4:14–22. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rao RV, Hermel E, Castro-Obregon S, del
Rio G, Ellerby LM, Ellerby HM and Bredesen DE: Coupling endoplasmic
reticulum stress to the cell death program. Mechanism of caspase
activation. J Biol Chem. 276:33869–33874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Foskett JK, White C, Cheung KH and Mak DO:
Inositol trisphosphate receptor Ca2+ release channels.
Physiol Re. 87:593–658. 2007. View Article : Google Scholar
|
|
20
|
Papp S, Dziak E, Michalak M and Opas M: Is
all of the endoplasmic reticulum created equal? The effects of the
heterogeneous distribution of endoplasmic reticulum
Ca2+-handling proteins. J Cell Biol. 160:475–479. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giampietri C, Petrungaro S, Conti S,
Facchiano A, Filippini A and Ziparo E: Cancer microenvironment and
endoplasmic reticulum stress response. Mediators Inflamm.
2015:4172812015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang M and Kaufman RJ: The impact of the
endoplasmic reticulum protein-folding environment on cancer
development. Nat Rev Cancer. 14:581–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yadav RK, Chae SW, Kim HR and Chae HJ:
Endoplasmic reticulum stress and cancer. J Cancer Prev. 19:75–88.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lemus L and Goder V: Regulation of
endoplasmic reticulum-associated protein degradation (ERAD) by
Ubiquitin. Cells. 3:824–847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bertolotti A, Zhang Y, Hendershot LM,
Harding HP and Ron D: Dynamic interaction of BiP and ER stress
transducers in the unfolded-protein response. Nat Cell Biol.
2:326–332. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fisher LW and Fedarko NS: Six genes
expressed in bones and teeth encode the current members of the
SIBLING family of proteins. Connect Tissue Res. 44(Suppl 1): 33–40.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bellahcène A, Castronovo V, Ogbureke KU,
Fisher LW and Fedarko NS: Small integrin-binding ligand N-linked
glycoproteins (SIBLINGs): Multifunctional proteins in cancer. Nat
Rev Cancer. 8:212–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ogbureke KU, Nikitakis NG, Warburton G,
Ord RA, Sauk JJ, Waller JL and Fisher LW: Up-regulation of SIBLING
proteins and correlation with cognate MMP expression in oral
cancer. Oral Oncol. 43:920–932. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ogbureke KU, Abdelsayed RA, Kushner H, Li
L and Fisher LW: Two members of the SIBLING family of proteins,
DSPP and BSP, may predict the transition of oral epithelial
dysplasia to oral squamous cell carcinoma. Cancer. 116:1709–1717.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anunobi CC, Koli K, Saxena G, Banjo AA and
Ogbureke KU: Expression of the SIBLINGs and their MMP partners in
human benign and malignant prostate neoplasms. Oncotarget.
7:48038–48049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ogbureke KU, Weinberger PM, Looney SW, Li
L and Fisher LW: Expressions of matrix metalloproteinase-9 (MMP-9),
dentin sialophosphoprotein (DSPP), and osteopontin (OPN) at
histologically negative surgical margins may predict recurrence of
oral squamous cell carcinoma. Oncotarget. 3:286–298. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saxena G, Koli K, de la Garza J and
Ogbureke KU: Matrix metalloproteinase 20-dentin sialophosphoprotein
interaction in oral cancer. J Dent Res. 94:584–593. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Joshi R, Tawfik A, Edeh N, McCloud V,
Looney S, Lewis J, Hsu S and Ogbureke KU: Dentin
sialophosphoprotein (DSPP) gene-silencing inhibits key tumorigenic
activities in human oral cancer cell line, OSC2. PLoS One. 5. pp.
e139742010, View Article : Google Scholar
|
|
34
|
Gkouveris I, Nikitakis NG, Aseervatham J
and Ogbureke KUE: The tumorigenic role of DSPP and its potential
regulation of the unfolded protein response and ER stress in oral
cancer cells. Int J Oncol. 53:1743–1751. 2018.PubMed/NCBI
|
|
35
|
Murtas D, Maric D, De Giorgi V, Reinboth
J, Worschech A, Fetsch P, Filie A, Ascierto ML, Bedognetti D, Liu
Q, et al: IRF-1 responsiveness to IFN-γ predicts different cancer
immune phenotypes. Br J Cancer. 109:76–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma Z, Qin H and Benveniste EN:
Transcriptional suppression of matrix metalloproteinase-9 gene
expression by IFN-gamma and IFN-beta: Critical role of STAT-1alpha.
J Immunol. 167:5150–5159. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Knüpfer MM, Knüpfer H, Jendrossek V, Van
Gool S, Wolff JE and Keller E: Interferon-gamma inhibits growth and
migration of A172 human glioblastoma cells. Anticancer Res.
21A:3989–3994. 2001.
|
|
38
|
Cheng SM, Xing B, Li JC, Cheung BK and Lau
AS: Interferon-gamma regulation of TNFalpha-induced matrix
metalloproteinase 3 expression and migration of human glioma T98G
cells. Int J Cancer. 121:1190–1196. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chawla-Sarkar M, Lindner DJ, Liu YF,
Williams BR, Sen GC, Silverman RH and Borden EC: Apoptosis and
interferons: Role of interferon-stimulated genes as mediators of
apoptosis. Apoptosis. 8:237–249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yonekura N, Yokota S, Yonekura K, Dehari
H, Arata S, Kohama G and Fujii N: Interferon-gamma downregulates
Hsp27 expression and suppresses the negative regulation of cell
death in oral squamous cell carcinoma lines. Cell Death Differ.
10:313–322. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gadkaree SK, Fu J, Sen R, Korrer MJ, Allen
C and Kim YJ: Induction of tumor regression by intratumoral STING
agonists combined with anti-programmed death-L1 blocking antibody
in a preclinical squamous cell carcinoma model. Head Neck.
39:1086–1094. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu B, Shu Y and Liu P: INF-γ sensitizes
head and neck squamous cell carcinoma cells to chemotherapy-induced
apoptosis and necroptosis through up-regulation of Egr-1. Histol
Histopathol. 29:1437–1443. 2014.PubMed/NCBI
|
|
43
|
Ning Y, Riggins RB, Mulla JE, Chung H,
Zwart A and Clarke R: IFNgamma restores breast cancer sensitivity
to fulvestrant by regulating STAT1, IFN regulatory factor 1,
NF-kappaB, BCL2 family members, and signaling to caspase-dependent
apoptosis. Mol Cancer Ther. 9:1274–1285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tan H, Zeng C, Xie J, Alghamdi NJ, Song Y,
Zhang H, Zhou A and Jin D: Effects of interferons and
double-stranded RNA on human prostate cancer cell apoptosis.
Oncotarget. 6:39184–39195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen J, Zhao J, Chen L, Dong N, Ying Z,
Cai Z, Ji D, Zhang Y, Dong L, Li Y, et al: STAT1 modification
improves therapeutic effects of interferons on lung cancer cells. J
Transl Med. 13:2932015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu W, Hu M, Wang Y, Sun B, Guo Y, Xu Z,
Li J and Han B: Overexpression of interleukin-18 protein reduces
viability and induces apoptosis of tongue squamous cell carcinoma
cells by activation of glycogen synthase kinase-3β signaling. Oncol
Rep. 33:1049–1056. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dong D, Ni M, Li J, Xiong S, Ye W, Virrey
JJ, Mao C, Ye R, Wang M, Pen L, et al: Critical role of the stress
chaperone GRP78/BiP in tumor proliferation, survival, and tumor
angiogenesis in transgene-induced mammary tumor development. Cancer
Res. 68:498–505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fu Y, Wey S, Wang M, Ye R, Liao CP,
Roy-Burman P and Lee AS: Pten null prostate tumorigenesis and AKT
activation are blocked by targeted knockout of ER chaperone
GRP78/BiP in prostate epithelium. Proc Natl Acad Sci USA.
105:19444–19449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Daneshmand S, Quek ML, Lin E, Lee C, Cote
RJ, Hawes D, Cai J, Groshen S, Lieskovsky G, Skinner DG, et al:
Glucose-regulated protein GRP78 is up-regulated in prostate cancer
and correlates with recurrence and survival. Hum Pathol.
38:1547–1552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cook KL, Shajahan AN, Wärri A, Jin L,
Hilakivi-Clarke LA and Clarke R: Glucose-regulated protein 78
controls cross-talk between apoptosis and autophagy to determine
antiestrogen responsiveness. Cancer Res. 72:3337–3349. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee E, Nichols P, Spicer D, Groshen S, Yu
MC and Lee AS: GRP78 as a novel predictor of responsiveness to
chemotherapy in breast cancer. Cancer Res. 66:7849–7853. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Firczuk M, Gabrysiak M, Barankiewicz J,
Domagala A, Nowis D, Kujawa M, Jankowska-Steifer E, Wachowska M,
Glodkowska-Mrowka E, Korsak B, et al: GRP78-targeting subtilase
cytotoxin sensitizes cancer cells to photodynamic therapy. Cell
Death Dis. 4:e7412013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang J, Jiang Y, Jia Z, Li Q, Gong W,
Wang L, Wei D, Yao J, Fang S and Xie K: Association of elevated
GRP78 expression with increased lymph node metastasis and poor
prognosis in patients with gastric cancer. Clin Exp Metastasis.
23:401–410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Son SM, Byun J, Roh SE, Kim SJ and
Mook-Jung I: Reduced IRE1α mediates apoptotic cell death by
disrupting calcium homeostasis via the InsP3 receptor. Cell Death
Dis. 5:e11882014. View Article : Google Scholar
|
|
55
|
Kanekura K, Ma X, Murphy JT, Zhu LJ, Diwan
A and Urano F: IRE1 prevents endoplasmic reticulum membrane
permeabilization and cell death under pathological conditions. Sci
Signal. 8:ra622015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fribley AM, Miller JR, Brownell AL,
Garshott DM, Zeng Q, Reist TE, Narula N, Cai P, Xi Y, Callaghan MU,
et al: Celastrol induces unfolded protein response-dependent cell
death in head and neck cancer. Exp Cell Res. 330:412–422. 2015.
View Article : Google Scholar
|
|
57
|
Liu X, Lv Z, Zou J, Liu X, Ma J, Wang J,
Sa N, Jing P and Xu W: Afatinib down-regulates MCL-1 expression
through the PERK-eIF2α-ATF4 axis and leads to apoptosis in head and
neck squamous cell carcinoma. Am J Cancer Res. 6:1708–1719.
2016.
|
|
58
|
Fujimoto A, Kawana K, Taguchi A, Adachi K,
Sato M, Nakamura H, Ogishima J, Yoshida M, Inoue T, Nishida H, et
al: Inhibition of endoplasmic reticulum (ER) stress sensors
sensitizes cancer stem-like cells to ER stress-mediated apoptosis.
Oncotarget. 7:51854–51864. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Koumenis C: ER stress, hypoxia tolerance
and tumor progression. Curr Mol Med. 6:55–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Axten JM, Medina JR, Feng Y, Shu A,
Romeril SP, Grant SW, Li WH, Heerding DA, Minthorn E, Mencken T, et
al: Discovery of
7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H
-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a
potent and selective first-in-class inhibitor of protein kinase R
(PKR)-like endoplasmic reticulum kinase (PERK). J Med Chem.
55:7193–7207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bobrovnikova-Marjon E, Grigoriadou C,
Pytel D, Zhang F, Ye J, Koumenis C, Cavener D and Diehl JA: PERK
promotes cancer cell proliferation and tumor growth by limiting
oxidative DNA damage. Oncogene. 29:3881–3895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Vandewynckel YP, Laukens D, Geerts A,
Bogaerts E, Paridaens A, Verhelst X, Janssens S, Heindryckx F and
Van Vlierberghe H: The paradox of the unfolded protein response in
cancer. Anticancer Res. 33:4683–4694. 2013.PubMed/NCBI
|
|
63
|
Lin JH, Li H, Yasumura D, Cohen HR, Zhang
C, Panning B, Shokat KM, Lavail MM and Walter P: IRE1 signaling
affects cell fate during the unfolded protein response. Science.
318:944–949. 2007. View Article : Google Scholar : PubMed/NCBI
|




















