Introduction
The growth of primary prostate cancer (PC) is
dependent on androgens (1).
Androgen ablation therapy through chemical castration with a
luteinizing hormone-releasing hormone agonist, in combination with
non-steroidal androgen receptor (AR) antagonists, is often used to
impede the growth of PC (2,3).
Although there is an initial regression in cancer growth, PC almost
always progresses to a more aggressive castration-resistant form,
which can be accompanied by the appearance of bone metastases
(4).
A tumor biomarker of PC, i.e., prostate-specific
membrane antigen (PSMA), is expressed in primary carcinomas and an
increase in PSMA expression has been shown to be associated with
tumor grade, pathological stage and aneuploidy (5). PSMA expression is also upregulated
with the transition from androgen-sensitive PC to
castration-refractory PC (6).
Since PSMA is predominantly expressed in the prostate epithelium
and the neovasculature of solid tumors (7,8), it
can be an excellent candidate in PSMA-targeted therapy.
Bortezomib (BZ; PS-341) is a boronic acid dipeptide
that inhibits 26S proteasome activity (9). The antitumor effects of BZ have been
extensively studied in multiple myeloma (MM) cells. BZ has been
shown to inhibit the proliferation of and induce the apoptosis of
MM cells in vitro, and in an MM murine model via the
inactivation of nuclear factor-κB (NF-κB) (10-13).
BZ has also been shown to overcome drug resistance and potentiate
the anticancer effects of conventional therapeutic agents (14-16).
Moreover, a previous clinical study using BZ in individuals with
relapsed, refractory MM have demonstrated objective responses, some
of which were complete responses (17). Although BZ appears to be a
promising therapeutic agent for MM, its activity against
non-hematological malignancies, including PC remains to be
elucidated, regardless of its potent anticancer effects on MM
cells.
The results of our preliminary experiments to
evaluate the effects of BZ treatment on PC cells suggested that BZ
exerts an inhibitory effect on PSMA expression (data not shown).
Therefore, the detailed functions of BZ in PC cells should be
defined. It was previously observed that docetaxel (DOC) exerts a
suppressive effect on AR expression, but not on PSMA expression
(18). Thus, distinguishing
between the effects of DOC and BZ on protein expression is an
intriguing line of investigation.
In this study, we examined the effects of BZ on
protein expression in PC cells and compared its anticancer effects
on the growth of PC cells treated with BZ, DOC, or a combination of
both.
Materials and methods
Cell lines and reagents
The human PC cell lines, LNCaP, CWR22Rv1, MDA-PCa-2b
and LAPC-4, were purchased from the American Type Culture
Collection (ATCC, Manassas, VA, USA). The LNCaP and CWR22Rv1 cells
were maintained in RPMI-1640 medium supplemented with 2 mM
L-glutamine, 1% penicillin-streptomycin, and 10% heat-inactivated
fetal bovine serum (FBS) (all from Invitrogen/Thermo Fisher
Scientific, Waltham, MA, USA). The MDA-PCa-2b cells were grown in
F12K medium containing 2 mM L-glutamine, 1%
penicillin-streptomycin, 20% heat-inactivated FBS, 25 ng/ml cholera
toxin (Sigma-Aldrich, St. Louis, MO, USA), 10 ng/ml epidermal
growth factor (BD Biosciences, Franklin Lakes, NJ, USA), 5
µM phosphoethanolamine, 100 pg/ml hydrocortisone, 45 nM
selenious acid and 5 µg/ml insulin (all from Sigma-Aldrich).
The LAPC-4 cells were maintained in IMDM medium supplemented with 2
mM L-glutamine, 1% penicillin-streptomycin and 5% heat-inactivated
FBS. All the cell lines were kept at 37°C in a 5% CO2
atmosphere. DOC was purchased from Sanofi-Aventis (Bridgewater, NJ,
USA). BZ was purchased from Tronto Research Chemicals (North York,
ON, Canada).
RNA interference
Small interfering RNA (siRNA) duplexes specific to
AR and PSMA and random siRNA were purchased from Dharmacon
(Lafayette, CO, USA). The sequence of AR-specific siRNA (AR-siRNA)
corresponds to the human AR site was 5′-GACUCAGCUGCCCCAUCCA-3′)
(19). The PSMA-specific siRNA
(PSMA-siRNA) is a custom product (siGENOME Duplex D-005881-04-0050,
Human FOLH1, NM_004476). A non-targeting siRNA (NT-siRNA)
(5′-CCUACGCCACCAAUUUCGU-3′) was used as a control for the RNA
interference experiments (20).
Following overnight incubation of the suspended cells at 37°C
transfected with 10-40 nM of AR- or PSMA-siRNA or NT-siRNA using
Lipofectamine RNAiMAX reagent (Invitrogen/Thermo Fisher Scientific)
according to the manufacturer's instructions, the media were
changed with fresh media and the cells were incubated at 37°C with
or without the drugs for the time indicated in the Figures and
Figure legends.
Cell cycle analysis
All cell lines were first incubated in 6-well plates
(2.5x105 cells/well) overnight and then transfected with
10 nM of AR-siRNA and/or 10 nM of PSMA-siRNA for 72 h.
Subsequently, the cells were prepared for cell cycle analysis using
the Cell Cycle Phase Determination kit (Cayman Chemical Co., Ann
Arbor, MI, USA) according to the manufacturer's instructions.
Briefly, the cells were trypsinized, collected and centrifuged to
the pellet. The cell pellet was fixed in fixative included in the
kit for at least 2 h at -20°C prior to propidium iodide (PI)
staining after being washed 2 times with assay buffer also in the
kit. After the fixed cells were centrifuged at 500 x g for 5 min
and the fixative was thoroughly removed, the cell pellet was
suspended in PI solution and incubated for 30 min at room
temperature in the dark. The samples were subjected to FACScan flow
cytometer and analyzed using CellQuest software (both from BD
Biosciences).
Western blot analysis
The cells were lysed with Cell Lysis Buffer (Cell
Signaling Technology, Danvers, MA, USA) containing 1 mM
phenylmethylsulphonyl fluoride (EMD Chemicals, Gibbstown, NJ, USA).
Equal amounts of proteins (40-60 µg for each) determined by
the Lowry method (DC protein assay reagents) were applied to each
well on a 10% Tris-HCl gel (both from Bio-Rad Laboratories,
Hercules, CA, USA). The proteins were transferred onto Immobilon-P
Membranes (Millipore, Billerica, MA, USA), after which the filters
were washed with 15 ml of Tris-buffered saline (Bio-Rad
Laboratories) with Tween-20 (TBST) containing 5% bovine serum
albumin (Sigma-Aldrich) 3 times for 5 min each at room temperature,
and probed with solutions containing the following reagents: Mouse
monoclonal anti-PSMA antibody J591 (generated and purified in Dr
Neil Bander's laboratory) (dilution 1:4,000) (8), mouse monoclonal antibody anti-AR
(sc-7305) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) (dilution
1:100), goat polyclonal antibody anti-glyceraldehyde-3-phosphate
dehydrogenase (sc-31915) (GAPDH; Santa Cruz Biotechnology)
(dilution 1:100). The temperature and duration of the incubation
with the primary and secondary antibodies were 4°C overnight, and
room temperature for 1 h, respectively. After these steps, proteins
were detected using ECL Plus Western blotting detection reagents
(GE Healthcare, Chicago, IL, USA).
AR and PSMA DNA transfection
For all transfections, the LNCaP, CWR22Rv1 and
MDA-PCa-2b cells were seeded at 2x105 cells/well in 1 ml
of complete media in 12-well plates or 3.5x105
cells/well in 2 ml of complete media in 6-well plates on the day
before transfection. Subsequently, 1.3 µg (for 12-well) or
3.4 µg (for 6-well) of AR DNA, or 1.0 µg (for
12-well) or 2.5 µg (for 6-well) of PSMA DNA were mixed with
1 µl (for 12-well) or 2.5 µl (for 6-well) of PLUS
reagent (Invitrogen/Thermo Fisher Scientific) in 200 µl (for
12-well) or 500 µl (for 6-well) of serum-free media
(Opti-MEM® I Reduced Serum Media; Invitrogen/Thermo
Fisher Scientific) for 10 min. A total of 4 µl (for 12-well)
or 11.25 µl (for 6-well) of Lipofectamine LTX
(Invitrogen/Thermo Fisher Scientific) was then added, mixed and
incubated for an additional 25 min before the mixture of DNA, PLUS
reagent and Lipofectamine LTX was added dropwise to the cells. The
cells were then incubated for 48 h at 37°C, 5% CO2 prior
to the addition of 10 nM of DOC to the cells transfected with AR
DNA or 20 nM of BZ to the cells transfected with PSMA DNA.
Following treatment with DOC or BZ for an additional 48 h, the
cells in the 12-well plates were subjected to cell counting by flow
cytometry, and those in the 6-well plates were subjected to western
blot analysis.
For cell counting, the cells were first incubated
overnight in 24-well plates (4x104 cells/well) and then
treated for various periods of times with several methods. The
media from each well were then discarded. Subsequently, 300
µl of 0.1% trypsin was placed in each well for 8 min at 37°C
followed by 700 µl of fresh media containing 10% FBS, and
the cells were collected. A total of 50 µl of SPHERO™
(Spherotech, Lake Forest, IL, USA) was then added to each sample,
and the cells were then subjected to flow cytometry (BD
Biosciences). The data were analyzed using CellQuest software (BD
Biosciences) according to the manufacturer's instructions.
Cell viability assay
MTT assay was used to assess cell viability
following treatment of the PC cells. The cells were seeded in
96-well plates (5x103 cells/well), grown overnight, and
then treated for various periods of time as indicated in the
results and/or figure legends. Subsequently, 10 µl of MTT
reagent (R&D Systems, Minneapolis, MN, USA) were added to each
well. The plates were incubated for approximately 4 h at 37°C. When
purple precipitate was clearly visible grossly, 100 µl of
detergent reagent (R&D Systems) was added to all wells,
including the control wells. The color change was measured at 570
nm with a reference wavelength of 650 nm on a Spectramax 340PC
Microplate Reader (Molecular Devices Corporation, Sunnyvale, CA,
USA) following overnight incubation at room temperature.
Apoptotic cell detection
The LNCaP and CWR22Rv1 cells were seeded in 6-well
plates (1x105/well), grown overnight, and then treated
with 5 nM of DOC and/or 10 nM of BZ for 72 h. Following treatment,
the cells trypsinized were collected and centrifuged at 1,000 x g
for 5 min at room temperature, and then resuspended in 500
µl of binding buffer (MBL International, Woburn, MA, USA).
Subsequently, 5 µl of Annexin V-conjugated fluorescein
isothiocyanate (FITC) and 5 µl of PI (both from MBL
International) were added into each binding buffer containing the
suspended cells. Following a 5-min incubation at room temperature
in the dark, the cells were subjected to FACScan flow cytometer (BD
Biosciences). The data were analyzed using CellQuest software (BD
Biosciences) according to the manufacturer's instructions.
Statistical analysis
All values in bar graphs are expressed as the means
± standard deviation, and variables for different groups were
compared using one-way analysis of variance (ANOVA) with Tukey's
post hoc test, and a value of P<0.05 was considered to indicate
a statistically significant difference. Statistical analyses were
performed with JMP version 8.0 (SAS Institute, Cary, NC, USA).
Results
Effect of AR- and/or PSMA-siRNA on cell
growth and protein expression
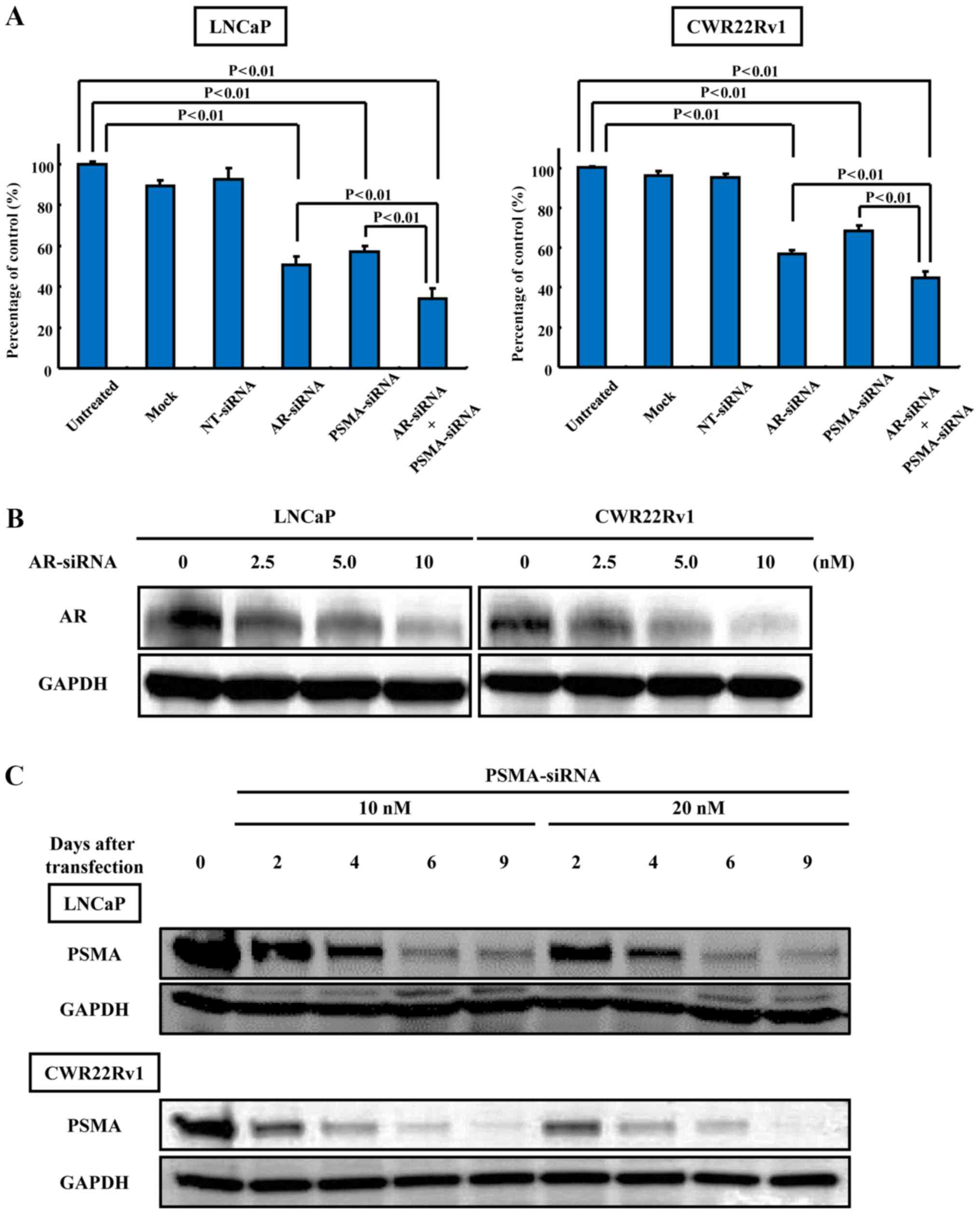
The knockdown effect of AR- or PSMA-siRNA
transfection on cell growth and protein expression in the LNCaP and
CWR22Rv1 cells was examined. The knockdown of both AR and PSMA
suppressed cell growth more significantly (33.89±5.42 and
45.00±3.00%) than AR alone (50.50±4.36 and 56.92±1.81%) or PSMA
alone (57.21±2.43 and 68.18±2.93%) in the LNCaP and CWR22Rv1 cells,
respectively (Fig. 1A). There was
also a significant difference in growth between the cells
transfected with AR- or PSMA-siRNA or both and the untreated cells
in the LNCaP and CWR22Rv1 cells (Fig.
1A). No statistically significant difference in cell growth
between the AR-siRNA-transfected cells and PSMA-siRNA-transfected
cells was observed (Fig. 1A). The
expression levels of AR and PSMA were sufficiently downregulated
following the knockdown of AR or PSMA using siRNA specific to AR or
PSMA (Fig. 1B and C).
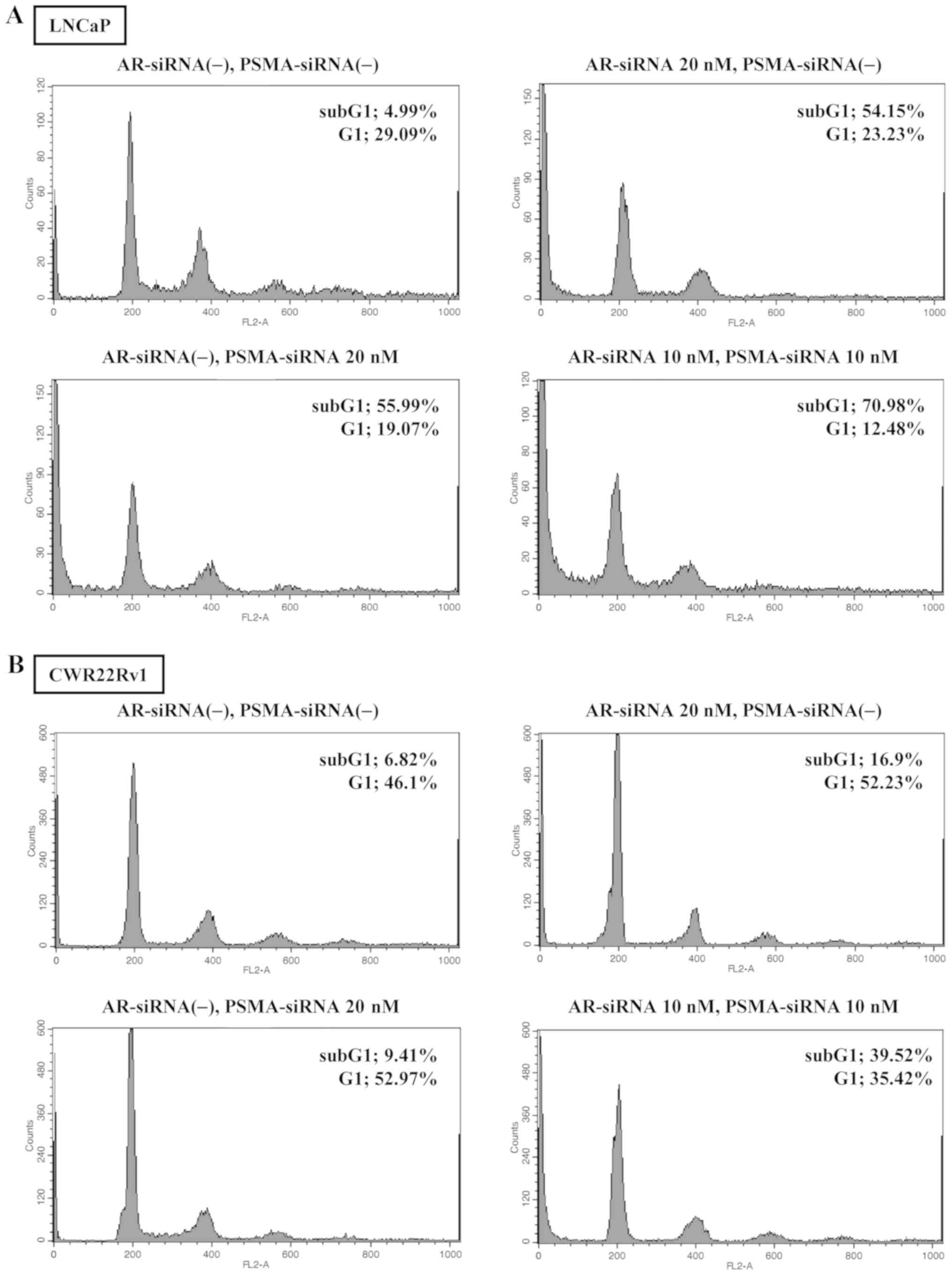
Cell cycle analysis in cells treated with
AR- and/or PSMA-siRNA transfection
The percentage of sub-G1 in the cells transfected
with AR-siRNA alone (20 nM), or PSMA-siRNA alone (20 nM), or
AR-siRNA (10 nM) plus PSMA-siRNA (10 nM) for 72 h was 54.15, 55.99
and 70.98%, respectively in the LNCaP cells, and 16.90, 9.41 and
39.52%, respectively in the CWR22Rv1 cells (Fig. 2A and B).
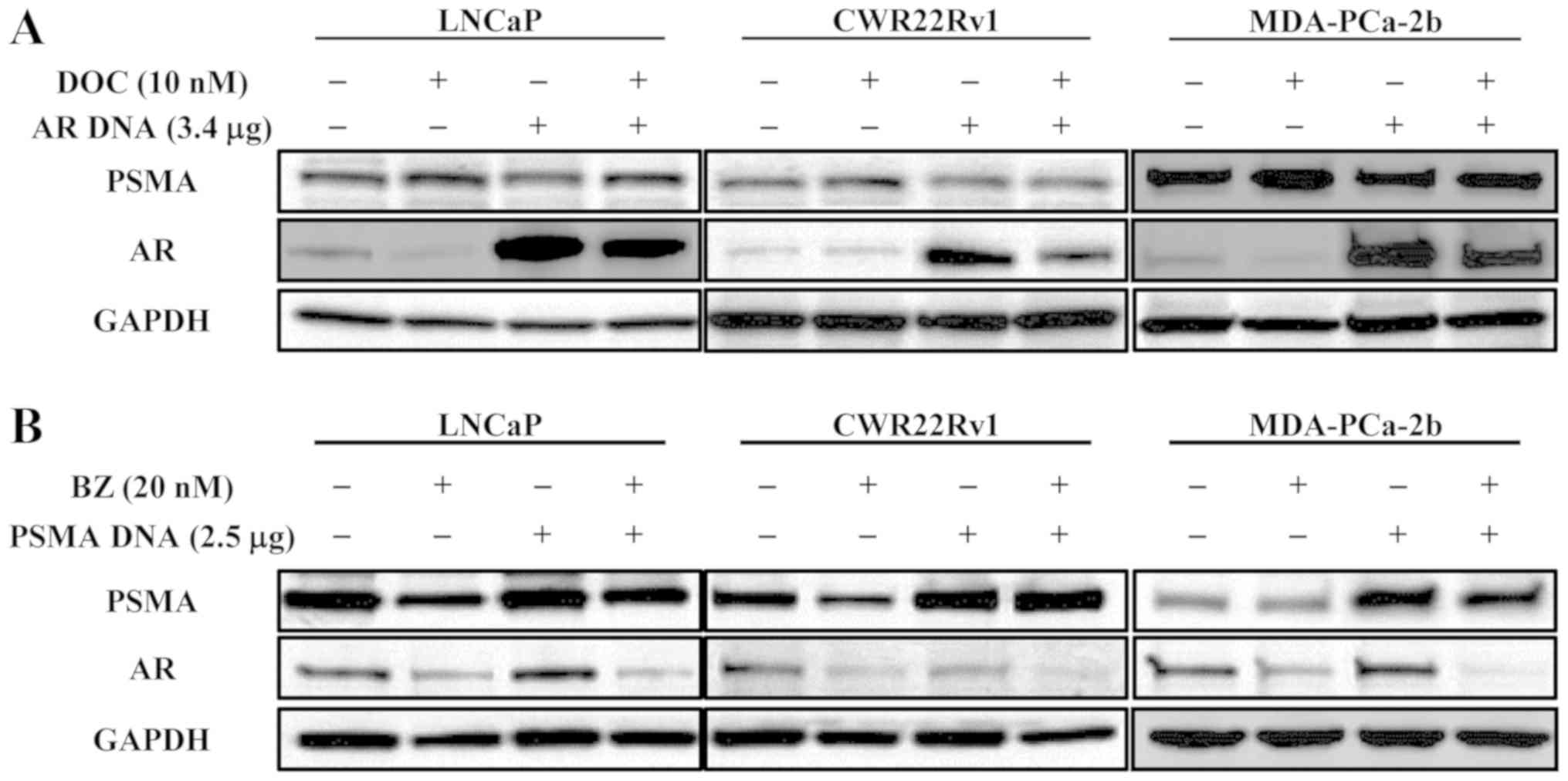
Effect of DOC on protein expression in
cells transfected with AR DNA and that of BZ on protein expression
in those transfected with PSMA DNA
The effects of DOC on the LNCaP, CWR22Rv1 and
MDA-PCa-2b cells, and on the cells transfected to highly express AR
were examined. Even under the condition of AR upregulation, DOC
inhibited the expression of AR, but not that of PSMA, in all of the
cell lines tested (Fig. 3A). The
effects of BZ were also examined using the same cell lines
transfected with PSMA DNA. BZ decreased both AR and PSMA expression
in normal cells and in the cells transfected to upregulate PSMA in
every cell line tested (Fig.
3B).
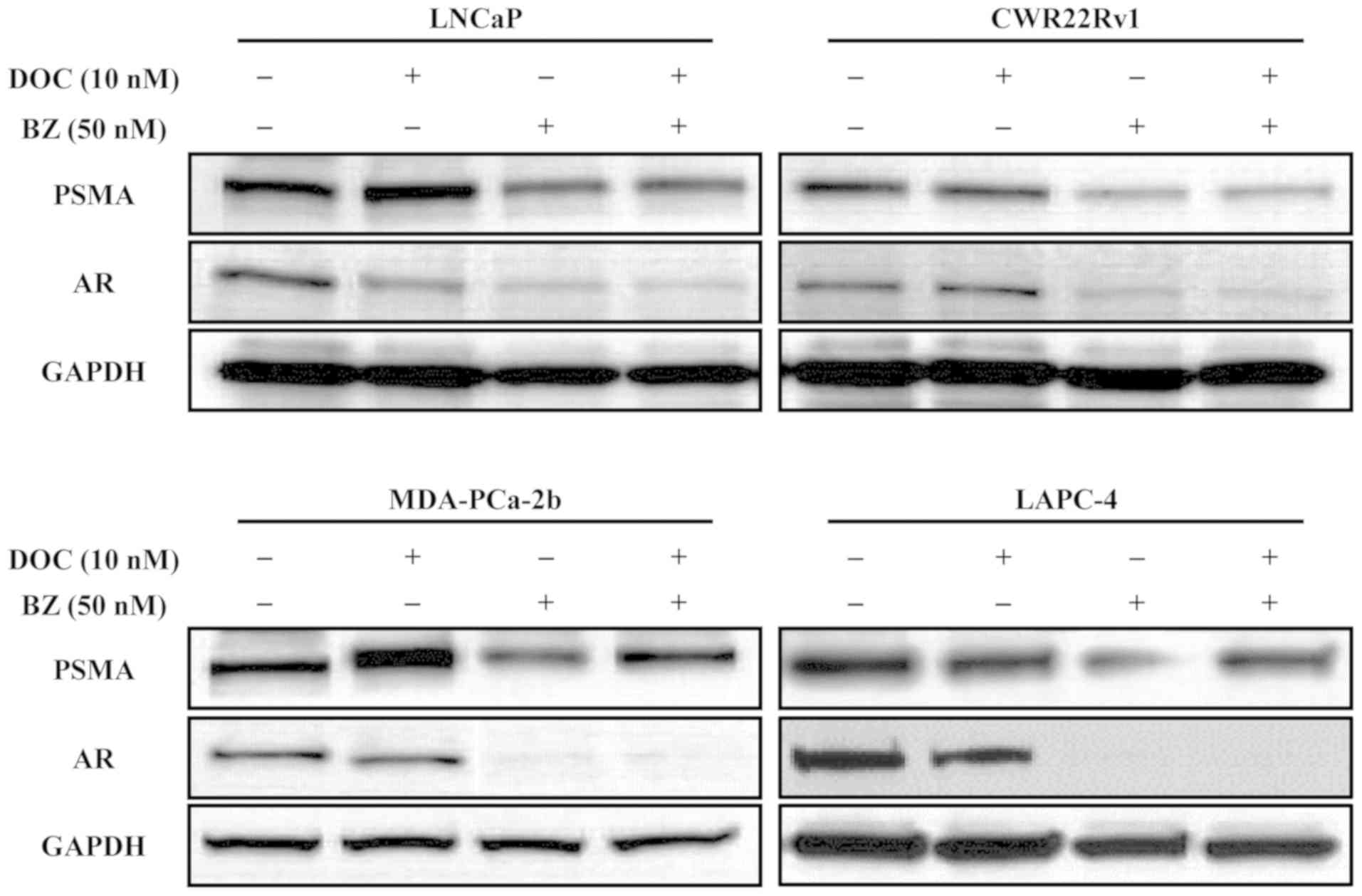
Effect of DOC and/or BZ on the expression
of AR and PSMA
The effects of DOC and/or BZ on the expression of
proteins, including AR and PSMA in the LNCaP, CWR22rv1 and
MDA-PCa-2b cells incubated with 10 nM DOC, and/or 50 nM BZ for 48 h
were evaluated. DOC inhibited the expression of AR in all the cell
lines tested in a dose-dependent manner; however, it did not
suppress PSMA expression in any of the cell lines tested (Fig. 4). On the other hand, BZ inhibited
the expression of both AR and PSMA expression in all the cell lines
tested (Fig. 4).
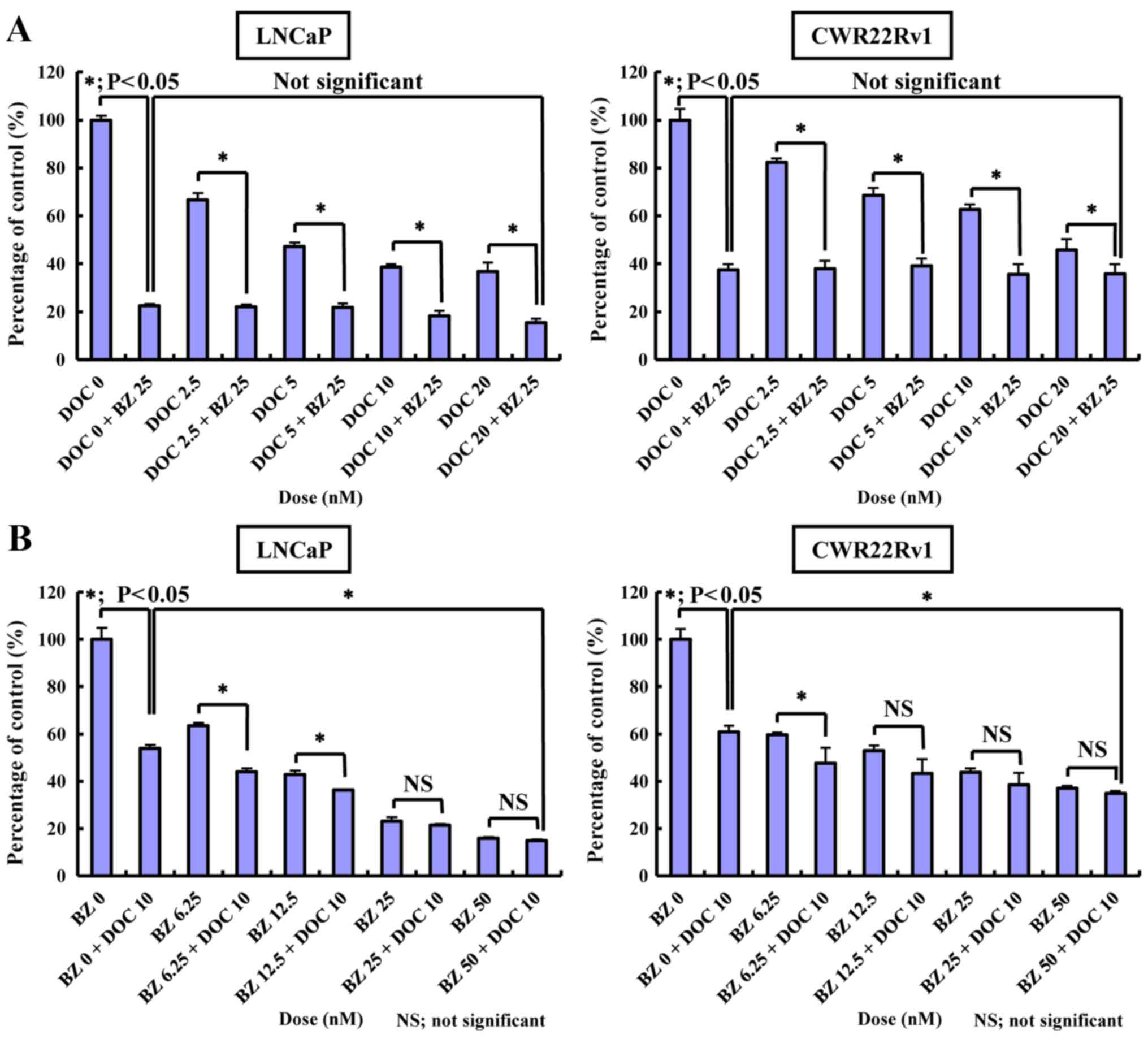
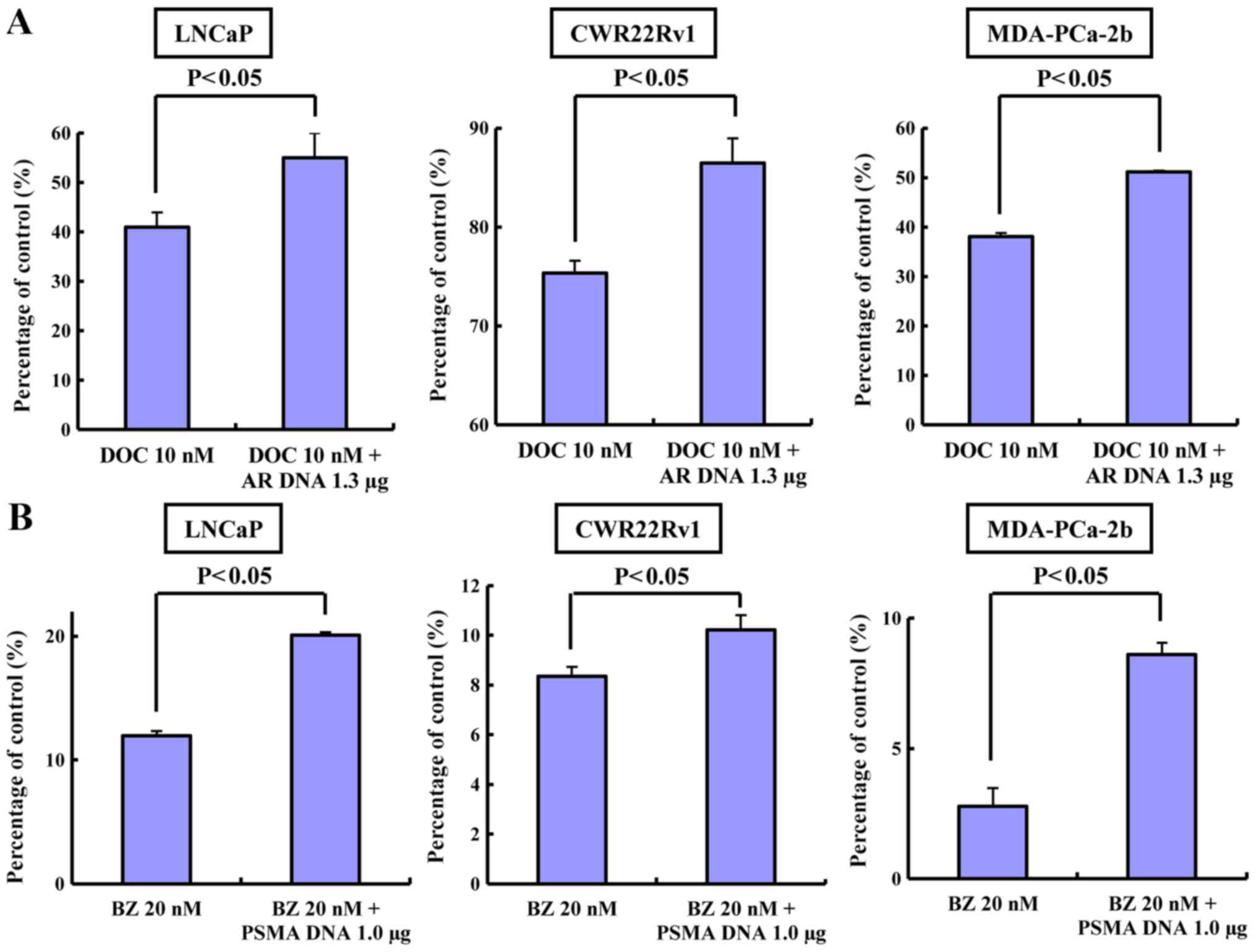
Inhibitory effect of BZ and/or DOC on
normal cells or those transfected to upregulate AR or PSMA
The inhibitory effects of BZ and/or DOC on normal
cells or cells transfected to overexpress AR or PSMA were also
evaluated. The results of MTT assay revealed that BZ itself exerted
a significantly potent anticancer effect on the cell lines tested
with or without the administration of DOC (Fig. 5). Cell growth was not inhibited
compared to the normal cells in all the cell lines transfected to
overexpress AR or PSMA, which were respectively treated with DOC or
BZ (Fig. 6A and B).
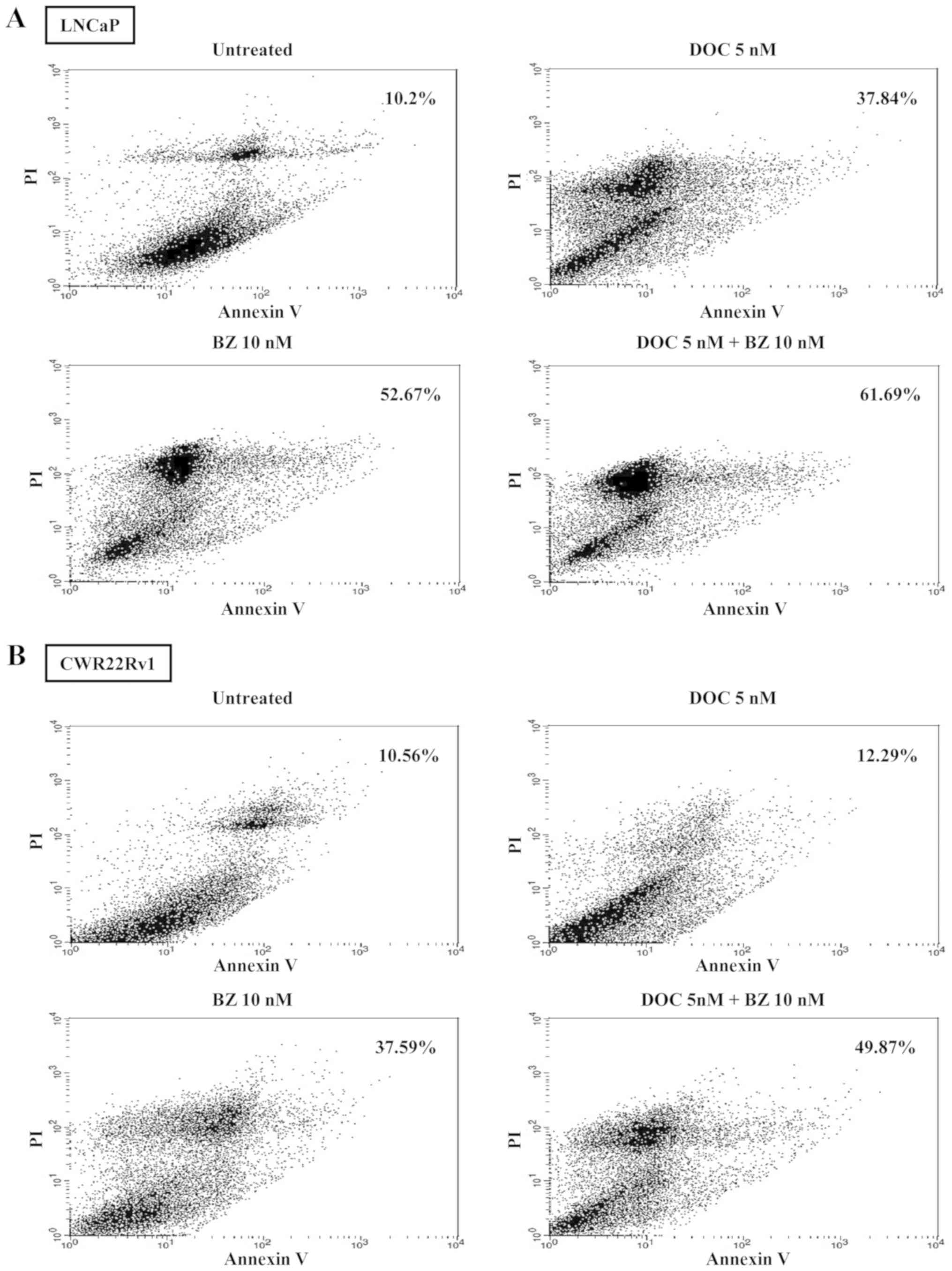
Apoptotic cell detection in cells treated
with BZ and/or DOC
The percentages of late apoptotic cells detected
using Annexin V and propidium iodide staining, which were used with
DOC alone (5 nM), BZ alone (10 nM), or DOC (5 nM) plus BZ (10 nM)
for 72 h, were 37.84, 52.67 and 61.69%, respectively in the LNCaP
cells and 12.29, 37.59 and 49.87%, respectively in the CWR22Rv1
cells (Fig. 7).
Discussion
Previous research suggests that PSMA expression is
modulated inversely by the expression level of androgens. In other
words, PSMA expression is upregulated when AR expression is
downregulated and vice versa (6,21).
It was previously confirmed that saporin-conjugated, anti-PSMA
antibodies have potent and selective anticancer effects on
PSMA-expressing PC cells in vitro and in vivo
(22). The same effects were
observed by immunohistochemistry in the downregulation of PSMA,
particularly in inoculated tumors (22). These results indicate that along
with decreased levels of PSMA, AR expression may be downregulated.
Consequently, the viability of PC cells could be significantly
reduced by the simultaneous knockdown of PSMA and AR. As stated in
this study in the Introduction, BZ was found to exert an inhibitory
effect on PSMA expression in our preliminary experiments. The
present study verified whether the anticancer effect of BZ is
dependent on decreased expression levels of both AR and PSMA.
Based on these previous findings, this study
investigated the anticancer effect of BZ on PC cell lines compared
to DOC alone or BZ plus DOC after confirming the inhibitory effect
of siRNA on cell growth, the expression of PSMA as well as AR, and
the cell cycles (Figs. 1 and
2). The results suggested that the
simultaneous knockdown of AR and PSMA exerted sufficient growth
inhibitory effects on PC cells. These phenomena were tested and
confirmed using the cells transfected to overexpress AR or PSMA to
determine whether DOC exerts an inhibitory effect on AR expression
or whether BZ exerts a suppressive effect on PSMA expression even
in the cells transfected to upregulate AR or PSMA expression
(Figs. 3 and 4). These findings were similar to those
observed with both AR- and PSMA-siRNA on cell growth, protein
expression and cell cycle analysis observed in Figs. 1 and 2. Based on these findings, a bona-fide
inhibitory effect on cell growth in PC cells was examined. We do
assert that BZ has an inhibitory effect on the expression of both
AR and PSMA. However, the expression levels of AR and PSMA are
inversely correlated. When AR expression is upregulated, PSMA
expression is downregulated (6).
Therefore, we did not use cell lines transfected with AR DNA for
examining the effect of BZ on AR expression, as we suspected that
PSMA expression would be relatively low in such cell lines with the
effect of BZ not sufficiently significant. The effect of BZ alone
on cell growth was found to be sufficiently potent when compared to
the effect of DOC alone or to that of the combined administration
of both drugs on LNCaP and CWR22Rv1 cells. Furthermore, BZ
inhibited the growth of the two cell lines in a dose-dependent
manner (Fig. 5). We believe that
AR and PSMA play essential roles in enabling cells to survive the
treatment of anticancer drugs, such as DOC and BZ (23-26).
In fact, we demonstrated that cells transfected with AR DNA or PSMA
DNA were resistant to the inhibitory effects of DOC and BZ on the
growth of the cells (Fig. 6). In
detecting apoptotic cells by fluorescence-activated cell sorting
(FACS) analysis, the apoptosis-inducing effect on the cells treated
with BZ alone was close to that observed with treatment with BZ
plus DOC, but more potent than that observed with treatment with
DOC alone (Fig. 7). The dose of BZ
and DOC tested was designated appropriately in this series of
experiments we conducted, and was within a normal limitation
(16,18). Based on these findings, BZ induced
the apoptosis of the cells expressing AR, as well as PSMA by
suppressing both the expression of both proteins, and the
apoptosis-inducing effect of BZ was similar to that observed with
DOC plus BZ.
BZ is a highly selective, reversible inhibitor of
the 26S proteasome that is recommended for single-agent use in the
treatment of patients with multiple myeloma (9-13).
BZ has been known to have a variety of mechanisms influencing the
physiology of cells, which indicates that exposure to BZ has the
exposure to BZ results in the stabilization of p21, p27 and p53, as
well as the pro-apoptotic Bid and Bax proteins, caveolin-1 and
inhibitor κB-α, which prevents the activation of NF-κB-induced cell
survival pathways. BZ can also promote the activation of
proapoptotic c-Jun-NH2 terminal kinase, as well as the endoplasmic
reticulum stress response. Thus, this drug exerts potent anticancer
effects on various types of malignant tumors (27-29).
Although a relatively high expression level of p21
and p27 have been found in cells treated with BZ, other proteins
such as p53, Bid, Bax and c-Jun-NH2 terminal kinase have been found
to be expressed weakly even in the cells treated with BZ alone
(data not shown). However, DOC has been known to be an effective
chemotherapeutic drug against PC. As far as we investigated its
anticancer function, this drug has an inhibitory effect on AR
expression, not PSMA (18).
Therefore, the anticancer effect of BZ may be attributed to its
downregulating effect against both AR and PSMA.
Resistance to chemotherapy can be attributed to
mechanisms specific to PC biology, general mechanisms common to
other tumor types, drug pharmacokinetics, such as continued
androgen-AR signaling and upregulation of pro-survival cellular
pathways, or to the role of angiogenesis and immune mechanisms in
the tumor microenvironment (30).
Therefore, BZ should be used in combination with other anticancer
agents. BZ has reportedly demonstrated anticancer effects when used
in combination with other chemotherapeutic drugs, such as
etoposide, for PC cell lines and has been reported to sensitize
human PC cells to radiation effects when administered with DOC
(31,32). Other studies have reported the
synergistic anticancer effects of BZ when it is administered in
combination with various agents on PC cell lines (33,34).
In addition to PC, BZ has reportedly been shown to exert a
synergistic anticancer effect on ovarian cancer and multiple
myeloma when administered with a histone deacetylase inhibitor or
daratumumab (a human CD38-directed monoclonal antibody) plus
dexamethasone (35,36). In the near future, BZ may be
prescribed in combination with targeted molecular drugs or immune
checkpoint inhibitors. Although a report indicating the single use
of proteasome inhibitor exerting an anticancer effect in the
treatment of PC exists (37), the
fact that the use of BZ to prevent biochemical recurrence if used
together with antiandrogen drugs introduced in another study should
also be considered (38).
Nevertheless, further research is still required to overcome these
limitations.
There are a few limitations to this study. First,
the anticancer effect of BZ alone was found to be as close as that
of BZ plus DOC. This reason for this may be that DOC occasionally
exerts an upregulating effect on PSMA, which was not found
previously, but DOC could theoretically induce the upregulation of
PSMA by downregulating AR expression. Similar results to the ones
found in the present study have been reported (39). In fact, further investigation
includes whether the synergistic effect of BZ plus other anticancer
drugs or chemical agents have a more potent effect on PC cells.
Second, the mechanism of AR and PSMA downregulation induced by BZ
was not described. Furthermore, we could not clarify why the
expression of AR and PSMA were inversely correlated, but this
phenomenon remained consistent from our previous study to the
present study (18).
In conclusion, the results of this study suggest
that the knockdown of PSMA expression, as well as the suppression
of AR expression inhibits the growth of treated PC cells. BZ, which
has the same effect on the expression of AR and PSMA, is promising
if applied to clinical use for molecular targeted therapy. The
inhibition of PSMA, as well as AR severely inhibits the growth of
PC cells. The proteasome inhibitor, BZ, has the same inhibitory
effect on the expression of AR and PSMA as siRNA specific to AR or
PSMA. Our results from cells transfected with AR DNA or PSMA DNA
would also be more relevant as BZ and/or DOC may be used for
patients with aggressive PC. This chemotherapeutic agent shows
promise as a molecularly targeted drug for the treatment of PC, and
BZ plus other anticancer drugs or chemical agents may be required
for more potent anticancer effect.
Funding
This study was funded by a research grant from Weill
Cornell Medical College.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KK and HL were involved in the conception and design
of the study; KK performed the experiments; KK analyzed the data;
KK drafted the manuscript; KK and HL reviewed and edited the
manuscript. Both authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Huggins C and Hodges CV; Studies on
prostatic cancer: I The effect of castration, of estrogen and
androgen injection on serum phosphatases in metastatic carcinoma of
the prostate CA. Cancer J Clin. 22:232–240. 1972. View Article : Google Scholar
|
|
2
|
Brawer MK, Crawford ED, Labrie F,
Mendoza-Valdes A, Miller PDPD and Petrylak DP: Androgen deprivation
and other treatments for advanced prostate cancer. Rev Urol.
3(Suppl 2): S59–S68. 2001.
|
|
3
|
Labrie F: Medical castration with LHRH
agonists: 25 years later with major benefits achieved on survival
in prostate cancer. J Androl. 25:305–313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View
Article : Google Scholar
|
|
5
|
Ross JS, Sheehan CE, Fisher HAG, Kaufman
RP Jr, Kaur P, Gray K, Webb I, Gray GS, Mosher R and Kallakury BV:
Correlation of primary tumor prostate-specific membrane antigen
expression with disease recurrence in prostate cancer. Clin Cancer
Res. 9:6357–6362. 2003.PubMed/NCBI
|
|
6
|
Wright GL Jr, Grob BM, Haley C, Grossman
K, Newhall K, Petrylak D, Troyer J, Konchuba A, Schellhammer PF and
Moriarty R: Upregulation of prostate-specific membrane antigen
after androgen-deprivation therapy. Urology. 48:326–334. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang SS, Keefe DS, Bacich DJ, Reuter VE,
Heston WD and Gaudin PB: Advances in brief prostate-specific
membrane antigen is produced in tumor. Clin Cancer Res.
5:2674–2681. 1999.PubMed/NCBI
|
|
8
|
Liu H, Moy P, Kim S, Xia Y, Rajasekaran A,
Navarro V, Knudsen B and Bander NH: Monoclonal antibodies to the
extracellular domain of prostate-specific membrane antigen also
react with tumor vascular endothelium. Cancer Res. 57:3629–3634.
1997.PubMed/NCBI
|
|
9
|
Adams J: Proteasome inhibitors as new
anticancer drugs. Curr Opin Oncol. 14:628–634. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
LeBlanc R, Catley LP, Hideshima T,
Lentzsch S, Mitsiades CS, Mitsiades N, Neuberg D, Goloubeva O, Pien
CS, Adams J, et al: Proteasome inhibitor PS-341 inhibits human
myeloma cell growth in vivo and prolongs survival in a murine
model. Cancer Res. 62:4996–5000. 2002.PubMed/NCBI
|
|
11
|
Hideshima T, Chauhan D, Richardson P,
Mitsiades C, Mitsiades N, Hayashi T, Munshi N, Dang L, Castro A,
Palombella V, et al: NF-κB as a therapeutic target in multiple
myeloma. J Biol Chem. 277:16639–16647. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitsiades N, Mitsiades CS, Poulaki V,
Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA,
Treon SP, et al: Molecular sequelae of proteasome inhibition in
human multiple myeloma cells. Proc Natl Acad Sci USA.
99:14374–14379. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Orlowski RZ, Stinchcombe TE, Mitchell BS,
Shea TC, Baldwin AS, Stahl S, Adams J, Esseltine DL, Elliott PJ,
Pien CS, et al: Phase I trial of the proteasome inhibitor PS-341 in
patients with refractory hematologic malignancies. J Clin Oncol.
20:4420–4427. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mitsiades N, Mitsiades CS, Richardson PG,
Poulaki V, Tai YT, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph
M, et al: The proteasome inhibitor PS-341 potentiates sensitivity
of multiple myeloma cells to conventional chemotherapeutic agents:
Therapeutic applications. Blood. 101:2377–2380. 2003. View Article : Google Scholar
|
|
15
|
Ma MH, Yang HH, Parker K, Manyak S,
Friedman JM, Altamirano C, Wu ZQ, Borad MJ, Frantzen M, Roussos E,
et al: The proteasome inhibitor PS-341 markedly enhances
sensitivity of multiple myeloma tumor cells to chemotherapeutic
agents. Clin Cancer Res. 9:1136–1144. 2003.PubMed/NCBI
|
|
16
|
Hideshima T, Richardson P, Chauhan D,
Palombella VJ, Elliott PJ, Adams J and Anderson KC: The proteasome
inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes
drug resistance in human multiple myeloma cells. Cancer Res.
61:3071–3076. 2001.PubMed/NCBI
|
|
17
|
Richardson PG, Barlogie B, Berenson J,
Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina
M, Alexanian R, et al: A phase 2 study of bortezomib in relapsed,
refractory myeloma. N Engl J Med. 348:2609–2617. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuroda K, Liu H, Kim S, Guo M, Navarro V
and Bander NH: Docetaxel down-regulates the expression of androgen
receptor and prostate-specific antigen but not prostate-specific
membrane antigen in prostate cancer cell lines: Implications for
PSA surrogacy. Prostate. 69:1579–1585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Compagno D, Merle C, Morin A, Gilbert C,
Mathieu JR, Bozec A, Mauduit C, Benahmed M and Cabon F:
SIRNA-directed in vivo silencing of androgen receptor inhibits the
growth of castration-resistant prostate carcinomas. PLoS One.
2:e10062007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee UJ, Choung SR, Prakash KV, Lee EJ, Lee
MY, Kim YJ, Han CW and Choi YC: Dual knockdown of p65 and p50
subunits of NF-kappaB by siRNA inhibits the induction of
inflammatory cytokines and significantly enhance apoptosis in human
primary synoviocytes treated with tumor necrosis factor-alpha. Mol
Biol Rep. 35:291–298. 2008. View Article : Google Scholar
|
|
21
|
Israeli RS, Powell CT, Corr JG, Fair WR
and Heston WD: Expression of the prostate-specific membrane
antigen. Cancer Res. 54:1807–1811. 1994.PubMed/NCBI
|
|
22
|
Kuroda K, Liu H, Kim S, Guo M, Navarro V
and Bander NH: Saporin toxin-conjugated monoclonal antibody
targeting prostate-specific membrane antigen has potent anticancer
activity. Prostate. 70:1286–1294. 2010.PubMed/NCBI
|
|
23
|
Bravaccini S, Puccetti M, Bocchini M,
Ravaioli S, Celli M, Scarpi E, De Giorgi U, Tumedei MM, Raulli G,
Cardinale L, et al: PSMA expression: A potential ally for the
pathologist in prostate cancer diagnosis. Sci Rep. 8:42542018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Minner S, Wittmer C, Graefen M, Salomon G,
Steuber T, Haese A, Huland H, Bokemeyer C, Yekebas E, Dierlamm J,
et al: High level PSMA expression is associated with early PSA
recurrence in surgically treated prostate cancer. Prostate.
71:281–288. 2011. View Article : Google Scholar
|
|
25
|
Khurana N, Talwar S, Chandra PK, Sharma P,
Abdel-Mageed AB, Mondal D and Sikka SC: Sulforaphane increases the
efficacy of anti-androgens by rapidly decreasing androgen receptor
levels in prostate cancer cells. Int J Oncol. 49:1609–1619. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Komura K, Jeong SH, Hinohara K, Qu F, Wang
X, Hiraki M, Azuma H, Lee GS, Kantoff PW and Sweeney CJ: Resistance
to docetaxel in prostate cancer is associated with androgen
receptor activation and loss of KDM5D expression. Proc Natl Acad
Sci USA. 113:6259–6264. 2016. View Article : Google Scholar
|
|
27
|
Williams S, Pettaway C, Song R, Papandreou
C, Logothetis C and McConkey DJ: Differential effects of the
proteasome inhibitor bortezomib on apoptosis and angiogenesis in
human prostate tumor xenografts. Mol Cancer Ther. 2:835–843.
2003.PubMed/NCBI
|
|
28
|
Papandreou CN and Logothetis CJ:
Bortezomib as a potential treatment for prostate cancer. Cancer
Res. 64:5036–5043. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Codony-Servat J, Tapia MA, Bosch M, Oliva
C, Domingo-Domenech J, Mellado B, Rolfe M, Ross JS, Gascon P,
Rovira A, et al: Differential cellular and molecular effects of
bortezomib, a proteasome inhibitor, in human breast cancer cells.
Mol Cancer Ther. 5:665–675. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lohiya V, Aragon-Ching JB and Sonpavde G:
Role of chemotherapy and mechanisms of resistance to chemotherapy
in metastatic castration-resistant prostate cancer. Clin Med
Insights Oncol. 10(Suppl 1): 57–66. 2016.PubMed/NCBI
|
|
31
|
Aras B and Yerlikaya A: Bortezomib and
etoposide combinations exert synergistic effects on the human
prostate cancer cell line PC-3. Oncol Lett. 11:3179–3184. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cao W, Shiverick KT, Namiki K, Sakai Y,
Porvasnik S, Urbanek C and Rosser CJ: Docetaxel and bortezomib
downregulate Bcl-2 and sensitize PC-3-Bcl-2 expressing prostate
cancer cells to irradiation. World J Urol. 26:509–516. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Christian PA, Thorpe JA and Schwarze SR:
Velcade sensitizes prostate cancer cells to TRAIL induced apoptosis
and suppresses tumor growth in vivo. Cancer Biol Ther. 8:73–80.
2009. View Article : Google Scholar
|
|
34
|
Thorpe JA, Christian PA and Schwarze SR:
Proteasome inhibition blocks caspase-8 degradation and sensitizes
prostate cancer cells to death receptor-mediated apoptosis.
Prostate. 68:200–209. 2008. View Article : Google Scholar
|
|
35
|
Janyst K, Janyst M, Siernicka M and Lasek
W: Synergistic antitumor effects of histone deacetylase inhibitor
scriptaid and bortezomib against ovarian cancer cells. Oncol Rep.
39:1999–2005. 2018.PubMed/NCBI
|
|
36
|
Iida S, Ichinohe T, Shinagawa A, Suzuki K,
Takezako N and Aoki M: Safety and efficacy of daratumumab in
combination with bortezomib and dexamethasone in Japanese patients
with relapsed or refractory multiple myeloma. Int J Hematol.
107:460–467. 2018. View Article : Google Scholar
|
|
37
|
Wehenkel M, Ban JO, Ho YK, Carmony KC,
Hong JT and Kim KB: A selective inhibitor of the immunoproteasome
subunit LMP2 induces apoptosis in PC-3 cells and suppresses tumour
growth in nude mice. Br J Cancer. 107:53–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kraft AS, Garrett-Mayer E, Wahlquist AE,
Golshayan A, Chen CS, Butler W, Bearden J and Lilly M: Combination
therapy of recurrent prostate cancer with the proteasome inhibitor
bortezomib plus hormone blockade. Cancer Biol Ther. 12:119–124.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hainsworth JD, Meluch AA, Spigel DR,
Barton J Jr, Simons L, Meng C, Gould B and Greco FA: Weekly
docetaxel and bortezomib as first-line treatment for patients with
hormone-refractory prostate cancer: A Minnie Pearl Cancer Research
Network phase II trial. Clin Genitourin Cancer. 5:278–283. 2007.
View Article : Google Scholar : PubMed/NCBI
|