Introduction
Salivary adenoid cystic carcinoma (SACC) is one of
the most common malignancies of the salivary glands and is
characterized by slow indolent growth, frequent local recurrences,
a high incidence of metastasis and a poor prognosis (1-3). A
total of 40-60% of patients with SACC suffer distant metastasis
even when treated with surgical resection and intensive
radiotherapy (4,5). The lung is the most common distant
metastasis site, followed by bone, liver and brain (6). However, no effective treatment is
available to inhibit or reduce lung metastases (6-8).
Thus, there is an urgent need to find novel treatments to suppress
SACC tumour metastasis, especially lung metastases.
MYB [the gene that encodes transcriptional
activator Myb (MYB)]-NFIB (the gene that encodes nuclear
factor 1 B-type) fusion occurred in 119/232 (51%) of SACCs, and
MYB mRNA overexpression was detected in 119/136 (88%) of
SACCs (9-15), indicating that MYB may serve an
important role in the occurrence and development of SACC. MYB is
associated with the development of tumours, including leukaemia,
pancreatic cancer, breast cancer and prostate cancer (16-18).
However, whether MYB is associated with the development and
metastasis of SACC is not clear (19).
Epithelial-mesenchymal transition (EMT) is a typical
event in SACC metastasis (20,21).
Changes in cellular morphology and phenotypic characteristics
facilitate epithelial cell transformation into cells with
mesenchymal features, which gain an invasive phenotype and stronger
motility (22-24). During EMT, the expression of cell
adhesion molecules, such as cadherin-1 (E-cadherin, encoded by
CDH1), decrease, and the expression of mesenchymal markers,
including vimentin (encoded by VIM) and cadherin-2
(N-cadherin, encoded by CDH2), increase (25). MYB has been reported to enhance
breast cancer metastasis by activating the Wnt/catenin β-1
(β-catenin)/Axin-2 signalling pathway, which is one of the most
important pathways that leads to EMT activation. However, whether
MYB is associated with EMT during SACC metastasis is unclear. Based
on the aforementioned studies, the authors of the current study
aimed to explore the effect of MYB on the development and lung
metastasis of SACC, and to analyse the association of MYB and EMT
with SACC metastasis.
Materials and methods
Human tissue samples
A total of 10 human SACC tissues and 10 paired
normal submandibular gland (SMG) tissues were used for the
microarray analysis. They were collected from patients with SACC at
the Peking University School and Hospital of Stomatology (Beijing,
China) between August 2015 and April 2016. A total of 50 human SACC
and 41 SMG tissues collected between June 2008 and September 2013
at the Peking University School and Hospital of Stomatology were
also used to analyse MYB expression in tissues. Patients had
not undergone radiation therapy or chemotherapy. The tumours were
classified according to the histological classification of salivary
gland tumours proposed by the World Health Organization (26). The clinicopathological data are
summarized in Table I. The study
was approved by the Ethics Committee of Peking University School
and Hospital of Stomatology (permit no. PKUSSIRB-201522040).
 | Table ICorrelation between
clinicopathological variables and MYB expression in patients with
salivary adenoid cystic carcinoma. |
Table I
Correlation between
clinicopathological variables and MYB expression in patients with
salivary adenoid cystic carcinoma.
| Variables (n) | | MYB expression
| χ2 | P-value |
|---|
| Total (n) | Low (n) | High |
|---|
| Age (years) | | | | 0.786 | 0.375 |
| <42 | 18 | 6 | 12 | | |
| ≥42 | 32 | 7 | 25 | | |
| Sex | | | | 1.708 | 0.191 |
| Male | 23 | 8 | 15 | | |
| Female | 27 | 5 | 22 | | |
| Tumour size | | | | 3.814 | 0.051 |
| <4 cm | 31 | 11 | 20 | | |
| ≥4 cm | 19 | 2 | 17 | | |
| Clinical stage | | | | 4.372 | 0.037 |
| I/II | 26 | 10 | 16 | | |
| I II/IV | 24 | 3 | 21 | | |
| Site | | | | 1.418 | 0.234 |
| Major salivary
gland | 13 | 5 | 8 | | |
| Minor salivary
gland | 37 | 8 | 29 | | |
| Pathological
type | | | | 7.031 | 0.030 |
| Cribriform | 13 | 6 | 7 | | |
| Tubular | 25 | 7 | 18 | | |
| Solid | 12 | 0 | 12 | | |
| Lymph node
metastasis | | | | 0.191 | 0.662 |
| Absent | 44 | 11 | 33 | | |
| Present | 6 | 2 | 4 | | |
| Perineural
invasion | | | | 0.082 | 0.775 |
| Absent | 17 | 4 | 13 | | |
| Present | 33 | 9 | 24 | | |
| Lung
metastasis | | | | 5.418 | 0.020 |
| Absent | 33 | 12 | 21 | | |
| Present | 17 | 1 | 16 | | |
| Local regional
recurrence | | | | 0.090 | 0.764 |
| Absent | 21 | 5 | 16 | | |
| Present | 29 | 8 | 21 | | |
Microarray gene expression analysis
Total RNA was extracted from the 10 human SACC and
10 paired SMG tissues using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) following the manufacturer's
protocol. Then the RNA was purified using the RNeasy Midi kit
(Qiagen Sciences, Inc., Gaithersburg, MD, USA). Hybridization was
performed by Shanghai Biotechnology Corporation (Shanghai, China)
using The Shbio Human ceRNA microarray v1.0 (for mRNA/circular
RNA/long non-coding RNA detection) and the SurePrint Human miRNA
microarray v21.0 (Agilent Technologies, Inc., Santa Clara, CA,
USA), which contained 18,853 mRNA probes per array product. Genes
with fold changes >2 and P<0.05 were identified as
differentially expressed genes, of which 15 were identified.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from tissues or cells with
the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The PrimeScript™ RT kit
(cat. no. DRR037A; Takara Biotechnology Co., Ltd., Dalian, China)
was used to synthesize the cDNA using 1 µg total RNA,
according to the manufacturer's protocol. All primer sequences
(sense/antisense) were as follows: MYB:
5′-GCCAATTATCTCCCGAATCGA-3′/5′-ACCAACGTTTCGGACCGTA-3′; CDH1:
5′-GACCGGTGCAATCTTCAAA-3′/5′-TTGACGCCGAGAGCTACAC-3′;
vimentin: 5′-GACGCCATCAACACCGAGTT-3′/5′-CTTTGTCGTTGG
TTAGCTGGT-3′; G1/S-specific cyclin-D1 (encoded by CCND1):
5′-GATCAAGTGTGACCCGGACT-3′/5′-TCC TCCTCTTCCTCCTCCTC-3′;
cyclin-dependent kinase inhibitor 1 (encoded by p21):
5′-TGCCCAAGCTCTACCTTCC-3′/5′-CAGGTCCACATGGTCTTCCT-3′; induced
myeloid leukemia cell differentiation protein Mcl-1 (encoded by
MCL1):
5′-TGCATCGAACCATTAGCAGA-3′/5′-TCCTGATGCCACCTTCTAGG-3′;
intercellular adhesion molecule 1 (encoded by ICAM1):
5′-CACAGTCACCTATGGCAACG-3′/5′-GTGTCTCCTGGCTCTGGTTC-3′; vascular
endo-thelial growth factor A (VEGF, encoded by VEGFA):
5′-CTTGCCTTGCTGCTCTACCT-3′/5′-AGCTGCGCTGATAGACA TCC-3′; matrix
metalloproteinase-7 (MMP-7, encoded by MMP7):
5′-GTCTCGGAGGAGATGCTCAC-3′/5′-GGAATG TCCCATACCCAAAG-3′; MMP9:
5′-GCCTGGCACATAG TAGGCCC-3′/5′-CTTCCTAGCCAGCCGGCATC-3′; and
GAPDH: 5′-CCATGGAGAAGGCTGGG-3′/5′-CAAAGTTG TCATGGATGACC-3′.
Quantification of mRNA expression was performed with the FastStart
Universal SYBR Green Master (ROX) reagent (Roche Diagnostics,
Basel, Switzerland) using the ABI 7500 Sequence Detection System
(Thermo Fisher Scientific, Inc.). The amplification conditions were
as follows: 50°C for 2 min, 95°C for 10 min, and 40 cycles at 95°C
for 15 sec and 60°C for 1 min, followed by a dissociation curve
stage to check amplification specificity. The mRNA expression
levels of the target genes were normalized to GAPDH and
calculated using the ΔCT and ΔΔCT methods (27).
Cell lines and transfection
The SACC-83 cell line originated from the ACC tissue
of a 26-year-old female patient's sublingual gland in November 1983
(28). The SACC-LM cell line
exhibited enhanced lung metastatic behaviour and were isolated
in vivo after injecting SACC-83 cells into the tail veins of
immunodeficient mice (21-23). The SACC-83 and SACC-LM cell lines
were collected by SLL and kept at Peking University School and
Hospital of Stomatology. The pSMG cells were derived from a
4-year-old boy patient's sublingual gland in November 2016
(29). The pSMG cell line was
collected by ZHD and kept at Peking University School and Hospital
of Stomatology. SACC-83 and SACC-LM cells were maintained in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS;
both Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified
atmosphere containing 5% CO2. The pSMG cells were
cultured with DMEM/F12 (1:1 mixture; Gibco; Thermo Fisher
Scientific, Inc.) containing 2.5% FBS, trace element mix (Gibco;
Thermo Fisher Scientific, Inc.), 20 nM sodium selenite, 5
µg/ml transferrin (Gibco; Thermo Fisher Scientific, Inc.),
1.1 µM hydrocortisone, 0.1 µM retinoic acid, 2.0 nM
T3, 8.4 ng/ml cholera toxin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), 5 µg/ml insulin, 80 ng/ml epidermal growth factor
(both Gibco; Thermo Fisher Scientific, Inc.), extra glutamine
(final concentration, 5 mM), 50 µg/ml gentamicin sulfate and
1 µg/ml amphotericin B (Gibco; Thermo Fisher Scientific,
Inc.).
The lentiviral vector pHBLV-CMV-GFP-T2A-puro, empty
(negative control) lentiviral vector and auxiliary transfection
reagent, Polybrene, were purchased from Shanghai GenePharma Co.,
Ltd. (Shanghai, China). The MYB overexpressing lentiviral
vector or empty lentiviral vector with a virus titre of
1×108 TU/ml were transfected into SACC-83 cells. The
multiplicity of infection was 50. After 72 h of transfection,
SACC-83 cells were incubated in RPMI-1640 medium containing 3
µg/ml puromycin for 72 h to select cells that had been
successfully transfected, and then the cells were cultured in
RPMI-1640 medium containing 1.5 µg/ml puromycin for 2 weeks
to obtain MYB overexpressing (MYB OE) or negative control
(NC) SACC-83 cells. The GFP-positive cells were sorted using BD
FACS Aria II (BD Bioscience, San Jose, CA, USA) and cultured in
RPMI-1640 medium supplemented with 10% FBS at 37°C in a humidified
atmosphere containing 5% CO2. Monoclonal cell lines that
stably overexpressed MYB (M1, M2 and M3 cells) and
monoclonal cell lines successfully transfected with the empty
lentiviral vector (Vector1, Vector2 and Vector3 cells) were
obtained. Untransfected SACC-83 cells served as control (BLK)
cells. Cell morphology under bright field and fluorescence was
captured using an Eclipse TE2000-U fluorescence microscope (Nikon
Corporation, Tokyo, Japan).
To knockdown MYB expression, SACC-83 and
SACC-LM cells were transfected with 50 nM small interfering
(si)RNAs specific for MYB (siMYB) or negative control siRNAs
(siNC; Guangzhou RiboBio Co., Ltd., Guangzhou, China) for 24 h
using riboFECT™ CP Transfection kit (cat. no. C10511-1; Guangzhou
RiboBio Co., Ltd.) according to the manufacturer's protocol. The
MYB siRNA sequences are as follows: 5′-GGT
CGAACAGGAAGGTTAT-3′ (siMYB-1) and 5′-CAACCGA GAATGAGCTAAA-3′
(siMYB-2). The NC siRNA sequence is 5′-TTTCTCCGAACGTGTCACG-3′
(siNC).
Experimental animals
A total of 13 female non-obese diabetic/severe
combined immunodeficiency (NOD/SCID) mice (5-6 weeks old) were
purchased from Beijing Vital River Laboratory Animal Technology
Co., Ltd. (Beijing, China). All animal experiments complied with
the ARRIVE guidelines, and were conducted in accordance with the UK
Animals (Scientific Procedures) Act, 1986 and associated guidelines
as well as the EU Directive 2010/63/EU for animal experiments
(30-32). These experiments were approved by
the Peking University Institutional Animal Care and Use Committee
(permit no. LA2015099).
Western blot analysis
SACC-83, NC, MYB OE, siNC, siMYB-1 and siMYB-2 cells
were seeded in 100 mm dishes at 2×106 cells/dish and
incubated in RPMI-1640 medium supplemented with 10% FBS at 37°C in
a humidified atmosphere containing 5% CO2 for 48 h.
Cells were harvested, washed with ice-cold PBS and dissolved in
ice-cold radioimmunoprecipitation assay buffer (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) containing a
protease inhibitor cocktail (Applygen Technologies, Inc., Beijing,
China). The protein concentration was measured with a Pierce™
Bicinchoninic Acid Protein Assay kit (Thermo Fisher Scientific,
Inc.), following the manufacturer's protocol. The protein extracts
(40 µg/lane) derived from each sample were separated by
SDS-PAGE on 10-15% gels and electroblotted onto polyvinylidene
fluoride membranes. The membranes were blocked with 5% non-fat milk
in TBST (20 mM Tris, 137 mM NaCl and 0.1% Tween-20, pH 7.4) for 1 h
at room temperature and then incubated with the following primary
antibodies: Anti-MYB (cat. no. ab45150; Abcam, Cambridge, MA, USA),
anti-E-cadherin (cat. no. 3195), anti-N-cadherin (cat. no. 13116),
anti-vimentin (cat. no. 5741), anti-actin, aortic smooth muscle
(α-SMA, encoded by ACTA2; cat. no. 19245), anti-β-catenin (cat. no.
8480; all Cell Signaling Technology, Inc., Danvers, MA, USA) and
anti-β-actin (cat. no. ZM0001; OriGene Technologies, Inc., Beijing,
China). Primary antibodies (1:1,000) were incubated for at least 1
h at 4°C. Then, the membrane was washed extensively with TBST and
incubated with secondary antibodies (1:10,000) for 1 h at room
temperature. Horseradish peroxidase-conjugated goat anti-rabbit
(cat. no. ZB-2301) or anti-mouse (cat. no. ZB-2305; both OriGene
Technologies, Inc.) IgG antibodies were used as the secondary
antibodies. The immunocomplexes were detected using SuperEnhanced
chemiluminescence detection kit (cat. no. P1030-100; Applygen
Technologies, Inc.). β-actin was used as the internal control. All
bands were quantified using Adobe Photoshop CS5 Extended 12.0.3
×32, which was purchased from Adobe Systems Incorporated (San Jose,
CA, USA). The MYB bands of SACC cells used in this experiment
exhibited two bands; the two bands were counted in all the
statistics. Three independent experiments with three biological
replicates each were performed.
Cell proliferation assay
Cell proliferation was assessed by counting viable
cells with Cell Counting Kit-8 (CCK8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan). SACC-83, NC, MYB OE, Vector3,
M1, M2, M3 cells and siMYB-1-, siMYB-2- and control
siRNA-transfected cells were seeded into 96-well plates at a
density of 3,000 cells/well in 100 µl RPMI-1640 medium. At
24, 48 and 72 h, the absorbance of each well was measured at 450 nm
using the ELx808 Absorbance Microplate Reader (BioTek Instruments,
Inc., Winooski, VT, USA). For SACC-83, NC, MYB OE, Vector3, M1, M2,
M3 cells, cell proliferation was compared by calculating the
relative cell number because the initial density of the seeded
cells was difficult to control. Relative cell number = optical
density (OD) 450 value per time point / OD 450 values at 0 h. The
data presented are representative of one of three replicates.
In vitro wound closure assay
After 12 h of FBS deprivation, confluent cell
monolayers of BLK, Vector3, M1, M2 and M3 cells were scratched with
a 200-µl pipette tip to create wounded areas with widths of
400-600 µm. Images were captured 0 and 1 h after scratching
under an Eclipse TE2000-U fluorescence microscope (Nikon
Corporation). The migrated distance was quantified by Integrated
Performance Primitives software (version 6.0.0.260; Intel
Corporation, Santa Clara, CA, USA). Three independent experiments
were performed with three biological replicates each.
Transwell migration and invasion
assays
To analyse Transwell migration and invasion, cell
inserts (8.0-µm pore size; EMD Millipore, Billerica, MA,
USA) were coated with (invasion) or without (migration) Matrigel
(BD Bioscience). SACC-83, NC, MYB OE, Vector1, Vector2, Vector3,
M1, M2 and M3 cell or siMYB-1-, siMYB-2- and siNC-transfected
SACC-83 and SACC-LM cells were seeded at a density of
5×104 cells/well in RPMI-1640 medium without serum in
the upper chamber. The lower chamber contained RPMI-1640 medium
supplemented with 10% FBS. After 16 h of incubation, the cells on
the upper surface of the insert were wiped off. The cells on the
lower surface of the insert were fixed with 95% ethanol for 30 min
at room temperature, stained with 1% crystal violet for 40 min at
room temperature and counted under a BX51 fluorescence microscope
(Olympus Corporation, Tokyo, Japan) at a magnification of ×20.
Every experiment was repeated independently at least three
times.
Mouse lung metastasis model
NOD/SCID mice were injected in the tail vein with
4×106 MYB OE (n=7) or NC cells (n=6). After 8 weeks, the
mice were sacrificed and their lungs were collected. After the
lungs were fixed with 4% paraformaldehyde for 24 h at room
temperature, the lungs was embedded in paraffin and then cut into
4-µm-thick sections. The sections were used for hematoxylin
and eosin staining.
Briefly, the sections were deparaffinized and
rehydrated at 37°C with xylene I, xylene II and xylene III for 20
min each; then absolute ethanol I and absolute ethanol II for 10
min each; and finally 95, 80 and 70% ethanol for 10, 4 and 2 min,
respectively. The sections were wash with deionized water three
times for 3 min each time, then stained with hematoxylin for 2-3
min at room temperature. Next, differentiation was performed with
hydrochloric acid alcohol for 1 sec, and then the sections were
rinsed with deionized water for 10 min and stained with eosin for 8
min at room temperature. Gradient dehydration was performed at room
temperature with 70, 80, 90, 95 and 100% ethanol for 2, 2, 3, 3 and
10 min, respectively, then with xylene I, xylene II and xylene III
for 10 min each. The sections were sealed with neutral gum and
placed in a ventilated room at room temperature overnight. The
stained sections were evaluated by an experienced pathologist from
the Department of Oral Pathology, Peking University School and
Hospital of Stomatology.
Statistical analysis
All statistical analyses were conducted with SPSS
(version 19.0; IBM Corp., Armonk, NY, USA). All in vitro
experiments were performed at least three times and numerical data
are presented as mean ± standard deviation of three independent
experiments. P-values were calculated by a two-tailed unpaired
Student's t-test or one-way analysis of variance with Bonferroni
post-test correction. The results were confirmed in at least three
independent experiments. P<0.05 indicated that the difference
between groups was statistically significant. The Pearson
correlation coefficient was calculated to evaluate the correlation
of MYB with E-cadherin or vimentin.
Results
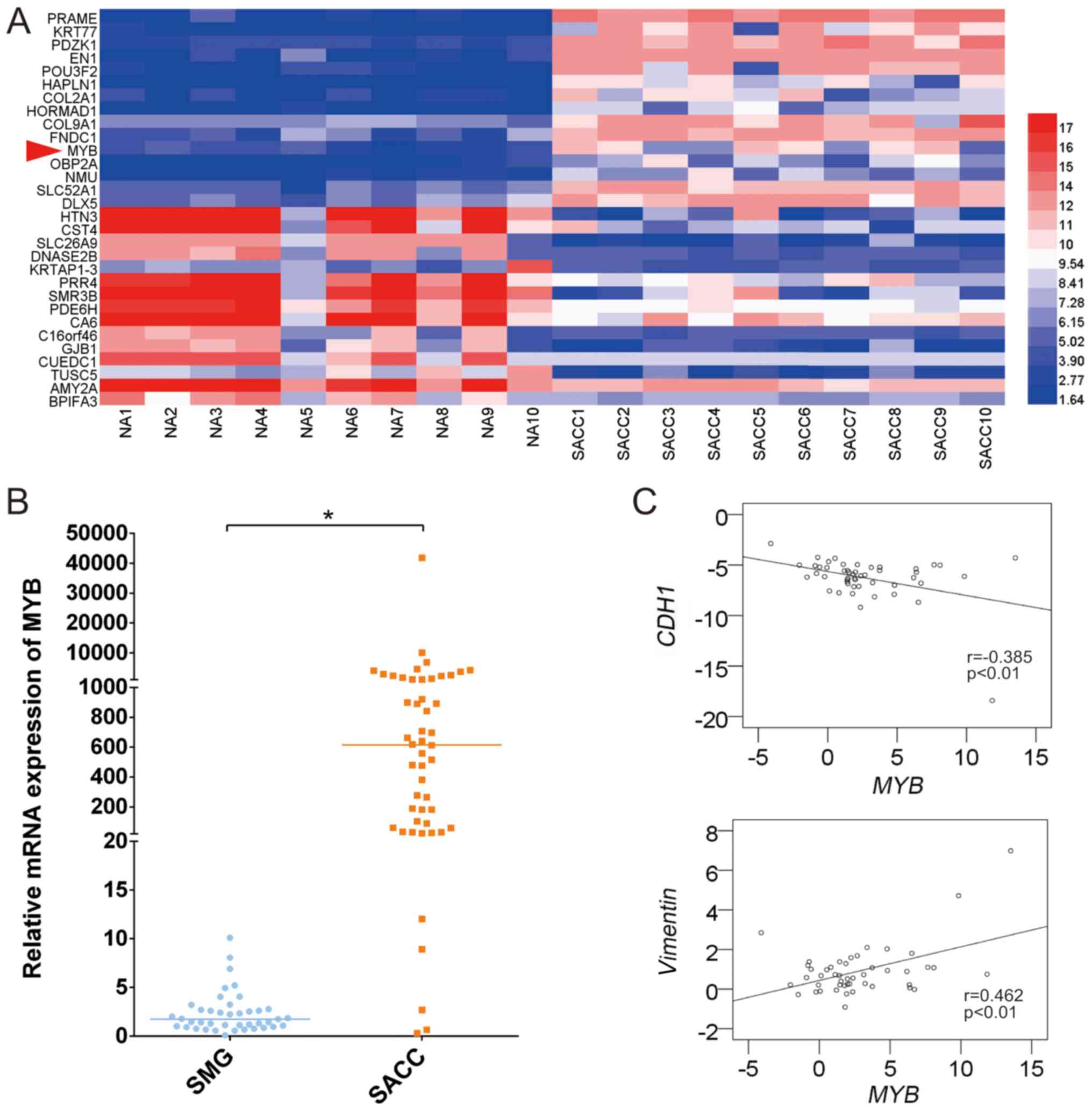
MYB is overexpressed in SACC tissue
samples and associated with the potential for metastasis in
clinical cases
As presented in Fig.
1A, MYB was 11th among the top 15 upregulated mRNAs in
the 10 human SACC and paired SMG tissues in the microarray
analysis. The mRNA expression levels of MYB and
EMT-associated markers were also measured in 50 fresh frozen SACC
tissues and 41 normal fresh frozen SMG tissues using RT-qPCR.
MYB expression was significantly higher in the SACC tissues
compared with the normal SMG tissues, with a high expression rate
of 90% (Fig. 1B). The
clinicopathological features of the SACC patients are stratified in
Table I. A total of 50 SACC
patients were classified into the high or low MYB group
depending on the median MYB level. High MYB
expression was correlated with the pathological type, lung
metastasis and clinical stage. Although high MYB expression
did not significantly correlate with tumour size, the P-value was
close to the threshold. No correlation was identified between
MYB expression and the other parameters, including age, sex,
site, lymph node metastasis, perineural invasion and local regional
recurrence.
CDH1 and vimentin expression was also
measured by RT-qPCR in these SACC tissues, and the correlation of
MYB with CDH1 and vimentin was analysed. MYB
tended to be significantly and negatively correlated with CDH1, and
significantly and positively correlated with vimentin
(Fig. 1C).
MYB is overexpressed in SACC cells and
modulates cell proliferation in vitro
To explore the biological role of MYB in SACC
cells, MYB expression was measured in SACC-83 cells, primary
cells derived from a human SMG tissue (pSMG cells) and a subset of
SACC cells that exhibited high lung metastasis (SACC-LM cells).
MYB mRNA expression levels were significantly higher and
MYB protein expression levels were markedly higher in the
SACC-83 and SACC-LM cells compared with in the pSMG cells (Fig. 2A). MYB mRNA expression
levels were significantly higher and MYB protein expression
levels were markedly higher in the SACC-LM cells compared with the
SACC-83 cells, indicating that MYB is associated with SACC
oncogenesis and metastasis.
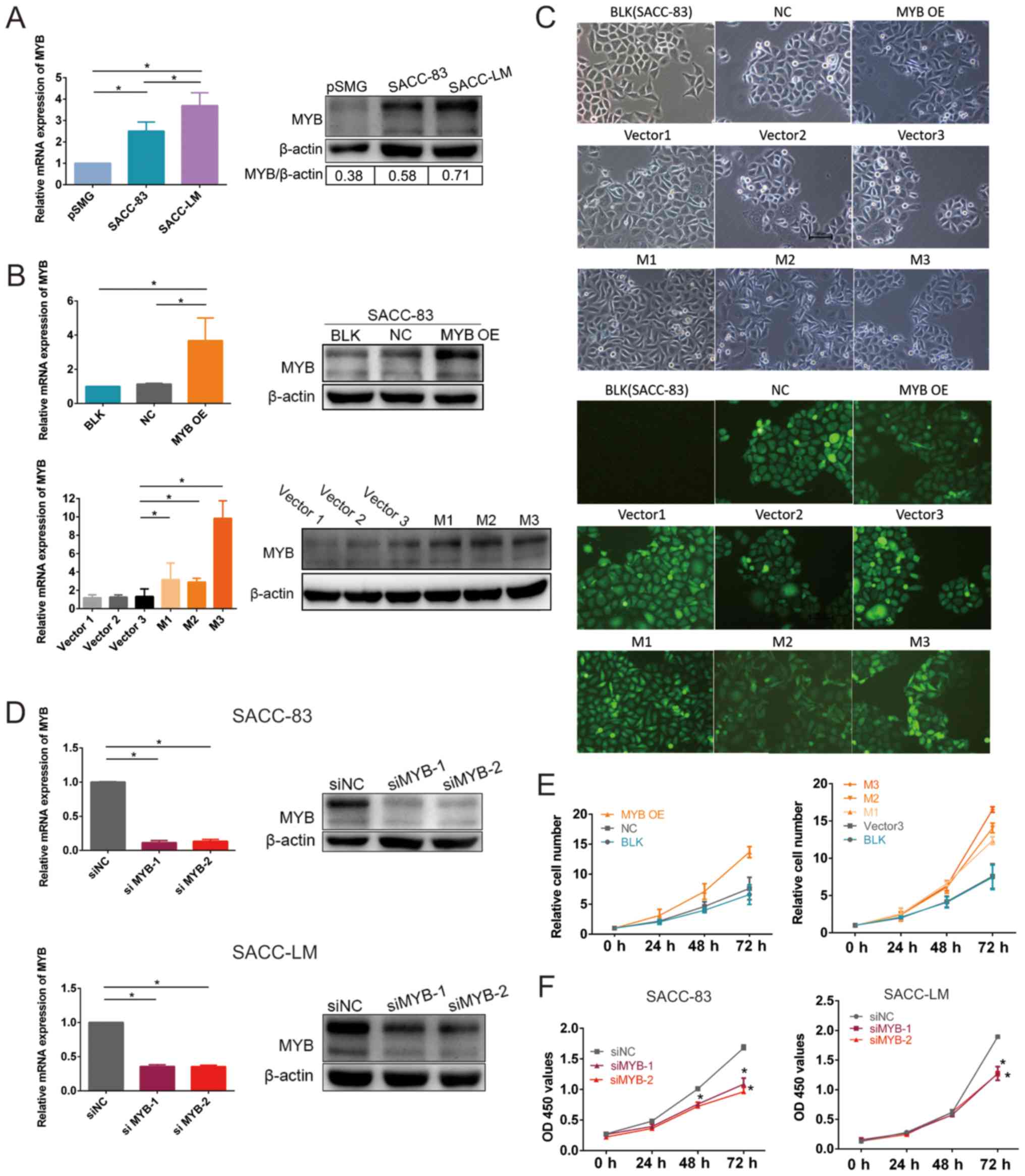 | Figure 2MYB is overexpressed in SACC cells,
which promotes SACC cell proliferation, and its knockdown inhibits
SACC cell proliferation. (A) RT-qPCR analysis of MYB mRNA
expression and western blot analysis of MYB protein expression in
pSMG, SACC-83 and SACC-LM cells (*P<0.05 as defined).
(B) RT-qPCR analysis of MYB mRNA expression and western blot
analysis of MYB protein expression in transfected SACC-83 cells
(*P<0.05 as defined). (C) Fluorescence microscopy in
transfected SACC-83 and BLK cells. (D) RT-qPCR analysis of
MYB mRNA expression and western blot analysis of MYB protein
expression in siNC-, siMYC-1- and siMYC-2-transfected SACC-83 and
SACC-LM cells (*P<0.05 as defined). (E) Cell
proliferation of the transfected SACC-83 cells, including BLK, NC,
MYB OE, Vector3, M1, M2 and M3 cells. Cell proliferation was
assessed using the Cell Counting Kit-8 assay and compared by
calculating the relative cell number. Relative cell number = OD 450
values per time point / OD 450 values of 0 h. (F) Proliferation of
siNC-, siMYC-1- and siMYC-2-transfected SACC-83 and SACC-LM cells
was assessed using the Cell Counting Kit-8 assay. Data are
presented as mean ± standard deviation of three independent
experiments (*P<0.05 vs. siNC). RT-qPCR, reverse
transcription-quantitative polymerase chain reaction analysis;
pSMG, primary cells derived from the human submandibular gland;
SACC, salivary adenoid cystic carcinoma; MYB,
transcriptional activator Myb gene; NC, cells transfected with an
empty vector; MYB OE, MYB overexpressing cells; M, cells
derived from MYB overexpressing cells; Vector, cells derived
from NC cells; si, small interfering RNA; siNC, cells transfected
with a negative control siRNA; siMYB, cells transfected with a
siRNA against MYB; BLK, untransfected cells; OD, optical
density. |
MYB mRNA expression levels were significantly
higher in the MYB OE cells compared with NC and BLK cells,
demonstrating that MYB overexpressing vector transfection
was stable (Fig. 2B). MYB
protein expression levels were markedly higher in the MYB OE cells
compared with NC and BLK cells. The MYB mRNA expression
levels were significantly higher in the M1, M2 and M3 cells
compared with the Vector3 cells, while protein expression levels
were markedly higher in the M1, M2 and M3 cells compared with the
Vector3 cells. Fibroblastic morphological changes of the cells
undergoing EMT were identified in MYB overexpressing cells.
Microscopic observation revealed the cobblestone-like structure of
BLK, NC, Vector1, Vector2 and Vector3 cells. MYB OE, M1, M2 and M3
cells exhibited varying degrees of fibroblastic changes (Fig. 2C). Approximately 90% of MYB OE, M1
and M2 cells and ~40% of M3 cells lost their original polygonal
phenotype and acquired a fibroblastic phenotype. MYB
expression was knocked down in the SACC-83 and SCC-LM cell lines
using siMYB-1 and siMYB-2 (Fig.
2D). The MYB mRNA expression levels were significantly
lower and MYB protein expression levels were markedly lower
in the siMYB-1 and siMYB-2 cells compared with the siNC cells.
The proliferation ability of MYB OE, M1, M2 and M3
cells was markedly higher compared with control cells at 72 h
(Fig. 2E). The cell proliferation
assay demonstrated that SACC cell growth was inhibited in the two
cell lines (Fig. 2F). The
proliferation was significantly attenuated in siMYB-1 and siMYB-2
cells compared with the siNC cells. Together, these findings
support a growth-promoting role for MYB in SACC cells.
MYB promotes SACC cell migration and
invasion in vitro
To investigate the effect of MYB expression
on the aggressiveness of SACC cells, the effect of MYB on
cell migration and invasion was studied. The wound healing and
Transwell migration assays demonstrated that the migration of M1,
M2 and M3 cells was significantly higher compared with the Vector3
cells (Fig. 3A). Additionally, the
Transwell invasion assay demonstrated that the invasive ability of
the MYB OE cells was significantly greater compared with that of
the NC and BLK cells (Fig. 3B).
Similarly, the M1, M2 and M3 cells had significantly higher
invasive abilities compared with the Vector1, Vector2 and Vector3
cells, respectively.
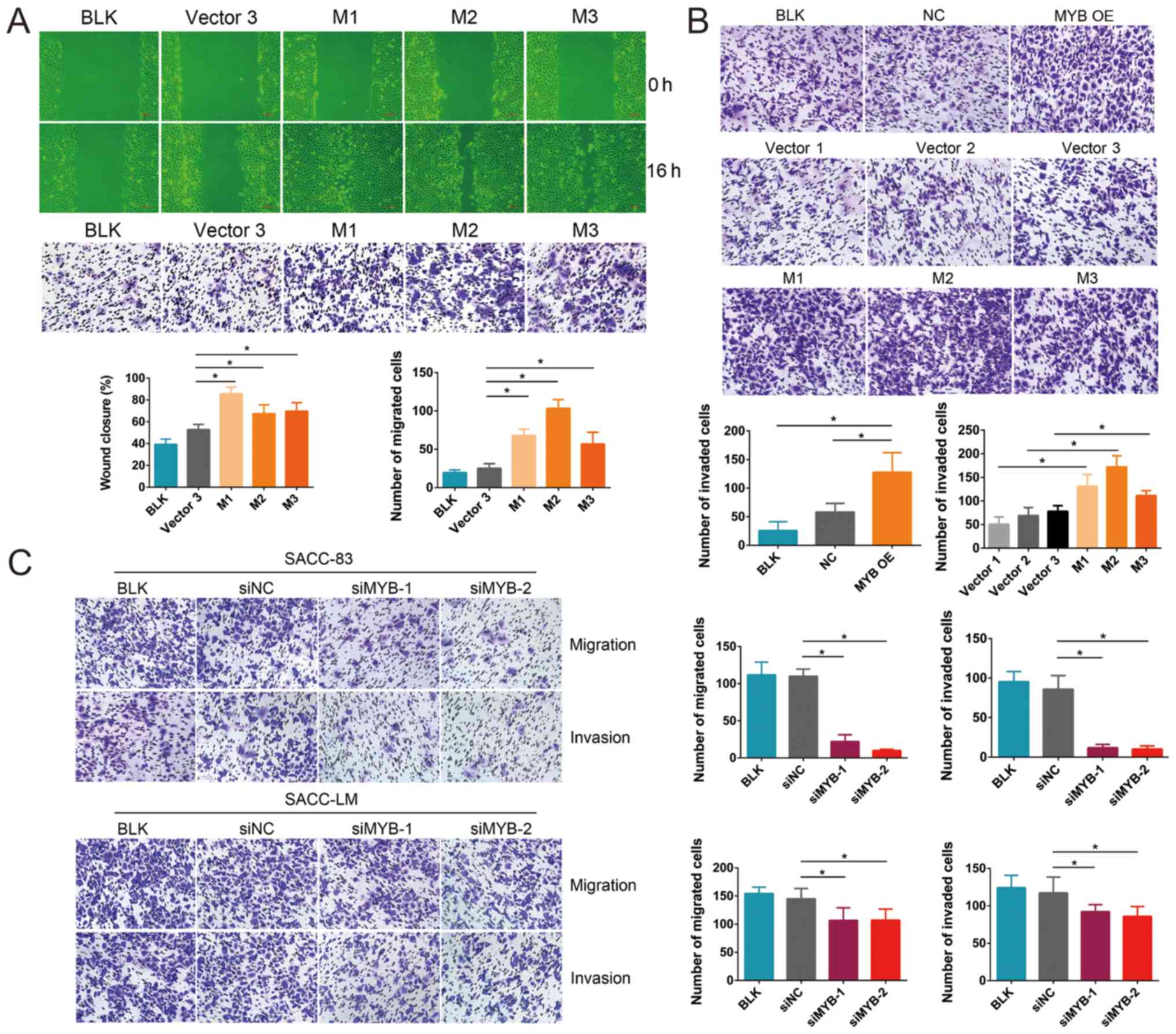 | Figure 3MYB overexpression promotes
and MYB knockdown inhibits the migration and invasion of
SACC cells. (A) Wound closure and Transwell migration assays of
M1-, M2-, M3- and Vector3-transfected, and BLK SACC-83 cells. (B)
Transwell invasion assay of transfected SACC-83 cells and BLK
SACC-83 cells. (C) Transwell migration and invasion assays of
siNC-, MYB-1- and MYB-2-transfected, and BLK SACC-83 cells. Data
are presented as mean ± standard deviation of three independent
experiments (*P<0.05). SACC, salivary adenoid cystic
carcinoma; MYB, transcriptional activator Myb gene; NC,
cells transfected with an empty vector; M, cells derived from
MYB overexpressing cells; Vector, cells derived from NC
cells; si, small interfering RNA; siNC, cells transfected with a
negative control siRNA; siMYB, cells transfected with a siRNA
against MYB; BLK, untransfected cells. |
In contrast, the knockdown of MYB by siMYB-1
and siMYB-2 in the SACC-83 and SACC-LM cells significantly
inhibited cell migration and invasion compared with the siNC cells
(Fig. 3C). These results indicated
that MYB overexpres-sion promoted SACC cell migration and
invasion, whereas MYB knockdown inhibited SACC cell
migration and invasion.
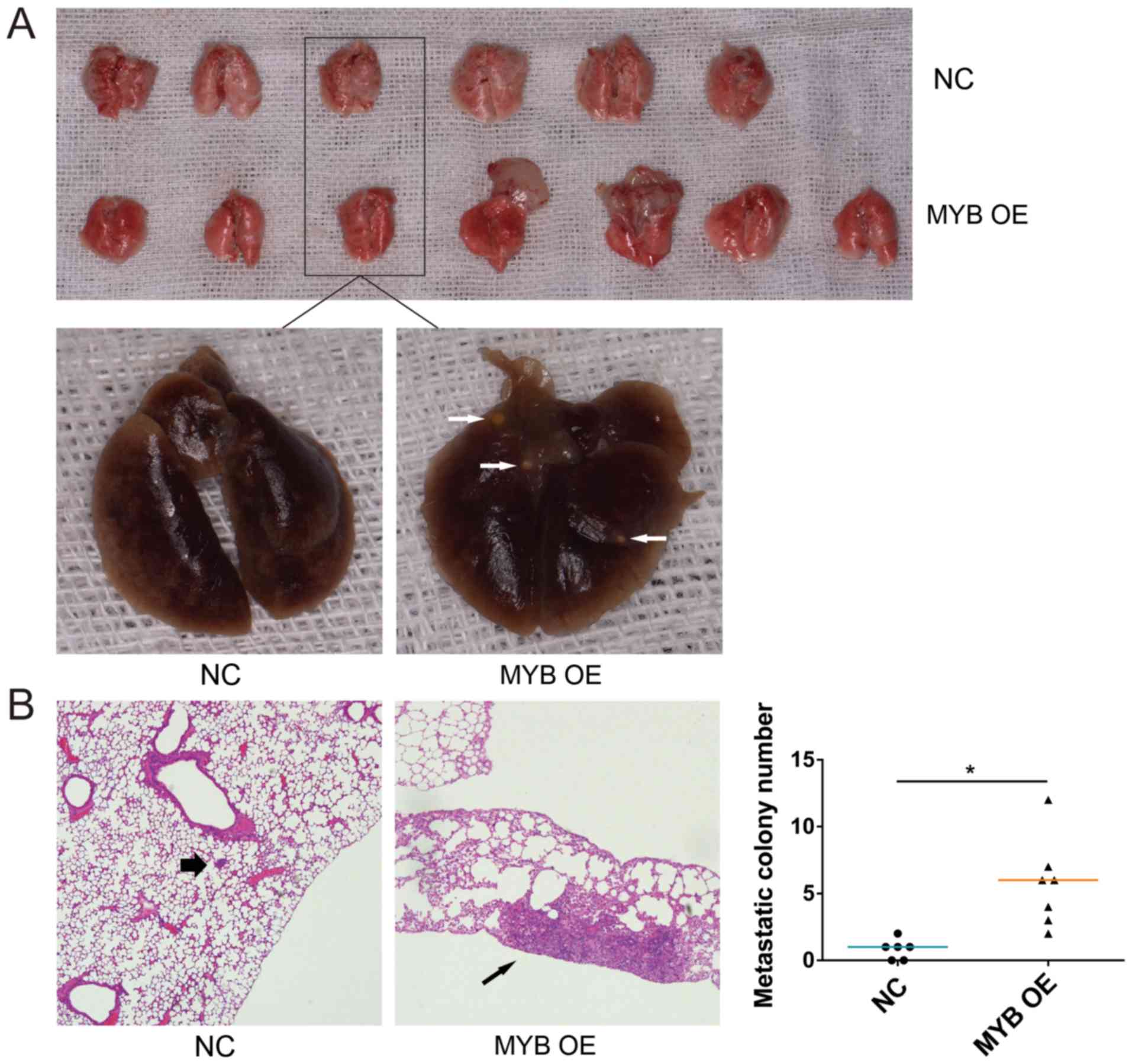
MYB promotes SACC lung metastasis in
vivo
To investigate the effect of MYB on SACC lung
metastasis, MYB OE and NC cells were injected into NOD/SCID mice
through the tail vein. The lung tissues were collected from all
mice after 8 weeks. The mice injected with MYB OE cells had visible
tumour nodules, which were not observed in the NC cell-injected
mice (Fig. 4A). The number of
tumour nodules in the lungs of the mice injected with MYB OE cells
was significantly greater compared with that of the NC cells based
on hematoxylin and eosin staining of the lung tissues (Fig. 4B). These results indicated that MYB
promoted SACC lung metastasis in vivo.
MYB modulates the expression of genes
associated with proliferation and metastasis
To explore the molecular mechanism underlying
MYB-induced tumour promotion and metastasis, genes associated with
tumour progression and metastasis were examined in NC, MYB OE, siNC
and siMYB-1 cells by RT-qPCR. Significant changes were identified
in five of the 22 genes (data not shown). The expression of
CCND1 and MCL1, which are associated with cell
proliferation (33,34), was upregulated following MYB
overexpression, whereas p21 expression was downregulated
(Fig. 5A). ICAM1, VEGFA,
MMP7 and MMP9, which are involved in cell migration and
invasion (24,25,35,36),
were upregulated following MYB overexpression. In addition,
CCND1, MCL1, ICAM1, VEGFA, MMP7 and MMP9 were
downregulated, whereas p21 was upregulated following MYB
knockdown (Fig. 5B). Taken
together, these findings indicate that MYB promotes the
growth, migration and invasion of SACC cells by regulating the
expression of genes involved in proliferation and metastasis.
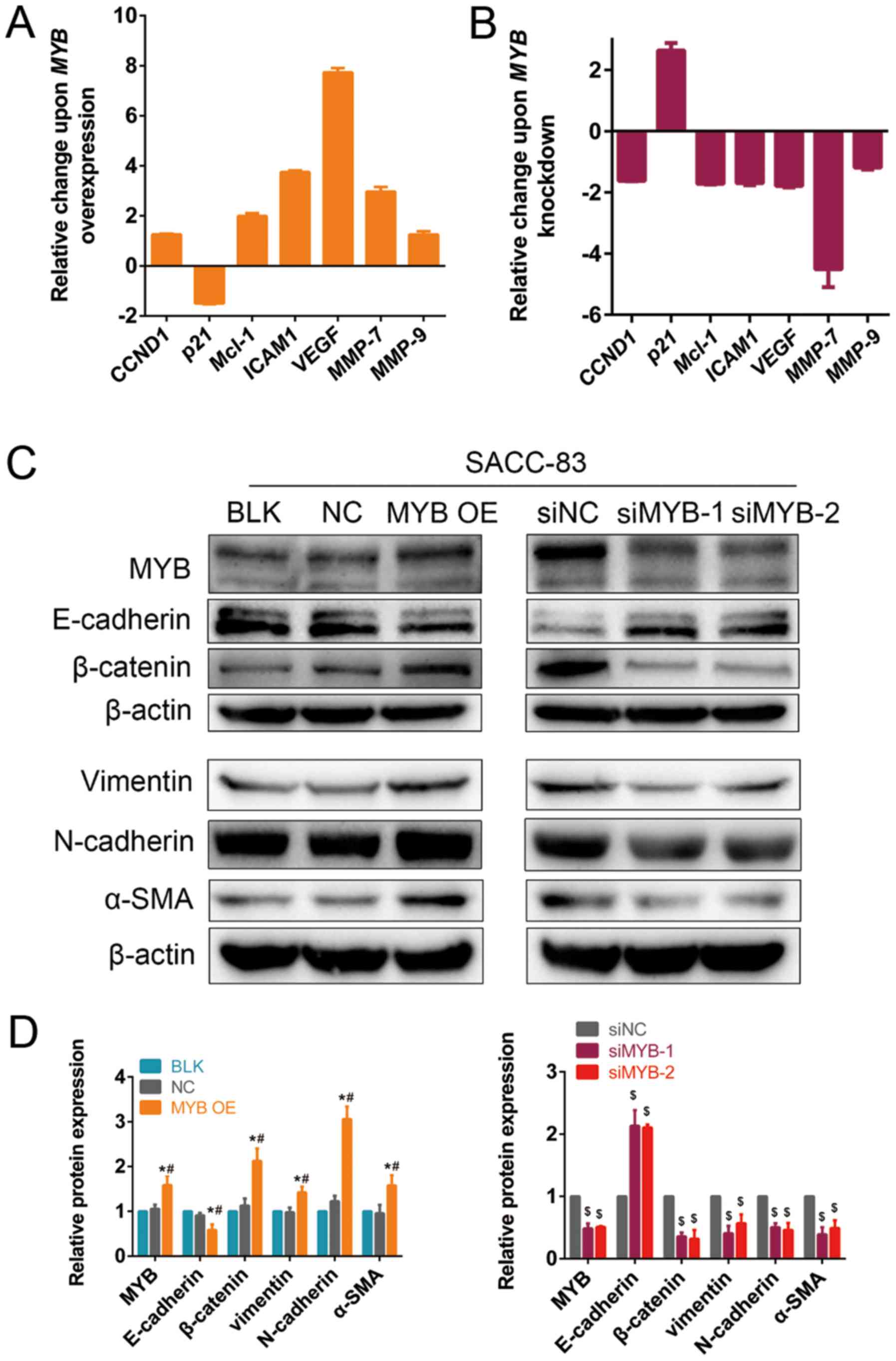 | Figure 5MYB modulates the expression of genes
associated with proliferation and metastasis. Quantification of
RT-qPCR analysis expression following MYB (A) overexpression
and (B) knockdown in SACC-83 cells. (C) Western blotting of
transfected and BLK SACC-83 cells. (D) Quantification of protein
bands in transfected and BLK SACC-83 cells. Data are presented as
mean ± standard deviation of three independent experiments
(*P<0.05 vs. NC; #P<0.05 vs. BLK;
$P<0.05 vs. siNC). SACC, salivary adenoid cystic
carcinoma; MYB, transcriptional activator Myb gene; MYB,
transcriptional activator Myb protein; NC, cells transfected with
an empty vector; si, small interfering RNA; siNC, cells transfected
with a negative control siRNA; siMYB, cells transfected with a
siRNA against MYB; BLK, untransfected cells; CCND1,
G1/S-specific cyclin-D1 gene; p21, cyclin-dependent kinase
inhibitor 1 gene; MCL1, myeloid leukemia cell
differentiation protein Mcl-1 gene; ICAM1, intercellular
adhesion molecule 1 gene; VEGFA, vascular endothelial growth
factor A gene; MMP, matrix metal-loproteinase gene;
E-cadherin, cadherin-1; β-catenin, catenin β-1; N-cadherin,
cadherin-2; α-SMA, actin, aortic smooth muscle. |
MYB regulates EMT in SACC cells
As mentioned previously, MYB tends to be negatively
correlated with the epithelial marker, E-cadherin, and positively
correlated with the mesenchymal marker, vimentin. The expression of
E-cadherin in the MYB OE group was significantly downregulated
following MYB overexpression compared with the BLK and NC
groups, whereas N-cadherin, vimentin and α-SMA expression was
significantly upregulated (Fig. 5C and
D). β-catenin, which is the key molecule of the canonical Wnt
signalling pathway (18), was also
significantly upregulated following MYB overexpression
compared with the BLK and NC groups, suggesting that MYB may
regulate EMT via the Wnt signalling pathway. Additionally,
E-cadherin was significantly upregulated, and N-cadherin, vimentin,
α-SMA and β-catenin were significantly downregulated in the
siMYB-1- and siMYB-1-transfected cells compared with the siNC
group.
Discussion
In the current study, MYB expression was
measured in 50 fresh frozen SACC tissues and 41 fresh frozen SMG
tissues, and the clinicopathological features of the SACC patients
were stratified. In particular, it was determined that MYB was
closely associated with lung metastasis and was associated with the
pathological tumour type in patients with SACC (14,18).
MYB expression was significantly greater in SACC tissues
compared with normal SMG tissues, with an expression rate of 90%.
This result was consistent with that of previous studies (12,14),
but the proportion of MYB overexpression seemed to be higher
in the current study. These results evidently suggest that MYB
serves an important role in SACC.
To explore the effect of MYB in SACC, overexpression
and knockdown of MYB in SACC cells was performed and cell
proliferation was explored using CCK8. The results demonstrated
that upregulation of MYB increased the relative number of
cells, which was consistent with previous findings in prostate and
pancreatic cancer (37,38). Knockdown of MYB
significantly inhibited cell proliferation, which was similar to
findings for acute myeloid leukaemia, chronic myeloid leukaemia and
colon cancer (39,40). Mechanistically, previous studies
demonstrated that MYB induced the growth of tumour cells
through the promotion of cell cycle progression, which was
associated with cell proliferation (41,42).
It has been suggested that MYB is capable of regulating various
genes responsible for tumorigenesis in multiple cancers (17,35,43,44).
Using RT-qPCR, the authors of the current study revealed that MYB
may improve cell proliferation by enhancing cell cycle progression
through CCND1 upregulation and p21 downregulation.
Additionally, MYB may decrease apoptosis by upregulating
MCL1 expression, which encodes proteins belonging to the
Bcl-2 family (34).
Apart from its effect on tumour cell growth, MYB
also has been revealed to promote a variety of phenotypes
associated with the migration and invasion of cancer cells
(18,41,42,45,46).
Similarly, in the current study, MYB significantly enhanced SACC
cell migration and invasion in in vivo and in vitro
experiments. Previous studies demonstrated that VEGF promoted
metastasis via angiogenesis (24,25,47).
The current study demonstrated that VEGFA was upregulated in
MYB overexpressing SACC cells, indicating that MYB could
increase the metastasis of SACC cells by increasing VEGFA
expression. In addition, MYB was demonstrated to increase the
ICAM1 level, which encodes intercellular adhesion molecules
that can promote tumour metastasis (48). MMP7 and MMP9 are
involved in angiogenesis and tumor metastasis (45,49).
The current study revealed that MMP-7 and MMP-9 were upregulated in
MYB overexpressing SACC cells, indicating that MYB could
increase the metastasis of SACC cells by increasing MMP7 and
MMP9 expression. However, the corresponding protein levels
of these RNAs were not assessed in the current study, which is a
limitation.
Tumour metastasis depends on the occurrence of EMT,
including decreased expression of cell adhesion molecules, such as
E-cadherin, and increased expression of mesenchymal markers,
including N-cadherin and vimentin (22-24).
In SACC tissues, MYB tended to be negatively correlated with
CDH1 and positively correlated with vimentin.
Additionally, the authors revealed that, in SACC cells, MYB
upregulated EMT-associated markers, including vimentin, N-cadherin
and α-SMA, which was similar to the results of previous reports
investigating breast cancer (18,50).
These results indicated that MYB regulated SACC metastasis by
promoting the EMT.
In conclusion, MYB regulated
proliferation-associated molecules, including CCND1, p21 and
MCL1, in SACC cells to enhance cell proliferation. MYB also
regulated ICAM1, VEGFA, MMP7, MMP9 and EMT-associated
markers, including E-cadherin, vimentin, N-cadherin and α-SMA, in
SACC cells to increase metastasis. In addition, MYB promoted SACC
lung metastasis in a xenograft mouse model. Specifically, it was
determined that MYB was closely associated with lung metastasis and
the pathological tumour type in patients with SACC, which has
rarely been reported. The results of the current study suggest that
MYB may be an important therapeutic target for SACC.
Abbreviations:
|
MYB
|
transcriptional activator Myb
|
|
CCND1
|
the gene encoding G1/S-specific
cyclin-D1
|
|
ACC
|
adenoid cystic carcinoma
|
|
EMT
|
epithelial-mesenchymal transition
|
|
SMG
|
submandibular gland
|
|
p21
|
the gene encoding cyclin-dependent
kinase inhibitor 1
|
|
ICAM1
|
the gene encoding intercellular
adhesion molecule 1
|
|
VEGF
|
vascular endothelial growth factor
A
|
Funding
The current study was supported by the National
Natural Science Foundation of China (grant nos. 81272966 and
81272967).
Availability of data and materials
The datasets used and/or analysed during the
current study are available from the corresponding author upon
reasonable request.
Authors' contributions
LHX, XYG and SLL conceived and designed the
experiments. LHX and FZ performed the experiments and acquired the
data. LHX, WWY and CWC analysed and interpreted the data. LHX, ZHD
and MF performed the statistical analysis. LHX wrote the
manuscript. All authors edited the final manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The collection and use of human tissue samples was
approved by the Ethics Committee of Peking University School and
Hospital of Stomatology (Beijing, China; permit no.
PKUSSIRB-201522040). All animal experiments complied with the
ARRIVE guidelines and were performed in accordance with the U.K.
Animals (Scientific Procedures) Act, 1986 and associated guidelines
and the EU Directive 2010/63/EU for animal experiments. These
experiments were approved by the Peking University Institutional
Animal Care and Use Committee (Beijing, China; permit no.
LA2015099). Written informed consent was provided by all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Lei Chen from
the Department of Endocrinology (Peking University First Hospital,
Beijing, China) for proofreading the article.
References
|
1
|
Kokemueller H, Eckardt A, Brachvogel P and
Hausamen JE: Adenoid cystic carcinoma of the head and neck - a 20
years experience. Int J Oral Maxillofac Surg. 33:25–31. 2004.
View Article : Google Scholar
|
|
2
|
Gondivkar SM, Gadbail AR, Chole R and
Parikh RV: Adenoid cystic carcinoma: A rare clinical entity and
literature review. Oral Oncol. 47:231–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Weert S, Bloemena E, van der Waal I,
de Bree R, Rietveld DH, Kuik JD and Leemans CR: Adenoid cystic
carcinoma of the head and neck: A single-center analysis of 105
consecutive cases over a 30-year period. Oral Oncol. 49:824–829.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu J, Shao C, Tan ML, Mu D, Ferris RL and
Ha PK: Molecular biology of adenoid cystic carcinoma. Head Neck.
34:1665–1677. 2012. View Article : Google Scholar
|
|
5
|
Grimm M, Henopp T, Hoefert S, Schaefer F,
Kluba S, Krimmel M and Reinert S: Multiple osteolytic lesions of
intraosseous adenoid cystic carcinoma in the mandible mimicking
apical periodontitis. Int Endod J. 45:1156–1164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wal JE, Becking AG, Snow GB and van der
Waal I: Distant metastases of adenoid cystic carcinoma of the
salivary glands and the value of diagnostic examinations during
follow-up. Head Neck. 24:779–783. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang Y, Yu T, Fu X, Chen J, Liu Y, Li C,
Xia Y, Zhang Z and Li L: EGFR inhibition prevents in vitro tumor
growth of salivary adenoid cystic carcinoma. BMC Cell Biol.
14:132013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jaso J and Malhotra R: Adenoid cystic
carcinoma. Arch Pathol Lab Med. 135:511–515. 2011.PubMed/NCBI
|
|
9
|
Persson M, Andrén Y, Mark J, Horlings HM,
Persson F and Stenman G: Recurrent fusion of MYB and NFIB
transcription factor genes in carcinomas of the breast and head and
neck. Proc Natl Acad Sci USA. 106:18740–18744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brill LB II, Kanner WA, Fehr A, Andrén Y,
Moskaluk CA, Löning T, Stenman G and Frierson HF Jr: Analysis of
MYB expression and MYB-NFIB gene fusions in adenoid cystic
carcinoma and other salivary neoplasms. Mod Pathol. 24:1169–1176.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Persson M, Andrén Y, Moskaluk CA, Frierson
HF Jr, Cooke SL, Futreal PA, Kling T, Nelander S, Nordkvist A,
Persson F, et al: Clinically significant copy number alterations
and complex rearrangements of MYB and NFIB in head and neck adenoid
cystic carcinoma. Genes Chromosomes Cancer. 51:805–817. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitani Y, Li J, Rao PH, Zhao YJ, Bell D,
Lippman SM, Weber RS, Caulin C and El-Naggar AK: Comprehensive
analysis of the MYB-NFIB gene fusion in salivary adenoid cystic
carcinoma: Incidence, variability, and clinicopathologic
significance. Clin Cancer Res. 16:4722–4731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brayer KJ, Frerich CA, Kang H and Ness SA:
Recurrent fusions in MYB and MYBL1 define a common, transcription
factor-driven oncogenic pathway in salivary gland adenoid cystic
carcinoma. Cancer Discov. 6:176–187. 2016. View Article : Google Scholar :
|
|
14
|
West RB, Kong C, Clarke N, Gilks T,
Lipsick JS, Cao H, Kwok S, Montgomery KD, Varma S and Le QT: MYB
expression and translocation in adenoid cystic carcinomas and other
salivary gland tumors with clinicopathologic correlation. Am J Surg
Pathol. 35:92–99. 2011. View Article : Google Scholar :
|
|
15
|
Argyris PP, Wetzel SL, Greipp P, Wehrs RN,
Knutson DL, Kloft-Nelson SM, García JJ and Koutlas IG: Clinical
utility of myb rearrangement detection and p63/p40
immunophenotyping in the diagnosis of adenoid cystic carcinoma of
minor salivary glands: A pilot study. Oral Surg Oral Med Oral
Pathol Oral Radiol. 121:282–289. 2016. View Article : Google Scholar
|
|
16
|
Belloni E, Shing D, Tapinassi C, Viale A,
Mancuso P, Malazzi O, Gerbino E, Dall'Olio V, Egurbide I, Odero MD,
et al: In vivo expression of an aberrant MYB-GATA1 fusion induces
leukemia in the presence of GATA1 reduced levels. Leukemia.
25:733–736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramsay RG and Gonda TJ: MYB function in
normal and cancer cells. Nat Rev Cancer. 8:523–534. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Jin K, van Pelt GW, van Dam H, Yu X,
Mesker WE, Ten Dijke P, Zhou F and Zhang L: c-Myb enhances breast
cancer invasion and metastasis through the Wnt/β-catenin/Axin2
pathway. Cancer Res. 76:3364–3375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Costa AF, Altemani A, García-Inclán C,
Fresno F, Suárez C, Llorente JL and Hermsen M: Analysis of MYB
oncogene in transformed adenoid cystic carcinomas reveals distinct
pathways of tumor progression. Lab Invest. 94:692–702. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong L, Wang YX, Li SL, Yu GY, Gan YH, Li
D and Wang CY: TGF-beta1 promotes migration and invasion of
salivary adenoid cystic carcinoma. J Dent Res. 90:804–809. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gibbons DL and Creighton CJ: Pan-cancer
survey of epithelial-mesenchymal transition markers across the
Cancer Genome Atlas. Dev Dyn. 247:555–564. 2018. View Article : Google Scholar
|
|
22
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY,
Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, et al: Bmi1 is
essential in Twist1-induced epithelial-mesenchymal transition. Nat
Cell Biol. 12:982–992. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thiery JP, Acloque H, Huang RYJ and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang WW, Yang LQ, Zhao F, Chen CW, Xu LH,
Fu J, Li SL and Ge XY: Epiregulin promotes lung metastasis of
salivary adenoid cystic carcinoma. Theranostics. 7:3700–3714. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Levene A: International Histological
Classification of Tumours No. 7 Histological Typing of Salivary
Gland Tumours. J Clin Pathol. 26:8951973. View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(- Delta C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
28
|
Li SL: Establishment of a human cancer
cell line from adenoid cystic carcinoma of the minor salivary
gland. Zhonghua Kou Qiang Yi Xue Za Zhi. 25:29–31. 1990.In
Chinese.
|
|
29
|
Du ZH, Li SL, Ge XY, Yu GY and Ding C:
Comparison of the secretory related molecules expression in stem
cells from the pulp of human exfoliated deciduous teeth and dental
pulp stem cells. Zhonghua Kou Qiang Yi Xue Za Zhi. 53:741–747.
2018.In Chinese. PubMed/NCBI
|
|
30
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. Osteoarthritis
Cartilage. 20:256–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hollands C: The Animals (scientific
procedures) Act 1986. Lancet. 2:32–33. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grignaschi G, Redaelli V, Luzi F and
Fornasier M: The bodies in charge of animal welfare: What they do
and what they could do? Front Physiol. 9:3912018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ramos-García P, Gil-Montoya JA, Scully C,
Ayén A, González-Ruiz L, Navarro-Triviño FJ and González-Moles MA:
An update on the implications of cyclin D1 in oral carcinogenesis.
Oral Dis. 23:897–912. 2017. View Article : Google Scholar
|
|
34
|
Xiang W, Yang CY and Bai L: MCL-1
inhibition in cancer treatment. OncoTargets Ther. 11:7301–7314.
2018. View Article : Google Scholar
|
|
35
|
Quintana AM, Liu F, O'Rourke JP and Ness
SA: Identification and regulation of c-Myb target genes in MCF-7
cells. BMC Cancer. 11:302011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang H: Matrix metalloproteinase-9
(MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent
advances. Sensors (Basel). 18:182018. View Article : Google Scholar
|
|
37
|
Srivastava SK, Bhardwaj A, Arora S, Singh
S, Azim S, Tyagi N, Carter JE, Wang B and Singh AP: MYB is a novel
regulator of pancreatic tumour growth and metastasis. Br J Cancer.
113:1694–1703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Srivastava SK, Bhardwaj A, Singh S, Arora
S, McClellan S, Grizzle We, Reed E and Singh AP: Myb overexpression
overrides androgen depletion-induced cell cycle arrest and
apoptosis in prostate cancer cells, and confers aggressive
malignant traits: Potential role in castration resistance.
Carcinogenesis. 33:1149–1157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pattabiraman DR and Gonda TJ: Role and
potential for therapeutic targeting of MYB in leukemia. Leukemia.
27:269–277. 2013. View Article : Google Scholar
|
|
40
|
Cheasley D, Pereira L, Sampurno S, Sieber
O, Jorissen R, Xu H, Germann M, Yuqian Y, Ramsay RG and Malaterre
J: Defective Myb function ablates cyclin E1 expression and perturbs
intestinal carcinogenesis. Mol Cancer Res. 13:1185–1196. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ku DH, Wen SC, Engelhard A, Nicolaides NC,
Lipson KE, Marino TA and Calabretta B: c-myb transactivates cdc2
expression via Myb binding sites in the 5′-flanking region of the
human cdc2 gene. J Biol Chem. 268:2255–2259. 1993.PubMed/NCBI
|
|
42
|
Nakata Y, Shetzline S, Sakashita C, Kalota
A, Rallapalli R, Rudnick SI, Zhang Y, Emerson SG and Gewirtz AM:
c-Myb contributes to G2/M cell cycle transition in human
hematopoietic cells by direct regulation of cyclin B1 expression.
Mol Cell Biol. 27:2048–2058. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu F, Lei W, O'Rourke JP and Ness SA:
Oncogenic mutations cause dramatic, qualitative changes in the
transcriptional activity of c-Myb. Oncogene. 25:795–805. 2006.
View Article : Google Scholar
|
|
44
|
Gravina GL, Mancini A, Marampon F,
Colapietro A, Delle Monache S, Sferra R, Vitale F, Richardson PJ,
Patient L, Burbidge S, et al: The brain-penetrating CXCR4
antagonist, PRX177561, increases the antitumor effects of
bevacizumab and sunitinib in preclinical models of human
glioblastoma. J Hematol Oncol. 10:52017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Knopfová L, Beneš P, Pekarčíková L,
Hermanová M, Masařík M, Pernicová Z, Souček K and Smarda J: c-Myb
regulates matrix metalloproteinases 1/9, and cathepsin D:
Implications for matrix-dependent breast cancer cell invasion and
metastasis. Mol Cancer. 11:152012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen RX, Xia YH, Xue TC and Ye SL:
Transcription factor c-Myb promotes the invasion of hepatocellular
carcinoma cells via increasing osteopontin expression. J Exp Clin
Cancer Res. 29:1722010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Oka N, Soeda A, Inagaki A, Onodera M,
Maruyama H, Hara A, Kunisada T, Mori H and Iwama T: VEGF promotes
tumorigenesis and angiogenesis of human glioblastoma stem cells.
Biochem Biophys Res Commun. 360:553–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sluiter N, de Cuba E, Kwakman R, Kazemier
G, Meijer G and Te Velde EA: Adhesion molecules in peritoneal
dissemination: Function, prognostic relevance and therapeutic
options. Clin Exp Metastasis. 33:401–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lauer-Fields JL, Whitehead JK, Li S,
Hammer RP, Brew K and Fields GB: Selective modulation of matrix
metalloproteinase 9 (MMP-9) functions via exosite inhibition. J
Biol Chem. 283:20087–20095. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sala A: c-MYB and TGFβ: EMT's dynamic duo
in breast cancer. Cell Cycle. 11:172012. View Article : Google Scholar
|