Introduction
The most common cancer type worldwide in women is
breast cancer, with an estimated >2 million new breast cancer
cases diagnosed in 2018, worldwide (1). Triple negative breast cancer (TNBC)
is the most aggressive and invasive subtype, and account for ~20%
of all breast cancer cases. TNBCs lack expression of the estrogen
receptor (ER) and progesterone receptor (PR), and do not express
the human epidermal growth factor receptor-2 (HER2) gene. As a
result of this, there are no effective targeted therapies for
patients with TNBC that are currently available as standard
treatments, although some are under trial. The CDK4/6 inhibitor,
Palbociclib, has been approved by the U.S. Food and Drug
Administration for the treatment of metastatic breast cancer, but
there is not sufficient clinical data to support its effectiveness
in patients with TNBC (2). TNBC
tumors are typically of a higher grade and exhibit a higher
proliferative and metastatic rate compared with hormone
receptor-positive cancers, resulting in a lower 5-year survival
rate than that of other breast cancer subtypes (3).
Circular (circ)RNAs are a novel class of non-coding
RNAs, which have often been overlooked as they were considered to
be by-products of the splicing process (4). However, with sequencing technology
development, the importance of circRNAs is becoming more widely
accepted, with their characteristics including high abundance and
tissue‑specific expression (5,6).
According to circFunBase, there are >7,000 circRNAs with
ascribed functions (7). A major
reported function of circRNAs is the binding of microRNAs
(miRNAs/miRs) to serve as endogenous 'sponge' molecule for miRNAs
and RNA-binding proteins, resulting in the regulation of gene
expression (8,9). They are also reported to regulate
other RNA levels via complementary base pairing (10). CircRNAs can regulate protein
activity and abundance via direct interaction with target proteins
(8,11). Consequently, circRNAs can serve as
disease biomarkers. Reflecting these broad functions, circRNAs
influence various physiological and pathological processes,
including tumorigenesis (12).
miRNAs have been demonstrated to target a range of mRNA transcripts
which regulate the proliferation, migration and apoptosis of cancer
cells (13) and circRNAs may
influence this function. For example, circRNA CDR1 was reported to
inhibit miR-7 and regulate EGFR and IGF-1R expression in colorectal
cancer, inhibiting its progression (14). In addition, blocking miR-7 via CDR1
also regulated CCNE1 and PIK3CD expression in hepatocellular
carcinoma, inhibiting both proliferation and invasion.
The Hippo signaling pathway is a regulator of tissue
growth during organ development and regeneration, and exhibits
considerable interspecies conservation (15). Transcriptional coactivator with
PDZ-binding motif (TAZ), is a key downstream effector of the Hippo
signaling pathway, which serves a major role in regulating cell
number and differentiation, organ growth control, stem cell
function and tissue development and regeneration (16,17).
In TAZ‑deficient mice, mammary gland branching was decreased in
post-pubertal virgin animals, suggesting an association between TAZ
and the number of basal and luminal cells in the mammary gland
(18). It is common to see
elevated expression and nuclear translocation of TAZ in breast
cancer (19). Moreover, the
activity of TAZ in TNBC has been reported to be significantly
higher compared with all other breast cancer subtypes (20,21).
Increased TAZ expression is associated with a more invasive breast
cancer phenotype, which promotes cell transformation and
epithelial-mesenchymal transition (22). Thus, targeting TAZ may represent a
potential avenue for the treatment of TNBC.
In the present study research, it was revealed via
dual-luciferase reporter assays, that hsa_circ_0091074 [a circRNA
arising from the X‑inactive specific transcript (XIST) genomic
region] directly binds miR-1297 via its 3'UTR. Furthermore,
miR-1297 targeted TAZ to suppress proliferation and migration, and
regulate the cell cycle of TNBC cells.
Materials and methods
Cell culture
The human breast cancer cell lines MDA-MB-231, MCF-7
and MDA-MB-468 were purchased from the Chinese Academy of Sciences.
These cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.),
penicillin (100 U/ml) and streptomycin (100 µg/ml) (PS; Enpromise,
Hangzhou, China). Human normal breast cell line MCF-10A was bought
from Shanghai Zhongqiao Xinzhou Biotechnology Co., Ltd. MCF-10A
cells were cultured in Mammary Epithelial Cell Medium (MEpiCm,
ScienCell, Research Laboratories, Inc.). Cells were incubated at
37̊C and supplemented with 5% CO2. MDA-MB-231 and MCF-7
were subcultured every 2-3 days, and MDA-MB-468 and MCF-10A were
subcultured every 4-5 days.
Transfection assays
Hsa-miR-1297 mimic (miR-1297; forward, 5′-UUC AAG
UAA UUC AGG UG-3′ and reverse, 5′-CCU GAA UUA CUU GAA UU-3′),
hsa-miR-1297 inhibitor (miR-1297 in; forward, 5′-CAG UAC UUU UGU
GUA GUA CAA-3′ and reverse, 5′-GUA CUACACAAAAGUACUGUU-3′), negative
control mimic (NC; forward, 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and
reverse, 5′-ACG UGA CAC GUU GGG AGA ATT-3′) and negative control
inhibitor (NC in; forward, 5′-CAG UAC UUU UGU GUA GUA CAA-3′ and
reverse, 5′-GUA CUA CAC AAA AGU ACU GTT-3′) were chemically
synthesized by Guangzhou RiboBio Co., Ltd. Small interfering RNA
(siRNA) targeting TAZ (siRNA-TAZ; forward, 5′-GGC CAG AGA UAU UUC
CUU ATT-3′ and reverse, 5′-UAA GGA AAU AUC UCU GGC CTT-3′) was
chemically synthesized by Sangon Biotech Co., Ltd. siRNA targeting
hsa_circ_0091074 (si-circ; forward, 5′-AGG AUA GCU GAA UGA AAU CUU
TT-3′ and reverse, 5′-AAG AUU UCA UUC AGC UAU CCU TT-3′) and siRNA
negative control (si-NC; forward, 5′-UUC UC CGA ACG UGU CAC GUT
T-3′ and reverse, 5′-ACG UGA CAC GUU CGG AGA AT T-3′) were
chemically synthesized by Integrated Biotech Solutions. Cells were
added into 6-well plates, at a density of
~2.5x104/cm2 and cultured with DMEM,
supplemented with FBS and PS. When the cell confluency reached
30‑50%, the culture medium was replaced with DMEM without FBS and
antibiotics, and 0.8 µmol miR-1297, miR-1297 in, NC, NC in or
si-TAZ were added to the cells, along with 4 µl
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer′s instructions.
After 4-6 h of incubation, the medium was replaced by DMEM with 10%
FBS and 5 ml PS, and all the cells were incubated at 37̊C in a cell
incubator supplemented with 5% CO2 for 48 h, before
being harvested for analysis of biological function and protein
expression.
Reverse transcription‑quantitative
(RT‑q)PCR
Total RNA was extracted from MDA-MB-231, MDA-MB-468,
MCF-7 and MCF-10A cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), as well as the
transfected MDA‑MB‑231 and MDA-MB-468 cells, according to the
manufacturer's protocol. cDNA was generated via reverse
transcription using the PrimeScript RT-PCR kit in accordance with
the manufacturer's instructions (Takara Bio, Inc.). Subsequently,
qPCR was performed on a 7900HT Fast RT-PCR instrument (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The primer sequences
used were as follows: miR-1297 forward, 5'-UUC AAG UAA UUC AGG
UG-3′ and reverse, 5′-CAG TGC GTG TCG TGG AGT-3′; U6 forward,
5′-CTC GCT TCG GCA GCA CA-3′ and reverse, 5′-AAC GCT TCA CGA ATT
TGC GT-3′. The amplification protocol was as follows: Initial
denaturation for 3 min at 95̊C, followed by 40 cycles of
denaturation at 95̊C for 3 sec, annealing at 65̊C for 30 sec and
elongation at 72̊C for 20 sec. U6 was used as the reference gene.
The RT-qPCR results were analyzed with the
2‑∆∆Cq method (23).
Colony formation assay
MDA-MB-231 and MDA-MB-468 cells were first cultured
in six‑well plates. When cell confluency reached 30-50%, the cells
were transfected with miR-1297, miR-1297 in, NC, NC in, si-circ or
si-NC as described above. After 48 h, the treated cells were then
harvested and plated into a six-well plate at a density of 500
cells/well. The plates were incubated at 37̊C and 5% CO2
for 7 to 10 days, with the medium changed every three days. When
the colonies were visible, the medium was removed and the plates
were washed three times with phosphate buffered saline (PBS) and
allowed to dry. The dried colonies were fixed using 95% ethanol for
15 min at room temperature, then dried and stained with 0.1%
crystal violet solution for 15 min at room temperature. Finally,
the colonies were washed with water three times, dried and
immediately imaged. Colonies were counted using a light microscope
(magnification, x20).
MTT assay
MDA-MB-231 and MDA-MB-468 cells were transfected
with miR-1297, miR-1297 in, NC, NC in, si-circ or si-NC as
previously described. Then the transfected cells were seeded into
96-well plates at a density of 500 cells/well. Cell viability was
estimated using an MTT assay kit (Sangon Biotech Co., Ltd.) at 24,
48, 72, 96 and 120 h, according to the manufacturer's instructions.
After 4 h incubation in MTT reagent at 37̊C and 5% CO2,
the medium was replaced with 150 µl dimethyl sulfoxide at room
temperature (DMSO; Sangon Biotech Co., Ltd.). The absorbance of
each sample was measured at 490 nm using a microplate
spectrophotometer (BioTek Instruments, Inc.), after 10 min of
agitation on a shaking table.
Wound healing assay
MDA-MB-231 cells were stored in 6-well plates, and
transfected with miR-1297, miR-1297 in, NC or NC in with a range of
constructs as indicated. When the cells were ~90% confluent, a
scratch was generated in the cell monolayer by drawing a 200 µl
pipette tip over the surface of each well, holding the tip
perpendicular to the plate. The monolayers were washed three times
with PBS and cultured with DMEM medium without FBS or PS.
Wound-healing was observed under a light microscope (magnification,
x20) and cells were imaged at 0, 24 and 36 h in the same position
to observe cell movement.
Cell cycle assay
In total, 1x106 MDA-MB-231 cells were
collected 36 h after transfection with miR-1297, NC, si-TAZ or
si-NC, washed and centrifuged at 1,200 x g for 5 min at room
temperature. The cells were suspended with pre-cooled PBS, and
fixed in 75% pre‑cooled ethanol at 4˚C. After 24 h, the cells were
centrifuged at 1,200 x g for 5 min at 4˚C. The supernatants were
then removed and RNase (0.1 g/l) and a total of 300 µl (0.05 g/l)
propidium iodide (PI) staining solution were added to each sample
to stain nuclear DNA. The cells were incubated for 30 min at room
temperature in the dark. Cell cycle distribution was analyzed using
a FACSCantoTM II flow cytometer (BD Biosciences).
Western blotting
MDA-MB-231 and MDA-MB-468 cells were cultured and
transfected with miR-1297, miR-1297 in, NC, NC in, si-TAZ and
si-NC, as described above. The cells were washed three times with
4̊C pre‑cooled PBS. Whole cell protein extracts were prepared by
lysis of cells using cold RIPA buffer (100 µl/well, Beyotime
Institute of Biotechnology). After incubation on ice for 30 min,
the samples were centrifuged at 12,000 x g for 30 min at 4̊C.
Supernatants were transferred to fresh tubes, and the concentration
was measured using a bicinchoninic acid (BCA) Protein assay kit
(Beyotime Institute of Biotechnology), according to the
manufacturer's instruction. A total of 30 µg/lane of of each of the
protein samples was denatured with 6x sodium dodecyl sulfate (SDS)
loading buffer (Beyotime Institute of Biotechnology) at 100̊C for
10 min. Protein samples were separated by electrophoresis on a 10%
polyacrylamide SDS gel (Beyotime Institute of Biotechnology) and
transferred onto 0.45 µm nitrocellulose membranes (Beyotime
Institute of Biotechnology). Following 60 min of blocking with 5%
fat-free milk in double distilled H2O in room
temperature, membranes were incubated with primary antibodies in
antibody diluent (Beyotime Institute of Biotechnology) overnight at
4˚C. Blots were washed three times with PBST (PBS containing 0.1%
Tween 20; Sangon Biotech Co., Ltd), each time for 10 min, and
incubated for 1 h with the anti-rabbit or mouse horseradish
peroxidase-conjugated secondary antibody, as appropriate (cat. no.
sc-2357; 1:2,000; Santa Cruz Biotechnology, Inc.). After three 10
min washes with PBST, immunoreactive protein bands were detected
using an Odyssey Scanning system (LI-COR Biosciences). The density
of the bands was measured using ImageStudio5.2 (LI-COR
Biosciences). The following primary antibodies and dilutions were
used: Matrix metalloproteinase MMP2 (cat. no. ARG40620; 1:1,000;
Arigo Biolaboratories), MMP9 (cat. no. ARG22191; 1:1,000; Arigo
Biolaboratories), CDK4 (cat. no. ab137675; 1:2,000; Abcam), CDK6
(cat. no. ab151247; 1:2,000; Abcam), Cyclin D1 (cat. no. ab226977;
1:2,000; Abcam), β-actin (cat. no. sc-81178; 1:2,000, Santa Cruz
Biotechnology, Inc.), TAZ (cat. no. BS71120; 1:1,000; Bioworld
Technology, Inc.) and TEA domain transcription factor 4 (TEAD4 cat.
no. BS70599; 1:1,000; Bioworld Technology, Inc.).
Dual‑luciferase reporter assay
293T cells (Shanghai Institute of Biochemistry and
Cell Biology) were seeded in 48-well plates and incubated at 37̊C
in a cell incubator supplemented with 5% CO2. When cell
confluency reached 80%, DMEM medium was replaced with medium
without FBS or PS (250 µl/well). PmirGLO-hsa_circ_0091074-miR-1297
mutant and wild type reporter plasmids, psicheck-2/TAZ 3′-UTR
mutant and wild type reporter plasmids were purchased from
Integrated Biotech Solutions. 293T cells were co-transfected with
pmirGLO-hsa_circ_0091074-miR-1297 mutant or wild type reporter
plasmid, and miR-1297 or NC vector using Lipofectamine®
2000 reagent. These reagents were added to Opti-MEM (Life
Technologies, Inc.) medium according to the manufacturer's
instruction. 293T cells were co-transfected with psicheck-2/TAZ
3′-UTR wild type or mutant reporter plasmids, and miR-1297 or NC
using Lipofectamine® 2000 reagent according to the
manufacturer's instruction. After 24 h, firefly and Renilla
luciferase activities were measured using a Dual-luciferase
Reporter Assay (Promega Corporation), according to the
manufacturer's protocol. Firefly luciferase activity was normalized
to the Renilla control, and the ratio of
firefly/Renilla activity was recorded.
Database analysis
StarBase v2.0 (http://starbase.sysu.edu.cn/starbase2/mirCircRNA.php),
which identifies RNA‑RNA and RNA-protein interaction networks, was
used to predict interactions between circRNAs and miRNAs (24). CircBase (http://www.circbase.org) was used to search for the
sequence of circRNAs (25).
KMplotter (https://kmplot.com/analysis/index.php?p=service&cancer=breast_mirna)
is a webtool that combines data from the Gene Expression Omnibus
(26), European Genome-Phenome
Archive (27), The Cancer Genome
Atlas (TCGA) (28) and Pubmed, and
uses Kaplan-Meier survival analysis to determine the association
between expression levels of potential prognostic biomarkers and
clinical outcome in a range of cancers, including breast cancer
(29). KMplotter was used to
evaluate the association between miR-1297 or TAZ expression and
breast cancer survival in TCGA datasets. TargetScan 7.2 (http://www.targetscan.org/vert_72/) was used to
predict potential target genes of miRNAs (30).
Statistical analysis
Data were obtained from at least three independent
experiments, and are presented as the mean ± standard deviation
(SD). Differences were considered significant for P‑values <0.05
using the paired or unpaired Student's t-test and one-way ANOVA
with Dunnett's post-hoc test. The Log-rank test was used to analyze
survival in patients with breast cancer. GraphPad Prism software
version 7.0 (GraphPad Software, Inc.) was used to conduct
statistical analyses.
Results
Reciprocal association between
hsa_circ_0091074 and miR‑1297 expression in breast cancer
cells
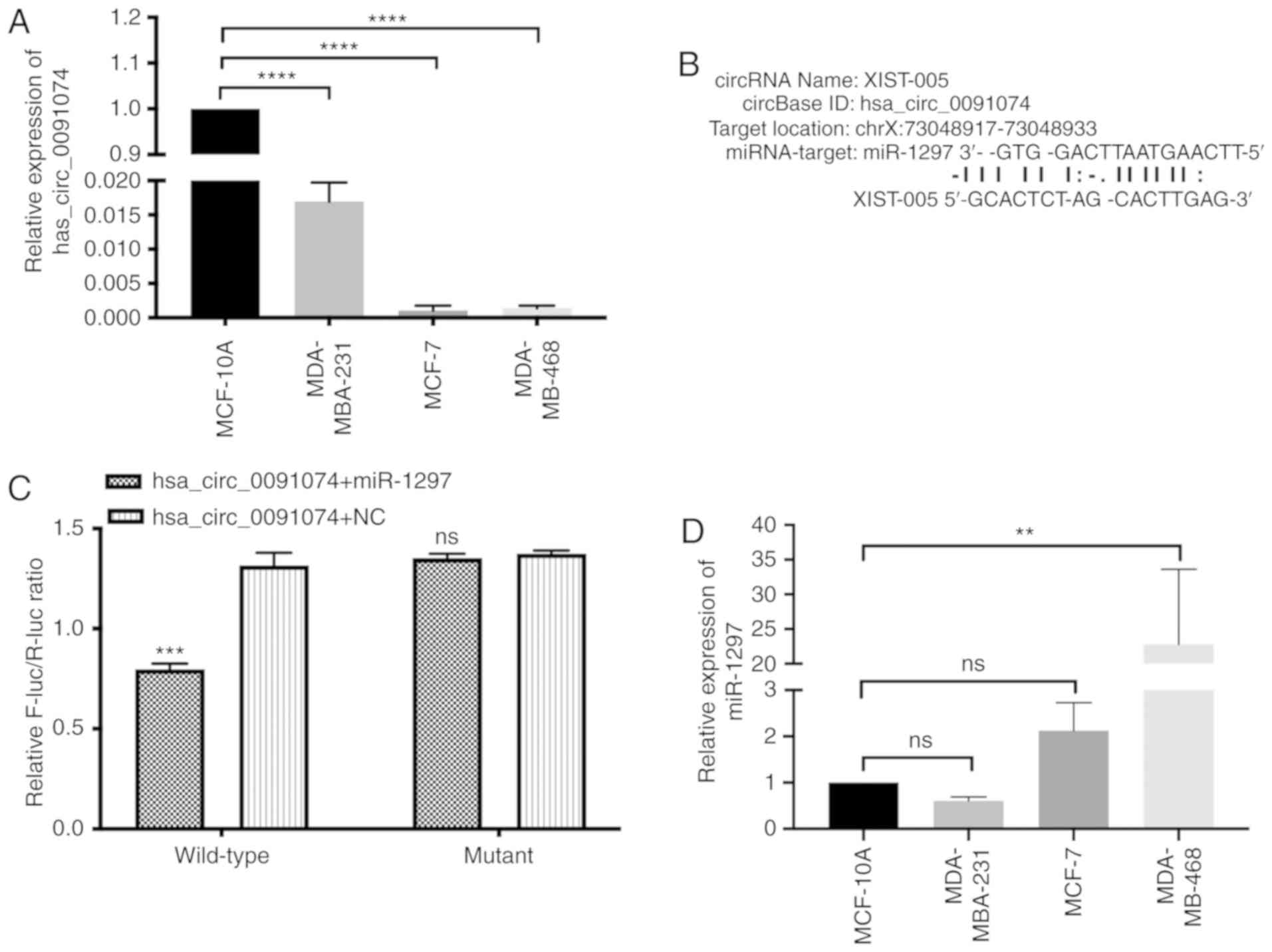
A screen of circRNAs revealed that hsa_circ_0091074
is expressed at low levels in MDA-MB-231, MCF-7 and MDA-MB-468
breast cancer cells, compared with the MCF-10A transformed normal
breast cell line, which harbors a homozygous deletion of the CDKN2A
tumor suppressor gene (Fig. 1A)
(31). StarBase v2.0 analysis
predicted that miR-1297 was a potential miRNA-target of
hsa_circ_0091074, as displayed in Fig.
1B. A dual-luciferase reporter assay, using constructs
containing wild-type and mutant sequences and spanning the
predicted binding sites in hsa_circ_0091074, revealed a 40%
reduction in luciferase activity following transfection with the
wild-type hsa_circ_0091074 + miR-1297 reporter, and no significant
change with the mutant sequence-miR-1297 combination, compared with
the control (Fig. 1B and C). As
shown in Fig. 1D, miR-1297 is
highly expressed in MCF-7 and MDA-MB-468 cells, while its
expression is lower in MDA‑MB‑231 and MCF‑10A cells. There was no
significance difference between MCF-7 and MDA-MB-231 compared with
MCF-10A cells. The current results indicated an inverse association
between the expression levels of hsa_circ_0091074 and miR-1297.
This indicates that hsa_circ_0091074 may serve as a 'sponge' RNA,
targeting miR-1297.
Knockdown of hsa_circ_0091074 inhibits
proliferation and viability of MDA‑MB‑231 cells
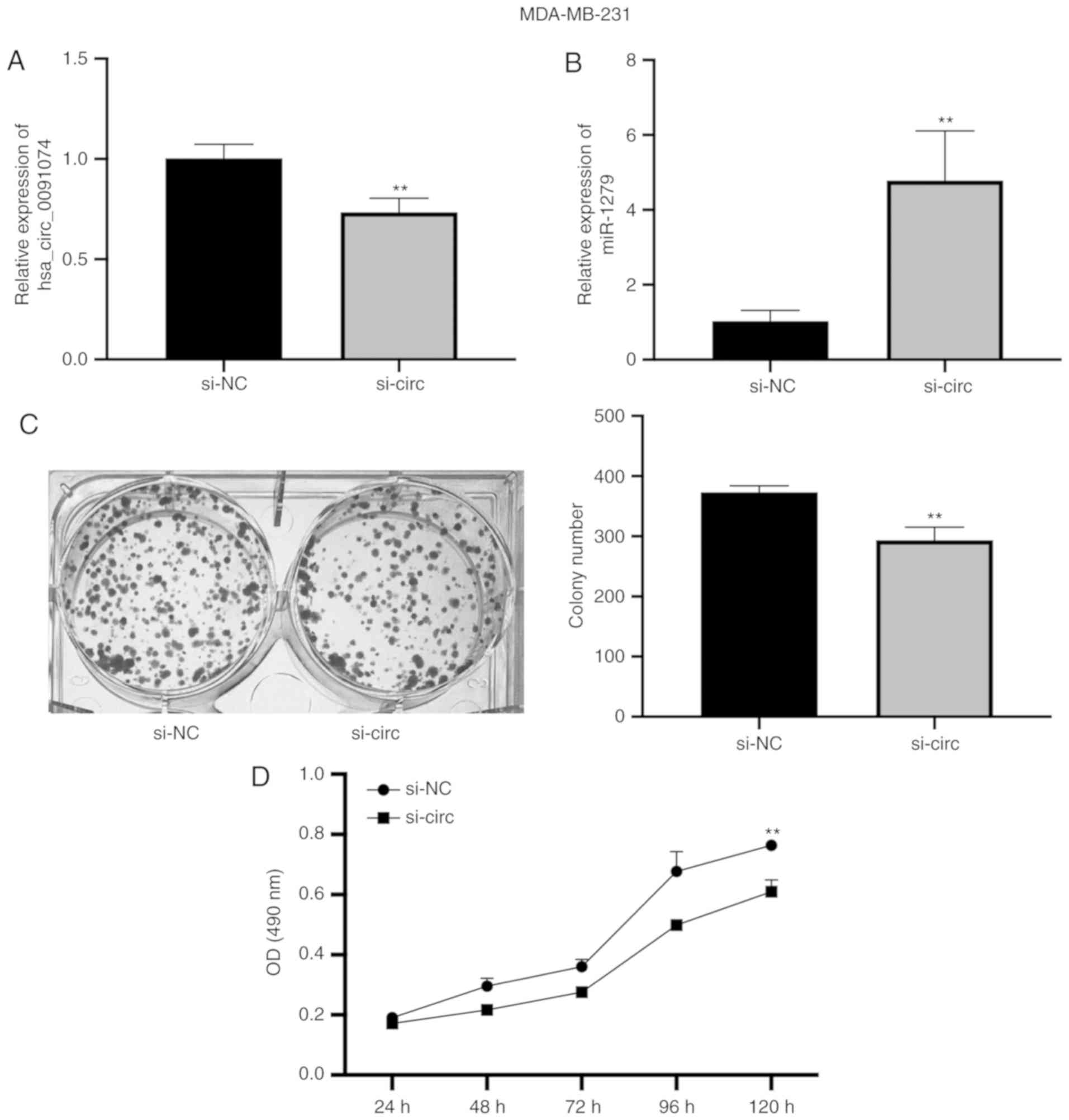
Hsa_circ_0091074 was knocked down in MDA-MB-231
cells and the expression of miR-1297 was measured by RT-qPCR. The
knockdown of hsa_circ_0091074 resulted in a significant increase in
the level of miR-1297 (Fig. 2A and
B). Cell proliferation and viability were estimated in colony
formation and MTT assays. Knockdown of hsa_circ_0091074 reduced the
colony number and lowered MTT activity compared with the NC group
(Fig. 2C and D). This indicates
that inhibiting hsa_circ_0091074 suppresses the proliferation and
viability of MDA-MB-231 cells.
miR‑1297 directly inhibits the expression
of TAZ
To explore the potential consequences of
downregulating miR-1297 in TNBC cells, a search was performed to
predict putative miR-1297 targets using the database TargetScan
7.2. Notably, although hsa_circ_0091074 (which is predicted to bind
miR-1297) arises from the genomic region encoding XIST RNA,
miR-1297 was not predicted to bind XIST RNA. However, the analysis
predicted that TAZ was a potential target of miR‑1297, and this was
confirmed using a Dual‑luciferase reporter assay. Constructs
containing wild-type and mutant sequences spanning the predicted
binding sites of miR-1297 were combined in the assay with TAZ. This
revealed a highly significant 35% decrease in luciferase activity
when the wild type miR-1297 construct was transfected with TAZ,
whereas no change in activity was observed after transfection with
the NC sequence (Fig. 3A).
MDA-MB-231 and MDA-MB-468 cells were transfected with the miR-1297
mimic, NC mimic and inhibitor (in) fragments, and the expression
levels of miR-1297 were evaluated by RT-qPCR (Fig. 3B). Moreover, the expression of
hsa_circ_0091074 in MDA-MB-231 was measured using RT-qPCR (Fig. 3C). After transfection with the
miR-1297 mimic, the expression level of miR-1297 in the cell lines
was significantly increased, and miR‑1297 inhibitor markedly
decreased the miR-1297 level, compared with the relevant negative
controls. Western blotting analysis (Fig. 3D), revealed that overexpression of
miR-1297 downregulated TAZ protein levels while transfection with
the miR-1297 inhibitor resulted in a small but significant increase
in TAZ protein levels, compared with the negative control
inhibitor, in both MDA-MB-231 and MDA-MB-468 cells. Furthermore,
miR-1297 also decreased the expression of TAZ transcriptional
cofactor TEAD4, while miR-1297 inhibitor increased TEAD4 expression
in MDA-MB-231 and MDA-MB-468 TNBC cells. In summary, miR-1297
downregulates TAZ expression and this influences TEAD4 expression
in MDA-MB-231 and MDA-MB-468 cells. Given the association between
the Hippo pathway in cancer invasion and metastasis, downregulation
of components of this pathway by miR-1297 represents a potential
mechanism for tumor suppression via the targeting of this
molecule.
Positive association between miR‑1297 and
recurrence‑free survival time in TNBC
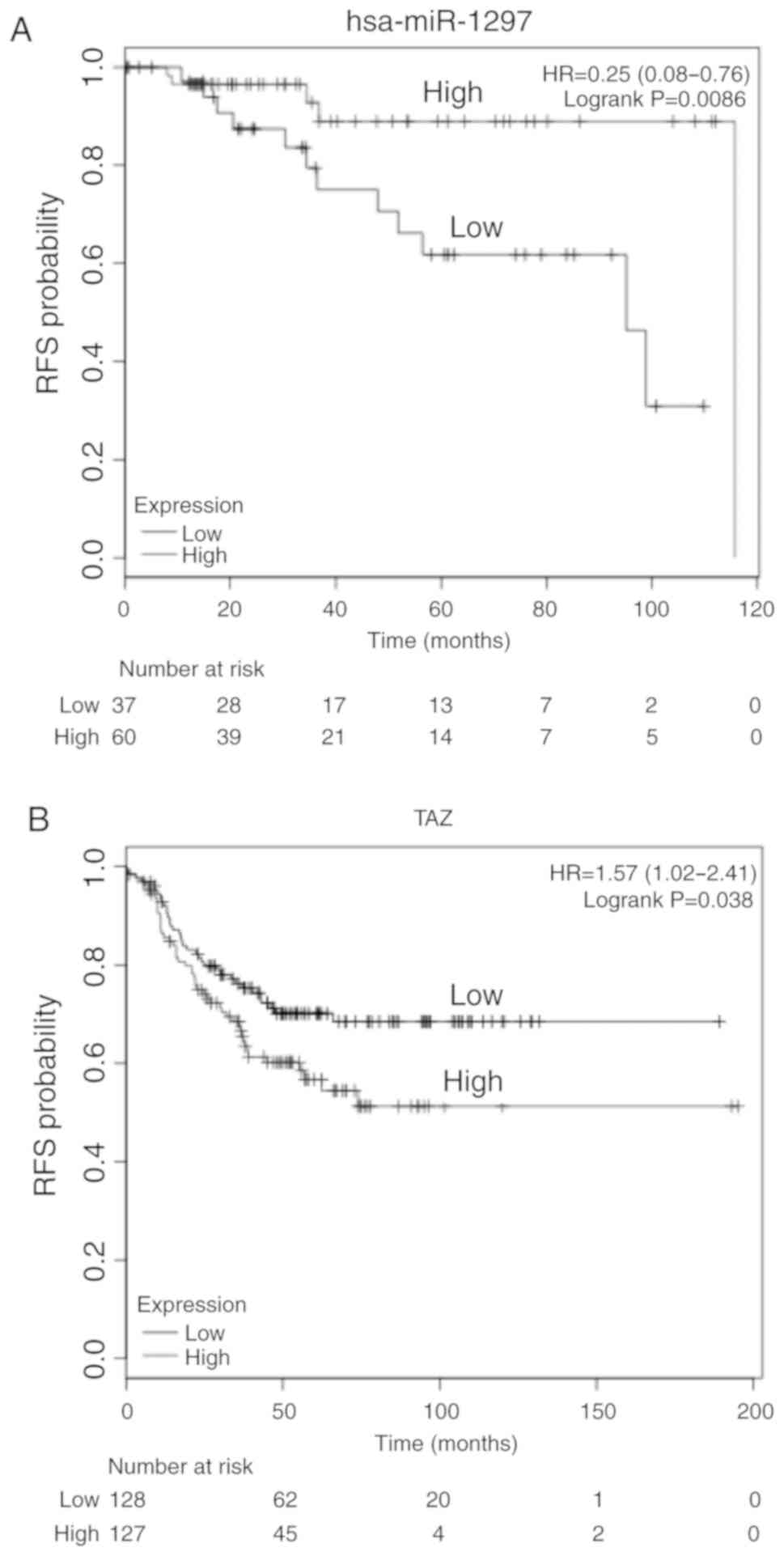
To investigate the potential role of miR-1297 and
TAZ in TNBC, the association between miR-1297 or TAZ expression and
survival was investigated in TCGA breast cancer cohort using the
online tool KMplotter. The patients were grouped according to the
median expression of miR-1297 or TAZ, and then the two groups were
compared by Cox regression, and a Kaplan-Meier plot was drawn
(29). This revealed that high
expression of miR-1297 was associated with a more favorable
survival time compared with the TNBC cases in which miR-1297
expression was lower (Fig. 4A).
Moreover, high expression of TAZ in patients with TNBC was
associated with poorer survival compared with patients with low
miR-1297 expression levels (Fig.
4B). The results indicate that miR-1297 may be a tumor
suppressor and that TAZ may be an oncogene in TNBC.
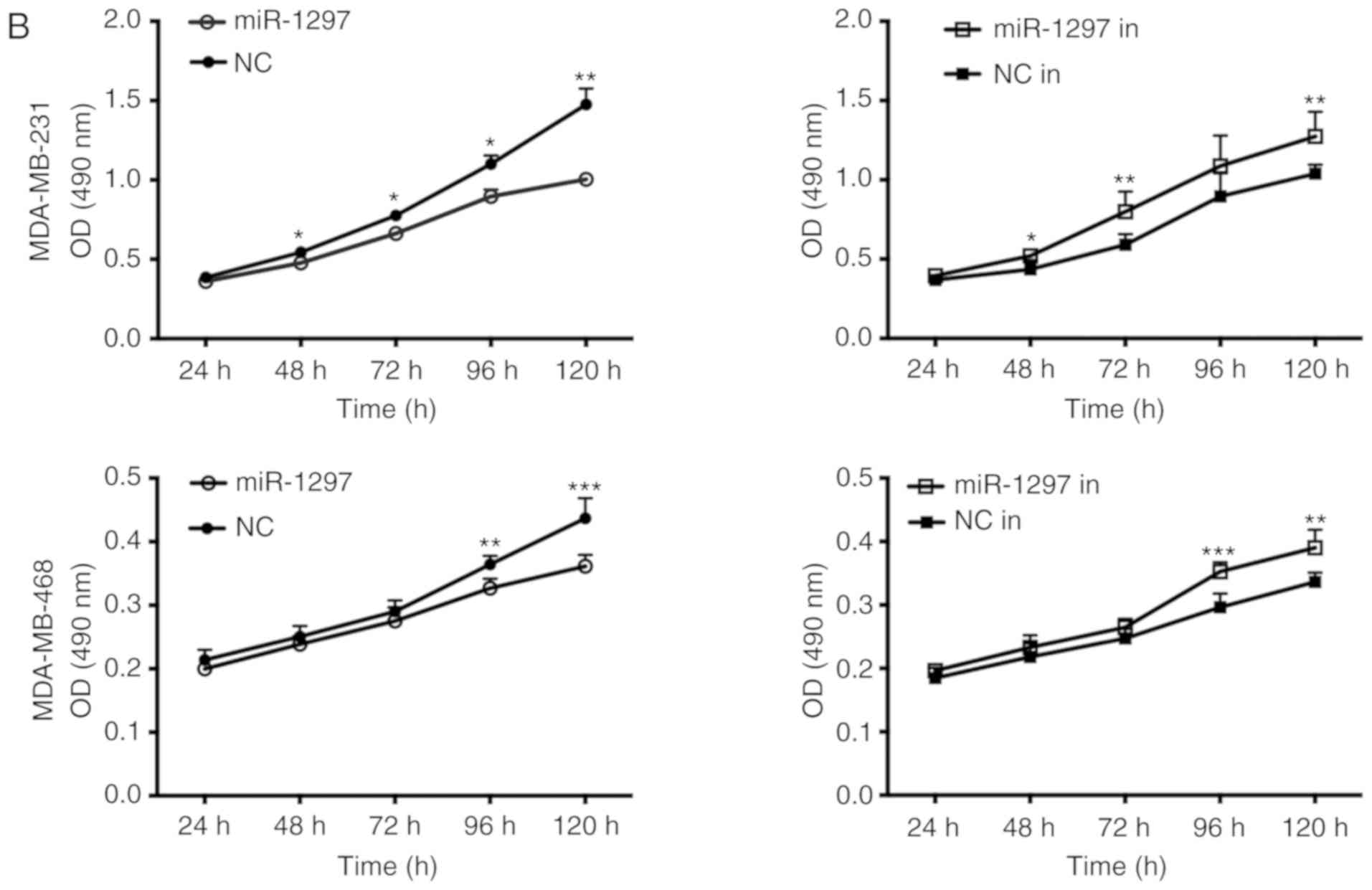
miR‑1297 inhibits proliferation and
viability of triple negative breast cancer cells
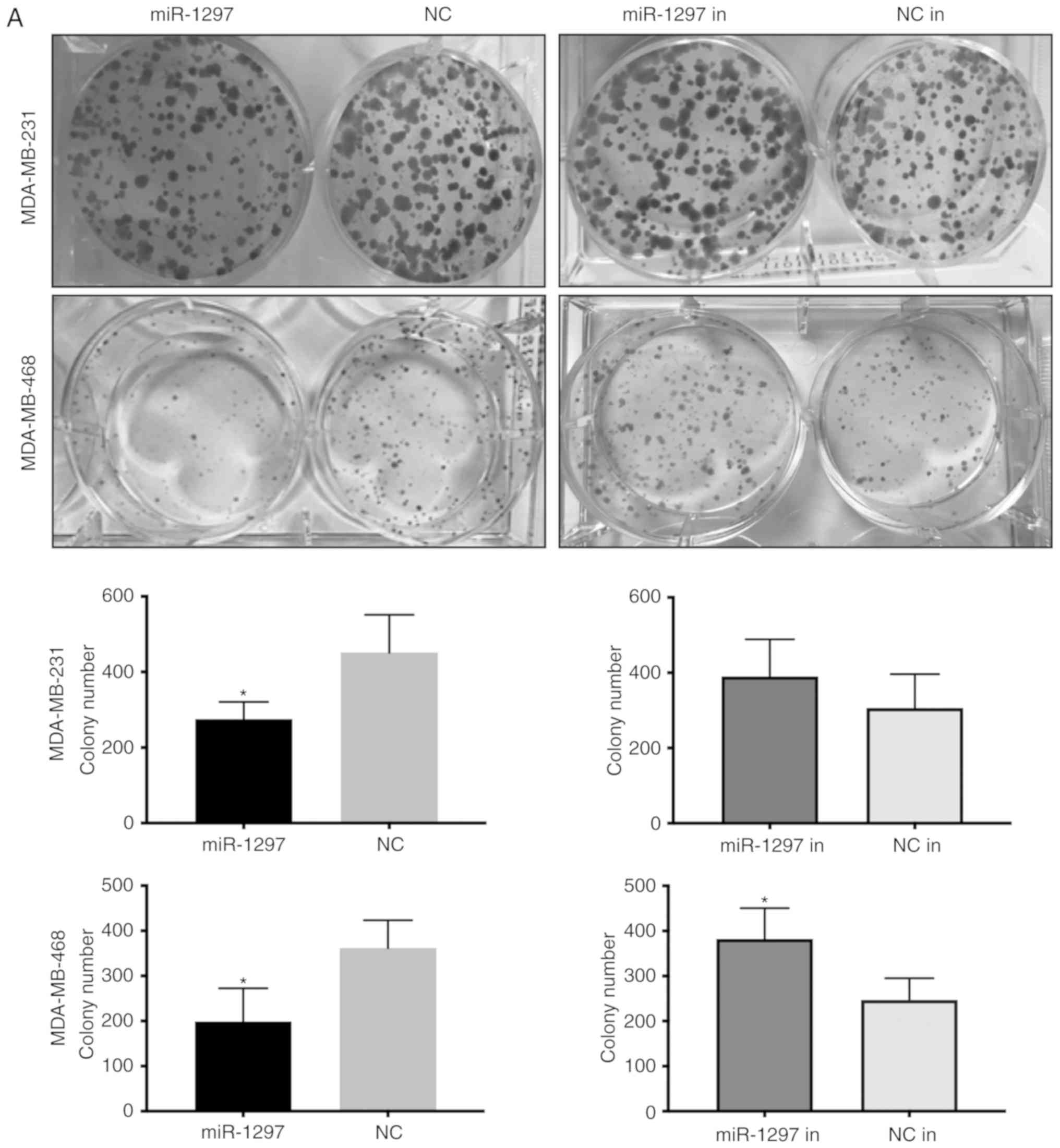
miR-1297 and miR-1297 inhibitor were overexpressed
in MDA-MB-231 and MDA-MB-468 cells, and cell proliferation was
estimated via colony formation and viability was estimated using
MTT assays (Fig. 5A and B).
miR-1297 decreased the colony number and lowered MTT activity,
while transfection with miR-1297 inhibitor caused the opposite
results; however, the difference of miR-1297 inhibitor in
MDA-MB-231 cells was not significant. This revealed that
overexpression miR-1297 inhibits cell proliferation and viability,
while knockdown of miR-1297 promoted cell proliferation and
viability in both cell lines. These results further support the
finding that miR‑1297 suppresses the proliferation and viability of
TNBC cells.
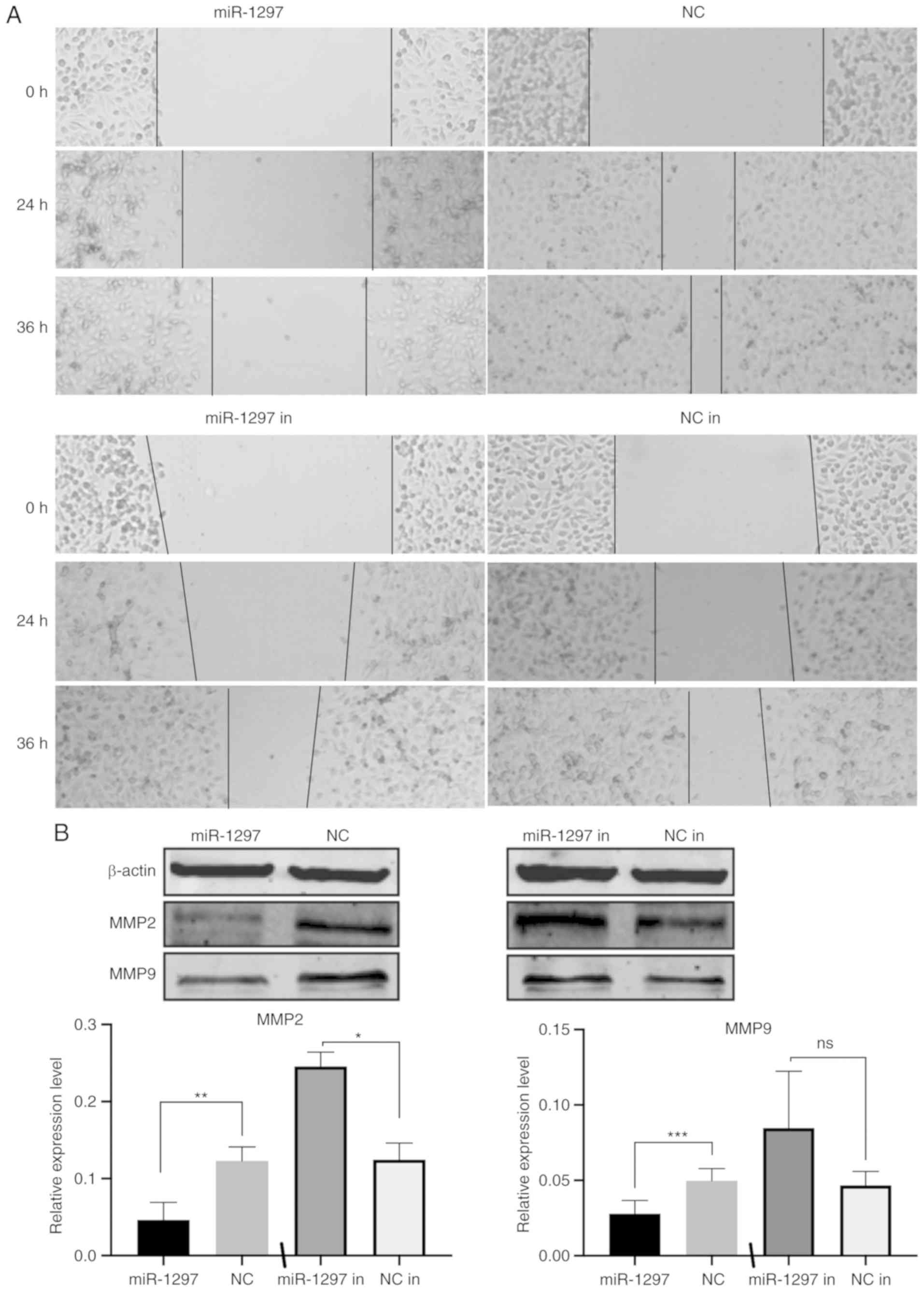
miR‑1297 inhibits the migration of
MDA‑MB‑231 cells
The influence of miR‑1297 on TNBC migration was
tested by over-expressing either the miR-1297 mimic and miR-1297
inhibitor in MDA-MB-231 cells, and performing a wound healing assay
(Fig. 6A). Cells overexpressing
miR-1297 migrated markedly slower than the control group. After 24
and 36 h, limited migration was seen in the miR-1297 overexpression
group, while the wound area in the control group was almost
completely filled. Although a small decrease in migration was also
seen with the addition of miR-1297 inhibitor, this was not markedly
different compared with the control group. Given that expression of
miR-1297 in MDA-MB-231 cells is already low, it was expected that
miR-1297 knockdown resulted in limited effect in these cells.
Western blotting analysis (Fig.
6B), revealed that miR-1297 inhibited the expression of the
migration markers MMP2 and MMP9, in MDA-MB-231 cells. These results
provided further evidence that miR-1297 inhibits cell migration in
MDA-MB-231 cells.
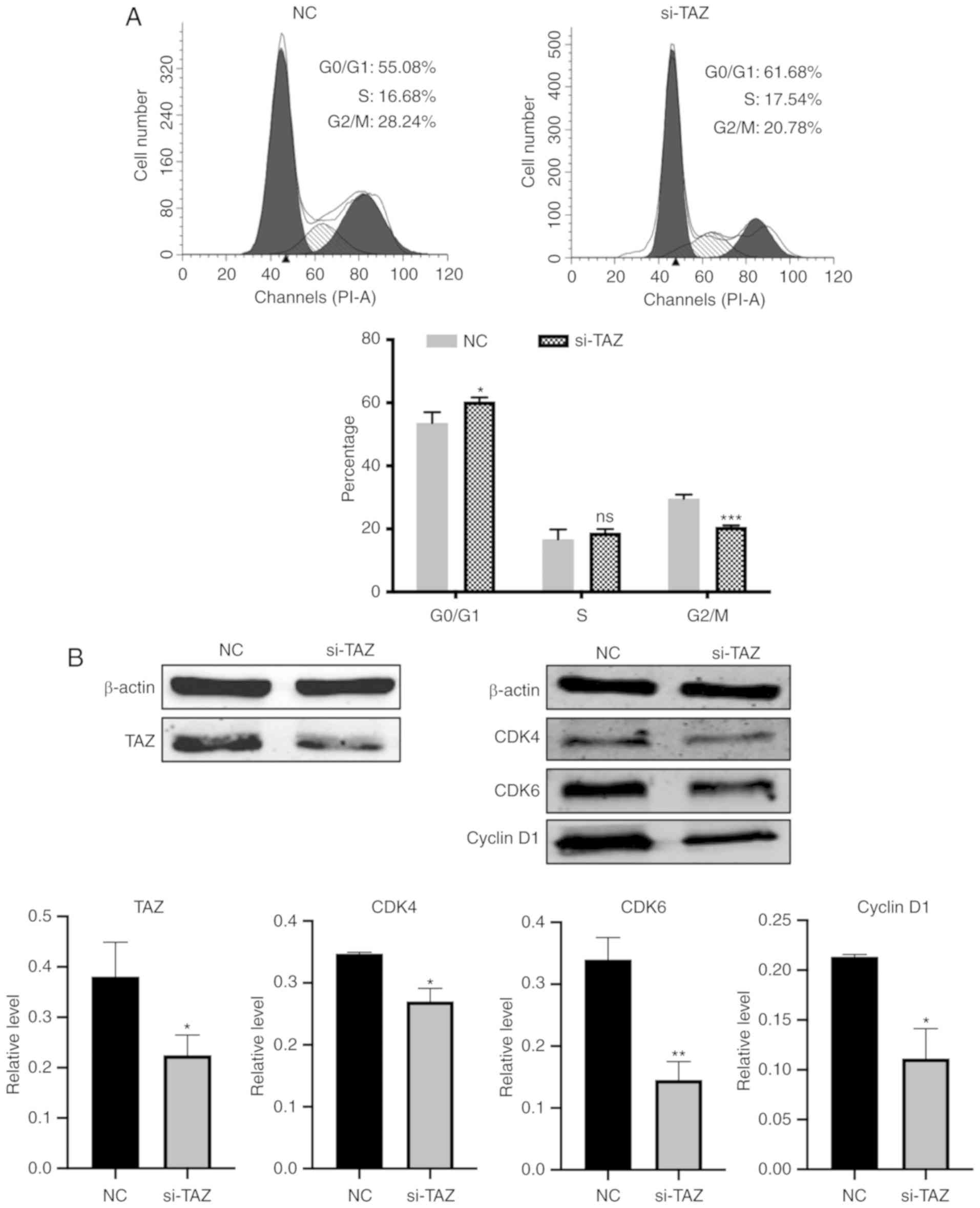
siRNA‑TAZ regulates the cell cycle in
MDA‑MB‑231 cells
To assess whether inhibition of cell proliferation
by miR-1297 could be mediated by overexpressing miR-1297 or
knockdown of TAZ, miR-1297, siRNA-TAZ and NC were transfected in
MDA-MB-231 cells, and cell cycle distribution was analyzed by flow
cytometry. The percentage of G0/G1 phase
cells (61.68%) increased in the si-TAZ group, compared with the
control group (55.08%). Meanwhile, the percentage of S phase cells
(17.54%) exhibited no significant difference compared with NC group
(16.68%). The percentage of G2/M phase cells decreased
in the siRNA-TAZ group (20.78%) compared with the control group
(28.24%) (Fig. 7A). Overexpressing
miR-1297 also caused a 2% increase in the
G0/G1 phase population compared with the
control, but this difference was not statistically significant
(Fig. S1). TAZ protein expression
was notably reduced after transfection with siRNA-TAZ in MDA-MB-231
cells (Fig. 7B). The cell cycle
markers CDK4, CDK6 and Cyclin D1 were also significantly decreased
at the protein level following transfection with siRNA-TAZ. In
summary, knockdown of TAZ expression regulated the
G0/G1 and G2/M phase of MDA-MB-231
cells, consistent with a reduction in cell proliferation.
Discussion
Globally, the incidence of breast cancer has been
increasing rapidly in recent years (32). TNBC is an aggressive subtype of
breast cancer that exhibits high genomic heterogeneity and commonly
develops resistance to existing targeted therapies (33). Consequently, when compared with
other subtypes, the outcomes for patients with TNBC are often poor.
For example, patients diagnosed with TNBC exhibit significantly
less favorable disease-free and overall survival rates compared
with those with other breast cancer types (34).
A major function of circRNAs is the negative
regulation of miRNA via binding miRNA, serving as a molecular
sponge and thus decreasing the cellular abundance (35). In the present study, it was
revealed that there was variable expression of hsa_circ_0091074
between different breast cell lines. It was predicted that miR-1297
was a potential target for hsa_circ_0091074. This was supported by
the finding that there is an overall inverse association between
the expression of hsa_circ_0091074 and miR-1297 in breast cell
lines, which was most significant in the cancer cell lines, and
less apparent in the transformed normal line. Silencing
hsa_circ_0091074 resulted in an increased abundance of miR-1297,
further supporting the inverse association between hsa_circ_0091074
and miR-1297. Using a Dual-luciferase reporter assay, it was
confirmed that hsa_circ_0091074 binds miR‑1297 by targeting its
3'UTR. Data are not currently available to compare the levels of
hsa_circ_0091074 and miR-1297 in a breast cancer cohort. Therefore,
it is necessary to examine whether the association between the two
RNAs is observed in breast cancers, and whether there is an
association with overall outcome.
MicroRNAs have gained interest due to their
involvement in a plethora of different physiological processes,
where they act as regulators of gene expression via
post-transcriptional gene silencing. There is considerable interest
in manipulating the levels of miRNAs that target tumor suppressor
genes, as a potential therapeutic approach for a range of cancer
types (36,37). The role of miR-1297 in cancers is
controversial; miR-1297 has been reported to serve as a tumor
suppressor in hepatocellular carcinoma by targeting HMGA2 (38), and in gastric cancer by targeting
HMGB2 (39). The current study
suggests that miR-1297 acts as a tumor suppressor in TNBC by
inhibiting the oncogene TAZ. However, miR-1297 has also been
reported to act as a cancer promoting factor by targeting the tumor
suppressor gene PTEN in non-small cell lung cancer, laryngeal
squamous cell carcinoma and testicular germ cell tumors (40-42)
and in the MCF-7 and MDA-MB-453 breast cancer cell lines (43). In the present study, it was
revealed that that high expression of miR-1297 was positively
associated with survival in patients with TNBC using data from
TCGA, and this result is consistent with a tumor suppressor role.
However, further analysis revealed that higher miR-1297 was
associated with a less favorable outcome in ER-positive cases in
the same cohort. This analysis indicates that miR-1297 acts as a
tumor suppressor in TNBC while it serves as an oncogene in
ER-positive breast cancer. A range of approaches were employed to
study the impact of silencing miR-1297, in both MDA-MB-231 and
MDA-MB-436 cells, and the effects seen were proportional to the
basal expression of miR-1297. In MDA-MB-231 cells, silencing of
miR-1297 resulted in a 21% increase in proliferation, whereas in
MDA-MB-436 cells there was a 35% increase, indicating an inverse
association between miR-1297 expression and cell proliferation.
The Dual‑luciferase assay confirmed that the
oncogene TAZ is a target gene of miR-1297, which significantly
decreased luciferase activity in the wild-type group. Certain
specific miRNAs simultaneously exert both oncogenic and tumor
suppressive effects by silencing tumor suppressive and oncogenic
mRNAs, respectively (44).
Yes-associated protein (YAP) and TAZ have higher expression levels
in TNBC compared with other breast cancer subtypes, providing a
possible explanation for the differential association of miR-1297
with outcome in ER-positive and triple negative breast cancer
(45).
As a member of the Hippo pathway, TAZ is an
important oncogene in the human growth and developmental processes,
with an established role as a key regulator of organ size and
tissue homeostasis (46). The role
of Hippo in suppressing contact inhibition and modulating cell
proliferation, apoptosis and stemness, has also implicated it in
cancer (47). YAP/TAZ upregulation
induces cell proliferation, inflammation, acquisition of cancer
stem cell features, metastasis formation, suppression of anoikis,
reduced apoptosis and drug resistance (48). Therefore, given the high expression
level of YAP/TAZ in TNBC, by targeting the Hippo pathway kinase TAZ
and inhibiting its expression, miR-1297 was demonstrated to have an
important function in TNBC. Although overexpression of miR‑1297 did
not have a significant effect on cell cycle, a small increase in
G0/G1 fraction was observed, suggesting that
miR‑1297 influenced but did not significantly decrease TAZ
expression. Moreover, there was a lag in time between the increase
in miR-1297 expression, decrease in TAZ protein expression and the
functional outcome, such that a greater effect may be observed
after more sustained exposure to miR-1297. It should be noted that
direct suppression of TAZ also resulted in a relatively small
change in cell cycle distribution.
Taken together, the present study revealed that
hsa_circ_0091074 can serve as a miRNA sponge to bind miR-1297, and
also aimed to elucidate the function of miR-1297 in TNBC. Via MTT,
colony forming and wound healing assays, it was revealed that
miR-1297 inhibited the proliferation and migration of TNBC cells.
Moreover, it was demonstrated that miR-1297 directly regulates the
expression of TAZ and influences the protein expression of its
transcriptional cofactor TEAD4, of the Hippo pathway. The current
results also indicate that miR-1297 serves as a tumor suppressor in
TNBC and TAZ is an oncogene, which is upregulated in TNBC cells.
CircRNA sponges targeting such miRNAs may serve as a novel method
to achieve this regulation for the treatment of TNBC in the future.
Future research should focus on the biological functions of
circRNAs and miRNAs and their association with the Hippo
pathway.
Supplementary Data
Funding
The present study was supported by the Shanghai
Science and Technology Commission (grant no. 17411967200) and the
Shanghai Municipal Commission of Health and Family Planning (grant
no. 201640097).
Availability of data and materials
The datasets analyzed during the current study are
available in The Cancer Genome Atlas repository (https://www.cancer.gov/tcga).
Authors' contributions
JH designed, performed the experiment and wrote the
manuscript. CJ co-performed the experiments and analysed data. KH
performed western blot assays and analysed the data. XW performed
RT-qPCR and analysed the data of them. XD acquired data by
performing cell transfection, colony formation assays and counted
colony numbers, then analysed and interpreted this data. JL
performed the cell transfection and MTT assays, and analysed and
interpreted this data. DG assisted in interpreting the data and was
a major contributor in drafting the manuscript and revising it
critically before final submission. LF helped to perform the
experiment and contributed to the design of experiments. All
authors read and approved the final version of the manuscript to be
published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Ferlay J, Ervik M, Lam F, Colombet M, Mery
L, Piñeros M, Znaor A, Soerjomataram I and Bray F: global Cancer
Observatory: Cancer Today. IARC; Lyon: 2018, https://gco.iarc.fr/today.
Accessed March 8, 2019.
|
|
2
|
Kwapisz D: Cyclin-dependent kinase 4/6
inhibitors in breast cancer: Palbociclib, ribociclib, and
abemaciclib. Breast Cancer Res Treat. 166:41–54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Woodcock CC, Huang Y, Woodcock SR,
Salvatore SR, Singh B, Golin-Bisello F, Davidson NE, Neumann CA,
Freeman BA and Wendell SG: Nitro-fatty acid inhibition of triple
negative breast cancer cell viability, migration, invasion and
tumor growth. J Biol Chem. 293:1120–1137. 2018. View Article : Google Scholar
|
|
4
|
Cocquerelle C, Mascrez B, Hétuin D and
Bailleul B: Mis-splicing yields circular RNA molecules. FASEB J.
7:155–160. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qu S, Yang X, Li X, Wang J, Gao Y, Shang
R, Sun W, Dou K and Li H: Circular RNA: A new star of noncoding
RNAs. Cancer Lett. 365:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meng X, Hu D, Zhang P, Chen Q and Chen M:
CircFunBase: A database for functional circular RNAs. Database
(Oxford) 2019. baz0032019.
|
|
8
|
Zhang Y, Yang L and Chen LL:
Characterization of circular RNAs. Methods Mol Biol. 1402:215–227.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:3843882013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hansen TB, Wiklund ED, Bramsen JB,
Villadsen SB, Statham AL, Clark SJ and Kjems J: miRNA-dependent
gene silencing involving Ago2-mediated cleavage of a circular
antisense RNA. EMBO J. 30:4414–4422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xin Z, Ma Q, Ren S, Wang G and Li F: The
understanding of circular RNAs as special triggers in
carcinogenesis. Brief Funct Genomics. 16:80–86. 2017.
|
|
12
|
Rybak‑Wolf A, Stottmeister C, Glažar P,
Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss
R, et al: Circular RNAs in the mammalian brain are highly abundant,
conserved, and dynamically expressed. Mol Cell. 58:870–885. 2015.
View Article : Google Scholar
|
|
13
|
Meng X, Zhu Y, Tao L, Zhao S and Qiu S:
miR-590-3p mediates melatonin-induced cell apoptosis by targeting
septin 7 in the human osteoblast cell line hFOB 1.19. Mol Med Rep.
17:7202–7208. 2018.PubMed/NCBI
|
|
14
|
Tang W, Ji M, He G, Yang L, Niu Z, Jian M,
Wei Y, Ren L and Xu J: Silencing CDR1as inhibits colorectal cancer
progression through regulating microRNA-7. Onco Targets Ther.
10:2045–2056. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buglioni S, Vici P, Sergi D, Pizzuti L, Di
Lauro L, Antoniani B, Sperati F, Terrenato I, Carosi M, Gamucci T,
et al: Analysis of the hippo transducers TAZ and YAP in cervical
cancer and its microenvironment. Oncoimmunology. 5:e11601872016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Royer C, Koch S, Qin X, Zak J, Buti L,
Dudziec E, Zhong S, Ratnayaka I, Srinivas S and Lu X: ASPP2 links
the apical lateral polarity complex to the regulation of YAP
activity in epithelial cells. PLos One. 9:e1113842014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou X, Su J, Feng S, Wang L, Yin X, Yan J
and Wang Z: Antitumor activity of curcumin is involved in
down-regulation of YAP/TAZ expression in pancreatic cancer cells.
Oncotarget. 7:79076–79088. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi P, Feng J and Chen C: Hippo pathway in
mammary gland development and breast cancer. Acta Biochim Biophys
Sin (Shanghai). 47:53–59. 2015. View Article : Google Scholar
|
|
19
|
Plouffe SW, Hong AW and Guan KL: Disease
implications of the Hippo/YAP pathway. Trends Mol Med. 21:212–222.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bartucci M, Dattilo R, Moriconi C,
Pagliuca A, Mottolese M, Federici G, Benedetto AD, Todaro M, Stassi
G, Sperati F, et al: TAZ is required for metastatic activity and
chemoresistance of breast cancer stem cells. Oncogene. 34:681–690.
2015. View Article : Google Scholar
|
|
21
|
Cordenonsi M, Zanconato F, Azzolin L,
Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR,
Poletti A, et al: The Hippo transducer TAZ confers cancer stem
cell-related traits on breast cancer cells. Cell. 147:759–772.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Passaniti A, Brusgard JL, Qiao Y, Sudol M
and Finch-Edmondson M: Roles of RUNX in Hippo pathway signaling.
Adv Exp Med Biol. 962:435–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42(Database Issue): D92–D97. 2014. View Article : Google Scholar
|
|
25
|
Glažar P, Papavasileiou P and Rajewsky N:
circBase: A database for circular RNAs. RNA. 20:1666–1670. 2014.
View Article : Google Scholar
|
|
26
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res. 41(Database Issue):
D991–D995. 2013. View Article : Google Scholar
|
|
27
|
Lappalainen I, Almeida-King J, Kumanduri
V, Senf A, Spalding JD, Ur-Rehman S, Saunders G, Kandasamy J,
Caccamo M, Leinonen R, et al: The European Genome-phenome Archive
of human data consented for biomedical research. Nat Genet.
47:692–695. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomczak K, Czerwińska P and Wiznerowicz M:
The Cancer Genome Atlas (TCGA): An immeasurable source of
knowledge. Contemp Oncol (Pozn). 19:A68–A77. 2015.
|
|
29
|
Lánczky A, Nagy Á, Bottai G, Munkácsy G,
Szabó A, Santarpia L and Győrffy B: miRpower: A web‑tool to
validate survival‑associated miRNAs utilizing expression data from
2178 breast cancer patients. Breast Cancer Res Treat. 160:439–446.
2016. View Article : Google Scholar
|
|
30
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar :
|
|
31
|
Cowell JK, LaDuca J, Rossi MR, Burkhardt
T, Nowak NJ and Matsui S: Molecular characterization of the t(3;9)
associated with immortalization in the MCF10A cell line. Cancer
Genet Cytogenet. 163:23–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Beatty A, Fink LS, Singh T, Strigun A,
Peter E, Ferrer CM, Nicolas E, Cai KQ, Moran TP, Reginato MJ, et
al: Metabolite profiling reveals the glutathione biosynthetic
pathway as a therapeutic target in triple-negative breast cancer.
Mol Cancer Ther. 17:264–275. 2018. View Article : Google Scholar
|
|
34
|
Chen LL, Zhang ZJ, Yi ZB and Li JJ:
MicroRNA-211-5p suppresses tumour cell proliferation, invasion,
migration and metastasis in triple-negative breast cancer by
directly targeting SETBP1. Br J Cancer. 117:78–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Verduci L, Strano S, Yarden Y and Blandino
G: The circ RNA-micro RNA code: Emerging implications for cancer
diagnosis and treatment. Mol Oncol. 13:669–680. 2019.PubMed/NCBI
|
|
36
|
Hosseinahli N, Aghapour M, Duijf PH and
Baradaran B: Treating cancer with microRNA replacement therapy: A
literature review. J Cell Physiol. 233:5574–5588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shah MY, Ferrajoli A, Sood AK,
Lopez-Berestein G and Calin GA: microRNA therapeutics in cancer-an
emerging concept. EBioMedicine. 12:34–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Liang H and Jiang X: MiR-1297
promotes apoptosis and inhibits the proliferation and invasion of
hepatocellular carcinoma cells by targeting HMGA2. Int J Mol Med.
36:1345–1352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Gao J, Tian W, Li Y and Zhang J:
Long non-coding RNA MALAT1 drives gastric cancer progression by
regulating HMGB2 modulating the miR-1297. Cancer Cell Int.
17:442017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bu W and Luo T: miR-1297 promotes cell
proliferation of non-small cell lung cancer cells: Involving in
PTEN/Akt/Skp2 signaling pathway. DNA Cell Biol. 36:976–982. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li X, Wang HL, Peng X, Zhou HF and Wang X:
miR-1297 mediates PTEN expression and contributes to cell
progression in LSCC. Biochem Biophys Res Commun. 427:254–260. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang NQ, Zhang J, Tang QY, Guo JM and Wang
GM: miRNA-1297 induces cell proliferation by targeting phosphatase
and tensin homolog in testicular germ cell tumor cells. Asian Pac J
Cancer Prev. 15:6243–6246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu C, Liu Z, Li X, Tang X, He J and Lu S:
MicroRNA-1297 contributes to tumor growth of human breast cancer by
targeting PTEN/PI3K/AKT signaling. Oncol Rep. 38:2435–2443. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Svoronos AA, Engelman DM and Slack FJ:
OncomiR or tumor suppressor? The duplicity of microRNAs in cancer.
Cancer Res. 76:3666–3670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu J, Ye L, Li Q, Wu X, Wang B, Ouyang Y,
Yuan Z, Li J and Lin C: Synaptopodin-2 suppresses metastasis of
triple-negative breast cancer via inhibition of YAP/TAZ activity. J
Pathol. 244:71–83. 2018. View Article : Google Scholar
|
|
46
|
Yu FX, Zhao B and Guan KL: Hippo pathway
in organ size control, tissue homeostasis, and cancer. Cell.
163:811–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park JH, Shin JE and Park HW: The role of
hippo pathway in cancer stem cell biology. Mol Cells. 41:83–92.
2018.PubMed/NCBI
|
|
48
|
Ferraiuolo M, Verduci L, Blandino G and
Strano S: Mutant p53 protein and the hippo transducers YAP and TAZ:
A critical oncogenic node in human cancers. Int J Mol Sci. 18:pii:
E961. 2017.PubMed/NCBI
|