Hodgkin's lymphoma (HL) was first described in the
year 1832 by Thomas Hodgkin, a British pathologist following the
autopsies of 7 patients with lymphadenopathy and splenomegaly
(1). Between the years 2014 and
2018, the prevalence of new HL cases was 26 individuals per million
males and females. In addition, between 2015 and 2019, the annual
mortality rate due to HL was 3 individuals per million males and
females. Notably, the 5-year relative survival rate from 2011 to
2017 was 88.3% (data from Surveillance, Epidemiology and End
Results, https://seer.cancer.gov/archive/csr/1975_2017/). It is
estimated that HL accounts for ~10% of newly diagnosed lymphoma
cases in the United States (8,480 of 85,720 cases), with a
mortality rate of 4.6% (970 of 20,910 cases) (2).
Classical HL (cHL) is a highly curable malignancy
treated with standard chemotherapy or chemoradiotherapy. However,
there is significant percentage of patients, particularly those
with advanced cHL, who will relapse or become refractory to initial
therapy; however, the treatment options for relapsed or refractory
(R/R) cHL are suboptimal (3-5).
Salvage high-dose chemotherapy followed by autologous hematopoietic
stem cell transplantation (ASCT) in patients who are sensitive to
chemotherapy has been the standard therapy for patients with R/R
cHL and has been shown to achieve 50% curability (6-9).
With an improved understanding of cHL biology and its tumor
microenvironment, novel agents with marked efficacy have been
developed, several of which have been approved by the US Food and
Drug Administration (FDA) for patients with R/R cHL. Given the
success of novel therapies for R/R cHL, these approaches have been
explored or are being evaluated in other settings, including in
combination with chemotherapy as frontline therapy, or
consolidation following ASCT. Significant progress has been made in
determining which patients benefit the most from these therapies
and when to administer them. The present review summarizes the key
clinical developments of novel therapies for HL and discusses the
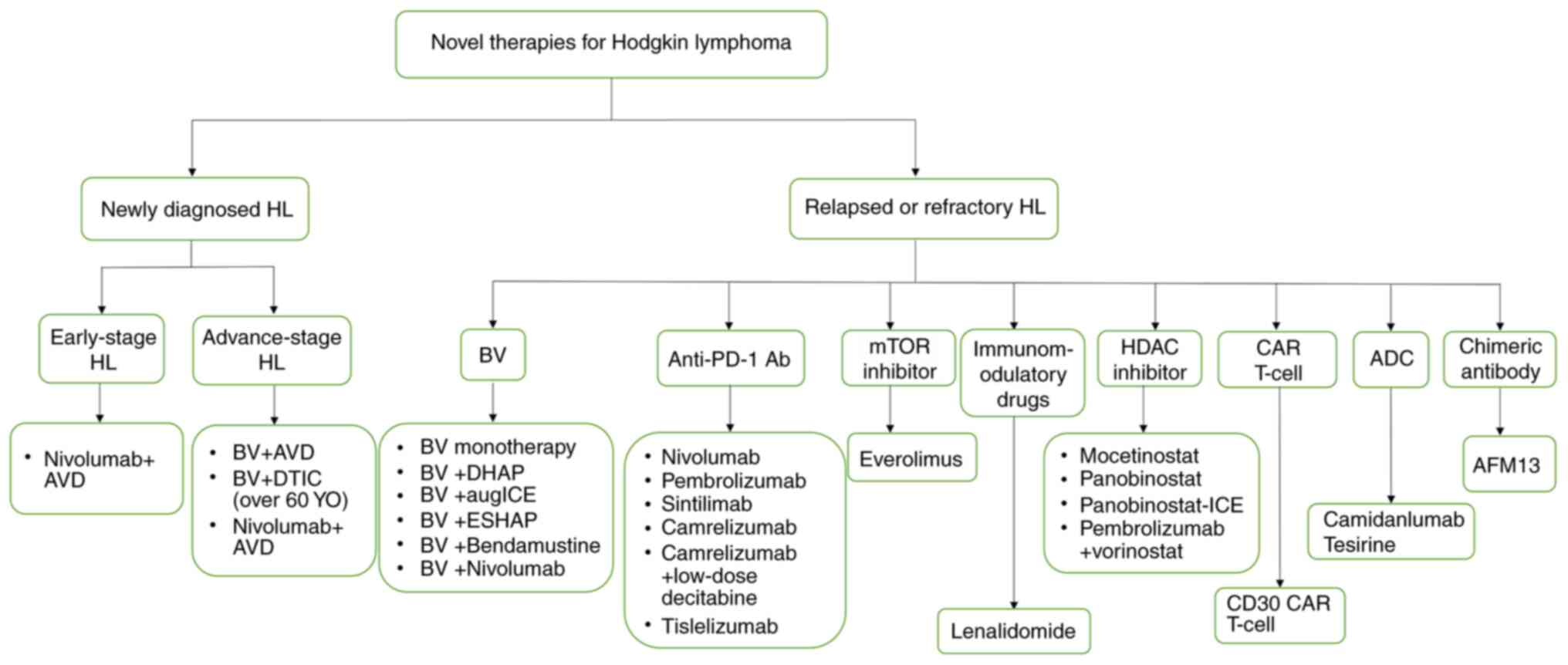
future directions in HL therapy (Fig.
1).
The malignant cells in HL are comprised of Hodgkin
and Reed-Sternberg (HRS) cells, which can be pathognomonic,
multinucleate giant cells or large mononuclear cells (10). CD30, a member of the TNF receptor
superfamily, is a surface antigen that is characteristically
expressed on HRS cells. CD30 has a restricted expression in normal
tissues, rendering it an ideal therapeutic target for cHL (11-14).
Although several anti-CD30 antibodies, including anti-CD30
bispecific antibodies, anti-CD30 immunotoxins or anti-CD30
radiolabeled with iodine-131 have been evaluated in patients with
R/R CD30-expressing lymphomas, the results have not been
encouraging (15-20). BV is an antibody-drug conjugate
(ADC) containing the potent antimitotic drug, monomethylauristatin
E (MMAE), which is attached to the anti-CD30 monoclonal antibody,
cAC10, through a cleavable dipeptide linker. After the ADC is
internalized through receptor-mediated endocytosis, the linker is
exposed to proteolytic enzymes inside of the CD30-positive cells,
followed by the release of MMAE. Intracellular concentrations of
the released drug are high over a prolonged period of time;
however, the amount of effluxed drug is also sufficient to exert
bystander activity on surrounding CD30-negative cells (21).
Although multi-agent chemotherapies, including the
combination of doxorubicin, bleomycin, vinblastine and dacarbazine
(ABVD) can cure ~70-80% of patients with advanced-stage HL
(4,22,23),
the ABVD regimen is often associated with severe bleomycin-induced
pulmonary toxicities which can be life-threatening (24-26).
A phase I, a dose-escalation trial compared the efficacies of BV
combined with ABVD or with doxorubicin, vinblastine and dacarbazine
(AVD) as first-line therapy for treatment-naïve patients with
advanced-stage HL. The complete remission (CR) rate was almost
similar in the BV + ABVD and BV + AVD (95 vs. 96%) groups, although
an unacceptable number of patients (44%) in the BV + ABVD group
presented significant pulmonary toxicities, which was not
experienced by any of the patients in the BV + AVD group (27). Long-term follow-up analysis
revealed that the BV + AVD regimen had an estimated 5-year
failure-free survival and overall survival (OS) of 92 and 100%,
respectively (Table I) (28). Subsequently, a large randomized
phase 3 study, ECHELON-1, reported a significant improvement in the
2-year modified progression-free survival (PFS) rates following
treatment with BV + AVD as compared to ABVD (82.1 vs. 77.2%;
P=0.04) for patients with stage III/IV cHL, and a decrease in the
number of deaths that were not statistically significant from 28
deaths to 39 deaths (Table I)
(29). According to the long-term
follow-up results, the 3-year PFS rates in the BV + AVD and ABVD
arms were 83.1 and 76.0%, respectively, where the BV + AVD regimen
was favored by 7.1% (P=0.005) (30). Recent results have shown that the
5-year PFS rates in BV + AVD and ABVD groups were 82.2 and 75.3%,
respectively (P=0.0017). Importantly, the BV + AVD group had fewer
secondary malignancies than the ABVD group (31). Another study found that a
combination of 1.8 mg/kg BV and 375 mg/m2 dacarbazine
for up to 12 cycles was active and well-tolerated in patients with
treatment-naïve advanced HL aged >60 years, with an objective
response rate (ORR) of 100% and a CR of 62%. The median PFS was
17.9 months at a median observation time of 21.6 months (32).
For patients with R/R HL, salvage chemotherapy
followed by ASCT has been the standard treatment with a cure rate
of ~50% (7-9). A randomized, double-blind phase 3
trial, AETHERA, established BV as an effective consolidation
therapy following ASCT in patients with cHL at high risk of relapse
or progression. The median PFS by an independent review in the BV
group (42.9 months) was superior to the placebo group (24.1
months), after patients received 16 cycles of 1.8 mg/kg BV or
placebo intravenously every 3 weeks, starting 30-45 days following
transplantation (33). Even at the
5-year follow-up, sustained PFS was found to favor the BV group.
The 5-year PFS rates in the BV and placebo groups were 59 and 41%,
respectively. Notably, patients with >2 risk factors with BV
exhibited a significantly higher 5-year PFS than patients who
received the placebo and patients who received BV as early
consolidation delayed time to second subsequent therapy (34).
A pivotal phase II clinical trial demonstrated that
the ORR and CR rates recorded for 102 patients with R/R HL after
failed ASCT, who received a dose of 1.8 mg/kg BV every 3 weeks for
up to 16 cycles, were 75 and 34%, respectively (35). At the 5-year follow-up, the
estimated OS rate was 41% and the PFS rate was 22%. Among the 34
patients with CR, 6 patients who underwent a consolidative
allogeneic stem cell transplantation following BV treatment had
estimated 5-year PFS and OS rates of 67 and 83%, respectively,
while the remaining 28 non-transplant patients with CR had
estimated 5-year PFS and OS rates of 48 and 60%, respectively. The
median OS and PFS were not attained in patients with CR (36). Based on these findings, BV appears
to be an effective option not only as a consolidation therapy
following ASCT, but also as a useful therapy after the failure of
ASCT.
In patients with R/R HL, BV has been evaluated in
combination with traditional salvage chemotherapy. A phase II
transplant BRaVE study was conducted to investigate the efficacy
and safety of BV plus dexamethasone, cisplatin and cytarabine
(DHAP) followed by high-dose chemotherapy (HDC) and autologous
peripheral blood stem-cell transplantation (auto-PBSCT). According
to the [18F] fluorodeoxyglucose-positron emission tomography
(PET)-computed tomography (CT) results, 81% of the patients
achieved metabolic CR (mCR) before HDC/auto-PBSCT, and 5 patients
achieved metabolic partial remission and of which 4 converted to
mCR after HDC/auto-PBSCT. The 2-year PFS and OS were 74 and 95%,
respectively (37). A phase 2
trial that validated the improved curability following high-dose
therapy (HDT)/ASCT treatment reported that of the patients with R/R
HL who received the PET-based sequential salvage therapy with BV
followed by augmented ifosamide, carboplatin and etoposide, 76%
achieved a PET-negative status (38). The long-term results of a trial
from the Spanish GELTAMO Group demonstrated that the combination of
BV and ESHAP on R/R HL achieved ORR of 91%, including 70% CR prior
to transplant. Following a subsequent ASCT, a CR of 82%, a PFS of
71%, and an OS of 91% was recorded at a median follow-up of 27
months (39).
The combination of BV and bendamustine has been
confirmed as a highly potent salvage therapy leading to a high
response prior to ASCT in patients with R/R HL. In a phase 1/2
trial, 55 patients with R/R HL received BV (1.8 mg/kg) on day 1 and
bendamustine (90 mg/m2) on days 1 and 2 every 3 weeks
for up to six cycles followed by ASCT and/or BV monotherapy for up
to 16 cycles. Following a median of two cycles of combination
therapy, the ORR was 92.5%, with 73.6% of patients achieving CR
(40). The OS at 3 years was 93%
with no difference between patients who with ASCT or without ASCT;
the PFS at 3 years was 60.3%, 67.1% for patients with ASCT and
40.4% without ASCT (41). Notably,
a combination regimen of BV and bendamustine could safely achieve a
high overall and complete response, serving as a potential and
efficacious alternative to platinum-based chemotherapy before ASCT,
even in heavily pre-treated patients with R/R HL (42). Of all patients who were
administered a regimen consisting of 1.8 mg/kg BV on day 1 combined
with 120 mg/m2 bendamustine (bendamustine supercharge)
per day on days 2 and 3 every 3 weeks for a total of four courses,
80% could accomplish a Deauville 5-point score of ≤2, which is an
important indicator of favorable efficacy post-ASCT, and a 2-year
PFS rate of 93.7%. Notably, bendamustine was increased to a higher
dose and the timing of bendamustine was subsequently modified
according to the preclinical theory that bendamustine administered
after BV may exert a synergistic effect (43). Numerous clinical results from Italy
have also shown the combination of BV and bendamustine to be a
promising and effective salvage treatment with a manageable
toxicity profile in patients with R/R HL (44,45).
Given these noteworthy results, BV plus chemotherapy may be an
efficacious therapy as a bridge to SCT for the improvement of
curability in patients with R/R HL.
Peripheral neuropathy is the most common toxicity
associated with BV, accounting for 67% of toxicity cases, which
often results in dose reduction and/or treatment discontinuation
(34,36). When combined with systemic
chemotherapy, including AVD, ESHAP or bendamustine, myelotoxicity
with different grades, particularly neutropenia, frequently
occurred (29,39,42).
Granulocyte colony-stimulating factor primary prophylaxis may be
effective for patients to reduce this toxicity (30,40).
Of note, infusion-related reactions (IRRs) were reported in more
than half of patients treated with BV and bendamustine and the
majority of IRRs occurred during cycle two of combination therapy.
Therefore, high-dose corticosteroid and antihistamine premedication
were prophylactically prescribed with combination therapy; however,
this approach only decreased the severity of IRRs and did not
appreciably affect the incidence (40).
Programmed death 1 (PD-1) and its ligands, PD ligand
1 (PD-L1) and PD ligand 2 (PD-L2), exhibit inhibitory signals to
regulate the balance between T-cell activation, tolerance and
immunopathology. After the clearance of pathogens and tumors, PD-1
is required for the induction and maintenance of T-cell tolerance,
where PD-L1 can limit effector T-cell responses and protect against
immune-mediated tissue damage. Although T-cells can recognize the
antigens present in tumors, the immunological clearance of tumors
rarely occurs, which is partly due to immune suppression of the
tumor microenvironment. The expression of PD-L1 on tumors
contributes to this immunological suppression (46). The expression of PD-1 is markedly
elevated in tumor-infiltrating T-cells of HL, while that of PD-L
expression is upregulated on HRS cells (47). In cHL, chromosome 9p24.1
alterations have been shown to increase PD-L1 expression and
further promote their induction via the copy number-dependent Janus
kinase (JAK)2/signal transducer and activator of transcription
(STAT) signaling pathway (48,49).
Another mechanism of PD-L1 overexpression in cHL involves an
Epstein-Barr virus infection (50). Due to these two mechanisms (9p24.1
amplification and Epstein-Barr virus infection), PD-1/PD-L1
blockade has been an ideal treatment for cHL.
Nivolumab was the first anti-PD-1 antibody approved
by the FDA for R/R cHL. In a heavily pre-treated population of
patients with cHL, of whom 78% relapsed after ASCT and 78% relapsed
after BV, all patients received 3 mg/kg nivolumab every 2 weeks.
The ORR of nivolumab was 87%, with a CR of 17% and partial
remission (PR) of 70%. Responses were durable, with 86% PFS at 6
months (51). Findings from the
multicohort single-arm phase II trial, CheckMate 205, suggested
that nivolumab may be associated with a favorable safety profile
and long-term benefits across a wide range of patients with R/R cHL
(Table II) (52). In that study, 243 patients were
divided to three cohorts due to treatment history, including 63
patients in the BV-naïve (cohort A), 80 in the BV received after
autologous hematopoietic cell transplantation (auto-HCT) (cohort
B), and 100 in the BV received before and/or after auto-HCT (cohort
C). Following a median follow-up of 18 months, the ORR was 69%
overall, including 16% of patients achieving CR and 53% achieving
PR. The ORRs were 65, 68 and 73% in cohorts A, B and C,
respectively, with CR in 29, 13 and 12% of patients, respectively.
The median duration of response (DOR) and median PFS were 16.6 and
14.7 months, respectively, and the median OS was not reached. The
response rates and median PFS were comparable in patients who
received BV after or only before auto-HCT (52). In addition, the 5-year PFS and OS
were 18 and 71%, respectively. It appears feasible to terminate the
use of nivolumab after 1 year of CR and restart therapy upon
disease progression (53).
Subsequently, the results from cohort D of the CheckMate 205 trial
revealed that nivolumab monotherapy followed by nivolumab plus
doxorubicin, vinblastine and dacarbazine (N-AVD) was a safe and
efficacious regimen for newly diagnosed, advanced-stage cHL. The
cohort had a total of 51 patients who received 4 doses of nivolumab
monotherapy, followed by 12 doses of N-AVD; doses administered
every 2 weeks, and nivolumab (240 mg) was administered
intravenously. The ORR was 84%, with 67% CR and a 9-month modified
PFS of 92%. Patients with a higher PD-L1 expression on HRS cells
tended to have more favorable responses to nivolumab monotherapy
(P=0.096), and significantly deeper and more durable responses to
N-AVD (P=0.041) (54). Recently,
nivolumab and AVD was evaluated for patients with early-stage
unfavorable HL in a randomized phase 2 German Hodgkin Study Group
NIVAHL trial (55). A total of 109
patients were randomly assigned (1:1) to receive either a
concomitant treatment with four cycles of N-AVD or sequential
treatment with four doses of nivolumab, two cycles of N-AVD, and
two cycles of AVD. For both groups, a consolidating 30-Gy
involved-site radiotherapy (IS-RT) was scheduled post-systemic
treatment. At interim evaluation after two cycles of N-AVD or four
doses of nivolumab monotherapy, the ORR was 100 and 96%, with CR in
87 and 51%, respectively. Following treatment, the CR was 90 and
94% in the concomitant treatment and sequential treatment, with a
12-month PFS of 100 and 98%, respectively (55).
In addition to the high response rates achieved with
nivolumab and BV monotherapies, their combination has also been
reported to be well-tolerated and highly effective as a first
salvage therapy in patients with R/R HL. An ORR of 82% and a CR of
61% were recorded for the combination, which was higher than BV or
nivolumab monotherapy in R/R HL. Importantly, the responses were
achieved in an outpatient setting, where nausea, fatigue and
infusion-related reactions were the most common adverse events
(AEs) and differed from toxicities associated with traditional
salvage chemotherapy (56). Based
on these clinical trial results, nivolumab not only exhibits
impressive responses in R/R HL, but also exhibits notable efficacy
in addition to AVD in newly diagnosed patients.
In comparison to pembrolizumab, nivolumab (3 mg/kg,
every 2 weeks) had higher mean incidences of all-grade AEs and AEs
of grade ≥3 (57). When nivolumab
was administered as monotherapy, the most common drug-related AEs
of any grade were fatigue, diarrhea and IRRs and most common grade
3 or 4 drug-related AEs were elevated lipase, neutropenia and
elevated levels of alanine aminotransferase (ALT). A few patients
discontinued treatment primarily due to pneumonitis and autoimmune
hepatitis (52). When combined
with multi-agent chemotherapy, such as AVD, hematologic AEs of
grade ≥3 most commonly occurred, which warrants caution
particularly in patients over the age of 60 (54,55).
In another combination regimen of BV and nivolumab, a relatively
higher proportion of patients (44%) experienced IRRs mostly during
cycle 2 of the study therapy, most of which were grade 1 or 2
(56).
Pembrolizumab is a fully humanized IgG4/κ anti-PD-1
monoclonal antibody. A large phase II trial, KEYNOTE-087, which
enrolled 210 patients with R/R cHL, demonstrated that 69 patients
relapsed after ASCT followed by BV (cohort 1), 81 patients relapsed
after salvage chemotherapy and BV without ASCT (cohort 2) and 60
patients relapsed after ASCT without BV (cohort 3). All patients
received pembrolizumab 200 mg once every 3 weeks without
premedication for a maximum of 24 months. According to the blinded
independent central review, the ORR and CR were 69.0 and 22.4%,
respectively. In cohorts 1, 2 and 3, the ORRs were 73.9, 64.2 and
70.0%, respectively, while the CRs were 21.7, 24.7 and 20.0%,
respectively (58). With a median
of 39.5 months of follow-up, pembrolizumab continued to exhibit
efficacious and durable antitumor activity in patients with R/R
cHL, as the ORR was 71% with a 27.6% CR and a 43.3% PR. The overall
median PFS was 13.6 months, and the PFS of cohorts 1, 2 and 3 were
16.4, 11.1 and 19.4 months, respectively. The median OS was not
reached in the total population or any cohort. Notably, 17 patients
received an additional 17 cycles of pembrolizumab (second-course)
as they experienced disease progression upon discontinuing
pembrolizumab after achieving an initial confirmed CR post-6 months
of treatment. The second-course treatment could re-induce remission
in most patients who previously reached CR, including 31.3% of
patients in CR and 37.5% of patients in PR (59). Additionally, pembrolizumab has been
demonstrated as effective with an acceptable safety profile in
patients with R/R cHL after ASCT. The PFS at 18 months was 82% and
OS was 100% (60). The most common
treatment-related AEs (TRAEs) were hypothyroidism and pyrexia. And
the most common grade 3 or 4 TRAEs were neutropenia, dyspnea and
diarrhea (58). For transplant
eligible R/R cHL patients, pembrolizumab plus gemcitabine,
vinorelbine and liposomal doxorubicin (pembro-GVD) as second-line
therapy achieved 100% of patients with ORR and 95% with CR in a
phase II study. Among the 38 evaluable patients, 36 (95%) patients
received HDT/AHCT and all transplanted patients were in remission
at a median post-transplant follow-up of 13.5 months. The majority
of AEs were grade 1 or 2, and few grade 3 AEs included rash (n=1),
elevated AST/ALT (n=4), mucositis (n=2), neutropenia (n=4) and
hyperthyroidism (n=1) (61).
Sintilimab, a highly selective and fully humanized
anti-PD-1 monoclonal antibody, was evaluated in a phase II trial,
ORIENT-1, which involved 96 adult patients from 18 hospitals in
China with R/R cHL who had received two or more lines of therapy
(62). All the patients received
sintilimab at 200 mg administered intravenously over a period of
30-60 min, once every 3 weeks. In the full analysis set (n=92), the
ORR and CR were 80.4 and 34%, respectively, with 18% of patients
exhibiting mCR according to PET-CT scans, and 27% exhibiting CR on
contrast-enhanced CT scans. The PFS at 6 months was 77.6% by the
cut-off date, and the median PFS was not attained. All patients
experienced at least one treatment-emergent AE, the majority of
which were grade 1 or 2, and 25% of patients had grade 3 or 4 AEs.
The most common TRAE was pyrexia (41%), and the drug-related severe
AEs were pneumonitis (3%), lung infection (3%) and infusion
reaction (2%) (62).
Camrelizumab (SHR-1210) is a humanized high-affinity
IgG4 anti-PD-1 monoclonal antibody that has exhibited promising
antitumor efficacies with manageable toxicities in clinical trials
(63-65). In a phase II study, 75 patients who
had failed to achieve remission status, experienced progression
following ASCT or had received at least 2 prior lines of systemic
chemotherapies were administered camrelizumab at 200 mg every 2
weeks. With a median follow-up of 12.9 months, the ORR was 76%,
with a CR and PR of 28 and 48%, respectively. According to the
independent review committee assessment, the 12-month PFS rate was
66.5% and the median OS was not reached (66). Notably, low-dose decitabine, a
hypomethylating agent, in addition to camrelizumab can lead to a
significantly higher CR rate than camrelizumab alone in patients
with R/R cHL. Even for patients who relapsed or were refractory to
prior anti-PD-1 monotherapy such as nivolumab and pembrolizumab,
there were still 52% of patients who benefited from the combination
of decitabine and camrelizumab, with 28% achieving CR (67). It is worth noting that the most
common treatment-related AE was cutaneous reactive capillary
endothelial proliferation with all grade 1 or grade 2, both in
monotherapy group (84%) or combined with decitabine (87%). The
pathological results from a few patients indicated the benign
proliferation of endothelial cells in the lesion tissue (66,67).
It has been reported that Fcϒ receptor compromises
the antitumor activity of anti-PD-1 antibodies as the activity of
anti-PD-1 antibodies are Fcϒ receptor-independent (68). Tislelizumab is an investigational
humanized IgG4 monoclonal antibody binding to the extracellular
domain of human PD-1 with high specificity and affinity. In
addition, tislelizumab was specifically engineered to minimize Fcϒ
receptor binding on macrophages, which may abrogate
antibody-dependent phagocytosis. In a multicenter, single-arm,
phase 2 study, 70 patients with R/R cHL after the failure of or
ineligible of ASCT were enrolled and treated with tislelizumab at
200 mg intravenously every 3 weeks. With a median follow-up of 33.8
months, the ORR was 87.1% and CR was 67.1%. The 3-year OS and PFS
rates were 84.8 and 40.8%, respectively. While 97.1% of patients
experienced treatment-emergent AEs (TEAEs) of any grade, 41.4%
experienced grade ≥3 TEAEs. The most common TEAEs were pyrexia
(57.1%), upper respiratory tract infection (38.6%), hypothyroidism
(37.1%), weight gain (34.3%), cough (21.4%), a decrease in white
blood cell count (21.4%) and an increase in ALT levels (20.0%).
TEAEs leading to treatment discontinuation occurred in 6 (8.6%)
patients, including pneumonitis in two patients, and focal
segmental glomerulosclerosis, organizing pneumonia, psychomotor
skills impaired and seizure in one patient. Correlative biomarker
analysis identified that Fcϒ receptor I-expressing macrophages had
no observed impact on either the CR or PFS rate achieved with
tislelizumab. Patients with a shorter PFS were associated with
'B-cell marker' cluster including CD19, CD22,
CD72 and CD79B genes, along with interferon
regulatory factors, including IRF1, IRF2,
IRF3, IRF8 and IRF9 (69,70).
T-cell immunoglobulin and ITIM domain (TIGIT) is an
inhibitory receptor exclusively expressed on lymphocytes including
cytotoxic T-cells, helper T-cells, regulatory T-cells and natural
killer (NK) cells. The primary ligand of TIGHT is CD155, which is
expressed in healthy tissues including monocytes, dendritic cells
and endothelial cells, as well as in cancer cells (71-73).
Based on these insights, TIGIT may be a potential target for
patients with HL. A phase I, multicenter, dose-escalation/expansion
study, SCNTGT-001, is currently underway to investigate the safety
and preliminary efficacy of SEA-TGT, an effector-function enhanced
human monoclonal antibody targeting TIGIT, in multiple relapsed,
refractory or progressive metastatic solid tumors including cHL
(74).
It has been demonstrated that the JAK-mediated
signaling pathway is upregulated in several patients with HL
(75), and its blockade can
inhibit HL cell proliferation. In addition, the genomic
amplification of 9p24.1, which includes the JAK2 locus, is commonly
observed in HL and results in the activation of STAT6 that
stimulates tumor cell growth (76,77).
Ruxolitinib is the first potent and selective inhibitor of JAK1/2
that can be administered orally. In a phase II study on 32
evaluable patients with R/R HL, ruxolitinib (15 or 20 mg) was
administered twice daily. Following six cycles, the ORR was 9.4%,
with the optimal ORR being 18.8%. The median DOR, median PFS and
median OS were 7.7, 3.5 and 27.1 months, respectively. A total of
40 AEs were observed in 14/33 patients (42.4%) and 25 of which were
grade ≥3. All AEs were considered to be related to ruxolitinib,
with anemia being the most common. Other main causes of AEs of
grade ≥3 included lymphopenia and infections (78). Another clinical study, involving 13
patients with R/R HL who received ruxolitinib at 20 mg twice daily
every 28 days, reported that the disease control rate was 54%,
including 1 patient with CR, 5 patients with PR and 1 patient with
stable disease (SD). JAK2 amplification via FISH analysis was shown
in 4 patients with HL with PR or SD. The median PFS was 3.6 months
and the median OS was not reached within the median follow-up of
37.0 months. Treatment-related AEs were reported in 14 patients
(73.6%), although the majority of events were mild (grade 1 or 2)
(79). Based on these results,
ruxolitinib exhibits a long-term clinical activity with mild
toxicity, which may be combined with other regimens in the
future.
Preclinical evidence has indicated that
phosphatidyl-inositide 3 kinase (PI3K) and its substrate Akt are
constitutively activated in HL-derived cell lines. Moreover,
several downstream effectors of Akt signaling, including glycogen
synthase kinase 3 and mammalian target of rapamycin (mTOR)
substrates 4E-BP1 and p70 S6 kinase, have also been found to be
phosphorylated in HL cells (80).
Everolimus, an oral mTOR inhibitor, has been confirmed to exert an
antitumor effect in HL cells (81). A phase II clinical trial reported
that 10 mg everolimus daily was administered to 57 patients that
had relapsed following HDT/ASCT and/or a gemcitabine-, vinorelbine-
or vinblastine-containing regimen. The ORR was 45.6%, including
8.8% of patients in CR and 36.8% of patients in PR. The median PFS
was 8.0 months, with 12% of patients having a response duration
>1 year. The most common TRAEs were thrombocytopenia, fatigue,
anemia, rash and stomatitis (82).
Another phase I/II multicenter trial conducted by the German
Hodgkin Study Group evaluated the effect of adding everolimus to
the standard DHAP towards improving the CR rate of reinduction
chemotherapy. Although the addition of everolimus to DHAP was
feasible, the efficacy of the combinatorial therapy failed to
achieve an improvement (83).
Lenalidomide, a thalidomide analogue, exhibits
multiple mechanisms of action, including the direct induction of
apoptosis in malignant cells, antiangiogenic effects and indirectly
affects the tumor microenvironment, such as the activation of NK
cells and T-cells (84-86). It has been long recognized that the
critical cHL pathogenesis is scant HRS cells surrounded by the
tumor microenvironment. In a phase II trial, 38 heavily pre-treated
patients were administered lenalidomide at 25 mg daily on days 1-21
of a 28-day cycle until the occurrence of an unacceptable AE or
disease progression. Among these patients, 33 patients had received
a stem cell transplantation and had a median number of four prior
therapies. The results revealed an ORR of 19%, a cytostatic ORR of
33%, a median PFS of 4 months, and a median OS of 20 months. The
treatment was well-tolerated, with hematological toxicities being
the most common grade 3 or 4 AE (87). Another phase I study that enrolled
patients aged ≥60 years with early unfavorable- or advanced-stage
HL who received 4-8 cycles of AVD and lenalidomide in escalation
with overdose control confirmed ORRs of 67 and 94% with a
lenalidomide dose of 20 and 25 mg, respectively. Although the
results demonstrated that this combination was highly effective and
feasible, with the 3-year estimates for PFS and OS being 69.7 and
83.8%, it caused severe hematological acute toxicities, suggesting
that this may not be an ideal regimen in older patients with HL
(88). Since both everolimus and
lenalidomide have exhibited clinical efficacies as single agents in
patients with R/R HL and non-HL, a phase I/II trial attempted to
evaluate the activity this combination at the Mayo Clinic. The ORR
in the cHL cohort of 10 patients was 25%, with 2 patients each
obtaining CR and PR, respectively (89).
HDACs are involved in multiple important cell
functions, including cell cycle progression, angiogenesis, cell
differentiation and apoptosis, and immunity. Therefore, HDAC
inhibitors can be used as an antitumor therapy against a broad
spectrum of hematologic and solid neoplasms (90,91).
Mocetinostat, an oral isotype-selective HDAC inhibitor, was
evaluated in R/R HL with two different dose cohorts (85 and 110
mg). A total of 51 patients received mocetinostat three times
weekly for every 28 days a cycle. Of these, 81% of patients who
completed at least two cycles of therapy exhibited a reduction in
tumor measurements, and the ORRs were 35 and 21% for the 110 and 85
mg dose cohorts, respectively. There were 4 patients that succumbed
during the study, all in the 110 mg cohort, with two of these
deaths considered to be treatment-related. Mocetinostat, at a dose
of 85 mg, demonstrated improved tolerance without a reduced
efficacy and should be used for developing a single agent in the
future (92). Panobinostat, a
potent pan-deacetylase inhibitor, was administered at 40 mg orally
three times a week in 129 patients with heavily pre-treated cHL. A
total of 96 patients (74%) had tumor reductions with an ORR of 27%,
a CR of 4% and a PR of 23%. However, not all patients responded to
the immediately preceding panobinostat and the median time to
response was 2.3 months. In addition, the DOR was 6.9 months and
the median PFS was 6.1 months. Gastrointestinal AEs were generally
grade 1 and 2 and most common grade 3 and 4 toxicities were
manageable hematological AEs, primarily thrombocytopenia (93). The results from a phase 2 study
that evaluated the efficacy of vorinostat in R/R HL were not
encouraging, with an ORR of 4% and a median PFS of 4.8 months
(94). The preliminary results
from a phase I trial of pembrolizumab plus vorinostat in patients
with R/R HL revealed that the combination produced objective
responses with an ORR and a CR of 100 and 44%, including patients
who had a disease progression before an anti-PD1 treatment
(95).
Several studies have demonstrated that HDAC
inhibitors can synergize the antitumor effects of chemotherapeutic
agents in HL cell lines (96-98).
A small number of patients with R/R cHL were recruited to evaluate
the efficacy and safety of panobinostat in combination with
ifosfamide, carboplatin, etoposide (P-ICE) in a phase I/phase II
study. The results revealed that P-ICE exhibited an excellent
response, with a CR of 82% in the P-ICE arm compared with 67% in
the ICE arm, but with increased myelosuppression (99). Another combination of panobinostat
and lenalidomide in patients with R/R HL was evaluated in a phase
I/II trial. However, the recorded efficacy was limited with an ORR
of 16.7% and a median PFS of 3.8 months, and severe AEs, such as
neutropenia and febrile neutropenia, indicating that further
evaluation was not warranted (100).
The antibody-drug conjugate, ADCT-301 (camidanlumab
tesirine), is composed of an anti-CD25 monoclonal antibody
conjugated to a pyrrolobenzodiazepine dimer toxin. As CD25 is
expressed on the cell surface of a number of lymphoma types,
including cHL, a phase I clinical trial was conducted to evaluate
the efficacy of camidanlumab tesirine in patients with R/R cHL
(101). The study enrolled 60
patients with the median number of prior therapies being five
(range, 2-15). The ORR and CR in 55 patients were 69.1 and 43.6%,
respectively. The recommended dose of camidanlumab tesirine was 45
µg/kg every 3 weeks with an ORR of 80.8% and a CR of 50%.
The ORR was 80.8% for patients who had previously received BV and
80.0% for those who had received both checkpoint inhibitors and BV.
The ORR was 85.7% for those who received a checkpoint inhibitor, BV
and a hematopoietic cell transplant. The median PFS and DOR were
6.7 and 7.7 months, respectively. The most common grade 3 and 4
TEAEs were liver dysfunction (36.7%), maculopapular rash (13.3%),
anemia (8.3%) and thrombocytopenia (5.0%) (101).
AFM13 is the first bispecific and tetravalent
chimeric antibody that can specifically recruit NK cells by binding
to CD16A and targeting CD30 expressed on tumor cells. In a phase 1
clinical study, AFM13 was administered to 28 patients with heavily
pre-treated R/R HL with doses ranging from 0.01 to 7 mg/kg, where
doses >1.5 mg/kg exhibited more potent efficacy (102). The maximum tolerated dose was not
reached. The overall disease control was 61.5%, achieving a PR of
11.5% and a SD of 50%. Of the 7 patients who had received BV as the
most recent therapy, 6 patients had SD after AFM13 treatment. Of
note, the majority of AEs were mild to moderate, including fever
(53.6%), chills (39.3%), headache (28.6%), nausea (17.9%),
nasopharyngitis (17.9%), infusion reaction (14.3%), rash (14.3%),
vomiting (14.3%) and pneumonia (14.3%) (102).
The combination of AFM13 and pembrolizumab is
currently being evaluated as a potent and well-tolerated salvage
regimen in patients with R/R HL. A phase 1b clinical trial enrolled
30 patients with R/R HL who had a median age of 34 years and a
median number of prior therapies of four. All patients had
previously failed standard treatments including BV, while 13 had BV
as their most recent therapy. In the 23 patients with maximum
administered dose, the ORR and CR were 87 and 35%, respectively.
The most common AEs were IRRs (80%), rash (30%), pyrexia (23%),
nausea (23%), diarrhea (20%), fatigue (17%), headache (17%) and
elevated aspartate aminotransferase (13%), and elevated alanine
aminotransferase (10%); however, the majority of IRRs were
manageable without treatment discontinuations (103).
CAR T-cell therapy for hematological malignancies
has been a breakthrough advancement in recent years. CARs are
recombinant antigen receptors that contain an antigen recognition
domain and a T-cell signaling domains (104-106). Therefore, CD30 CAR T-cell therapy
is another method which can be used to specifically target the
surface antigen CD30 of HL, apart from BV. In a phase I clinical
trial, 18 patients with heavily pre-treated R/R cHL were infused
with a mean of 1.56×107 (range, 1.1-2.1) CAR T-cells/kg
after conditioning regimens. The PFS was 6 months and 7 patients
achieved PR with 6 patients with SD (Table III). The CD30 CAR T-cell infusion
was safe and tolerable. The most common treatment-related AEs
included nausea/vomiting (27.8%) and urticarial-like rash (11.1%)
(107). When compared to the
results from the study by Wang et al (107), which used lymphodepletion before
CAR T-cell infusion based on the more general practice, that study
demonstrated the direct effects of CD30 CAR T-cells as a major
strength. The optimal responses observed mainly occurred in
patients with low soluble CD30, since CD30 is present in a soluble
form in the plasma of HL patients with advanced/aggressive disease
(108), suggesting that the
affinity of the single-chain variable fragment (scFv) and a lower
burden of disease may be important. Additionally, that study
proposed that CD30 CAR T-cells may synergize PD1/PD-L1 blockade
(109).
Another study evaluated the efficacy and safety of
CD30 CAR T-cell therapy in 9 patients with R/R CD30+
lymphoma (110). The study
enrolled 6 patients with HL and 3 patients with anaplastic large
cell lymphoma who were administered a median dose of
1.4×107/kg CD30 CAR T-cells. The results were promising,
with 7 patients achieving CR at the first visit and a median PFS of
13 months. Moreover, 3 patients with CR continued to be in
remission for >2 years. A total of 5 patients with HL,
refractory to anti-PD-1 antibody treatment were infused with
anti-PD-1 antibody again; one relapsed patient regained a CR status
and the other 4 patients sustained CR for at least a further 8
months, which indicated a synergistic effect of CD30 CAR T-cell
therapy with the subsequent anti-PD-1 antibody treatment. Most AEs
were mild, and it was reported that patients with a greater tumor
burden may exhibit a more severe cytokine release syndrome (CRS)
(110).
In a phase I/II clinical trial, 41 patients with
heavily treated R/R HL received autologous CD30 CAR T-cell therapy.
The median number of prior therapies was 7, including BV, immune
checkpoint inhibitor and stem cell transplantation. The dose levels
of CD30 CAR T-cells ranged from 1×108/m2 to
2×108/m2. Although 10 patients (24%)
developed CRS, all reported events were grade 1 and all patients
recovered without tocilizumab and/or steroids. Some patients
experienced prolonged cytopenias, particularly thrombocytopenia
without significant complications. The ORR was 62% and the CR was
51%. The 1-year OS and 1-year PFS were 94 and 36%, respectively.
Notably, CD30 CAR T-cells at the dose of
2×108/m2 after fludarabine-based
lymphodepletion exhibited notable efficacy with no significant
toxicity (111).
A pilot study reported the results of 5 patients
undergoing the successful manufacturing of non-viral RNA
anti-CD19-directed CAR-modified T-cells (CART19), on the hypothesis
that some circulating CD19+ B cells are putative HRS
stem cells (112) and cytokines
produced by CART19 potentially changing the tumor microenvironment
(113). This non-viral RNA CART19
was manufactured by transfecting T-cells with messenger RNA using
electroporation, resulting in transient expression of CAR, which
limited the potential for AEs. There were no severe toxicities with
transient response (114).
Advances in HL treatment have significantly improved
patient survival. While radiotherapy and chemotherapy have been the
primary regimens for HL for decades, HSCT is considered a salvage
therapy for R/R HL (115),
although it is associated with high relapse rates (40%) (116). With a better understanding of HL
and its associated tumor microenvironment, the antibody-drug
conjugate, BV and immune checkpoint inhibitors have exhibited
marked antitumor efficacies in R/R cHL. A combination of anti-PD-1
antibodies and BV may be an effective treatment option for patients
who are untreated, localized and intolerant to chemotherapy. For
patients who are untreated with advanced-stage HL and are eligible
to receive anti-PD-1 antibodies, AVD combined with anti-PD-1
antibody for six cycles may be effective. Patients can also receive
a combination of AVD and BV therapy to avoid the toxicity of
bleomycin. Patients experiencing a first relapse are encouraged to
receive salvage chemotherapy followed by ASCT. In addition,
administering anti-PD-1 antibodies may be a therapeutic option for
refractory patients, while BV may be used in patients who are
contradictory to anti-PD-1 antibodies. Patients who have failed
both anti-PD-1 antibody and BV, can choose from other targeted
therapies including lenalidomide, PI3K/mTOR inhibitors, HDAC
inhibitor, CD25 antibody-drug conjugate or anti-CD30 CAR T-cell
therapy. The integration of these novel strategies into early lines
of therapy may prove beneficial to achieve higher curability,
sustained benefits and manageable toxicity. In addition to these
therapies, other agents with various mechanisms also demonstrate a
certain level of efficacy. Notably, CD30 CAR T-cell therapy
exhibits potent clinical activity in R/R HL and is well-tolerable
with manageable toxicity. However, further studies are required to
focus on developing a personalized regimen for each patient, in
order to make it easier to select the optimal treatment with
appropriate timing and minimal the long-term toxicity.
Not applicable.
YC and XS conceived and designed the study. YC wrote
the manuscript. XD, LX, JZ, XZ, NL and XS revised the manuscript.
All authors have read and approved the final manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
No funding was received.
|
1
|
Hodgkin: On some morbid appearances of the
absorbent glands and spleen. Med Chir Trans. 17:68–114. 1832.
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnson P, Federico M, Kirkwood A, Fossa
A, Berkahn L, Carella A, d'Amore F, Enblad G, Franceschetto A,
Fulham M, et al: Adapted treatment guided by interim PET-CT scan in
advanced Hodgkin's lymphoma. N Engl J Med. 374:2419–2429. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Viviani S, Zinzani PL, Rambaldi A,
Brusamolino E, Levis A, Bonfante V, Vitolo U, Pulsoni A, Liberati
AM, Specchia G, et al: ABVD versus BEACOPP for Hodgkin's lymphoma
when high-dose salvage is planned. N Engl J Med. 365:203–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Radford J, Illidge T, Counsell N, Hancock
B, Pettengell R, Johnson P, Wimperis J, Culligan D, Popova B, Smith
P, et al: Results of a trial of PET-directed therapy for
early-stage Hodgkin's lymphoma. N Engl J Med. 372:1598–1607. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sureda A, Constans M, Iriondo A, Arranz R,
Caballero MD, Vidal MJ, Petit J, López A, Lahuerta JJ, Carreras E,
et al: Prognostic factors affecting long-term outcome after stem
cell transplantation in Hodgkin's lymphoma autografted after a
first relapse. Ann Oncol. 16:625–633. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Majhail NS, Weisdorf DJ, Defor TE, Miller
JS, McGlave PB, Slungaard A, Arora M, Ramsay NK, Orchard PJ,
MacMillan ML, et al: Long-term results of autologous stem cell
transplantation for primary refractory or relapsed Hodgkin's
lymphoma. Biol Blood Marrow Transplant. 12:1065–1072. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sirohi B, Cunningham D, Powles R, Murphy
F, Arkenau T, Norman A, Oates J, Wotherspoon A and Horwich A:
Long-term outcome of autologous stem-cell transplantation in
relapsed or refractory Hodgkin's lymphoma. Ann Oncol. 19:1312–1319.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith SD, Moskowitz CH, Dean R, Pohlman B,
Sobecks R, Copelan E, Andresen S, Bolwell B, Maragulia JC, Vanak
JM, et al: Autologous stem cell transplant for early
relapsed/refractory Hodgkin lymphoma: Results from two transplant
centres. Br J Haematol. 153:358–363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shanbhag S and Ambinder RF: Hodgkin
lymphoma: A review and update on recent progress. CA Cancer J Clin.
68:116–132. 2018. View Article : Google Scholar :
|
|
11
|
Durkop H, Latza U, Hummel M, Eitelbach F,
Seed B and Stein H: Molecular cloning and expression of a new
member of the nerve growth factor receptor family that is
characteristic for Hodgkin's disease. Cell. 68:421–427. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiarle R, Podda A, Prolla G, Gong J,
Thorbecke GJ and Inghirami G: CD30 in normal and neoplastic cells.
Clin Immunol. 90:157–164. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horie R and Watanabe T: CD30: Expression
and function in health and disease. Semin Immunol. 10:457–470.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schwab U, Stein H, Gerdes J, Lemke H,
Kirchner H, Schaadt M and Diehl V: Production of a monoclonal
antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's
disease and a subset of normal lymphoid cells. Nature. 299:65–67.
1982. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hartmann F, Renner C, Jung W and
Pfreundschuh M: Anti-CD16/CD30 bispecific antibodies as possible
treatment for refractory Hodgkin's disease. Leuk Lymphoma.
31:385392. View Article : Google Scholar
|
|
16
|
Falini B, Bolognesi A, Flenghi L, Tazzari
PL, Broe MK, Stein H, Dürkop H, Aversa F, Corneli P, Pizzolo G, et
al: Response of refractory Hodgkin's disease to monoclonal
anti-CD30 immunotoxin. Lancet. 339:1195–116. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schnell R, Staak O, Borchmann P, Schwartz
C, Matthey B, Hansen H, Schindler J, Ghetie V, Vitetta ES, Diehl V
and Engert A: A Phase I study with an anti-CD30 ricin A-chain
immunotoxin (Ki-4.dgA) in patients with refractory CD30+ Hodgkin's
and non-Hodgkin's lymphoma. Clin Cancer Res. 8:1779–1786.
2002.PubMed/NCBI
|
|
18
|
Borchmann P, Schnell R, Fuss I, Manzke O,
Davis T, Lewis LD, Behnke D, Wickenhauser C, Schiller P, Diehl V
and Engert A: Phase 1 trial of the novel bispecific molecule
H22xKi-4 in patients with refractory Hodgkin lymphoma. Blood.
100:3101–3107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schnell R, Dietlein M, Staak JO, Borchmann
P, Schomaecker K, Fischer T, Eschner W, Hansen H, Morschhauser F,
Schicha H, et al: Treatment of refractory Hodgkin's lymphoma
patients with an iodine-131-labeled murine anti-CD30 monoclonal
antibody. J Clin Oncol. 23:4669–4678. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Forero-Torres A, Leonard JP, Younes A,
Rosenblatt JD, Brice P, Bartlett NL, Bosly A, Pinter-Brown L,
Kennedy D, Sievers EL and Gopal AK: A Phase II study of SGN-30
(anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large
cell lymphoma. Br J Haematol. 146:171–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okeley NM, Miyamoto JB, Zhang X, Sanderson
RJ, Benjamin DR, Sievers EL, Senter PD and Alley SC: Intracellular
activation of SGN-35, a potent anti-CD30 antibody-drug conjugate.
Clin Cancer Res. 16:888–897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gordon LI, Hong F, Fisher RI, Bartlett NL,
Connors JM, Gascoyne RD, Wagner H, Stiff PJ, Cheson BD,
Gospodarowicz M, et al: Randomized phase III trial of ABVD versus
Stanford V with or without radiation therapy in locally extensive
and advanced-stage Hodgkin lymphoma: An intergroup study
coordinated by the Eastern Cooperative Oncology Group (E2496). J
Clin Oncol. 31:684–691. 2013. View Article : Google Scholar :
|
|
23
|
Federico M, Luminari S, Iannitto E,
Polimeno G, Marcheselli L, Montanini A, La Sala A, Merli F,
Stelitano C, Pozzi S, et al: ABVD compared with BEACOPP compared
with CEC for the initial treatment of patients with advanced
Hodgkin's lymphoma: Results from the HD2000 Gruppo Italiano per lo
Studio dei Linfomi Trial. J Clin Oncol. 27:805–811. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yagoda A, Mukherji B, Young C, Etcubanas
E, Lamonte C, Smith JR, Tan CT and Krakoff IH: Bleomycin, an
antitumor antibiotic. Clinical experience in 274 patients. Ann
Intern Med. 77:861–870. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duggan DB, Petroni GR, Johnson JL, Glick
JH, Fisher RI, Connors JM, Canellos GP and Peterson BA: Randomized
comparison of ABVD and MOPP/ABV hybrid for the treatment of
advanced Hodgkin's disease: Report of an intergroup trial. J Clin
Oncol. 21:607–614. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoskin PJ, Lowry L, Horwich A, Jack A,
Mead B, Hancock BW, Smith P, Qian W, Patrick P, Popova B, et al:
Randomized comparison of the stanford V regimen and ABVD in the
treatment of advanced Hodgkin's Lymphoma: United Kingdom National
Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J
Clin Oncol. 27:5390–5396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Younes A, Connors JM, Park SI, Fanale M,
O'Meara MM, Hunder NN, Huebner D and Ansell SM: Brentuximab vedotin
combined with ABVD or AVD for patients with newly diagnosed
Hodgkin's lymphoma: A phase 1, open-label, dose-escalation study.
Lancet Oncol. 14:1348–1356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Connors JM, Ansell SM, Fanale M, Park SI
and Younes A: Five-year follow-up of brentuximab vedotin combined
with ABVD or AVD for advanced-stage classical Hodgkin lymphoma.
Blood. 130:1375–1377. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Connors JM, Jurczak W, Straus DJ, Ansell
SM, Kim WS, Gallamini A, Younes A, Alekseev S, Illés Á, Picardi M,
et al: Brentuximab Vedotin with Chemotherapy for Stage III or IV
Hodgkin's Lymphoma. N Engl J Med. 378:331–344. 2018. View Article : Google Scholar
|
|
30
|
Straus DJ, Dlugosz-Danecka M, Alekseev S,
Illes A, Picardi M, Lech-Maranda E, Feldman T, Smolewski P, Savage
KJ, Bartlett NL, et al: Brentuximab vedotin with chemotherapy for
stage III/IV classical Hodgkin lymphoma: 3-year update of the
ECHELON-1 study. Blood. 135:735–742. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Straus DJ, Długosz-Danecka M, Connors JM,
Alekseev S, Illés Á, Picardi M, Lech-Maranda E, Feldman T,
Smolewski P, Savage KJ, et al: Brentuximab vedotin with
chemotherapy for stage III or IV classical Hodgkin lymphoma
(ECHELON-1): 5-year update of an international, open-label,
randomised, phase 3 trial. Lancet Haematol. 8:e410–e421. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Friedberg JW, Forero-Torres A, Bordoni RE,
Cline VJM, Patel Donnelly D, Flynn PJ, Olsen G, Chen R, Fon A, Wang
Y, et al: Frontline brentuximab vedotin in combination with
dacarbazine or bendamustine in patients aged >/=60 years with
HL. Blood. 130:2829–2837. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moskowitz CH, Nademanee A, Masszi T, Agura
E, Holowiecki J, Abidi MH, Chen AI, Stiff P, Gianni AM, Carella A,
et al: Brentuximab vedotin as consolidation therapy after
autologous stem-cell transplantation in patients with Hodgkin's
lymphoma at risk of relapse or progression (AETHERA): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
385:1853–1862. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moskowitz CH, Walewski J, Nademanee A,
Masszi T, Agura E, Holowiecki J, Abidi MH, Chen AI, Stiff P,
Viviani S, et al: Five-year PFS from the AETHERA trial of
brentuximab vedotin for Hodgkin lymphoma at high risk of
progression or relapse. Blood. 132:2639–2642. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Younes A, Gopal AK, Smith SE, Ansell SM,
Rosenblatt JD, Savage KJ, Ramchandren R, Bartlett NL, Cheson BD, de
Vos S, et al: Results of a pivotal phase II study of brentuximab
vedotin for patients with relapsed or refractory Hodgkin's
lymphoma. J Clin Oncol. 30:2183–2189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen R, Gopal AK, Smith SE, Ansell SM,
Rosenblatt JD, Savage KJ, Connors JM, Engert A, Larsen EK, Huebner
D, et al: Five-year survival and durability results of brentuximab
vedotin in patients with relapsed or refractory Hodgkin lymphoma.
Blood. 128:1562–1566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kersten MJ, Driessen J, Zijlstra JM,
Plattel WJ, Morschhauser F, Lugtenburg PJ, Brice P, Hutchings M,
Gastinne T, Liu R, et al: Combining brentuximab vedotin with
dexamethasone, high-dose cytarabine and cisplatin as salvage
treatment in relapsed or refractory Hodgkin lymphoma: The phase II
HOVON/LLPC Transplant BRaVE study. Haematologica. 106:1129–1137.
2021. View Article : Google Scholar :
|
|
38
|
Moskowitz AJ, Schoder H, Yahalom J, McCall
SJ, Fox SY, Gerecitano J, Grewal R, Hamlin PA, Horwitz S, Kobos R,
et al: PET-adapted sequential salvage therapy with brentuximab
vedotin followed by augmented ifosamide, carboplatin, and etoposide
for patients with relapsed and refractory Hodgkin's lymphoma: A
non-randomised, open-label, single-centre, phase 2 study. Lancet
Oncol. 16:284–292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Garcia-Sanz R, Sureda A, de la Cruz F,
Canales M, Gonzalez AP, Pinana JL, Rodriguez A, Gutierrez A,
Domingo-Domenech E, Sanchez-Gonzalez B, et al: Brentuximab vedotin
and ESHAP is highly effective as second-line therapy for Hodgkin
lymphoma patients (long-term results of a trial by the Spanish
GELTAMO Group). Ann Oncol. 30:612–620. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
LaCasce AS, Bociek RG, Sawas A, Caimi P,
Agura E, Matous J, Ansell SM, Crosswell HE, Islas-Ohlmayer M,
Behler C, et al: Brentuximab vedotin plus bendamustine: A highly
active first salvage regimen for relapsed or refractory Hodgkin
lymphoma. Blood. 132:40–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
LaCasce AS, Bociek RG, Sawas A, Caimi P,
Agura E, Matous J, Ansell SM, Crosswell HE, Islas-Ohlmayer M,
Behler C, et al: Three-year outcomes with brentuximab vedotin plus
bendamustine as first salvage therapy in relapsed or refractory
Hodgkin lymphoma. Br J Haematol. 189:e86–e90. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
O'Connor OA, Lue JK, Sawas A, Amengual JE,
Deng C, Kalac M, Marchi E, Turenne I, Lichtenstein R, Rojas C, et
al: Brentuximab vedotin plus bendamustine in relapsed or refractory
Hodgkin's lymphoma: An international, multicentre, single-arm,
phase 1-2 trial. Lancet Oncol. 19:257–266. 2018. View Article : Google Scholar
|
|
43
|
Picardi M, Della Pepa R, Giordano C,
Pugliese N, Mortaruolo C, Trastulli F, Rascato MG, Cappuccio I,
Raimondo M, Memoli M, et al: Brentuximab vedotin followed by
bendamustine supercharge for refractory or relapsed Hodgkin
lymphoma. Blood Adv. 3:1546–1552. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Broccoli A, Argnani L, Botto B, Corradini
P, Pinto A, Re A, Vitolo U, Fanti S, Stefoni V and Zinzani PL;
Fondazione Italiana Linfomi ONLUS: First salvage treatment with
bendamustine and brentuximab vedotin in Hodgkin lymphoma: A phase 2
study of the Fondazione Italiana Linfomi. Blood Cancer J.
9:1002019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Iannitto E, Romano A, Scalzulli PR,
Bonanno V, Scalone R, Chiarenza A, Pirosa MC, Caruso AL, Minoia C,
Mantuano S, et al: Brentuximab vedotin in association with
bendamustine in refractory or multiple relapsed Hodgkin lymphoma. A
retrospective real-world study. Eur J Haematol. 104:581–587. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yamamoto R, Nishikori M, Kitawaki T, Sakai
T, Hishizawa M, Tashima M, Kondo T, Ohmori K, Kurata M, Hayashi T
and Uchiyama T: PD-1-PD-1 ligand interaction contributes to
immunosuppressive microenvironment of Hodgkin lymphoma. Blood.
111:3220–3224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Roemer MG, Advani RH, Ligon AH, Natkunam
Y, Redd RA, Homer H, Connelly CF, Sun HH, Daadi SE, Freeman GJ, et
al: PD-L1 and PD-L2 genetic alterations define classical Hodgkin
lymphoma and predict outcome. J Clin Oncol. 34:2690–2697. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Green MR, Monti S, Rodig SJ, Juszczynski
P, Currie T, O'Donnell E, Chapuy B, Takeyama K, Neuberg D, Golub
TR, et al: Integrative analysis reveals selective 9p241
amplification, increased PD-1 ligand expression, and further
induction via JAK2 in nodular sclerosing Hodgkin lymphoma and
primary mediastinal large B-cell lymphoma. Blood. 116:3268–3277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Green MR, Rodig S, Juszczynski P, Ouyang
J, Sinha P, O'Donnell E, Neuberg D and Shipp MA: Constitutive AP-1
activity and EBV infection induce PD-L1 in Hodgkin lymphomas and
posttransplant lymphoproliferative disorders: Implications for
targeted therapy. Clin Cancer Res. 18:1611–1618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ansell SM, Lesokhin AM, Borrello I,
Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry
D, Freeman GJ, et al: PD-1 blockade with nivolumab in relapsed or
refractory Hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015.
View Article : Google Scholar :
|
|
52
|
Armand P, Engert A, Younes A, Fanale M,
Santoro A, Zinzani PL, Timmerman JM, Collins GP, Ramchandren R,
Cohen JB, et al: Nivolumab for Relapsed/Refractory classic Hodgkin
lymphoma after failure of autologous hematopoietic cell
transplantation: Extended follow-up of the multicohort single-arm
phase II CheckMate 205 trial. J Clin Oncol. 36:1428–1439. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ansell S, Bröckelmann P, von Keudell G,
Lee HJ, Santoro A, Zinzani PL, Collins G, Cohen J, De Boer JP,
Kuruvilla J, et al: HL-398: Five-year overall survival from the
CheckMate 205 study of nivolumab for relapsed or refractory (R/R)
classical Hodgkin lymphoma (cHL). Clin Lymphoma Myeloma Leuk.
21(Suppl 1): S373–S374. 2021. View Article : Google Scholar
|
|
54
|
Ramchandren R, Domingo-Domenech E, Rueda
A, Trneny M, Feldman TA, Lee HJ, Provencio M, Sillaber C, Cohen JB,
Savage KJ, et al: Nivolumab for Newly diagnosed Advanced-Stage
classic Hodgkin lymphoma: Safety and efficacy in the phase II
CheckMate 205 Study. J Clin Oncol. 37:1997–12007. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Brockelmann PJ, Goergen H, Keller U,
Meissner J, Ordemann R, Halbsguth TV, Sasse S, Sökler M, Kerkhoff
A, Mathas S, et al: Efficacy of Nivolumab and AVD in early-stage
unfavorable classic Hodgkin lymphoma: The randomized phase 2 german
Hodgkin study group NIVAHL Trial. JAMA Oncol. 6:872–880. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Herrera AF, Moskowitz AJ, Bartlett NL,
Vose JM, Ramchandren R, Feldman TA, LaCasce AS, Ansell SM,
Moskowitz CH, Fenton K, et al: Interim results of brentuximab
vedotin in combination with nivolumab in patients with relapsed or
refractory Hodgkin lymphoma. Blood. 131:1183–1194. 2018. View Article : Google Scholar :
|
|
57
|
Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan
X, Shen C, Duma N, Vera Aguilera J, Chintakuntlawar A, et al:
Treatment-related adverse events of PD-1 and PD-L1 inhibitors in
clinical trials: A systematic review and Meta-analysis. JAMA Oncol.
5:1008–1019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen R, Zinzani PL, Fanale MA, Armand P,
Johnson NA, Brice P, Radford J, Ribrag V, Molin D, Vassilakopoulos
TP, et al: Phase II study of the efficacy and safety of
pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J
Clin Oncol. 35:2125–2132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zinzani PL, Lee HJ, Armand P, Johnson N,
Brice P, Radford J, Ribrag V, Molin D, Radford J, Ribrag V, et al:
Three-year Follow-up of Keynote-087: Pembrolizumab monotherapy in
relapsed/refractory classic Hodgkin lymphoma. Blood. 134:2402019.
View Article : Google Scholar
|
|
60
|
Armand P, Chen YB, Redd RA, Joyce RM, Bsat
J, Jeter E, Merryman RW, Coleman KC, Dahi PB, Nieto Y, et al: PD-1
blockade with pembrolizumab for classical Hodgkin lymphoma after
autologous stem cell transplantation. Blood. 134:22–29. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Moskowitz AJ, Shah G, Schöder H, Ganesan
N, Drill E, Hancock H, Ganesan N, Drill E, Hancock H, Davey T, et
al: Phase II trial of pembrolizumab plus gemcitabine, vinorelbine,
and liposomal doxorubicin as second-line therapy for relapsed or
refractory classical Hodgkin lymphoma. J Clin Oncol. 39:3109–3117.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shi Y, Su H, Song Y, Jiang W, Sun X, Qian
W, Zhang W, Gao Y, Jin Z, Zhou J, et al: Safety and activity of
sintilimab in patients with relapsed or refractory classical
Hodgkin lymphoma (ORIENT-1): A multicentre, single-arm, phase 2
trial. Lancet Haematol. 6:e12–e9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fang W, Yang Y, Ma Y, Hong S, Lin L, He X,
Xiong J, Li P, Zhao H, Huang Y, et al: Camrelizumab (SHR-1210)
alone or in combination with gemcitabine plus cisplatin for
nasopharyngeal carcinoma: Results from two single-arm, phase 1
trials. Lancet Oncol. 19:1338–1350. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mo H, Huang J, Xu J, Chen X, Wu D, Qu D,
Wang X, Lan B, Wang X, Xu J, et al: Safety, anti-tumour activity,
and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody
in advanced solid tumours: A dose-escalation, phase 1 study. Br J
Cancer. 119:538–545. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Huang J, Xu B, Mo H, Zhang W, Chen X, Wu
D, Qu D, Wang X, Lan B, Yang B, et al: Safety, activity, and
biomarkers of SHR-1210, an Anti-PD-1 antibody, for patients with
advanced esophageal carcinoma. Clin Cancer Res. 24:1296–1304. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Song Y, Wu J, Chen X, Lin T, Cao J, Liu Y,
Zhao Y, Jin J, Huang H, Hu J, et al: A Single-arm, multicenter,
phase II study of camrelizumab in relapsed or refractory classical
Hodgkin lymphoma. Clin Cancer Res. 25:7363–7369. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Nie J, Wang C, Liu Y, Yang Q, Mei Q, Dong
L, Li X, Liu J, Ku W, Zhang Y, et al: Addition of low-dose
decitabine to Anti-PD-1 antibody camrelizumab in
relapsed/refractory classical Hodgkin lymphoma. J Clin Oncol.
37:1479–1489. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dahan R, Sega E, Engelhardt J, Selby M,
Korman AJ and Ravetch JV: FcγRs modulate the anti-tumor activity of
antibodies targeting the PD-1/PD-L1 axis. Cancer Cell. 28:285–295.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Song Y, Gao Q, Zhang H, Fan L, Zhou J, Zou
D, Li W, Yang H, Liu T, Wang Q, et al: Treatment of relapsed or
refractory classical Hodgkin lymphoma with the anti-PD-1,
tislelizumab: Results of a phase 2, single-arm, multicenter study.
Leukemia. 34:533–542. 2020. View Article : Google Scholar :
|
|
70
|
Song Y, Gao Q, Zhang H, Fan L, Zhou J, Zou
D, Li W, Yang H, Liu T, Wang Q, et al: Tislelizumab for
relapsed/refractory classical Hodgkin lymphoma: 3-year follow-up
and correlative biomarker analysis. Clin Cancer Res. 28:1147–1156.
2022. View Article : Google Scholar
|
|
71
|
Boles KS, Vermi W, Facchetti F, Fuchs A,
Wilson TJ, Diacovo TG, Cella M and Colonna M: A novel molecular
interaction for the adhesion of follicular CD4 T cells to
follicular DC. Eur J Immunol. 39:695–703. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yu X, Harden K, Gonzalez LC, Francesco M,
Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, et al: The
surface protein TIGIT suppresses T cell activation by promoting the
generation of mature immunoregulatory dendritic cells. Nat Immunol.
10:48–57. 2009. View Article : Google Scholar
|
|
73
|
Stanietsky N, Simic H, Arapovic J, Toporik
A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, et al:
The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell
cytotoxicity. Proc Natl Acad Sci USA. 106:17858–17863. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Garralda E, Sanborn RE, Minchom AR, Davar
D, Curigliano G, Ribrag V, Mehta A, Foss FM, Zain JM, Forero-Torres
A and Ansell SM: SGNTGT-001: A phase 1 study of SEA-TGT, an
effector-function enhanced monoclonal antibody (mAb), in advanced
malignancies (trial in progress). J Clin Oncol. 39(15_Suppl):
TPS26572021. View Article : Google Scholar
|
|
75
|
Navarro A, Diaz T, Martinez A, Gaya A,
Pons A, Gel B, Codony C, Ferrer G, Martinez C, Montserrat E and
Monzo M: Regulation of JAK2 by miR-135a: Prognostic impact in
classic Hodgkin lymphoma. Blood. 114:2945–2951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Meier C, Hoeller S, Bourgau C, Hirschmann
P, Schwaller J, Went P, Pileri SA, Reiter A, Dirnhofer S and
Tzankov A: Recurrent numerical aberrations of JAK2 and deregulation
of the JAK2-STAT cascade in lymphomas. Mod Pathol. 22:476–487.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Joos S, Granzow M, Holtgreve-Grez H,
Siebert R, Harder L, Martín-Subero JI, Wolf J, Adamowicz M, Barth
TF, Lichter P and Jauch A: Hodgkin's lymphoma cell lines are
characterized by frequent aberrations on chromosomes 2p and 9p
including REL and JAK2. Int J Cancer. 103:489–495. 2003. View Article : Google Scholar
|
|
78
|
Van Den Neste E, André M, Gastinne T,
Stamatoullas A, Haioun C, Belhabri A, Reman O, Casasnovas O,
Ghesquieres H, Verhoef G, et al: A phase II study of the oral
JAK1/JAK2 inhibitor ruxolitinib in advanced relapsed/refractory
Hodgkin lymphoma. Haematologica. 103:840–848. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kim SJ, Yoon DH, Kang HJ, Hong JY, Lee HS,
Oh SY, Shin HJ, Kong JH, Yi JH, Sakamoto K, et al: Ruxolitinib
shows activity against Hodgkin lymphoma but not primary mediastinal
large B-cell lymphoma. BMC Cancer. 19:10802019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Dutton A, Reynolds GM, Dawson CW, Young LS
and Murray PG: Constitutive activation of phosphatidyl-inositide 3
kinase contributes to the survival of Hodgkin's lymphoma cells
through a mechanism involving Akt kinase and mTOR. J Pathol.
205:498–506. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jundt F, Raetzel N, Muller C, Calkhoven
CF, Kley K, Mathas S, Lietz A, Leutz A and Dörken B: A rapamycin
derivative (everolimus) controls proliferation through
down-regulation of truncated CCAAT enhancer binding protein {beta}
and NF-{kappa} B activity in Hodgkin and anaplastic large cell
lymphomas. Blood. 106:1801–1807. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Johnston PB, Pinter-Brown LC, Warsi G,
White K and Ramchandren R: Phase 2 study of everolimus for relapsed
or refractory classical Hodgkin lymphoma. Exp Hematol Oncol.
7:122018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gillessen S, Hüttmann A, Vucinic V, Müller
H, Plütschow A, Viardot A, Topp MS, Kobe C, Böll B, Eichenauer DA,
et al: Reinduction therapy with everolimus in combination with
dexamethasone, high-dose cytarabin and cisplatinum in patients with
relapsed or refractory classical Hodgkin lymphoma: An experimental
phase I/II multicentre trial of the German Hodgkin Study Group
(GHSG HD-R3i). Br J Haematol. 196:606–616. 2022. View Article : Google Scholar
|
|
84
|
Kotla V, Goel S, Nischal S, Heuck C, Vivek
K, Das B and Verma A: Mechanism of action of lenalidomide in
hematological malignancies. J Hematol Oncol. 2:362009. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bartlett JB, Dredge K and Dalgleish AG:
The evolution of thalidomide and its IMiD derivatives as anticancer
agents. Nat Rev Cancer. 4:314–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Marriott JB, Muller G, Stirling D and
Dalgleish AG: Immunotherapeutic and antitumour potential of
thalidomide analogues. Expert Opin Biol Ther. 1:675–682. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Fehniger TA, Larson S, Trinkaus K, Siegel
MJ, Cashen AF, Blum KA, Fenske TS, Hurd DD, Goy A, Schneider SE, et
al: A phase 2 multicenter study of lenalidomide in relapsed or
refractory classical Hodgkin lymphoma. Blood. 118:5119–5125. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Boll B, Plutschow A, Burkle C, Atta J,
Pfreundschuh M, Feuring-Buske M, Vogelhuber M, Sökler M, Eichenauer
DA, Thielen I, et al: Doxorubicin, vinblastine, dacarbazine and
lenalidomide for older Hodgkin lymphoma patients: Final results of
a German Hodgkin Study Group (GHSG) phase-I trial. Br J Haematol.
185:42–52. 2019. View Article : Google Scholar
|
|
89
|
Padrnos L, Ernst B, Dueck AC, Kosiorek HE,
Ginos BF, Toro A, Johnston PB, Habermann TM, Leis JF, Mikhael JR,
et al: A Novel Combination of the mTORC1 inhibitor everolimus and
the immunomodulatory drug lenalidomide produces durable responses
in patients with heavily pretreated relapsed lymphoma. Clin
Lymphoma Myeloma Leuk. 18:664–672.e2. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Marks PA and Xu WS: Histone deacetylase
inhibitors: Potential in cancer therapy. J Cell Biochem.
107:600–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lane AA and Chabner BA: Histone
deacetylase inhibitors in cancer therapy. J Clin Oncol.
27:5459–5468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Younes A, Oki Y, Bociek RG, Kuruvilla J,
Fanale M, Neelapu S, Copeland A, Buglio D, Galal A, Besterman J, et
al: Mocetinostat for relapsed classical Hodgkin's lymphoma: An
open-label, single-arm, phase 2 trial. Lancet Oncol. 12:1222–1228.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Younes A, Sureda A, Ben-Yehuda D, Zinzani
PL, Ong TC, Prince HM, Harrison SJ, Kirschbaum M, Johnston P,
Gallagher J, et al: Panobinostat in patients with
relapsed/refractory Hodgkin's lymphoma after autologous stem-cell
transplantation: Results of a phase II study. J Clin Oncol.
30:2197–2203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kirschbaum MH, Goldman BH, Zain JM, Cook
JR, Rimsza LM, Forman SJ and Fisher RI: A phase 2 study of
vorinostat for treatment of relapsed or refractory Hodgkin
lymphoma: Southwest Oncology Group Study S0517. Leuk Lymphoma.
53:259–262. 2012. View Article : Google Scholar
|
|
95
|
Herrera AF, Chen L, Popplewell LL, Budde
LE, Mei M, Armenian SH, Darrah J, Nikolaenko L, Chen RW, Peters L,
et al: Preliminary results from a phase I trial of pembrolizumab
plus vorinostat in patients with relapsed or refractory diffuse
large B-cell lymphoma, follicular lymphoma, and Hodgkin lymphoma.
Blood. 134:7592019. View Article : Google Scholar
|
|
96
|
Buglio D, Mamidipudi V, Khaskhely NM,
Brady H, Heise C, Besterman J, Martell RE, MacBeth K and Younes A:
The class-I HDAC inhibitor MGCD0103 induces apoptosis in Hodgkin
lymphoma cell lines and synergizes with proteasome inhibitors by an
HDAC6-independent mechanism. Br J Haematol. 151:387–396. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lemoine M, Derenzini E, Buglio D, Medeiros
LJ, Davis RE, Zhang J, Ji Y and Younes A: The pan-deacetylase
inhibitor panobinostat induces cell death and synergizes with
everolimus in Hodgkin lymphoma cell lines. Blood. 119:4017–4025.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Georgakis GV, Yazbeck VY, Li Y and Younes
A: The histone deacetylase inhibitor vorinostat (SAHA) induces
apoptosis and cell cycle arrest in Hodgkin lymphoma (HL) cell lines
by altering several survival signaling pathways and synergizes with
doxorubicin, gemcitabine and bortezomib. Blood. 108:22602006.
View Article : Google Scholar
|
|
99
|
Hu B, Younes A, Westin JR, Turturro F,
Claret L, Feng L, Fowler N, Neelapu S, Romaguera J, Hagemeister FB,
et al: Phase-I and randomized phase-II trial of panobinostat in
combination with ICE (ifosfamide, carboplatin, etoposide) in
relapsed or refractory classical Hodgkin lymphoma. Leuk Lymphoma.
59:863–870. 2018. View Article : Google Scholar
|
|
100
|
Maly JJ, Christian BA, Zhu X, Wei L,
Sexton JL, Jaglowski SM, Devine SM, Fehniger TA, Wagner-Johnston
ND, Phelps MA, et al: A Phase I/II Trial of panobinostat in
combination with lenalidomide in patients with relapsed or
refractory Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk.
17:347–353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Hamadani M, Collins GP, Samaniego F, Spira
AI, Davies A, Radford J, Caimi P, Menne T, Boni J, Cruz H, et al:
Phase 1 study of Adct-301 (Camidanlumab Tesirine), a novel
pyrrolobenzodiazepine-based antibody drug conjugate, in
relapsed/refractory classical Hodgkin lymphoma. Blood. 132(Suppl
1): S9282018. View Article : Google Scholar
|
|
102
|
Rothe A, Sasse S, Topp MS, Eichenauer DA,
Hummel H, Reiners KS, Dietlein M, Kuhnert G, Kessler J, Buerkle C,
et al: A phase 1 study of the bispecific anti-CD30/CD16A antibody
construct AFM13 in patients with relapsed or refractory Hodgkin
lymphoma. Blood. 125:4024–4031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Bartlett NL, Chen RW, Domingo-Domenech E,
Forero-Torres A, Garcia-Sanz R, Armand P, Devata S, Rodriguez
Izquierdo A, Lossos IS, Reeder CB, et al: A Phase 1b study
investigating the combination of the tetravalent bispecific NK cell
engager AFM13 and pembrolizumab in patients with
relapsed/refractory Hodgkin lymphoma after brentuximab vedotin
failure: Updated safety and efficacy data. Blood. 132:16202018.
View Article : Google Scholar
|
|
104
|
Kochenderfer JN and Rosenberg SA: Treating
B-cell cancer with T cells expressing anti-CD19 chimeric antigen
receptors. Nat Rev Clin Oncol. 10:267–2676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Sadelain M, Brentjens R and Riviere I: The
basic principles of chimeric antigen receptor design. Cancer
Discov. 3:388–3898. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Johnson LA and June CH: Driving
gene-engineered T cell immunotherapy of cancer. Cell Res. 27:38–58.
2017. View Article : Google Scholar :
|
|
107
|
Wang CM, Wu ZQ, Wang Y, Guo YL, Dai HR,
Wang XH, Li X, Zhang YJ, Zhang WY, Chen MX, et al: Autologous T
cells expressing CD30 chimeric antigen receptors for relapsed or
refractory Hodgkin lymphoma: An open-label phase I trial. Clin
Cancer Res. 23:1156–1166. 2017. View Article : Google Scholar
|
|
108
|
Zanotti R, Trolese A, Ambrosetti A, Nadali
G, Visco C, Ricetti MM, Benedetti F and Pizzolo G: Serum levels of
soluble CD30 improve International Prognostic Score in predicting
the outcome of advanced Hodgkin's lymphoma. Ann Oncol.
13:1908–1914. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ramos CA, Ballard B, Zhang H, Dakhova O,
Gee AP, Mei Z, Bilgi M, Wu MF, Liu H, Grilley B, et al: Clinical
and immunological responses after CD30-specific chimeric antigen
receptor-redirected lymphocytes. J Clin Invest. 127:3462–3471.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang D, Zeng C, Xu B, Xu JH, Wang J, Jiang
LJ, Wang QX, Li CR, Wang N, Huang L, et al: Anti-CD30 chimeric
antigen receptor T cell therapy for relapsed/refractory
CD30+ lymphoma patients. Blood Cancer J. 10:82020.
View Article : Google Scholar
|
|
111
|
Ramos CA, Grover NS, Beaven AW, Lulla PD,
Wu MF, Ivanova A, Wang T, Shea TC, Rooney CM, Dittus C, et al:
Anti-CD30 CAR-T cell therapy in relapsed and refractory Hodgkin
lymphoma. J Clin Oncol. 38:3794–3804. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Jones RJ, Gocke CD, Kasamon YL, Miller CB,
Perkins B, Barber JP, Vala MS, Gerber JM, Gellert LL, Siedner M, et
al: Circulating clonotypic B cells in classic Hodgkin lymphoma.
Blood. 113:5920–5926. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Beatty GL, Haas AR, Maus MV, Torigian DA,
Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, et al:
Mesothelin-specific chimeric antigen receptor mRNA-engineered T
cells induce anti-tumor activity in solid malignancies. Cancer
Immunol Res. 2:112–1120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Svoboda J, Rheingold SR, Gill SI, Grupp
SA, Lacey SF, Kulikovskaya I, Suhoski MM, Melenhorst JJ, Loudon B,
Mato AR, et al: Nonviral RNA chimeric antigen receptormodified T
cells in patients with Hodgkin lymphoma. Blood. 132:1022–1026.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Nademanee A, O'Donnell MR, Snyder DS,
Schmidt GM, Parker PM, Stein AS, Smith EP, Molina A, Stepan DE,
Somlo G, et al: High-dose chemotherapy with or without total body
irradiation followed by autologous bone marrow and/or peripheral
blood stem cell transplantation for patients with relapsed and
refractory Hodgkin's disease: Results in 85 patients with analysis
of prognostic factors. Blood. 85:1381–1390. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Burroughs LM, O'Donnell PV, Sandmaier BM,
Storer BE, Luznik L, Symons HJ, Jones RJ, Ambinder RF, Maris MB,
Blume KG, et al: Comparison of outcomes of HLA-matched related,
unrelated, or HLA-haploidentical related hematopoietic cell
transplantation following nonmyeloablative conditioning for
relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow
Transplant. 14:1279–1287. 2008. View Article : Google Scholar : PubMed/NCBI
|