Introduction
Gemcitabine is a key drug for pancreatic and biliary
tract cancer. For transportation past the cell membrane,
gemcitabine is phosphorylated to its mononucleotide moiety by
deoxycytidine kinase (dCK), a rate-limiting enzyme involved in the
salvage of deoxyribonucleosides that provides deoxynucleotide
triphosphates for replicative and repair DNA synthesis (1). dCK expression is associated with
prolonged survival after adjuvant gemcitabine for pancreatic
adenocarcinoma (2,3). Previously, we reported that the
gallbladder carcinoma cell lines with dCK expression were sensitive
to gemcitabine treatment (4).
However, gemcitabine is not widely used for the
treatment of esophageal carcinoma. At present, few studies are
availabe regarding the use of gemcitabine treatment in esophageal
cancer and most of the targets involved adenocarcinoma (5–8).
Furthermore, no studies regarding dCK expression of esophageal
squamous cell carcinoma (ESCC) patients have been reported thus
far. In the present study, dCK expression in ESCC was analyzed and
compared to the clinocopathological characteristics of the
patients.
Patients and methods
Patient characteristics and tissue
microarray
A squamous cell carcinoma tissue microarray was
produced using ESCC, laryngeal and pharyngeal SCC, uterine/cervical
SCC and oral SCC. Tumor areas were selected with matched
hematoxylin and eosin (H&E)-stained slides and marked directly
on the donor block. The cylindrical tissue sample was cored
(diameter, 0.6 mm) from the selected region in the donor block and
extruded directly into the recipient block. Sections (4 μm)
were cut with a microtome and transferred to glass slides
(Fisherbrand, Superfrost Plus, Thermo Fisher Scientific, Waltham,
MA, USA) (9,10). In total, 114 ESCC patients who
underwent esophagectomies between 1990 and 2008 were included in
this array (Fig. 1).
Immunohistochemistry
A rabbit anti-dCK polyclonal antibody (LS-B1825,
Lifespan Biosciences, Seattle, WA, USA) was used at a dilution of
1:200. Glass slides with the primary antibodies were incubated on
an optimized titer and diluted using universal blocking reagent
(BioGenex, Fremont, CA, USA) for 60 min. After washing three times
with phosphate-buffered saline (PBS), the slides were incubated for
30 min with biotinylated secondary antibodies (Vector Laboratories,
Bulingame, CA, USA) diluted to 1:250 by universal blocking reagent.
The slides were then washed three times in PBS and incubated for 45
min with the avidin-biotin complex method reagent (Vectastain Elite
ABC kit; Vector Laboratories). The reaction products were rinsed
twice with PBS, placed in 0.05 M Tris-HCl buffer (pH 7.5) for 5 min
and developed with liquid 3,3’-diaminobenzidine (Dako, Glostrup,
Denmark) for 3 min. Thereafter, the slides were washed twice with
distilled water, lightly counterstained with Mayer’s hematoxylin,
dehydrated, cleared and mounted with a resinous mounting medium.
Procedures were carried out at room temperature (10).
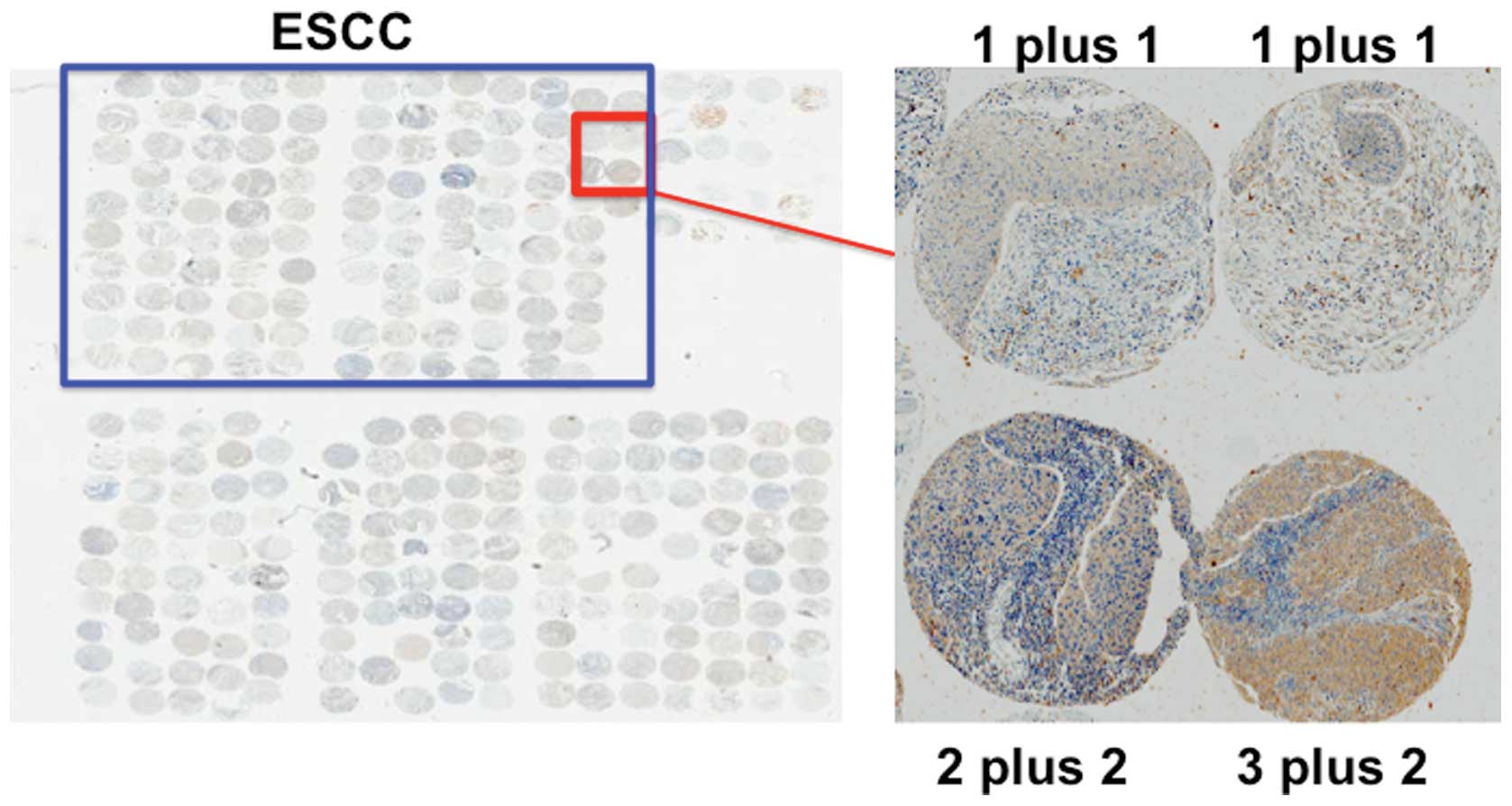
Immunohistochemical analysis
Two investigators analyzed the expression of each
gene independently and scored the intensity of expression as 0, no
expression; 1, weak expression; 2, moderate expression or 3, strong
expression. They also scored the distribution of expression as 0,
none; 1, 1–50% of tumor cells; or 2, 50–100% of tumor cells. On the
basis of the total score, each patient was then classified into the
low expression group (lower group: total score of 0–3) or high
expression group (higher/upper group: total score of 4–5).
Statistical analysis
The Chi-square test, Fisher’s exact test and
Student’s t-test were used to compare clinicopathological data. The
overall survival (OS) rate and the cause-specific survival (CSS)
rate after surgery were calculated for each group by the
Kaplan-Maier method and differences were assessed by the log-rank
test. P<0.05 was considered to indicate a statistically
significanct difference. Analyses were performed using JMP 9.0
software (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
Out of 114 spots of ESCC, 84 spots were diagnosed as
appropriate for the evaluation. Of these, 8 patients received
preoperative chemotherapy and were not eligible. The remaining 76
ESCC patients (67 male and 9 female patients; average age, 64.2
years old) were analyzed in this study. These patients underwent R0
resections. TNM stage (version 6) of the patients was as follows:
stage 1, 10; stage 2a, 15; stage 2b, 10; stage 3, 36 and stage 4,
7. All M1 were distant lymph node metastasis with no organ
metastasis and were surgically removed. Forty-one patients received
postoperative cisplatin based chemotherapy.
dCK expression in ESCC patients and its
prognostic impact
Forty-one patients were positive for dCK and 35
patients were negative for dCK (Fig.
2). There was a significant association between dCK expression
and gender (P=0.01). However, there was only a minor association
between dCK expression and depth of tumor, lymph node metastasis or
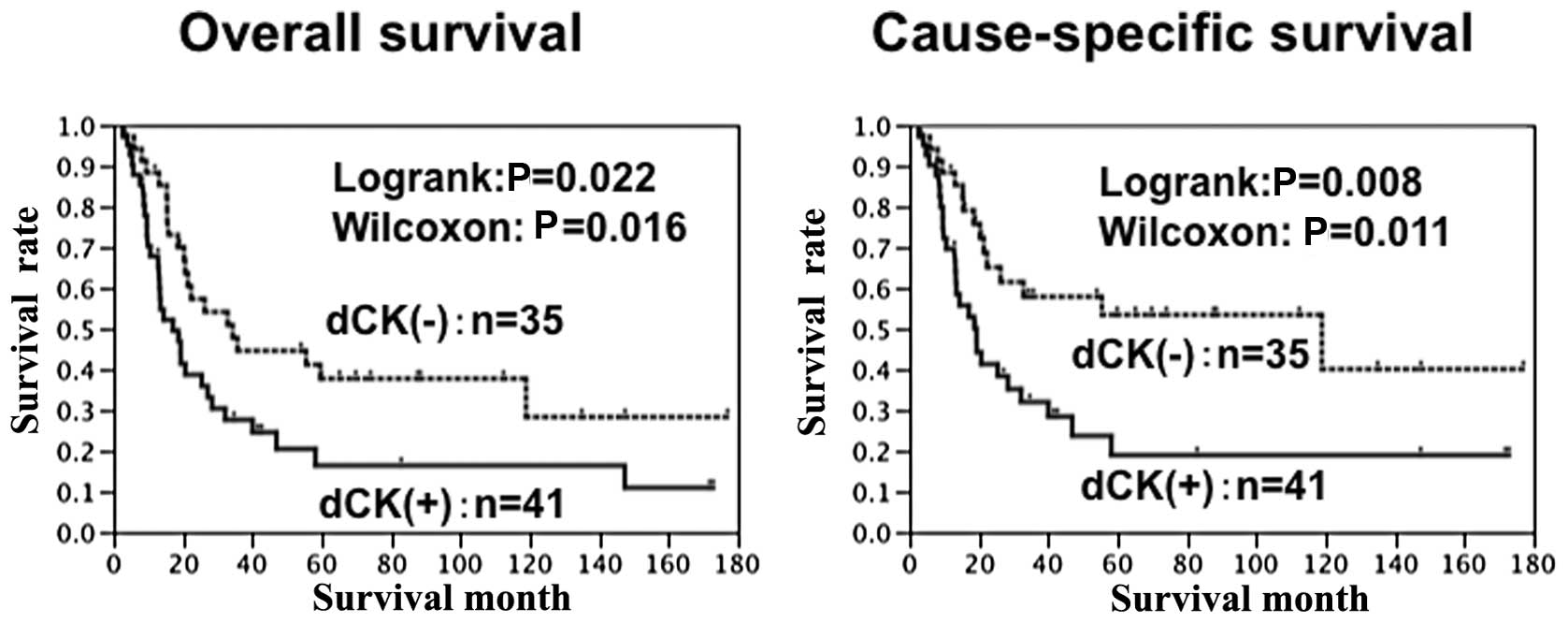
pathological stage (P=0.19, P=0.14 and 0.10 respectively) (Table I). The prognosis of the patients
with a high expression of dCK was significantly worse than that of
the patients with a low expression of dCK (P=0.022) (Fig. 3). Although dCK expression was not
an independent prognostic factor regarding overall survival, dCK
expression was an independent prognostic factor regarding
cause-specific prognosis (risk ratio 2.2, P=0.031) (Tables II and III).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variables | dCK (+) | dCK (−) | P-value |
|---|
| Age, years | 62.5±11.0 | 66.2±8.0 | 0.10 |
| Gender | | | 0.01 |
| Male | 40 | 27 | |
| Female | 1 | 8 | |
| Depth of tumor | | | 0.19 |
| T1 | 5 | 11 | |
| T2 | 7 | 6 | |
| T3 | 23 | 13 | |
| T4 | 6 | 5 | |
| Lymph node
metastasis | | | 0.14 |
| N0 | 11 | 15 | |
| N1 | 30 | 20 | |
| Distant
metastasisa | | | 0.44 |
| M0 | 36 | 33 | |
| M1 | 5 | 2 | |
| TNM stage | | | 0.10 |
| 1 | 3 | 7 | |
| 2a | 7 | 7 | |
| 2b | 3 | 7 | |
| 3 | 23 | 12 | |
| 4 | 5 | 2 | |
| Histological
type | | | 0.55 |
| Well-mod | 32 | 30 | |
| Por | 9 | 5 | |
| Adjuvant
chemotharapy | | | 0.38 |
| No | 17 | 18 | |
| Yes | 24 | 17 | |
 | Table II.Correlation between patient
characteristics and the overall prognosis in ESCC, assessed by
univariate and multivariate analyses. |
Table II.
Correlation between patient
characteristics and the overall prognosis in ESCC, assessed by
univariate and multivariate analyses.
| Variables | Univariate analysis
| Multivariate analysis
|
|---|
| P-value | Risk ratio | 95% CI | P-value |
|---|
| Age (>65
years) | 0.717 | 1.46 | 0.75–2.86 | 0.262 |
| Gender (male) | 0.160 | 1.04 | 0.37–3.42 | 0.941 |
| T (>2) | 0.001 | 1.65 | 0.84–3.42 | 0.151 |
| N (positive) | <0.001 | 2.23 | 1.03–5.16 | 0.041 |
| M (positive) | 0.006 | 2.25 | 0.85–5.34 | 0.098 |
| Histological type
(por) | 0.263 | 0.44 | 0.18–0.98 | 0.044 |
| Adjuvant chemotherapy
(yes) | 0.347 | 1.55 | 0.81–2.99 | 0.186 |
| dCK (positive) | 0.041 | 1.83 | 0.96–3.59 | 0.065 |
 | Table III.Correlation between patient
characteristics and cause-specific prognosis in ESCC. Univariate
and multivariate analyses. |
Table III.
Correlation between patient
characteristics and cause-specific prognosis in ESCC. Univariate
and multivariate analyses.
| Variables | Univariate analysis
| Multivariate analysis
|
|---|
| P-value | Risk ratio | 95% CI | P-value |
|---|
| Age (>65
years) | 0.911 | 1.53 | 0.73–3.25 | 0.261 |
| Gender (male) | 0.199 | 0.88 | 0.27–3.41 | 0.840 |
| T (>2) | <0.001 | 2.27 | 1.02–5.43 | 0.044 |
| N (positive) | 0.002 | 1.66 | 0.70–4.22 | 0.254 |
| M (positive) | 0.001 | 2.69 | 0.99–6.74 | 0.053 |
| Histological type
(por) | 0.276 | 0.42 | 0.15–1.01 | 0.053 |
| Adjuvant chemotherapy
(yes) | 0.016 | 1.99 | 0.96–4.17 | 0.064 |
| dCK (positive) | 0.008 | 2.34 | 1.12–5.10 | 0.022 |
Discussion
Results of the present study suggest an association
of gender and dCK expression. Sebastiani et al also reported
that dCK expression in male patients was higher than that in female
patients (11). Thus, dCK
expression may be associated with gender, smoking or alcohol.
Gemcitabine is a key drug for pancreatic and biliary
tract cancer. However, gemcitabine is not widely used for the
treatment of esophageal carcinoma, and a limited number of studies
have focused on gemcitabine treatment in esophageal cancer
(5–8). Findings of those studies suggest that
gemcitabine alone or as gemcitabine-cisplatin combination were
tolerable. However, gemcitabine with irinotecan or gemcitabine with
paclitaxel was highly toxic (12,13).
Furthermore, there were no additional survival benefits. Thus,
gemcitabine was not a standard treatment regimen for esophageal
cancer. However, most of their targets involved adenocarcinoma.
(Table IV).
 | Table IV.Clinical studies for gemcitabine in
esophageal cancer. |
Table IV.
Clinical studies for gemcitabine in
esophageal cancer.
| Author (Refs.) | Study drug | No. of patients
a | Histology
| Med OS (M) | CR | PR | RR (%) | Cytotoxity (%) |
|---|
| SCC | ADC | Other |
|---|
| Sandler et
al (5) | Gem | 21 (17) | 6 | 14 | 1 | 5 | 0 | 0 | 0.0 | Grade 3–4 anemia
(10.5)
Granulocytopenia (21) |
| Urba et al
(6) | Gem+CDDP | 64 (64) | 10 | 52 | 2 | 7.3 | - | - | - | Neutropenia
(31) |
| Kroep et al
(7) | Gem+CDDP | 36 (34) | 12 | 24 | 0 | 9.8 | 2 | 12 | 41.0 | Neutropenia
(83)
Thrombocytopenia (67) |
| Millar et al
(15) | Gem+CDDP | 42 (32) | 14 | 28 | 0 | 11 | 3 | 16 | 45.0 (SCC>ADC,
71 vs. 33 P<0.04) | Neutropenia
(37) |
| Morgan-Meadows
et al (8) | Gem+5-FU, LV | 35 | 3 | 32 | 0 | 9.8 (1 year;
37.1%) | 1 | 10 | 31.4 | Neutropenia
(58) |
| Wiliamson et
al (12) | Gem+IRI | 57 | - | - | - | 6.3 | - | - | - | 4 TRD, neutropenia
(35)
Thrombocytopenia (16) |
| Lowy et al
(13) | Gem+PTX +
(FP+radiation) | 29 | 3 | 26 | 0 | 3 years; 36% | 4 | 11 | 52.0 | Increase in
postoperative complications |
| Huang et al
(16) | Gem+CDDP | 38 | 38 | 0 | 0 | 10 (1 year
36.8%) | 2 | 14 | 42.1 | Leucopenia
(44.7) |
By contrast, a phase I study for solid malignancy
revealed that 4 cases with response to treatment were ESCC or
transitional cell carcinoma (14).
Furthermore, Millar et al (15) suggested that the response rate
appears to be greater in patients with squamous cell carcinoma
compared to those with adenocarcinoma. Huang et al (16) revealed that a cisplatin-gemcitabine
regimen was manageable and had significant efficacy in patients
with ESCC as improved survival time was observed. Findings of the
abovementioned reports suggested that gemcitabine may be more
effective against ESCC as compared to esophageal adenocarcinoma
(Table IV). Thus, although our
results suggest that ESCC with dCK-positive patients have a worse
prognosis, gemcitabine treatment is expected to improve the
prognosis of ESCC patients. However, to confirm the usefulness of
dCK for gemcitabine treatment in ESCC, prospective clinical trials
should be performed based on dCK expression.
In conclusion, results of the present study suggest
that dCK expression is a prognostic factor for ESCC patients.
Therefore, dCK-positive ESCC patients may be optimal targets for
gemcitabine treatment.
Acknowledgements
Research grants were received from the
Japanese Ministry of Education, Culture, Sports, Science and
Technology (MEXT/JSPS KAKENHI Grant no. B:23390320), as well as
from the Japan Society for the Promotion of Science (JSPS) (Funding
Program for World-Leading Innovative R&D on Science and
Technology (FIRST Program).
References
|
1.
|
McDonagh EM, Whirl-Carrillo M, Garten Y,
Altman RB and Klein TE: From pharmacogenomic knowledge acquisition
to clinical applications: the PharmGKB as a clinical
pharmacogenomic biomarker resource. Biomark Med. 5:795–806.
2011.PubMed/NCBI
|
|
2.
|
Fujita H, Ohuchida K, Mizumoto K, et al:
Gene expression levels as predictive markers of outcome in
pancreatic cancer after gemcitabine-based adjuvant chemotherapy.
Neoplasia. 12:807–817. 2010.PubMed/NCBI
|
|
3.
|
Maréchal R, Mackey JR, Lai R, et al:
Deoxycitidine kinase is associated with prolonged survival after
adjuvant gemcitabine for resected pancreatic adenocarcinoma.
Cancer. 116:5200–5206. 2010.PubMed/NCBI
|
|
4.
|
Sekine S, Shimada Y, Nagata T, et al:
Establishment and characterization of a new human gallbladder
carcinoma cell line. Anticancer Res. 32:3211–3218. 2012.PubMed/NCBI
|
|
5.
|
Sandler AB, Kindler HL, Einhorn LH, et al:
Phase II trial of gemcitabine in patients with previously untreated
metastatic cancer of the esophagus or gastroesophageal junction.
Ann Oncol. 11:1161–1164. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Urba SG, Chansky K, van Veldhuizen PJ, et
al: Gemcitabine and cisplatin for patients with metastatic or
recurrent esophageal carcinoma: a southwest oncology group study.
Invest New Drugs. 22:91–97. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kroep JR, Pinedo HM, Giaccone G, Van
Bochove A, Peters GJ and Van Groeningen CJ: Phase II study of
cisplatin preceding gemcitabine in patients with advanced
oesophageal cancer. Ann Oncol. 15:230–235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Morgan-Meadows S, Mulkerin D, Berlin JD,
et al: A phase II trial of gemcitabine 5-fluorouracil and
leucovorin in advanced esophageal carcinoma. Oncology. 69:130–134.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Fukuoka J, Fujii T, Shih JH, et al:
Chromatin remodeling factors and BRM/BRG1 expression as prognostic
indicators in non-small cell lung cancer. Clin Cancer Res.
10:4314–4324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Nagata T, Shimada Y, Sekine S, et al:
Prognostic significance of NANOG and KLF4 for breast cancer. Breast
Cancer. Apr. 17–2012.(E-pub ahead of print).
|
|
11.
|
Sebastiani V, Ricci F, Rubio-Viqueira B,
et al: Immunohistochemical and genetic evaluation of deoxycytidine
kinase in pancreatic cancer: relationship to molecular mechanisms
of gemcitabine resistance and survival. Clin Cancer Res.
12:2492–2497. 2006. View Article : Google Scholar
|
|
12.
|
Williamson SK, McCoy SA, Gandara DR, et
al: Phase II trial of gemcitabine plus irinotecan in patients with
esophageal cancer: a Southwest Oncology Group (SWOG) Trial. Am J
Clin Oncol. 29:116–122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lowy AM, Firdaus I, Roychowdhury D, et al:
A phase II study of sequential neoadjuvant gemcitabine and
paclitaxel, radiation therapy with cisplatin and 5-fluorouracil and
surgery in locally advanced esophageal carcinoma. Am J Clin Oncol.
29:555–561. 2006. View Article : Google Scholar
|
|
14.
|
Fleming DR, Glisson SD, Bhupalam L,
Michelson GD, Goldsmith GH and LaRocca RV: Phase I study of
paclitaxel and day 1/day8 gemicitabine in patients with solid
malignancies. Am J Clin Oncol. 23:349–352. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Millar J, Scullin P, Morrison A, et al:
Phase II study of gemcitabine and cisplatin in locally
advanced/metastatic oesophageal cancer. Br J Cancer. 93:1112–1116.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Huang J, Fan QX, Chen L, et al: Long-term
outcomes of gemcitabine and cisplatin in patients with recurrent or
metastatic esophageal squamous cell carcinoma: a phase II trial.
Chin Med J (Engl). 124:4012–4017. 2011.PubMed/NCBI
|