Introduction
Lung cancer is the leading cause of cancer-related
mortality, resulting in over one million deaths annually worldwide
(1). Non-small-cell lung cancer
(NSCLC) accounts for >80% of lung cancer cases. However,
approximately two-thirds of patients have inoperable locally
advanced (stage IIIB) or metastatic (stage IV) disease at the time
of diagnosis, with a 1-year survival rate of <20% (2,3). It
was demonstrated that the integrated basic treatment with
chemotherapy is crucial in advanced NSCLC. Platinum-based doublet
regimens are considered to be the standard treatment for advanced
NSCLC (4–7). With the development of
third-generation cytotoxic agents, such as taxanes, gemcitabine and
vinorelbine, doublet chemotherapies consisting of platinum plus a
third-generation agent are currently considered to be the standard
regimens and are recommended as first-line chemotherapy for
advanced NSCLC by the American Society of Clinical Oncology (ASCO)
and the National Comprehensive Cancer Net (5,8,9).
Vinorelbine was the first agent to demonstrate a survival benefit
when combined with cisplatin and it consequently became a standard
regimen for the first-line treatment of NSCLC (10). Docetaxel was the first drug
approved for the second-line treatment of NSCLC (5). Docetaxel plus cisplatin (DC)
treatment was shown to have better survival benefits compared with
vinorelbine plus cisplatin (VC) treatment (11) and may therefore be used as a
first-line agent in combination with platinum. Although
third-generation anticancer drugs in combination with cisplatin may
have the best efficacy in terms of longer survival and milder
toxicity profiles, their use is currently controversial (5,12).
Consequently, we conducted a systemic overview on published phase
II and III randomized controlled trials (RCTs) comparing VC and DC
in the first-line treatment of advanced NSCLC, with study endpoints
such as tumor response rate, overall survival and toxicity.
Materials and methods
Search strategy
An electronic search was conducted through PubMed,
the Cochrane Central Register of Controlled Trials (CENTRAL),
EMBASE and the Chinese Biomedical Literature database (CBM) up to
May, 2013, for trials comparing VC to DC in the management of
advanced NSCLC. The following terms were used: ‘non-small-cell lung
cancer’, ‘carcinoma, non-small-cell lung’, ‘chemotherapy’ and
‘randomized controlled trials’. The language of the publication and
year of publication were not considered to be limitations. The
reference lists of the original and review articles were also
investigated for additional literature.
Inclusion criteria
Studies were considered eligible if they compared VC
to DC chemotherapy for advanced NSCLC. The patients involved were
required to have pathological or cytological confirmation of
advanced (stage IIIB/IV) NSCLC, with a performance status of 0–2 on
the World Health Organization (WHO) scale, or a Karnofsky
performance status of ≥80%. Only the full-published studies (RCTs)
were selected, whereas conference or meeting abstracts were
excluded. The quality of the trials was assessed using the
three-question instrument described by Jadad et al (13). The quality scores are listed in
Table I.
 | Table I.Baseline characteristics of the 9
trials comparing VC with DC in the treatment of advanced
non-small-cell lung cancer. |
Table I.
Baseline characteristics of the 9
trials comparing VC with DC in the treatment of advanced
non-small-cell lung cancer.
| Patient no. | Treatment
regimen | Mean age (years) | Disease stage
(%IIIB/IV) | Quality scores | Year (refs.) |
|---|
| 404 | Vin 25
mg/m2 d1, 8, 15 and 22 + cispl 100 mg/m2
d1a | 61 | 33/67 | 3 | 2003 (11) |
| 408 | Doc 75
mg/m2 d1 + cispl 75 mg/m2 d1 | 61 | 33/67 | | |
| 118 | Vin 30
mg/m2 d1, 8 + cispl 100 mg/m2 d1 | 57 | 0/100 | 3 | 2005 (15) |
| 115 | Doc 75
mg/m2 d1 + cispl 100 mg/m2 d1 | 58 | 0/100 | | |
| 33 | Vin 25
mg/m2 d1, 8 + cispl 20 mg/m2 d1–3 | 56 | 46/54 | 2 | 2006 (16) |
| 26 | Doc 37.5
mg/m2 d1, 8 + cispl 20 mg/m2 d1–3 | 55 | 27/73 | | |
| 48 | Vin 25
mg/m2 d1, 8 + cispl 60 mg/m2 d1 | 65 | 17/83 | 3 | 2007 (17) |
| 46 | Doc l60
mg/m2 d1 + cispl 60 mg/m2 d1 | 60 | 20/80 | | |
| 45 | Vin 30
mg/m2 d1, 8 + cispl 25 mg/m2 d1–3 | 51 | 58/42 | 3 | 2007 (18) |
| 42 | Doc 75
mg/m2 d1 + cispl 30 mg/m2 d1–3 | 47 | 60/40 | | |
| 33 | Vin 25
mg/m2 d1, 8 + cispl 75 mg/m2 d1 | - | 55/45 | 2 | 2007 (19) |
| 34 | Doc 75
mg/m2 d1 + cispl 75 mg/m2 d1 | - | 59/41 | | |
| 35 | Vin 25
mg/m2 d1, 8 + cispl 27 mg/m2 d1–3 | 62 | 63/37 | 2 | 2007 (20) |
| 32 | Doc 37.5
mg/m2 d1, 8 + cispl 27 mg/m2 d1–3 | 61 | 63/37 | | |
| 190 | Vin 30
mg/m2 d 1iv, 80 mg/m2 d8 oral + cispl 80
mg/m2 d1 | 59 | 20/80 | 3 | 2009 (21) |
| 191 | Doc 75
mg/m2 d1 + cispl 75 mg/m2 d1 | 62 | 15/85 | | |
| 44 | Vin 30
mg/m2 d1, 8 + cispl 80 mg/m2 d1 | 62 | 20/80 | 2 | 2009 (22) |
| 42 | Doc 75
mg/m2 d1 + cispl 75 mg/m2 d1 | 61 | 19/81 | | |
Data extraction
The following information was independently
extracted: first author, year of publication, quality scores,
number of patients, chemotherapy regimens, mean age, percentage of
stage IIIB and IV disease, overall RR, 1- and 2-year survival and
specific toxicity data, such as leucopenia, neutropenia,
thrombocytopenia, anemia, nausea and vomiting and diarrhea.
Disagreements were resolved through discussion with an independent
expert. The characteristics of the meta-analysis for each treatment
group were assessed as follows: overall response rate, overall 1-
and 2-year survival and number of patients with grade 3/4 specific
toxicity data, such as leucopenia, neutropenia, thrombocytopenia,
anemia, nausea and vomiting and diarrhea. Since they are considered
milestones in survival result analyses of NSCLC chemotherapy, 1-
and 2-year survival were selected as primary endpoints. An attempt
was made to contact the authors of each unpublished study on
whether there had been any update of the trial following its
presentation. The response was evaluated according to the Response
Evaluation Criteria in Solid Tumors (14) or the WHO criteria and classified as
complete response (CR), partial response (PR), stable disease and
progressive disease. Overall response was defined as the sum of CR
and PR. Toxicity profiles were graded according to the National
Cancer Institute Common Toxicity Criteria or the WHO criteria.
Statistical analysis
The analyses were tested by pairwise comparisons
between the VC arm of the identified trials and the respective DC
arm. The relative risk (RR) for overall response to treatment, 1-
and 2-year survival and the odds ratio (OR) for different types of
toxicity were calculated using Review Manager software, version
5.0.3 (The Cochrane Collection, Oxford, UK). P<0.05 was
considered to indicate a statistically significant difference. An
RR of >1 reflected a favorable outcome in the VC arm regarding
response and 1- or 2-year survival rate; an OR of >1 indicated a
higher toxicity in the VC arm. The heterogeneity of the studies was
also assessed and P<0.1 was defined as heterogenous. If the test
indicated heterogeneity across studies, the random effects model
(Der Simonian and Laird) was selected. Otherwise, we used the fixed
effects model (Mantel-Haenszel) to analyze two treatment groups.
All toxicities were analyzed in the trials stating the relative
toxicities in a per-patient manner; trials not reporting on the
relative toxic effects or reporting in a different way (e.g.,
reporting toxicities per treatment cycle) were excluded from the
toxic effects evaluation.
Results
Characteristics of the included
trials
A total of 9 RCTs that met the inclusion criteria
were selected (11,15–22),
of which 7 trials were phase II (15–20,22)
and the remaining were phase III RCTs (11,21).
The details of these trials are summarized in Table I. Randomization was stated in all
trials; however, only 5 described the detailed methods of
randomization. None of the trials were double-blind and all trials
reported withdrawals and drop-outs. Overall, 1,886 patients were
randomized to receive VC or DC chemotherapy (950 and 936 patients,
respectively).
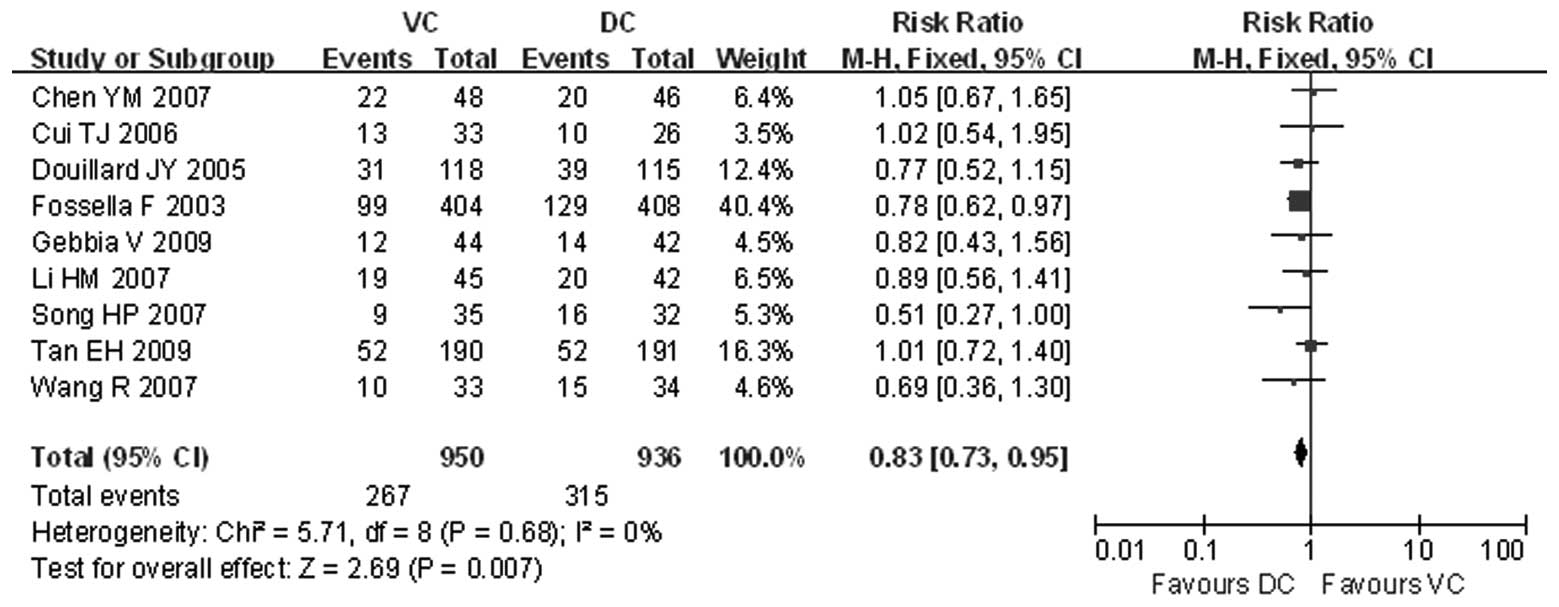
Response rate
The number of the cases achieving an overall
response was presented in all the trials. The intention-to-treat
analysis demonstrated that the overall response rate of the VC
group was 28.11% and that of the DC group was 33.65%. The patients
receiving DC therapy exhibited a significantly higher response rate
(RR=0.83, 95% CI: 0.73–0.95 and P<0.05) (Fig. 1). There was no heterogeneity
between the compared groups (χ2=5.71; P=0.68;
I2=0%).
Survival
One-year survival data were available for 7 of the 9
trials (11,17–21),
including a total of 1,741 patients (Fig. 2). The 1-year survival rates of the
VC and DC group were comparable (RR=0.90, 95% CI: 0.81–1.01 and
P=0.07) and there was no heterogeneity (χ2=2.08; P=0.91;
I2=0%). Furthermore, as shown in Fig. 2, patients treated with the DC
regimen benefited from a significant reduction in the risk of
mortality within the first 2 years (RR=0.65, 95% CI: 0.50–0.84 and
P=0.001), as shown in the 2-year survival analysis of 4 trials
(11,15,19,20).
Toxicity
All trials provided toxicity profile results. The
adverse effects of chemotherapy were described as number of cases
experiencing grade 3/4 toxicity. The most frequently reported
toxicities included leucopenia, neutropenia, thrombocytopenia,
anemia, nausea and vomiting and diarrhea (Table II). VC chemotherapy was more
frequently associated with grade 3/4 leucopenia, anemia and
vomiting (OR=1.26, 95% CI: 1.02–1.54 and P<0.05; OR=3.40; 95%
CI: 2.42–4.76 and P<0.05; and OR=1.58, 95% CI: 1.14–2.20 and
P<0.05, respectively), whereas patients receiving DC
chemotherapy were more prone to grade 3/4 diarrhea (OR=0.31, 95%
CI: 0.18–0.55 and P<0.0001). However, the incidence of
neutropenia, thrombocytopenia and nausea were not significantly
different between the two groups (OR=1.46, 95% CI: 0.93–2.29 and
P=0.10; OR=1.69, 95% CI: 0.97–2.96 and P=0.06; and OR=0.94; 95% CI:
0.37–2.38 and P=0.90, respectively).
 | Table II.Summary of grade 3/4 toxicities in VC
and DC for advanced non-small-cell lung cancer. |
Table II.
Summary of grade 3/4 toxicities in VC
and DC for advanced non-small-cell lung cancer.
| Toxicity | No. of studies | No. of cases
| Test of homogeneity
| OR (95% CI) | P-value |
|---|
| VC | DC | I2
(%) | P-value |
|---|
| Leucopenia | 8 | 338/822 | 298/817 | 21 | 0.26 | 1.26 (1.02,
1.54)a | 0.03 |
| Neutropenia | 6 | 561/829 | 524/830 | 65 | 0.01 | 1.46 (0.93,
2.29)b | 0.10 |
|
Thrombocytopenia | 8 | 33/907 | 19/898 | 0 | 0.88 | 1.69 (0.97,
2.96)a | 0.06 |
| Anemia | 6 | 146/686 | 51/683 | 48 | 0.09 | 3.40 (2.42,
4.76)a | <0.0001 |
| Nausea | 4 | 88/752 | 72/758 | 77 | 0.004 | 0.94 (0.37,
2.38)b | 0.90 |
| Vomiting | 6 | 99/831 | 66/832 | 47 | 0.10 | 1.58 (1.14,
2.20)a | 0.006 |
| Diarrhea | 6 | 15/829 | 49/826 | 0 | 0.71 | 0.31 (0.18,
0.55)a | <0.0001 |
Discussion
NSCLC is a highly malignant disease exhibiting short
survival times in the advanced stages. Improving the treatment for
advanced NSCLC has proven to be challenging. Several NSCLC
meta-analyses have been published over the last decade (6,23,24).
These studies helped to determine a doublet chemotherapy consisting
of platinum plus a third-generation agent as the gold standard in
the treatment of NSCLC (5,6,8,9,12).
In this study, we evaluated agents considered to be the gold
standard according to current ASCO guidelines and may therefore be
clinically useful in selecting the appropriate treatment for
patients with advanced NSCLC.
We observed that patients receiving DC therapy
exhibited higher response and 2-year survival rates compared to
those who received VC therapy; however there was no significant
difference in the 1-year survival rate between the VC and DC
groups. Since second-line treatment may affect survival, the
unbalanced post-study treatment may have had an impact on the
survival analysis of our study. We also observed that VC as well as
DC may cause hematological and digestive adverse events, although
the VC group was prone to develop leucopenia, anemia and vomiting,
whereas the DC group was more likely to develop severe diarrhea.
There were no significant differences in the incidence of
neutropenia, thrombocytopenia and nausea between the two
groups.
One of the major issues with the available data on
treatment for advanced NSCLC is the lack of quality of life (QoL)
analyses. Although 4 trials in this meta-analysis included a formal
QoL assessment, the assessment scales used, including EuroQoL
Five-Dimensional Questionnaire (11), Lung Cancer Symptom Scale (11,17,21)
and EORTC QLQ-C30 (22), were
different; therefore, the data could not be pooled. The QoL scores
were not significantly different between the two groups in any of
the trials, although the TAX 326 study demonstrated that the DC
regimen relieved the symptoms and improved QoL compared to the VC
regimen, according to the EuroQoL Five-Dimensional Questionnaire.
Since the primary role of chemotherapy in patients with advanced
NSCLC is palliative, the effect on patients’ QoL is crucial in
determining the overall value of new therapy.
Although there is no evidence that the DC regimen
improves QoL compared to the VC regimen, our meta-analysis
demonstrated that the DC regimen exhibits certain advantages over
the VC regimen as first-line treatment for advanced NSCLC. The DC
regimen was also associated with a more favorable safety profile
compared to the VC regimen. These findings may be helpful when
selecting the appropriate treatment for advanced NSCLC, with the
aim of improving the response and survival rates, without
increasing toxicity. Recently, with the advances in the research of
cancer cell signal transduction, molecular targeted therapy has
emerged as a treatment option; such regimens may provide a
potential platform on which to add targeted therapy for first-line
treatment in the future. Lynch et al (25) performed a phase III trial including
676 patients with advanced NSCLC without restrictions posed by
histology or epidermal growth factor receptor expression, in which
treatment with taxane plus carboplatin alone was compared to taxane
plus carboplatin with cetuximab, confirming a remarkable increase
in the overall response rate in taxane plus carboplatin with
cetuximab over taxane plus carboplatin alone. The difference in
overall survival favored cetuximab, although it did not reach a
statistical significance. The results of that study cannot be
applied to all patients with advanced NSCLC, as it excluded
patients with previous infusion reactions to chimerized/murine
monoclonal antibodies, history of acute myocardial infarction,
higher than grade 2 peripheral neuropathy and inadequate
hematological, hepatic or renal functions. However, such patients
represent a substantial population of patients with advanced NSCLC
and viable alternatives are required to improve their
treatment.
This meta-analysis had certain limitations that
should be considered. Our study was limited by the number and
quality of the available RCTs. Although it may be difficult for
phase II studies to produce reliable survival data, no significant
heterogeneity was observed in the response rate or in the 1- and
2-year survival rates among the trials included in the analysis.
This result of the 2-year survival analysis supports the decision
to include all randomized phase II or III trials with prospectively
recorded 2-year survival data. Furthermore, the survival data at 2
years of follow-up and some adverse effects were lacking in several
trials, which may have led to a biased estimate.
In conclusion, this meta-analysis revealed that DC
therapy exhibited a marginally better response rate and 2-year
survival rate and a milder toxicity profile compared to VC.
Therefore, the former may be the better choice for patients with
advanced NSCLC. However, these results need to be interpreted with
caution, as the outcome of these meta-analyses on the basis of
summary data derived from the literature may be affected by several
biases.
Acknowledgements
This study was funded by the National
Natural Science Foundation of China (no. 81071808), the Anhui
Provincial Key Science and Technology Project (no. 12010402126),
the Natural Science Foundation of Anhui Higher Education
Institutions of China (no. KJ2012A284) and the Anhui Provincial
Program for Industry Innovative Research Team of Cancer
Immunotherapy and Nutrition Diagnosis and Therapy.
References
|
1.
|
Parkin DM, Bray FJ and Pisani P: Global
cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2.
|
DeVita VT, Lawrence TS and Rosenberg SA:
DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of
Oncology. 9th Edition. Lippincott Williams & Wilkins;
Philadelphia: 2009
|
|
3.
|
Montazeri A, Gillis CR and McEwen J:
Quality of life in patients with lung cancer: a review of
literature from 1970 to 1995. Chest. 113:467–481. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Abratt RP and Hart GJ: 10-year update on
chemotherapy for non-small cell lung cancer. Ann Oncol. 17(Suppl
5): v33–v36. 2006.PubMed/NCBI
|
|
5.
|
Pfister DG, Johnson DH, Azzoli CG, et al:
American Society of Clinical Oncology: American Society of Clinical
Oncology treatment of unresectable non-small-cell lung cancer
guideline: update 2003. J Clin Oncol. 22:330–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
No authors listed:. Chemotherapy in
non-small cell lung cancer: a meta-analysis using updated data on
individual patients from 52 randomised clinical trials. Non-small
Cell Lung Cancer Collaborative Group. BMJ. 311:899–909. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Pujol JL, Barlesi F and Daurèsa JP: Should
chemotherapy combinations for advanced non-small cell lung cancer
be platinum-based? A meta-analysis of phase III randomized trials.
Lung Cancer. 51:335–345. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ettinger DS, Bepler G, Bueno R, et al:
Non-small cell lung cancer clinical practice guidelines in
oncology. J Natl Compr Canc Netw. 4:548–582. 2006.PubMed/NCBI
|
|
9.
|
Azzoli CG, Baker S Jr, Temin S, et al:
American Society of Clinical Oncology: American Society of Clinical
Oncology Clinical Practice Guideline update on chemotherapy for
stage IV non-small-cell lung cancer. J Clin Oncol. 27:6251–6266.
2009. View Article : Google Scholar
|
|
10.
|
Le Chevalier T, Brisgand D, Douillard JY,
et al: Randomized study of vinorelbine and cisplatin versus
vindesine and cisplatin versus vinorelbine alone in advanced
non-small-cell lung cancer: results of a European multicenter trial
including 612 patients. J Clin Oncol. 12:360–367. 1994.
|
|
11.
|
Fossella F, Pereira JR, von Pawel J, et
al: Randomised, multinational phase III study of docetaxel plus
platinum combinations versus vinorelbine plus cisplatin for
advanced non-small-cell lung cancer: the TAX 326 study group. J
Clin Oncol. 21:3016–3024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Goffin J, Lacchetti C, Ellis PM, et al:
First-line systemic chemotherapy in the treatment of advanced
non-small cell lung cancer: a systematic review. J Thorac Oncol.
5:260–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Jadad AR, Moore RA, Carroll D, et al:
Assessing the quality of reports of randomized clinical trials: is
blinding necessary? Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar
|
|
14.
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
15.
|
Douillard JY, Gervais R, Dabouis G, et al:
Sequential two-line strategy for stage IV non-small-cell lung
cancer: docetaxel-cisplatin versus vinorelbine-cisplatin followed
by cross-over to single-agent docetaxel or vinorelbine at
progression: final results of a randomised phase II study. Ann
Oncol. 16:81–89. 2005. View Article : Google Scholar
|
|
16.
|
Cui TJ, Liu ZH, Chen Z, et al: Comparisons
among four chemo-therapy regimens for advanced non-small cell lung
cancer. J Oncol (Chinese). 12:136–138. 2006.
|
|
17.
|
Chen YM, Perng RP, Shih JF, et al: A
randomized phase II study of docetaxel or vinorelbine in
combination with cisplatin against inoperable, chemo-naïve
non-small-cell lung cancer in Taiwan. Lung Cancer. 56:363–369.
2007.
|
|
18.
|
Li HM, Li HX, Liu KW, et al: Three
chemotherapy regimens including cisplatin for advanced non-small
cell lung cancer. J Shandon Univ China (Health Sci). 45:499–502.
2007.
|
|
19.
|
Wang R, Wang YL and Wang QC: A comparison
of docetaxel plus cisplatin and vinorelbine plus cisplatin in 67
cases with advanced non-small-cell lung cancer. Bulletin Chin
Cancer. 16:476–477. 2007.
|
|
20.
|
Song HP, Qiu WS, Xu JH, et al: First-line
chemotherapy of docetaxel/cisplatin for advanced non-small cell
lung cancer. Chin J Clin Oncol. 34:388–390. 2007.
|
|
21.
|
Tan EH, Rolski J, Grodzki T, et al: Global
Lung Oncology Branch trial 3 (GLOB3): final results of a randomised
multinational phase III study alternating oral and i.v. vinorelbine
plus cisplatin versus docetaxel plus cisplatin as first-line
treatment of advanced non-small-cell lung cancer. Ann Oncol.
20:1249–1256. 2009. View Article : Google Scholar
|
|
22.
|
Gebbia V, Lorusso V, Galetta D, et al:
First-line cisplatin with docetaxel or vinorelbine in patients with
advanced non-small-cell lung cancer: a quality of life directed
phase II randomized trial of Gruppo Oncologico Italia Meridionale.
Lung Cancer. 69:218–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Auperin A, LePechoux C, Pignon JP, et al:
Concomitant radio-chemotherapy based on platin compounds in
patients with locally advanced non-small cell lung cancer (NSCLC):
a meta-analysis of individual data from 1,764 patients. Ann Oncol.
17:473–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
D’Addario G, Pintilie M, Leighl NB, et al:
Platinum-based versus non-platinum-based chemotherapy in advanced
non-small-cell lung cancer: a meta-analysis of the published
literature. J Clin Oncol. 23:2926–2936. 2005.PubMed/NCBI
|
|
25.
|
Lynch TJ, Patel T, Dreisbach L, et al:
Cetuximab and first-line taxane/carboplatin chemotherapy in
advanced non-small-cell lung cancer: results of the randomized
multicenter phase III trial BMS099. J Clin Oncol. 28:911–917. 2010.
View Article : Google Scholar : PubMed/NCBI
|