Introduction
Colorectal cancer (CRC) is the third most common
type of cancer worldwide (1). The
treatment options for CRC are currently limited, with poor efficacy
and marked variation in the therapeutic outcomes among patients. In
the majority of cases, surgical resection remains the only curative
treatment option; however, it involves costly and invasive
procedures with considerable limitations. Therefore, the design of
non-invasive and effective therapies is of prime importance.
Over the last decade, extensive research has focused
on multiple synthetic chemotherapeutic agents that are
non-invasive, but display low selectivity and severe adverse
effects (2). The cornerstone of
adjuvant and palliative chemotherapy for CRC is currently
5-fluorouracil (5-FU) (3).
However, 5-FU has been associated with several side effects,
including myelotoxicity (4),
gastrointestinal disturbances (5),
cardiotoxicity (6) and
hepatotoxicity (7). Those
limitations prompted investigators to design a more effective and
safe drug, which may enhance the therapeutic benefits for CRC
patients.
Epidemiological studies have consistently documented
high incidence rates of CRC in Australia, New Zealand, Europe and
North America. By contrast, low incidence rates of CRC have been
reported in Africa and South-Central Asia, where the intake of
fruits and vegetables is high (8).
These epidemiological findings suggest the possibility of a causal
association between habitual dietary intake and the incidence of
CRC. Furthermore, these observations were substantiated by the
conclusion of the World Cancer Research Fund, which reported an
inverse association between the dietary intake of fruits or
vegetables, flavonoid-rich diets and the incidence of CRC (9).
The basic chemical structure of flavonoids is based
on a C6-C3-C6 system with a chromane ring bearing a second aromatic
B ring in position 2, 3 or 4. Flavonoids are polyphenolic compounds
present in a free state or as glycosides, usually O-glycosides,
with the sugar moiety generally bound to the aglycone hydroxyl (OH)
group at C-7 or, occasionally, C-3. The typical sugar moieties
include D-glucose and L-rhamnose. Over 6,000 plant flavonoids have
been described and classified into at least 10 chemical groups
according to structural patterns (10). However, laboratory and
epidemiological studies have focused mainly on bioflavonoids,
including rutin, quercetin, chrysin, hesperetin and hesperidin,
grouped under the following three flavonoid subgroups: flavonols,
flavones and flavanones (Fig. 1).
Flavonols, such as quercetin and its glycoside, rutin, are the most
abundant flavonoids in foods and are found in leafy vegetables,
apples, onions and berries. Flavones, such as chrysin, are found in
honey and propolis and in low concentrations in fruits, vegetables
and certain beverages. Flavanones, such as hesperetin and its
glycoside, hesperidin, also known as citrus flavonoids, are found
in citrus fruits and their juices (11).
Although a broad spectrum of biological activities
has been identified for these flavonoids, no study has yet
investigated their anticolon cancer activity in depth. The proposed
protective role of flavonoids against tumor development may prevail
in the intestinal tract due to direct exposure to intestinal
epithelia. Therefore, this study aimed to investigate the potential
anticolon cancer activity of these structurally diverse flavonoids
in comparison to that of 5-FU and delineate the association between
structural characteristics and anticolon cancer activity. Such
findings may simplify drug design to allow safer and more effective
anticolon cancer pharmaceuticals to be synthesized.
Materials and methods
Materials and reagents
Dulbecco’s modified Eagle’s medium (DMEM) was
obtained from Gibco-BRL (Invitrogen Life Technologies Inc., Grand
Island, NY, USA). Non-essential amino acids and fetal calf serum
(FCS) were purchased from Hyclone (Logan, UT, USA). Penicillin and
streptomycin were purchased from Amresco (Solon, OH, USA). The
flavonoids were previously isolated at the Department of
Pharmacognosy, Faculty of Pharmacy, Mansoura University (Mansoura,
Egypt) and identified by ultraviolet, infrared, mass and nuclear
magnetic resonance spectroscopy. The purity of flavonoids was
>99%, assessed by high-performance liquid chromatography. Plates
of 96 wells were purchased from Corning Inc. (Cambridge, MA,
USA).
Cell culture
The Caco-2 human colon adenocarcinoma cell line was
purchased from American Type Culture Collection (HTB-37; Rockville,
MD, USA). The Caco-2 cells were cultured in DMEM containing
D-glucose (4.5 g/l), NaHCO3 (3.7 g/l) and supplemented
with 10% FCS, penicillin (100 U/ml) and streptomycin (100 μg/ml),
in an atmosphere of 5% CO2 and 95% relative humidity at
37°C. All the cells used in this study were between passages 50 and
62.
Drug treatment
The concentration of the investigated flavonoids was
50–250 μM. The flavonoids were dissolved in 100% dimethylsulfoxide
(DMSO; Sigma, St. Louis, MO, USA) and diluted to their final
concentrations in serum-free media. In all the experiments, the
control cells were incubated with DMSO alone. The final
concentration of DMSO was maintained at 0.2% w/v. The cells were
incubated with flavonoids or 5-FU for 24, 48 and 72 h.
Cytotoxicity assay
Cytotoxicity was assessed with the MTT assay,
according to the manufacturer’s recommendations (Roche Diagnostics
GmbH, Mannheim, Germany). The experiments were repeated 3 times and
data are expressed as means ± standard deviation (SD). This assay
relies on the ability of metabolically active viable cells to
reduce a yellow tetrazolium salt (MTT; Sigma) to a purple formazan
product. This reaction occurs when mitochondrial reductase enzymes
are active. The cells were grown in 96-well plates
(1×104/200 μl/well). Following incubation with the
reagents, the medium was removed and the cells were treated with 20
μl of MTT (5 mg/ml) for 3 h at 37°C. Subsequently, 100 μl DMSO was
added to each well. The solubilized formazan product was
spectrophotometrically quantified using a PowerWave XS microplate
reader (BioTek, Winooski, VT, USA) at 540 nm.
Statistical analysis
Data are presented as means ± SD. Statistical
comparisons between groups were performed by one-way analysis of
variance (ANOVA) followed by post hoc Tukey’s test (Statistica;
StatSoft Inc., Tulsa, OK, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Dose-viability response of 5-FU
The anticolon cancer activities of the selected
flavonoids were evaluated in a cell-based assay using Caco-2 cells
and compared to that of 5-FU, a drug extensively used in adjuvant
and palliative chemotherapy for CRC. The dose response and time
course cytotoxicity of the standard agent, 5-FU, were initially
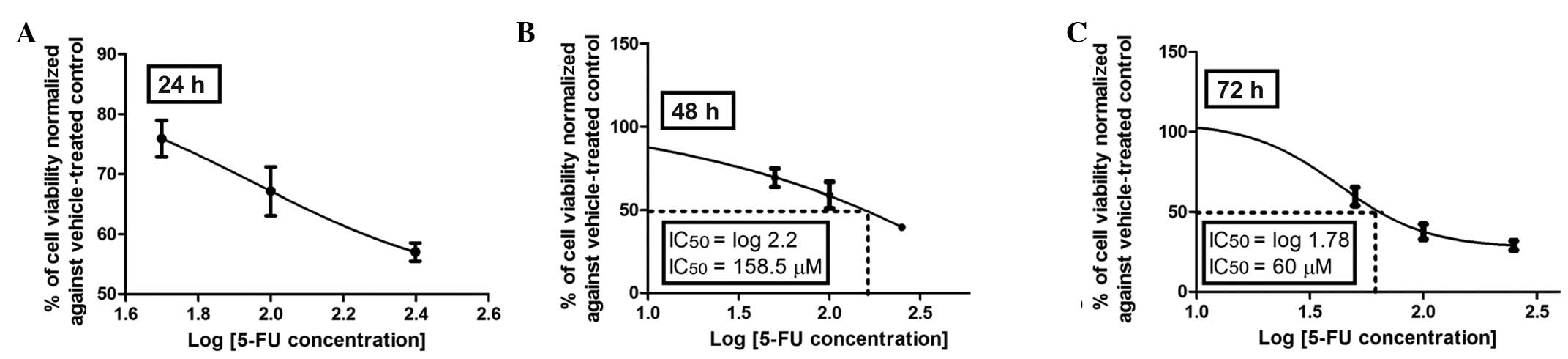
evaluated with the MTT assay. As shown in Fig. 2A–C, the dose-response effect of
5-FU was more evident after 72 h of incubation with an inhibitory
concentration (IC50) of 60 μM compared to that at 48 h
of incubation with an IC50 of 158.5 μM, whereas at 24 h
an IC50 was not reached with 5-FU at any of the
concentrations investigated (50–250 μM). Therefore, 72 h was
selected as the incubation period to assess the dose-viability
response of the investigated flavonoids (Figs. 3–6).
Anticancer potency of flavonoids
Among the investigated flavonoids in this cell-based
assay, flavone, chrysin (Fig. 3A and
B) and 3-hydroxyflavonol (Fig.
4A) exhibited the highest anticancer potencies, which were
superior or comparable to that of 5-FU. By contrast, quercetin was
not active, even at concentrations up to 100 μM (Fig. 4B). Notably, hesperetin (Fig. 5A) exhibited a moderate
IC50 that was significantly lower compared to that of
quercetin, but higher compared to those of flavone and chrysin.
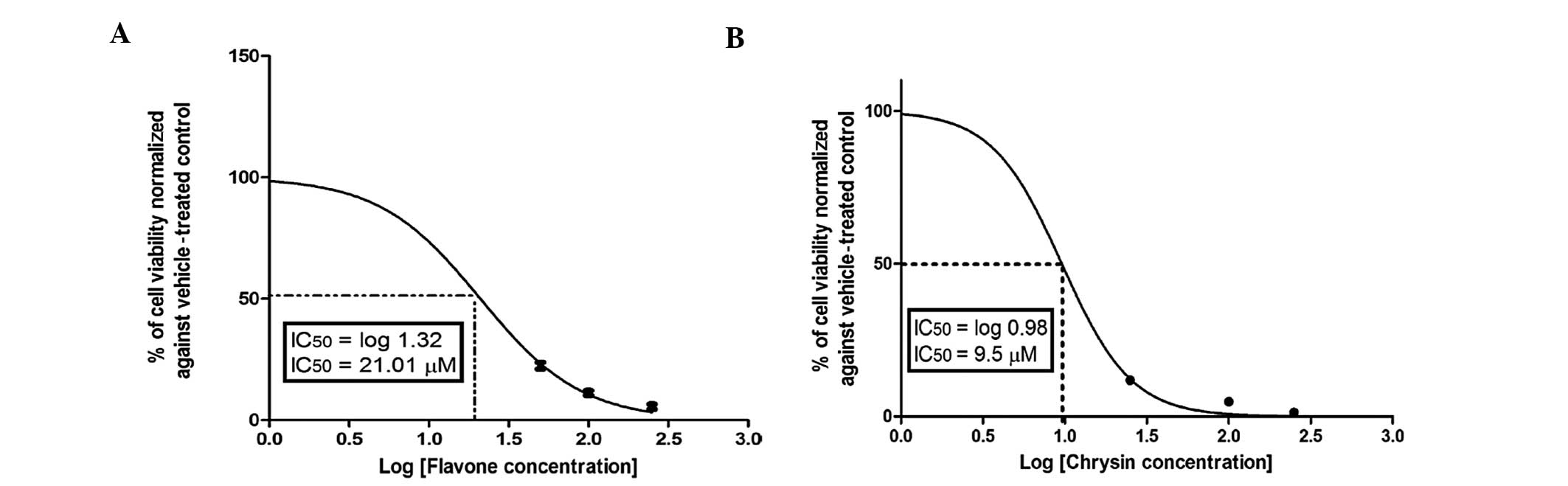
Cytotoxicity of flavone
The dose-viability response (counted as percentage
of control, 0 μM) in Caco-2 cells during 72 h of incubation with
different concentrations of flavone, the form commonly found in
fresh plants, which is then converted to different flavonoid
derivatives by benzopyrene hydroxylases, is shown in Fig. 1. Flavone exerted a strong cytotoxic
effect against Caco-2 cells in a dose-dependent manner, with an
IC50 of 21.01±1.3 μM.
Association between structural
characteristics of flavonoids and anticancer activity
In light of the anticancer effects of flavone, we
sought to determine whether the addition of functional groups
modifies its anticancer activity. The addition of OH groups in ring
A significantly enhanced the anticolon cancer activity of flavone,
as shown in Fig. 3B for chrysin.
Chrysin, which has 2 OH groups in ring A, caused 50% inhibition of
colon cancer cell proliferation at a concentration of 9.5±2.2 μM,
which was significantly (P<0.01) lower compared to that of
flavone. However, introducing a OH group in ring C caused a
significant decrease in the anticancer activity of flavone, as
shown in Fig. 4A for
3-hydroxyflavone. Furthermore, the addition of a OH group in ring B
resulted in a marked decrease in the anticancer activity of
flavonoids, as shown in Figs. 4B
and 5A for quercetin and
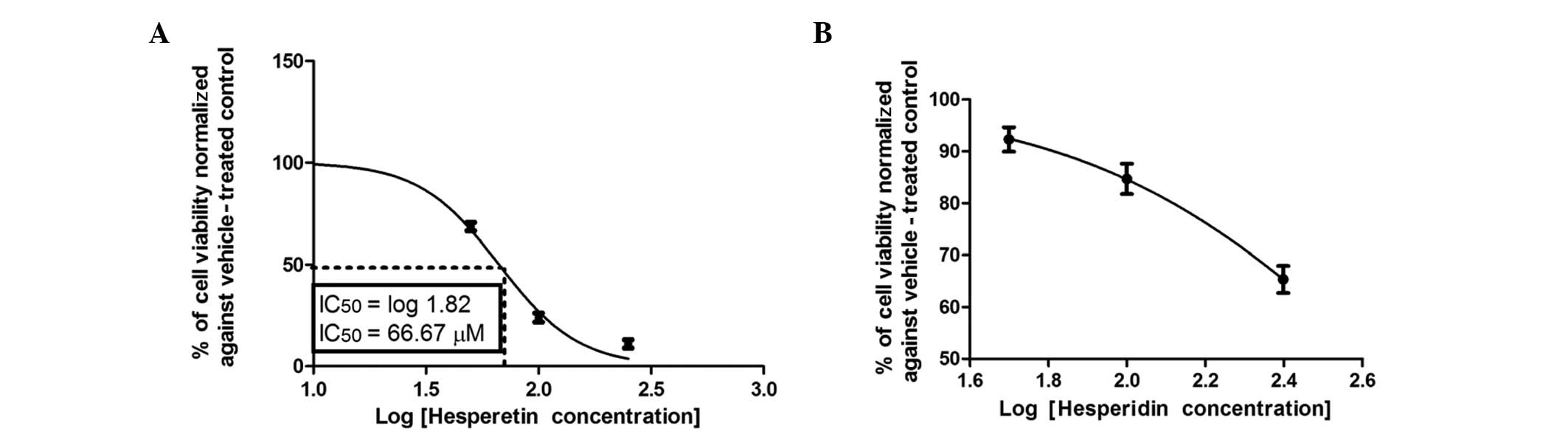
hesperetin, respectively. Hesperetin, which has a OH group in ring
B, caused a 50% reduction in the viability of Caco-2 cells after 72
h of incubation at a concentration of 66.67±1.5 μM (Fig. 5A). The addition of the second OH
group in ring B resulted in a significant decrease in anticolon
cancer activity, as illustrated by quercetin, which caused a 50%
inhibition of cell viability after 72 h of incubation at a
concentration of 195±2.5 μM (Fig.
4B). Furthermore, the addition of sugar moieties at position 3
exerted a greater effect on the anticolon cancer activity of
quercetin. This is clearly manifested in rutin, in which the
cytotoxic activity was significantly diminished over the applied
concentration range (Fig. 4C). The
addition of sugar moieties at position 7 in ring A distinctly
reduced the anticolon cancer effect of hesperitin over the
concentration range, as manifested in hesperidin (Fig. 5B).
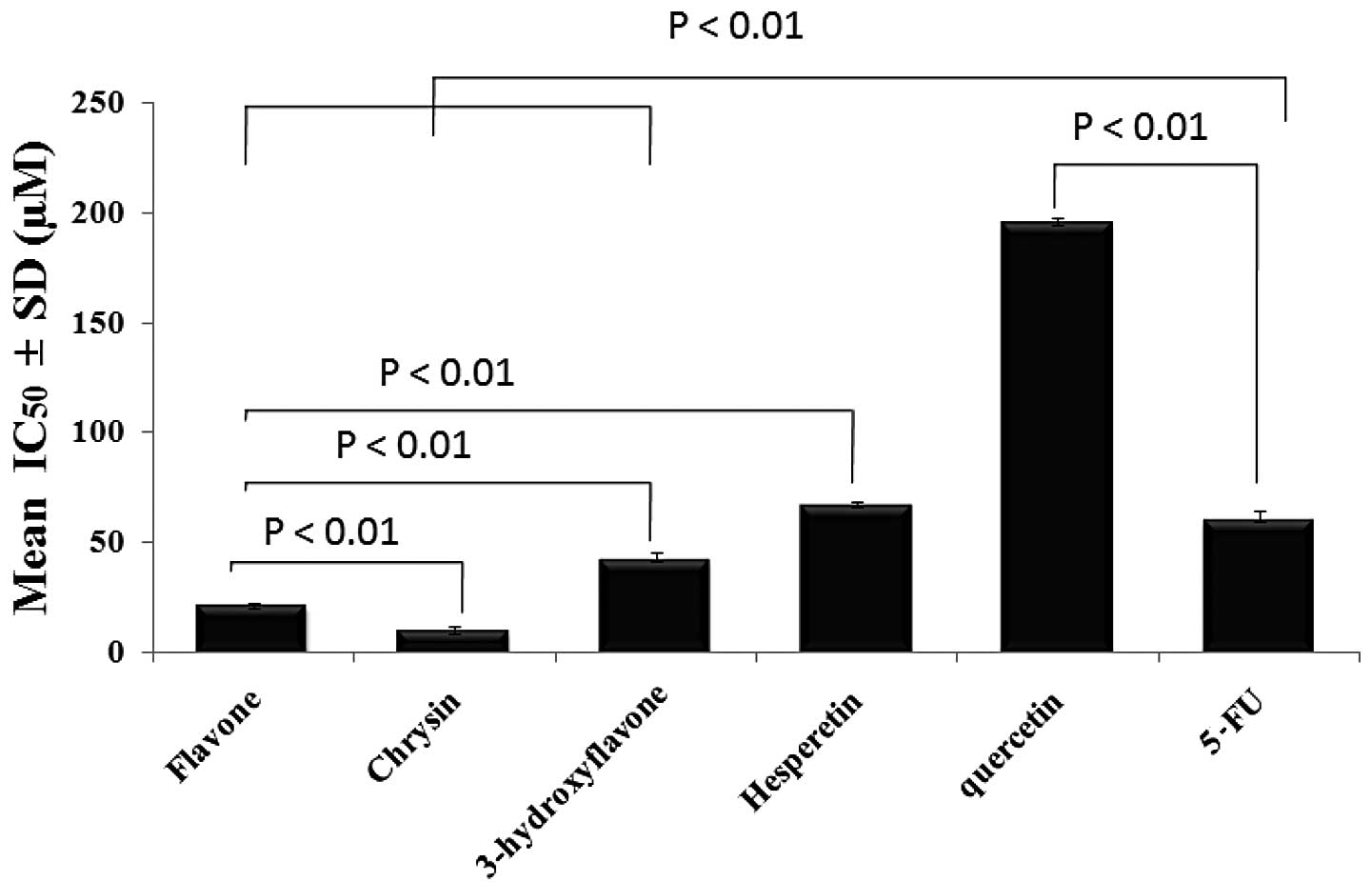
One-way ANOVA was used to evaluate the statistical
significance of the difference in the anticolon cancer effect at
IC50 for each flavonoid after an incubation period of 72
h and the means were found to be significantly different across the
samples (Fig. 6).
Discussion
Effective therapies for colon cancer, which is
considered to be a disease of the civilization, have long been the
focus of investigation. Despite the clinical success of several
newly developed anticolon cancer agents, there are certain
limitations, such as high peripheral neurotoxicity, complicated
synthesis procedures and drug resistance conferred by multidrug
resistance transporters. Therefore, there has been an increasing
interest in identifying novel anticolon cancer agents with
alternative modes of action and improved pharmacological profiles,
particularly reduced toxicity. The re-emphasis on natural products
in drug discovery was the subject of recent cancer research and
development and was shown to be a valuable, effective and
inexpensive approach (12,13). In the present study, the use of
naturally-derived Free-B-Ring flavonoids as potential anticolon
cancer agents was investigated.
Free-B-Ring flavonoids are benzo-γ-pyrone
derivatives found in a number of medicinal plant species and
possess a plethora of biological and pharmacological properties.
Over several years, the development and use of these flavonoids and
their derivatives for the prevention and treatment of cancer has
been a focus of investigation. Chrysin, the most abundant
Free-B-Ring flavonoid in honey, was found to potentiate the
antiproliferative effect of various chemotherapeutic agents
(14). Furthermore, chrysin was
found to be capable of inhibiting cell proliferation and inducing
apoptosis in human cervical carcinoma (15), leukemia (16), esophageal squamous cell carcinoma
(17), malignant glioma, breast
carcinoma (18) and prostate
cancer cell lines (19). To extend
our knowledge of the antineoplastic role of chrysin, the present
study demonstrated the ability of chrysin to induce colon cancer
cell death more efficiently compared to 5-FU.
Additionally, flavones and 3-hydroxyflavone are
among the Free-B-Ring flavonoid derivatives that were investigated
in this study and exhibited more efficient anticolon cancer
activities compared to that of 5-FU. Given these properties,
Free-B-Ring flavonoids may be promising anticolon cancer agents, a
hypothesis originating partly from a previous study demonstrating
that baicalein (5,6,7-trihydroxyflavone) exerted an
antiproliferative effect on Caco-2 cells (20). That initial study was further
supported by the observation that flavone increased early and late
apoptosis parameters in Caco-2 cells (21). Additionally, those findings were
reinforced by more recent studies, demonstrating that
5,7-dihydroxy-3,6,8-trimethoxy flavone displayed a potent activity
against the more differentiated carcinomas of the colon compared to
its flavonol isomer, 5-hydroxy-6,7,8-trimethoxy-3-flavonol
(22). Taken together, those
findings have formed the concept of Free-B-Ring flavonoids as
potential anticolon cancer agents and several mechanisms have been
proposed regarding their anticancer activity. One such mechanism
refers to the ability of these agents to inhibit DNA topoisomerase
II (23), whereas another refers
to their ability to produce free radicals and, consequently, cleave
DNA (24).
In concordance with previous findings, the potent
cytotoxicity of three Free-B-Ring flavonoids against Caco-2 cells
was observed in our study. However, to the best of our knowledge,
our study is the first to report that, although the basic chemical
structures of various flavonoids are similar, the specific
functional groups attached at specific positions, particularly the
OH group, may confer significantly different bioactivities to the
resulting compounds. Chrysin, which has 2 OH groups in the A ring,
was the most active compound to arrest Caco-2 cell survival
followed by flavone, 3-hydroxyflavone, hesperitin, quercetin,
hesperidin and finally rutin. In addition, we demonstrated the
causal association between the structural characteristics and the
activity of these flavonoids, as the acquisition of the OH group in
the B ring significantly decreased their anticancer activity; in a
similar manner, glycosylation was also associated with a marked
decrease in anticancer activity.
In conclusion, with the ongoing demand for novel
drug-like lead compounds against CRC, the significant anticancer
activity of Free-B-Ring flavonoids compared to standard therapy
renders them potential leads to future anticolon cancer agents.
References
|
1
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar
|
|
2
|
Dhorajiya BD, Ibrahim AS, Badria FA and
Dholakiya BZ: Design and synthesis of novel nucleobase-based
barbiturate derivatives as potential anticancer agents. Med Chem
Res. August;2013.1 View Article : Google Scholar
|
|
3
|
Quasar Collaborative Group. Gray R,
Barnwell J, McConkey C, Hills RK, Williams NS and Kerr DJ: Adjuvant
chemotherapy versus observation in patients with colorectal cancer:
a randomised study. Lancet. 370:2020–2029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi EH, Ok HE, Yoon Y, Magnuson BA, Kim
MK and Chun HS: Protective effect of anthocyanin-rich extract from
bilberry (Vaccinium myrtillus L.) against myelotoxicity
induced by 5-fluorouracil. Biofactors. 29:55–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meta-Analysis Group In Cancer. Toxicity of
fluorouracil in patients with advanced colorectal cancer: effect of
administration schedule and prognostic factors. Meta-Analysis Group
In Cancer. J Clin Oncol. 16:3537–3541. 1998.PubMed/NCBI
|
|
6
|
Jensen SA, Hasbak P, Mortensen J and
Sørensen JB: Fluorouracil induces myocardial ischemia with
increases of plasma brain natriuretic peptide and lactic acid but
without dysfunction of left ventricle. J Clin Oncol. 28:5280–5286.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choti MA: Chemotherapy-associated
hepatotoxicity: do we need to be concerned? Ann Surg Oncol.
16:2391–2394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
World Cancer Research Fund/American
Institute for Cancer Research. Food, Nutrition and the Prevention
of Cancer: a Global Perspective. American Institute for Cancer
Research, Washington, DC: AICR; pp. 134–136. 1997
|
|
10
|
Heim KE, Tagliaferro AR and Bobilya DJ:
Flavonoid antioxidants: chemistry, metabolism and
structure-activity relationships. J Nutr Biochem. 13:572–584. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao LH, Jiang YM, Shi J, Tomas-Barberan
FA, Datta N, Singanusong R and Chen SS: Flavonoids in food and
their health benefits. Plant Foods Hum Nutr. 59:113–122. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koehn FE: Natural products and cancer drug
discovery. Humana Press; New York, NY: 2013, View Article : Google Scholar
|
|
13
|
Badria FA and Ibrahim AS: Evaluation of
natural anthracene-derived compounds as antimitotic agents. Drug
Discov Ther. 7:84–89. 2013.PubMed/NCBI
|
|
14
|
Gao AM, Ke ZP, Shi F and Chen H: Chrysin
enhances sensitivity of BEL-7402/ADM cells to doxorubicin by
suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem Biol Interact.
206:100–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang T, Chen X, Qu L, Wu J, Cui R and
Zhao Y: Chrysin and its phosphate ester inhibit cell proliferation
and induce apoptosis in HeLa cells. Bioorg Med Chem. 12:6097–6105.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khoo BY, Chua SL and Balaram P: Apoptotic
effects of chrysin in human cancer cell lines. Int J Mol Sci.
11:2188–2199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Q, Zhao XH and Wang ZJ: Cytotoxicity
of flavones and flavonols to a human esophageal squamous cell
carcinoma cell line (KYSE-510) by induction of G2/M arrest and
apoptosis. Toxicol In Vitro. 23:797–807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lirdprapamongkol K, Sakurai H, Abdelhamed
S, Yokoyama S, Maruyama T, Athikomkulchai S, Viriyaroj A, Awale S,
Yagita H, Ruchirawat S, Svasti J and Saiki I: A flavonoid chrysin
suppresses hypoxic survival and metastatic growth of mouse breast
cancer cells. Oncol Rep. 30:2357–2364. 2013.PubMed/NCBI
|
|
19
|
Samarghandian S, Afshari JT and Davoodi S:
Chrysin reduces proliferation and induces apoptosis in the human
prostate cancer cell line PC-3. Clinics (Sao Paulo). 66:1073–1079.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuntz S, Wenzel U and Daniel H:
Comparative analysis of the effects of flavonoids on proliferation,
cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J
Nutr. 38:133–142. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wenzel U and Daniel H: Early and late
apoptosis events in human transformed and non-transformed
colonocytes are independent on intracellular acidification. Cell
Physiol Biochem. 14:65–76. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomas CM, Wood RC III, Wyatt JE,
Pendleton MH, Torrenegra RD, Rodriguez OE, Harirforoosh S,
Ballester M, Lightner J, Krishnan K and Ramsauer VP:
Anti-neoplastic activity of two flavone isomers derived from
Gnaph alium elegans and Achyrocline
bogotensis. PLoS One. 7:e398062012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Austin CA, Patel S, Ono K, Nakane H and
Fisher LM: Site-specific DNA cleavage by mammalian DNA
topoisomerase II induced by novel flavone and catechin derivatives.
Biochem J. 282:883–889. 1992.PubMed/NCBI
|
|
24
|
Marozienė A, Nemeikaitė-Čėnienė A,
Vidžiūnaitė R and Čėnas N: Correlation between mammalian cell
cytotoxicity of flavonoids and the redox potential of phenoxyl
radical/phenol couple. Acta Biochim Pol. 59:299–305.
2012.PubMed/NCBI
|