Introduction
Lung cancer remains the leading cause of
cancer-related mortality worldwide. Non-small-cell lung cancer
(NSCLC) is the predominant histological type of lung cancer and
~70.0% of all NSCLC patients have advanced-stage IIIB or IV disease
at diagnosis. Platinum-based chemotherapy is currently the standard
treatment for advanced NSCLC; however, almost all the patients
treated by initial chemotherapy alone eventually develop a
relapse.
Erlotinib, a selective epidermal growth factor
receptor (EGFR)-tyrosine kinase inhibitor (TKI), is currently
recommended as second- or third-line standard treatment in patients
with NSCLC (1). The presence of
activating somatic mutations in the EGFR gene has been shown to be
a predictor of the response to treatment with EGFR-TKIs (2) and first-line EGFR-TKI therapy for
patients with EGFR mutation-positive NSCLC was shown to improve the
progression-free survival (PFS) compared to standard platinum-based
chemotherapy (3–6). However, the results of subgroup
analyses in the BR21 and SATURN trials suggest that erlotinib may
also be beneficial to patients with EGFR wild-type (WT) NSCLC
(1,7).
While assessing the efficacy of erlotinib in
patients with EGFR WT NSCLC, the sensitivity of the method(s) used
for the EGFR mutation analysis may affect the results of the
evaluation. Although direct DNA sequencing has been widely used for
EGFR mutation analysis, several new techniques, such as the peptide
nucleic acid-locked nucleic acid (PNA-LNA) polymerase chain
reaction (PCR) clamp method and the Scorpion Amplification
Refractory Mutation System (S-ARMS) assay are currently available
(8,9). Kim et al (10) reported a higher sensitivity of the
PNA-LNA clamp method as compared to direct DNA sequencing for the
detection of EGFR mutations in patients with NSCLC. In their study,
the EGFR mutation positivity rate in 240 NSCLC patients was 34.6%
when assessed by the PNA-LNA clamp method, but only 26.3% when
assessed by direct DNA sequencing. Therefore, it is possible that
erlotinib is found to be considerably less effective in patients
with EGFR WT NSCLC, when the EGFR genotype is confirmed by highly
sensitive methods, such as the PNA-LNA clamp method.
In addition, the predictive value of KRAS mutations
for the efficacy of erlotinib in patients with EGFR WT NSCLC has
not been fully elucidated. It was previously suggested that the
presence of KRAS mutations may predict a poor response to EGFR-TKI
therapy in patients with NSCLC (11). However, the EGFR mutation status
may be a confounding factor in the analysis of the predictive value
of KRAS mutations, since KRAS and EGFR mutations exhibit a strong
negative correlation and EGFR mutation is a predictor of the
response to EGFR-TKI therapy. Therefore, further evaluation of the
predictive value of KRAS mutations in patients with EGFR WT NSCLC
is required.
Based on these findings, we conducted a multicenter
phase II trial of erlotinib for previously treated patients with
EGFR WT NSCLC. The primary endpoint of this study was to assess the
efficacy and safety of erlotinib in patients with EGFR WT NSCLC, as
confirmed by the PNA-LNA clamp method, which is a highly sensitive
method for EGFR mutation analysis. Preplanned reevaluation of the
EGFR and KRAS mutation status as exploratory endpoints was
performed using the S-ARMS assay in this study.
Patients and methods
Study design
This study was a multicenter, open-label,
single-arm, phase II trial conducted in Japan. The study protocol
was approved by the Central Japan Lung Study Group (CJLSG) Protocol
Review Committee and the Institutional Review Board of each center
as the CJLSG 0903 trial. The study was performed in accordance with
the principles laid out in the Declaration of Helsinki and is
registered with the University Hospital Medical Information Network
in Japan (no. 000002692). The primary endpoint was the objective
response rate (ORR) and the secondary endpoints were disease
control rate (DCR), PFS, overall survival (OS) and safety.
Moreover, if residual samples were available, we performed a
preplanned reevaluation of the EGFR mutation status and KRAS
mutation analysis with the S-ARMS assay as a secondary
endpoint.
Eligibility criteria
Pretreated stage IIIB/IV NSCLC patients were
assessed regarding their eligibility for enrollment in this study.
The main inclusion criteria were as follows: Pathologically proven
NSCLC; EGFR WT genotype confirmed by the PNA-LNA PCR clamp method;
history of one or two prior chemotherapies, including at least one
platinum-based chemotherapy; age ≥20 years; Eastern Cooperative
Oncology Group performance status (PS) of 0–2; adequate bone
marrow, hepatic and renal function; at least one measurable lesion
as defined by the Response Evaluation Criteria in Solid Tumors
(RECIST), version 1.1 (12); life
expectancy of ≥3 months; and patient willingness to provide written
informed consent. The main exclusion criteria were as follows:
Pulmonary disorders, such as interstitial lung disease,
pneumoconioses, or active radiation pneumonitis; severe eye
disorders; and massive pleural or pericardial effusion.
EGFR genotype testing for
eligibility
The PNA-LNA PCR clamp method was used for
confirmation of the EGFR mutation status in the NSCLC patients
prior to enrollment. This method is a highly sensitive and simple
procedure for the detection of 13 known EGFR mutations (8). For this study, we enrolled patients
with the WT allele of EGFR in all 13 mutation sites. A total of 5
tissue slides (5-μm) or pleural effusion cytology samples
containing tumor cells were used for the analysis. Tissue slides
were prepared from tumor cell-rich sections of formalin-fixed
paraffin-embedded tumor samples. In Japan, the PNA-LNA PCR clamp
method is commercially available and performed by the Mitsubishi
Chemical Medience Corporation (Tokyo, Japan).
Screening of tumors for the KRAS genotype
and reanalysis of the EGFR mutation status using the S-ARMS
assay
Following completion of patient enrollment, the
tumor samples available for KRAS mutation analysis and EGFR
mutation reanalysis were collected. DNA was extracted at the
laboratory of the Department of Respiratory Medicine, Nagoya
University Graduate School of Medicine, using the QIAamp DNA Mini
kit (Qiagen, Tokyo, Japan), followed by quantitation of the DNA.
According to a previous report, the PNA-LNA PCR clamp method and
the S-ARMS assay exhibit an equally high sensitivity for the
detection of the EGFR mutation status (13). Therefore, we prioritized KRAS
mutation screening if the amount of DNA available was not
sufficient for evaluation of both the KRAS and EGFR mutation status
by the S-ARMS assay. S-ARMS analysis for the detection of EGFR
mutation was performed using the EGFR Mutation RGQ PCR kit (Qiagen,
Manchester, UK) and S-ARMS analysis for evaluation of the KRAS
mutation status was performed using the KRAS PCR kit (Qiagen,
Manchester), which is able to detect 7 mutations in codons 12 and
13 of the KRAS gene.
Treatment
Oral erlotinib was administered at a dose of 150 mg
daily until disease progression or development of unacceptable
toxicity. The erlotinib dose was reduced (first reduction to 100 mg
daily and second reduction to 50 mg daily) or treatment was
interrupted in the event of any grade 3 non-hematological toxicity.
Dose escalation was not permitted. In the event of development of
interstitial lung disease (ILD) of any grade or any grade 4
toxicity, the protocol was discontinued.
Efficacy and safety evaluation
Tumor response was assessed in accordance with
RECIST, version 1.1 (12). The
baseline assessment included chest and upper abdominal computed
tomography (CT), head CT or magnetic resonance imaging and bone
scintigraphy or 18F-fluorodeoxyglucose-positron emission
tomography. Assessment of the tumor response was performed every 4
weeks during the first 8 weeks, every 8 weeks during the subsequent
40 weeks and every 12 weeks thereafter. In this study, the
definition of stable disease (SD) required a duration of ≥8 weeks.
PFS was defined as the time from the date of study enrollment until
the date of objectively determined progressive disease (PD) or
death due to any cause or the date of the last follow-up. OS was
defined as the time from the date of study enrollment until death
due to any cause or the date of last follow-up. Toxicity was
evaluated using the Common Toxicity Criteria for Adverse Events
(version 3.0).
Statistical analysis
The primary endpoint was the ORR and the sample size
for the trial was calculated using Simon’s two-stage design.
Assuming that a response rate of 18.0% indicates potential
usefulness, while a rate of 6.8% is the lower limit of interest,
with α=0.05 and β=0.20, the estimated accrual number was 49
patients. In this study, the rate of the lower limit of interest
was adopted based on the ORRs of docetaxel reported in previous
phase III studies (14,15). Among these, ≥7 responders were
required for this therapy to be considered worthy of further
evaluation. We selected a target sample number of 54, to allow for
5 dropouts. The differences in ORR according to histology were
analyzed using the Mantel extension test adjusted for PS and M
factor (M0, M1a and M1b). A stratified log-rank test adjusted for
these factors was used to evaluate the difference in PFS according
to histology. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
Between February, 2010 and April, 2012, a total of
55 patients were enrolled. A review of the data indicated that 2 of
the patients enrolled in this study did not fulfill the eligibility
criteria listed in the study protocol and the remaining 53 patients
were included in the analysis as evaluable. The characteristics of
the 53 patients are summarized in Table I. The median age of the patients
was 67 years (range, 47–77 years). The histological subtypes were
non-squamous cell carcinoma (non-SCC) in 44 patients
[adenocarcinoma, 40 patients; and not otherwise specified (NOS), 4
patients] and SCC in 9 patients. The number of prior chemotherapies
was 1 in 26 patients (49.0%) and 2 in the remaining 27 patients
(51.0%).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Patient no. (%)
(n=53) |
|---|
| Age, years |
| Median | 67 |
| Range | 47–77 |
| Gender |
| Male | 43 (81.0) |
| Female | 10 (19.0) |
| Smoking status |
| Never | 7 (13.0) |
| Former/current | 46 (87.0) |
| Histology |
| Adenocarcinoma | 40 (75.0) |
| Squamous cell
carcinoma | 9 (17.0) |
| NOS | 4 (8.0) |
| No. of prior
chemotherapies |
| 1 | 26 (49.0) |
| 2 | 27 (51.0) |
| Stage |
| IIIB | 2 (4.0) |
| IV |
| M1a | 16 (30.0) |
| M1b | 35 (66.0) |
| ECOG PS |
| 0 | 23 (43.4) |
| 1 | 24 (45.3) |
| 2 | 6 (11.3) |
Efficacy
The median treatment duration was 51 days (range,
5–404 days). Of the 53 eligible patients, partial response (PR) was
obtained in 6 patients (4 with adenocarcinoma and 2 with SCC),
yielding an ORR of 11.3% (95% confidence interval (CI): 4.3–23.0).
SD was observed in 9 patients and the DCR was 28.3% (95% CI:
16.8–42.3). The ORR according to the histology was 9.1% (95% CI:
2.5–21.7) in patients with non-SCC and 22.2% (95% CI: 2.8–60.0) in
patients with SCC. The difference in the ORR between these two
groups was not statistically significant (P=0.29, Mantel extension
test). A summary of the tumor responses is provided in Table II. At the time of the analysis, 48
patients (91.0%) had developed disease progression and 34 (64.0%)
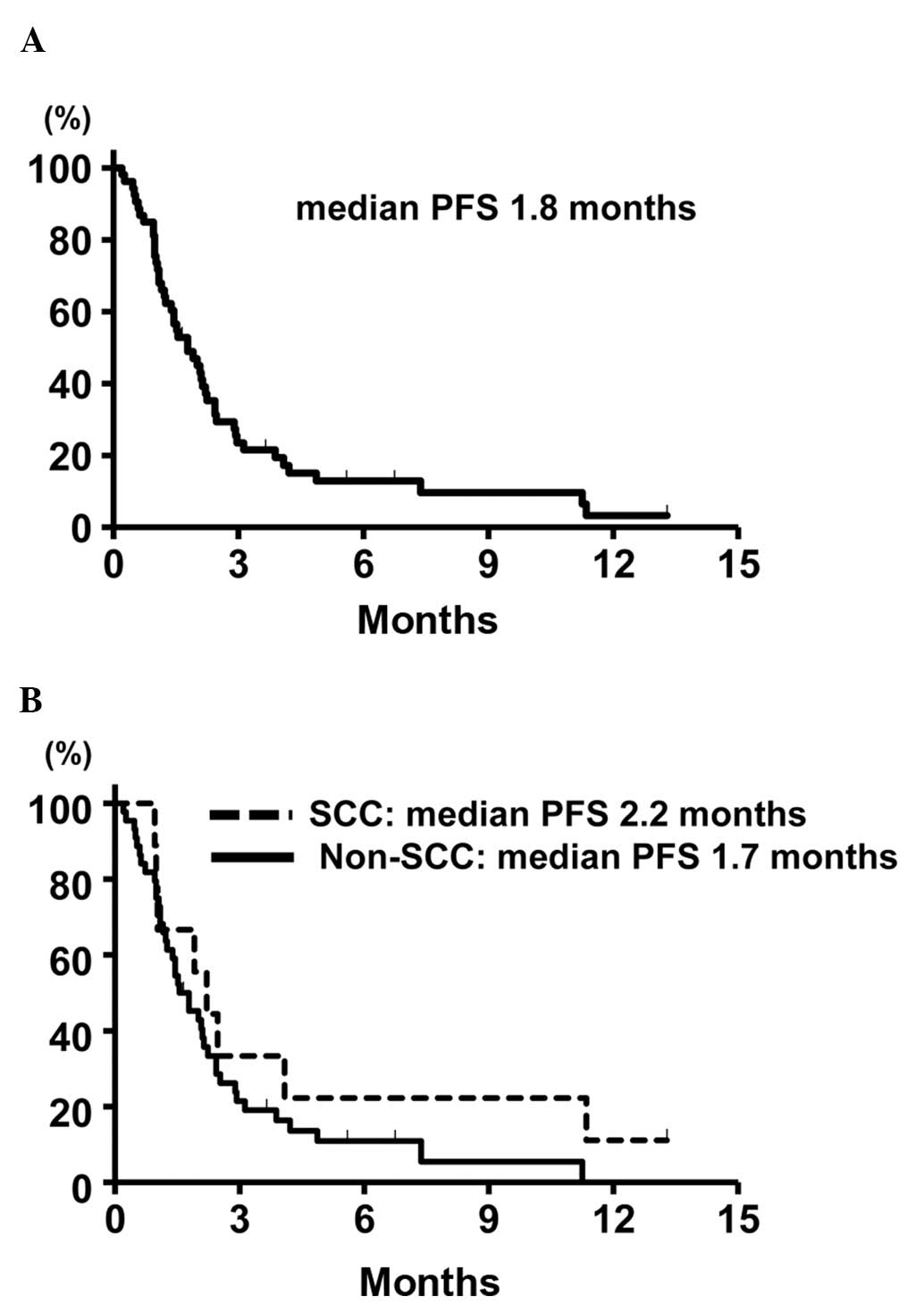
had succumbed to the disease. The median PFS of the entire patient
cohort was 1.8 months (95% CI: 1.2–2.3). The median PFS in the
patients with non-SCC and SCC was 1.7 months (95% CI: 1.2–2.1), and
2.2 months (95% CI: 1.0–11.3), respectively, without a
statistically significant difference (P=0.54, stratified log-rank
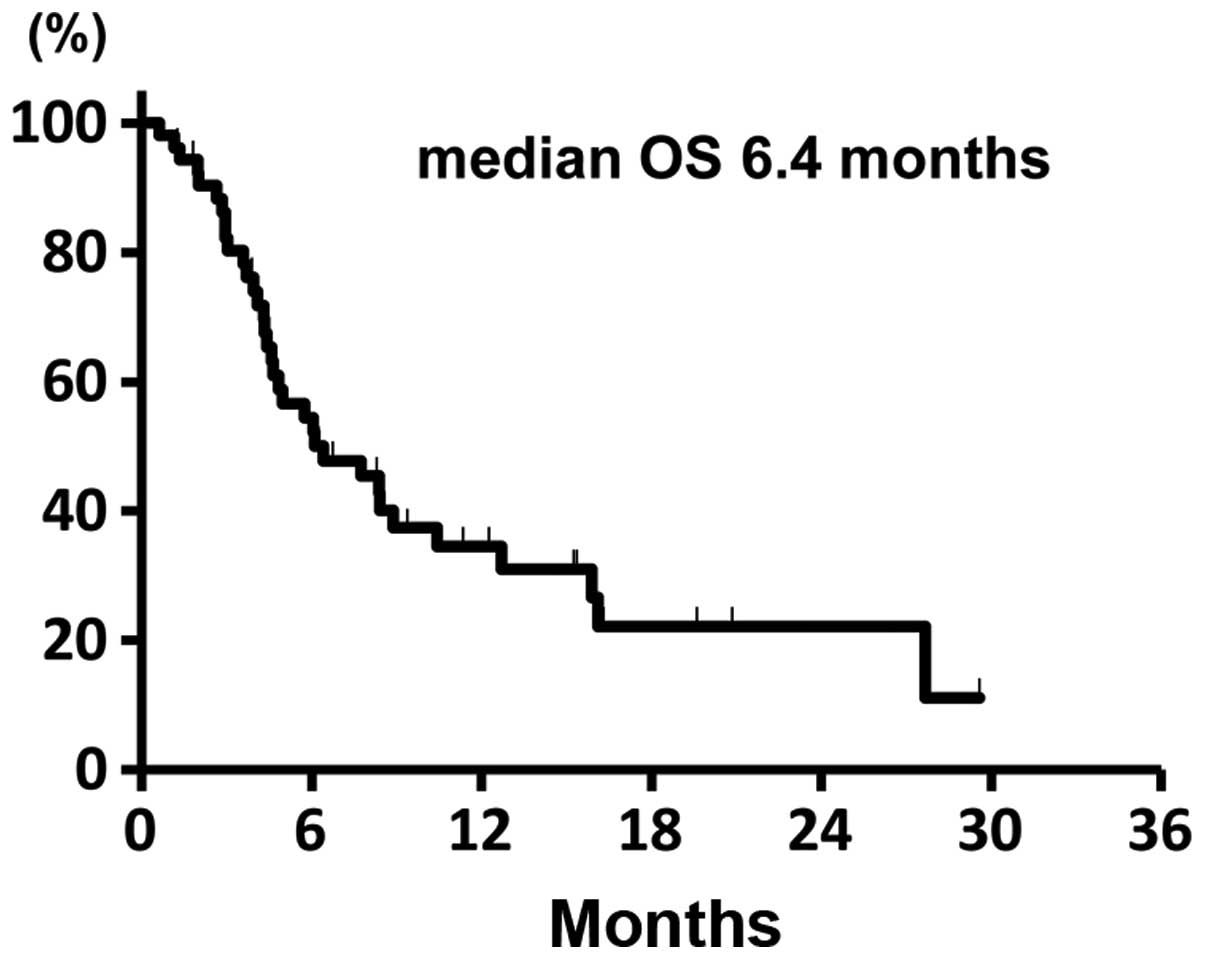
test). The Kaplan-Meier survival curve for PFS is shown in Fig. 1. The median OS was 6.4 months (95%
CI: 4.5–10.4) and the Kaplan-Meier survival curve for OS is shown
in Fig. 2. The median OS in the
patients with 1 and 2 prior chemotherapies was 8.5 and 5.5 months,
respectively.
 | Table IITumor response. |
Table II
Tumor response.
| Type of response | Total (n=53) | Non-SCC (n=44) | SCC (n=9) |
|---|
| CR | 0 | 0 | 0 |
| PR | 6 | 4 | 2 |
| SD | 9 | 7 | 2 |
| PD | 37 | 32 | 5 |
| NE | 1 | 1 | 0 |
| ORR, % (95% CI) | 11.3 (4.3–23.0) | 9.1 (2.5–21.7) | 22.2 (2.8–60.0) |
| DCR, % (95% CI) | 28.3 (16.8–42.3) | 25.0 (13.2–40.3) | 44.4 (13.7–78.8) |
Safety
The adverse events are summarized in Table III. The major adverse events were
rash in 81.1% of the patients (11.3% ≥grade 3) and anorexia in
47.1% (9.4% ≥grade 3). No grade 3 or 4 hematological adverse events
were observed. Grade 3–5 ILD was reported in 3 patients (5.6%) and
grade 5 ILD possibly related to erlotinib in 2 patients (3.8%). In
the 2 patients with grade 5 ILD, the baseline chest CT revealed
carcinomatous lymphangitis and lung cancer progression was
concurrently detected by chest CT at the time of development of the
ILD.
 | Table IIIAdverse events in the patients
(n=53). |
Table III
Adverse events in the patients
(n=53).
| Adverse events | Grade (patient
no.) | % of patients with
grade 3–4 toxicity |
|---|
|
|---|
| 1 | 2 | 3 | 4 |
|---|
| Skin rash | 11 | 26 | 6 | 0 | 11.3 |
| Diarrhea | 16 | 1 | 1 | 0 | 1.9 |
| Anorexia | 14 | 6 | 5 | 0 | 9.4 |
| Nausea | 4 | 2 | 0 | 0 | 0.0 |
| Vomiting | 1 | 1 | 1 | 0 | 1.9 |
| Fatigue | 8 | 7 | 3 | 0 | 5.7 |
| Stomatitis | 10 | 2 | 1 | 0 | 1.9 |
| Ocular
disorders | 2 | 0 | 1 | 0 | 1.9 |
| ALT increased | 7 | 4 | 1 | 2 | 5.7 |
| AST increased | 10 | 4 | 1 | 2 | 5.7 |
| Amy increased | 0 | 1 | 0 | 1 | 1.9 |
| Leukopenia | 1 | 1 | 0 | 0 | 0.0 |
|
Thrombocytopenia | 6 | 0 | 0 | 0 | 0.0 |
| ILD | 2 | 0 | 1 | 0 | 5.6a (G3–5) |
EGFR mutation reanalysis with the S-ARMS
assay and KRAS mutation screening
Samples from 26 patients (49% of the eligible
patients) were available for EGFR mutation reanalysis. Of these,
only 1 patient with adenocarcinoma was found to be EGFR
mutation-positive (exon 19 deletion) NSCLC with the S-ARMS assay
and this patient exhibited a PR. In the remaining 25 patients, EGFR
WT was reconfirmed by the S-ARMS assay and two of these patients
exhibited a PR. The ORR was 8.0% in the NSCLC patients with EGFR WT
as confirmed by both the PNA-LNA PCR clamp method and the S-ARMS
assay.
The KRAS mutation status was screened by the S-ARMS
assay in samples obtained from 44 patients, of which DNA
amplification was unsuccessful in 2. KRAS mutation screening was
successfully performed in the samples from the remaining 42
patients (79.0% of eligible patients). Of these 42 patients, 4
(9.1%) were found to be KRAS mutation-positive. The characteristics
of these 4 patients and the sites of the KRAS mutations are listed
in Table IV. As regards treatment
response, PD was observed in all 4 patients. By contrast, the ORR
and median PFS in the patients with KRAS WT NSCLC were 6.9% and 1.9
months, respectively.
 | Table IVKRAS mutation-positive patients. |
Table IV
KRAS mutation-positive patients.
| Case | Gender | Smoking status | Smoking index | Amino acid
change | Best overall
response |
|---|
| 1 | Male | Former | 1020 | Gly12Ala
(GGT>GCT) | PD |
| 2 | Male | Current | 1020 | Gly12Cys
(GGT>TGT) | PD |
| 3 | Male | Current | 1000 | Gly12Ala
(GGT>GCT) | PD |
| 4 | Male | Former | 1520 | Gly12Cys
(GGT>TGT) | PD |
Discussion
In this study, we evaluated the efficacy and safety
of erlotinib in pretreated patients with NSCLC harboring EGFR WT as
confirmed by the PNA-LNA clamp method, which is reported as being
highly sensitive. This study did not meet the primary endpoint
based on the reported ORRs of docetaxel in previous studies,
although erlotinib treatment was associated with an ORR of
11.3%.
Two recent phase III studies reported the
inferiority of erlotinib compared to docetaxel regarding ORR and
PFS in EGFR WT NSCLC patients (16,17).
Based on these results, including the findings of our study, it
appears that docetaxel should be preferred as second-line therapy,
if not used as a part of first-line platinum based combination
therapy.
However, there remains the clinical question of
whether erlotinib should not be used for EGFR WT NSCLC in any-line
setting. In our opinion, erlotinib monotherapy may be an viable
option in pretreated patients with EGFR WT NSCLC following failure
of docetaxel treatment for the following reasons: First, EGFR WT
was reconfirmed by the S-ARMS assay in 25 of the 26 patient samples
examined in this study, of which 2 (8.0%) achieved a PR. Our
results suggested that erlotinib may still be effective against
EGFR WT NSCLC, even when the EGFR mutation status is confirmed by
two different highly sensitive methods.
Second, a discordance in the EGFR mutation status
between the PNA-LNA clamp method and S-ARMS assay was observed in 1
patient in this study. Although large, tumor cell-rich samples are
required for accurate EGFR mutation analysis, we cannot, in
general, obtain surgically resected specimens from advanced NSCLC
patients in clinical practice. Fukui et al (18) verified the accuracy of the EGFR
mutation analysis in small samples by high-resolution melting
analysis, which has also been reported to be a highly sensitive
method. In that study, the results of DNA sequencing combined with
laser capture microdissection in paired surgically resected
specimens revealed a few false-negative results in small samples.
Those data suggested that it may be difficult to determine the EGFR
mutation status with complete accuracy in small tissue samples,
irrespective of the sensitivity of the method used. Therefore, if
we do not use erlotinib for EGFR WT NSCLC in any-line setting, we
may miss the opportunity to attempt elrotinib treatment for
patients with a false-negative EGFR mutation result. This may also
lead to loss of the significant survival benefit obtained from
EGFR-TKI therapy for EGFR mutation-positive NSCLCs.
We succeeded in obtaining 42 samples (79% of the
eligible patients) for KRAS mutation screening. KRAS mutations were
detected in 4 of the 42 patients screened (9.5%) and all the KRAS
mutation-positive patients exhibited PD. In a phase III study
conducted to compare erlotinib and pemetrexed, none of the patients
with KRAS mutation-positive NSCLC responded to erlotinib treatment,
which was similar to the findings of our study (19). These results should be interpreted
with caution, as we could not exclude the KRAS mutation status as a
potential prognostic factor. However, the presence of KRAS mutation
may be useful as a negative predictive factor, at least regarding
response to erlotinib therapy, in patients with EGFR WT NSCLC.
We performed a subgroup analysis according to
histological subtype. In patients with EGFR mutated NSCLC, the
efficacy of EGFR-TKIs for SCC appeared to be lower compared to that
for non-SCC (20). However, SCC
histology may not be associated with poor efficacy of erlotinib in
patients with EGFR WT NSCLC based on our results. Molecular
biomarkers, such as KRAS, may be required to select suitable
candidates for erlotinib treatment among patients with EGFR WT
NSCLC.
The toxicity profile of erlotinib in this study, in
terms of the incidence/grade of skin rash, diarrhea and
hematological toxicities, was consistent with previous reports.
However, grade 3–5 ILD was reported in 3 patients (5.8%). In a
large-scale surveillance study conducted in Japan, the incidence of
ILD was also higher compared to that reported by the BR21 and
SATURN trials (1,7,21).
Further studies are required to determine whether there are ethnic
differences in the incidence of ILD, as suggested by a previous
study (22).
In conclusion, this study did not meet the primary
endpoint, although erlotinib was found to be moderately effective
in pre-treated patients with EGFR WT NSCLC, even when the EGFR
mutational status was confirmed by the highly sensitive PNA-LNA
clamp PCR method.
Acknowledgements
This study was supported by the Central Japan Lung
Study Group (CJLSG), a non-profit organization supported by
unrestricted donations from the following pharmaceutical companies:
Chugai Pharmaceutical Co., Ltd. (Tokyo, Japan); Shionogi & Co.,
Ltd. (Osaka, Japan); Daiichi Sankyo Co., Ltd. (Tokyo, Japan);
Dainippon Sumitomo Pharma Co., Ltd. (Osaka, Japan); Janssen
Pharmaceutical K.K. (Tokyo, Japan); Eli Lilly Japan K.K. (Kobe,
Japan); Taisho Toyama Pharmaceutical Co., Ltd.; Meiji Seika Pharma
Co., Ltd.; MSD K.K.; Bayer Holding Ltd.; Astellas Pharma Inc. and
Nippon Boehringer Ingelheim Co., Ltd. (all from Tokyo, Japan). Dr
Morise reported receiving honoraria for lecturing from Chugai
Pharmaceutical Co.; Dr Taniguchi has served as a member of the
advisory boards at Chugai Pharmaceutical Co., Boehringer Ingelheim
and Shionogi & Co., Ltd.; Dr Saka reported receiving two grants
from Chugai Pharmaceutical Co., which were paid to Nagoya Medical
Center (Nagoya, Japan); Dr Hase reported receiving honoraria for
lecturing from Chugai Pharmaceutical Co., Pfizer Inc. (New York
City, NY, USA) and Astra Zeneca Co. (London, UK); Dr Ando reported
having a board membership at Chugai Pharmaceutical Co.; Dr Kondo
reported receiving honoraria for lecturing from Chugai
Pharmaceutical Co.; Dr Saito received research funding from Chugai
Pharmaceutical Co.; Dr. Hasegawa reported receiving honoraria for
lecturing from Chugai Pharmaceutical Co. and receiving a grant from
Chugai-Pharmaceutical Co. that was paid to Nagoya University
References
|
1
|
Shepherd FA, Rodrigues Pereira J, et al;
National Cancer Institute of Canada Clinical Trials Group.
Erlotinib in previously treated non-small-cell lung cancer. N Engl
J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paez JG, Janne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maemondo M, Inoue A, Kobayashi K, et al:
Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitsudomi T, Morita S, Yatabe Y, et al:
Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): an open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar
|
|
5
|
Zhou C, Wu YL, Chen G, et al: Erlotinib
versus chemotherapy as first-line treatment for patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase
3 study. Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosell R, Carcereny E, Gervais R, et al:
Erlotinib versus standard chemotherapy as first-line treatment for
European patients with advanced EGFR mutation-positive
non-small-cell lung cancer (EURTAC): a multicentre, open-label,
randomised phase 3 trial. Lancet Oncol. 13:239–246. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cappuzzo F, Ciuleanu T, Stelmakh L, et al:
Erlotinib as maintenance treatment in advanced non-small-cell lung
cancer: a multicentre, randomised, placebo-controlled phase 3
study. Lancet Oncol. 11:521–529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nagai Y, Miyazawa H, Huqun, et al: Genetic
heterogeneity of the epidermal growth factor receptor in non-small
cell lung cancer cell lines revealed by a rapid and sensitive
detection system, the peptide nucleic acid-locked nucleic acid PCR
clamp. Cancer Res. 65:7276–7282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kimura H, Fujiwara Y, Sone T, et al: High
sensitivity detection of epidermal growth factor receptor mutations
in the pleural effusion of non-small cell lung cancer patients.
Cancer Sci. 97:642–648. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HJ, Lee KY, Kim YC, et al: Detection
and comparison of peptide nucleic acid-mediated real-time
polymerase chain reaction clamping and direct gene sequencing for
epidermal growth factor receptor mutations in patients with
non-small cell lung cancer. Lung Cancer. 75:321–325. 2012.
View Article : Google Scholar
|
|
11
|
Linardou H, Dahabreh IJ, Kanaloupiti D, et
al: Assessment of somatic k-RAS mutations as a mechanism associated
with resistance to EGFR-targeted agents: a systematic review and
meta-analysis of studies in advanced non-small-cell lung cancer and
metastatic colorectal cancer. Lancet Oncol. 9:962–972. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
13
|
Goto K, Satouchi M, Ishii G, et al: An
evaluation study of EGFR mutation tests utilized for non-small-cell
lung cancer in the diagnostic setting. Ann Oncol. 23:2914–2919.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fossella FV, DeVore R, Kerr RN, et al:
Randomized phase III trial of docetaxel versus vinorelbine or
ifosfamide in patients with advanced non-small-cell lung cancer
previously treated with platinum-containing chemotherapy regimens.
The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol.
18:2354–2362. 2000.
|
|
15
|
Shepherd FA, Dancey J, Ramlau R, et al:
Prospective randomized trial of docetaxel versus best supportive
care in patients with non-small-cell lung cancer previously treated
with platinum-based chemotherapy. J Clin Oncol. 18:2095–2103.
2000.
|
|
16
|
Garassino MC, Martelli O, Broggini M, et
al; TAILOR trialists. Erlotinib versus docetaxel as second-line
treatment of patients with advanced non-small-cell lung cancer and
wild-type EGFR tumours (TAILOR): a randomised controlled trial.
Lancet Oncol. 14:981–988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawaguchi T, Ando M, Asami K, et al:
Randomized phase III trial of erlotinib versus docetaxel as second-
or third-line therapy in patients with advanced non-small cell lung
cancer : Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin
Oncol. May 19–2014.(Epub ahead of print).
|
|
18
|
Fukui T, Ohe Y, Tsuta K, et al:
Prospective study of the accuracy of EGFR mutational analysis by
high-resolution melting analysis in small samples obtained from
patients with non-small cell lung cancer. Clin Cancer Res.
14:4751–4757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karampeazis A, Voutsina A, Souglakos J, et
al: Pemetrexed versus erlotinib in pretreated patients with
advanced non-small cell lung cancer: a Hellenic Oncology Research
Group (HORG) randomized phase 3 study. Cancer. 119:2754–2764. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hata A, Katakami N, Yoshioka H, et al: How
sensitive are epidermal growth factor receptor-tyrosine kinase
inhibitors for squamous cell carcinoma of the lung harboring EGFR
gene-sensitive mutations? J Thorac Oncol. 8:89–95. 2013. View Article : Google Scholar
|
|
21
|
Nakagawa K, Kudoh S, Ohe Y, et al:
Postmarketing surveillance study of erlotinib in Japanese patients
with non-small-cell lung cancer (NSCLC): an interim analysis of
3488 patients (POLARSTAR). J Thorac Oncol. 7:1296–1303. 2012.
View Article : Google Scholar
|
|
22
|
Yoshioka H, Hotta K, Kiura K, et al;
Okayama Lung Cancer Study Group. A phase II trial of erlotinib
monotherapy in pretreated patients with advanced non-small cell
lung cancer who do not possess active EGFR mutations: Okayama Lung
Cancer Study Group trial 0705. J Thorac Oncol. 5:99–104. 2010.
View Article : Google Scholar : PubMed/NCBI
|