Introduction
Pancreatic cancer remains one of the most aggressive
and intractable malignant tumors and is currently the fourth
leading cause of cancer-related mortality worldwide (1). Due to its extremely high invasive and
metastatic potential, pancreatic cancer is usually diagnosed at an
advanced stage, leaving no adequate time window for effective
systemic therapies and often recurs even following curative
surgical excision (2). Despite
significant advances in cancer therapy, including surgery,
radiation, chemotherapy, or a combination of these approaches, the
overall pancreatic cancer mortality rate has not been significantly
reduced (3–5). Therefore, novel therapeutic
approaches are required to improve the prognosis of pancreatic
cancer patients.
A hypoxic tumor microenvironment is critical for
tumor development, invasion and metastasis. Hypoxia-inducible
factor-1 (HIF-1) plays a key role in cellular adaptation to
hypoxia. Although HIF-1 is usually strongly suppressed by
post-translational mechanisms under normoxic conditions, the
activation of HIF-1 during hypoxia may upregulate hypoxia-induced
gene expression, promote clonal selection of viable hypoxic tumor
cells and drive the tumor toward a more aggressive phenotype,
leading to invasion and metastasis (6,7).
Recent studies confirmed that elevated levels of hypoxia-induced
gene expression have been found in pancreatic cancer cells,
suggesting that HIF-1α plays a crucial role in malignant tumor
progression (8,9). However, the molecular mechanisms
through which the hypoxic microenvironment promotes pancreatic
cancer invasion and metastasis have not been fully elucidated.
As is the case for the majority of solid malignant
tumors, pancreatic cancer development is a multistep process,
involving uncontrolled tumor growth, angiogenesis, detachment from
the matrix, invasion and metastasis. Tumor invasion and metastasis
have been shown to involve proteolytic degradation of the
extracellular matrix, which is accomplished primarily by members of
the matrix metalloproteinase (MMP) family. MMPs have been
implicated in a number of physiological and pathological processes,
including cellular migration, angiogenesis and invasion and
metastasis of tumor cells (10).
Indeed, MMPs are continuously overexpressed in a variety of
malignant solid tumors and are involved in extracellular matrix
destruction, thereby contributing to tumor invasion and metastasis
(11,12). Among those MMPs, membrane-type MMPs
(MT-MMPs) constitute a specific subtype, conducting pericellular
proteolysis, which is considered to be an important step in
pericancerous tissue remodeling (13,14).
MT-MMPs, which constitute a subfamily of six
distinct membrane-associated MMPs, are Zn2+-binding
endopeptidases that degrade various components of the extracellular
matrix. MT2-MMP (also referred to as MMP-15), was the second member
of the MT-MMP subfamily to be discovered, was originally isolated
from a human lung cDNA library (15) and has been found to be
constitutively expressed at low levels in normal tissues, but
highly expressed in malignant tumors (16–19).
MT2-MMP has been identified as a powerful modulator of the
pericellular environment, promoting tumor growth, angiogenesis,
invasion and metastasis (20).
Moreover, MT2-MMP was also characterized as a new element in the
anti-apoptotic pathway network of human tumor cells (21). Therefore, MT2-MMP has been
considered to be particularly important for the malignant behavior
of cancer cells.
Although we previously demonstrated that the
upregulation of MT2-MMP in response to hypoxia is promoted by
HIF-1α in cancer cells (22), the
available information on the association of the expression of
MT2-MMP and HIF-1α with clinical outcomes is limited. This study
was undertaken to investigate the regional expression of MT2-MMP
and HIF-1α in pancreatic cancer, study the association of MT2-MMP
and HIF-1α expression with tumor progression and assess relevant
clinicopathological factors for patient survival.
Materials and methods
Patients and surgical specimens
A total of 78 patients with pancreatic cancer who
underwent pancreatectomy at the Department of Pancreatic Surgery,
Union Hospital, Huazhong University of Science and Technology
(HUST; Wuhan, China) between December, 2005 and September, 2010
were included in this retrospective study. The surgical specimens
were collected, immediately placed in liquid nitrogen and stored at
−80°C until analysis. The diagnosis of all the cases was confirmed
based on the World Health Organization classification, staged
according to the tumor-node-metastasis (TNM) classification and
were reviewed by two pathologists (23). Clinicopathological parameters,
including age at surgery, gender, location of tumor, size of tumor,
perineural invasion, vascular invasion, lymph node status, distant
metastasis, TNM stage and tumor differentiation, were retrieved
from patient records. The overall survival time was defined as the
time from surgical resection to date of last follow-up or death.
The analysis of human tissues was approved by the Human Research
Ethics Committee of HUST and all the patients provided written
informed consent on the use of their clinical specimens for medical
research.
Immunohistochemistry (IHC)
The expression of MT2-MMP, HIF-1α and CD34 was
determined using Histostain-Plus kits (Zymed Laboratories, San
Francisco, CA, USA), as previously described (24). In brief, serial tissue sections (4
μm) from formalin-fixed and paraffin-embedded specimens were
deparaffinized in xylene and rehydrated. All the sections were then
immersed in 3% hydrogen peroxide for 30 min to block endogenous
peroxidase activity. After neutralization of endogenous peroxidase,
sections on glass slides were preincubated in blocking serum and
were then incubated overnight at 4°C with anti-MT2-MMP antibody
(Abcam, Inc., Cambridge, MA, USA), anti-HIF-1α antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and monoclonal anti-CD34
antibody (Boster Biotechnology, Wuhan, China) at a 1:50 dilution.
After three washes in phosphate-buffered saline (PBS), the sections
were incubated for 2 h with biotinylated anti-mouse IgG, washed
three times with PBS, incubated with avidin-biotin-peroxidase
complex for 1 h and again washed for 10 min with PBS. The reaction
products were visualized with 3,3′-diaminobenzidine
tetrahydrochloride and the slides were counterstained with
hematoxylin. A consecutive section from each specimen processed
with normal mouse IgG (Boster Biotechnology) was used as a negative
control.
The slides were evaluated twice at different times
by two independent investigators who were blinded to the
pathological characteristics. The staining intensity of MT2-MMP and
HIF-1α expression was classified semi-quantitatively by the
percentage of the stained cells and the intensity of the staining
into four grades as follows: absent (grade 1), weak (grade 2),
moderate (grade 3) and strong (grade 4). Grade 3 and 4 specimens
were considered to be overexpressing MT2-MMP and HIF-1α and were
recorded as positive results in the statistical analysis. To
compare the regional expression of MT2-MMP and HIF-1α in pancreatic
cancer, their expression was evaluated in serial sections of
selected cases exhibiting MT2-MMP and HIF-1α expression.
To visualize the microvessels, sections stained with
anti-CD34 antibody were examined under a light microscope (Olympus
Corporation, Tokyo, Japan) and the hotspots containing a large
number of microvessels were identified at a low magnification
(x100). Five randomly selected hotspots were observed and the
number of microvessels was counted manually (magnification, ×200).
The microvascular density (MVD) was calculated by counting
CD34-positive vascular endothelial cells, using the method
previously described (25) and
expressed as number of vessels per 0.74 mm2
(magnification, ×200).
Cell culture and hypoxic treatment
PANC-1, BxPC-3 and AsPC-1 pancreatic cancer cells
were maintained in Dulbecco’s modified Eagle’s medium supplemented
with 10% fetal calf serum, 100 mg/ml penicillin and 100 mg/ml
streptomycin and cultured at 37°C in a 5% CO2 incubator.
For the stably transfected cells, 600 mg/ml G418 (Sigma-Aldrich,
St. Louis, MO, USA) was added into the medium. For hypoxic
treatment, the cells were exposed to hypoxic conditions of 1%
O2, 5% CO2 and 94% N2, which was
created by a hypoxic chamber (Billups-Rothenberg, Inc., San Diego,
CA, USA). The medium was switched to serum-free medium (Invitrogen
Life Technologies, Carlsbad, CA, USA) before the cells were
subjected to hypoxia and control cultures were grown in serum-free
medium under normoxia in a 5% CO2 incubator (Forma
Scientific Co., Marietta, OH, USA). After incubation for the
desired periods, the cells were harvested for subsequent
experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was isolated from each sample
using the TRIzol reagent (Invitrogen Life Technologies) and
subjected to DNase treatment. cDNA was prepared using the TaqMan
Reverse Transcription Reagents kit (Applied Biosystems, Foster
City, CA, USA) according to the manufacturer’s instructions. The
following reaction mixture was used for all the RT-qPCR samples: 1X
of iQ SYBR-Green supermix (Bio-Rad, Hercules, CA, USA), 200 nmol/l
of each primer and 2.5 μl of cDNA in a total volume of 25 μl. The
reactions were amplified and analyzed using the ABI-7000 system
(Applied Biosystems). The MT2-MMP primers were 5′-CTTCGG
CTTTATGGCTACCT-3′ (forward) and 5′-AAGGTCAGA TGGTGGTTGTTC-3′
(reverse), whereas the primers used for HIF-1α amplification were
5′-CATCTCCATCTCCTACCC ACA-3′ (forward) and 5′-CTCAAAGCGACAGATAAC
ACG-3′ (reverse). β-actin was used as an internal control and the
data were averaged from three individual experiments.
Western blotting
The cells were treated under normoxia or hypoxia and
lysed by lysis buffer [50 mM Tris (pH 7.2), 1% Triton X-100, 0.5%
sodium deoxycholate, 0.1% sodium dodecyl sulphate (SDS), 500 mM
NaCl, 10 mM MgCl2 with 1 mM phenylmethanesulfonyl
fluoride]. The proteins were separated by SDS-PAGE at 100 V for 1 h
and transferred onto polyvinylidene difluoride membranes
(Millipore, Billerica, MA, USA). Non-specific binding sites were
blocked with 5% milk in Tris-buffered saline with Tween-20 [120 mM
Tris-HCl (pH 7.4), 150 mM NaCl and 0.05% Tween-20] for 1 h at room
temperature. The membranes were incubated with primary antibody
against HIF-1α or MT2-MMP overnight at 4°C. The membranes were
washed three times and incubated with horseradish
peroxidase-conjugated secondary antibody and the proteins were
visualized by ECL Western Blotting substrate (Pierce Biotechnology,
Inc., Rockford, IL, USA). β-actin was used as an internal control
and the data were averaged from three individual experiments.
Luciferase activity assay
A 956-bp MT2-MMP promoter construct, corresponding
to the sequence from −855 to +101 (relative to the transcriptional
start site) of the 5′-flanking region of the human MT2-MMP gene,
was generated from human genomic DNA using F1 (5′-TTTGCTAGCAAG
TGAGTCCGTGGAAATGATAG-3′) and R1 (5′-TTTCTC
GAGCGCCTGGGAAGAATGAAGAC-3′) as forward and reverse primers,
respectively. The cDNA of MT2-MMP was amplified by PCR and
sequenced, then cloned into the pcDNA3.1/histidine (His) vector
(Invitrogen Life Technologies) to yield a His-tagged MT2-MMP. The
full-length HIF-1α expression plasmid HA-HIF-1α-pcDNA3 was
purchased from Addgene, Inc. (www.addgene.org;
Cambridge MA, USA). Each promoter construct was co-transfected with
the pRL-TK plasmid into subconfluent (80–90%) monolayer cells using
Lipofectamine 2000 (Invitrogen Life Technologies) according to the
manufacturer’s protocol. The transfection efficiency was determined
as the percentage of control green fluorescent protein-expressing
cells counted by flow cytometry. After 4–6 h of transfection, the
cells were washed and allowed to recover overnight in fresh medium.
Approximately 12 h later, the transfected cells were incubated
under normoxia or hypoxia for 12 h. Luciferase activity detection
was performed using the Dual-Luciferase Reporter Assay system
(Promega Corporation, Madison, WI, USA) according to the
manufacturer’s instructions.
Statistical analysis
All the statistical analyses were performed using
the IBM SPSS Statistics version 20.0 (IBM Corp., New York, NY,
USA). The significance of the difference between the incidence of
MT2-MMP and HIF-1α expression and several clinical and pathological
parameters was assessed by the χ2 test or the
Mann-Whitney t-test. The Pearson’s correlation was used to evaluate
the association between MT2-MMP and HIF-1α by computing the
correlation coefficient (r) and corresponding P-values. The Cox
proportional hazards regression model was used for multivariate
analyses. The Fisher’s exact test was applied to assess the
association between categorical variables. The overall survival
time was calculated using the Kaplan-Meier method from the date of
surgery to the date of death from pancreatic cancer and compared by
the log-rank test. All the P-values were based on two-tailed
statistical analysis and P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients
All the patients were diagnosed with pancreatic
cancer and none had received radiotherapy or chemotherapy prior to
surgery. The demographic data and tumor characteristics are
summarized in Table I. The
patients included 45 men and 33 women, with a median age of 54
years (range, 23–78 years). At the time of last clinical follow-up
(September, 2012), 15 patients (19.2%) remained alive. The majority
of the patients (73.1%) had undergone radical surgery, whereas 21
patients (27.9%) received palliative surgery. If indicated, the
patients received standard gemcitabine-based chemotherapy,
according to their clinical stage and performance status. No
significant difference was noted between the MT2-MMP and HIF-1α
expression with respect to clinicopathological parameters such as
age at surgery, gender, location of tumor, size of tumor,
perineural invasion, vascular invasion, lymph node status, distant
metastasis, TNM stage and differentiation (P>0.05, Table I).
 | Table IPatient characteristics and frequency
of MT2-MMP and HIF-1α expression in pancreatic cancer. |
Table I
Patient characteristics and frequency
of MT2-MMP and HIF-1α expression in pancreatic cancer.
| | MT2-MMP | HIF-1α |
|---|
| |
|
|
|---|
| Variables | Total, n (%) | Negative, n (%) | Positive, n (%) | P-value | Negative, n (%) | Positive, n (%) | P-value |
|---|
| Age at surgery,
years | | | | 0.598 | | | 0.237 |
| <60 | 46 (59) | 20 (44) | 26 (56) | | 22 (48) | 24 (52) | |
| ≥60 | 32 (41) | 12 (38) | 20 (62) | | 11 (34) | 21 (66) | |
| Gender | | | | 0.251 | | | 0.656 |
| Male | 45 (58) | 16 (36) | 29 (64) | | 20 (44) | 25 (56) | |
| Female | 33 (42) | 16 (49) | 17 (51) | | 13 (40) | 20 (60) | |
| Tumor location | | | | 0.475 | | | 0.686 |
| Head | 50 (64) | 22 (44) | 28(56) | | 22 (44) | 28 (56) | |
| Body and tail | 28 (36) | 10 (36) | 18 (64) | | 11 (39) | 17 (61) | |
| Tumor size, cm | | | | 0.233 | | | 0.174 |
| <2 | 50 (64) | 23 (46) | 27 (54) | | 24 (48) | 26 (52) | |
| ≥2 | 28 (36) | 9 (32) | 19 (68) | | 9 (32) | 19 (68) | |
| Perineural
invasion | | | | 0.475 | | | 0.174 |
| Absent | 50 (64) | 22 (44) | 28 (56) | | 24 (48) | 26 (52) | |
| Present | 28 (36) | 10 (36) | 18 (64) | | 9 (32) | 19 (68) | |
| Vascular
invasion | | | | 0.208 | | | 0.052 |
| Absent | 63 (81) | 28 (44) | 35 (56) | | 30 (48) | 33 (52) | |
| Present | 15 (19) | 4 (27) | 11 (74) | | 3 (20) | 12 (80) | |
| Lymph node
status | | | | 0.673 | | | 0.359 |
| Absent | 54 (69) | 23 (43) | 31 (57) | | 21 (39) | 33 (61) | |
| Present | 24 (31) | 9 (38) | 15 (62) | | 12 (50) | 12 (50) | |
| Distant
metastasis | | | | 0.944 | | | 0.399 |
| Absent | 68 (87) | 28 (41) | 40 (59) | | 30 (44) | 38 (56) | |
| Present | 10 (13) | 4 (40) | 6 (60) | | 3 (30) | 7 (70) | |
| TNM stage | | | | 0.536 | | | 0.538 |
| I+II | 48 (62) | 21 (44) | 27 (56) | | 19 (40) | 29 (60) | |
| III+IV | 30 (38) | 11 (37) | 19 (63) | | 14 (47) | 16 (53) | |
| Cell
differentiation | | | | 0.251 | | | 0.061 |
| I+II | 33 (42) | 16 (48) | 17 (52) | | 18 (54) | 15 (46) | |
| III+IV | 45 (58) | 16 (36) | 29 (64) | | 15 (33) | 30 (67) | |
| Total no. of
patients | 78 (100) | 32 (41) | 46 (59) | | 33 (42) | 45 (58) | |
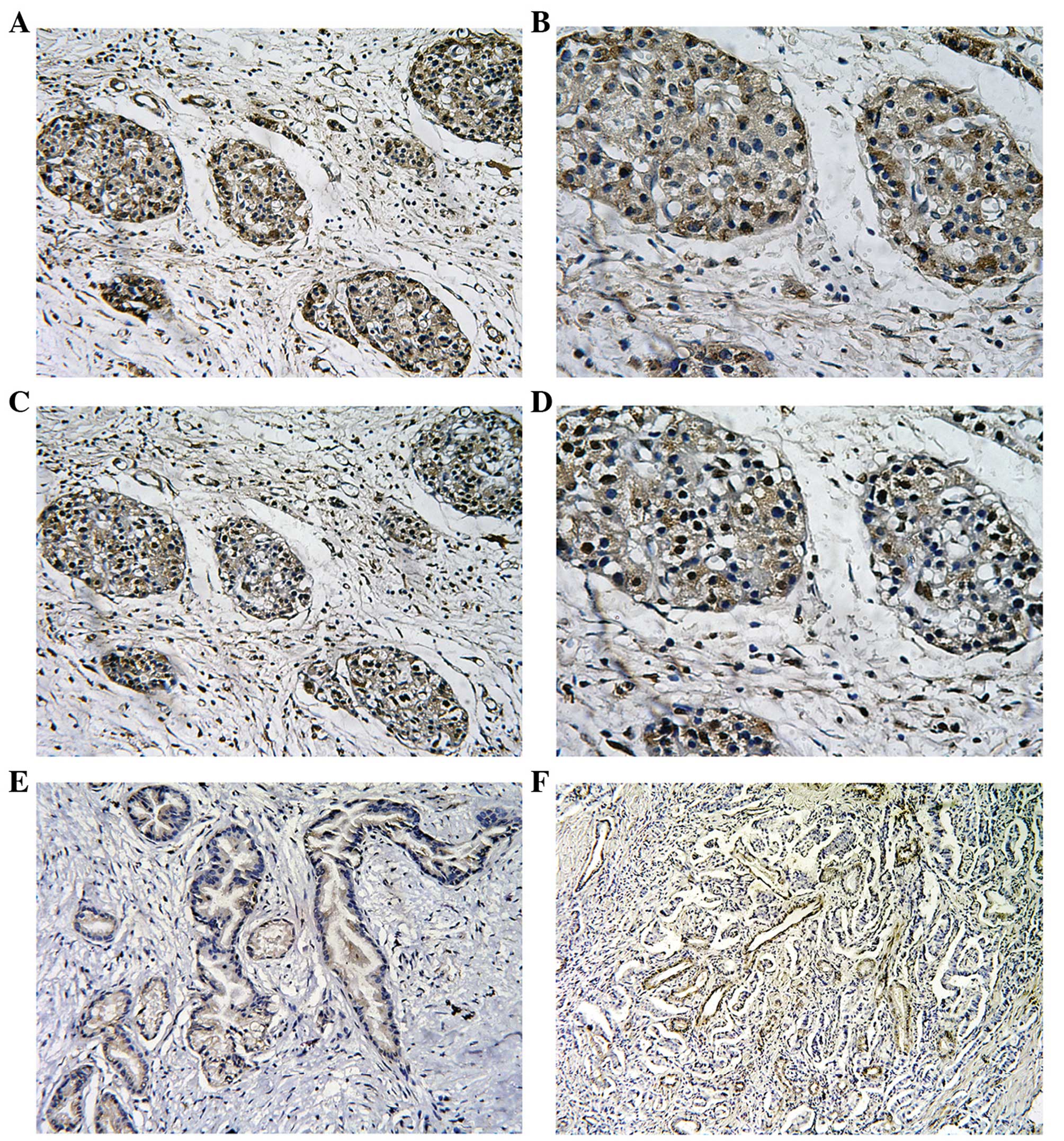
Expression of MT2-MMP and HIF-1α in
pancreatic cancer
The expression of the MT2-MMP and HIF-1α proteins
was assessed by IHC in tissue specimens from 78 patients with
pancreatic cancer. The expression of the MT2-MMP protein was mainly
localised to the plasma membrane and cytoplasm of tumor cells,
whereas the nuclei of stromal cells (including fibroblasts and
endothelial cells) were also occasionally stained. By contrast, the
distribution of HIF-1α expression was predominantly reflected by
diffuse staining of the nucleus and/or cytoplasm of the tumor cells
(Fig. 1). Of the 78 tissue
specimens, 46 were positive (59%) and 32 were negative (41%) for
MT2-MMP expression, whereas 45 tissue specimens (58%) were positive
for HIF-1α expression. Of note, we observed that the expression of
HIF-1α and MT2-MMP overlapped when MT2-MMP expression was compared
to HIF-1α expression in consecutive sections of the same tumor,
suggesting a spatially coincident expression (Fig. 1). In addition, the positive
expression of CD34 was mainly presented as brownish or
brownish-yellow granules in the cytoplasm of microvascular
endothelial cells.
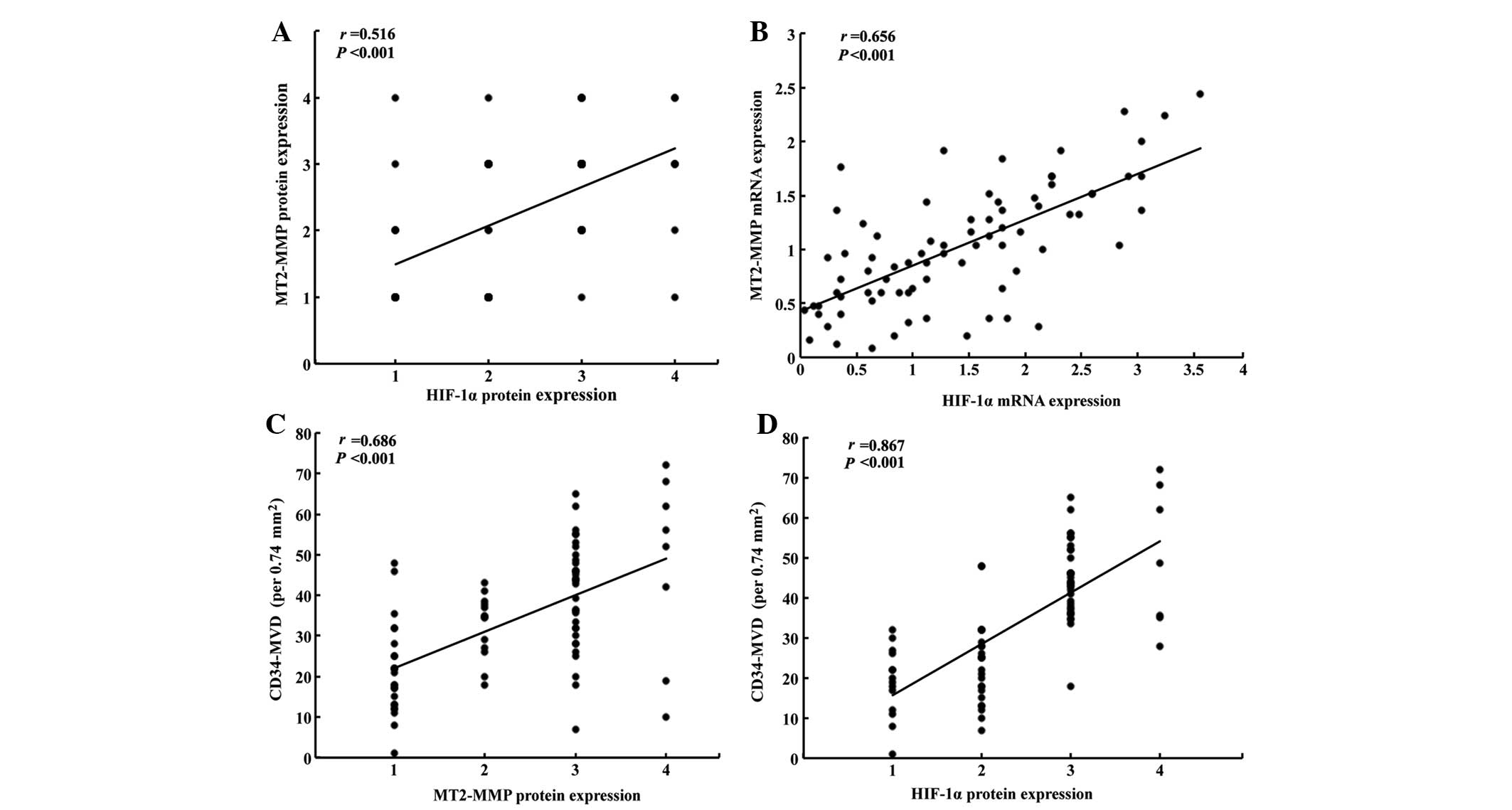
Correlation between MT2-MMP, HIF-1α
expression and CD34-MVD in pancreatic cancer
Using the Spearman’s rank correlation coefficient,
the immunostaining of MT2-MMP was confirmed to be significantly
correlated with that of HIF-1α in pancreatic cancer tissues
(r=0.516, P<0.001; Fig. 2A).
Furthermore, we confirmed that the expression of HIF-1α mRNA was
also correlated with that of MT2-MMP (r=0.656, P<0.001; Fig. 2B). The CD34-MVD in the
MT2-MMP-positive tumors was 40.7±15.4/0.74 mm2, which
was significantly higher compared to that in MT2-MMP-negative
tumors (24.2±10.8/0.74 mm2; P<0.001). The CD34-MVD in
HIF-1α-positive tumors was 39.4±14.9/0.74 mm2, which was
significantly higher compared to that in HIF-1α-negative tumors
(26.4±14.2/0.74 mm2; P<0.001). Furthermore, we also
observed that CD34-MVD was significantly associated with the
expression of MT2-MMP (r=0.686, P<0.001; Fig. 2C) and HIF-1α (r=0.867, P<0.001;
Fig. 2D) in pancreatic cancer.
Hypoxia induces MT2-MMP expression in
pancreatic cancer cells
Based on the correlation of MT2-MMP and HIF-1α
expression in pancreatic cancer tissues, we investigated the
expression of MT2-MMP in response to hypoxia in three pancreatic
cancer cell lines, namely PANC-1, BxPC-3 and AsPC-1. The levels of
MT2-MMP expression were determined using RT-qPCR and western
blotting when the cells were exposed to hypoxia (1% O2)
for 0, 12 and 24 h. The MT2-MMP expression at the mRNA and protein
levels was markedly increased in a time-dependent manner following
exposure to hypoxia for 12 h (Fig.
3). Moreover, the increased MT2-MMP expression was accompanied
by a markedly increased HIF-1α expression. Furthermore, the MT2-MMP
promoter luciferase reporter was cotransfected with pRL-TK plasmids
into the indicated cells and exposed to either normoxia or hypoxia
for 12 h; the data indicated that hypoxia induces MT2-MMP
expression in pancreatic cancer cells.
 | Figure 3Hypoxia induces membrane type-2 matrix
metalloproteinase (MT2-MMP) expression in pancreatic cancer. (A)
Pancreatic cancer cells were exposed to hypoxia (1% O2)
at different time-points and the relative mRNA levels of MT2-MMP
and hypoxia-inducible factor-1α (HIF-1α) expression were determined
by quantitative polymerase chain reaction. (B) The densitometric
analysis of MT2-MMP and HIF-1α mRNA expression was performed by
BandScan 5.0 software (Glyko Inc., Novato, CA, USA). The results
are presented as the relative mRNA levels of MT2-MMP and HIF-1α to
β-actin. Columns, duplicate determinations in one representative
experiment of three; bars, standard deviation (SD).
*P<0.05, compared to the prior timepoints. (C)
Protein levels of MT2-MMP and HIF-1α after exposure to hypoxia (1%
O2) at different time-points were analyzed by western
blotting in pancreatic cancer cells. (D) The densitometric analysis
was preformed and the results were presented as the relative
protein levels of MT2-MMP and HIF-1α to β-actin. Columns, duplicate
determinations in one representative experiment of three; bars, SD.
*P<0.05, compared to the prior timepoints. (E) The
MT2-MMP promoter luciferase reporter was cotransfected with pRL-TK
plasmids into the indicated cells and exposed to either normoxia or
hypoxia for 12 h; the cells were then extracted and luciferase
activity was detected. The results were expressed as means ± SD and
represent three independent experiments. *P<0.05,
compared to control. |
Correlation between MT2-MMP, HIF-1α
expression and postoperative prognosis
Upon univariate analysis with the Cox proportional
hazards model, MT2-MMP-positive patients exhibited a significantly
poorer survival compared to MT2-MMP-negative patients (P<0.001,
Table II). The overall survival
rate of MT2-MMP-positive and MT2-MMP-negative patients was found to
be 4 and 41%, respectively, and TNM stage was positively correlated
with a poor prognosis (P=0.026). Furthermore, the multivariate
analysis using the Cox regression model indicated that MT2-MMP
expression was an independent predictor of an unfavorable prognosis
[P=0.005; hazard ratio (HR)=2.445; 95% confidence interval (CI):
1.316–4.544], as were the presence of vascular invasion (P=0.012;
HR=3.427; and 95% CI: 1.622–7.241), TNM stage (P=0.021; HR=1.862;
and 95% CI: 1.098–3.156), cell differentiation (P=0.009; HR=2.082;
and 95% CI: 1.200–3.610) and HIF-1α expression (P=0.001; HR=3.120;
and 95% CI: 1.600–6.082) (Table
III). Moreover, the Kaplan-Meier survival curves demonstrated
that the positive expression of the MT2-MMP protein was associated
with a shorter disease-free survival (log-rank = 22.369,
P<0.001, Fig. 4A), as was the
positive expression of the HIF-1α protein (log-rank = 33.017,
P<0.001, Fig. 4B).
 | Table IICorrelation of MT2-MMP and HIF-1α
expression with overall survival of patients with pancreatic
cancer. |
Table II
Correlation of MT2-MMP and HIF-1α
expression with overall survival of patients with pancreatic
cancer.
| | Status, no.
(%) | | |
|---|
| |
| | |
|---|
| Variables | Total, no.
(n=78) | Alive (n=15) | Deceased
(n=63) | Overall survival
rate (%) | P-value |
|---|
| Age at surgery,
years | | | | | 0.621 |
| <60 | 46 | 8 (17) | 38 (83) | 17 | |
| ≥60 | 32 | 7 (22) | 25 (78) | 22 | |
| Gender | | | | | 0.052 |
| Male | 45 | 12 (27) | 33 (73) | 27 | |
| Female | 33 | 3 (9) | 30 (91) | 9 | |
| Tumor location | | | | | 0.407 |
| Head | 50 | 11 (22) | 39 (78) | 22 | |
| Body and tail | 28 | 4 (14) | 24 (86) | 14 | |
| Tumor size, cm | | | | | 0.153 |
| <2 | 50 | 12 (24) | 38 (76) | 24 | |
| ≥2 | 28 | 3 (11) | 25 (89) | 11 | |
| Perineural
invasion | | | | | 0.407 |
| Absent | 50 | 11 (22) | 39 (78) | 22 | |
| Present | 28 | 4 (14) | 24 (86) | 14 | |
| Vascular
invasion | | | | | 0.933 |
| Absent | 63 | 12 (19) | 51 (81) | 19 | |
| Present | 15 | 3 (20) | 12 (80) | 20 | |
| Lymph node
status | | | | | 0.104 |
| Absent | 54 | 13 (24) | 41 (76) | 24 | |
| Present | 24 | 2 (8) | 22 (92) | 8 | |
| Distant
metastasis | | | | | 0.947 |
| Absent | 68 | 13 (19) | 55 (81) | 19 | |
| Present | 10 | 2 (20) | 8 (80) | 20 | |
| TNM stage | | | | | 0.026 |
| I+II | 48 | 13 (27) | 35 (73) | 27 | |
| III+IV | 30 | 2 (7) | 28 (93) | 7 | |
| Cell
differentiation | | | | | 0.336 |
| I+II | 33 | 8 (24) | 25 (76) | 24 | |
| III+IV | 45 | 7 (16) | 38 (84) | 16 | |
| MT2-MMP
expression | | | | | <0.001 |
| Negative | 32 | 13 (41) | 19 (59) | 41 | |
| Positive | 46 | 2 (4) | 44 (96) | 4 | |
| HIF-1α
expression | | | | | <0.001 |
| Negative | 33 | 13 (39) | 20 (61) | 39 | |
| Positive | 45 | 2 (4) | 43 (96) | 4 | |
 | Table IIIMultivariate Cox analysis of overall
survival of 78 patients with pancreatic cancer. |
Table III
Multivariate Cox analysis of overall
survival of 78 patients with pancreatic cancer.
| Variables | Univariate analysis
P-value | Multivariate
analysis |
|---|
|
|---|
| P-value | Hazard ratio | 95% CI |
|---|
| Age at surgery,
years | 0.621 | 0.149 | - | - |
| Gender | 0.052 | 0.598 | - | - |
| Tumor location | 0.407 | 0.299 | - | - |
| Tumor size, cm | 0.153 | 0.230 | - | |
| Perineural
invasion | 0.407 | 0.453 | - | - |
| Vascular
invasion | 0.933 | 0.012 | 3.427 | 1.622–7.241 |
| Lymph node
status | 0.104 | 0.959 | - | - |
| Distant
metastasis | 0.947 | 0.429 | - | - |
| TNM stage | 0.026 | 0.021 | 1.862 | 1.098–3.156 |
| Cell
differentiation | 0.336 | 0.009 | 2.082 | 1.200–3.610 |
| MT2-MMP | <0.001 | 0.005 | 2.445 | 1.316–4.544 |
| HIF-1α | <0.001 | 0.001 | 3.120 | 1.600–6.082 |
Discussion
Tumor progression requires increased adaptation to a
hypoxic microenvironment. Tumor hypoxia is an important
characteristic of advanced pancreatic cancer, with a negative
effect on patient prognosis. The evaluation of cellular responses
to hypoxia may be of clinical relevance in a prognostic and
predictive way and may also help treatment adaptation (26). The transcription factor HIF-1 plays
a central role in mediating this process. Inhibition of HIF-1
expression may lead to prevention of cell proliferation and growth,
induction of cell apoptosis and arrest of tumor progression
(27). MT2-MMP is known to play an
important role in angiogenesis, tumor invasion and metastasis
through the breakdown of the extracellular matrix (20). Although our previous study in
vitro demonstrated the induction of MT2-MMP by HIF-1α in cancer
cells under hypoxia (22), to the
best of our knowledge, the association of MT2-MMP expression and
HIF-1α has not yet been assessed in pancreatic cancer.
In the present study, a significant association
between MT2-MMP and HIF-1α expression was reported in an IHC study
of pancreatic cancer. The expression of MT2-MMP was diffuse in
pancreatic cancer tissues, unlike that of HIF-1α, which was focal
and heterogeneous. When MT2-MMP expression was compared to that of
HIF-1α in consecutive sections of same tumor, almost all areas
exhibiting MT2-MMP staining also exhibited HIF-1α staining,
although HIF-1α staining was found in certain areas without MT2-MMP
staining. Furthermore, we observed that MT2-MMP expression was
markedly increased at the mRNA as well as the protein level,
accompanied by increased levels of HIF-1α expression when subjected
to hypoxia. Those results suggested that HIF-1α may play a
regulatory role in the expression of MT2-MMP in pancreatic
cancer.
HIF-1α binds to hypoxia response elements in the
promoter regions of HIF-1-targeted genes, thereby activating a
large number of downstream genes involved in cell proliferation,
differentiation, apoptosis, energy metabolism, tumor invasion and
metastasis. In the present study, the MT2-MMP promoter luciferase
reporter indicated that hypoxia induces MT2-MMP expression in
pancreatic cancer cells though direct binding of HIF-1α to the
MT2-MMP promoter. Herein, these findings support the regulation of
MT2-MMP expression by HIF-1α in pancreatic cancer.
It is well recognized that angiogenesis is essential
for the growth and progression of pancreatic cancer. MT2-MMP plays
a vital role in the turnover of the basement membrane and exerts a
significant effect on angiogenesis (20,28,29).
The CD34-MVD is a reliable index of tumor angiogenesis and reflects
the potentiality of pancreatic cancer progression and prognosis
(30,31). In this study, we found that
patients with a high level of CD34-MVD exhibited an inferior
survival duration; furthermore, CD34-MVD was significantly
associated with MT2-MMP expression in pancreatic cancer.
Interestingly, a positive correlation was identified between
MT2-MMP and HIF-1α expression. Therefore, MT2-MMP expression
induced by hypoxia appears to play a critical role in tumor
development by enhancing tumor aggressiveness and malignant
potential through the induction of processes such as
neovascularization.
Considering the superior prognostic significance of
MT2-MMP in the multivariate analysis, MT2-MMP expression status may
be a more reliable marker for predicting tumor aggressiveness
associated with tumor hypoxia. A growing body of evidence
specifically implicates MT2-MMP in pancreatic cancer characterized
by local invasion and a propensity to metastasize (32). MT2-MMP-positive patients exhibited
a significantly poorer survival compared to MT2-MMP-negative
patients.
The key findings of this study are that the
overexpression of MT2-MMP is induced by hypoxia marker HIF-1α in
pancreatic cancer and that MT2-MMP overexpression is an independent
predictor of poor clinical outcome and decreased survival.
Therefore, the hypoxia-related marker MT2-MMP may be of significant
value as a prognostic and predictive marker and as a potential
therapeutic target in pancreatic cancer.
Acknowledgements
This study was supported by grants from the
Scientific Research Fund of Sichuan Provincial Health Department,
China (nos. 110207 and 130138) and the Young Doctor Fund of Sichuan
Academy of Medical Sciences and Sichuan Provincial People’s
Hospital, China (no. 30305030562).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Traverso LW: Pancreatic cancer: surgery
alone is not sufficient. Surg Endosc. 20(Suppl 2): S446–S449. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frigeri M, De Dosso S, Castillo-Fernandez
O, Feuerlein K, Neuenschwander H and Saletti P: Chemotherapy in
patients with advanced pancreatic cancer: too close to death?
Support Care Cancer. 21:157–163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vladov N, Takorov I, Kazarov K, et al:
Surgical potentialities for the treatment of pancreatic cancer.
Hepatogastroenterology. 59:280–283. 2012.PubMed/NCBI
|
|
5
|
Sultana A, Cox T, Ghaneh P and Neoptolemos
JP: Adjuvant therapy for pancreatic cancer. Recent Results Cancer
Res. 196:65–88. 2012. View Article : Google Scholar
|
|
6
|
Cavadas MA, Nguyen LK and Cheong A:
Hypoxia-inducible factor (HIF) network: insights from mathematical
models. Cell Commun Signal. 11:422013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evans CE, Branco-Price C and Johnson RS:
HIF-mediated endothelial response during cancer progression. Int J
Hematol. 95:471–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shibaji T, Nagao M, Ikeda N, et al:
Prognostic significance of HIF-1 alpha overexpression in human
pancreatic cancer. Anticancer Res. 23:4721–4727. 2003.PubMed/NCBI
|
|
9
|
Cheng ZX, Sun B, Wang SJ, et al: Nuclear
factor-κB-dependent epithelial to mesenchymal transition induced by
HIF-1α activation in pancreatic cancer cells under hypoxic
conditions. PLoS One. 6:e237522011.
|
|
10
|
Malemud CJ: Matrix metalloproteinases
(MMPs) in health and disease: an overview. Front Biosci.
11:1696–1701. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato H and Takino T: Coordinate action of
membrane-type matrix metalloproteinase-1 (MT1-MMP) and MMP-2
enhances pericellular proteolysis and invasion. Cancer Sci.
101:843–847. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou C and Petroll WM: MMP regulation of
corneal keratocyte motility and mechanics in 3-D collagen matrices.
Exp Eye Res. 121:147–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Zucker S, Pulkoski-Gross A, et al:
Conversion of stationary to invasive tumor initiating cells (TICs):
role of hypoxia in membrane type 1-matrix metalloproteinase
(MT1-MMP) trafficking. PLoS One. 7:e384032012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shaverdashvili K, Wong P, Ma J, Zhang K,
Osman I and Bedogni B: MT1-MMP modulates melanoma cell
dissemination and metastasis through activation of MMP2 and RAC1.
Pigment Cell Melanoma Res. 27:287–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka M, Sato H, Takino T, Iwata K, Inoue
M and Seiki M: Isolation of a mouse MT2-MMP gene from a lung cDNA
library and identification of its product. FEBS Lett. 402:219–222.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fillmore HL, VanMeter TE and Broaddus WC:
Membrane-type matrix metalloproteinases (MT-MMPs): expression and
function during glioma invasion. J Neurooncol. 53:187–202. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Davidson B, Goldberg I, Berner A, et al:
Expression of membrane-type 1, 2, and 3 matrix metalloproteinases
messenger RNA in ovarian carcinoma cells in serous effusions. Am J
Clin Pathol. 115:517–524. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Docampo MJ, Cabrera J, Rabanal RM and
Bassols A: Expression of matrix metalloproteinase-2 and -9 and
membrane-type 1 matrix metalloproteinase in melanocytic tumors of
dogs and canine melanoma cell lines. Am J Vet Res. 72:1087–1096.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen L, Di D, Luo G, et al: Immunochemical
staining of MT2-MMP correlates positively to angiogenesis of human
esophageal cancer. Anticancer Res. 30:4363–4368. 2010.PubMed/NCBI
|
|
20
|
Ito E, Yana I, Fujita C, et al: The role
of MT2-MMP in cancer progression. Biochem Biophys Res Commun.
393:222–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abraham R, Schafer J, Rothe M, Bange J,
Knyazev P and Ullrich A: Identification of MMP-15 as an
anti-apoptotic factor in cancer cells. J Biol Chem.
280:34123–34132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu S, Zhou Y, Wang L, et al:
Transcriptional upregulation of MT2-MMP in response to hypoxia is
promoted by HIF-1α in cancer cells. Mol Carcinog. 50:770–780.
2011.PubMed/NCBI
|
|
23
|
Sobin LH and Fleming ID: TNM
Classification of Malignant Tumors, fifth edition (1997). Union
Internationale Contre le Cancer and the American Joint Committee on
Cancer. Cancer. 80:1803–1804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elias JM, Margiotta M and Gaborc D:
Sensitivity and detection efficiency of the peroxidase
antiperoxidase (PAP), avidin-biotin peroxidase complex (ABC), and
peroxidase-labeled avidin-biotin (LAB) methods. Am J Clin Pathol.
92:62–67. 1989.
|
|
25
|
Ikeda N, Nakajima Y, Tokuhara T, et al:
Clinical significance of aminopeptidase N/CD13 expression in human
pancreatic carcinoma. Clin Cancer Res. 9:1503–1508. 2003.PubMed/NCBI
|
|
26
|
Hu Y, Liu J and Huang H: Recent agents
targeting HIF-1α for cancer therapy. J Cell Biochem. 114:498–509.
2013.
|
|
27
|
Chen C and Yu Z: siRNA targeting
HIF-1alpha induces apoptosis of pancreatic cancer cells through
NF-kappaB-independent and -dependent pathways under hypoxic
conditions. Anticancer Res. 29:1367–1372. 2009.
|
|
28
|
Plaisier M, Koolwijk P, Hanemaaijer R, et
al: Membrane-type matrix metalloproteinases and vascularization in
human endometrium during the menstrual cycle. Mol Hum Reprod.
12:11–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Quick RE, Dunlap JA and Jessen JR:
Expression analysis of zebrafish membrane type-2 matrix
metalloproteinases during embryonic development. Gene Expr
Patterns. 12:254–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamahatsu K, Matsuda Y, Ishiwata T, Uchida
E and Naito Z: Nestin as a novel therapeutic target for pancreatic
cancer via tumor angiogenesis. Int J Oncol. 40:1345–1357.
2012.PubMed/NCBI
|
|
31
|
Nanashima A, Shibata K, Nakayama T, et al:
Relationship between microvessel count and clinicopathological
characteristics and postoperative survival in patients with
pancreatic carcinoma. Hepatogastroenterology. 59:1964–1969.
2012.
|
|
32
|
Iki K, Tsutsumi M, Kido A, et al:
Expression of matrix metalloproteinase 2 (MMP-2), membrane-type 1
MMP and tissue inhibitor of metalloproteinase 2 and activation of
proMMP-2 in pancreatic duct adenocarcinomas in hamsters treated
with N-nitrosobis(2-oxopropyl)amine. Carcinogenesis. 20:1323–1329.
1999. View Article : Google Scholar : PubMed/NCBI
|