Introduction
Uterine cervical cancer is the second most common
type of cancer in females globally (1). According to the Globocan project, the
disease has one of the greatest incidences of female mortalities,
despite the effective screening system (2). Since cervical cancer is accepted as a
radiosensitive tumor, ionizing radiation is the most frequently
used treatment modality against the disease. Therefore, cellular
radiosensitivity is a long-term research focus in the field of
radiation oncology and biology as it has a clear effect on the
outcome of therapy (3). The
tumors, however, are not equally sensitive to radiation (4).
Traditionally, radioresistant tumors have been
mainly designated from the histopathological viewpoint (5). Glassy cell carcinoma and small cell
carcinoma, including neuroendocrine tumors of the cervix, are
generally regarded as radioresistant tumors. These specific
histological types, however, are rather rare in cervical neoplasms.
Furthermore, there may be significant variation in radiosensitivity
even within the same histological type. Thus, tumor histology may
not be a crucial determinant of radiosensitivity.
The initial damage should be a major determinant of
cell radiosensitivity (6). By
contrast, flow cytometry (FC) is a technique for the rapid analysis
of DNA content, phenotype expression and the sorting of cells for
further studies. FC allows quantitative measurements on single
cells or cellular constituents at an extremely high speed rate. It
is also feasible to monitor the effects of radiation on the cell
cycle distribution following DNA staining of mammalian cells
(7, 8). Since the formation of DNA
double-strand breaks is considered to be critical for the cytocidal
effect of radiation therapy (9–12),
identifying the underlying molecular processes that results in
radioresistance may lead to novel radiosensitising strategies.
A newly developed microscope-based laser scanning
cytometer (LSC) offers a number of advantages over FC (13, 14). LSC can assess the DNA index (DI) in
hypocellular materials, even on cytological smear slides (15).
Cell necrobiology incorporates the life processes
associated with morphological, biochemical and molecular changes
that predispose, precede and accompany cell death, and assess the
consequences and tissue response to cell death (16). The aim of the present study was to
discern the radiation-induced initial damage that leads to cancer
cell death by necrobiological observation, including cytological
morphology and LSC.
Materials and methods
Patients
Seven patients with locally advanced uterine
cervical carcinoma were treated in the Kurume University Hospital
(Kurume, Fukuoka, Japan) between June 2008 and June 2009. The
patient characteristics are shown in Table I. Subsequent to obtaining informed
consent, two patients received external beam radiotherapy alone at
a dose of 1.8 Gy per day by linac 10 MeV X-ray, and the remaining
five patients underwent concurrent chemo-radiotherapy consisting of
5 mg/body of cisplatin prior to the same dose of radiation therapy.
A total dose of 50 Gy was administered. The patient age ranged from
38 to 74 years (mean, 58 years), and their tumors were classified
as six stage IIIb diseases and one stage IVa cancer, according to
the International Federation of Gynecology and Obstetrics staging
criteria (17). The tumor
histologies were equally non-keratinizing squamous cell
carcinoma.
 | Table ICharacteristics of seven patients with
inoperable cervical cancer. |
Table I
Characteristics of seven patients with
inoperable cervical cancer.
| Patients | Age, years | FIGO stage | Tumor size, mm | Treatments | Clinical
response | Prognosis | PFS, months |
|---|
| 1 | 38 | IIIb | 59 | RT | PR | DOD | 7 |
| 2 | 48 | IIIb | 68 | CCRT | CR | AWD | 24 |
| 3 | 57 | IIIb | 59 | CCRT | PR | NED | – |
| 4 | 53 | IIIb | 52 | CCRT | CR | NED | – |
| 5 | 45 | IIIb | 32 | CCRT | PR | NED | – |
| 6 | 70 | IIIb | 74 | CCRT | CR | DOD | 3 |
| 7 | 74 | IVa | 50 | RT | CR | AWD | 24 |
Preparation for the Papanicolau
staining and DNA index
To exhibit the therapeutic responses, the response
criteria offered by the UICC (Union Internationale Contre le
Cancer) were used for the evaluable lesions (18). To assess the effects of radiation
on tumor cells, cervical smears were obtained following each
radiation therapy using a cotton-tipped stick, rinsed into
serum-free medium (RPMI-1640) and fixed in 95% ethanol prior to
Papanicolau (Pap) staining. The radiation-induced morphological
changes were evaluated by routine cytological examination.
For the cytometric observation, the Pap smear
specimens were decolorized and dipped in propidium iodine (PI)
solution, which was composed of 25 µg/ml PI in phosphate-buffered
saline containing 0.1% RNase (Sigma-Aldrich, St. Louis, MO, USA),
and stained again with fluorochrome and PI. For the cellular DNA
content analysis, a laser scanning cytometer (LSC 101; Olympus Co.,
Tokyo, Japan) was used. At least 500 cancer cells were measured per
sample.
To determine the DI, human leucocytes from freshly
collected blood were used as a standard. A DI of 3.0 indicates DNA
tetraploid. In the present study, tumors that were 3.0 < DI <
30 were classified as near-tetraploid cases and distinguished from
DNA aneuploid tumors (DI>3.0). P<0.05 was considered to
indicate a statistically significant difference. All the patients
provided written informed consent according to the institutional
regulations. The study was approved by the Ethics Committee of the
Department of Gynecology, Oita Prefecture Saiseikai Hita Hospital
(Hita, Oita, Japan).
Results
Patient characteristics
Clinical responses to the radiation therapy are
demonstrated in Table I, showing a
response rate of 100% [four complete responses (CRs) and three
partial responses (PRs)]. Three CR cases remained with no evidence
of disease, and two PR cases remained with disease, showing a
disease-free survival rate of 42.7%.
Radiation-induced morphological damage
of cancer cells with cytoplasmic vacuolization
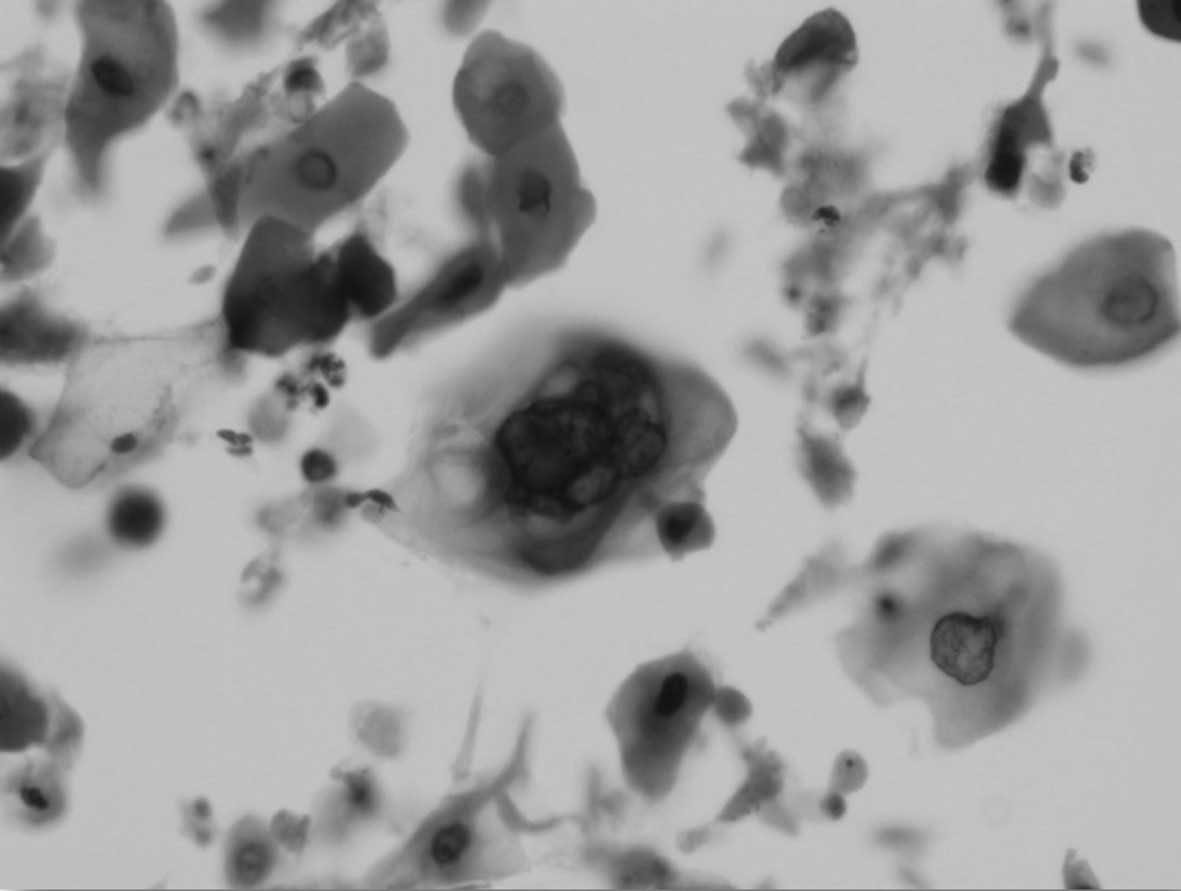
In the cytology of all cases, a characteristic
feature of the radiation effect was observed, exhibiting
intracytoplasmic vacuolization (Fig.
1). These morphological changes emerged at cumulative doses
between 7.2 and 14.4 Gy. Evidently, radiation that was <7.2 Gy
did not cause any discernible changes in cancer cell cytology.
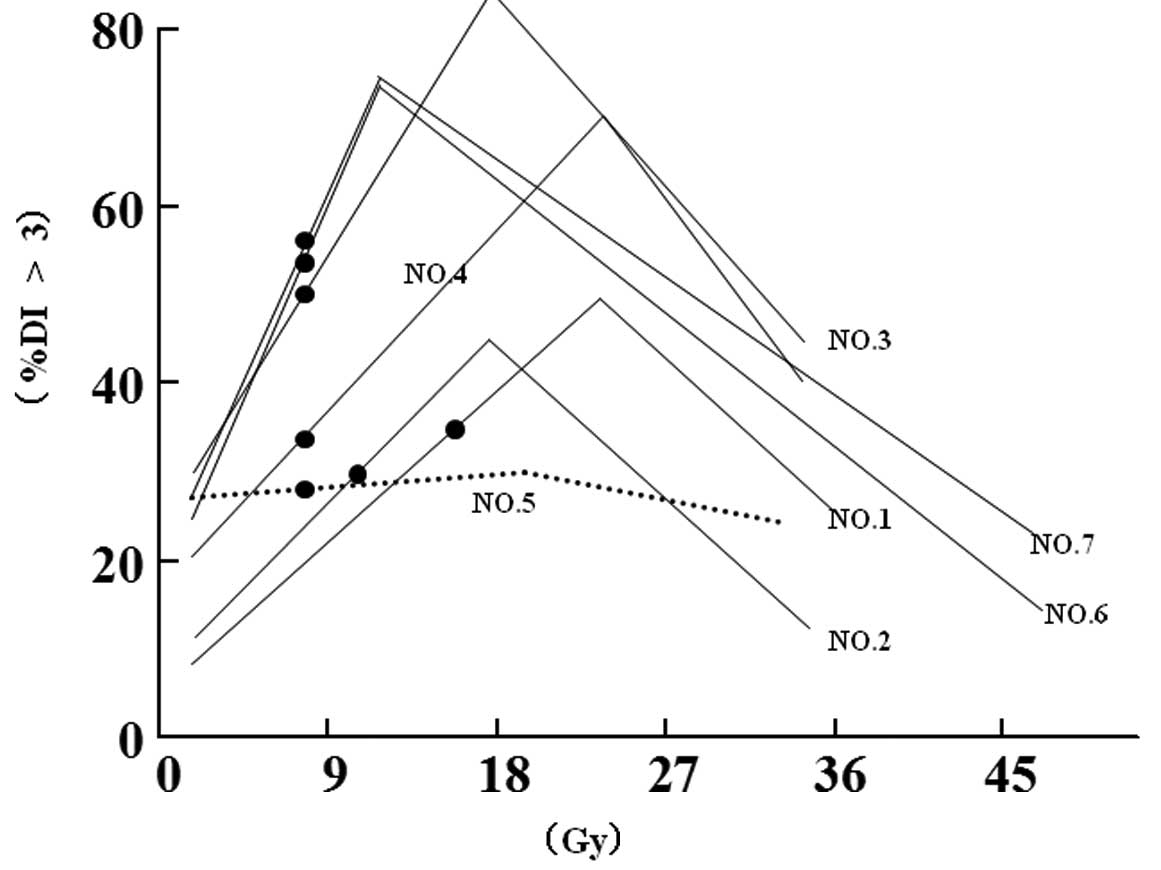
DNA content analysis
The DNA content analysis by LSC revealed six out of
seven cases (85.7%, P<0.05), showing the percentage of cells
having a DI value >3. The increase in DNA content was observed
immediately following the start of radiation therapy, although the
values were varied in each case (Fig.
2).
Discussion
Although ionizing radiotherapy is a key strategy and
has >80 years history in the treatment of cervical cancer, the
crucial determinant of radiosensitivity of the tumor remains
unknown (3, 4, 19).
Thus, understanding how to identify the treatment-induced initial
damage of cancer cells is essential for further therapeutic plans
in cancer therapy. An assay with the ability to predict the
radiosensitivity of tumors may provide a useful tool for the
further individualization of radiotherapy of cancer patients
(20). The prognostic significance
of the fraction of survival following 2 Gy of radiation (SF2) is
crucial in the treatment of head and neck cancer (21). However, the methods to determine
SF2 can take ≤4 weeks and are therefore not clinically
practical.
To improve the treatment strategy, the early
evaluation of therapeutic responses should be performed. The
current response criteria, including that of the UICC, are only
used for the evaluation of the treatment results. Radiation damages
can be observed as cellular degeneration by cytology. However,
these are late events in the treatment course. The importance of a
more prompt evaluation is critical with regards to clinical
decision making.
The impacts of radiation on cervical cancer cells
resulted in a significant elevation of the DNA content level in six
out of seven cases. Radiation causes a division delay dominated by
G2 arrest in the cell cycle. The delay is likely a mechanism
allowing the cell to repair its DNA damage. Ionizing radiation can
also induce polyploidization in a cancer cell line (22). Furthermore, radiation-induced
apoptosis is morphologically identified by an increase in
cytoplasmic granularity, chromatin condensation, membrane blebbing,
cell shrinkage and the formation of distinctive nuclear bodies.
These radiation effects should attribute to the change of DNA
content.
Currently, there are a number of studies reporting
on the concern of the radiation impacts on the molecular structure
of cancer cells by novel techniques, including cytometry and LSC,
revealing the precise mechanism involved in radiation effects.
Despite the notable technical advance in elucidation of the
molecular mechanism of the radiation effects, the results obtained
remain to be utilized in clinical decision making. Rapid analyses
of radiation-induced molecular changes by LSC are promising,
although certain changes remain to be resolved, and this can lead
to the ‘real-time judgement’ of the radiosensitivity of the tumor,
and aid in making a treatment decision in the clinical
practice.
Acknowledgements
The present study was supported by the Supporting
Fund of Obstetrics and Gynecology of the Kurume University. The
authors would like to thank C.T. Kazuko Eguchi for her technical
support of Pap and PI staining.
References
|
1
|
Waqqoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu Q and Hill RP: Radiosensitivity,
apoptosis and repair of DNA double-strand breaks in
radiation-sensitive Chinese hamster ovary cell mutants treated at
different dose rates. Radiat Res. 146:636–645. 1996. View Article : Google Scholar
|
|
4
|
Weichselbaum RR, Dahleberg W and Little
JB: Inherently radioresistant cells exist in some human tumors.
Proc Natl Acad Sci USA. 82:4732–4735. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steel GG, McMillan TJ and Peacock JH: The
radiobiology of human cells and tissues. In vitro radiosensitivity.
The picture has changed in the 1980s. Int J Radiat Biol.
56:525–537. 1989.
|
|
6
|
Ruiz de Almodóvar JM, Núñez MI, McMillan
TJ, Olea C, Mort C, Villalobos M, Pedraza V and Steel GG: Initial
radiation-induced DNA damage in human tumour cell lines: a
correlation with intrinsic cellular radiosensitivity. Br J Cancer.
69:457–462. 1994.PubMed/NCBI
|
|
7
|
Kamentsky LA and Melamed MR:
Spectrophotometer cell sorter. Science. 156:1364–1365. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baatout S and Derradji H: Cytometric
methods to analyze radiation effects. J Biol Regul Homeost Agents.
18:101–105. 2004.PubMed/NCBI
|
|
9
|
Ward JF: The yield of DNA double-strand
breaks produced intracellulary by ionizing radiation: a review. Int
J Radiat Biol. 57:1141–1150. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brenner DJ and Ward JF: Constraints on
energy deposition and target size of multiply damaged sites
associated with DNA double-strand breaks. Int J Radiat Biol.
61:737–748. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nikjoo H, O'Neill P, Wilson WE and
Goodhead DT: Computational approach for determining the spectrum of
DNA damage induced by ionizing radiation. Radiat Res. 156:577–583.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Datta K, Jaruga P, Dizdarglu M, Neumann RD
and Winters TA: Molecular analysis of base damage clustering
associated with a site-specific radiation-induced DNA double-strand
break. Radiat Res. 166:767–781. 2006.
|
|
13
|
Kamentsky LA and Kamentsky LD:
Microscope-based multiparameter laser scanning cytometer yielding
data comparable to flow cytometry data. Cytometry. 12:381–387.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kamentsky LA, Burger DE, Gershman RJ,
Kamentsky LD and Luther E: Slide-based laser scanning cytometry.
Acta Cytol. 41:123–143. 1997.PubMed/NCBI
|
|
15
|
Martin-Reay DG, Kamentsky LA, Weinberg DS,
Hollister KA and Cibas ES: Evaluation of a new slide-based laser
scanning cytometer for DNA analysis of tumors. Comparison with flow
cytometry and image analysis. Am J Clin Pathol. 102:432–438.
1994.PubMed/NCBI
|
|
16
|
Darzynkiewicz Z, Juan G, Li X, Gorczyca W,
Murakami T and Traganos F: Cytometry in cell necrobiology: analysis
of apoptosis and accidental cell death (necrosis). Cytometry.
27:1–20. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petignat P and Roy M: Diagnosis and
management of cervical cancer. BMJ. 335:765–768. 2007. View Article : Google Scholar
|
|
18
|
Monfardini S, Brunner K, Crowther D, Olive
D, MacDonald J, Eckhardt S and Whitehouse J: Manua. of Cancer
Chemotherapy3rd. Union Internationale Contre le Cancer; Geneva:
1981
|
|
19
|
Gao Y, Ma JL and Song LP: The evaluation
of older patients with cervical cancer. Clin Interv Aging.
8:783–788. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bentzen SM and Hendry JH: Variability in
the radiosensitivity of normal cells and tissues. Report from a
workshop organised by the European Society for Therapeuic Radiology
and Oncology in Edinburgh, UK, 19 September 1998. Int J Radiat
Biol. 75:513–517. 1999. View Article : Google Scholar
|
|
21
|
Björk-Eriksson T, West C, Karlsson E and
Mercke C: Tumor radiosensitivity (SF2) is a prognostic factor local
control in head and neck cancers. Int J Radiat Oncol Biol Phys.
46:13–19. 2000.PubMed/NCBI
|
|
22
|
Baatout S, Derradji H, Jacquet P, Ooms D,
Michaux A and Mergeay M: Enhanced radiation-induced apoptosis of
cancer cell lines after treatment with resveratrol. Int J Mol Med.
13:895–902. 2004.PubMed/NCBI
|