Introduction
Gastric cancer is one of the most common causes of
cancer-related mortality worldwide (1). Despite the decline in incidence and
mortality rates over the last two decades, >40% of gastric
cancer patients present with advanced-stage disease at diagnosis
(2).
Several randomized trials demonstrated that
palliative chemotherapy may relieve gastric cancer-related
symptoms, prolong survival and improve the quality of life compared
to best supportive care; therefore, it is offered as a routine
treatment option to patients with a satisfactory performance status
(3–5).
A meta-analysis demonstrated that combination chemotherapy,
particularly with three-drug combinations, is superior to
monotherapy (6).
Since encouraging survival outcomes and better
quality of life have been obtained with the docetaxel + cisplatin +
5-fluorouracil (5-FU) regimen (DCF) in several studies, this
regimen has been widely used to treat advanced-stage gastric cancer
(7,8).
However, these studies reported that the incidence of grade 3–4
toxicity with DCF was higher compared to that with other
combination regimens; therefore, this regimen has not been
established as standard chemotherapy for advanced gastric cancer
(AGC).
Although a number of different chemotherapeutic
agents have been tested in AGC patients, there is currently no
globally accepted standard chemotherapeutic regimen for the
treatment of AGC. In addition, despite the introduction of
new-generation chemotherapeutic agents and the significant increase
in the proportion of patients receiving palliative chemotherapy
over the last few years, overall survival (OS) has not increased in
AGC patients (9).
Thus, first-line chemotherapy should be extensively
investigated in these patients, to determine the optimal
chemotherapeutic regimens that will improve patient survival and
quality of life, with reduced toxicity.
In numerous phase II studies, combination
chemotherapy with 5-FU, folinic acid (leucovorin; LV) and
oxaliplatin (FOLFOX regimens), has exhibited considerable antitumor
activity and a tolerable toxicity profile in AGC patients using
different doses and schedules (10–14).
To address this issue, a retrospective analysis was
conducted comparing baseline characteristics and treatment results
with the oxaliplatin + 5-FU + LV regimen [modified (m)FOLFOX-6] and
the DCF regimen in previously untreated patients with AGC.
Patients and methods
Patients
A total of 126 patients with AGC (unresectable or
metastatic), who were treated with DCF or mFOLFOX-6 as first-line
chemotherapy between June, 2010 and August, 2014 at the Department
of Medical Oncology, Faculty of Medicine, Trakya University
(Edirne, Turkey), were retrospectively reviewed. Patients who had
received prior treatment, or exhibited insufficient hematological,
hepatic and renal functions, were excluded from the analysis.
This retrospective study was approved by the
Institutional Review Board of the Trakya University.
Treatment
In the DCF arm (n=72), the patients received 75
mg/m2 docetaxel and 75 mg/m2 cisplatin as an
intravenous (i.v.) infusion on day 1 and 750 mg/m2/day
5-FU as a continuous infusion for 5 days. The DCF protocol was
repeated every 3 weeks, for up to 6 cycles. In the DCF arm, 55
(76.4%) of the patients received prophylactic granulocyte
colony-stimulating factor (G-CSF) 48–72 h following completion of
chemotherapy.
In the mFOLFOX6 arm (n=54), the patients received 85
mg/m2 oxaliplatin and 400 mg/m2 LV as an i.v.
infusion over 2 h and a 5-FU bolus of 400 mg/m2 as a
10-min infusion, followed by 2,400 mg/m2 5-FU as a 46-h
continuous infusion. The mFOLFOX-6 protocol was repeated every 2
weeks, for up to 12 cycles. Chemotherapy was continued until
disease progression, unacceptable toxicity, patient refusal or the
physician's decision. Demographic, medical and toxicity data were
obtained from the medical and chemotherapy charts.
The performance status of the patients was estimated
according to the Eastern Cooperative Oncology Group performance
status (ECOG PS; http://ecog-acrin.org/resources/ecog-performance-status)
scale.
Response to treatment
Response evaluation was performed every 8–12 weeks
according to the Response Evaluation Criteria in Solid Tumors,
version 1.1 (15) and the adverse
events were graded according to the National Cancer Institute
Common Terminology Criteria for Adverse Events, version 4.0
(16).
The time to progression (TTP) was measured from
treatment initiation until the first evidence of disease
progression. The OS was measured from treatment initiation until
death or last control date. If a patient had succumbed to presumed
progressive disease in the absence of radiographic evidence of
progression, the date of death was used as the date of disease
progression.
Statistical analysis
The baseline characteristics of the mFOLFOX-6 and
DCF groups were compared by the χ2 test (for categorical
variables) or the two-sample t-test (for continuous variables). The
Kaplan-Meier method was used to provide median point estimates, TTP
and median OS, and the confidence intervals (CIs) were calculated
with the Greenwood's formula. The log-rank test was used to
determine the statistical significance of the differences between
the groups. Survival curves were created with IBM SPSS software,
version 20.0 (IBM Corp., Armonk, NY, USA). Safety analyses were
performed using descriptive statistics. P<0.05 was considered to
indicate statistically significant differences.
Results
Patient characteristics
A total of 126 patients were enrolled in this study,
54 and 72 of whom were treated with mFOLFOX-6 and DCF,
respectively. The median follow-up was 12.1 months and it was not
significantly different between the two groups (P=0.08). According
to the ECOG PS scale, 28 (51.9%) of the patients in the mFOLFOX-6
arm and 11 (15.3%) of the patients in the DCF arm had a PS of 2
(P<0.0001). The baseline characteristics of the patients
according to the first-line regimen are summarized in Table I.
 | Table I.Patient characteristics (n=126). |
Table I.
Patient characteristics (n=126).
| Characteristics | Chemotherapeutic
regimen | P-value |
|---|
|
|---|
|
mFOLFOX-6a,
no. (%) (n=54) | DCFb, no. (%) (n=72) |
|---|
| Age (years) |
|
| 0.103 |
|
Median | 58.5 | 56.0 |
|
|
Range | 32–80 | 27–78 |
|
| Gender |
|
| 0.590 |
| Male | 42 (77.8) | 53 (73.6) |
|
|
Female | 12 (22.2) | 19 (26.4) |
|
| ECOG PS |
|
| <0.0001 |
| 0–1 | 26 (48.1) | 61 (84.7) |
|
| 2 | 28 (51.9) | 11 (15.3) |
|
| Disease status |
|
| 0.700 |
| Locally
advanced | 3 (10) | 2 (7.1) |
|
|
Metastatic | 27 (90) | 26 (92.9) |
|
| Radical
gastrectomy | 13 (24.1) | 19 (26.4) |
|
| Any
palliative surgery | 19 (35.2) | 14 (19.4) |
|
| Adjuvant
treatment |
|
| 0.950 |
| No | 41 (75.9) | 55 (76.4) |
|
| Yes | 13 (24.1) | 17 (23.6) |
|
| No. of metastatic
sites |
|
| 0.720 |
| Locally
advanced | 5 (9.3) | 4 (5.6) |
|
| 1 | 31 (57.4) | 44 (61.1) |
|
| ≥2 | 18 (33.3) | 24 (33.3) |
|
| Organs most
commonly involved |
|
|
|
|
Liver | 26 (48.1) | 39 (54.2) | 0.500 |
|
Peritoneum | 16 (29.6) | 23 (31.9) | 0.170 |
|
Lung | 17 (31.5) | 15 (20.8) | 0.780 |
| Presence of
ascites | 13 (24.1) | 4 (5.6) | 0.003 |
Response to treatment
Patients were treated with a median of 10 and 6
cycles of mFOLFOX-6 and DCF, respectively.
The overall response rate (ORR) was 37.0 and 40.3%
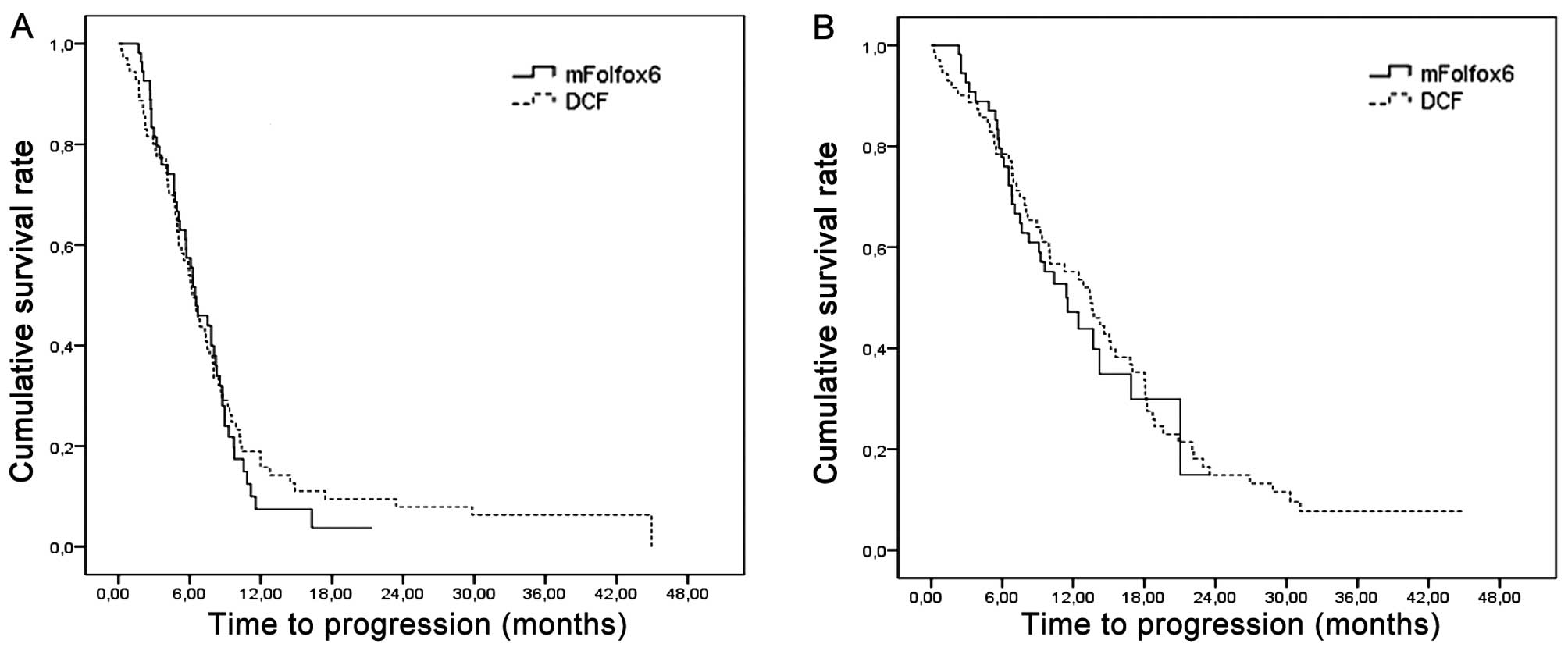
in the mFOLFOX-6 and DCF arms, respectively (P=0.72) (Table II). The median TTP was 6.5 (95% CI:
4.8–8.1) and 6.2 (95% CI: 5.2–7.2) months in the mFOLFOX-6 and DCF
arms, respectively (P=0.70) and the median OS was 11.4 (95% CI:
7.9–14.9) and 13.5 (95% CI: 10.2–16.8) months in the mFOLFOX-6 and
DCF arms, respectively (P=0.72) (Fig.
1).
 | Table II.Response to treatment according to
chemotherapeutic regimen. |
Table II.
Response to treatment according to
chemotherapeutic regimen.
|
Characteristics | Chemotherapeutic
regimen | P-value |
|---|
|
|---|
|
mFOLFOX-6a, no. (%) (n=54) | DCFb, no. (%) (n=72) |
|---|
| Complete
response | 2 (3.7) | 3 (4.2) | 0.72 |
| Partial
response | 18 (33.3) | 26 (36.1) |
|
| Stable disease | 18 (33.3) | 28 (38.9) |
|
| Progressive
disease | 16 (29.7) | 15 (20.8) |
|
Toxicities
The most commonly observed grade 3–4 hematological
toxicity was neutropenia in both arms. The rate of grade 3–4
neutropenia did not differ significantly between the two arms
(33.3% in the mFOLFOX-6 arm vs. 31.9% in the DCF arm; P=0.860). The
rate of febrile neutropenia also did not differ significantly
between the two arms (1.9% in the mFOLFOX-6 arm vs. 9.7% in the DCF
arm; P=0.07).
However, in the DCF arm, 55 (76.4%) of the patients
received primary G-CSF prophylaxis subcutaneously for 5 days. In
the subgroup analysis, the incidence of grade 3–4 neutropenia was
significantly higher among patients in the DCF arm who had not
received G-CSF prophylaxis (18.2 vs. 76.5%, P<0.001). Febrile
neutropenia was also significantly more common among patients in
the DCF arm who had not received G-CSF prophylaxis (3.6 vs. 29.4%,
P=0.002). The rates of anemia and thrombocytopenia were similar
between the two arms.
The most commonly encountered grade 3–4
non-hematological toxicities were nausea/vomiting, mucositis and
diarrhea in both arms. Grade 3–4 nausea-vomiting was more frequent
with DCF (20.8%) compared with mFOLFOX-6 (7.4%) (P=0.037). Grade
3–4 diarrhea was also more frequent with DCF (19.4%) compared with
mFOLFOX-6 (5.6%) (P=0.024). The treatment-related toxicities are
summarized in Table III. Dose
reduction was required in 15 (27.8%) and 28 (38.9%) patients in the
mFOLFOX-6 and DCF arms, respectively (P=0.19). Dose delays of at
least 7 days were required in 15 (27.8%) and 17 (23.6%) patients in
the mFOLFOX-6 and DCF arms, respectively (P=0.6). Treatment
discontinuation due to toxicity was required in 2 (3.7%) and 9
(12.3%) patients in the mFOLFOX-6 and DCF arms, respectively
(P=0.08). Treatment-related mortality was reported in 2 (3.7%) and
4 (5.6%) patients in the mFOLFOX-6 and DCF arms, respectively
(P=0.62) (data not shown).
 | Table III.Toxicities according to the NCI CTC
2.0. criteria. |
Table III.
Toxicities according to the NCI CTC
2.0. criteria.
| Grade 3–4 adverse
events | Chemotherapeutic
regimen | P-value |
|---|
|
|---|
|
mFOLFOX-6a, no. (%) (n=54) | DCFb, no. (%) (n=72) |
|---|
|
Non-hematological |
|
|
|
|
Nausea-vomiting | 4 (7.4) | 15 (20.8) | 0.037 |
|
Diarrhea | 3 (5.6) | 14 (19.4) | 0.024 |
|
Stomatitis | 4 (7.4) | 11 (15.3) | 0.180 |
|
Peripheral neuropathy | 3 (5.6) | 3 (4.2) | 0.710 |
| Hematological |
|
|
|
|
Neutropenia | 18 (33.3) | 23 (31.9) | 0.860 |
| Febrile
neutropenia | 1 (1.9) | 7 (9.7) | 0.070 |
|
Anemia | 2 (3.7) | 5 (6.9) | 0.430 |
|
Thrombocytopenia | 3 (5.6) | 5 (6.9) | 0.750 |
Discussion
Although AGC is considered to be relatively
chemosensitive, systemic chemotherapy for patients with gastric
cancer exerts a limited effect on OS. The majority of the patients
have received palliative chemotherapy in recent years; however, OS
did not increase as expected in patients with metastatic gastric
cancer (9). In addition, there is
currently no globally accepted chemotherapeutic regimen due to
concerns regarding the toxicity of chemotherapy and the
inconsistency in treatment response. In the face of the limited
progress in the treatment options for AGC, the therapeutic trend is
toward improved clinical efficacy and a more acceptable toxicity
profile.
Thus, we aimed to investigate the efficacy and
safety of mFOLFOX-6 and DCF as first-line regimens in AGC. To the
best of our knowledge, this study is the first to compare the
efficacy and safety of these two regimens as the first-line
treatment of AGC in the English literature.
In this study, we observed that DCF and mFOLFOX-6
were associated with similar ORR, TTP and OS, with a different
toxicity profile in the first-line stetting for patients with
AGC.
The DCF regimen has been widely used for the
treatment of AGC, with encouraging survival outcomes and improved
quality of life, as reported by several recent studies; in these
studies, the ORR was reported to be 36.6–43%, the TTP was 4.6–5.6
months and the OS was 9.2–10.4 months (7,8,17). In the present study, the DCF regimen
exhibited good efficacy, with an ORR of 40.3%, a median TTP of 6.2
months and a median OS of 13.5 months. Thus, our efficacy results
for the DCF arm were consistent with the literature.
Although DCF is commonly used in as first-line
chemotherapy in metastatic gastric cancer worldwide, its
tolerability is low due to toxicity. Therefore, evaluation of
treatment benefits against chemotherapy-related toxicities is
required and patients eligible for combination chemotherapy should
be carefully selected.
In several trials, novel chemotherapeutic agents,
such as capecitabine, taxanes, irinotecan and oxaliplatin, have
been tested in AGC over the last few decades (18–20). In
several studies conducted over the last decade, a number of
different FOLFOX regimens have exhibited satisfactory clinical
activity and acceptable toxicity in patients with AGC. The
effectiveness of a variety of FOLFOX-6 regimens in the treatment of
AGC has been recently evaluated, with a reported ORR of 40.2–48% a
TTP of 5.4–6.2 months and an OS of 8.6–13 months (11,21–25). In
the present study, the mFOLFOX-6 regimen exhibited good efficacy,
with an ORR of 37.0%, a median TTP of 6.5 months and a median OS of
11.4 months. The results of the present study were similar to those
previously reported by studies investigating FOLFOX-6 (11,21–25).
In terms of results, there was no significant
difference between the DCF and mFOLFOX-6 arms; the ORR and efficacy
data were comparable to the results of previous studies
investigating the DCF and mFOLFOX regimens (7,8,17,21–25).
As regards toxicity, the two regimens were
associated with a manageable toxicity profile. In the DCF arm, the
incidence of grade 3–4 nausea/vomiting and diarrhea was
significantly higher compared with that in the mFOLFOX-6 arm. The
rate of grade 3–4 neutropenia was similar between the two arms. The
lower hematological toxicity rates in the DCF arm of this study may
be explained by 76.4% of the patients in the DCF arm receiving
primary G-CSF prophylaxis. In the V325 trial, the rates of grade
3–4 neutropenia and febrile neutropenia were reported to be 82 and
29%, respectively (7). In the
subgroup analysis of the present study, grade 3–4 neutropenia and
febrile neutropenia were significantly more common among patients
in the DCF arm not receiving primary G-CSF prophylaxis; our results
were similar to those of the V325 study.
The benefits of administering primary G-CSF
prophylaxis in conjunction with docetaxel-based chemotherapy have
been reported by phase 3 trials in breast cancer patients. When
comparing patients receiving docetaxel-based combination regimens,
a significant reduction in the incidence of febrile neutropenia and
other neutropenia-related complications was observed in patients
receiving docetaxel-based combination regimens with primary
prophylactic G-CSF (26,27). Recent European and American guidelines
recommend the routine use of primary prophylaxis with G-CSF when
using chemotherapeutic regimens associated with a risk of febrile
neutropenia of ≥20%, such as DCF (28,29). The
results of the V325 trial support the use of G-CSF in conjunction
with the DCF protocol (7). In
previous studies using FOLFOX-6 regimens for AGC, the rates of
neutropenia, anemia and thrombocytopenia were 4.9–34.1, 1.2–20 and
0–7.3% respectively (21–25). The incidence of grade 3–4 adverse
effects in the mFOLFOX-6 arm was similar to that reported by
previous studies. Dose reduction, dose delays and treatment-related
mortality was similar between the two arms.
This study had certain limitations due to the
indirect comparison and retrospective design. First, the proportion
of patients with an ECOG PS of 2 was significantly higher in the
mFOLFOX-6 arm, although PS is not an accurate criterion for
evaluating the general status of cancer patients. However, our
results suggest that the mFOLFOX-6 regimen is an efficient and
tolerable treatment option for AGC patients with an ECOG PS of 2.
Second, adverse event data were limited to grade 3–4 toxicities. We
were unable to compare grade 1–2 toxicities due to insufficient
records in the medical charts. Finally, there was heterogeneity in
the DCF arm in terms of primary prophylaxis due to the physician's
decision. However, despite these limitations, the results of this
study may be considered as a major reference regarding the benefits
of G-CSF use in conjunction with the DCF regimen.
In conclusion, there was no statistically
significant difference between the DCF and mFOLFOX-6 arms in terms
of treatment results. The present study demonstrated that the
efficacy of mFOLFOX-6 was comparable to that of DCF in AGC patients
and the toxicity analysis revealed that DCF was associated with
worse non-hematological toxicities. Therefore, the mFOLFOX-6
regimen may be an effective and tolerable treatment option for AGC
patients with an ECOG PS of 2.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glimelius B, Ekström K, Hoffman K, et al:
Randomized comparison between chemotherapy plus best supportive
care with best supportive care in advanced gastric cancer. Ann
Oncol. 8:163–168. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murad AM, Santiago FF, Petroianu A, Rocha
PR, Rodrigues MA and Rausch M: Modified therapy with
5-fluorouracil, doxorubicin, and methotrexate in advanced gastric
cancer. Cancer. 72:37–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pyrhönen S, Kuitunen T, Nyandoto P and
Kouri M: Randomised comparison of fluorouracil, epidoxorubicin and
methotrexate (FEMTX) plus supportive care with supportive care
alone in patients with non-resectable gastric cancer. Br J Cancer.
71:587–591. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wagner AD, Grothe W, Haerting J, Kleber G,
Grothey A and Fleig WE: Chemotherapy in advanced gastric cancer: A
systematic review and meta-analysis based on aggregate data. J Clin
Oncol. 24:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
et al V325 Study Group: Phase III study of docetaxel and cisplatin
plus fluorouracil compared with cisplatin and fluorouracil as
first-line therapy for advanced gastric cancer: A report of the
V325 Study Group. J Clin Oncol. 24:4991–4997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roth AD, Fazio N, Stupp R, et al Swiss
Group for Clinical Cancer Research: Docetaxel, cisplatin, and
fluorouracil; docetaxel and cisplatin; and epirubicin, cisplatin,
and fluorouracil as systemic treatment for advanced gastric
carcinoma: A randomized phase II trial of the Swiss Group for
Clinical Cancer Research. J Clin Oncol. 25:3217–3223. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bernards N, Creemers GJ, Nieuwenhuijzen
GA, Bosscha K, Pruijt JF and Lemmens VE: No improvement in median
survival for patients with metastatic gastric cancer despite
increased use of chemotherapy. Ann Oncol. 24:3056–3060. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lordick F, Lorenzen S, Stollfuss J, et al:
Phase II study of weekly oxaliplatin plus infusional fluorouracil
and folinic acid (FUFOX regimen) as first-line treatment in
metastatic gastric cancer. Br J Cancer. 93:190–194. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Batran SE, Atmaca A, Hegewisch-Becker
S, et al: Phase II trial of biweekly infusional fluorouracil,
folinic acid, and oxaliplatin in patients with advanced gastric
cancer. J Clin Oncol. 22:658–663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeh YS, Tsai HL, Ma CJ, Wu DC, Lu CY, Wu
IC, Hou MF and Wang JY: A retrospective study of the safety and
efficacy of a first-line treatment with modified FOLFOX-4 in
unresectable advanced or recurrent gastric cancer patients.
Chemotherapy. 58:411–418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Vita F, Orditura M, Matano E, et al: A
phase II study of biweekly oxaliplatin plus infusional
5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment
of advanced gastric cancer patients. Br J Cancer. 92:1644–1649.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chao Y, Yeh KH, Chang CJ, Chen LT, Chao
TY, Wu MF, Chang CS, Chang JY, Chung CY, Kao WY, et al: Phase II
study of weekly oxaliplatin and 24-h infusion of high-dose
5-fluorouracil and folinic acid in the treatment of advanced
gastric cancer. Br J Cancer. 91:453–458. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: Revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cirillo M, Venturini M, Ciccarelli L,
Coati F, Bortolami O and Verlato G: Clinician versus nurse symptom
reporting using the National Cancer Institute-Common Terminology
Criteria for Adverse Events during chemotherapy: Results of a
comparison based on patient's self-reported questionnaire. Ann
Oncol. 20:1929–1935. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ajani JA, Fodor MB, Tjulandin SA,
Moiseyenko VM, Chao Y, Cabral Filho S, Majlis A, Assadourian S and
Van Cutsem E: Phase II multi-institutional randomized trial of
docetaxel plus cisplatin with or without fluorouracil in patients
with untreated, advanced gastric, or gastroesophageal
adenocarcinoma. J Clin Oncol. 23:5660–5667. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cunningham D, Starling N, Rao S, Iveson T,
Nicolson M, Coxon F, Middleton G, Daniel F, Oates J and Norman
ARUpper Gastrointestinal Clinical Studies Group of the National
Cancer Research Institute of the United Kingdom: Capecitabine and
oxaliplatin for advanced esophagogastric cancer. N Engl J Med.
358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet
Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moehler M, Kanzler S, Geissler M, Raedle
J, Ebert MP, Daum S, Flieger D, Seufferlein T, Galle PR and Hoehler
TArbeitsgemeinschaft Internistische Onkologie Germany: A randomized
multicenter phase II study comparing capecitabine with irinotecan
or cisplatin in metastatic adenocarcinoma of the stomach or
esophagogastric junction. Ann Oncol. 21:71–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo HY, Xu RH, Zhang L, et al: A pilot
study of oxaliplatin, fluorouracil and folinic acid (FOLFOX-6) as
first-line chemotherapy in advanced or recurrent gastric cancer.
Chemotherapy. 54:228–235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee HH, Hur H, Kim SH, Park AR, Kim W and
Jeon HM: Outcomes of modified FOLFOX-6 as first line treatment in
patients with advanced gastric cancer in a single institution;
retrospective analysis. Cancer Res Treat. 42:18–23. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Louvet C, André T, Tigaud JM, et al: Phase
II study of oxaliplatin, fluorouracil, and folinic acid in locally
advanced or metastatic gastric cancer patients. J Clin Oncol.
20:4543–4548. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cavanna L, Artioli F, Codignola C, et al:
Oxaliplatin in combination with 5-fluorouracil (5-FU) and
leucovorin (LV) in patients with metastatic gastric cancer (MGC).
Am J Clin Oncol. 29:371–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hwang WS, Chao TY, Lin SF, Chung CY, Chiu
CF, Chang YF, Chen PM and Chiou TJ: Phase II study of oxaliplatin
in combination with continuous infusion of
5-fluorouracil/leucovorin as first-line chemotherapy in patients
with advanced gastric cancer. Anticancer Drugs. 19:283–288. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vogel CL, Wojtukiewicz MZ, Carroll RR,
Tjulandin SA, Barajas-Figueroa LJ, Wiens BL, Neumann TA and
Schwartzberg LS: First and subsequent cycle use of pegfilgrastim
prevents febrile neutropenia in patients with breast cancer: A
multicenter, double-blind, placebo-controlled phase III study. J
Clin Oncol. 23:1178–1184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martín M, Lluch A, Seguí MA, et al:
Toxicity and health-related quality of life in breast cancer
patients receiving adjuvant docetaxel, doxorubicin,
cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and
cyclophosphamide (FAC): Impact of adding primary prophylactic
granulocyte-colony stimulating factor to the TAC regimen. Ann
Oncol. 17:1205–1212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aapro MS, Cameron DA, Pettengell R, et al:
European Organisation for Research and Treatment of Cancer (EORTC)
Granulocyte Colony-Stimulating Factor (G-CSF) Guidelines Working
Party: EORTC guidelines for the use of granulocyte
colony-stimulating factor to reduce the incidence of
chemotherapy-induced febrile neutropenia in adult patients with
lymphomas and solid tumours. Eur J Cancer. 42:2433–2453. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
National Comprehensive Cancer Network.
Practice Guidelines in Oncology: Myeloid growth factors. V.I.2014,
http://www.nccn.org/professionals/physician_gls/pdf/myeloid_growth.pdfAccessed.
December 20–2014
|