Introduction
Hepatocellular carcinoma (HCC) ranks as the third
cause of cancer-related fatality worldwide. An estimated 748,300
new HCC cases and 695,900 fatalities occurred worldwide in 2008
(1). Hepatitis B virus (HBV)
infection or HCV infection is one of the major risk factors for the
development of HCC, particularly in Eastern Asia and sub-Saharan
Africa. It has been estimated that HBV infection is associated with
50–80% of HCC cases worldwide. Increasing evidence indicates that
antiviral therapy with nucleos(t)ide analogue (NA) drugs is
effective in reducing the incidence of HCC in HBV-infected patients
(2).
Surgery, ablation and liver transplantation are the
potentially effective treatments for HCC, although the long-term
survival rate remains unsatisfactory, due to high recurrences in
36.8–78.0% of postoperative patients (3). Recently, a meta-analysis showed the
benefits of adjuvant NAs therapy following curative treatment of
HBV-related HCC based on the recurrence-free survival (RFS) and
overall survival (OS) (4). However,
this study did not show the exact 1-, 3- and 5-year RFS and OS, or
the HCC recurrence and mortality rates in patients following
surgery between the antiviral treatment and control groups. Five
NAs have been licensed to treat patients with HBV infection. Few
studies have described which type of NAs was the best for the HCC
patients. Based on these reasons, the present meta-analysis was
performed to focus on the effect of 1-, 3- and 5-year RFS and OS,
HCC recurrence and mortality rates between patients by adjuvant NAs
therapy following curative treatment of HBV-related HCC, and which
NA is the best for these patients.
Materials and methods
Literature search
The present study was performed according to the
recommendations of the PRISMA statement (5). Computerized searches were conducted on
Web of Science and PubMed until 1 November, 2014. The strategy was
based on MeSH terms combining with free text words. The detailed
search strategies were as follows: (HCC OR liver cancer OR hepatic
carcinoma OR hepatocellular carcinoma) AND [hepatectom* OR (liver*
OR hepatocellular* OR hepatic OR hepato-cellular and resection) OR
postoperative OR surgery] AND (nucleoside OR nucleotide and
analogue*) AND [lamivudine (LAM) OR adefovir (ADV) OR entecavir
(ETV) OR telbivudine OR tenofovir]. The reference lists of the
retrieved studies were also manually searched to identify more
qualified studies.
Inclusion and exclusion criteria
The inclusion criteria was as follows: i) Study
design: Non-randomized and randomized controlled trial (RCT)
studies were included; ii) study patients: Diagnosed with HBV
related-HCC; iii) therapy for HCC: Curative resection or ablation;
iv) antiviral treatment: Using NAs as regular therapy compared with
placebo or no treatment in the control group following curative
therapy of HCC; and v) results available on one of the following:
1-, 3- or 5-year RFS or OS after surgery with antiviral therapy,
HCC recurrence rate or mortality rate in the two groups. Exclusion
criteria were as follows: i) Primary HCC was treated with
palliative therapy (transarterial chemoembolization, radiation or
systemic chemotherapy); and ii) trials including participants
co-infected with other virus, such as HCV or human immunodeficiency
virus (HIV).
Quality assessment
The quality of the included studies was assessed
independently by two authors (Yuchen Zhou and Guosheng Yuan) using
the Newcastle-Ottawa Scale (NOS) (6)
for non-randomized studies. The NOS uses different tools for
non-randomized studies and consists of 3 parameters of quality:
Selection, comparability and exposure/outcome assessment. The NOS
assigns a maximum of 4 points for selection, 2 for comparability
and 3 for exposure/outcome. NOS scores of 1–3, 4–6 and 7–9 were
assigned for low, intermediate and high-quality studies,
respectively (7). Discrepancies were
settled by consensus following joint re-evaluation of the original
studies by the third author (Guangyao Zhou).
Data collection and statistical
analysis
For each eligible manuscript, the following
information was extracted: i) First author's name and year of
publication, the country of patients and duration of the follow-up;
ii) study design (randomized, case-control or cohort); iii) the
exact NAs for antiviral therapy, iv) the included number of
patients in the control and treatment group; v) the number of
patients between the two groups in RFS or OS in 1-, 3- and 5-year,
HCC recurrence or fatality; and vi) since numerous studies did not
report this information directly, Kaplan-Meier curves were read by
Engauge Digitizer version 4.1 (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm).
The relative ratio (RR) with a 95% confidence
interval (CI), using either a fixed-effect model or random-effect
model, was applied as a summary statistic for 1-, 3- and 5-year
RFS/OS, HCC recurrence and fatalities between the two groups. In
accordance with customary, an overall RR>1 favored the NAs group
in the survival rate and the control group in HCC recurrence and
mortality rate. The difference was considered to indicate a
statistical significance if the 95% CI of the RR did not overlap 1,
accompanied by P<0.05.
Potential publication bias was comprehensively
assessed by Begg's funnel plot and Egger's rank correlation test of
asymmetry. Publication bias was determined present when the P-value
was ≤0.10 by the Egger's or Begg's test. Sensitivity analyses were
used to evaluate the reliability of the results. All the
statistical analyses were performed using STATA version 11.0 (STATA
Corporation, College Station, TX, USA).
Results
Characteristics of included
studies
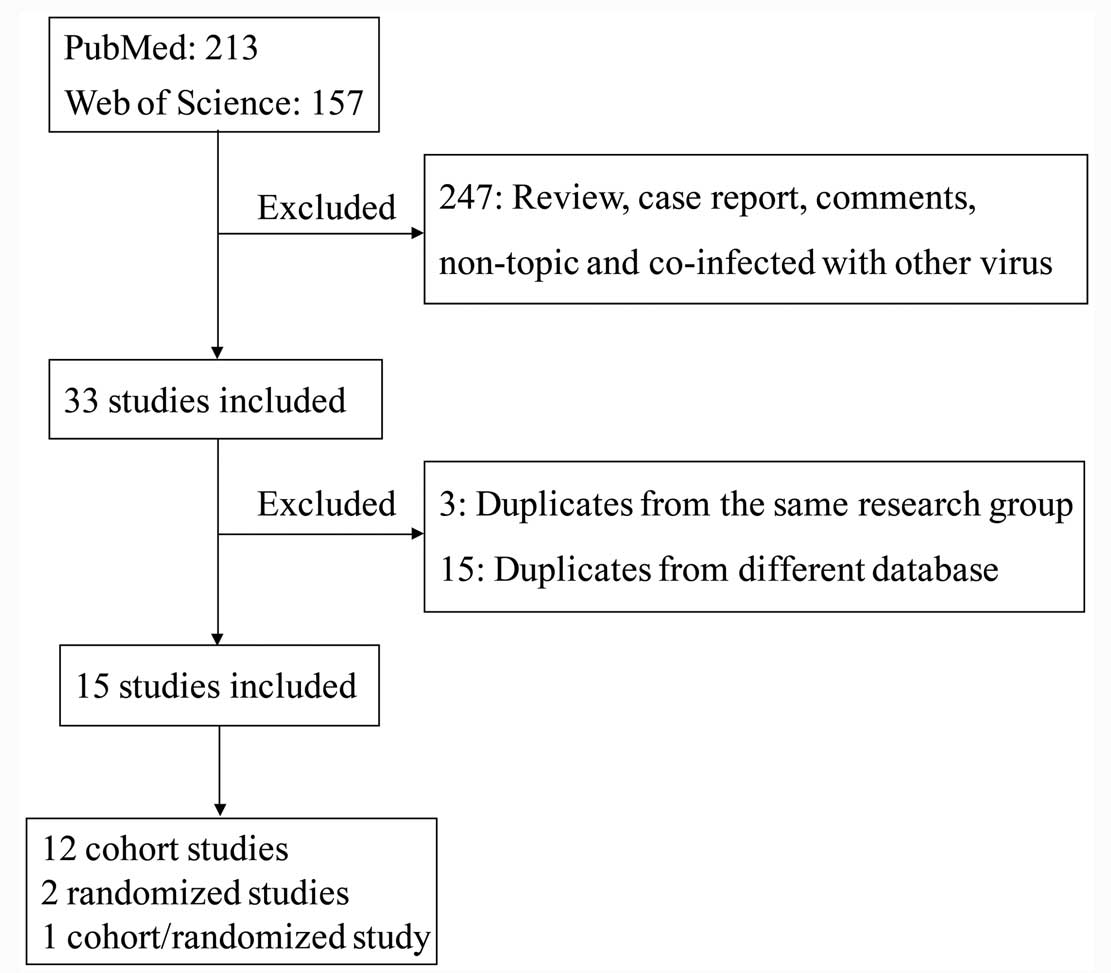
In total, 388 citations were identified from the
PubMed and Web of Science database. Following review by all the
authors, there were 15 studies (8–22) (13
cohorts, 1 randomized and 1 randomized combined with cohort) that
fulfilled the inclusion criteria (Fig.
1). The details are shown in Table
I. In total there were 7,019 subjects included, with 1,353
patients in the antiviral treatment group and 5,266 in the control
group. Based on the NOS scores, 13 of 14 studies (9 scores for 1
study, 8 scores for 6 studies and 7 scores for 6 studies) were of
high quality and the other study (6 scores) was acceptable. The
score of each study is presented in Table
I. As the included randomized studies were not double-blind
studies, these studies were low quality.
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
| Authors, year
(Refs.) | NOS scores | Data
collecteda | Study design | Cure for HCC | Adjuvant
treatment | Sample size, T/C |
|---|
| Kuzuya et
al, 2007 (17) | 7 | 1 | Cohort | Resection or
RFA | LAM (with ADV
rescue) | 141/141 |
| Kubo et al,
2007 (18) | 7 | 2 | Cohort | Resection | LAM (with ADV
rescue) | 81/82 |
| Yoshida et
al, 2008 (16) | 8 | 2 | Cohort | RFA | LAM (with ADV
rescue) | 215/402 |
| Koda et al,
2009 (14) | 7 | 2 | Cohort | Resection or
RFA | LAM (with ADV or
ETV rescue) | 99/32 |
| Chuma et al,
2009 (15) | 8 | 1 | Cohort | Resection or
RFA | LAM (with ADV or
ETV rescue) | 14/10 |
| Chan et al,
2011 (13) | 7 | 1 | Cohort | Resection | LAM or ETV | 16/33 |
| Hann et al,
2011 (12) | 9 | 1 | Cohort | Resection or
ablation | LAM, tenofovir or
ADV | 42/94 |
| Wu et al,
2012 (11) | 8 | 2 | Cohort | Resection | LAM, ETV,
telbivudin | 22/14 |
| Ke et al,
2013 (10) | 6 | 1 | Cohort | Resection | LAM | 9/6 |
| Yin et al,
2013 (8) | 8 | 2 | Cohort | Resection | LAM (with ADV or
ETV rescue) | 39/64 |
| Su et al,
2013 (9) | 8 | 2 | Cohort | Resection | LAM OR ETV | 62/271 |
| Huang et al,
2013 (20) | 7 | 1 | Cohort | Resection | ADV, ETV or
LAM | 518/4,051 |
| Nishikawa et
al, 2014 (19) | 8 | 1 | Cohort | Resection, RFA or
PCEI | LAM, ADV or
ETV | 865/175 |
| Li et al,
2010 (21) | 7 | 1 | Cohort | Resection | LAM (with or
without ADV) | 43/36 |
| Yin et al,
2013 (8) | Unclear bias | 2 | Randomized | Resection | LAM (with ADV or
ETV rescue) | 33/71 |
| Huang et al,
2013 (22) | Unclear bias | 1 | Randomized | Resection | ADV | 100/100 |
Effects of the intervention for the
RFS and OS
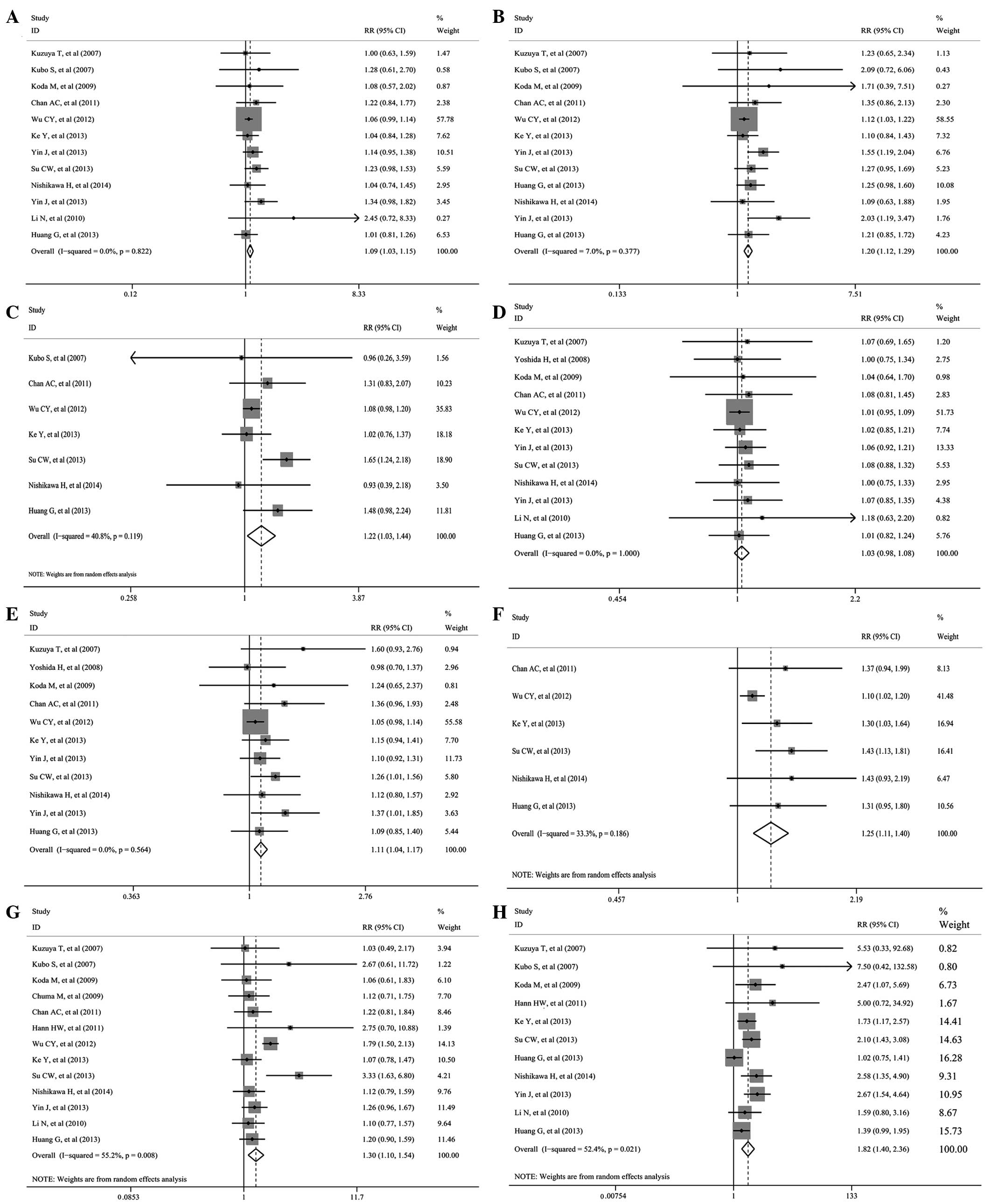
Pooling the data of 11 (8–11,13,14,17–19,21,22),
11 (8–11,13,14,17–20,22)
and 7 (9–11,13,18,19,22)
studies that assessed 1-, 3- and 5-year RFS (Table II and Fig.
2A–C) showed significant differences favoring NAs therapy (RR,
1.090; 95% CI, 1.030–1.153; P=0.003; RR, 1.202; 95% CI,
1.121–1.288; P<0.001; and RR, 1.219; 95% CI, 1.032–1.442;
P=0.02, respectively). The significant between-study heterogeneity
only existed in the pooled analysis of 5-year RFS
(I2=41.5%). In addition, as the randomized studies were
low quality, the included cohort studies were pooled and analyzed.
These studies also showed a significant benefit of 1- and 3-year
RFS (RR 1.087; 95% CI, 1.024–1.153; P=0.006 and RR 1.186; 95% CI,
1.104–1.273; P<0.001), while there was no significant difference
for the 5-year RFS (RR, 1.188; 95% CI, 0.994–1.142; P=0.058).
 | Table II.Pooled analysis of RFS and OS in 1-,
3- and 5-year. |
Table II.
Pooled analysis of RFS and OS in 1-,
3- and 5-year.
|
|
|
|
|
|
| Publication
bias |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Survival rate | Study design | No. of studies | RR (95% CI) | P-value | I2,
% | Eggers test | Beggs test | Model |
|---|
| RFS |
|
|
|
|
|
|
|
|
|
1-year | Cohort | 10 | 1.087
(1.024–1.153) | 0.006 |
0.0 | 0.102 | 0.152 | Fixed-effect |
|
|
Cohort/randomized | 11 | 1.090
(1.030–1.153) | 0.003 |
0.0 | 0.080 | 0.150 | Fixed-effect |
|
3-year | Cohort | 10 | 1.186
(1.104–1.273) | <0.001 |
0.0 | 0.060 | 0.474 | Fixed-effect |
|
|
Cohort/randomized | 12 | 1.202
(1.121–1.288) | <0.001 |
7.0 | 0.024 | 0.304 | Fixed-effect |
|
5-year | Cohort | 6 | 1.188
(0.994–1.420) | 0.058 | 41.5 | 0.644 | 0.707 | Random-effect |
|
|
Cohort/randomized | 7 | 1.219
(1.032–1.442) | 0.020 | 40.8 | 0.447 | 0.881 | Random-effect |
| OS |
|
|
|
|
|
|
|
|
|
1-year | Cohort | 10 | 1.028
(0.977–1.083) | 0.289 |
0.0 | 0.112 | 0.371 | Fixed-effect |
|
|
Cohort/randomized | 11 | 1.029
(0.980–1.081) | 0.249 |
0.0 | 0.082 | 0.631 | Fixed-effect |
|
3-year | Cohort | 9 | 1.096
(1.033–1.163) | 0.003 |
0.0 | 0.040 | 0.348 | Fixed-effect |
|
|
Cohort/randomized | 10 | 1.106
(1.045–1.171) | 0.001 |
0.0 | 0.019 | 0.213 | Fixed-effect |
|
5-year | Cohort | 5 | 1.252
(1.094–1.432) | 0.001 | 42.9 | 0.024 | 0.806 | Random-effect |
|
|
Cohort/randomized | 6 | 1.246
(1.110–1.400) | <0.001 | 33.3 | 0.009 | 0.452 | Random-effect |
Pooling the data of 11 (8–11,13,14,16,17,19,21,22),
10 (8–11,13,14,16,17,19,22)
and 6 (9–11,13,19,22)
studies that the assessed 3- and 5-year OS (Table II and Fig.
2D–F) showed significant differences favoring NAs therapy (RR,
1.106; 95% CI, 1.045–1.171; P=0.001; and RR, 1.246; 95% CI,
1.110–1.400; P<0.001), while no significant difference existed
in the 1-year OS (RR, 1.029; 95% CI, 0.980–1.081; P=0.249). The
significant between-study heterogeneity only existed in the pooled
analysis of 5-year OS (I2=33.3%). Additionally, as the
randomized studies were low quality, the included cohorts were
pooled and analyzed. These studies also showed the same results as
above (1-year OS: RR, 1.028; 95% CI, 0.977–1.083; P=0.289, 3-year
OS: RR, 1.096; 95% CI, 1.033–1.163; P=0.003 and 5-year OS: RR,
1.252; 95% CI, 1.094–1.432; P=0.001, respectively).
Effects of recurrence HCC and
fatalities
Pooling data of 13 (8–15,17–19,21,22)
and 11 (8–10,12,14,17–22)
studies that assessed the rate of recurrence HCC and fatalities
(Table III and Fig. 2G–H) showed a significantly higher rate
in the control group (RR, 1.301; 95% CI, 1.098–1.542; P=0.002; and
RR, 1.816; 95% CI, 1.399–2.358; P<0.001). The significant
between-study heterogeneity existed in the pooled analysis
(I2=55.2% and I2=52.4%). In addition, as the
randomized studies were low quality, the included cohorts were
pooled and analyzed. These studies also showed the same results as
above (recurrence HCC: RR, 1.328; 95% CI, 1.069–1.650; P=0.011; and
fatalities: RR, 1.840; 95% CI, 1.329–2.549; P<0.001).
 | Table III.Pooled analysis of recurrent HCC and
fatalities. |
Table III.
Pooled analysis of recurrent HCC and
fatalities.
|
|
|
|
|
|
| Publication
bias |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Outcome | Study design | No. of studies | RR (95% CI) | P-value | I2,
% | Eggers test | Beggs test | Model |
|---|
| Recurrent HCC | Cohort | 11 | 1.328
(1.069–1.650) | 0.011 | 60.5 | 0.723 | 0.213 | Random-effect |
| Fatalities |
Cohort/randomized | 13 | 1.301
(1.098–1.542) | 0.002 | 55.2 | 0.771 | 0.360 | Random-effect |
|
| Cohort | 9 | 1.840
(1.329–2.549) | <0.001 | 53.1 | 0.056 | 0.754 | Random-effect |
|
|
Cohort/randomized | 11 | 1.816
(1.399–2.358) | <0.001 | 52.4 | 0.029 | 0.350 | Random-effect |
Superior choice for antiviral
treatment
One cohort study was included in this analysis
(20). A total of 865 HBV-related HCC
patients received antiviral treatment at diagnosis or immediately
following surgery (adefovir at a dosage of 10 mg/day in 300
patients, entecavir at a dosage of 0.5 mg/day in 325 patients, and
lamivudine at a dosage of 100 mg/day in 240 patients). The 1-, 2-
and 3-year resistance rates were 0.9, 1.8 and 2.5% for the
entecavir group, 3.0, 8.3 and 12.0% for the adefovir group, and
21.7, 31.7 and 39.6% for the lamivudine group. The 3-year
disease-free survival for the entecavir group was also
significantly improved compared with the adefovir group and the
lamivudine group (HR, 0.810; 95% CI, 0.656–0.999; P=0.049; and HR,
0.737; 95% CI, 0.591–0.919; P=0.007).
Sensitivity analysis and publication
bias
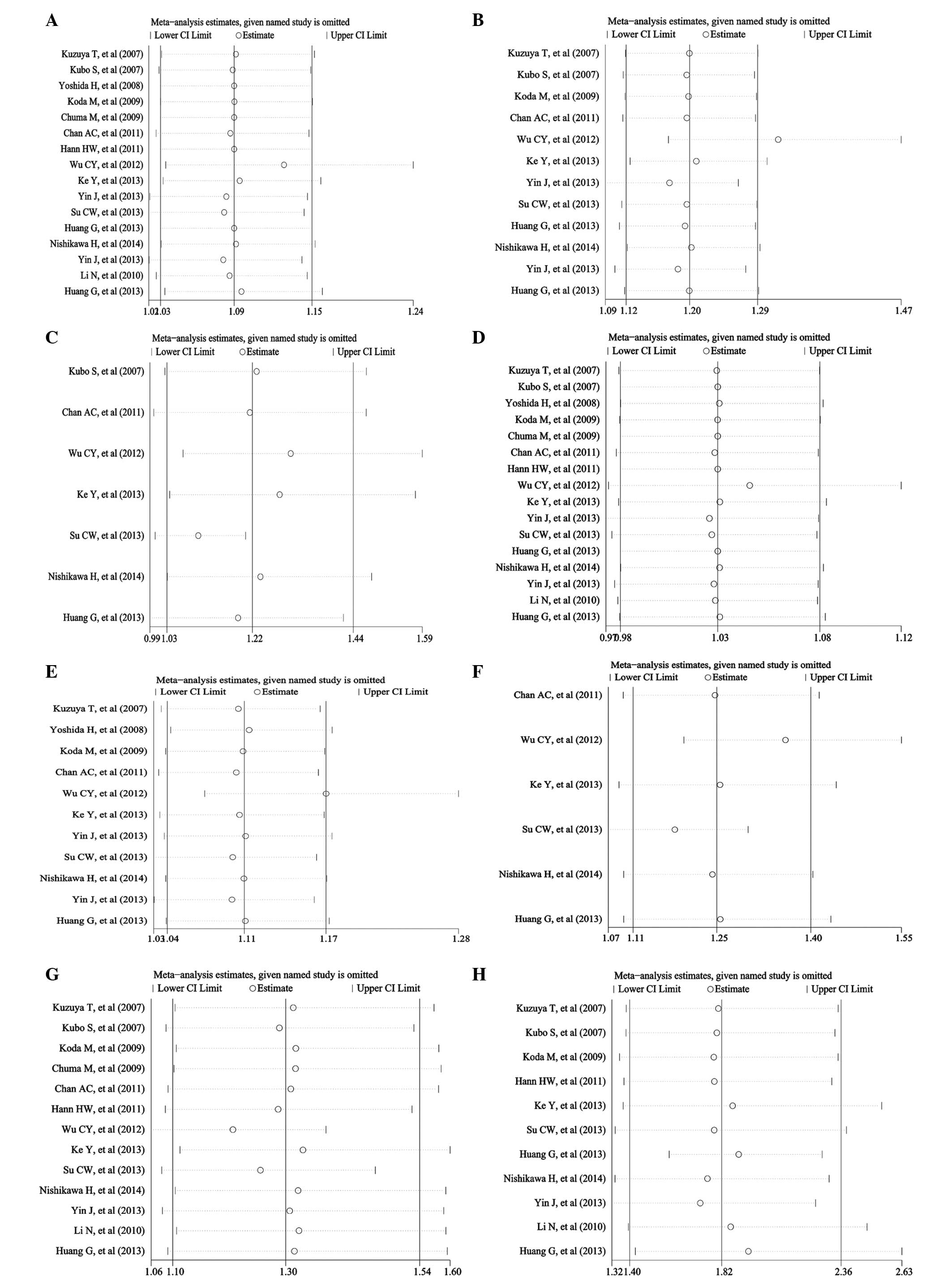
The sensitivity analyses were performed for the
pooled RR and 95% CI of the remaining researches by omitting each
of the included studies. The results did not change and remained
consistent with the pooled analyses as above (Fig. 3), except for the result of 5-year RFS,
which had a significant difference within the randomized study of
Huang et al (22), while there
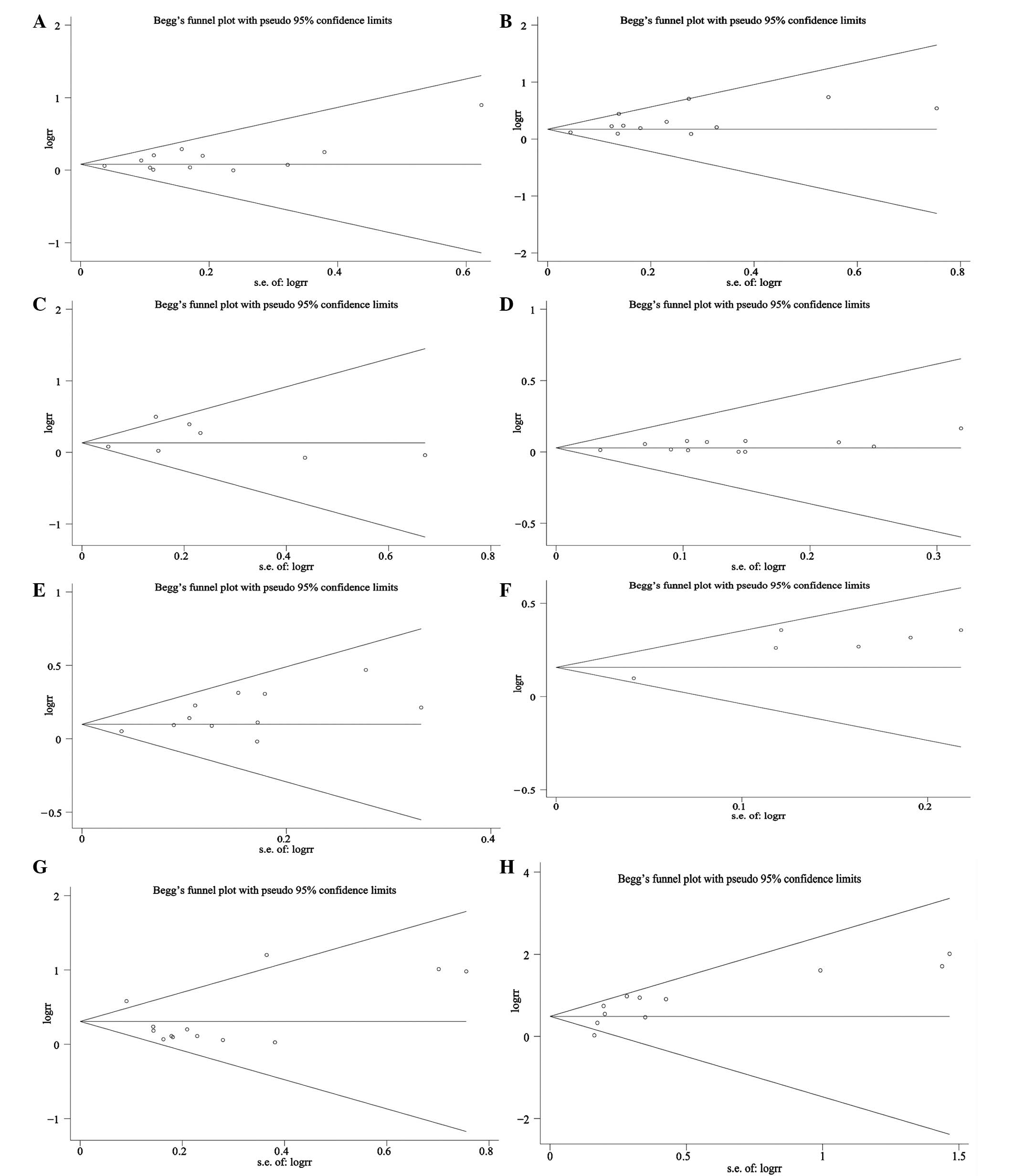
was no difference without the study. The results of publication
bias analyzed by the Begg's test and Egger's test are shown in
Tables I and II. Publication bias was found according to
the Begg's test and Egger's test in the 3-year RFS, OS and the rate
of fatalities (Tables II and
III and Fig. 4).
Discussion
In the present meta-analysis, 15 studies fulfilled
the criteria. The results showed the significant benefits of NAs
therapy for RFS, OS, recurrence HCC and fatalities, respectively.
Sensitivity analysis also confirmed the robustness of the
results.
In view of the established association between high
HBV DNA viral load and HCC recurrence and fatalities, inhibiting
HBV replication by antiviral therapy should theoretically be able
to prevent this condition. Recently, certain meta-analysis studies
have shown that postoperative antiviral therapy with NAs can reduce
HCC recurrence and mortality (23,24).
However, few analyses have shown that the antiviral treatment can
improve the survival rate in different years for patients following
curative treatment, except for the study in 2010 (25). Overall, the present pooled results
were similar with previous studies (23–25), which
revealed that NAs therapy can significantly delay the disease
progression of HBV-related HCC following resection. The results
from the study have demonstrated that NAs treatment following
curative resection of HBV-related HCC reduced recurrences,
mortality and improved the survival rate. The main beneficial
effect of NAs is associated with its prevention of viral
replication-related carcinogenesis. Additionally, suppression of
HBV replication could improve remnant liver function, which would
decrease the mortality due to liver failure and allow subsequently
aggressive treatment for recurrences (17). However, some of the pooled analysis,
such as 5-year RFS and 1-year OS, were insufficient. Although these
results had no clear difference between patients in the antiviral
treatment and control groups, they were nearly the cut-off value.
Therefore, more original studies are required to be conducted for
these purposes.
Only one study showed the different effects with
ETV, LAM and ADV (20). ETV is a
superior choice for HBV-related HCC patients following curative
treatment compared with the patients with LAM and ADV treatment,
which is based on the lower resistance rate and higher RFS. The
combination of ETV plus low-dose on-demand hepatitis B
immunoglobulin (HBIG) is effective with extremely low hepatitis B
recurrence following liver transplantation, compared with patients
on combination of LAM and HBIG (26).
As known, ETV is one of the first-line drugs for treatment of HBV
patients, even for patients with ADV resistance (27). For the patients with HBV-related
cirrhosis, ETV shows the higher efficacy in viral suppression and a
lower risk of antiviral resistance (28,29).
Treatment with ETV also showed that it could reduce the incidence
of HCC in HBV-infected patients (30). Although, only one study was included
in the present analysis, ETV may be the better choice compared with
LAM or ADV for HBV-related HCC patients following curative
treatment, without economic consideration.
Regarding the sensitivity analysis for the 5-year
RFS, two different results were observed. The significant
difference existed when the randomized study of Huang et al
(22) was pooled. By contrast, no
difference was observed. This may have been caused by two reasons.
First, the pooled analysis had heterogeneity, which impaired the
result. Second, the number of included patients was not sufficient.
Thus, more studies are required to be investigated in the
future.
Certain limitations of the study should be listed.
First, all the included studies were non-randomized trials except
two studies, but the results still showed significant benefits of
NAs therapy and were stable according to sensitivity analysis.
Second, significant between-study heterogeneity existed in the
pooled results of 5-year RFS, 5-year OS, recurrence HCC and
fatalities, which may be a result of the different patients, the
type of NAs and duration of treatment. In the present
meta-analysis, the pooled data neglected the differences, so a
random-effect model was applied. Third, a certain extent of
publication bias existed despite no statistical significance by
Egger's test, such as 3-year RFS, 3-year OS, 5-year OS and
fatalities, which may indicate a type of unpredictable report bias.
Fourth, certain data were transformed from a survival curve,
instead of as reported, which can lead to bias.
In conclusion, despite the limitations listed above,
the present study demonstrated beneficial effects of NAs therapy
following curative treatment of HBV-related HCC. ETV may be the
superior choice of antiviral treatment. Further studies should
focus on which type of NA drugs are beneficial for patients
following curative treatment of HBV-related HCC.
Acknowledgements
The present study was supported by grants from the
National Science Foundation of China (no. 81470856) and the Chinese
Foundation for Hepatitis Prevention and Control (no.
XJS20120601).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Papatheodoridis GV, Lampertico P,
Manolakopoulos S and Lok A: Incidence of hepatocellular carcinoma
in chronic hepatitis B patients receiving nucleos(t)ide therapy: A
systematic review. J Hepatol. 53:348–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Y, Sui C, Li B, Yin Z, Tan Y, Yang J
and Liu Z: Repeat hepatectomy for recurrent hepatocellular
carcinoma: A local experience and a systematic review. World J Surg
Oncol. 8:552010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun P, Dong X, Cheng X, Hu Q and Zheng Q:
Nucleot(s)ide analogues for hepatitis B virus-related
hepatocellular carcinoma after curative treatment: A systematic
review and meta-analysis. PLoS One. 9:e1027612014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med. 6:e10000972009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wells GA, Shea B, OConnell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses. Ottawa Hospital Research Institute, 2012. http://www.ohri.ca/programs/clinical_epidemiology/oxford.aspAccessed.
October 19–2009.
|
|
7
|
Zhang H, Zhou YP, Peng HJ, Zhang XH, Zhou
FY, Liu ZH and Chen XG: Predictive symptoms and signs of severe
dengue disease for patients with dengue fever: A meta-analysis.
Biomed Res Int. 2014:3593082014.PubMed/NCBI
|
|
8
|
Yin J, Li N, Han Y, Xue J, Deng Y, Shi J,
Guo W, Zhang H, Wang H, Cheng S, et al: Effect of antiviral
treatment with nucleotide/nucleoside analogs on postoperative
prognosis of hepatitis B virus-related hepatocellular carcinoma: A
two-stage longitudinal clinical study. J Clin Oncol. 31:3647–3655.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su CW, Chiou YW, Tsai YH, Teng RD, Chau
GY, Lei HJ, Hung HH, Huo TI and Wu JC: The influence of hepatitis B
viral load and pre-S deletion mutations on post-operative
recurrence of hepatocellular carcinoma and the tertiary preventive
effects by anti-viral therapy. PLoS One. 8:e664572013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ke Y, Ma L, You XM, Huang SX, Liang YR,
Xiang BD, Li LQ and Zhong JH: Antiviral therapy for hepatitis B
virus-related hepatocellular carcinoma after radical hepatectomy.
Cancer Biol Med. 10:158–164. 2013.PubMed/NCBI
|
|
11
|
Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu
MS and Lin JT: Association between nucleoside analogues and risk of
hepatitis B virus-related hepatocellular carcinoma recurrence
following liver resection. JAMA. 308:1906–1914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hann HW, Bergin D, Coben R and DiMarino
AJ: Prevention of new hepatocellular carcinoma with concomitant
antiviral therapy in chronic hepatitis B patients whose initial
tumor was successfully ablated. Int J Cancer. 128:739–742. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan AC, Chok KS, Yuen WK, Chan SC, Poon
RT, Lo CM and Fan ST: Impact of antiviral therapy on the survival
of patients after major hepatectomy for hepatitis B virus-related
hepatocellular carcinoma. Arch Surg. 146:675–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koda M, Nagahara T, Matono T, Sugihara T,
Mandai M, Ueki M, Ohyama K, Hosho K, Okano J, Kishimoto Y, et al:
Nucleotide analogs for patients with HBV-related hepatocellular
carcinoma increase the survival rate through improved liver
function. Intern Med. 48:11–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chuma M, Hige S, Kamiyama T, Meguro T,
Nagasaka A, Nakanishi K, Yamamoto Y, Nakanishi M, Kohara T, Sho T,
et al: The influence of hepatitis B DNA level and antiviral therapy
on recurrence after initial curative treatment in patients with
hepatocellular carcinoma. J Gastroenterol. 44:991–999. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida H, Yoshida H, Goto E, Sato T, Ohki
T, Masuzaki R, Tateishi R, Goto T, Shiina S, Kawabe T, et al:
Safety and efficacy of lamivudine after radiofrequency ablation in
patients with hepatitis B virus-related hepatocellular carcinoma.
Hepatol Int. 2:89–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuzuya T, Katano Y, Kumada T, Toyoda H,
Nakano I, Hirooka Y, Itoh A, Ishigami M, Hayashi K, Honda T, et al:
Efficacy of antiviral therapy with lamivudine after initial
treatment for hepatitis B virus-related hepatocellular carcinoma. J
Gastroenterol Hepatol. 22:1929–1935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kubo S, Tanaka H, Takemura S, Yamamoto S,
Hai S, Ichikawa T, Kodai S, Shinkawa H, Sakaguchi H, Tamori A, et
al: Effects of lamivudine on outcome after liver resection for
hepatocellular carcinoma in patients with active replication of
hepatitis B virus. Hepatol Res. 37:94–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishikawa H, Nishijima N, Arimoto A,
Inuzuka T, Kita R, Kimura T and Osaki Y: Effect of nucleoside
analog use in patients with hepatitis B virus-related
hepatocellular carcinoma. Hepatol Res. 44:608–620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang G, Yang Y, Shen F, Pan ZY, Fu SY,
Lau WY, Zhou WP and Wu MC: Early viral suppression predicts good
postoperative survivals in patients with hepatocellular carcinoma
with a high baseline HBV-DNA load. Ann Surg Oncol. 20:1482–1490.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li N, Lai EC, Shi J, Guo WX, Xue J, Huang
B, Lau WY, Wu MC and Cheng SQ: A comparative study of antiviral
therapy after resection of hepatocellular carcinoma in the
immune-active phase of hepatitis B virus infection. Ann Surg Oncol.
17:179–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang L, Li J, Yan J, Sun J, Zhang X, Wu M
and Yan Y: Antiviral therapy decreases viral reactivation in
patients with hepatitis B virus-related hepatocellular carcinoma
undergoing hepatectomy: A randomized controlled trial. J Viral
Hepat. 20:336–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou Y, Zhang Z, Zhao Y, Wu L and Li B:
Antiviral therapy decreases recurrence of hepatitis B virus-related
hepatocellular carcinoma after curative resection: A meta-analysis.
World J Surg. 38:2395–2402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong JS, Wong GL, Tsoi KK, Wong VW, Cheung
SY, Chong CN, Wong J, Lee KF, Lai PB and Chan HL: Meta-analysis:
The efficacy of anti-viral therapy in prevention of recurrence
after curative treatment of chronic hepatitis B-related
hepatocellular carcinoma. Aliment Pharmacol Ther. 33:1104–1112.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miao RY, Zhao HT, Yang HY, Mao YL, Lu X,
Zhao Y, Liu CN, Zhong SX, Sang XT and Huang JF: Postoperative
adjuvant antiviral therapy for hepatitis B/C virus-related
hepatocellular carcinoma: A meta-analysis. World J Gastroenterol.
16:2931–2942. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu TH, Chen CL, Lin CC, Wang CC, Chiu KW,
Yong CC, Liu YW and Eng HL: Section 14. Combination of entecavir
plus low-dose on-demand hepatitis B immunoglobulin is effective
with very low hepatitis B recurrence after liver transplantation.
Transplantation. 97:(Suppl 8). S53–S59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
European Association For The Study Of The
Liver, . EASL clinical practice guidelines: Management of chronic
hepatitis B virus infection. J Hepatol. 57:167–185. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim HR, Yim HJ, Kang S, Suh SJ, Kim SY,
Hyun JJ, Koo JS, Kim JH, Seo YS, Yeon JE, et al: Efficacy of
telbivudine compared with entecavir in hepatitis B virus-related
cirrhosis: 2 year follow-up data. Liver Int. 35:860–869. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye XG and Su QM: Effects of entecavir and
lamivudine for hepatitis B decompensated cirrhosis: Meta-analysis.
World J Gastroenterol. 19:6665–6678. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hosaka T, Suzuki F, Kobayashi M, Seko Y,
Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, et al:
Long-term entecavir treatment reduces hepatocellular carcinoma
incidence in patients with hepatitis B virus infection. Hepatology.
58:98–107. 2013. View Article : Google Scholar : PubMed/NCBI
|