Introduction
Multiple myeloma (MM) is a malignant neoplasm
characterized by the clonal proliferation of plasma cells in the
bone marrow that produce monoclonal protein (M protein) (1,2). Although
MM remains an incurable malignancy, the survival rates have
improved markedly (2). The first
notable improvement came following the introduction of autologous
stem cell transplantation (3,4). In addition, the use of drugs with
antiangiogenic, antiproliferative and immunomodulatory effects,
including thalidomide (5) and
lenalidomide (6,7), and proteasome inhibitors, including
bortezomib (8,9), were introduced (10). These agents have been combined with
melphalan, resulting in high response rates in patients with MM
(11–14).
Melphalan is a cytotoxic agent used extensively in
the treatment of MM (15,16). For decades, the combination of
melphalan with prednisolone provided the standard of care for
elderly patients with MM. Previously, a bortezomib plus oral
melphalan and prednisolone (VMP) regimen was demonstrated to exert
synergistic anticancer effects (17,18), and
thus this has become the current standard regimen for patients with
MM (11).
The oral bioavailability of melphalan is known to be
widely variable (19–21). For example, Sviland et al
(22) demonstrated that pretreatment
of the patients with the histamine 2 (H2) receptor
blocker, cimetidine, reduced the oral bioavailability of mephalan
by ~35%. This reduction is thought to stem from the poor absorption
of melphalan (19), since its
solubility is known to decrease under alkaline pH conditions
(23,24). However, this pharmacokinetic
interaction has not been conclusively demonstrated to be associated
with any reduced clinical efficacy. Furthermore, there is no
mention of any drug interactions between oral melphalan and gastric
antisecretory drugs in the medical package insert in Japan.
The aim of the present study was to assess whether
the clinical efficacy and toxicity of melphalan were influenced by
the concomitant use of gastric antisecretory drugs in patients with
MM receiving VMP therapy. The clinical significance of the
interaction between melphalan and gastric antisecretory drugs was
also discussed.
Patients and methods
Ethics statement
The present study was reviewed and approved by the
Institutional Review Boards of the Japan Community Health care
Organization Kyoto Kuramaguchi Medical Center (Kyoto, Japan; IRB
no.: 2015012602). Written patient consent was waived due to the
retrospective and observational nature of the study.
Study population and design
A retrospective study was performed at the Japan
Community Health care Organization Kyoto Kuramaguchi Medical Center
(Kyoto, Japan). A total of 12 patients with MM who received VMP
therapy between December 2011 and November 2014 were included.
However, two patients from this cohort were excluded from the
analysis, due to an inability to measure the level of M protein
throughout the first cycle of treatment. The remaining 10 patients
were divided into two groups: The concomitant (three patients) and
control (seven patients) groups, according to the additional use of
gastric antisecretory drugs, or the lack thereof. The VMP regimen
consisted of bortezomib (1.3 mg/m2) administered on days
1, 8, 15 and 22, and oral melphalan (6 mg/m2) and
prednisolone (40 mg/m2) administered on days 1–4 per one
cycle (35 days).
Data collection and definitions
Two parameters were used as measures of clinical
efficacy: (i) a reduction in the level of M protein; and (ii) the
best response according to the International Myeloma Working Group
criteria (25) throughout the course
of treatment. The hematological and gastrointestinal (GI) toxicity
of melphalan was assessed according to the Common Terminology
Criteria for Adverse Events version 4.0 (26), using the highest grade available
throughout the duration of the three-cycle treatment for the
analysis.
Statistical analysis
The data are expressed as the mean ± standard
deviation, or the median (range). Comparisons of the two groups
were performed using the unpaired Student's t-test (parametric) or
the Mann-Whitney U test (non-parametric). Differences between
observed and expected frequencies were evaluated using Fisher's
exact probability test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of the patients
Table I shows the
characteristics of the 10 patients enrolled in the present study.
Seven patients were not co-administered gastric antisecretory drugs
(control group), and three patients were co-administered gastric
antisecretory drugs (two patients were administered rabeprazole
sodium, one patient was administered famotidine) in the VMP regimen
(concomitant group). Patient no. 1 was changed to the VMP regimen
after having received oral melphalan and prednisolone therapy
during cycle 1. No significant differences were observed in the
characteristics of the patients between the control and concomitant
groups.
 | Table I.Characteristics of the patients. |
Table I.
Characteristics of the patients.
|
| Control | Concomitant |
|
|---|
|
|
|
|
|
|---|
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | P-value |
|---|
| Age (years) | 79 | 80 | 71 | 74 | 78 | 80 | 63 | 69 | 72 | 70 | 0.137a |
| Sex | F | M | M | M | F | F | F | M | F | F | 1.000b |
| Myeloma type | IgG | IgG | IgG | IgG | BJP | IgG | IgG | IgG | IgG | IgG | 1.000b |
| ISS stage | II | III | II | I | II | III | I | III | III | II | 0.888b |
| Previous treatment
(Number) | Yes (1) | No (0) | No (0) | No (0) | No (0) | No (0) | No (0) | No (0) | No (0) | No (0) | 1.000b |
Table II shows the
doses of chemotherapeutic agents in the VMP regimen in the control
and concomitant groups. The doses of melphalan were 4.6 and 5.5
mg/m2 in the control and concomitant groups,
respectively; the difference between the two doses was not
statistically significant. Furthermore, the doses of other
components used in the VMP regimen (bortezomib and prednisolone)
revealed no statistically significant differences between the
control and concomitant groups.
 | Table II.Doses of the chemotherapeutic agents
in the VMP regimen. |
Table II.
Doses of the chemotherapeutic agents
in the VMP regimen.
|
| Control (n=7) | Concomitant
(n=3) |
P-valuea |
|---|
| Bortezomib
(mg/m2) | 1.3 (1.0–1.3) | 1.3 (1.2–1.3) | 0.569 |
| Oral melphalan
(mg/m2) | 4.6 (3.4–8.6) | 5.5 (5.5–6.1) | 0.210 |
| Prednisolone
(mg/m2) | 30.0
(12.7–57.5) | 37.5
(27.7–38.0) | 0.909 |
Clinical efficacy
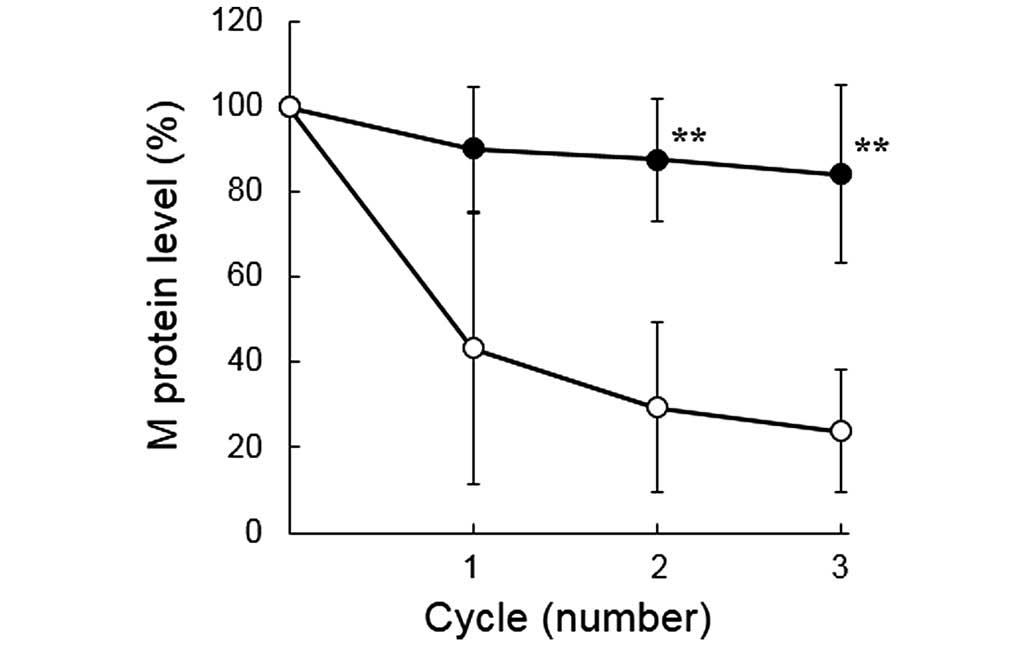
The levels of M protein over the duration of the
treatment period for the two groups are shown in Fig. 1. The levels of M protein in the
control group markedly decreased, depending on the number of
cycles; however, the levels in the concomitant group remained
largely unchanged. The levels of M protein in cycles 2 and 3 were
significantly higher in the concomitant group compared with the
control group (P<0.01).
The other index of clinical efficacy, i.e. the best
response in the three cycles, is summarized in Table III. All the patients in the control
group were graded as having a partial response (PR) to the drug
therapy, whereas all the patients in the concomitant group were
graded as having stable disease (SD). The best response to the VMP
regimen was significantly higher for the control group than for the
concomitant group.
 | Table III.Best response obtained in three
cycles of the VMP regimen. |
Table III.
Best response obtained in three
cycles of the VMP regimen.
|
| Response |
|
|---|
|
|
|
|
|---|
|
| PR | SD |
P-valuea |
|---|
| Control (n=7) | 7 | 0 | 0.008 |
| Concomitant
(n=3) | 0 | 3 |
|
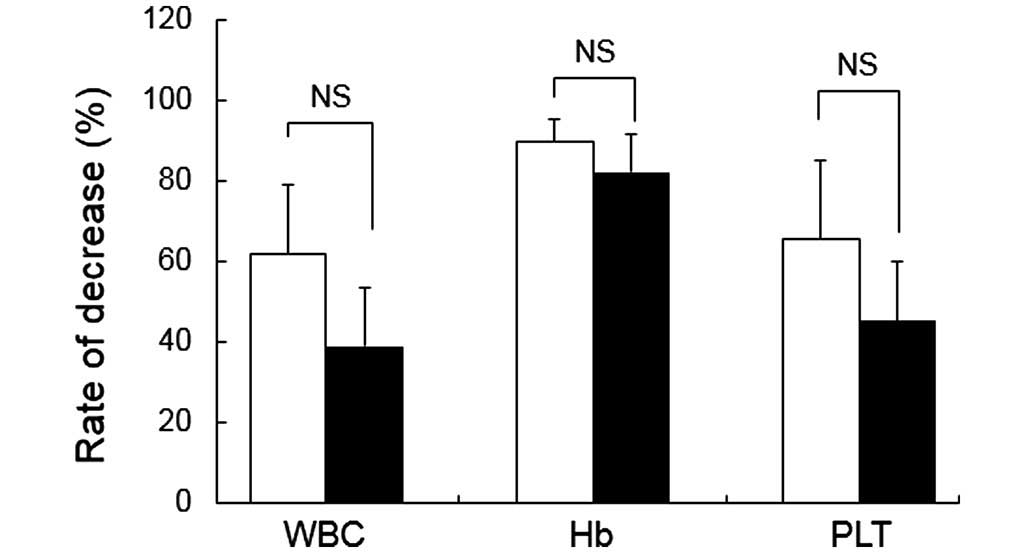
Hematological and GI toxicity
Table IV shows the
grade and frequency of selected hematological and GI toxicities.
Throughout each treatment cycle, the grade and frequency of
leukopenia, anemia and thrombocytopenia were comparable in the two
groups. Furthermore, no significant differences were observed in
the white blood cell count, hemoglobin level, or the platelet count
between the two groups (Fig. 2).
Grade 1 GI toxicities were observed in the control group,
manifesting as vomiting in one patient, and diarrhea and loss of
appetite in two other patients. No GI toxicity was observed in the
concomitant group.
 | Table IV.Hematological and gastrointestinal
toxicity. |
Table IV.
Hematological and gastrointestinal
toxicity.
|
| Grade | Control (n=7) | Concomitant
(n=3) |
P-valuea |
|---|
| Leukopenia | Grade 1/2 | 5 | 1 | 0.500 |
|
| Grade 3/4 | 2 | 2 |
|
| Anemia | Grade 1/2 | 5 | 1 | 0.500 |
|
| Grade 3/4 | 2 | 2 |
|
|
Thrombocytopenia | Grade 1/2 | 3 | 3 | 1.000 |
|
| Grade 3/4 | 1 | 0 |
|
| Vomit | Grade 1 | 1 | 0 | – |
| Diarrhea | Grade 1 | 2 | 0 | – |
| Appetite loss | Grade 1 | 2 | 0 | – |
Discussion
Limitations to the present study included its
retrospective small sample size and a lack of plasma melphalan
concentrations. However, the results in the present study are
sufficient to call attention to the interaction between melphalan
and gastric antisecretory drugs.
In the present study, three of the ten patients
received gastric antisecretory drugs in addition to VMP therapy. Of
the three patients in the concomitant group, two patients received
rabeprazole sodium and one received famotidine. With the exception
of the use of gastric antisecretory drugs, the control and the
concomitant groups were comparable in terms of the characteristics
of the patients (Table I) and the
doses of chemotherapeutic agents in the VMP regimen (Table II). However, the decrease in the
level of the M protein in treatment cycles 2 and 3 was
significantly greater in the control group (Fig. 1), indicating a more favorable clinical
response in that cohort. In addition, all the patients in the
control group were graded as having a PR to the drug therapy,
compared with the SD identified in the concomitant group (Table III). Taken together, these findings
suggested that the clinical efficacy of the VMP regimen against MM
decreases on co-administration of the gastric antisecretory drugs.
As for bortezomib, the pharmacokinetics, efficacy and safety of
bortezomib were not affected by co-administration of either the
proton-pump inhibitor, omeprazole (27), or the H2 receptor blocker,
lafutidine (28).
The hallmark adverse events of melphalan are known
to be hematological and GI toxicities. The present study disclosed
no significant difference in the analyses of hematological toxicity
between the control and concomitant groups (Fig. 2 and Table
IV), in stark contrast with the clinical efficacy findings
reported in the present study. These results are in agreement with
previous reports, which cited no significant differences in
hematological toxicity among patients receiving oral melphalan
following cimetidine pretreatment (22). Therefore, in spite of the decreased
bioavailability of melphalan in the concomitant group, the plasma
concentrations were likely to have remained within a range in which
myelosuppression would be predicted to occur. The observed
differences in GI toxicity (Table
IV) may be partly explained by the poorer solubility of
melphalan tablets with increasing gastric pH.
Taken together, these results suggested that the
interaction of the VMP regimen with gastric antisecretory drugs
resulted in a marked decrease in the clinical efficacy of
melphalan. As such, oral melphalan should not be co-administered
with gastric antisecretory drugs, and this drug interaction should
prominently feature in the medical package insert.
The mechanism of the interaction between melphalan
and gastric antisecretory drugs remains to be fully elucidated. For
example, melphalan was reported to be unstable at alkaline pH, and
the dissolution of melphalan tablets occurred more slowly at an
increased gastric pH (23).
Furthermore, Sviland et al (22) reported a 35.5% decrease in the oral
bioavailability of melphalan in patients pretreated with
cimetidine. Therefore, drugs that induce a potent and long-lasting
inhibition of gastric acid secretion [a pharmacological hallmark of
rabeprazole sodium (29,30) and famotidine] would predispose a
patient towards this interaction. Food intake was also reported to
decrease the absorption rate of oral melphalan (31,32). It is
notable that melphalan was administered before the meal in the
present study.
Taken together, the results in the present study
suggest that this drug interaction may arise from the poor
solubility of the melphalan tablets, brought about by an increase
in gastric pH caused by the concomitant use of gastric
antisecretory drugs, thereby resulting in a diminished clinical
efficacy of melphalan.
In conclusion, the interaction between oral
melphalan and gastric antisecretory drugs may result in a marked
decrease in its clinical efficacy in the treatment of MM. This drug
interaction possibly results from a reduction in the absorption of
melphalan caused by an increase in gastric pH. Due to the
aforementioned limitations of the current study, further research
is necessary to obtain definitive evidence for these
conclusions.
References
|
1
|
Raab MS, Podar K, Breitkreutz I,
Richardson PG and Anderson KC: Multiple myeloma. Lancet.
374:324–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harousseau JL and Moreau P: Autologous
hematopoietic stem-cell transplantation for multiple myeloma. N
Engl J Med. 360:2645–2654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimazaki C: Autologous stem cell
transplantation for multiple myeloma: History and future. Int J
Myeloma. 3:55–66. 2013.
|
|
5
|
Rajkumar SV, Blood E, Vesole D, Fonseca R
and Greipp PR: Eastern Cooperative Oncology Group: Phase III
clinical trial of thalidomide plus dexamethasone compared with
dexamethasone alone in newly diagnosed multiple myeloma: A clinical
trial coordinated by the Eastern cooperative oncology group. J Clin
Oncol. 24:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zonder JA, Crowley J, Hussein MA, Bolejack
V, Moore DF Sr, Whittenberger BF, Abidi MH, Durie BG and Barlogie
B: Lenalidomide and high-dose dexamethasone compared with
dexamethasone as initial therapy for multiple myeloma: A randomized
southwest oncology group trial (S0232). Blood. 116:5838–5841. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dimopoulos MA, Chen C, Spencer A,
Niesvizky R, Attal M, Stadtmauer EA, Petrucci MT, Yu Z, Olesnyckyj
M, Zeldis JB, et al: Long-term follow-up on overall survival from
the MM-009 and MM-010 phase III trials of lenalidomide plus
dexamethasone in patients with relapsed or refractory multiple
myeloma. Leukemia. 23:2147–2152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harousseau JL, Attal M, Avet-Loiseau H,
Marit G, Caillot D, Mohty M, Lenain P, Hulin C, Facon T, Casassus
P, et al: Bortezomib plus dexamethasone is superior to vincristine
plus doxorubicin plus dexamethasone as induction treatment prior to
autologous stem-cell transplantation in newly diagnosed multiple
myeloma: Results of the IFM 2005-01 phase III trial. J Clin Oncol.
28:4621–4629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Richardson PG, Sonneveld P, Schuster M,
Irwin D, Stadtmauer E, Facon T, Harousseau JL, Ben-Yehuda D, Lonial
S, Goldschmidt H, et al: Extended follow-up of a phase 3 trial in
relapsed multiple myeloma: Final time-to-event results of the APEX
trial. Blood. 110:3557–3560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ria R, Reale A and Vacca A: Novel agents
and new therapeutic approaches for treatment of multiple myeloma.
World J Methodol. 4:73–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harousseau JL, Palumbo A, Richardson PG,
Schlag R, Dimopoulos MA, Shpilberg O, Kropff M, Kentos A, Cavo M,
Golenkov A, et al: Superior outcomes associated with complete
response in newly diagnosed multiple myeloma patients treated with
nonintensive therapy: Analysis of the phase 3 VISTA study of
bortezomib plus melphalan-prednisone versus melphalan-prednisone.
Blood. 116:3743–3750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kapoor P, Rajkumar SV, Dispenzieri A,
Gertz MA, Lacy MQ, Dingli D, Mikhael JR, Roy V, Kyle RA, Greipp PR,
et al: Melphalan and prednisone versus melphalan, prednisone and
thalidomide for elderly and/or transplant ineligible patients with
multiple myeloma: A meta-analysis. Leukemia. 25:689–696. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Palumbo A, Hajek R, Delforge M, Kropff M,
Petrucci MT, Catalano J, Gisslinger H, Wiktor-Jędrzejczak W,
Zodelava M, Weisel K, et al: Continuous lenalidomide treatment for
newly diagnosed multiple myeloma. N Engl J Med. 366:1759–1769.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Palumbo A, Bringhen S, Rossi D, Cavalli M,
Larocca A, Ria R, Offidani M, Patriarca F, Nozzoli C, Guglielmelli
T, et al: Bortezomib-melphalan-prednisone-thalidomide followed by
maintenance with bortezomib-thalidomide compared with
bortezomib-melphalan-prednisone for initial treatment of multiple
myeloma: A randomized controlled trial. J Clin Oncol. 28:5101–5109.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alexanian R, Haut A, Khan AU, Lane M,
McKelvey EM, Migliore PJ, Stuckey WJ Jr and Wilson HE: Treatment
for multiple myeloma. Combination chemotherapy with different
melphalan dose regimens. JAMA. 208:1680–1685. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Combination chemotherapy versus melphalan
plus prednisone as treatment for multiple myeloma: An overview of
6,633 patients from 27 randomized trials. Myeloma Trialists'
Collaborative Group. J Clin Oncol. 16:3832–3842. 1998.PubMed/NCBI
|
|
17
|
Ma MH, Yang HH, Parker K, Manyak S,
Friedman JM, Altamirano C, Wu ZQ, Borad MJ, Frantzen M, Roussos E,
et al: The proteasome inhibitor PS-341 markedly enhances
sensitivity of multiple myeloma tumor cells to chemotherapeutic
agents. Clin Cancer Res. 9:1136–1144. 2003.PubMed/NCBI
|
|
18
|
Mitsiades N, Mitsiades CS, Richardson PG,
Poulaki V, Tai YT, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph
M, et al: The proteasome inhibitor PS-341 potentiates sensitivity
of multiple myeloma cells to conventional chemotherapeutic agents:
Therapeutic applications. Blood. 101:2377–2380. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Woodhouse KW, Hamilton P, Lennard A and
Rawlins MD: The pharmacokinetics of melphalan in patients with
multiple myeloma: An intravenous/oral study using a conventional
dose regimen. Eur J Clin Pharmacol. 24:283–285. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bosanquet AG and Gilby ED:
Pharmacokinetics of oral and intravenous melphalan during routine
treatment of multiple myeloma. Eur J Cancer Clin Oncol. 18:355–362.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ehrsson H, Eksborg S, Osterborg A,
Mellstedt H and Lindfors A: Oral melphalan
pharmacokinetics-relation to dose in patients with multiple
myeloma. Med Oncol Tumor Pharmacother. 6:151–154. 1989.PubMed/NCBI
|
|
22
|
Sviland L, Robinson A, Proctor SJ and
Bateman DN: Interaction of cimetidine with oral melphalan. A
pharmacokinetic study. Cancer Chemother Pharmacol. 20:173–175.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alberts DS, Chang SY, Chen HS, Evans TL
and Moon TE: Oral melphalan kinetics. Clin Pharmacol Ther.
26:737–745. 1979.PubMed/NCBI
|
|
24
|
Tabibi SE and Cradock JC: Stability of
melphalan in infusion fluids. Am J Hosp Pharm. 41:1380–1382.
1984.PubMed/NCBI
|
|
25
|
Durie BG, Harousseau JL, Miguel JS, Bladé
J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J,
Sonneveld P, et al: International Myeloma Working Group:
International uniform response criteria for multiple myeloma.
Leukemia. 20:1467–1473. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Common Terminology Criteria for Adverse
Events (CTCAE) Version 4.0, U.S. Department of Health and Human
Sciences. National Institutes of Health. National Cancer Institute
Published. May 28–2009.v4.03: June 14, 2010.
|
|
27
|
Quinn DI, Nemunaitis J, Fuloria J, Britten
CD, Gabrail N, Yee L, Acharya M, Chan K, Cohen N and Dudov A:
Effect of the cytochrome P450 2C19 inhibitor omeprazole on the
pharmacokinetics and safety profile of bortezomib in patients with
advanced solid tumours, non-Hodgkin's lymphoma or multiple myeloma.
Clin Pharmacokinet. 48:199–209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsukaguchi M, Shibano M, Matsuura A and
Mukai S: The protective effects of lafutidine for bortezomib
induced peripheral neuropathy. J Blood Med. 4:81–85. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dammann HG, Burkhardt F and Wolf N: The
effects of oral rabeprazole on endocrine and gastric secretory
function in healthy volunteers. Aliment Pharmacol Ther.
13:1195–1203. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adachi K, Katsube T, Kawamura A, Takashima
T, Yuki M, Amano K, Ishihara S, Fukuda R, Watanabe M and Kinoshita
Y: CYP2C19 genotype status and intragastric pH during dosing with
lansoprazole or rabeprazole. Aliment Pharmacol Ther. 14:1259–1266.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bosanquet AG and Gilby ED: Comparison of
the fed and fasting states on the absorption of melphalan in
multiple myeloma. Cancer Chemother Pharmacol. 12:183–186. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reece PA, Kotasek D, Morris RG, Dale BM
and Sage RE: The effect of food on oral melphalan absorption.
Cancer Chemother Pharmacol. 16:194–197. 1986. View Article : Google Scholar : PubMed/NCBI
|