Introduction
Nab-paclitaxel is a newly developed chemotherapy
drug, which is albumin-bound paclitaxel nanoparticles (1). Compared with ordinary paclitaxel,
nab-paclitaxel is highly soluble and can easily reach potential
target tumor tissue through the bloodstream, exerting its
broad-spectrum antitumor activity (2). It is now commonly used in breast cancer,
pancreatic cancer, lung cancer and gastric cancer (3–6). In
clinical practice, the infusion time for nab-paclitaxel is shorter
and the incidence of allergic reactions is notably lower compared
with ordinary paclitaxel, which significantly potentiates its
clinical efficacy (7). However, the
safety of this agent remains to be fully understood, and the side
effects have rarely been reported. Although there several previous
studies focussing on the side effect of this novel drug, and the
majority of the mentioned side effects were edema, heart failure,
asthenia, neutropenia and neuropathy (8–12), another
previous study reported a rare case of capillary leak syndrome and
pulmonary hypertension following treatment with nab-paclitaxel
(13). However, in consideration of
the limited clinical administration and short practice time,
certain rare side effects must exist, which may not have been
revealed in clinical trails. The present study reported an unusual
case of nab-paclitaxel-associated paralytic ileus. Although this
case accepted gemcitabine and nab-paclitaxel at the same time,
considering well-demonstrated side effects of gemcitabine (14), the present study deduced that the rare
paralytic ileus was associated with nab-paclitaxel. This case was
accurately diagnosed and recovered well following the effective
treatments. The present case provided additional evidence for the
probable adverse effect of this novel drug, which may further guide
our clinical practice.
Case report
A 65-year-old male with pancreatic cancer was
admitted to the Department of Medical Oncology, Changzheng Hospital
(Shanghai, China). He was diagnosed with pancreatic adenocarcinoma
and had undergone a radical surgery 17 months previously (Fig. 1). However, the patient suffered from
tumor recurrence and metastasis, according to a recent computed
tomography (CT) examination, which revealed tumor recurrence, and
metastasis to the spleen and retroperitoneal lymph node. According
to the latest research (4), the
present case study selected nab-paclitaxel plus gemcitabine as the
first-line chemotherapy (nab-paclitaxel, 130 mg/m2
intravenous infusion days 1 and 8 and gemcitabine 1,000
mg/m2 intravenous infusion days 1 and 8, every 3
weeks).
After 3 days of the second cycle of chemotherapy
(November 24th 2014), the patient complained of sudden
and sustained abdominal pain with bloating and reduced anal
exhaust. Physical examinations revealed abdominal distension,
cullen and lower abdominal tenderness, weak bowel sounds (0–1
beat/min) and positive shifting dullness. Laboratory examinations
reported negative blood and urine amylase. Furthermore, abdominal
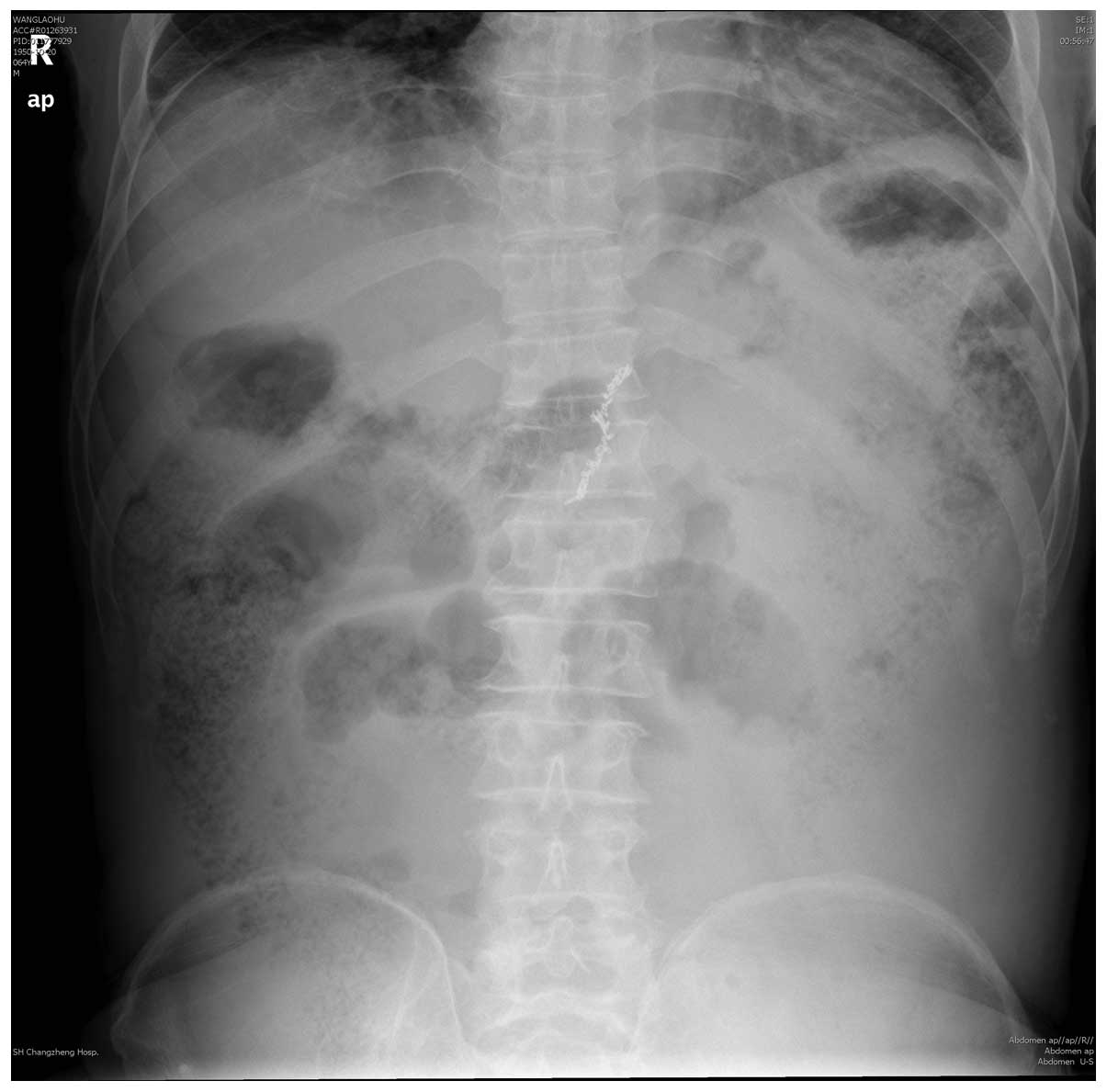
X-ray examination reported intestinal dilatation of product gas and
fluid (Fig. 2). Accordingly, the
patient was diagnosed with acute intestinal obstruction. The
patient was then fasted and administered conventional treatments,
including gastrointestinal decompression, gastrointestinal
secretion inhibition, fluid replacement, nutritional support and
enema.
The abdominal CT revealed no indications of other
acute abdominal diseases, including visceral perforation, rupture
or purulent infection, no significant expansion of tumor size or
location, or intestinal tumor metastasis. Serum tumor markers
declined and a serum potassium was normal. Therefore, the present
study hypothesized that the patient may be a rare case of
nab-paclitaxel-associated paralytic ileus rather than mechanical
resistance, blood flow obstruction or hypokalemia-associated
paralytic ileus. Methycobal was futher added to antagonize
potential nervous system toxicity caused by nab-paclitaxel.
Following active enema treatment, the patient passed a little
yellow watery stool on November 29th and defecated with abundant
amounts daily from then on. Bloating and abdominal pain were
relieved overtly. Flatus also recovered and bowel sounds returned
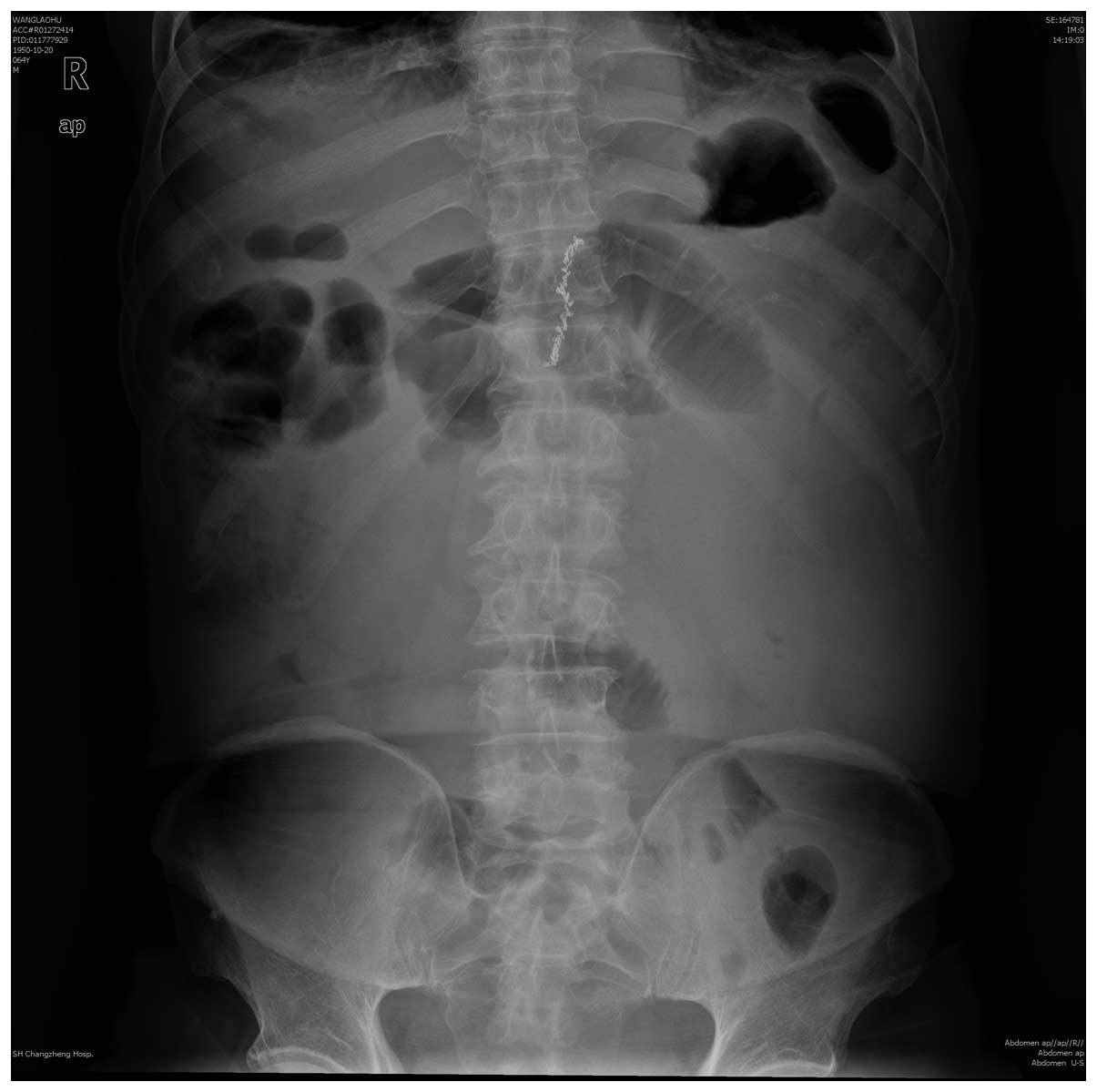
to 3 times/min. Another abdominal X-ray examination revealed that
the intestinal intraluminal stool shadow disappeared and only a
shadow of a small quantity of gas existed in the colon (Fig. 3). Clinical outcomes further supported
the diagnosis of paralytic ileus and the patient was discharged 3
days later. Unfortunately, this patient succumbed to mortality,
unrelated to the cancer, a week following discharge without any
anticancer therapy. Until mortality, his stool remained normal and
no more bowl obstruction occurred.
Discussion
Nab-paclitaxel is an albumin-bound paclitaxel
nanoparticle, and it is highly soluble and can easily reach
potential tumor tissues through the bloodstream to serve its
broad-spectrum antitumor activity. The drug contains no toxic
solvents, including polyoxyethylene castor oil or ethanol, which
may shorten the elapse of intravenous infusion and reduce the
incidence of allergic reactions (2).
Currently, the major reported adverse effects of
nab-paclitaxel include cardiac toxicity, nervous system toxicity,
muscle and joint pain, gastrointestinal reactions and hematological
toxicity (1), whereas bowel
obstruction is extremely rare. The present study presented for the
first time, to the best of our knowledge, a case of paralytic ileus
associated with nab-paclitaxel, which was eliminated following
active treatments.
The underlying mechanisms for intestinal obstruction
caused by nab-paclitaxel remain to be elucidated. According to
previous results from several clinical trials, the incidence of
neuropathy of nab-paclitaxel containing regimen ranged between 2.9
and 17% (4,15), however, all of the neuropathy occurred
in peripheral nerve, with no report of autonomic nerve involvement.
The present study hypothesized that autonomic nervous system
toxicity of nab-paclitaxel may be a probable contributing factor in
this case. Additionally, the addition of methycobal to conventional
treatment (16,17), which possesses trophic action of
nerve, further ameliorated the symptoms. The preventive usage of
methycobal may be a practical method to reduce the incidence of
neuropathy caused by nab-paclitaxel, however, this hypothesis
requires further confirmation in clinical practice and clinical
trials.
In conclusion, nab-paclitaxel is a novel
chemotherapy drug, for which the adverse effects remain to be fully
understood. The present study reported for the first time, to the
best of our knowledge, that nab-paclitaxel may lead to acute
intestinal obstruction in certain cases, and that the obstruction
may be induced by nab-paclitaxel-associated autonomic nervous
system toxicity. Enough attention to the autonomic nervous
toxicity, beside peripheral nervous toxicity, is required in
patients using this novel drug.
Acknowledgements
The present study received the support of the Young
Start-up Foundation of Changzheng Hospital (no. 2015CZQN07), the
Foundation of Shanghai Municipal Commission of health and family
planning (no. 201540174) and the Shanghai Natural Science
Foundation (no. 15ZR1414300).
References
|
1
|
Cecco S, Aliberti M, Baldo P, Giacomin E
and Leone R: Safety and efficacy evaluation of albumin-bound
paclitaxel. Expert Opin Drug Saf. 13:511–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yardley DA: Nab-Paclitaxel mechanisms of
action and delivery. J Control Release. 170:365–372. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yardley DA, Hart L, Bosserman L, Salleh
MN, Waterhouse DM, Hagan MK, Richards P, DeSilvio ML, Mahoney JM
and Nagarwala Y: Phase II study evaluating lapatinib in combination
with nab-paclitaxel in HER2-overexpressing metastatic breast cancer
patients who have received no more than one prior chemotherapeutic
regimen. Breast Cancer Res Treat. 137:457–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Socinski MA, Bondarenko I, Karaseva NA,
Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P and
Zhang H: Weekly nab-paclitaxel in combination with carboplatin
versus solvent-based paclitaxel plus carboplatin as first-line
therapy in patients with advanced non-small-cell lung cancer: Final
results of a phase III trial. J Clin Oncol. 30:2055–2062. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koizumi W, Morita S and Sakata Y: A
randomized Phase III trial of weekly or 3-weekly doses of
nab-paclitaxel versus weekly doses of Cremophor-based paclitaxel in
patients with previously treated advanced gastric cancer (ABSOLUTE
Trial). Jpn J Clin Oncol. 45:303–306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guarneri V, Dieci MV and Conte P:
Enhancing intracellular taxane delivery: Current role and
perspectives of nanoparticle albumin-bound paclitaxel in the
treatment of advanced breast cancer. Expert Opin Pharmacother.
13:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vishnu P and Roy V: Safety and efficacy of
nab-paclitaxel in the treatment of patients with breast cancer.
Breast Cancer (Auckl). 5:53–65. 2011.PubMed/NCBI
|
|
9
|
Matsuoka N, Hasebe H, Mayama T and Fukuchi
T: Sub-tenon injections of triamcinolone acetonide had limited
effect on cystoid macular edema secondary to nanoparticle
albumin-bound-paclitaxel (Abraxane). Case Rep Ophthalmol Med.
2015:1812692015.PubMed/NCBI
|
|
10
|
Rivera E and Cianfrocca M: Overview of
neuropathy associated with taxanes for the treatment of metastatic
breast cancer. Cancer Chemother Pharmacol. 75:659–670. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
John P, Butler H and Saif MW: Congestive
heart failure secondary to gemcitabine nab-paclitaxel in patients
with pancreatic cancer. Anticancer Res. 34:7267–7270.
2014.PubMed/NCBI
|
|
12
|
Rahman HT, Yeh S and Bergstrom CS: Cystoid
macular edema without leakage secondary to nab-Paclitaxel
(Abraxane): Clinical experience with intravitreal bevacizumab. J
Ocul Pharmacol Ther. 29:360–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gardini Casadei A, Aquilina M, Oboldi D,
Lucchesi A, Carloni S, Tenti E, Burgio MA, Amadori D and Frassineti
GL: Separate episodes of capillary leak syndrome and pulmonary
hypertension after adjuvant gemcitabine and three years later after
nab-paclitaxel for metastatic disease. BMC Cancer. 13:5422013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aapro MS, Martin C and Hatty S:
Gemcitabine-a safety review. Anticancer Drugs. 9:191–201. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alberts DS, Blessing JA, Landrum LM,
Warshal DP, Martin LP, Rose SL, Bonebrake AJ and Ramondetta LM:
Phase II trial of nab-paclitaxel in the treatment of recurrent or
persistent advanced cervix cancer: A gynecologic oncology group
study. Gynecol Oncol. 127:451–455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yagihashi S, Tokui A, Kashiwamura H,
Takagi S and Imamura K: In vivo effect of methylcobalamin on the
peripheral nerve structure in streptozotocin diabetic rats. Horm
Metab Res. 14:10–13. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ide H, Fujiya S, Asanuma Y, Tsuji M, Sakai
H and Agishi Y: Clinical usefulness of intrathecal injection of
methylcobalamin in patients with diabetic neuropathy. Clin Ther.
9:183–192. 1987.PubMed/NCBI
|