Introduction
Histopathological typing of tumors arising in the
lacrimal gland is generally similar to the classification of
salivary gland, although the frequency of occurrence of individual
types is different. Myoepithelial tumor (MET) is an uncommon
epithelial neoplasm of the lacrimal gland and was first described
in salivary gland and lacrimal gland by Sheldon et al and
Heathcote et al, respectively (1,2). MET has
been included in the World Health Organization (WHO) classification
of salivary gland tumours since 1991. The histogenesis of
myoepithelial tumor is currently regarded as tumor showing
morphologic and immunophenotypic evidence towards myoepithelial
cell (3). Herein, the authors report
the clinical, radiological, histopathologic, and
immunohistochemical features of lacrimal myoepithelial carcinoma
(MEC) arising in pleomorphic adenoma of the lacrimal gland.
Case report
A 68-year-old Thai female patient presented with
progressive painless proptosis in the right eye. For 12 years ago,
she had had swelling of the right upper eyelid. She underwent total
tumor removal for pleomorphic adenoma, tumor size 3 cm in greaest
dimension, with intact capsule and complete surgical resected
margin. For one year, 11 years later, she had noticed progressive
proptosis. Three months before surgery, she developed blindness of
the right eye with a large palpable mass in the superotemporal
aspect of the periocular area. Physical examination showed a visual
acuity of no light perception in the right eye and 20/32 in the
left eye. A firm mass was palpated in the superior temporal part of
the right orbit. There was proptosis and limited upward gaze of the
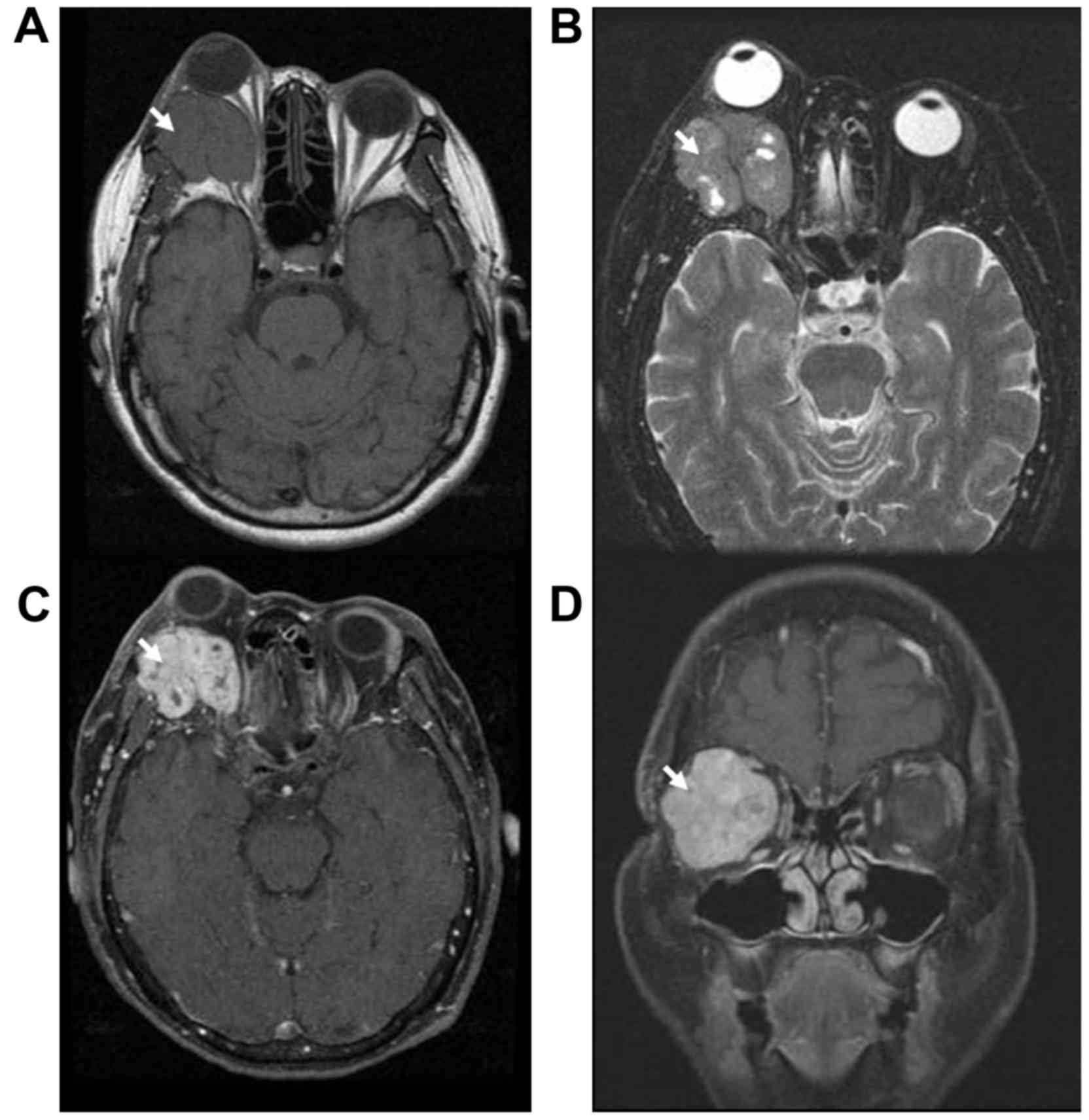
right eye. Magnetic resonance imaging (MRI) of the orbit revealed a
well-defined, lobulated, vivid inhomogeneous enhancing isosignal
T1W/slightly hypersignal T2W mass measuring 38×37×33 mm. The volume
was 30.626 cm3. It located at retrobulbar portion
involving extraconal-conal-intraconal spaces of the right orbit and
invading of the lateral bony wall laterally, displacing the eye
inferiorly, the optic nerve medially and the globe anteriorly
resulting exophthalmos (Fig. 1). No
regional lymphadenopathy was detected.
An incisional biopsy through the lateral orbitotomy
was performed, and the diagnosis of myoepithelial neoplasm of
uncertain malignant potential was made. Two months later,
exenteration of the right orbit was performed. Intraoperatively,
the tumor exhibited worrisome anatomic features in that is extended
into adjacent periocular soft tissue. The histopathologic diagnosis
was MEC arising in recurrent pleomorphic adenoma. Her postoperative
course was uneventful. The patient desired no further treatment.
Follow-up at 3 years revealed no evidence of tumor.
Pathologic findings
The resected specimen contained a firm gray-tan mass
measuring 40×40×35 mm. Cut surfaces were variably gray to
light-brown appearance. The mass had an infiltrative border not
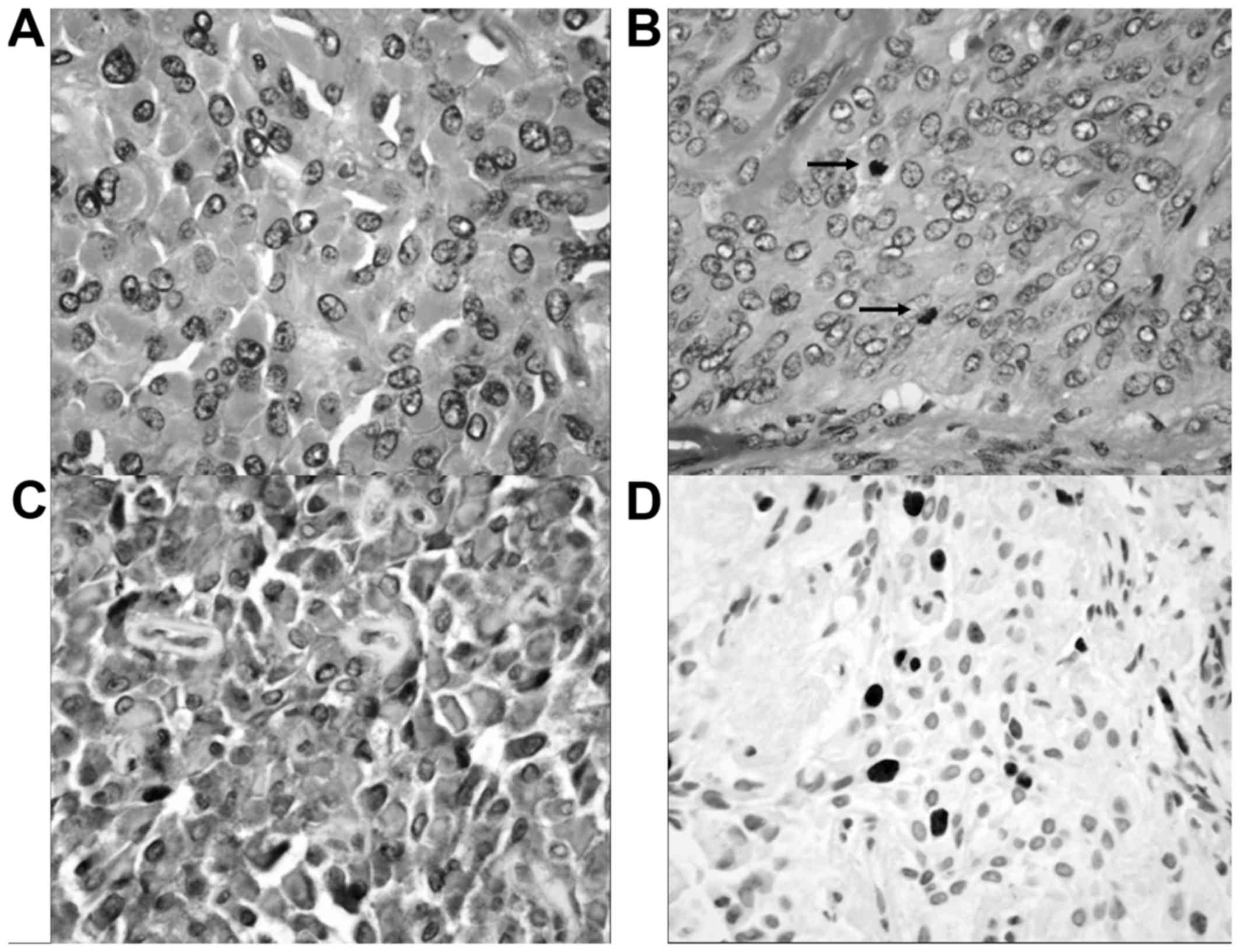
involving the margins of resection. Histopathologic examinations
revealed round to polygonal epithelioid cells with abundant
eosinophilic cytoplasm (Fig. 2).
Occasional cells had small amounts of spindle and plasmacytoid
appearance. The nuclei were round to oval with finely distributed
chromatin and small nucleoli. Cellular and nuclear pleomorphisms
were detected. Mitotic activity was 10/10 high-power fields (HPFs).
The tumor demonstrated focal infiltration into adjacent periocular
soft tissue. Angiolymphatic and neural invasions were not
identified. There was no intracytoplasmic mucin. There were small
foci of myxoid stroma, representing the residual pleomorphic
adenoma. Immunohistochemiscal stains of the epithelioid, spindle,
and plasmacytoid cells were diffuse positive reactivity for
cytokeratin AE1/AE3, S100 protein, vimentin, myogenin,
muscle-specific actin, and α-smooth muscle actin. The tumor cells
did not express sarcomeric actin, desmin, h-caldesmon, epithelial
membrane antigen (EMA), glial fibrillary acidic protein (GFAP),
HMB45, estrogen receptor, and progesterone receptor. The
proliferation (Ki67) of the tumor cells was 10.26%. The tumor was
completely excised. The pathologic diagnosis was lacrimal MEC
arising in recurrent pleomorphic adenoma.
Discussion
MET is an uncommon neoplasm composed of
histologically and immunohistochemically distinctive myoepithelial
cells (3). Most METs arise in the
salivary glands (3). Lacrimal METs
are uncommon. Including the authors' patient, 19 cases of lacrimal
MET have been reported (Table I). Of
these cases, nine cases (50%) were considered to be malignant MET
or MEC. The ages of patients range from 23 to 88 years with the
mean and median ages of 57.25 and 62.5 years, respectively
(2,4–18). The
average age of diagnosis of benign MET was younger than MEC
(50.50±22.13 vs. 60.62±23.84 years, P=0.495). The tumor sizes range
from 9 to 40 mm with the mean and median sizes of 28 and 30.5 mm,
respectively (2,4–18). The
average size of benign MET was smaller than MEC (24.60±10.78 cm vs.
30.43±7.89 cm, P=0.303). Male patients are more likely to have MEC
with a male to female ratio of 3:1 (P=0.049, Table II). Lacrimal METs usually remain
asymptomatic until they produce a mass effect. The most frequently
presenting symptoms are painless proptosis, progressive periorbital
swelling, diplopia, and blindness (2,4–18).
 | Table I.Summary of 19 reported cases of
lacrimal myoepithelial tumors. |
Table I.
Summary of 19 reported cases of
lacrimal myoepithelial tumors.
| Authors, year | Age (years) | Sex | Side | Size (mm) | Variant | Nature | (Refs.) |
|---|
| Heathcote et
al, 1990 | Middle | F | NA | 31×25×17 | Spindle | Benign | (2) |
| Herrera, 1990 | 68 | M | Left | 35×30×25 | Epithelioid | Malignant | (4) |
| Font et al,
1992 | 23 | F | Left | 30×25×17 | Spindle | Benign | (5) |
| Ni et al,
1992 | NA | NA | NA | NA | Spindle | Benign | (6) |
|
| NA | NA | NA | NA | Spindle | Benign |
|
| Grossniklaus et
al, 1997 | 76 | F | Right | 9×9×9 | Mixed | Benign | (7) |
| Okudela et al,
2000 | 34 | M | Right | 25×15×18 | Mixed | Malignant | (8) |
| Iida et al,
2001 | 77 | M | Left | NA | Spindle | Malignant | (9) |
| Bolzoni et al,
2005 | 46 | M | Right | 18×16×16 | Plasmacytoid | Benign | (10) |
| Pasquale et
al, 2005 | 57 | F | Left | 35×25×15 | Epithelioid | Benign | (11) |
| Wiwatwongwana et
al, 2009 | 84 | M | Left | 32×26×22 | Epithelioid | Malignant | (12) |
| Weis et al,
2009 | NA | NA | NA | NA | Mixed | Benign | (13) |
|
| NA | NA | NA | NA | Epithelioid | Malignant |
|
| Argyris et al,
2013 | 39 | F | Left | 16×11×13 | Epithelioid | Malignant | (14) |
| von Holstein et
al, 2013 | NA | NA | NA | NA | NA | Malignant | (15) |
| Eldesouky et
al, 2014 | NA | NA | NA | NA | NA | Benign | (16) |
| Moret et al,
2014 | 88 | M | Right | 35×17×25 | Spindle | Malignant | (17) |
| Rabade et al,
2014 | 27 | M | Right | 30×20 | Clear cell | Malignant | (18) |
| Present case | 68 | F | Right | 40×40×35 | Epithelioid | Malignant |
|
 | Table II.Clinicopathological characteristic of
19 reported cases of lacrimal myoepithelial tumors. |
Table II.
Clinicopathological characteristic of
19 reported cases of lacrimal myoepithelial tumors.
| Characteristics | Benign | Malignant | P-value |
|---|
| Mean age
(years) | 50.50±22.13 | 60.62±23.84 | 0.495 |
|
| (range, 23–76) | (range, 27–88) |
|
| M:F ratio | 1:4 | 3:1 | 0.049 |
| Right:left
ratio | 1:1 | 1:1 | 0.135 |
| Size (mm) | 24.60±10.78 | 30.43±7.89 | 0.303 |
| Histopathologic
variant |
|
| 0.046 |
|
Epithelioid | 1 | 5 |
|
|
Spindle | 4 | 2 |
|
|
Plasmacytoid | 1 | 0 |
|
|
Clear | 0 | 1 |
|
|
Mixed | 2 | 1 |
|
| NA | 1 | 1 |
|
The imaging procedures such as computed tomography,
and MRI may allow recognition of lacrimal METs. Imaging findings of
MET show vivid enhancing isosignal T1 W and hyper-, intermediate or
even hypointense T2 W (8,10). In the authors' case, the mass shows
typical MRI feature and invades the lateral wall of orbit. This
behavior suggests progression of slow growing malignant tumor.
The diagnosis of MET is based on histopathology and
immunohistochemical studies. The lacrimal MET can easily be
mistaken for variety tumors including atypical meningioma,
leiomyosarcoma, and metastatic amelanotic melanoma. Atypical
meningioma is excluded, as it does not immunohistochemically
express myogenin, muscle-specific actin, and alpha-smooth muscle
actin. Leiomyosarcoma with epithelioid feature does not demonstrate
immunoreactivity for S100 protein, and cytokeratin AE1/AE3.
Metastatic amelanotic melanoma may have a similar histopathology,
but the tumor cells typically show atypia, and usually locate in
the lymphovascular channels as well as there is no evidence of
primary lesion. Negative results of HMB45 immunohistochemical stain
may be helpful in excluding melanoma. Finally the definite
diagnosis is lacrimal MEC.
Histopathologically, MET is classified into four
subtypes composing of solid, trabecular, reticular, and mixed
pattern (3). Five cellular variants
are identified in MET: namely spindle, plasmacytoid, epithelioid,
clear, and mixed cell type (3,12,18).
Benign MET usually shows spindle cellular variant, whereas, MEC
usually shows epithelioid cellular variant (P=0.046). However,
different cell types and architectural patterns may be found within
the same tumor. In fact, most MECs are less monomorphic than benign
MET.
Most METs have benign course, however few reported
patients had malignant nature. Clear criteria for lacrimal MEC have
not been elaborated. On the basis of prior reports, it appears that
lacrimal MET displaying infiltrative, destructive growth, marked
hypercellularity, marked cellular pleomorphism, perineural
invasion, lymphovascular invasion, high mitotic activity or
necrosis should be regarded as indicating neoplasms with malignant
potential (5,8,9). METs
showing p53 expression and mitotic figure more than 7/10HPFs as
well as Ki67 labeling index more than 10% are indicatory for
malignancy (19). The authors
suggest lacrimal MET having a few above parameters should be
considered a tumor of malignant potential. Criteria for malignancy
of lacrimal MET should be used as same as salivary MET until
long-term clinicopathologic outcome data for a larger number of
lacrimal MECs become available. Additional investigations and
long-term follow-up are warranted to clarify the malignant
potential of lacrimal MET.
Malignant tumor can arise either de novo or develop
in a pleomorphic adenoma. Di Palma et al postulated that MEC
has a low-grade malignancy when it arises from a pleomorphic
adenoma, but may play more aggressive growth when it arises de novo
(20). To our knowledge, this is the
first reported case of MEC arising in recurrent pleomorphic adenoma
of the lacrimal gland.
Surgical excision remains the cornerstone of
management of the lacrimal neoplasms (21). Orbital exenteration is indicated
where the lacrimal neoplasm is extensive and the mass has
infiltrated beyond neoplastic capsule (21). Benign lacrimal tumors generally
behave in an indolent manner and generally do not recur after
complete wide surgical excision. However, malignant lacrimal
neoplasms appear to be more aggressive may recur and metastasize.
Close follow-up after wide surgical excision is recommended. The
surgery will be followed by radiotherapy, chemotherapy and
molecularly targeted agents, which classically belong to the
armamentarium of malignant neoplasm (21).
In conclusion, lacrimal MEC should be considered in
the differential diagnosis of lacrimal neoplasm. The application of
immunohistochemical investigation correlating with the clinical
presentation, intraoperative and radiological findings might help
in making the definite diagnosis.
References
|
1
|
Sheldon WH: So-called mixed tumors of the
salivary glands. Arch Pathol. 35:1–20. 1943.
|
|
2
|
Heathcote JG, Hurwitz JJ and Dardick I: A
spindle-cell myoepithelioma of the lacrimal gland. Arch Ophthalmol.
108:1135–1139. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bell D, Di Palma S, Katabi N, Schwartz MR,
Seethala R and Skálová A: Myoepithelial carcinomaWHO Classification
of Head and Neck Tumours. El-Naggar AK, John KC, Chan JRC, Grandis
JR, Takata T and Slootweg PJ: IARC Press; Lyon: pp. 174–175.
2017
|
|
4
|
Herrera GA: Light microscopic,
ultrastructural and immunocytochemical spectrum of malignant
lacrimal and salivary gland tumors, including malignant mixed
tumors. Pathobiology. 58:312–322. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Font RL and Garner A: Myoepithelioma of
the lacrimal gland: Report of a case with spindle cell morphology.
Br J Ophthalmol. 76:634–636. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ni C, Kuo PK and Dryja TP:
Histopathological classification of 272 primary epithelial tumours
of the lacrimal gland. Chin Med J (Engl). 105:481–485.
1992.PubMed/NCBI
|
|
7
|
Grossniklaus HE, Wojno TH, Wilson MW and
Someren AO: Myoepithelioma of the lacrimal gland. Arch Ophthalmol.
115:1588–1590. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okudela K, Ito T, Iida MI, Kameda Y,
Furuno K and Kitamura H: Myoepithelioma of the lacrimal gland:
Report of a case with potentially malignant transformation. Pathol
Int. 50:238–243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iida K, Shikishima K, Okido M, Sato S and
Masuda Y: A case of malignant myoepithelioma in the lacrimal gland.
Nippon Ganka Gakkai Zasshi. 105:42–46. 2001.(In Japanese).
PubMed/NCBI
|
|
10
|
Bolzoni A, Pianta L, Farina D and Nicolai
P: Benign myoepithelioma of the lacrimal gland: Report of a case.
Eur Arch Otorhinolaryngol. 262:186–188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pasquale S, Strianese D, Mansueto G and
Tranfa F: Epithelioid myoepithelioma of lacrimal gland. Virchows
Arch. 446:972005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wiwatwongwana D, Berean KW, Dolman PJ,
Rootman J and White VA: Unusual carcinomas of the lacrimal gland:
Epithelial-myoepithelial carcinoma and myoepithelial carcinoma.
Arch ophthalmol. 127:1054–1056. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weis E, Rootman J, Joly TJ, Berean KW,
Al-Katan HM, Pasternak S, Bonavolontà G, Strianese D, Saeed P,
Feldman KA, et al: Epithelial lacrimal gland tumors: Pathologic
classification and current understanding. Arch Ophthalmol.
127:1016–1028. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Argyris PP, Pambuccian SE, Cayci Z, Singh
C, Tosios KI and Koutlas IG: Lacrimal gland adenoid cystic
carcinoma with high-grade transformation to myoepithelial
carcinoma: Report of a case and review of literature. Head Neck
Pathol. 7:85–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
von Holstein SL, Therkildsen MH, Prause
JU, Stenman G, Siersma VD and Heegaard S: Lacrimal gland lesions in
Denmark between 1974 and 2007. Acta Ophthalmol. 91:349–354. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eldesouky MA, Elbakary MA, Sabik S and
Shareef MM: Lacrimal fossa lesions: A review of 146 cases in Egypt.
Clin Ophthalmol. 8:1603–1609. 2014.PubMed/NCBI
|
|
17
|
Moret A, Tabareau-Delalande F, Joly A, de
Muret A, Goga D and Laure B: Myoepithelial carcinoma of the
lacrimal gland. Rev Stomatol Chir Maxillofac Chir Orale.
115:172–177. 2014.(In French). PubMed/NCBI
|
|
18
|
Rabade NR and Goel NA: Clear cell
myoepithelial carcinoma ex pleomorphic adenoma. Indian J Pathol
Microbiol. 57:456–459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagao T, Sugano I, Ishida Y, Tajima Y,
Matsuzaki O, Konno A, Kondo Y and Nagao K: Salivary gland malignant
myoepithelioma: A clinicopathologic and immunohistochemical study
of ten cases. Cancer. 83:1292–1299. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Palma S and Guzzo M: Malignant
myoepithelioma of salivary glands: Clinicopathological features of
ten cases. Virchows Arch A Pathol Anat Histopathol. 423:389–396.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
von Holstein SL, Coupland SE, Briscoe D,
Le Tourneau C and Heegaard S: Epithelial tumours of the lacrimal
gland: A clinical, histopathological, surgical and oncological
survey. Acta Ophthalmol. 91:195–206. 2013. View Article : Google Scholar : PubMed/NCBI
|