Introduction
The Torricelli-Bernoulli sign (T-B sign) (1) is observed by computed tomography (CT)
when an ulcerated/necrotic submucosal gastrointestinal tumor
releases air bubbles into the intestinal lumen (2). Gastrointestinal stromal tumors (GISTs)
originate from Cajal cells (intestinal pacemaker cells) in the
interstitial tissues of the gastrointestinal wall. While more than
90% of GISTs express the receptor tyrosine kinase c-kit on the cell
surface, some tumors are negative for c-kit (3). Immunohistochemistry is needed to
confirm the diagnosis of GIST (4).
Approximately 70% of these tumors occur in the stomach, followed by
20–30% in the small intestine and 10% elsewhere in the intestines
(5,6). There are no characteristic
symptoms/signs of GIST, but it commonly causes fever, abdominal
pain, abdominal discomfort, intestinal bleeding, or intestinal
obstruction (7–11). We encountered a patient who had
perforated intestinal GIST associated with the T-B sign. We
performed a PubMed search for previous reports about perforated
intestinal GIST used the keywords ‘perforated GIST’, ‘free air’,
and ‘small intestine’, and we identified 6 articles (12–17). One
of these articles reported a mass with an air/fluid level, but did
not mention the T-B sign (17). We
also conducted a search including the term ‘T-B sign’, but could
not identify any additional literature. Accordingly, this rare case
of perforated GIST with the T-B sign is reported here.
Case report
Written informed consent was obtained from the
patient and his family for publication of this case report and the
accompanying images. The patient was a 75-year-old man with a
history of appendectomy, gastric ulcer, and old pulmonary
tuberculosis. He was being treated for diabetes at a local clinic.
In early March 2017, he developed acute abdominal pain and was
referred to Tokai University Oiso hospital. At the initial
examination, his height was 170 cm, weight was 65 kg, pulse rate
was 115/min (sinus rhythm), blood pressure was 115/85 mmHg, and
body temperature was 36.4°C. Conjunctival pallor suggesting anemia
was observed, but there was no jaundice. Rebound tenderness was
detected, particularly in the lower right abdomen. Due to
exacerbation of pain, acute abdomen was diagnosed. Laboratory tests
revealed a white blood cell (WBC) count of 19000/µl and elevation
of C-reactive protein (CRP) to 17.95 mg/dl. Hb was 8.8 g/dl,
indicating anemia. Blood glucose was 191 mg/dl and HbA1c was 8.3%.
CT scans revealed an intestinal mass lesion with so called T-B sign
which showed mass with an air/fluid level and free gas in the lower
right abdomen, findings corresponding to the T-B sign, as well as
another intestinal tumor with adjacent nodules that were considered
to represent peritoneal dissemination (Fig. 1). Based on these findings, a
perforated intestinal tumor with dissemination was diagnosed.
Emergency surgery was conducted via a midline lower abdominal
incision. A small amount of purulent opaque ascites was observed in
the lower right abdomen. There was an ileal tumor located about 30
cm from the ileocecal junction showing adhesion to the greater
omentum and peritoneal dissemination was observed in the
surrounding area. After partial resection of the small intestine,
part of the greater omentum was also resected and peritoneal
sampling was conducted. The postoperative clinical course was
uncomplicated and the patient was discharged at 11 days after
surgery. Examination of the resected specimen revealed an ileal
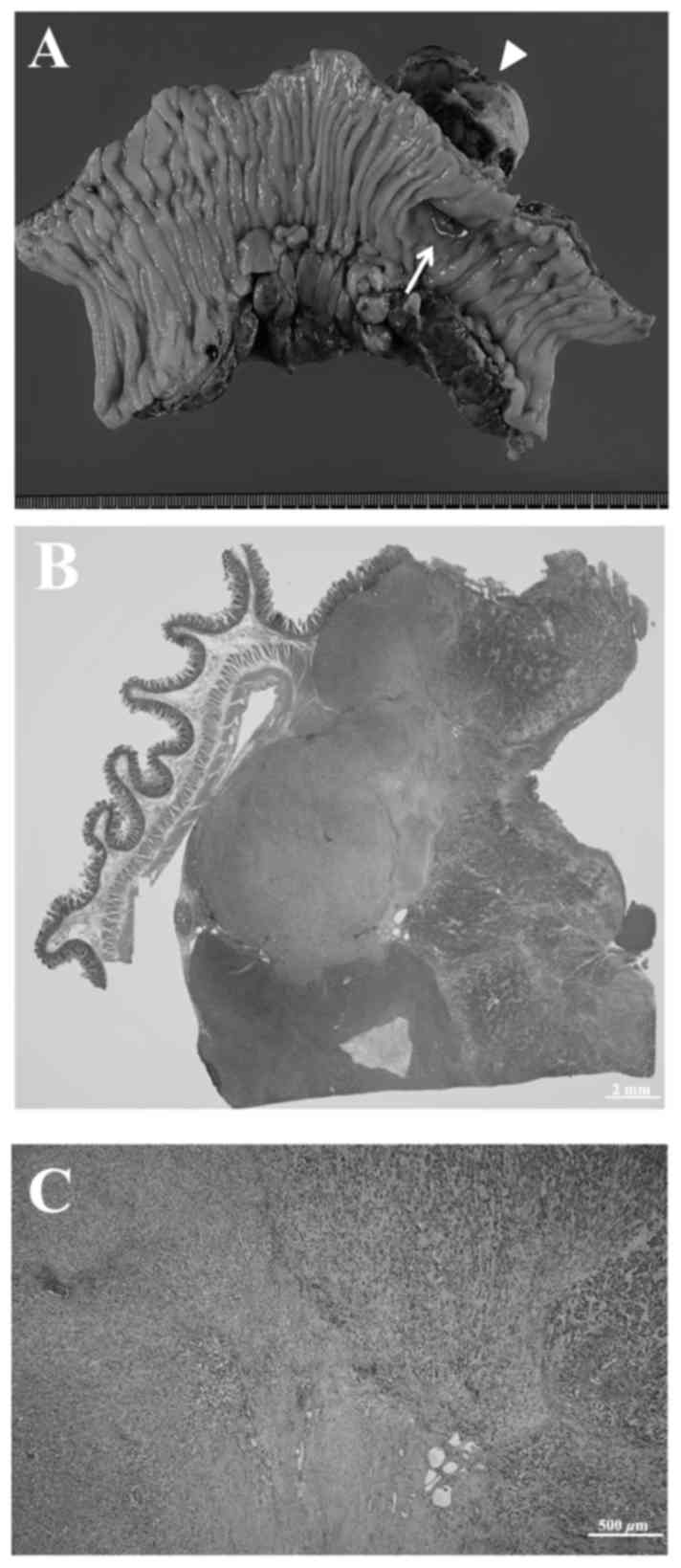
tumor with extramural growth and a diameter of 55 mm. Whitish
exudate was present on the serosal surface of the tumor and there
was a deep necrotic ulcer at the luminal surface (Fig. 2A). Multiple paraffin blocks were
prepared and processed in a routine manner. Then sections were cut
and were stained with hematoxylin and eosin or for
immunohistochemistry. Histopathological examination revealed a
submucosal tumor composed of proliferating spindle-shaped cells
with a mucoid interstitium. The tumor cells were epithelioid-like
and had strongly eosinophilic cytoplasm, with 5 mitotic figures per
50 high power fields. The serosal surface of the tumor was coated
with fibrin and inflammatory or necrotic exudate. These findings
were consistent with tumor perforation (Fig. 2B and C). The intraperitoneal masses
had similar cellular architecture to the primary tumor and were
diagnosed as peritoneal dissemination. Immunohistochemistry was
positive for CD34, c-kit, DOG1, and Ki-67 (MIB-1) in 15% of the
tumor cells, while SMA, S100, and desmin were negative. The tumor
was high risk according to the Miettinenn
classification/modified-Fletcher classification. According to the
Union for International Cancer Control (UICC)/TNM classification
(7th edition, 2009), the diagnosis was pT3, pN1, M0, Stage IV.
CT scanning and abdominal ultrasound were conducted
at one month postoperatively, with ultrasound detecting 3 hepatic
metastases <10 mm in diameter that were not confirmed by CT.
Oral administration of the tyrosine kinase inhibitor imatinib
resulted in disappearance of the peritoneal lesions, while cystic
degeneration of the metastatic liver tumors was seen on both CT and
abdominal ultrasound. Treatment is ongoing at 9 months after
surgery and there has been no evidence of recurrence.
Discussion
The Torricelli-Bernoulli (T-B) sign (1) is based on Torricelli's law proposed by
Evangelista Torricelli (18) and the
Bernoulli principle proposed by Daiel Bernoulli (19). This sign occurs when internal
necrosis of a submucosal tumor (leiomyosarcoma or GIST) is
associated with ulceration into the intestinal lumen, releasing a
stream of air bubbles that can be visualized by diagnostic imaging
(2). It is rare for an intestinal
GIST ≤5 cm in diameter to develop ulceration, and it has been
reported that the T-B sign generally occurs in patients with tumors
≥6 cm in size (20). Our patient's
tumor was only 5.5 cm in diameter, but the T-B sign was still
observed after it underwent necrosis.
While CT is most frequently used for preoperative
imaging in patients with intestinal tumors (21), MRI is also useful for the detection
of rectal GIST and liver metastasis (22). To confirm the diagnosis of GIST,
immunohistochemistry should be performed on a tumor specimen to
detect c-kit (a receptor tyrosine kinase protein encoded by kit)
and CD34, as well as using the monoclonal antibody DOG1 to stain
chloride ion channel protein, with the positive rate being ≥80–90%
for these markers (3,23). However, our patient had acute abdomen
and required emergency surgery, so biopsy of the lesion could not
be performed for diagnosis. Our patient's tumor showed positive
immunostaining for c-kit, CD34, and DOG1, confirming the diagnosis
of intestinal GIST (22).
GIST is considered to originate from Cajal cells in
the interstitial tissue of the gastrointestinal wall, which are
pacemaker cells that regulate intestinal motility (4). Approximately 25–35% of GISTs arise in
the small intestine, while the most frequent site is the stomach,
accounting for 60–70%. The colon, rectum, and esophagus are
uncommon sites for GIST (<10% each) (8). Small GISTs ≤2 cm in diameter are
usually asymptomatic and are generally found incidentally by
imaging examinations or during surgery for other diseases. Most of
these small tumors are benign (8)
There are no characteristic signs of intestinal GIST (22), but patients frequently develop fever,
abdominal pain, and abdominal discomfort. Other symptoms include
intestinal bleeding, fatigue related to anemia, and respiratory
distress (8–11). At diagnosis, ~50% of patients already
have liver metastasis and peritoneal dissemination (8,24,25). The
first-line treatment for GIST is surgery, if the tumor is
resectable (26,27). Our patient presented with tumor
perforation and acute abdomen, so emergency surgery with small
bowel resection was required. However, peritoneal dissemination was
observed during surgery (mainly in the lower right abdomen) and
liver metastases were detected by postoperative imaging.
Administration of imatinib, a first-generation
tyrosine kinase inhibitor was initiated after surgery (28). Follow-up imaging after initiation of
imatinib therapy revealed cystic degeneration of liver metastases
and disappearance of the peritoneal lesions, suggesting that this
treatment was effective. In patients with severe adverse reactions
to imatinib or those who show an insufficient response,
second-generation tyrosine kinase inhibitors like sunitinib or
regorafenib have been reported to be effective (29–31).
Preoperative diagnosis of intestinal GIST is quite
challenging and immunohistochemical staining is required for
confirmation. However, detection of the T-B sign on CT may be a
useful diagnostic clue in patients with a perforated submucosal
tumor.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets obtained and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
TT and TN conceived and designed this case report.
TT wrote the initial draft of the report. MT, TO and LFC acquired
the data in the surgical field. TO and KM acquired the data in the
diagnostic imaging. HS supervised the study and critically reviewed
the manuscript. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Written informed consent for surgery was obtained
from the patient.
Patient consent for publication
Written informed consent for publication of the
present report was obtained from the patient.
Competing interests
The authors declare that they have no conflicts of
interest.
Glossary
Abbreviations
Abbreviations:
|
GIST
|
gastrointestinal stromal tumor
|
|
T-B sign
|
Torricelli-Bernoulli sign
|
|
CT
|
computed tomography
|
References
|
1
|
Fortman BJ: Torricelli-Bernoulli sign in
an ulcerating gastric leiomyosarcoma. AJR Am J Roentgenol.
173:199–200. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sureka B, Bansal K and Arora A:
Torricelli-Bernoulli Sign in gastrointestinal stromal tumor. AJR Am
J Roentgenol. 205:W468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirota S, Isozaki K, Moriyama Y, Hashimoto
K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M,
et al: Gain-of-function mutations of c-kit in human
gastrointestinal stromal tumors. Science. 279:577–580. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kindblom LG, Remotti HE, Aldenborg F and
Meis-Kindblom JM: Gastrointestinal pacemaker cell tumor (GIPACT):
Gastrointestinal stromal tumors show phenotypic characteristics of
the interstitial cells of Cajal. Am J Pathol. 152:1259–1269.
1998.PubMed/NCBI
|
|
5
|
Miettinen M, Sarlomo-Rikala M and Lasota
J: Gastrointestinal stromal tumors: Recent advances in
understanding of their biology. Hum Pathol. 30:1213–1220. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Graadt van Roggen JF, van Velthuysen MLF
and Hogendoorn PCW: The histopathological differential diagnosis of
gastrointestinal stromal tumours. J Clin Pathol. 54:96–102. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ludwig DJ and Traverso LW: Gut stromal
tumors and their clinical behavior. Am J Surg. 173:390–394. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Connolly EM, Gaffney E and Reynolds JV:
Gastrointestinal stromal tumours. Br J Surg. 90:1178–1186. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors-definition, clinical, histological,
immunohistochemical, and molecular genetic features and
differential diagnosis. Virchows Arch. 438:1–12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ueyama T, Guo KJ, Hashimoto H, Daimaru Y
and Enjoji M: A clinicopathologic and immunohistochemical study of
gastrointestinal stromal tumors. Cancer. 69:947–955. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baheti AD, Shinagare AB, O'Neill AC,
Krajewski KM, Hornick JL, George S, Ramaiya NH and Tirumani SH:
MDCT and clinicopathological features of small bowel
gastrointestinal stromal tumours in 102 patients: a single
institute experience. Br J Radiol. 88:201500852015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Efremidou EI, Liratzopoulos N,
Papageorgiou MS, Romanidis K, Manolas KJ and Minopoulos GJ:
Perforated GIST of the small intestine as a rare cause of acute
abdomen: Surgical treatment and adjuvant therapy. Case report. J
Gastrointestin Liver Dis. 15:297–299. 2006.PubMed/NCBI
|
|
13
|
Misawa S, Takeda M, Sakamoto H, Kirii Y,
Ota H and Takagi H: Spontaneous rupture of a giant gastrointestinal
stromal tumor of the jejunum: A case report and literature review.
World J Surg Oncol. 12:1532014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shoji M, Yoshimitsu Y, Maeda T, Sakuma H,
Nakai M and Ueda H: Perforated gastrointestinal stromal tumor
(GIST) in a true jejunal diverticulum in adulthood: Report of a
case. Surg Today. 44:2180–2186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khuri S, Gilshtein H, Darawshy AA, Bahouth
H and Kluger Y: Primary small bowel GIST presenting as a
life-threatening emergency: A report of two cases. Case Rep Surg.
2017:18142542017.PubMed/NCBI
|
|
16
|
Alessiani M, Gianola M, Rossi S, Perfetti
V, Serra P, Zelaschi D, Magnani E and Cobianchi L: Peritonitis
secondary to spontaneous perforation of a primary gastrointestinal
stromal tumour of the small intestine: A case report and a
literature review. Int J Surg Case Rep. 6C:1–62. 2015.PubMed/NCBI
|
|
17
|
Sato K, Tazawa H, Fujisaki S, Fukuhara S,
Imaoka K, Hirata Y, Takahashi M, Fukuda S, Kuga Y, Nishida T, et
al: Acute diffuse peritonitis due to spontaneous rupture of a
primary gastrointestinal stromal tumor of the jejunum: A case
report. Int J Surg Case Rep. 39:288–292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Driver R: Toricelli's law: An ideal
example of an elementary ODE. Am Math Mon. 105:453–455. 1998.
View Article : Google Scholar
|
|
19
|
Stedman TL: Stedman's Medical Dictionary.
25th Edition. Williams & Wkjins; Baltimore: pp. 1821990
|
|
20
|
Nishida T, Kumano S, Sugiura T, Ikushima
H, Nishikawa K, Ito T and Matsuda H: Multidetector CT of high-risk
patients with occult gastrointestinal stromal tumors. AJR Am J
Roentgenol. 180:185–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amano M, Okuda T, Amano Y, Tajiri T and
Kumazaki T: Magnetic resonance imaging of gastrointestinal stromal
tumor in the abdomen and pelvis. Clin Imaging. 30:127–131. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grover S, Ashley SW and Raut CP: Small
intestine gastrointestinal stromal tumors. Curr Opin Gastroenterol.
28:113–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
West RB, Corless CL, Chen X, Rubin BP,
Subramanian S, Montgomery K, Zhu S, Ball CA, Nielsen TO, Patel R,
et al: The novel marker, DOG1, is expressed ubiquitously in
gastrointestinal stromal tumors irrespective of KIT or PDGFRA
mutation status. Am J Pathol. 165:107–113. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
DeMatteo RP, Lewis JJ, Leung D, Mudan SS,
Woodruff JM and Brennan MF: Two hundred gastrointestinal stromal
tumors: Recurrence patterns and prognostic factors for survival.
Ann Surg. 231:51–58. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Emory TS, Sobin LH, Lukes L, Lee DH and
O'Leary TJ: Prognosis of gastrointestinal smooth-muscle (stromal)
tumors: Dependence on anatomic site. Am J Surg Pathol. 23:82–87.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Demetri GD, von Mehren M, Antonescu CR,
Dematteo RP, Ganjoo KN, Maki RG, Pisters PWT, Raut CP, Riedel RF,
Schuetze S, Sundar HM, Trent JC and Wayne JD: NCCN Task Force
report: update on the management of patients with gastrointestinal
stromal tumors. J Natl Compr Canc Netw. 8 suppl 2:S1–S41; quiz
S42-S44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
ESMO/European Sarcoma Network Working
Group, . Gastrointestinal stromal tumors: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol.
23:vii49–vii55. 2012.PubMed/NCBI
|
|
28
|
Demetri GD, von Mehren M, Blanke CD, Van
den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA,
Singer S, Janicek M, et al: Efficacy and safety of imatinib
mesylate in advanced gastrointestinal stromal tumors. N Engl J Med.
347:472–480. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Demetri GD, van Oosterom AT, Garrett CR,
Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich
MC, Morgan JA, et al: Efficacy and safety of sunitinib in patients
with advanced gastrointestinal stromal tumour after failure of
imatinib: A randomised controlled trial. Lancet. 368:1329–1338.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wilhelm SM, Dumas J, Adnane L, Lynch M,
Carter CA, Schütz G, Thierauch KH and Zopf D: Regorafenib (BAY
73–4506): A new oral multikinase inhibitor of angiogenic, stromal
and oncogenic receptor tyrosine kinases with potent preclinical
antitumor activity. Int J Cancer. 129:245–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kajiura S, Hosokawa A, Nanjyo S, Nakada N,
Ando T and Sugiyama T: A case of a gastrointestinal stromal tumor
of the rectum effectively treated with continuously-administered
regorafenib after failure of imatinib and sunitinib. Nihon
Shokakibyo Gakkai Zasshi. 113:655–661. 2016.(In Japanese).
PubMed/NCBI
|